Abstract
Ionogels are hybrid materials comprising an ionic liquid confined within a polymer matrix. They have garnered significant interest due to their unique properties, such as high ionic conductivity, mechanical stability, and wide electrochemical stability. These properties make ionogels suitable for various applications, including energy storage devices, sensors, and solar cells. However, optimizing the electrochemical performance of ionogels remains a challenge, as the relationship between specific capacitance, ionic conductivity, and electrolyte solution concentration is yet to be fully understood. In this study, we investigate the impact of electrolyte solution concentration on the electrochemical properties of ionogels to identify the correlation for enhanced performance. Our findings demonstrate a clear relationship between the specific capacitance and ionic conductivity of ionogels, which depends on the availability of mobile ions. The reduced number of ions at low electrolyte solution concentrations leads to decreased ionic conductivity and specific capacitance due to the scarcity of a double layer, constraining charge storage capacity. However, at a 31 vol% electrolyte solution concentration, an ample quantity of ions becomes accessible, resulting in increased ionic conductivity and specific capacitance, reaching maximum values of 58 ± 1.48 μS/cm and 45.74 F/g, respectively. Furthermore, the synthesized ionogel demonstrates a wide electrochemical stability of 3.5 V, enabling diverse practical applications. This study provides valuable insights into determining the optimal electrolyte solution concentration for enhancing ionogel electrochemical performance for energy applications. It highlights the impact of ion pairs and aggregates on ion mobility within ionogels, subsequently affecting their resultant electrochemical properties.
1. Introduction
Stretchable and flexible electronic devices are emerging as new types of intelligent devices, which have grown enormously due to their real-time feedback, flexibility, and portability. Current flexible wearable devices can transmit, analyze and monitor human physiological signals in real time, presenting significant advantages [1]. Traditional sensors made of semiconductors and metals have restricted their application in wearable electronic devices due to poor stretchability and flexibility [2]. The fundamental aspect of the sensor is the establishment of deformation-responsive and conductive materials. The common practice for producing such materials is by incorporating electroactive materials into a polymer matrix, such as silver nanoparticles, graphene and carbon nanotubes [3,4].
Nevertheless, the escalation of ionogels has acted as a new initiative for application in energy [5] and wearable electronic devices. Ionogels are gels materials, whereas ionic liquids are constrained in a 3D crosslinking network through different interactions, forming desirable structures [6]. Ionogels maintain the interesting properties of ionic liquids, including high ionic conductivity, [6] wide electrochemical stability, resistance to evaporation and being highly tunable [7]. Thus, combining outstanding properties has made ionogel an exciting candidate for application in flexible solar cells [5] and other electronic devices [8].
The properties mentioned above result from the presence of ionic liquid within ionogel. Confinement of ionic liquids within ionogel adversely affects the electrochemical properties of ionogel, especially its ionic conductivity. The decrease in ionic conductivity is due to adding polymer, which will increase viscosity, thus prohibiting ion transportation [9]. Numerous techniques have been explored to boost ionogel performance, and one such method involves the addition of lithium salt. Research indicates that incorporating lithium salt can strengthen the electrochemical stability of ionogels. In addition, the dissolved lithium salt facilitates the conduction mechanism by establishing pathways for anions and cations, thereby increasing ionic conductivity [10]. To date, lithium salts have been incorporated in the synthesis of ionogel. For instance, Guillemin et al. studied the impact of adding lithium salts on the performance of an ionogel-based micro-supercapacitor [11]. The addition of lithium salt to ionogel notably boosts the energy density and capacitance of micro-supercapacitors (2.96 μWh cm−2 to 10.22 μWh cm−2), validating their use in high-temperature applications. Cheng et al. examined the impact of different lithium salts (LiOTf, CF3LiO3S and LiTfSI, LiC2NO4F6S2) on ionogel thermal stability and electrochemical performance [12]. The addition of lithium salts initiated the formation of a porous cross-linked structure, which is beneficial for ion transportation; thus, both lithium salt-incorporated ionogels have achieved outstanding ionic conductivity (10−3 S cm−1). Ogawa et al. proposed radical polymerization of ionic liquid in the presence of lithium salt and active material without solvent [13].
It has been demonstrated that the incorporation of lithium salt in ionogel leads to promising electrochemical properties of the ionogel. However, limited study has been conducted on understanding the interaction between lithium salt and ionic liquid. The possible interactions within ionogel are vital as these interactions may influence the performance of the ionogel. Hence, this study aims to comprehend the interaction between lithium salt and ionic liquid and the possible effect on the electrochemical performance of ionogel. In this study, the ionogel is synthesized through a non-aqueous sol-gel route. The 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonate) (EMIM TFSI) is an ionic liquid. Lithium trifluoromethyl sulfonate, LiTf acts as a lithium salt. In contrast, Tetraethyl orthosilicate (TEOS) and Poly(vinylidene fluoride)-co-hexafluoropropylene (PVDF-HFP) are used as silica precursor and polymer matrix, respectively. The interaction between LiTf and EMIM TFSI is investigated by manipulating the concentration of electrolyte solution in the ionogel, which is then analyzed with a series of material and electrochemical characterization studies.
2. Results and Discussion
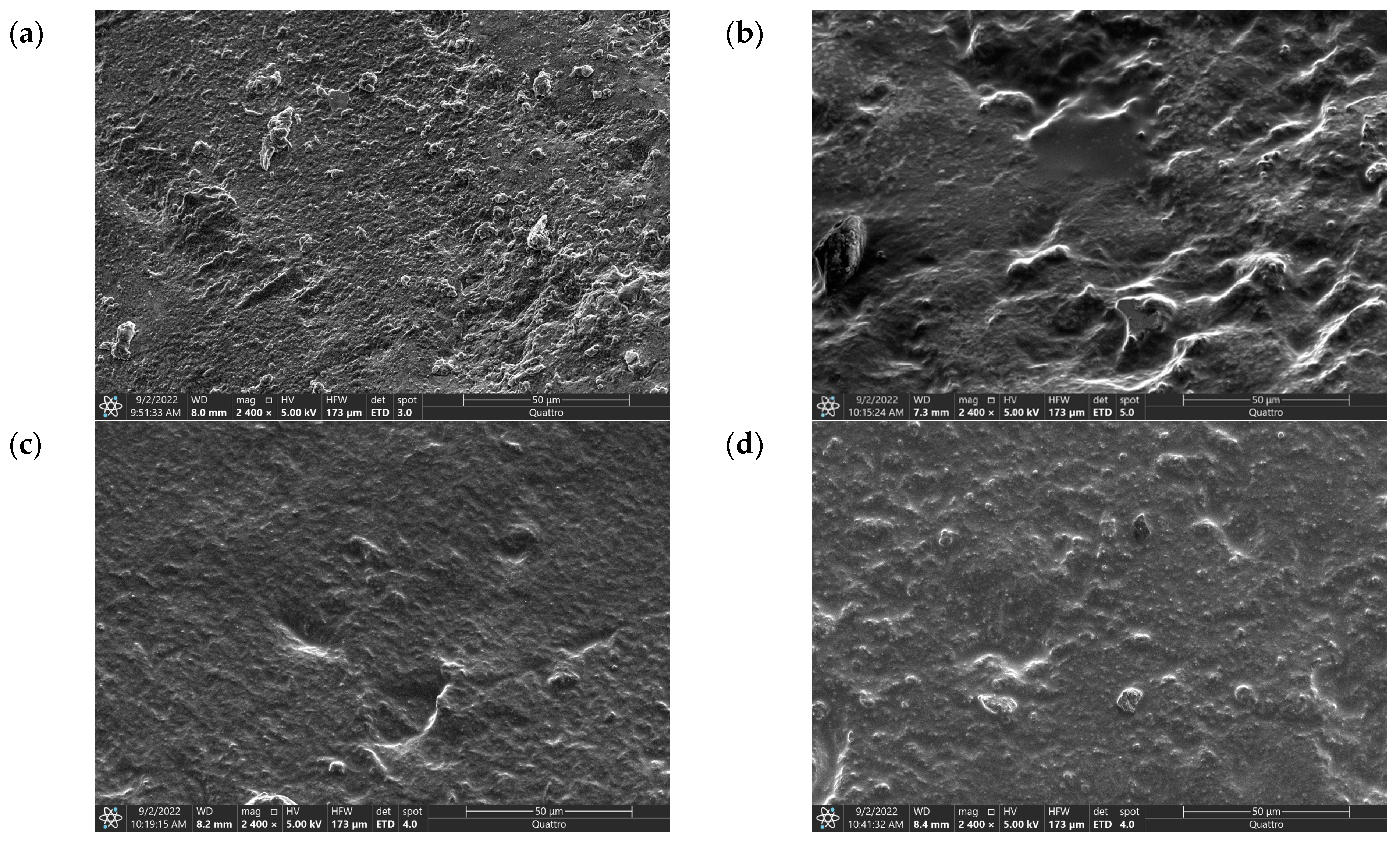
Scanning electron micrographs were used to study the morphology of ionogels. The ionogel was characterized by scanning electron microscopy, and the images were captured at the same magnification for comparison. Figure 1 shows the SEM images of different concentrations of electrolyte solution.
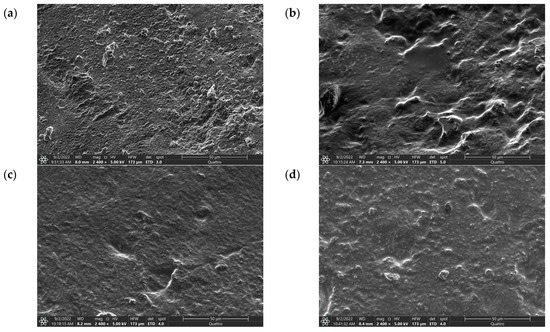
Figure 1.
SEM Images of (a) IG-25, (b) IG-28, (c) IG-31, (d) IG-38.
The SEM image of IG-25 shows a rough and uneven morphology. Besides the rough surfaces, several protrusions can be observed from the morphology of IG-25, while these protrusions exhibit a rough surface. This observation suggests the dominating characteristic of PVDF-HFP at low concentrations of electrolyte solution. As for IG-28, fewer protrusions have been observed on the surface of the ionogel. Instead, the protrusions have a smoother surface. This phenomenon could be due to the electrolyte solution being entrapped within the ionogel system. As the concentration of electrolyte solution increases, this forms an interconnected film within the polymer matrix. The 3D interconnected polymer–electrolyte solution structure implies the complete confinement of the electrolyte solution within the polymer matrix [14]. At 31 vol% of electrolyte solution, the effect of the electrolyte solution is more pronounced, whereby a smoother surface is exhibited on the morphology of ionogel. The presence of a smooth surface is ascribed to the addition of an electrolyte solution that reduces the crystallinity of the ionogel [15]. Furthermore, the smooth surface morphology also suggests a homogenously dispersed network [16]. Thus, it can be anticipated that the ionic conductivity will increase in the initial increment of electrolyte solution due to reduced crystallinity and a homogeneously dispersed network that provides a pathway for ion transportation. However, small aggregates can be observed at a concentration higher than 31 vol%. The formation of aggregates could be due to ionic liquid aggregation, which impedes ionic conductivity. Nevertheless, this will be further investigated and evidenced through XRD and FTIR.
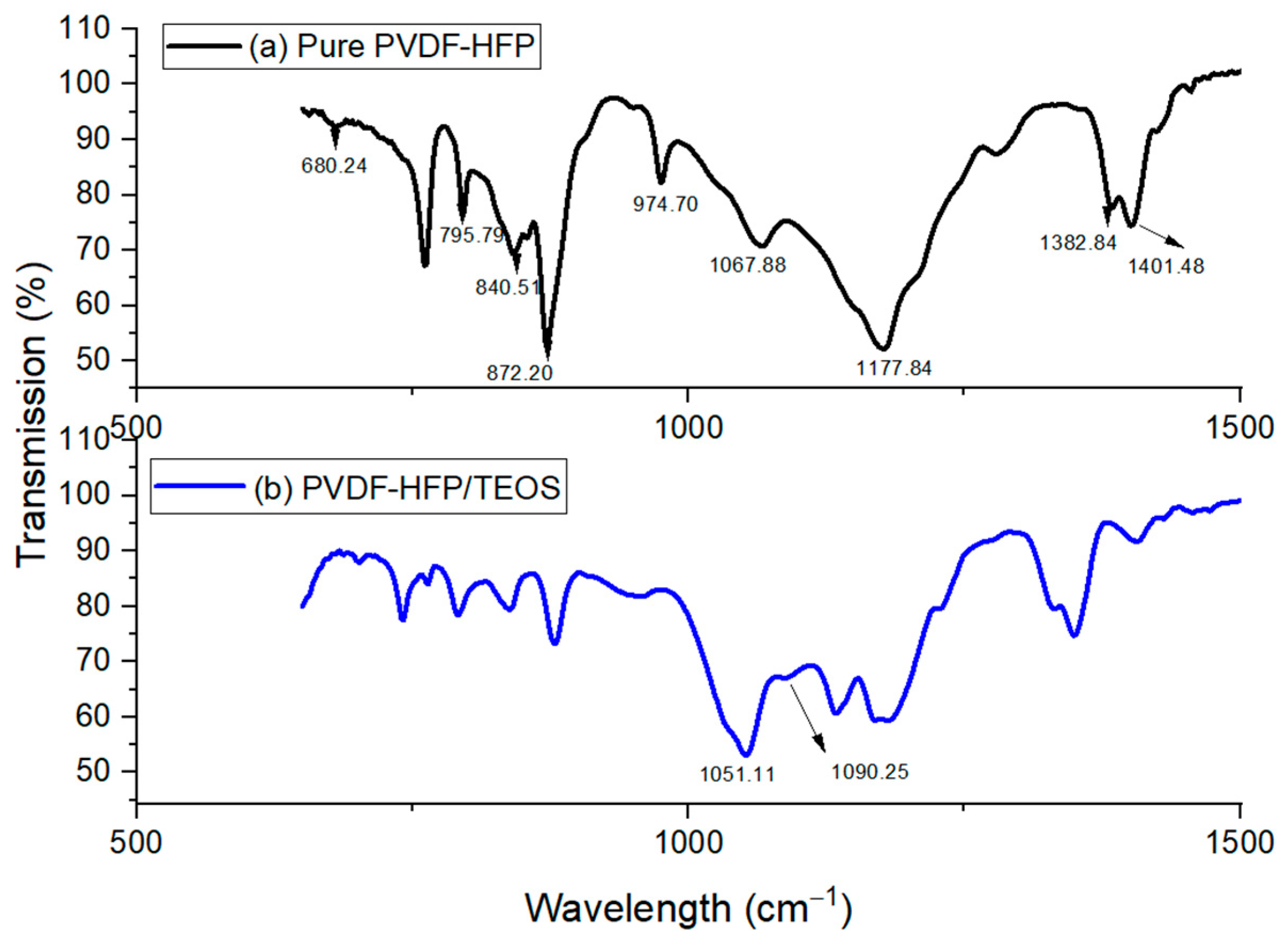
Fourier transform infrared spectroscopy (FTIR) characterization is performed on the samples to study the structural changes of the ionogel, which can be correlated to the electro-mechanical performances. Figure 2 shows the FTIR spectra of pure PVDF-HFP.
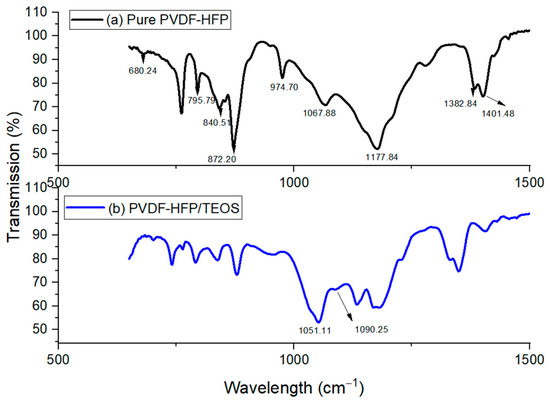
Figure 2.
FTIR Spectra for (a) Pure PVDF-HFP (b) PVDF-HFP/TEOS.
PVDF-HFP is an interesting polymer that displays different polymorphs, such as the alpha () phase, beta () phase and gamma () phase. The classification of phases is based on the chain conformation; for instance, the alpha phase is in the trans-gauche-trans-gauche’ conformation, while the beta phase has an all-trans planar zigzag conformation [17,18]. Efforts have been devoted to understanding the formation of varied phases, which is crucial in their wide extensive application. Meanwhile, this can be evidenced by the FTIR characterization. Bands were observed at 840.51 cm−1, 872.20 cm−1, 1067.88 cm−1, 1177.84 cm−1 and 1401.48 cm−1, respectively. These bands serve as an indication of the beta phase in PVDF-HFP [19]. Furthermore, the bands observed at 741.74 cm−1 and 840.51 cm−1 are accountable for the rocking vibration of CH2 [19]. Regarding vibration modes, the bands observed at 1401.48 cm−1 are due to the symmetric stretching vibrations of CH2. The IR bands confirm the mixed mode of combined CC and CF2 symmetric stretching observed at 872.20 cm−1 and 1067.88 cm−1 [19]. Apart from that, the characteristics of vibrational peaks observed at 1401.48 cm−1, 1177.84 cm−1 and 1067.88 cm−1 were due to the vibration of CF2 groups, swinging vibrations of CH2 and C-C-C bending vibration. In short, different bands are due to various vibrational modes and are also accountable for different phases, with bands observed at 1067.88 cm−1 non-polar crystalline phase. Meanwhile, a weak band is observed at the peak of 840.51 cm−1, which indicates a mixed mode of beta phase () of the PVDF-HFP [20,21].
Figure 2b shows the spectrum for PVDF-HFP with TEOS. A small peak was observed in the spectrum after the addition of TEOS, at a wavelength of 1090.25 cm−1; this is due to asymmetrical vibration stretching of Si-O-Si [20,22]. The appearance of a new peak implies the formation of silica particles within the PVDF-HFP matrix. Concerning the peak shifting, the initial peaks at 1052.97 cm−1 have been found to shift to 1051.11 cm−1, indicating the interaction of Si from SiO2 with the F of the CF3 group from PVDF-HFP. Moreover, the presence of SiO2 is also verified by observing absorption bands 990 cm−1 to 1135 cm−1, where a weak band can be observed near 957.93 cm−1; this indicates the weak bending vibration Si-OH. In addition, Si-O-Si asymmetric stretching vibration can be reported in the spectrum range of 838.65–764.10 cm−1.
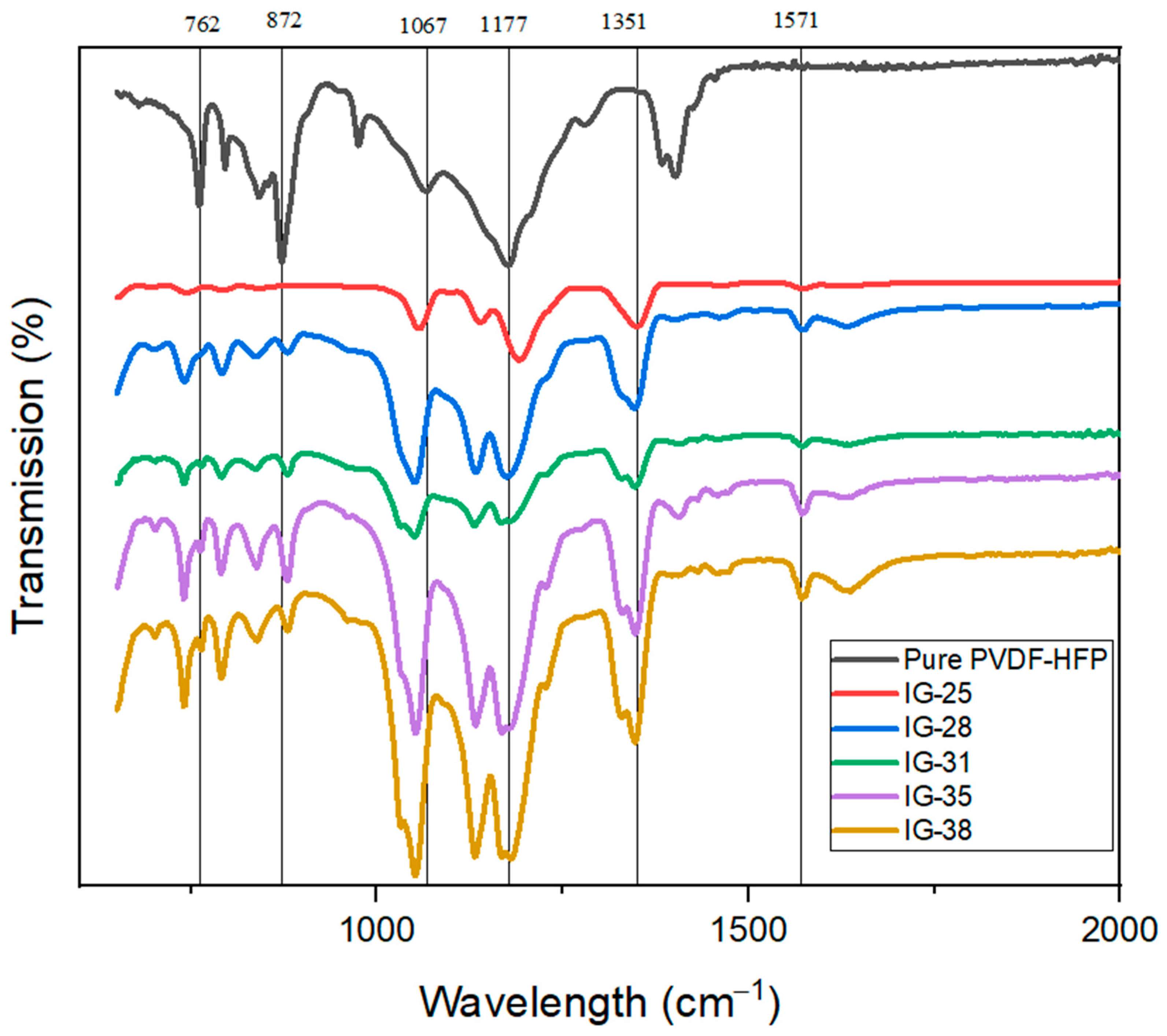
Figure 3 shows the FTIR spectra for pure PVDF-HFP and Ionogel with different concentrations of electrolyte solution. For the FTIR spectra for pure PVDF-HFP, two prominent peaks at 762.24 cm−1 and 872.19 cm−1 indicate the presence of the beta phase. However, after incorporating an electrolyte solution, both peaks exhibit a drastic reduction in intensity. The FTIR graphs show this peak’s intensity. Nevertheless, a slight shift is observed at peak 872.19 cm−1 after the incorporation of electrolyte solution where the peak is shifted to 879.65 cm−1. This phenomenon is consistent with previously reported findings, implying the presence and complexation of salt with the host polymer matrix. Furthermore, a peak at 1067.88 cm−1 was observable for pure PVDF-HFP, but this peak disappears for FTIR spectra of ionogel. Instead, for ionogel, with a new peak at 1054.83 cm−1, the presence of a new peak is also due to TFSI anion in the ES [23,24]. However, when the concentration of electrolyte solution reaches 31 vol%, a new shoulder peak (1028.75 cm−1) appears adjacent to the peak at 1054.83 cm−1. Nevertheless, peaks at band 1067.88 cm−1 and 1177.84 cm−1 remain in the FTIR spectra of the ionogel, showing good compatibility between an electrolyte solution and polymer matrix. The new peaks at 1347.43 cm−1 correspond to conformation, stretching and anti-stretching of the TFSI anion [25]. Moreover, a new peak is observed at 1571.09 cm−1 due to the stretching of EMIM+ cations, evidenced by the ionic liquid in ionogel. This peak becomes more dominant as the amount of the electrolyte solution increases [20,26,27].
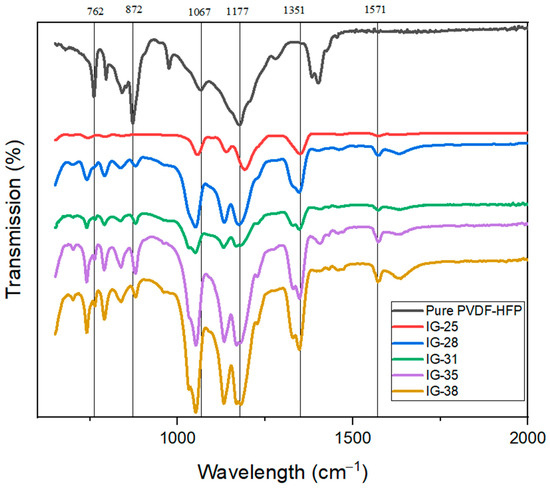
Figure 3.
FTIR spectra for Pure PVDF-HFP, IG-25, IG-28, IG-31, IF-35, IG-38.
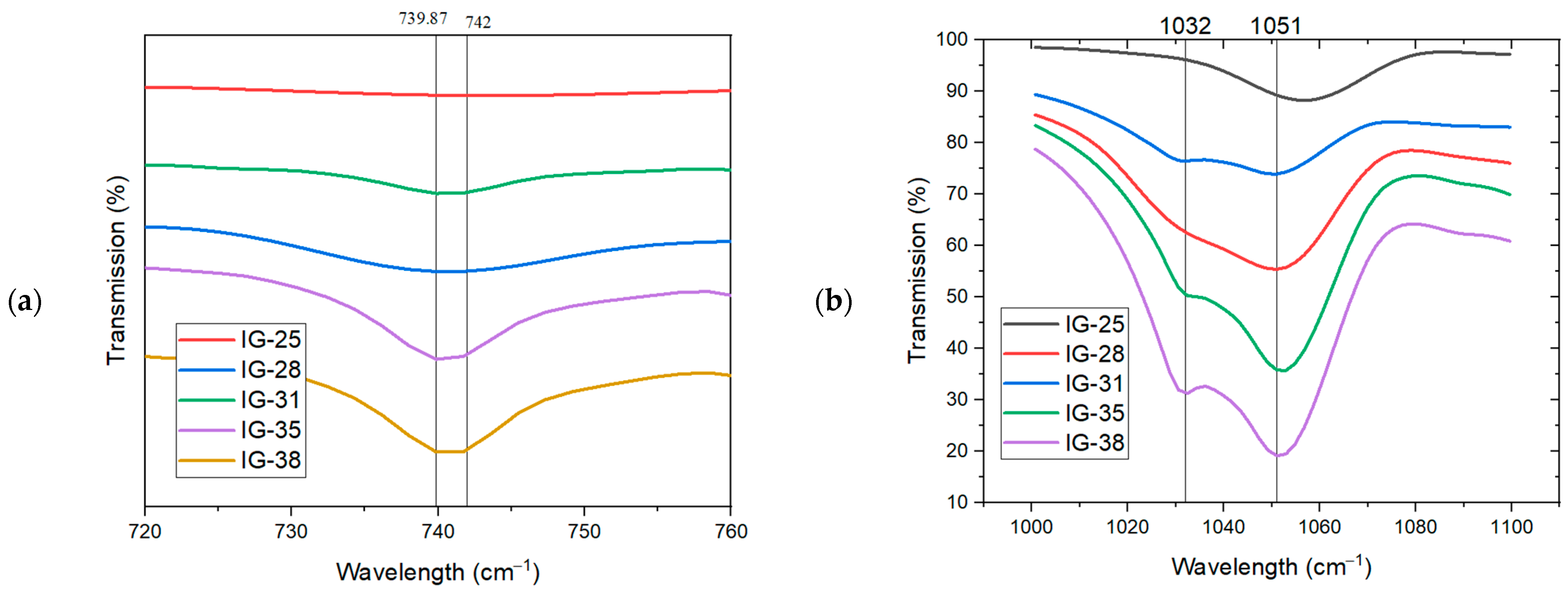
To further understand the presence of free ions or the formation of ion pairs, the FTIR spectra in the wavelength of 700 cm−1 to 770 cm−1 and 1000 cm−1 to 1100 cm−1 were displayed in Figure 4a. From Figure 4a, the wavelength at ~740 cm−1 is used to comprehend the availability of TFSI anion in the ionogel. As the concentration of the electrolyte solution increases from 25 vol% to 33 vol%, the peak at 739.87 cm−1 accounts for the increased availability of free TFSI- anions. Meanwhile, a second peak appears at a wavelength of 742 cm−1 due to the formation of ion pairs or ion aggregation in the ionogel. This peak becomes more pronounced when the electrolyte solution concentration reaches 38 vol%. As the concentration of the electrolyte solution continues to increase, the intensity of this peak is also enhanced, suggesting more ion pairs/ion aggregates present in the ionogel at a high concentration of electrolyte solution.
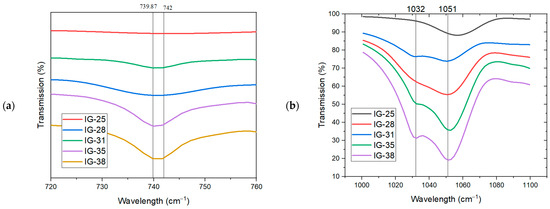
Figure 4.
FTIR Spectra in the wavelength of (a) 700 cm−1 to 760 cm−1 and (b) 1000 cm−1 to 1100 cm−1.
From Figure 4b, the peak at 1032 cm−1 is ascribed to the free ions present in the ionogel [21,28]. At low concentrations of electrolyte solution (25 vol%), this peak is faint, suggesting a scarcity of free ions in the ionogel. Further increment in the concentration of electrolyte solution results in the intensity enhancement of this peak, implying the presence of more free ions. On the contrary, as the concentration increases, a new peak appears at 1054 cm−1, resulting from forming ion pairs/ion aggregates. The intensity of this new peak eventually surpasses the peak at 1032 cm−1, which infers that there are more ion pairs/ion aggregates than free ions present in this ionogel. Thus, FTIR spectra suggest that at lower concentrations of electrolyte solution (25 vol% to 31 vol%), more free ions are available in the ionogel system. Nevertheless, further increasing the concentration of electrolyte solution, ions tend to interact with each other, forming ion pairs, or ion aggregates, as evidenced in the peak intensity at 742 cm−1 and 1054 cm−1, respectively.
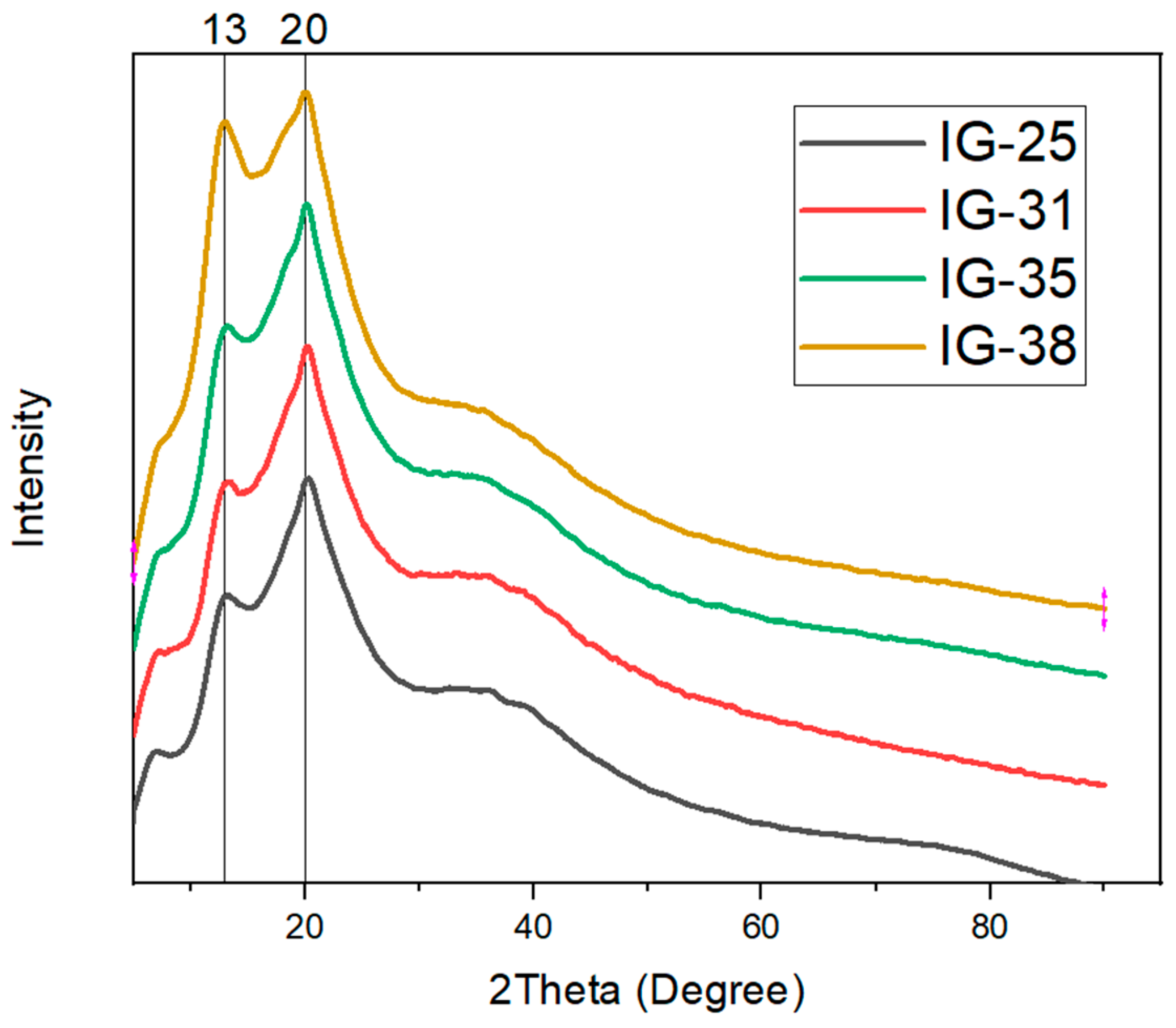
The X-ray diffraction characterization was performed to study the crystallinity of ionogel. The degree of crystallinity refers to the degree of the structural ordering within the ionogel. Figure 5 depicts the XRD curve for the ionogel.
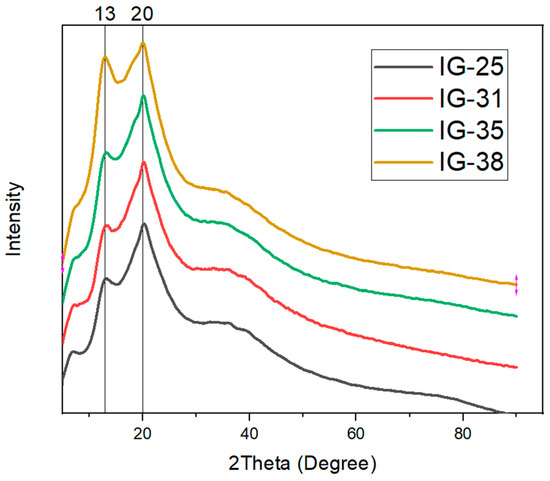
Figure 5.
XRD Curve for Ionogel with x-vol% of electrolyte solution.
Based on the XRD curves for all ionogels, two prominent peaks are observable at around 13° and 20°. In addition to the two distinct peaks, a broad hump is observed near 40°. The two prominent peaks are contributed by the -phase crystals and -phase crystals from PVDF-HFP, which demonstrate the semi-crystalline structure of PVDF-HFP [29]. However, the broad hump near 40° suggests the amorphous phase in the ionogel. As the concentration of electrolyte solution increases, one noticeable change is the enhancement of the broad peak near 40°. This phenomenon suggests that the increasing concentration of electrolyte solution enhances the amorphous phase of ionogel, which is favorable for the mobility of ions. This enhancement of the amorphous phase is ascribed to the Lewis acid-base reaction upon the addition of electrolyte solution, wherein the electrolyte solution acts as a Lewis acid. Simultaneously, the polymer matrix is considered Lewis’s base [30,31]. Besides the amorphous phase enhancement, the first peak (13°) has also increased. The intensity augmentation of the first peak is attributed to the high concentration of electrolyte solution; such a phenomenon evinces improved crystallinity [32]. Furthermore, the monophasic ionic liquid could also contribute to the observed phenomenon [33,34]. To illustrate, the degree of crystallinity is evaluated and tabulated in Table 1.

Table 1.
Degree of Crystallinity for different concentrations of Electrolyte Solution.
According to Table 1, the highest degree of crystallinity was shown by IG-25, followed by a drastic reduction when the concentration of electrolyte solution increased up to 31 vol%. This reveals that adding more electrolyte solution reduces the crystallinity of the ionogel, which is beneficial for ion transportation. However, beyond the concentration of 31 vol%, the degree of crystallinity continues to escalate and attain the highest degree of crystallinity (63.51%) at 38 vol%. Thus, adding electrolyte solution has enhanced the amorphous phase in the ionogel. At the same time, a further increase may result in increased crystallinity, which is undesired as this may lower the ionic conductivity.
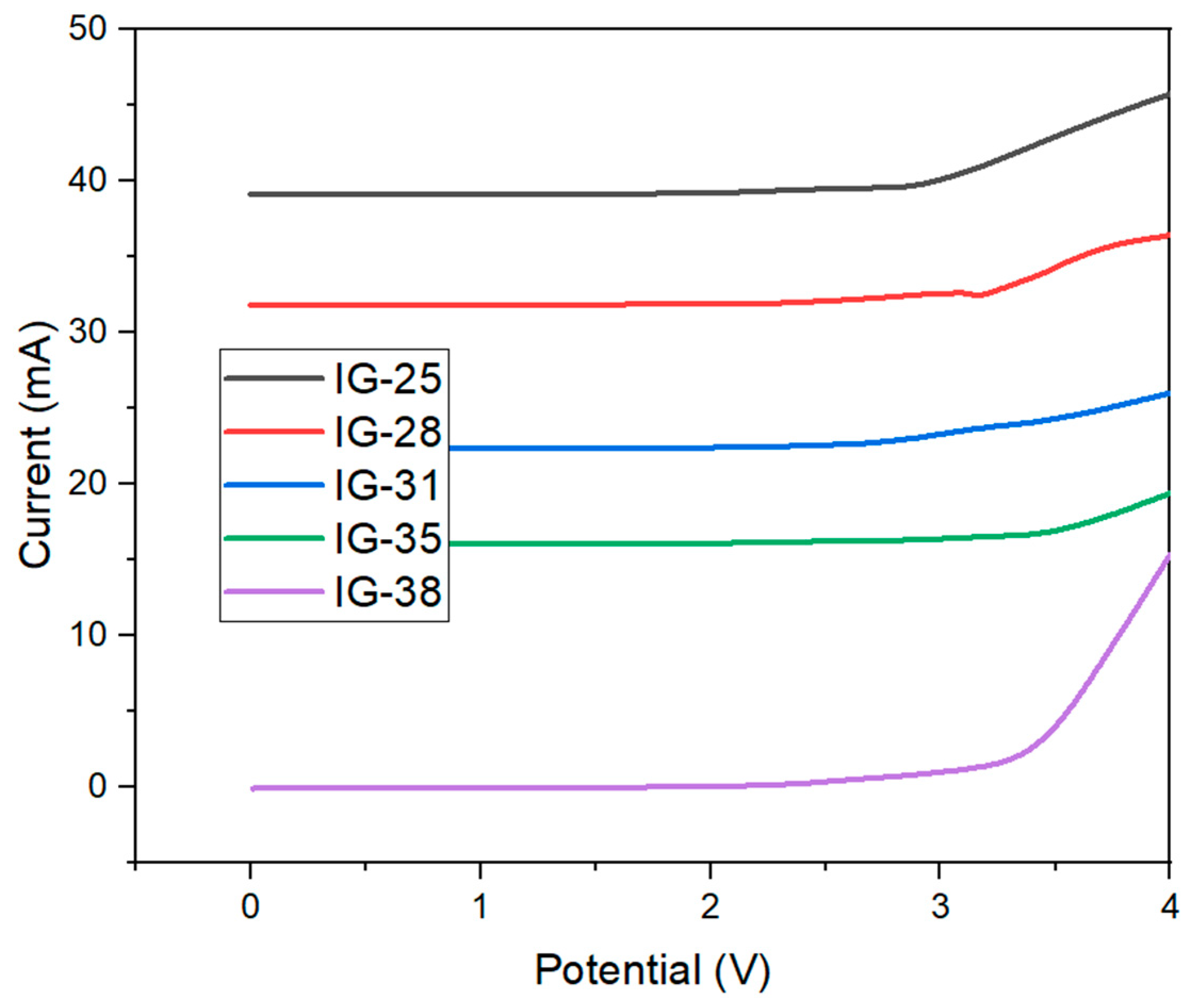
The electrochemical stability window (ESW) is an underlying consideration in evaluating the performance of ionogel as a solid electrolyte. Linear Sweep Voltammetry is a common technique to identify the electrochemical stability of window ionogel. Figure 6 shows the linear sweep voltammetry graph for all ionogels. The ionogel was tested in the voltage range of 0–5 V, as high voltage may lead to the decomposition of ionogel. All the ionogels showed a similar trend, whereby no current was captured when the voltage was applied, up until 3 V, where a drastic increase in the current is observed as the voltage is increased. The sudden spike in current is due to the decomposition of polymer electrolytes [35]. Similar LSV plots for ionogel have been reported [29,36,37,38]. The electrochemical stability window is identified by drawing a tangent on the uprise of the curve and intercepting the voltage axis [39].
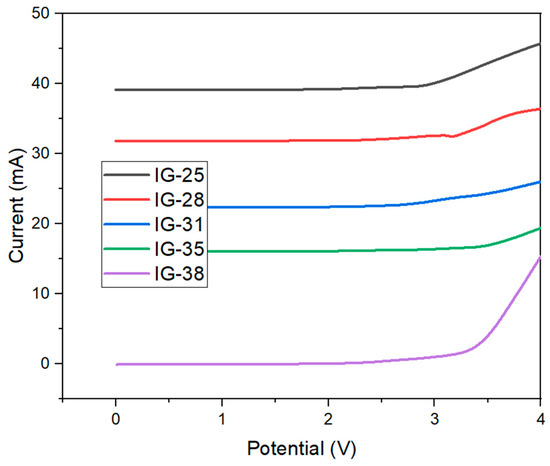
Figure 6.
Linear Sweep Voltammetry Plot of Ionogel.
The electrochemical stabilities are summarized in Table 2. From Table 2, it is observable that with the increase in the concentration of electrolytes solution, no substantial increment was observed in the electrochemical stability of ionogel. The results show that for electrolyte solutions in the range 25–38 vol%, the electrochemical stability is reported to be within the range of 3.00–3.50 V. To conclude, no significant current was recorded within 0–3.50 V, indicating that the synthesized ionogel has shown good electrochemical stability up to 3.50 V. The wide electrochemical stability window exhibited by ionogel has allowed it to be applied in photonic devices [40], common electrical double-layer capacitors and energy storage devices [41].

Table 2.
Electrochemical stability of Ionogel with different compositions of electrolyte solution.
Moreover, an increasing trend in electrochemical stability can be observed as the concentration of electrolyte solution increases. The maximum electrochemical stability, 3.50 V, is achieved by IG-31, followed by a subsequent decreasing trend. The electrochemical stability of ionogel is influenced by the ionic liquid due to strong interactions between anions and cations, effectively preventing the decomposition of ionogel at high voltages [42]. Hence, it can be deduced that the strongest electrostatic interaction exists at a concentration of 31 vol%, resulting in the widest electrochemical stability window.
Cyclic Voltammetry (CV) is an electrochemical characterization technique employed on 2 electrodes, or 3-electrode configurations, to characterize liquid electrolyte-based capacitors. This technique provides information on charge storage at the individual interfaces [43]. In this study, CV was applied to characterize the specific capacitance of the synthesized ionogels, which depends on the accessibility of the surface area and ion concentration in the ionogel.
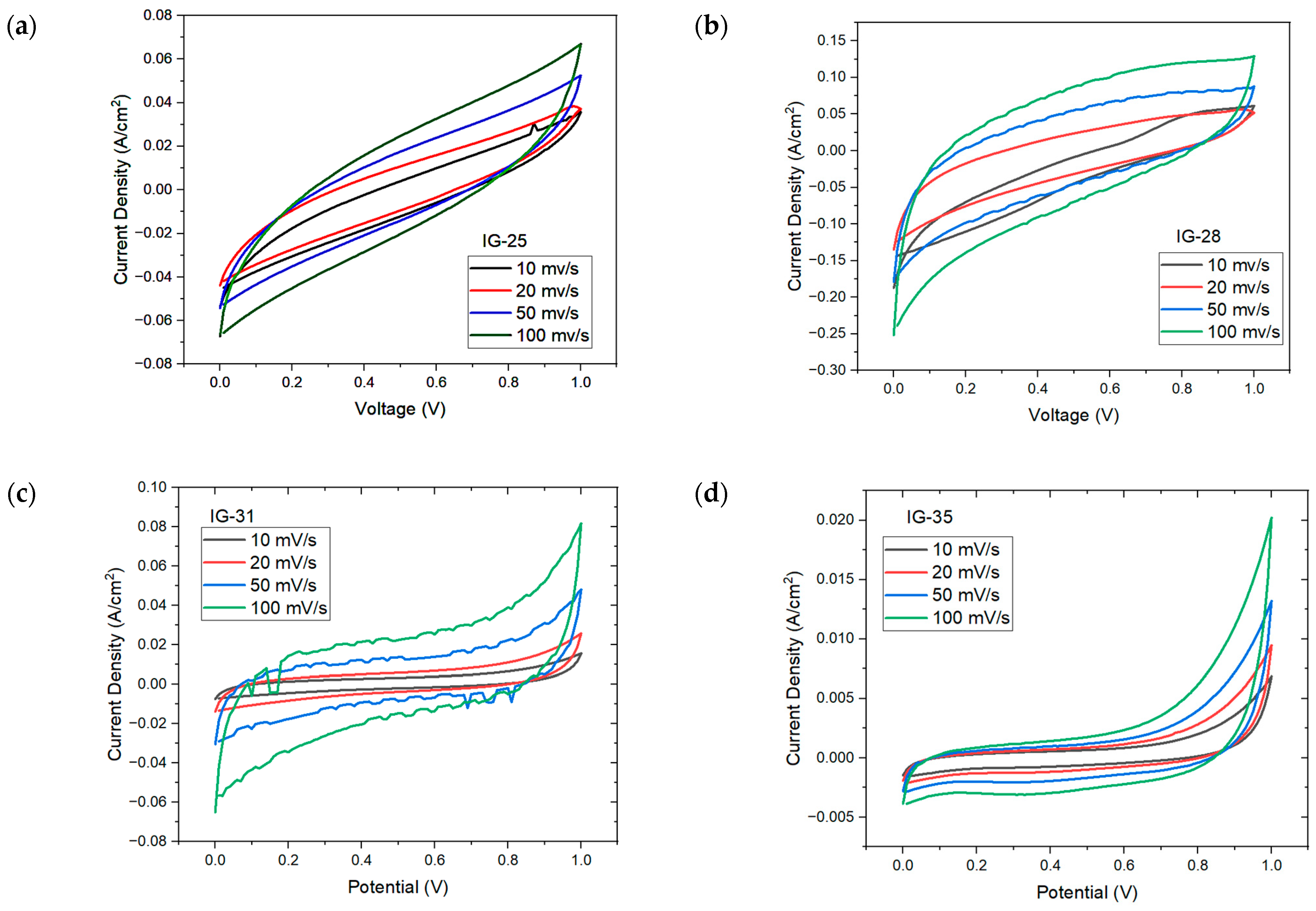
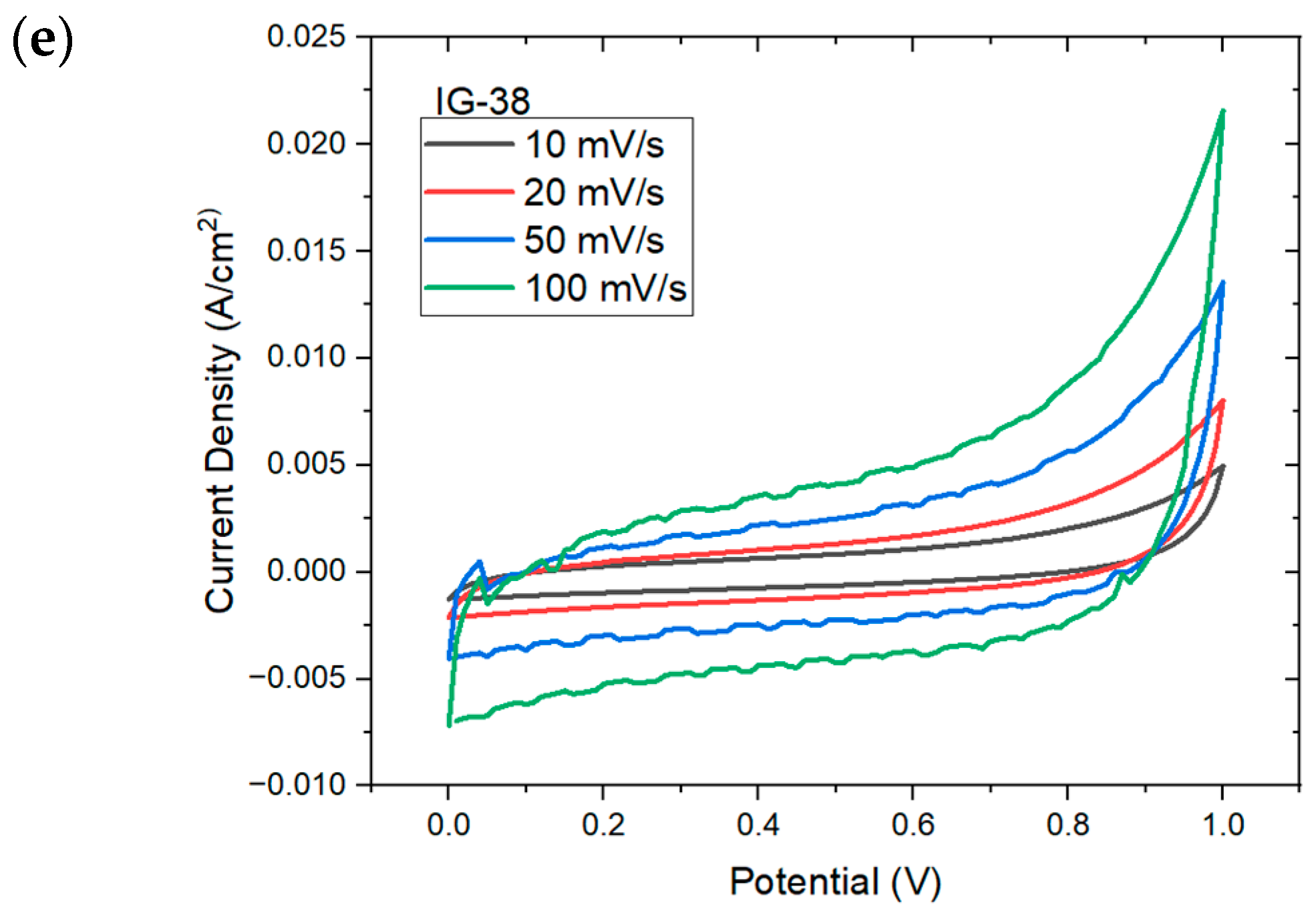
Figure 7 shows the CV plot for IG with varying concentration of electrolyte solution. At low concentration of electrolyte solution (less than 31 vol%), the CV plot displays an oval, ellipse-like shape, especially IG-25; this indicates the high instability and poor capacitive nature of ionogel, which is well reflected on the specific capacitance of IG-25 [44]. However, other than IG-25 and IG-28, all other figures show similar behavior, whereby a quasi-rectangular plot is observed; this implies excellent capacitive behavior and rapid charge propagation [45,46]. As the concentration of the electrolyte solution gradually increases, it can be observed that the CV plots approach rectangular shapes, up to 37 vol%. The presence of a rectangular CV plot implies pure double-layer capacitance. However, beyond this concentration, the CV plot drifts away from a rectangular shape. It was claimed that this results from the aggregation of ionic liquid. Meanwhile, the distorted CV plots could be due to the increasing internal resistance [47], Fontaine and Janani suggest that the deformed CV plots imply the resistive behavior of ionogel, behaving more like a resistor than a supercapacitor [48,49]. Moreover, the electrolyte–electrode charge transfer would also result in deviation from rectangular shape, while the reactions are considered pseudocapacitive provided that the charge transfer is reversible [50]. Furthermore, the increasing scan rates also increase the maximum current, which is one of the characteristics of capacitive material. In addition, no distinct peaks were observed in the CV plots due to internal resistance, implying a non-faradic process and charge double-layer at the electrode surface [51,52].
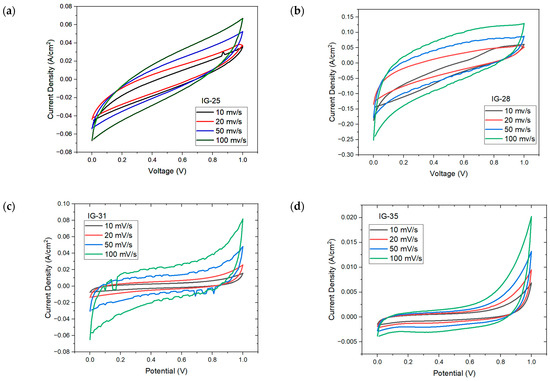
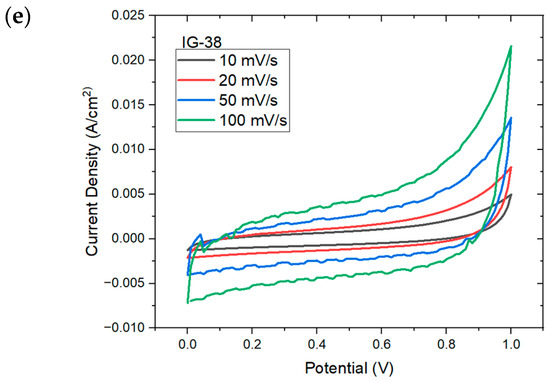
Figure 7.
CV plots for (a) IG-25, (b) IG-28, (c) IG-31, (d) IG-35 (e) IG-38.
The cyclic voltammetry plot is also used to evaluate the specific capacitance of ionogel. The specific capacitance reflects charge storage at the interfacial region due to the interaction between the ions (from the electrolyte) and electrons (in the electrode) under an applied electric field [53]. The specific capacitance of ionogel is obtained at each scan rate and tabulated in Table 3.

Table 3.
Specific Capacitance of ionogel with different concentrations of electrolyte solution at different scan rates.
Table 3 shows the highest specific capacitance value at 10 mV/s, where the specific capacitance ranges from 838.55 mF/g to 45.74 F/g, respectively. It is worth mentioning that the finding in specific capacitance is higher than the particular polymer capacitance electrolytes reported in the literature, which range only from about 1.7 F/g to 2.1 F/g in several studies [54,55,56]. The study reveals a decrease in specific capacitance with an increasing scan rate, possibly due to the charge per unit of time. Efficient ion conduction and double-layer formation at low scan rates lead to higher specific capacitance values [41]. At a high scan rate, there is insufficient time for the diffusion of ions to the surface of polymers, thus reducing specific capacitance [57,58].
To further elaborate, Specific capacitance is related to the storage capacity of the ionogel, indicating the number of free ions present in the ionogel [59]. At lower concentrations of electrolyte solution (below 31 vol%), there is a lack of charge-carrying ions for desorption and adsorption, thus resulting in low specific capacitance. However, at 31 vol% of the electrolyte solution, there is an adequate amount of ions present in the ionogel, and the surface area of the electrode can be fully exploited. This increased ion availability facilitates faster ionic responses. As a result, more ions diffuse and accumulate at the electrode–electrolyte interface, promoting double-layer formation and improving the ionogel’s specific capacitance [60].
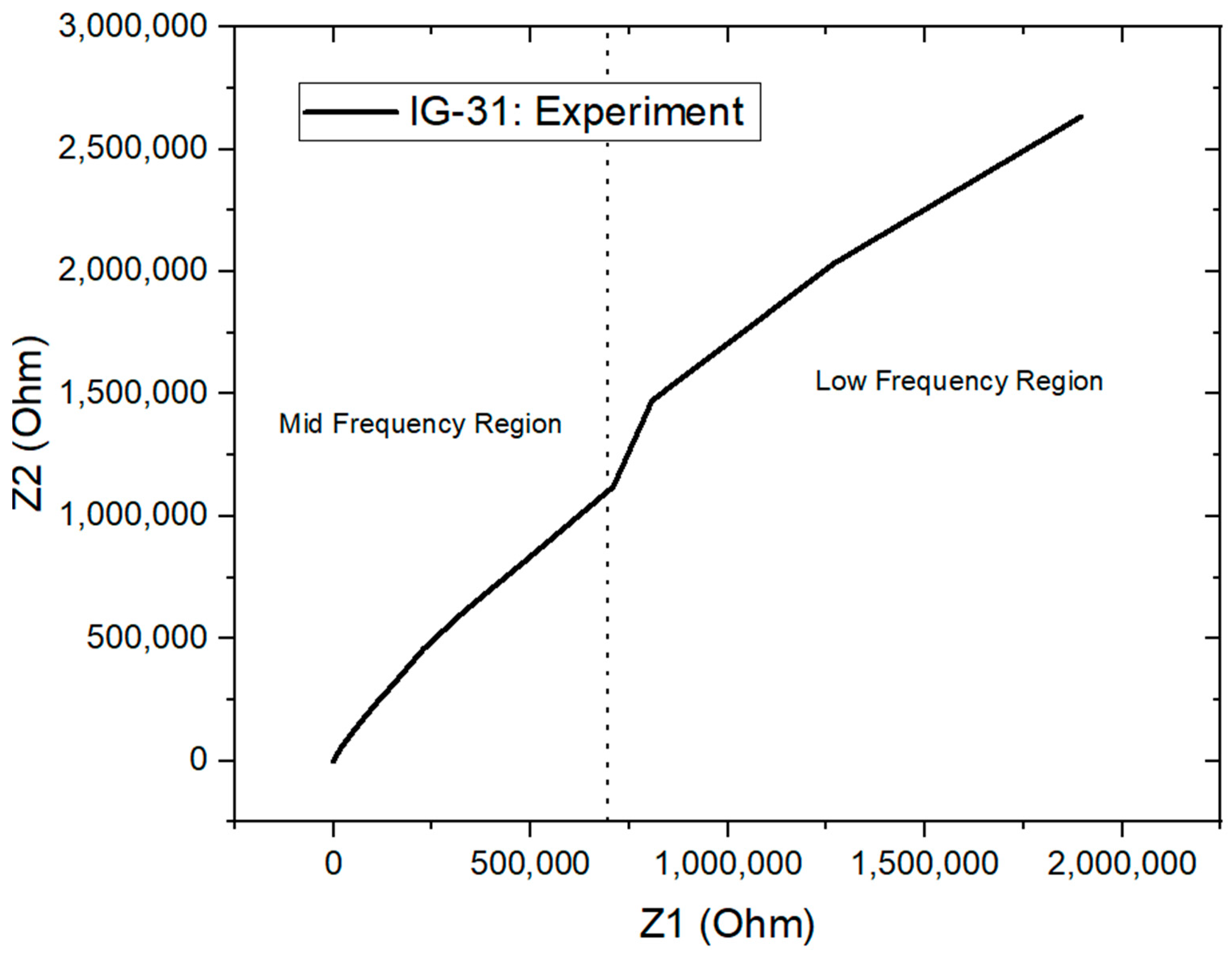
Electrochemical impedance spectroscopy (EIS) is performed on all the ionogel samples. This technique is considered definitive and effective in providing electrochemical characteristics of electrochemical systems. For instance, it provides information about solution resistance, double-layer capacitance, charge transfer process and charge transfer rate [61]. EIS is analyzed in a wide frequency range (0.01 Hz to 10 kHz) while the real and imaginary impedance is recorded, thus producing a Nyquist plot. The Nyquist plot can be differentiated into three regions according to their frequencies: the low-frequency region , mid-frequency region and ohmic region . The Nyquist plot for IG-31 is shown in Figure 8 with frequency region labelled.
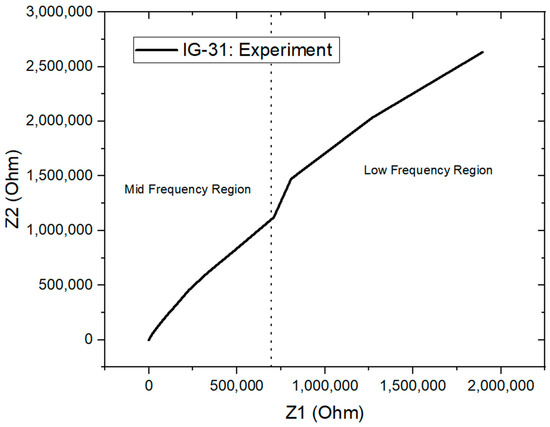
Figure 8.
Nyquist plot for IG-31.
The ohmic region indicates the internal resistance of the ionogel; this is the region where the impedance of the ionogel switches from inductive behavior to capacitive behavior. Moreover, the mid-frequency region from the Nyquist plot is of interest in this study to study the ionic conductivity of ionogel. The ionic conductivity of the ionogel is evaluated with the equation below:
The mid-frequency region shows the charge transfer process from the surface of the electrode to the bulk active material of the electrodes and from the electrolyte to the electrode’s surface area [62]. Relating this to the equation for ionic conductivity, the refers to the bulk resistance, which is determined from this region. The equivalent circuit model fits the Nyquist plot to obtain bulk resistance.
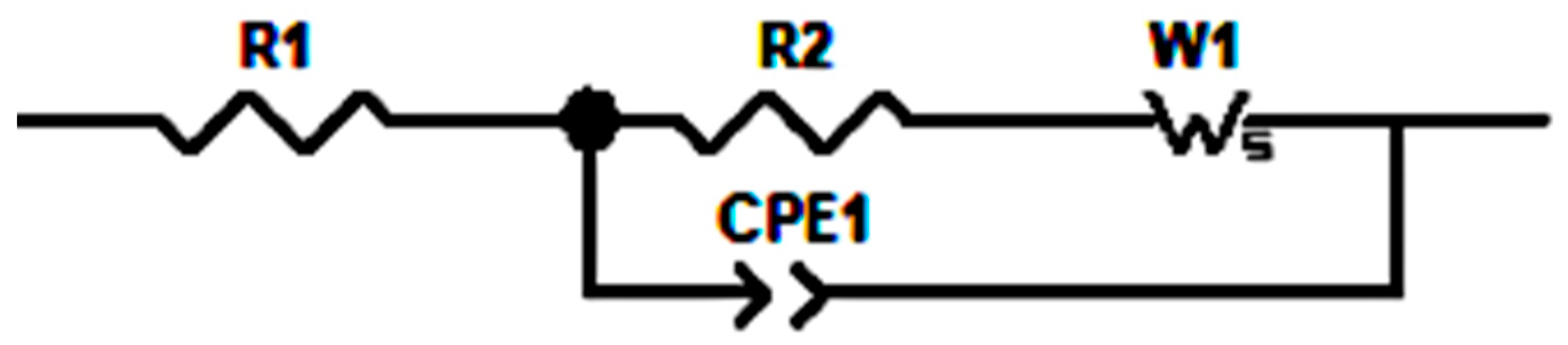
The equivalent circuit model (ECM) is the common approach to evaluating the Nyquist plot by fitting the plot. The commonly used elements in an equivalent circuit model are resistor, constant phase, and Warburg element. A constant phase element (CPE) relates to the dispersion effect and how it differs from capacitance. The Warburg element (W) is used to interpret the mechanism of the diffusion process in the low-frequency region. The Nyquist plot from IG-31 to IG-38 shows a similar trend. Thus, the equivalent circuits are identical, as shown in Scheme 1, while the fitted Nyquist plot for IG-31is displayed in Figure 8. In the high-frequency region of the Nyquist plot, the inductive effects are observed and often show non-ideal behavior [63,64]. Thus, a resistor (R1) describes this phenomenon. The arc represents the impedance due to charge transfer for the mid-frequency region. In this region, these attributes are due to the combination of impedances, which can be modelled by the parallel combination of a resistor (R2) and a constant phase element (CPE1) [65]. Hence, the parallel combination of resistor and CPE represents the mid-frequency range, while the Warburg element fits the diffusion processes in the low-frequency region. In this study, the Warburg element is placed in series to R2 but parallel to CPE1; this corresponds to the consideration of simple geometry. This arrangement is also analyzed by Huang et al. [66], where from a physical perspective, the Warburg element connects to R2 in series while being parallel to CPE1 is preferred. The diffusion process followed by the charge transfer reaction is considered parallel to the discharging and charging process of the double layer [65].
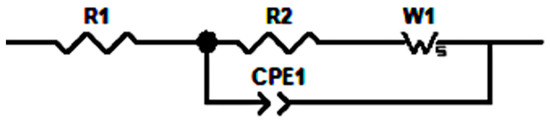
Scheme 1.
Equivalent Circuit Model.
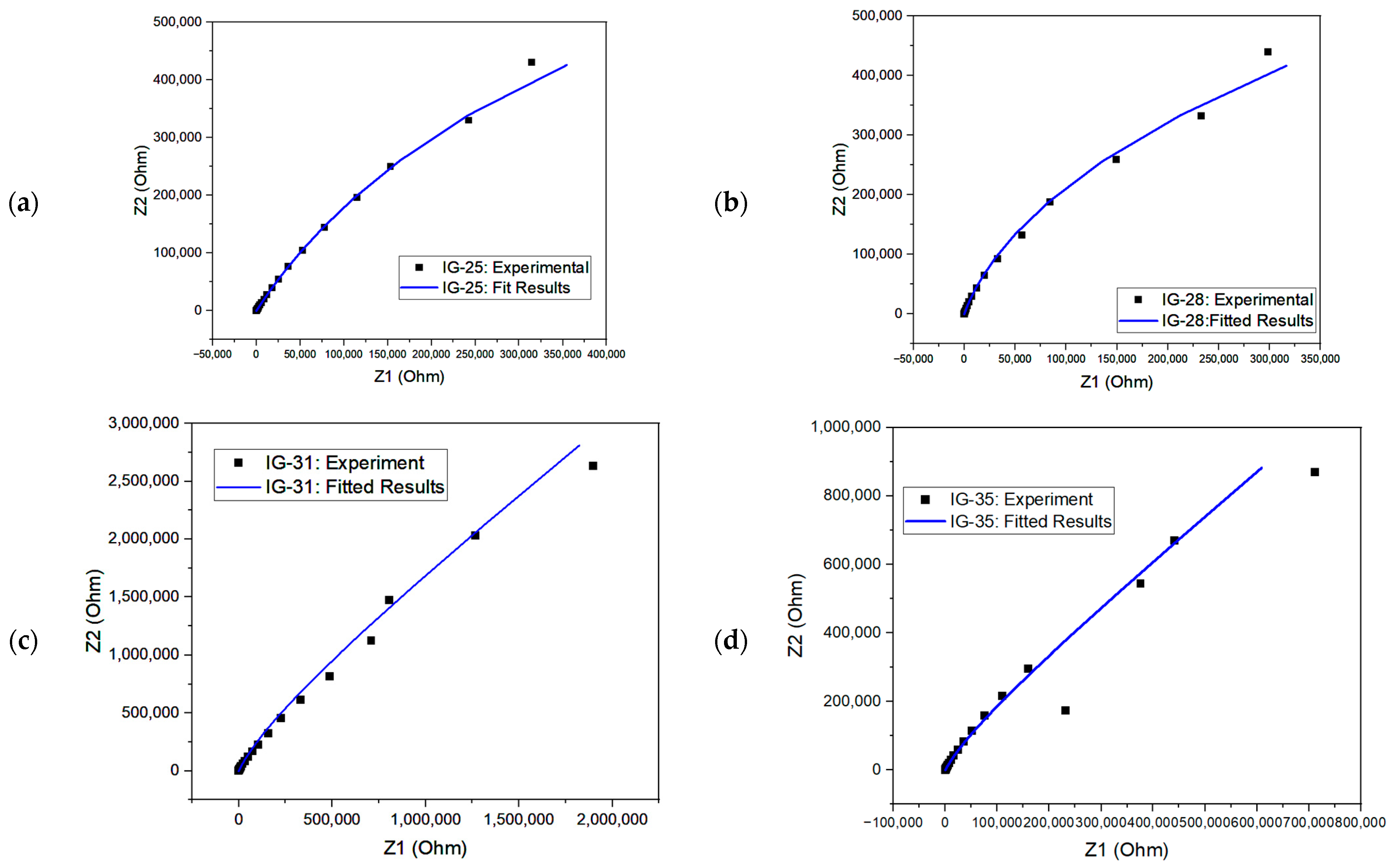
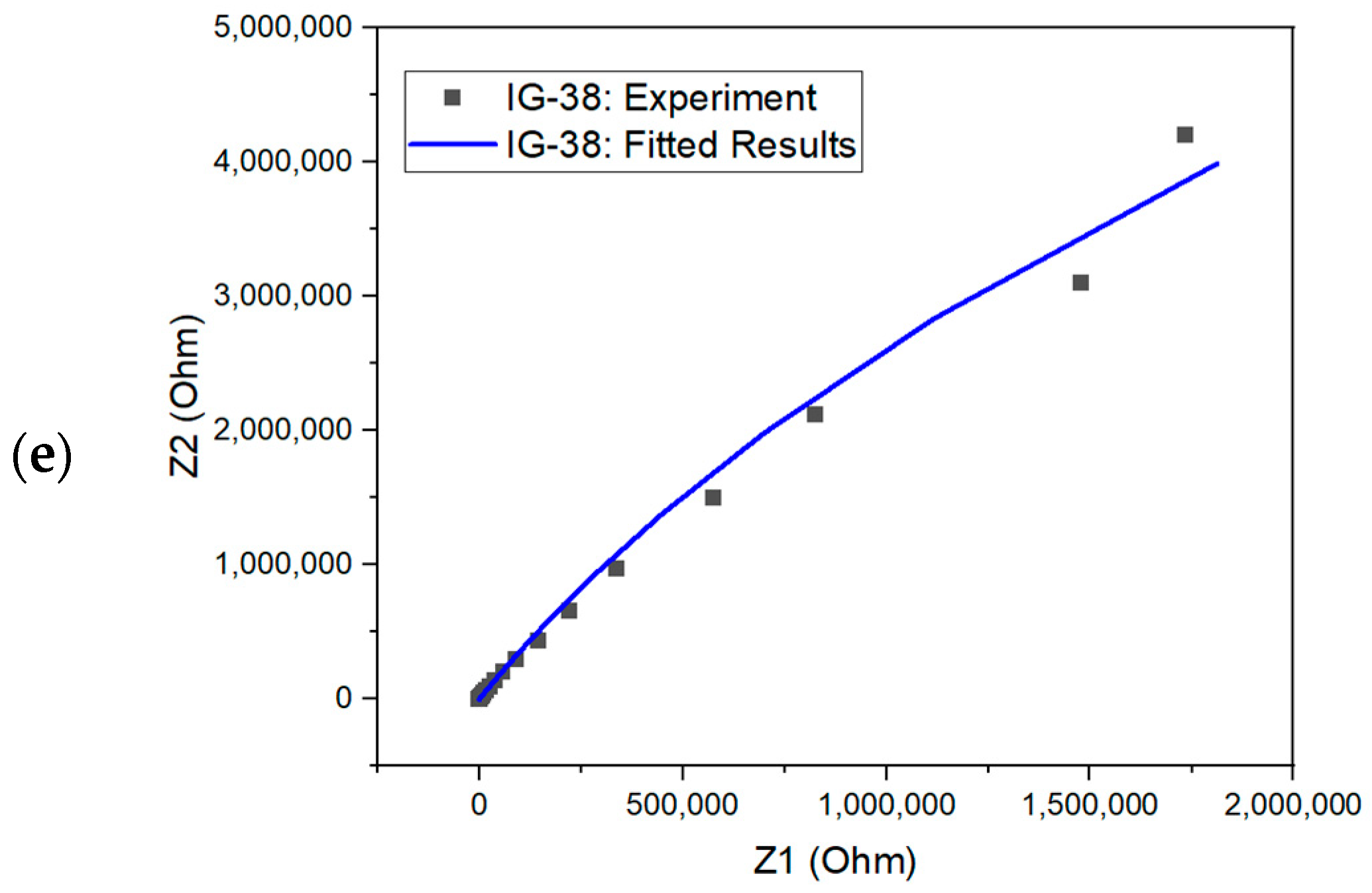
From the equivalent circuit, the second resistor element (R2) refers to bulk resistance; hence, this value can be used to evaluate the ionic conductivity of ionogel [67,68]. The Nyquist plot for fitted and experimental results is shown in Figure 9. The simulated results could fit almost all the Nyquist plots. One way to examine the accuracy of the fitted results is through the chi-square value. The chi-square value indicates the discrepancy between the modelled data and the measured value of impedance analysis, whereby a larger chi-square value implies a larger deviation degree [69]. The chi-square value of each fitted model is within the range , showing good fitting results.
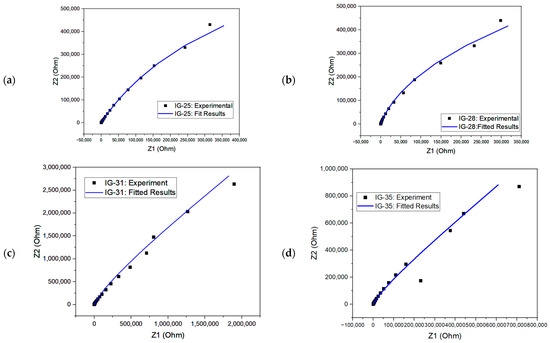
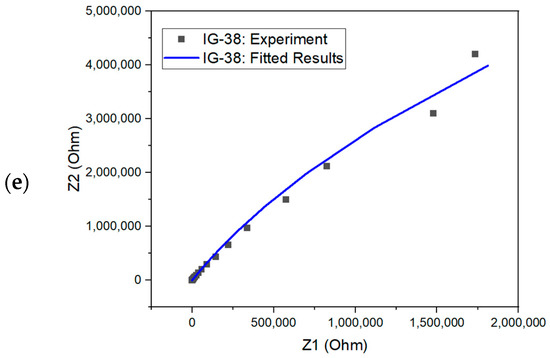
Figure 9.
Nyquist plot with fitted and experiment results for (a) IG-25, (b) IG-28, (c) IG-31, (d) IG-35 and (e) IG-38.
Ionic conductivity is one of the crucial parameters for ionogel, while high ionic conductivity permits a good performance rate at a high charge/discharge density. The ionic conductivities are also tabulated in Table 4. The ionic conductivity of ionogel exhibits consistent behavior as in specific capacitance and electrochemical stability, where the maximum ionic conductivity is achieved by IG-31, followed by a substantial decrease. This finding is consistent with SEM and XRD results. Based on the SEM image, good compatibility between ionic liquid and the electrolyte solution is shown for IG-31, showing smooth and porous morphology, which enables ion transport, thus attaining high ionic conductivity.

Table 4.
Ionic Conductivity of Ionogels.
Meanwhile, SEM results indicate that as the concentration of electrolyte solution increases, the porous morphology slowly disappears. As a result, ionic conductivity decreases as ion transportation relies on porous morphology. From the XRD analysis, IG-25 has the highest degree of crystallinity (72.46%). The degree of crystallinity gradually decreases as the concentration of electrolyte solution reaches 31 vol% (49.02%). Further increment of electrolyte solution leads to amplified crystallinity, whereby at 38 vol% of electrolyte solution, the degree of crystallinity has reached 63.51%. This XRD result is in complete unanimity with the ionic conductivity analysis of the ionogel. IG-25, which shows the highest crystallinity, impedes the transportation of ions, resulting in the lowest ionic conductivity (S/cm) of ionogel. IG-31 possesses the lowest degree of crystallinity, showing the highest ionic conductivity (S/cm).
The ionic conductivity value achieved by IG-31 is comparable to the values reported by recent literature; for instance, Barbosa et al. reported ionic conductivity of , including zeolite as filler, which is slightly lower than the values observed in our work [70]. Notably, in our work, the ionogel synthesized with a similar composition has achieved comparable ionic conductivity without adding any filler. This could be attributed to incorporating lithium salt, which provides more mobile ions. The observed result correlates with the findings from FTIR spectra, where the peak intensity (739 cm−1) increases as the concentration of electrolyte solution increases. Such a result is also in agreement with the results from cyclic voltammetry. A sufficient number of ions are available within the ionogel, corroborated by smooth and porous morphology, which assists the transport of ions, resulting in the highest ionic conductivity. However, a further increase in the electrolyte solution is accompanied by decreasing ionic conductivity. This is due to the formation of ion pairs which hinder the transport of ions. This is proven by a new peak in the FTIR spectra at wavelengths of 742 cm−1 and 1054 cm−1, respectively. In this study, the electrolyte solution is obtained by the dissolution of LiTf in EMIM TFSI. Lithium salts in ionic liquid could aid in enhancing the ionic conductivity of ionogel. Weak interaction exists within EMIM TFSI, while upon the addition of LiTf, this promotes the formation of ion pairs between Li+ ions and TFSI− when more lithium salt becomes available [71]. Thus, excessive Li+ ions will interact with TFSI−. This interaction results in the aggregation of Li+ ions and TFSI- ions, which is responsible for the increased viscosity, hence decreasing the ionic conductivity; such behavior is also consistent with previous findings in the literature [72,73,74].
3. Experimental Sections
3.1. Synthesis of Ionogel
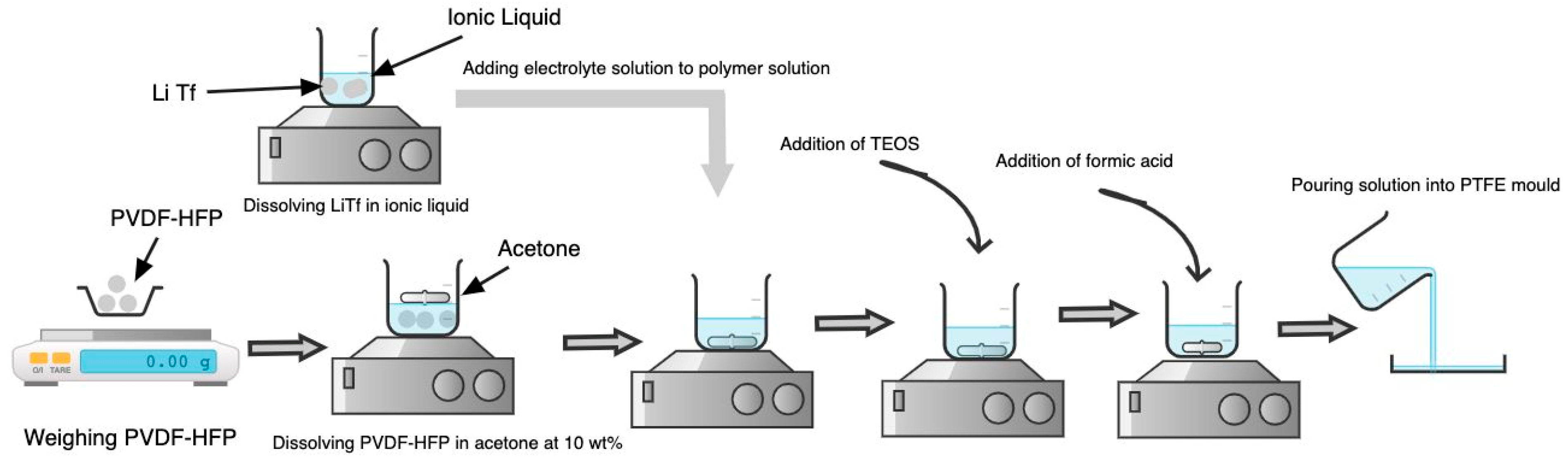
The synthesis of ionogel was based on the non-aqueous sol-gel route. Scheme 2 illustrates the synthesis route for ionogel. The polymer matrix, PVDF-HFP, was first dissolved in acetone at 10 wt% for 24 h for complete dissolution. Beyond 10 wt%, the polymer could not be dissolved. The LiTf was then dissolved in ionic liquid to produce an electrolyte solution. After completely dissolving the polymer, the electrolyte solution was added to the polymer solution under continuous stirring conditions for another 1 h. After constant stirring for 1 h, the silica precursor, TEOS, was added to the solution for further mixing. The TEOS to polymer matrix ratio was maintained at 75:25. This ratio produces ionogel with good mechanical and electrical properties.
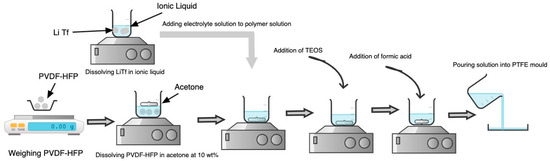
Scheme 2.
Synthesis of Ionogel.
Meanwhile, the formic acid was added to the solution as a catalyst. The molar ratio of formic acid to TEOS was maintained at 7.8:1. After adding formic acid, the solution was cast onto the mold and left in an oven at room temperature for 2 days.
3.2. Material Characterization
The surface morphology of ionogel is characterized using SEM. X-ray diffraction (XRD) patterns were determined from a Rigaku Single X-ray Diffractometer (RGS, Selangor, Malaysia). The FTIR characterization was performed using Agilent Cary 630N (DKSH, Sarawak, Malaysia). The electrochemical characterization of ionogel was performed by an IVIUM compact state 10800 (Palico Biotech, Singapore). XRD was performed for 2θ between 5 to 80° at a scan rate of 1°/min. The crystallinity of ionogel was examined through this analysis, and the degree of crystallinity was determined based on the equation below:
where and represent the area under the crystalline peak and the amorphous phase, respectively. The FTIR spectra were recorded in the wavelength of 600 cm−1 to 4000 cm−1 to inspect the conformational and structural changes of ionogel. The linear sweep voltammetry was performed in the range 0–5 V, with a 100 mV/s scan rate, to obtain the electrochemical stability of ionogel. The cyclic voltammetry was performed with different scan rates (10 mV/s, 20 mV/s, 50 mV/s and 100 mV/s), from 0 V to 1 V, while the specific capacitance of ionogel was determined based on the equation below:
The integration was performed to obtain the area under the cure, while m is the mass of the ionogel (g), represents the scan rate (mV/s) and V is the voltage range. Electrochemical impedance spectroscopy was performed with the frequency range to obtain impedance spectra. An equivalent circuit model was performed using the ivium software to identify the ionic conductivity of ionogel. An equivalent circuit model was performed using the ivium software to identify the ionic conductivity of ionogel. Thus, the equation below is used to evaluate ionic conductivity.
where σ, L and S are the conductivity (S cm−1), thickness (cm) and electrode area (cm2), respectively.
4. Conclusions
In conclusion, this study has demonstrated a correlation between the specific capacitance and ionic conductivity of ionogels, which is dependent on the availability of mobile ions. Low ionic conductivity is achieved at lower concentrations of electrolyte solution due to limited mobile ions. Conversely, a higher concentration of electrolyte solution leads to the formation of ion pairs which reduces the ionic conductivity of ionogel. This interaction hinders ion mobility and results in the formation of double-layer capacitance, ultimately leading to diminished specific capacitance and ionic conductivity. In this study, 31 vol% is identified as the effective concentration, as an ample quantity of ions becomes accessible, resulting in increased ionic conductivity and specific capacitance with maximum values of 58 ± 1.48 μS/cm and 45.74 F/g, respectively. This finding is consistent with the outcomes derived from FTIR spectra, which exhibit an intensified intensity at 739 cm−1. The ionogel also demonstrates a wide electrochemical stability of 3.5 V and attains electrochemical properties suitable for diverse practical applications.
Consequently, this study offers critical insights into determining the optimal electrolyte solution concentration for enhancing ionogel electrochemical performance. It highlights the impact of ion pairs and aggregates on ion mobility within ionogels, subsequently affecting their electrochemical properties. This is the first study to report such outstanding ionic conductivity and electrochemical stability at a relatively low electrolyte concentration of around 30% without incorporating any filler material. This groundbreaking finding highlights the potential of ionogels in various applications while presenting new avenues for future research in the field.
Author Contributions
J.W.S., N.K.E., S.D. and N.M.M.: Conceptualization, Data curation, Methodology, Writing—original draft, Writing—review & editing. N.K.E., S.D., N.M.M., C.I.L. and M.R.M.: Conceptualization, Supervision, Validation, Visualization, Writing—review & editing. M.K. and Y.S.T.—review & editing the manuscript. The manuscript was written through the contributions of all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Grant Scheme (grant number FRGS/1/2020/TK0/CURTIN/03/2; Project ID: 18019) from the Ministry of Higher Education (MOHE) Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the funding provided by the Fundametal Research Grant Scheme (FRGS/1/2020/TK0/CURTIN/03/2; Project ID: 18019) from the Ministry of Higher Education (MOHE) Malaysia. The authors also acknowledge the support provided by the Mechanical Engineering Department of Curtin University Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| CV | Cyclic Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| EMIM TFSI | 1-ethyl-3-methylimidazolium bis (trifluoromethyl sulfonate) |
| FTIR | Fourier transform infrared spectroscopy. |
| IG | Ionogel |
| IL | Ionic Liquid |
| LiTf | Lithium trifluoro methane sulfonate |
| LSV | Linear Sweep Voltammetry |
| PVDF-HFP | Poly(vinylidene fluoride)-co-hexafluoropropylene |
| SEM | Scanning Electron Microscopy |
| TEOS | Tetraethyl orthosilicate |
| XRD | X-ray diffraction |
References
- Ling, Y.; An, T.; Yap, L.W.; Zhu, B.; Gong, S.; Cheng, W. Disruptive, Soft, Wearable Sensors. Adv. Mater. 2020, 32, 1904664. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, J.; Cui, X.; Liu, X.; Chang, X.; Zhu, Y. Wearable Ionogel-Based Fibers for Strain Sensors with Ultrawide Linear Response and Temperature Sensors Insensitive to Strain. ACS Appl. Mater. Interfaces 2022, 14, 30268–30278. [Google Scholar] [CrossRef]
- Xiang, S.; Chen, S.; Yao, M.; Zheng, F.; Lu, Q. Strain sensor based on a flexible polyimide ionogel for application in high- and low-temperature environments. J. Mater. Chem. C 2019, 7, 9625–9632. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choo, Y.; Bilodeau, R.A.; Yuen, M.C.; Kaufman, G.; Shah, D.S.; Osuji, C.O.; Kramer-Bottiglio, R. Sustainable manufacturing of sensors onto soft systems using self-coagulating conductive Pickering emulsions. Sci. Robot. 2020, 5, eaay3604. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Li, R.; Wang, A.; Kang, J.; Wang, Z.; Bi, W.; Yang, Y.; Song, Y.; Dong, Q. Ionogel-perovskite matrix enabling highly efficient and stable flexible solar cells towards fully-R2R fabrication. Energy Environ. Sci. 2022, 15, 3439–3448. [Google Scholar] [CrossRef]
- Luo, Z.; Li, W.; Yan, J.; Sun, J. Roles of Ionic Liquids in Adjusting Nature of Ionogels: A Mini Review. Adv. Funct. Mater. 2022, 32, 2203988. [Google Scholar] [CrossRef]
- Clarke, C.J.; Matthews, R.P.; Brogan, A.P.S.; Hallett, J.P. Controlling surface chemistry and mechanical properties of metal ionogels through Lewis acidity and basicity. J. Mater. Chem. A 2021, 9, 4679–4686. [Google Scholar] [CrossRef]
- Lan, J.; Li, Y.; Yan, B.; Yin, C.; Ran, R.; Shi, L.-Y. Transparent Stretchable Dual-Network Ionogel with Temperature Tolerance for High-Performance Flexible Strain Sensors. ACS Appl. Mater. Interfaces 2020, 12, 37597–37606. [Google Scholar] [CrossRef]
- Bhandary, R.; Schönhoff, M. Polymer effect on lithium ion dynamics in gel polymer electrolytes: Cationic versus acrylate polymer. Electrochim. Acta 2015, 174, 753–761. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Singh, R.K. Lithium salt assisted enhanced performance of supercapacitor based on quasi solid-state electrolyte. J. Saudi Chem. Soc. 2018, 22, 838–845. [Google Scholar] [CrossRef]
- Guillemin, T.; Douard, C.; Robert, K.; Asbani, B.; Lethien, C.; Brousse, T.; Le Bideau, J. Solid-state 3D micro-supercapacitors based on ionogel electrolyte: Influence of adding lithium and sodium salts to the ionic liquid. Energy Storage Mater. 2022, 50, 606–617. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Zheng, R.; Zhang, D.; Zhang, H. Silica-based ionogel electrolyte with porous flower-like structure enables safer lithium ion battery. Appl. Surf. Sci. 2019, 485, 119–127. [Google Scholar] [CrossRef]
- Ogawa, H.; Mori, H. In-situ formation of poly(ionic liquid)s with ionic liquid-based plasticizer and lithium salt in electrodes for solid-state lithium batteries. Polymer 2019, 178, 121614. [Google Scholar] [CrossRef]
- Dutta, A.; Mishra, D.K.; Kundu, D.; Mahanta, U.; Jiang, S.P.; Silvester, D.S.; Banerjee, T. Examining the Electrochemical Nature of an Ionogel Based on the Ionic Liquid [P66614][TFSI] and TiO2: Synthesis, Characterization, and Quantum Chemical Calculations. Ind. Eng. Chem. Res. 2022, 61, 8763–8774. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Charge Carrier Relaxation in Different Plasticized PEO/PVDF-HFP Blend Solid Polymer Electrolytes. J. Phys. Chem. B 2017, 121, 5422–5432. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, P.; Shamsi, M.; Thelen, J.L.; Qian, W.; Truong, V.K.; Ma, J.; Hu, J.; Dickey, M.D. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 2022, 21, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Bowen, C.R. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: The impact of molecular-, micro- and macro-structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef]
- Badatya, S.; Kumar, A.; Sharma, C.; Srivastava, A.K.; Chaurasia, J.P.; Gupta, M.K. Transparent flexible graphene quantum dot-(PVDF-HFP) piezoelectric nanogenerator. Mater. Lett. 2021, 290, 129493. [Google Scholar] [CrossRef]
- Khurana, S.; Chandra, A. Ion conducting polymer-silica hybrid ionogels obtained via non-aqueous sol-gel route. Solid State Ion. 2019, 340, 115027. [Google Scholar] [CrossRef]
- Tafur, J.P.; Fernández Romero, A.J. Electrical and spectroscopic characterization of PVdF-HFP and TFSI—Ionic liquids-based gel polymer electrolyte membranes. Influence of ZnTf2 salt. J. Membr. Sci. 2014, 469, 499–506. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Thermal, electrical and structural studies on ionic liquid confined in ordered mesoporous MCM-41. J. Mater. Chem. A 2015, 3, 23809–23820. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, J.; Zhang, J.; Qiu, L.; Xu, D.; Zhang, H.; Han, X.; Sun, B.; Fu, G.; Zhang, Y.; et al. Bis-imidazolium based poly(ionic liquid) electrolytes for quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 18018–18024. [Google Scholar] [CrossRef]
- Li, R.; Fang, Z.; Wang, C.; Zhu, X.; Fu, X.; Fu, J.; Yan, W.; Yang, Y. Six-armed and dicationic polymeric ionic liquid for highly stretchable, nonflammable and notch-insensitive intrinsic self-healing solid-state polymer electrolyte for flexible and safe lithium batteries. Chem. Eng. J. 2022, 430, 132706. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly(vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. J. Colloid Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.P.; Hashmi, S.A. Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J. Power Sources 2009, 187, 627–634. [Google Scholar] [CrossRef]
- Sim, L.N.; Majid, S.R.; Arof, A.K. FTIR studies of PEMA/PVdF-HFP blend polymer electrolyte system incorporated with LiCF3SO3 salt. Vib. Spectrosc. 2012, 58, 57–66. [Google Scholar] [CrossRef]
- Asmara, S.N.; Kufian, M.Z.; Majid, S.R.; Arof, A.K. Preparation and characterization of magnesium ion gel polymer electrolytes for application in electrical double layer capacitors. Electrochim. Acta 2011, 57, 91–97. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Q.; Wang, D.; Ma, C.; Chen, Z.; Zhu, C.; Gao, Y.; Li, C. Decoupling the mechanical strength and ionic conductivity of an ionogel polymer electrolyte for realizing thermally stable lithium-ion batteries. J. Membr. Sci. 2020, 595, 117549. [Google Scholar] [CrossRef]
- Xing, C.; Li, B.; Zhang, J.; Su, P.; Pan, S.; Cao, Y.; Gao, E.; Zhang, H. Rational design of a pre-lithiated ionogel membrane with enhanced safety and electrochemical performances. Mater. Chem. Phys. 2021, 272, 124975. [Google Scholar] [CrossRef]
- Han, D.-D.; Liu, S.; Liu, Y.-T.; Zhang, Z.; Li, G.-R.; Gao, X.-P. Lithiophilic gel polymer electrolyte to stabilize the lithium anode for a quasi-solid-state lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, Y.; Ma, J.; Fan, M.; Zhang, S.; Wang, J. Ionic liquids for advanced materials. Mater. Today Nano 2022, 17, 100159. [Google Scholar] [CrossRef]
- Chen, N.; Xing, Y.; Wang, L.; Liu, F.; Li, L.; Chen, R.; Wu, F.; Guo, S. “Tai Chi” philosophy driven rigid-flexible hybrid ionogel electrolyte for high-performance lithium battery. Nano Energy 2018, 47, 35–42. [Google Scholar] [CrossRef]
- Yuan, C.; Zhu, X.; Su, L.; Yang, D.; Wang, Y.; Yang, K.; Cheng, X. Preparation and characterization of a novel ionic conducting foam-type polymeric gel based on polymer PVdF-HFP and ionic liquid [EMIM][TFSI]. Colloid Polym. Sci. 2015, 293, 1945–1952. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int. J. Hydrogen Energy 2019, 44, 8298–8306. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Su, Q.; Wu, M.; Que, X.; Liu, H. High-Power Bipolar Solid-State Batteries Enabled by In-Situ-Formed Ionogels for Vehicle Applications. ACS Appl. Mater. Interfaces 2022, 14, 5402–5413. [Google Scholar] [CrossRef]
- Taylor, M.E.; Clarkson, D.; Greenbaum, S.G.; Panzer, M.J. Examining the Impact of Polyzwitterion Chemistry on Lithium Ion Transport in Ionogel Electrolytes. ACS Appl. Polym. Mater. 2021, 3, 2635–2645. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Robust Succinonitrile Plastic Crystal-Based Ionogel for All-Solid-State Li-Ion and Dual-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4295–4304. [Google Scholar] [CrossRef]
- Dhatarwal, P.; Choudhary, S.; Sengwa, R.J. Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nanoparticles for the lithium ion batteries. Compos. Commun. 2018, 10, 11–17. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Abdullah, R.M.; Kadir, M.F.Z. A Promising Polymer Blend Electrolytes Based on Chitosan: Methyl Cellulose for EDLC Application with High Specific Capacitance and Energy Density. Molecules 2019, 24, 2503. [Google Scholar] [CrossRef]
- Hadi, J.M.; Aziz, S.B.; Nofal, M.M.; Hussen, S.A.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z.; Woo, H.J. Electrical, Dielectric Property and Electrochemical Performances of Plasticized Silver Ion-Conducting Chitosan-Based Polymer Nanocomposites. Membranes 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-N.; Jang, S.-J.; Kang, Y.C.; Roh, K.C. The effect of ILs as co-salts in electrolytes for high voltage supercapacitors. Sci. Rep. 2019, 9, 1180. [Google Scholar] [CrossRef]
- Pandey, G.P.; Kumar, Y.; Hashmi, S. Ionic liquid incorporated polymer electrolytes for supercapacitor application. Indian J. Chem. Sect. A 2010, 49A, 743–751. [Google Scholar]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Comparative Investigation of Activated Carbon Electrode and a Novel Activated Carbon/Graphene Oxide Composite Electrode for an Enhanced Capacitive Deionization. Materials 2020, 13, 5185. [Google Scholar] [CrossRef] [PubMed]
- Negre, L.; Daffos, B.; Turq, V.; Taberna, P.L.; Simon, P. Ionogel-based solid-state supercapacitor operating over a wide range of temperature. Electrochim. Acta 2016, 206, 490–495. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef]
- González-Gil, R.M.; Borràs, M.; Chbani, A.; Abitbol, T.; Fall, A.; Aulin, C.; Aucher, C.; Martínez-Crespiera, S. Sustainable and Printable Nanocellulose-Based Ionogels as Gel Polymer Electrolytes for Supercapacitors. Nanomaterials 2022, 12, 273. [Google Scholar] [CrossRef]
- Fontaine, O.; Touidjine, A.; Maréchal, M.; Bonhomme, C.; Ribot, F.; Geffroy, B.; Jousselme, B.; Sanchez, C.; Laberty-Robert, C. A one-pot route to prepare class II hybrid ionogel electrolytes. New J. Chem. 2014, 38, 2008–2015. [Google Scholar] [CrossRef]
- Janani, R.; Farmilo, N.; Roberts, A.; Sammon, C. Sol-gel synthesis pathway and electrochemical performance of ionogels: A deeper look into the importance of alkoxysilane precursor. J. Non-Cryst. Solids 2021, 569, 120971. [Google Scholar] [CrossRef]
- Wang, S.; Hsia, B.; Carraro, C.; Maboudian, R. High-performance all solid-state micro-supercapacitor based on patterned photoresist-derived porous carbon electrodes and an ionogel electrolyte. J. Mater. Chem. A 2014, 2, 7997–8002. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S. Electrical, structural, thermal and electrochemical properties of corn starch-based biopolymer electrolytes. Carbohydr. Polym. 2015, 124, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.F.Z.; Arof, A.K. Application of PVA–chitosan blend polymer electrolyte membrane in electrical double layer capacitor. Mater. Res. Innov. 2011, 15, s217–s220. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Brevik, I.; Hamsan, M.H.; Abdulwahid, R.T.; Majid, S.R.; Kadir, M.F.Z.; Hussen, S.A.; Abdullah, R.M. Characteristics of Glycerolized Chitosan: NH4NO3-Based Polymer Electrolyte for Energy Storage Devices with Extremely High Specific Capacitance and Energy Density Over 1000 Cycles. Polymers 2020, 12, 2718. [Google Scholar] [CrossRef]
- Lim, C.-S.; Teoh, K.H.; Liew, C.-W.; Ramesh, S. Capacitive behavior studies on electrical double layer capacitor using poly (vinyl alcohol)–lithium perchlorate based polymer electrolyte incorporated with TiO2. Mater. Chem. Phys. 2014, 143, 661–667. [Google Scholar] [CrossRef]
- Song, W.; Yang, L.; Sun, Z.; Li, F.; Du, S. Study on actuation enhancement for ionic-induced IL-cellulose based biocompatible composite actuators by glycerol plasticization treatment method. Cellulose 2018, 25, 2885–2899. [Google Scholar] [CrossRef]
- Villar-Chavero, M.M.; Domínguez, J.C.; Alonso, M.V.; Rigual, V.; Oliet, M.; Rodriguez, F. Viscoelastic properties of physical cellulosic bionogels of cholinium lysinate. Int. J. Biol. Macromol. 2019, 133, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.K.; Qader, I.B.; Alesary, H.F.; Kareem, J.H.; Ballantyne, A.D. Effect of Graphene Oxide and Temperature on Electrochemical Polymerization of Pyrrole and Its Stability Performance in a Novel Eutectic Solvent (Choline Chloride–Phenol) for Supercapacitor Applications. ACS Omega 2022, 7, 34326–34340. [Google Scholar] [CrossRef]
- Park, J.; Sun, J.-Y. Phase-Transitional Ionogel-Based Supercapacitors for a Selective Operation. ACS Appl. Mater. Interfaces 2022, 14, 23375–23382. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Tailoring of the structural, morphological, electrochemical, and dielectric properties of solid polymer electrolyte. Ionics 2019, 25, 1617–1632. [Google Scholar] [CrossRef]
- Liew, C.-W.; Ramesh, S.; Arof, A.K. Enhanced capacitance of EDLCs (electrical double layer capacitors) based on ionic liquid-added polymer electrolytes. Energy 2016, 109, 546–556. [Google Scholar] [CrossRef]
- Raistrick, I.D.; Macdonald, J.R.; Franceschetti, D.R. Theory. In Impedance Spectroscopy; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 21–105. [Google Scholar] [CrossRef]
- Abidin, S.Z.Z.; Ali, A.M.; Jaafar, N.K.; Yahya, M.Z.A. Electrical properties of cellulose acetate-based polymer gel electrolytes. AIP Conf. Proc. 2017, 1885, 020088. [Google Scholar] [CrossRef]
- Xiong, R.; Tian, J.; Mu, H.; Wang, C. A systematic model-based degradation behavior recognition and health monitoring method for lithium-ion batteries. Appl. Energy 2017, 207, 372–383. [Google Scholar] [CrossRef]
- Huang, Q.-A.; Shen, Y.; Huang, Y.; Zhang, L.; Zhang, J. Impedance Characteristics and Diagnoses of Automotive Lithium-Ion Batteries at 7.5% to 93.0% State of Charge. Electrochim. Acta 2016, 219, 751–765. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Huang, J.; Li, Z.; Liaw, B.Y.; Zhang, J. Graphical analysis of electrochemical impedance spectroscopy data in Bode and Nyquist representations. J. Power Sources 2016, 309, 82–98. [Google Scholar] [CrossRef]
- Faris, B.K.; Hassan, A.A.; Aziz, S.B.; Brza, M.A.; Abdullah, A.M.; Abdalrahman, A.A.; Abu Ali, O.A.; Saleh, D.I. Impedance, Electrical Equivalent Circuit (EEC) Modeling, Structural (FTIR and XRD), Dielectric, and Electric Modulus Study of MC-Based Ion-Conducting Solid Polymer Electrolytes. Materials 2022, 15, 170. [Google Scholar] [CrossRef]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Zhao, L.; Dai, H.; Pei, F.; Ming, P.; Wei, X.; Zhou, J. A Comparative Study of Equivalent Circuit Models for Electro-Chemical Impedance Spectroscopy Analysis of Proton Exchange Membrane Fuel Cells. Energies 2022, 15, 386. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Pinto, R.S.; Correia, D.M.; Fidalgo-Marijuan, A.; Gonçalves, R.; Ferdov, S.; Lanceros-Mendez, S.; Costa, C.M. Poly(vinylidene fluoride-co-hexafluoropropylene) based tri-composites with zeolite and ionic liquid for electromechanical actuator and lithium-ion battery applications. Electrochim. Acta 2022, 431, 141186. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Bazito, F.F.C.; Siqueira, L.J.A.; Ribeiro, M.C.C.; Torresi, R.M. Transport Coefficients, Raman Spectroscopy, and Computer Simulation of Lithium Salt Solutions in an Ionic Liquid. J. Phys. Chem. B 2008, 112, 2102–2109. [Google Scholar] [CrossRef]
- Asenbauer, J.; Ben Hassen, N.; McCloskey, B.D.; Prausnitz, J.M. Solubilities and ionic conductivities of ionic liquids containing lithium salts. Electrochim. Acta 2017, 247, 1038–1043. [Google Scholar] [CrossRef]
- Tong, J.; Wu, S.; von Solms, N.; Liang, X.; Huo, F.; Zhou, Q.; He, H.; Zhang, S. The Effect of Concentration of Lithium Salt on the Structural and Transport Properties of Ionic Liquid-Based Electrolytes. Front. Chem. 2020, 7, 945. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.C.; Zhang, Y.; Costa, L.T.; Maginn, E.J. A molecular dynamics study of lithium-containing aprotic heterocyclic ionic liquid electrolytes. J. Chem. Phys. 2018, 148, 193834. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).