Designing Nonconventional Luminescent Materials with Efficient Emission in Dilute Solutions via Modulation of Dynamic Hydrogen Bonds
Abstract
1. Introduction
2. Results
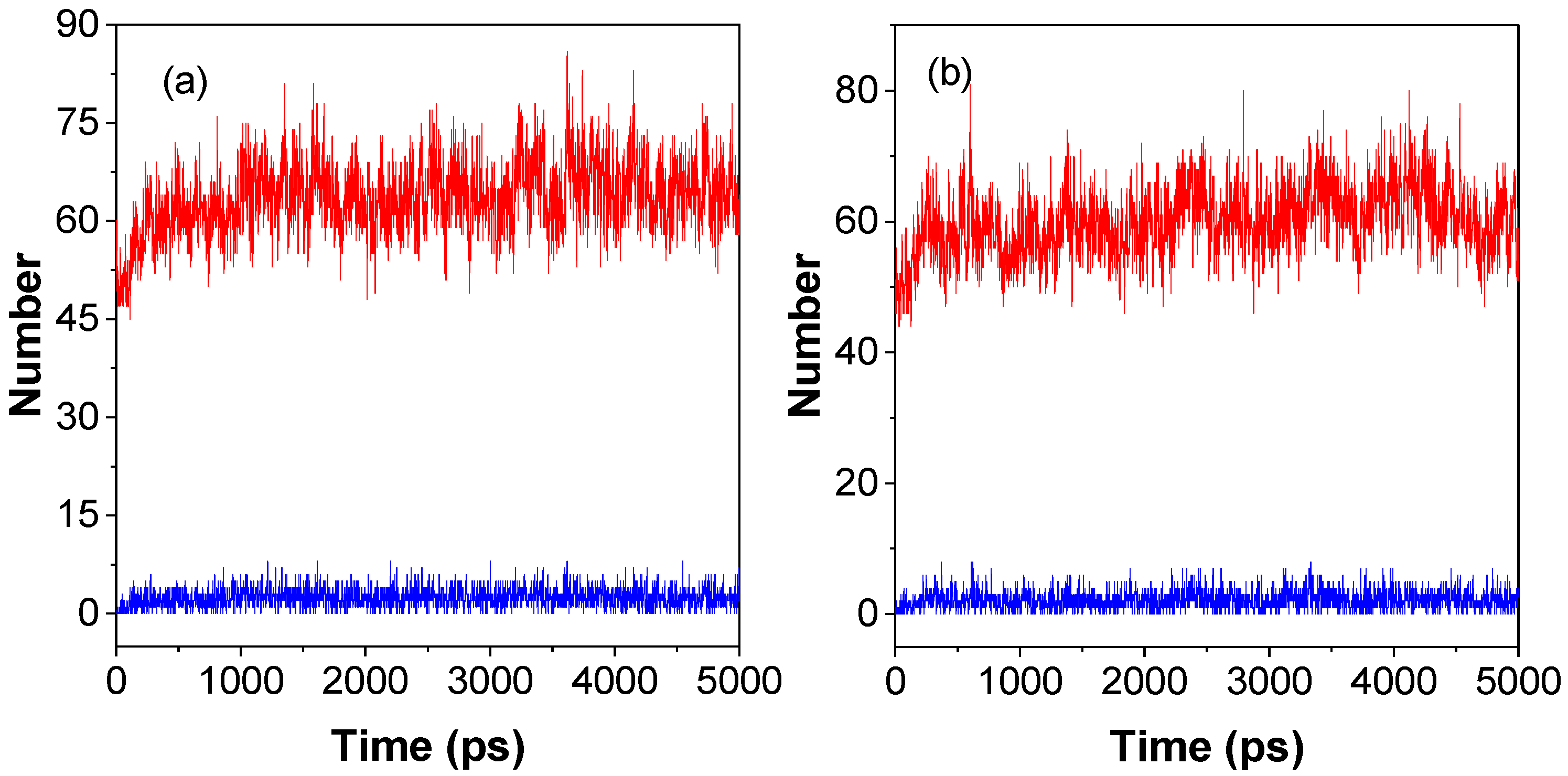
2.1. Molecular Dynamics Simulation
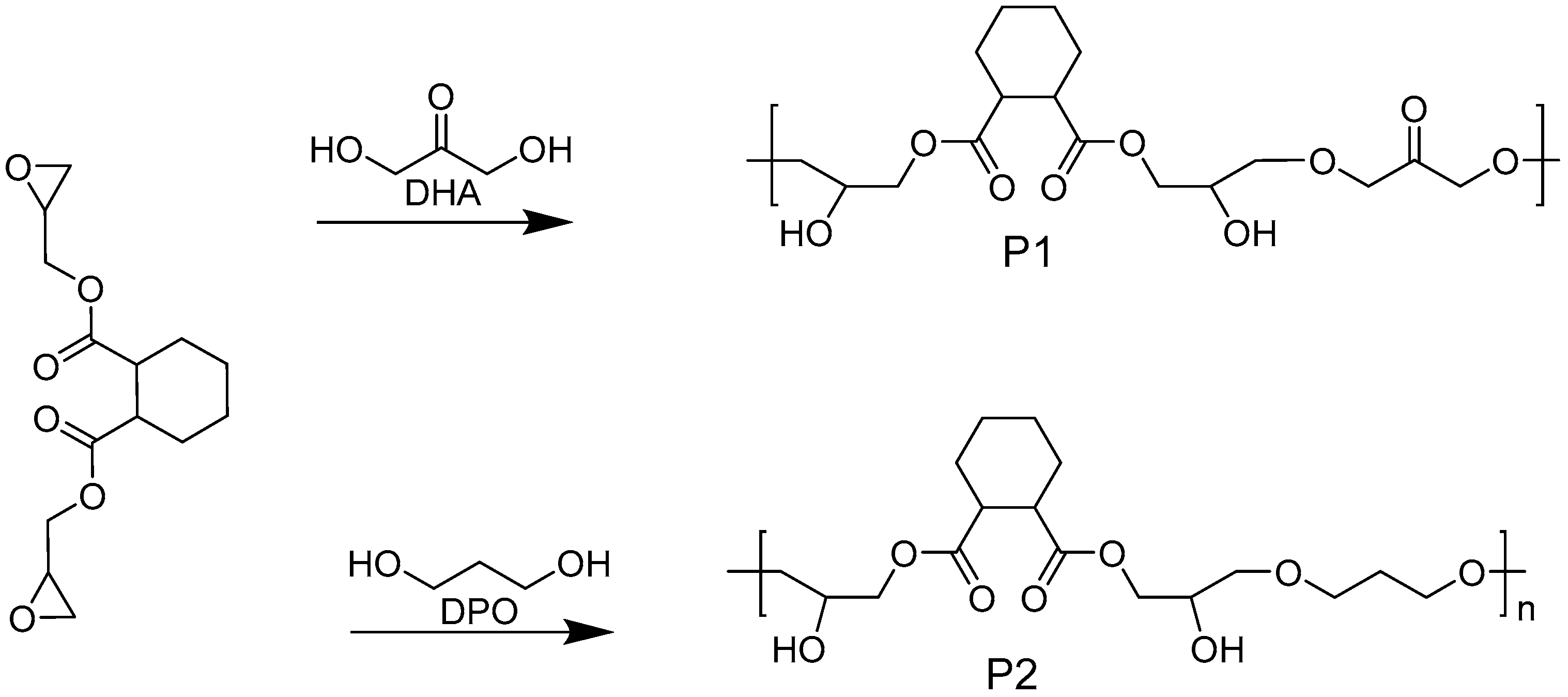
2.2. Synthesis
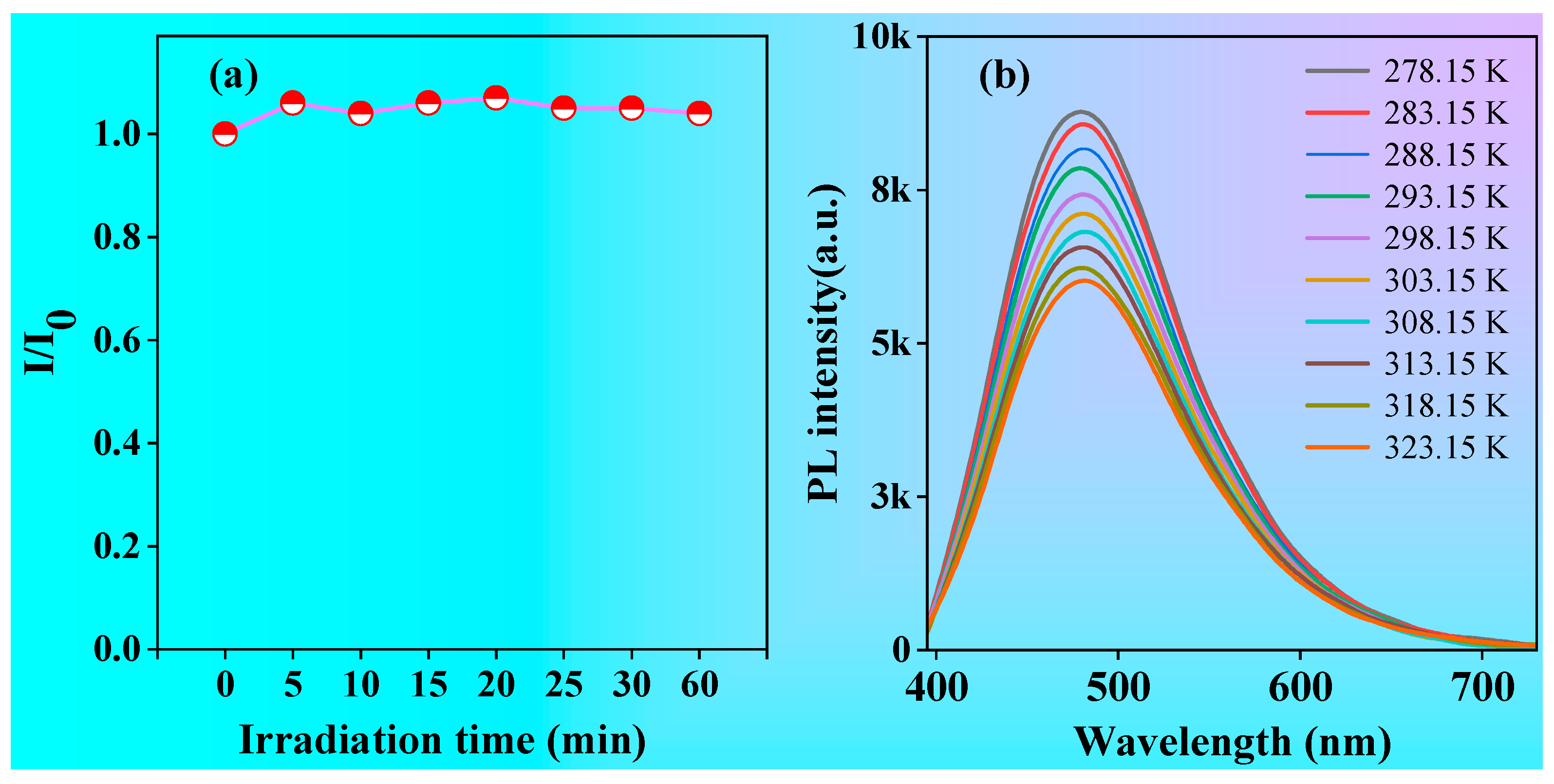
2.3. Optical Properties of P1 and P2 in Solution
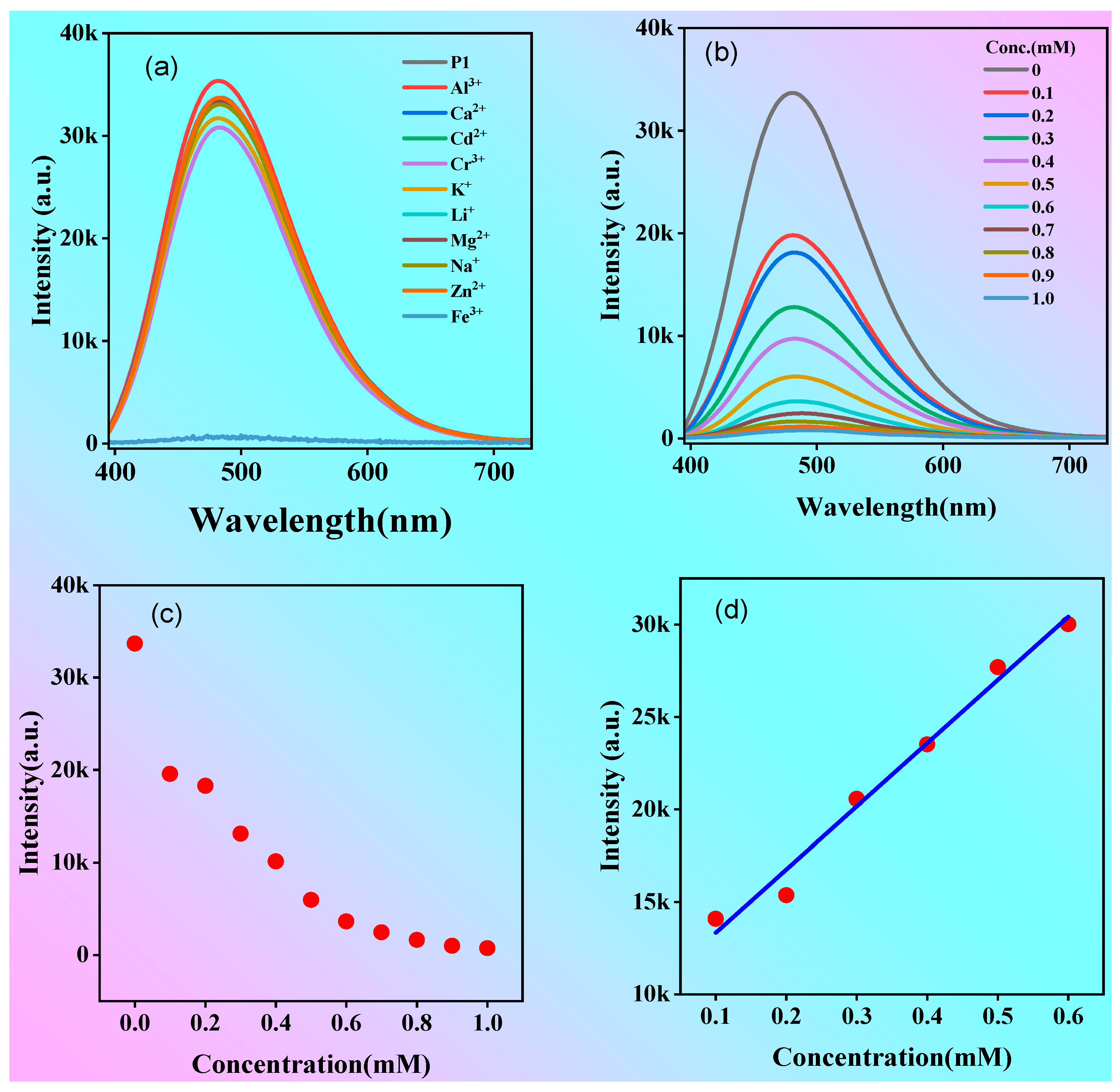
2.4. Polymer P1 for Iron Ion Detection
2.5. Cell Imaging Studies
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tang, M.-C.; Chan, M.-Y.; Yam, V.W.-W. Molecular Design of Luminescent Gold (III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials: Focus Review. Chem. Rev. 2021, 121, 7249–7279. [Google Scholar] [CrossRef]
- Bryden, M.A.; Zysman-Colman, E. Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 2021, 50, 7587–7680. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Li, X.; Shen, S.; Zhang, C.; Liu, M.; Lu, J.; Zhu, L. Small-molecule based thermally activated delayed fluorescence materials with dual-emission characteristics. Sci. China Chem. 2021, 64, 534–546. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-based AIE-active probes for sensing applications. ACS Appl. Mater. Interfaces 2017, 10, 12189–12216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, X.; Chen, Q.; Zeng, G.; Wu, Y.; Dong, X.; Zhang, G.; Li, Y.; Fan, X.; Wang, J. A new tetraphenylethylene based AIE probe for light-up and discriminatory detection of Cys over Hcy and GSH. Sens. Actuators B Chem. 2017, 252, 712–716. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Wu, H.; Song, S.; De Martino, M.T.; Pijpers, I.A.; Friedrich, H.; Abdelmohsen, L.K.; Williams, D.S.; van Hest, J.C. Photoactivated nanomotors via aggregation induced emission for enhanced phototherapy. Nat. Commun. 2021, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Kang, M.; Wu, Q.; Zhang, Z.; Wu, Y.; Li, C.; Li, K.; Wang, L.; Wang, D.; Tang, B.Z. Zwitterionic AIEgens: Rational Molecular Design for NIR-II Fluorescence Imaging-Guided Synergistic Phototherapy. Adv. Funct. Mater. 2021, 31, 2007026. [Google Scholar] [CrossRef]
- Feng, L.; Li, C.; Liu, L.; Wang, Z.; Chen, Z.; Yu, J.; Ji, W.; Jiang, G.; Zhang, P.; Wang, J. Acceptor planarization and donor rotation: A facile strategy for realizing synergistic cancer phototherapy via type I PDT and PTT. ACS Nano 2022, 16, 4162–4174. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R.; Wang, Z.; Zhou, Y.; Shen, Q.; Ji, W.; Dang, D.; Meng, L.; Tang, B.Z. Recent advances in luminescent materials for super-resolution imaging via stimulated emission depletion nanoscopy. Chem. Soc. Rev. 2021, 50, 667–690. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, Y.; Tian, H.; Yang, L.; Zhang, H.; Song, X.; Foley, J.W. Ratiometric Fluorescent Probe for Lysosomal pH Measurement and Imaging in Living Cells Using Single-Wavelength Excitation. Anal. Chem. 2017, 89, 7038–7045. [Google Scholar] [CrossRef]
- Zou, L.; Guo, S.; Lv, H.; Chen, F.; Wei, L.; Gong, Y.; Liu, Y.; Wei, C. Molecular design for organic luminogens with efficient emission in solution and solid-state. Dyes Pigm. 2022, 198, 109958. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef]
- Patel, R.; Zaveri, P.; Mukherjee, A.; Agarwal, P.K.; More, P.; Munshi, N.S. Development of fluorescent protein-based biosensing strains: A new tool for the detection of aromatic hydrocarbon pollutants in the environment. Ecotoxicol. Environ. Saf. 2019, 182, 109450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Z.; McGonigal, P.R.; Ye, R.; Liu, S.; Lam, J.W.Y.; Kwok, R.T.K.; Yuan, W.Z.; Xie, J.; Rogach, A.L.; et al. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292. [Google Scholar] [CrossRef]
- Tang, S.; Yang, T.; Zhao, Z.; Zhu, T.; Zhang, Q.; Hou, W.; Yuan, W.Z. Nonconventional luminophores: Characteristics, advancements and perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-traditional intrinsic luminescence: Inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Chen, X.; Luo, W.; Ma, H.; Peng, Q.; Yuan, W.Z.; Zhang, Y. Prevalent intrinsic emission from nonaromatic amino acids and poly(amino acids). Sci. China Chem. 2018, 61, 351–359. [Google Scholar] [CrossRef]
- Liao, P.; Huang, J.; Yan, Y.; Tang, B.Z. Clusterization-triggered emission (CTE): One for all, all for one. Mater. Chem. Front. 2021, 5, 6693–6717. [Google Scholar] [CrossRef]
- Zhang Yuan, W.; Zhang, Y. Nonconventional macromolecular luminogens with aggregation-induced emission characteristics. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 560–574. [Google Scholar] [CrossRef]
- Bauri, K.; Saha, B.; Banerjee, A.; De, P. Recent advances in the development and applications of nonconventional luminescent polymers. Polym. Chem. 2020, 11, 7293–7315. [Google Scholar] [CrossRef]
- Du, L.-L.; Jiang, B.-L.; Chen, X.-H.; Wang, Y.-Z.; Zou, L.-M.; Liu, Y.-L.; Gong, Y.-Y.; Wei, C.; Yuan, W.-Z. Clustering-triggered Emission of Cellulose and Its Derivatives. Chin. J. Polym. Sci. 2019, 37, 409–415. [Google Scholar] [CrossRef]
- Gong, Y.; Tan, Y.; Mei, J.; Zhang, Y.; Yuan, W.; Zhang, Y.; Sun, J.; Tang, B.Z. Room temperature phosphorescence from natural products: Crystallization matters. Sci. China Chem. 2013, 56, 1178–1182. [Google Scholar] [CrossRef]
- Studzian, M.; Pułaski, Ł.; Tomalia, D.A.; Klajnert-Maculewicz, B. Non-Traditional Intrinsic Luminescence (NTIL): Dynamic Quenching Demonstrates the Presence of Two Distinct Fluorophore Types Associated with NTIL Behavior in Pyrrolidone-Terminated PAMAM Dendrimers. J. Phys. Chem. C 2019, 123, 18007–18016. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Chen, Y.; Shen, Z.; Lahoz, A.; Stiriba, S.-E. Unprecedented Blue Intrinsic Photoluminescence from Hyperbranched and Linear Polyethylenimines: Polymer Architectures and pH-Effects. Macromol. Rapid Commun. 2007, 28, 1404–1409. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, B.; Zhu, C.; Xu, S.; Gong, Y.; Yuan, W.Z.; Zhang, Y. Clustering-Triggered Emission of Nonconjugated Polyacrylonitrile. Small 2016, 12, 6586–6592. [Google Scholar] [CrossRef]
- Chen, F.; Jin, Y.; Luo, J.; Wei, L.; Jiang, B.; Guo, S.; Wei, C.; Gong, Y. Poly-L-aspartic acid based nonconventional luminescent biomacromolecules with efficient emission in dilute solutions for Al3+ detection. Int. J. Biol. Macromol. 2023, 226, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yan, H.; Ding, F.; Bai, T.; Nie, Y.; Zhao, Y.; Feng, W.; Tang, B.Z. Multiring-induced multicolour emission: Hyperbranched polysiloxane with silicon bridge for data encryption. Mater. Chem. Front. 2020, 4, 1375–1382. [Google Scholar] [CrossRef]
- Niu, S.; Yan, H.; Li, S.; Xu, P.; Zhi, X.; Li, T. Bright blue photoluminescence emitted from the novel hyperbranched polysiloxane-containing unconventional chromogens. Macromol. Chem. Phys. 2016, 217, 1185–1190. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, Y.; Yan, H.; Liu, X. High-Efficiency Long-Wavelength Fluorescent Hyperbranched Polysiloxanes: Synthesis, Emission Mechanism, Information Encryption, and Film Preparation. Biomacromolecules 2022, 23, 4617–4628. [Google Scholar] [CrossRef]
- Fang, M.; Yang, J.; Xiang, X.; Xie, Y.; Dong, Y.; Peng, Q.; Li, Q.; Li, Z. Unexpected room-temperature phosphorescence from a non-aromatic, low molecular weight, pure organic molecule through the intermolecular hydrogen bond. Mater. Chem. Front. 2018, 2, 2124–2129. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Huang, Q.; Li, Z.; Tang, B.Z.; Mao, S. Molecular-level enhanced clusterization-triggered emission of nonconventional luminophores in dilute aqueous solution. Nat. Commun. 2023, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Guo, S.; Chen, F.; Jiang, B.; Wei, L.; Gong, Y.; Zhang, B.; Liu, Y.; Wei, C.; Tang, B.Z. Rational design strategies for nonconventional luminogens with efficient and tunable emission in dilute solution. Chem. Eng. J. 2023, 454, 140469. [Google Scholar] [CrossRef]
- Lindahl, E.; Abraham, M.J.; Hess, B.; van der Spoel, D. GROMACS 2020.6 Source Code. Available online: https://manual.gromacs.org/2020.6/download.html (accessed on 10 July 2021).
- Galindo-Murillo, R.; Robertson, J.C.; Zgarbová, M.; Šponer, J.; Otyepka, M.; Jurečka, P.; Cheatham, T.E., III. Assessing the Current State of Amber Force Field Modifications for DNA. J. Chem. Theory Comput. 2016, 12, 4114–4127. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Sobtop: A Tool of Generating Forcefield Parameters and GROMACS Topology File. Sobtop 1.0 (dev3.1). Available online: http://sobereva.com/soft/Sobtop (accessed on 1 August 2022).
- Van der Spoel, D.; van Maaren, P.J.; Larsson, P.; Tîmneanu, N. Thermodynamics of Hydrogen Bonding in Hydrophilic and Hydrophobic Media. J. Phy. Chem. B 2006, 110, 4393–4398. [Google Scholar] [CrossRef]
- Saha, B.; Ruidas, B.; Mete, S.; Mukhopadhyay, C.D.; Bauri, K.; De, P. AIE-active non-conjugated poly(N-vinylcaprolactam) as a fluorescent thermometer for intracellular temperature imaging. Chem. Sci. 2020, 11, 141–147. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, X.; Li, B.; Li, S.; Kong, X.Z. Fluorescence behavior and mechanisms of poly (ethylene glycol) and their applications in Fe3+ and Cr6+ detections, data encryption, and cell imaging. ACS Sustain. Chem. Eng. 2021, 9, 5166–5178. [Google Scholar] [CrossRef]
- Luo, W.; Yu, H.; Liu, Z.; Ou, R.; Guo, C.; Zhang, J. Construction and application of hybrid covalent adaptive network with non-conjugated fluorescence, self-healing and Fe3+ ion sensing. J Mater. Res. Technol. 2022, 19, 1699–1710. [Google Scholar] [CrossRef]
- Bai, L.; Yan, H.; Wang, L.; Bai, T.; Yuan, L.; Zhao, Y.; Feng, W. Supramolecular Hyperbranched Poly (amino ester) s with Homogeneous Electron Delocalization for Multi-Stimuli-Responsive Fluorescence. Macromol. Mater. Eng. 2020, 305, 2000126. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Q.; Li, B.; Li, S.; Kong, X.Z. Fluorescence Behavior and Emission Mechanisms of Poly (ethylene succinamide) and Its Applications in Fe3+ Detection and Data Encryption. Chin. J. Polym. Sci. 2023, 41, 129–142. [Google Scholar] [CrossRef]
- Qin, X.; Wang, S.; Luo, L.; He, G.; Sun, H.; Gong, Y.; Jiang, B.; Wei, C. AIE-active polyanetholesulfonic acid sodium salts with room-temperature phosphorescence characteristics for Fe3+ detection. RSC Adv. 2018, 8, 31231–31236. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, X.; Lin, W. Four-armed functional siloxane enables ratiometric unconventional fluorescence for the detection of ONOO−. Sens. Actuators B Chem. 2021, 331, 129462. [Google Scholar] [CrossRef]
- Wang, Q.; Li, B.; Cao, H.; Jiang, X.; Kong, X.Z. Aliphatic amide salt, a new type of luminogen: Characterization, emission and biological applications. Chem. Eng. J. 2020, 388, 124182. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, H.; Shi, L.; Guan, S.; Huang, W.; Xue, X.; Yang, H.; Jiang, L.; Jiang, B. Self-Catalyzed Hydroxyl-Yne Click Reaction: A Powerful Tool toward Red-Emitting Nontraditional Small Organic Luminogens. Eur. J. Org. Chem. 2023, 26, e202201419. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, S.; Gao, Y.; Cui, X. Seeking brightness from natural resources: Soy protein isolate and its multifunctional applications. Dyes Pigm. 2021, 196, 109768. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Jiang, B.; Gong, Y.; Jin, Y.; He, J.; Xie, H.; Guo, S.; Liu, Y. Designing Nonconventional Luminescent Materials with Efficient Emission in Dilute Solutions via Modulation of Dynamic Hydrogen Bonds. Molecules 2023, 28, 5240. https://doi.org/10.3390/molecules28135240
Tang X, Jiang B, Gong Y, Jin Y, He J, Xie H, Guo S, Liu Y. Designing Nonconventional Luminescent Materials with Efficient Emission in Dilute Solutions via Modulation of Dynamic Hydrogen Bonds. Molecules. 2023; 28(13):5240. https://doi.org/10.3390/molecules28135240
Chicago/Turabian StyleTang, Xuansi, Bingli Jiang, Yongyang Gong, Yuxin Jin, Jiao He, Huihong Xie, Song Guo, and Yuanli Liu. 2023. "Designing Nonconventional Luminescent Materials with Efficient Emission in Dilute Solutions via Modulation of Dynamic Hydrogen Bonds" Molecules 28, no. 13: 5240. https://doi.org/10.3390/molecules28135240
APA StyleTang, X., Jiang, B., Gong, Y., Jin, Y., He, J., Xie, H., Guo, S., & Liu, Y. (2023). Designing Nonconventional Luminescent Materials with Efficient Emission in Dilute Solutions via Modulation of Dynamic Hydrogen Bonds. Molecules, 28(13), 5240. https://doi.org/10.3390/molecules28135240





