Controllable Synthesis of ZnO Nanoparticles with Improved Photocatalytic Performance for the Degradation of Rhodamine B under Ultraviolet Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
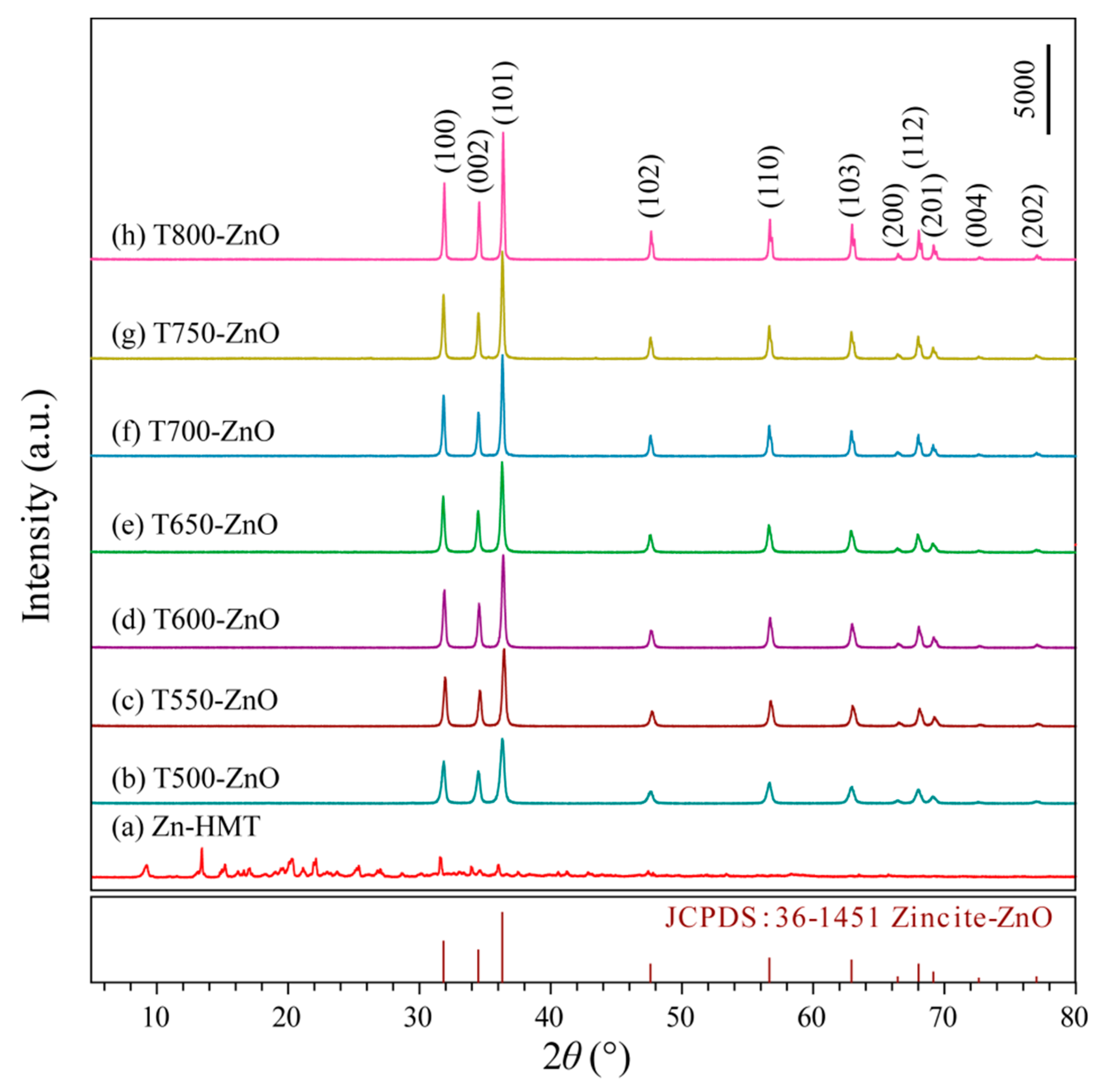
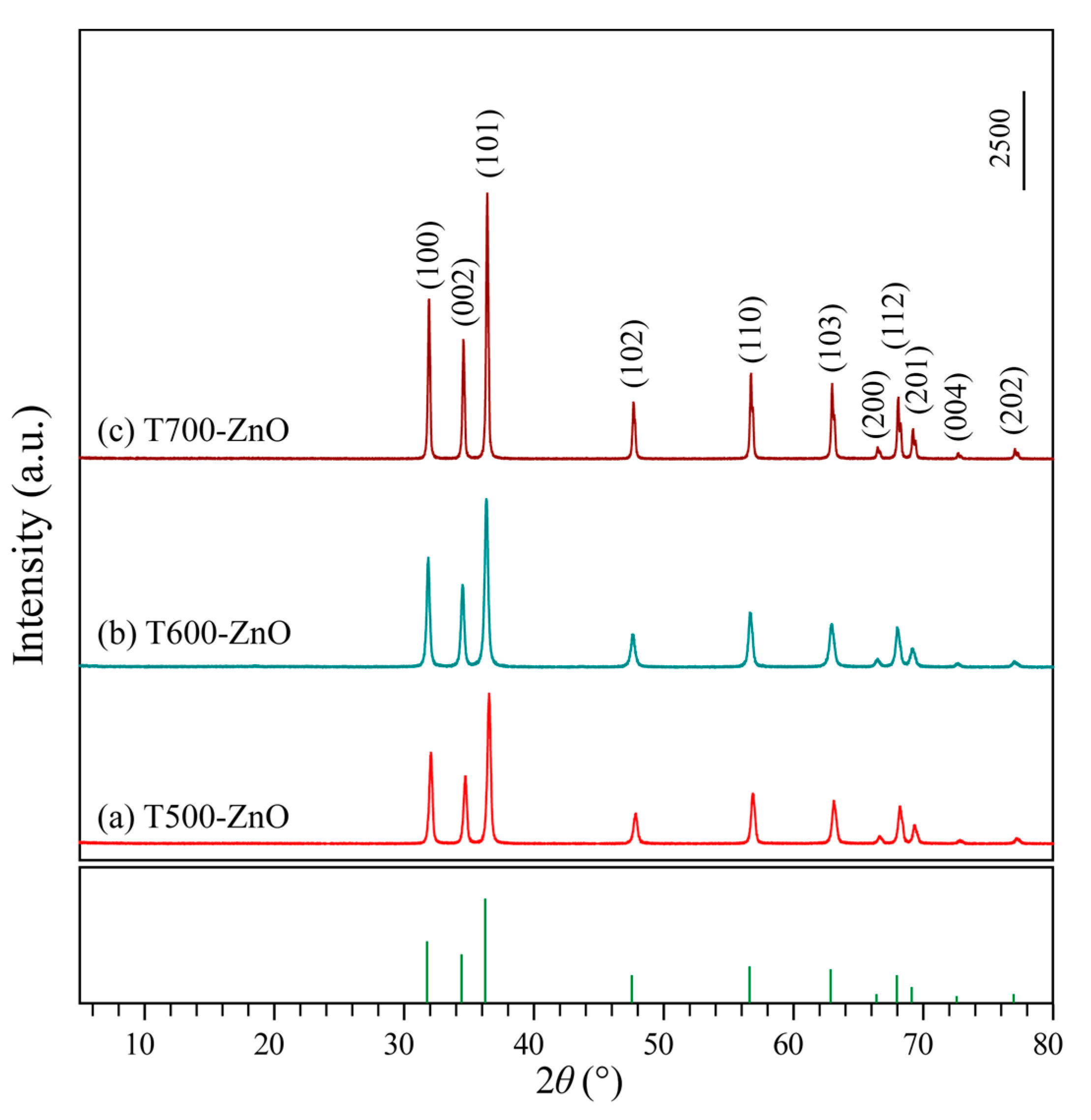
2.1. Structural Analysis
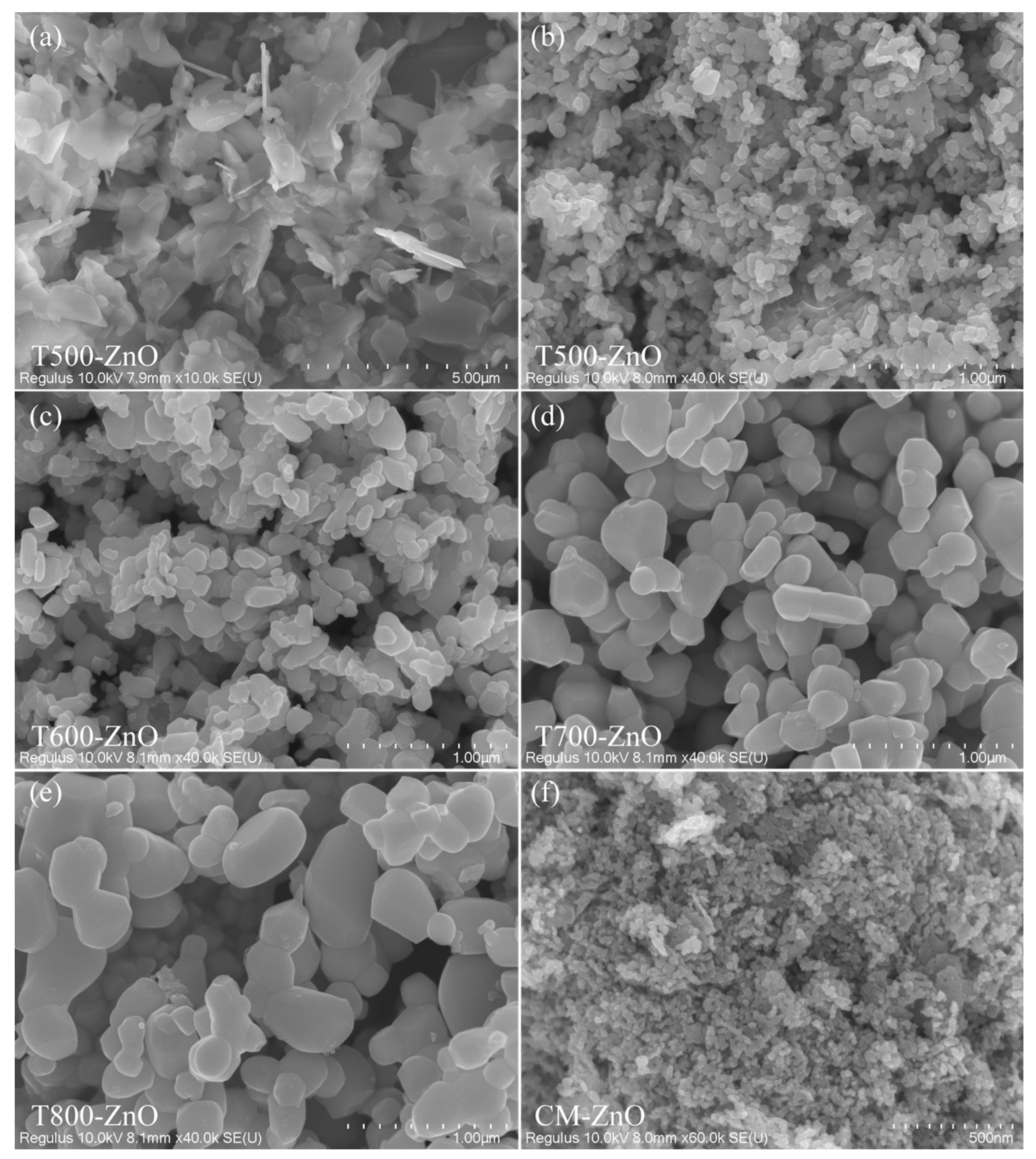
2.2. Morphological Analysis
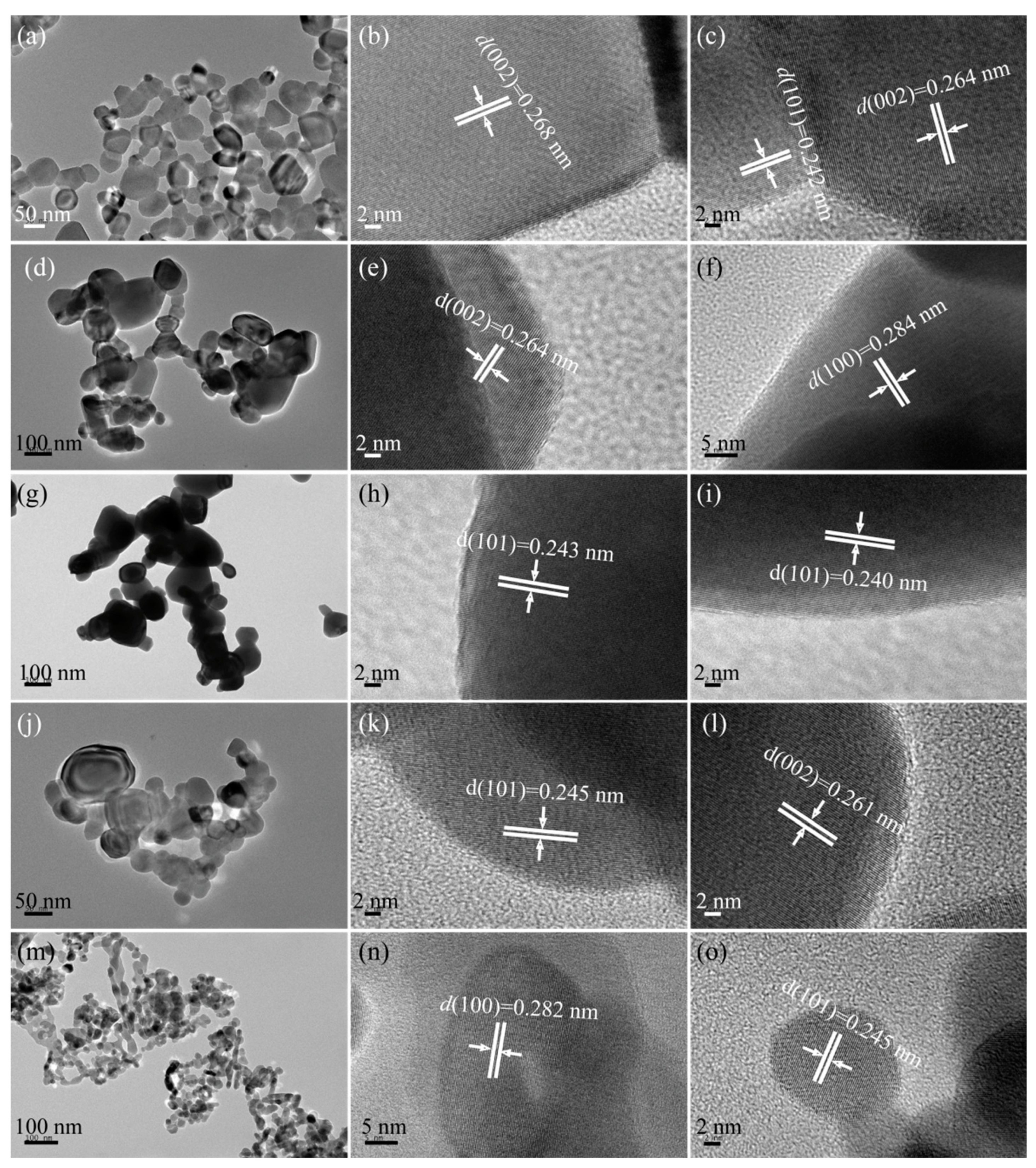
2.3. Microstructure Analysis
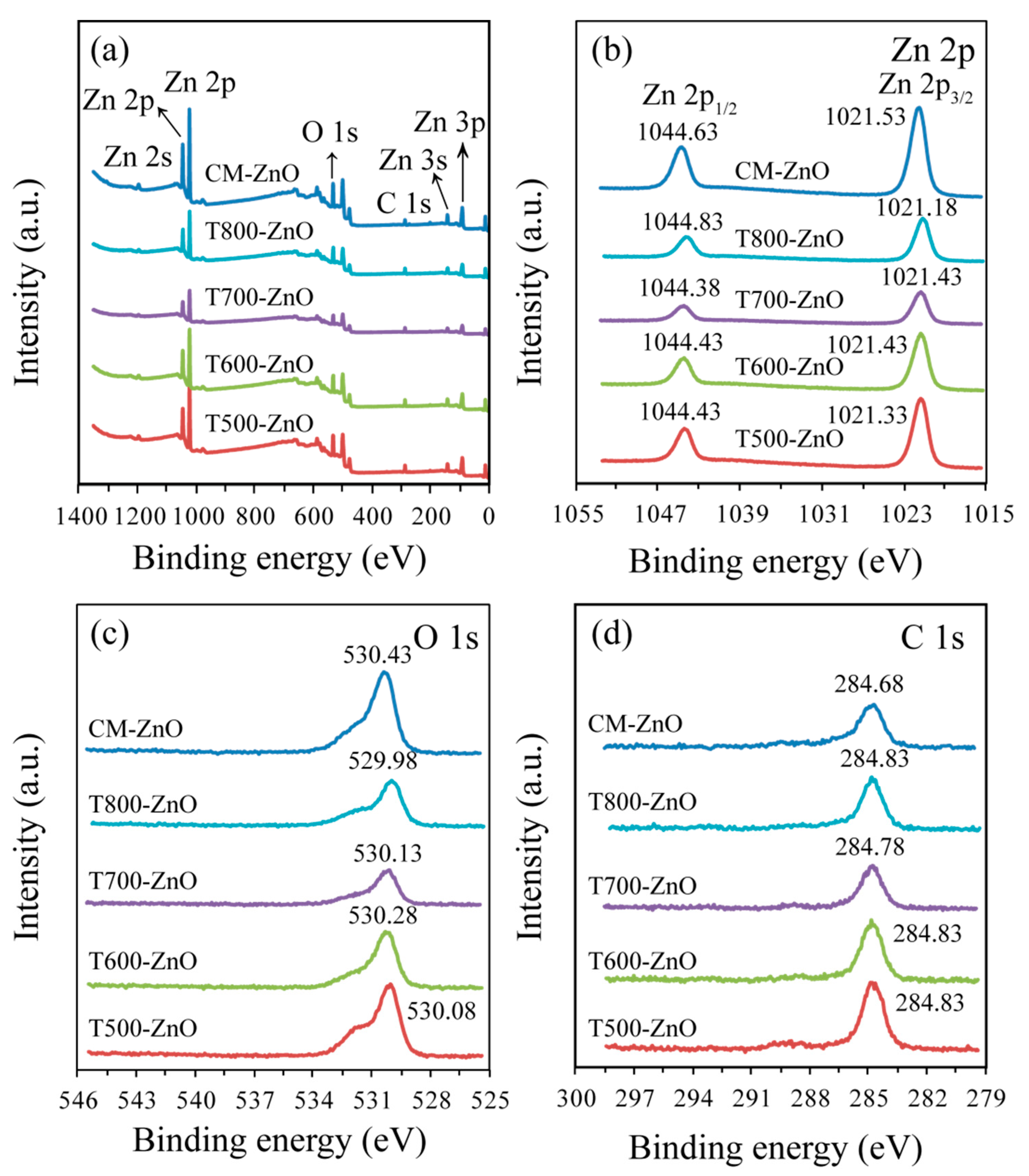
2.4. Surface and Interface Analysis
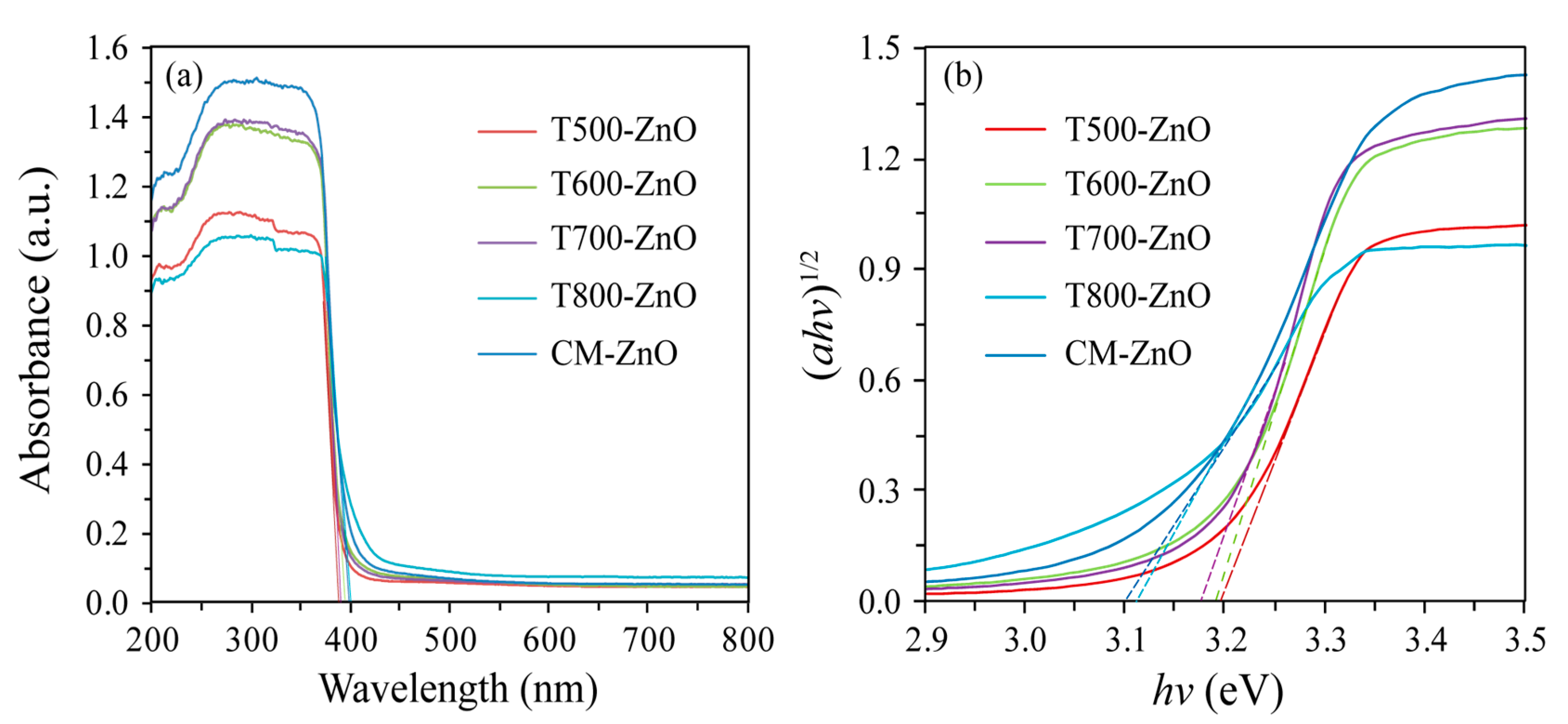
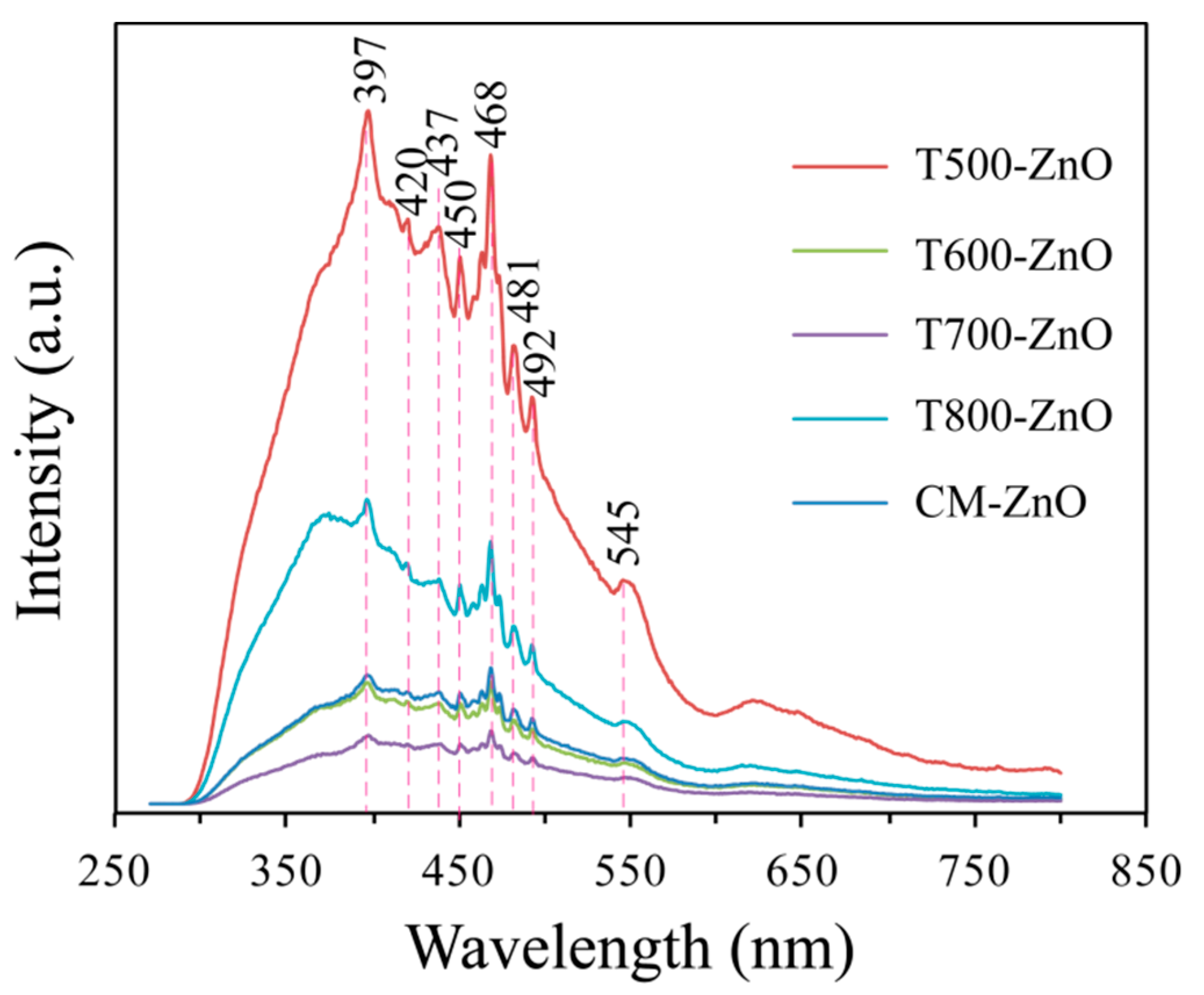
2.5. Optical Properties
2.6. Electrochemical Impedance Spectroscopy Analysis
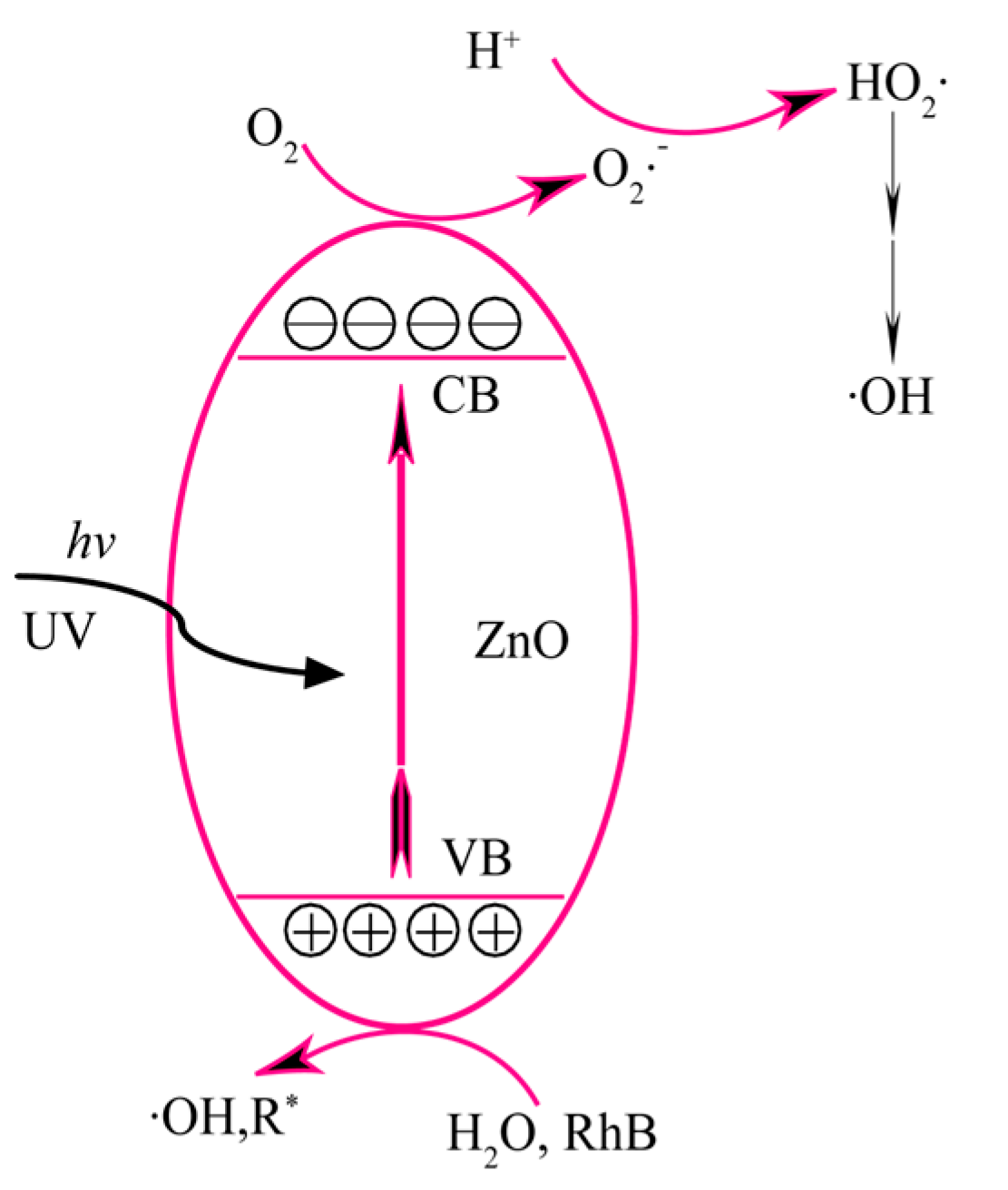
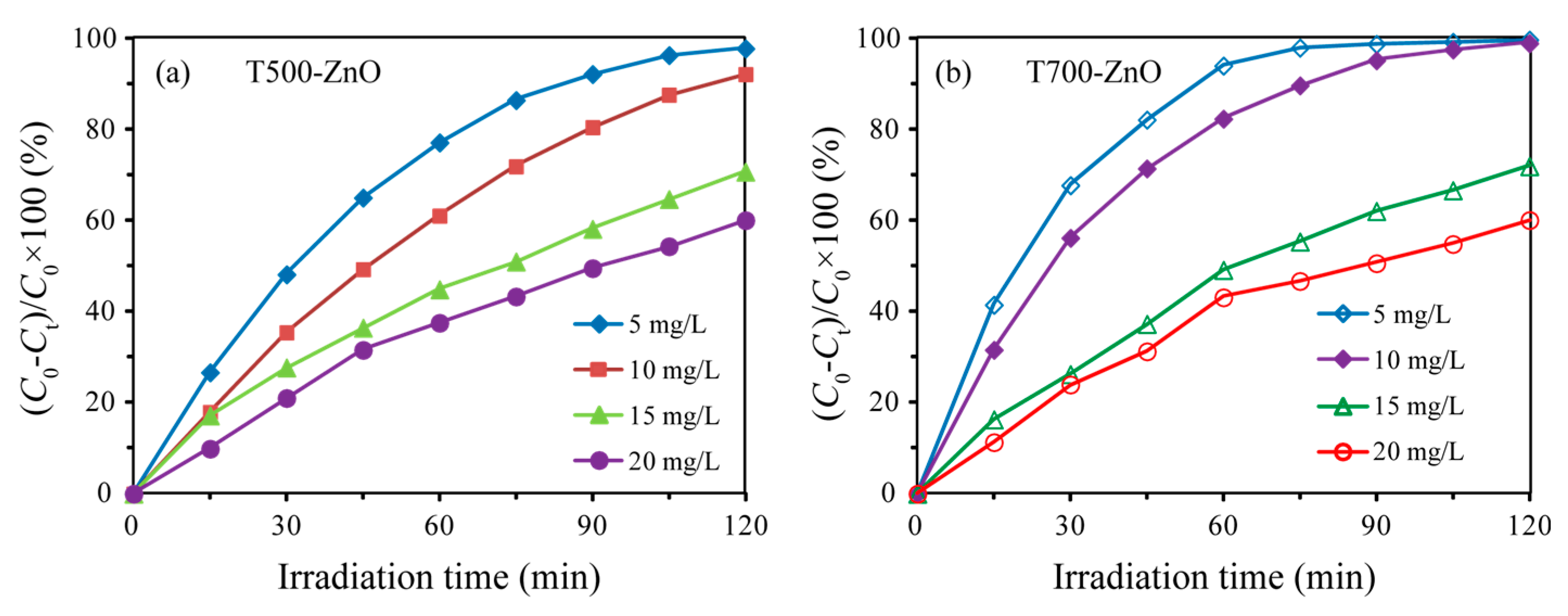
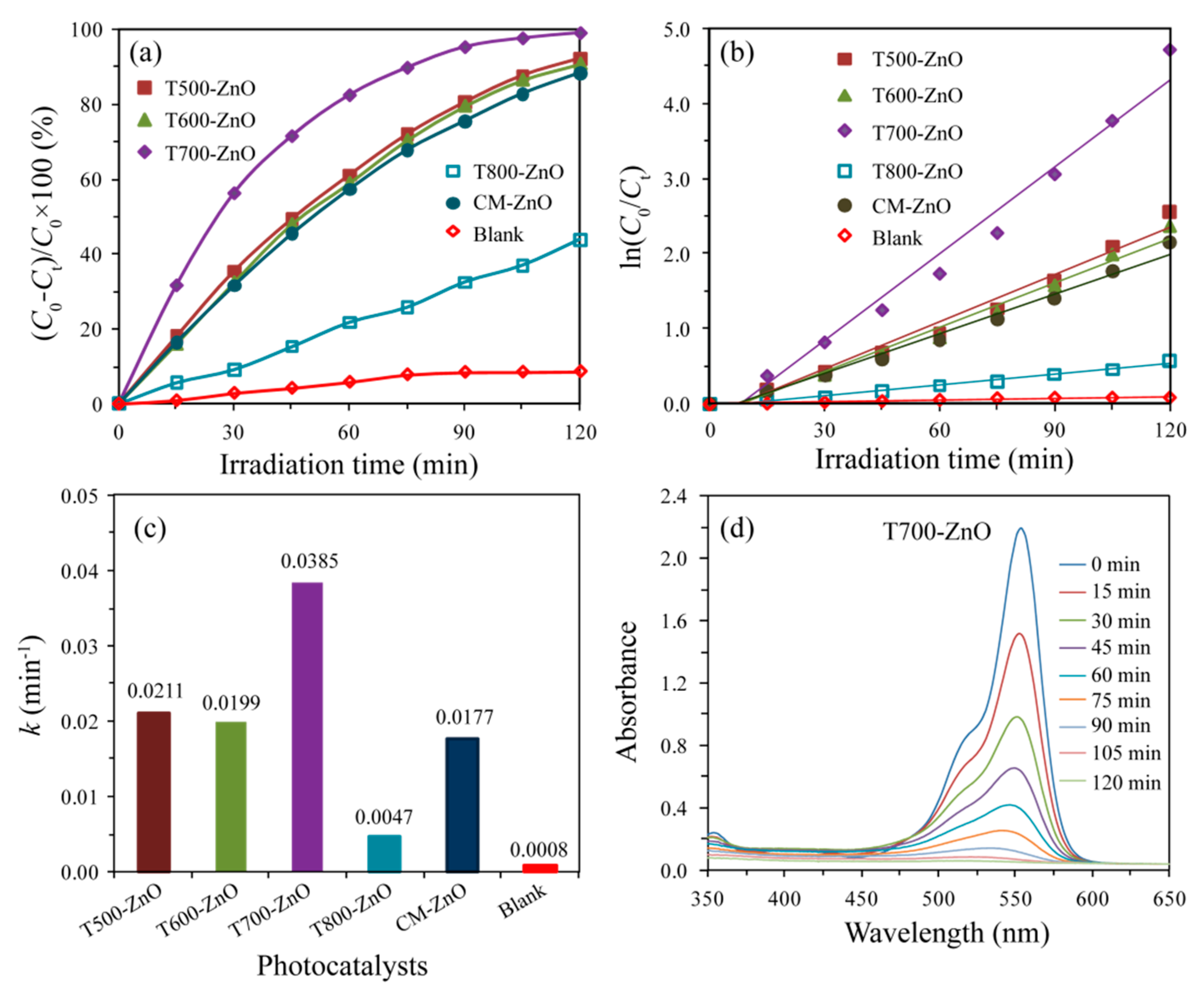
2.7. Photocatalytic Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZnO Nanoparticles
3.3. Characterization
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sher, M.; Javed, M.; Shahid, S.; Iqbal, S.; Qamar, M.A.; Bahadur, A.; Qayyumd, M.A. The controlled synthesis of g-C3N4/Cd-doped ZnO nanocomposites as potential photocatalysts for the disinfection and degradation of organic pollutants under visible light irradiation. RSC Adv. 2021, 11, 2025–2039. [Google Scholar] [CrossRef]
- Corrado, J.M.D.; Fernando, J.F.S.; Shortell, M.P.; Poad, B.L.J.; Blanksby, S.J.; Waclawik, E.R. ZnO colloid crystal facet-type determines both Au photodeposition and photocatalytic activity. ACS Appl. Nano Mater. 2019, 2, 7856–7869. [Google Scholar] [CrossRef]
- Oviedo, L.R.; Muraro, P.C.R.; Pavoski, G.; Espinosa, D.C.R.; Ruiz, Y.P.M.; Galembeck, A.; Rhoden, C.R.B.; Silva, W.L.D. Synthesis and characterization of nanozeolite from (agro)industrial waste for application in heterogeneous photocatalysis. Environ. Sci. Pollut. R. 2022, 29, 3794–3807. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.L.D.; Hamilton, J.W.J.; Sharma, P.K.; Dunlop, P.S.M.; Byrne, J.A.; Santos, J.H.Z.D. Agro and industrial residues: Potential raw materials for photocatalyst development. J. Photoch. Photobio. A 2021, 411, 113184. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, Z.; Yan, X.; Liao, Q. ZnO nanostructures in enzyme biosensors. Sci. China Mater. 2015, 58, 60–76. [Google Scholar] [CrossRef]
- Kim, D.W.; Leem, J.Y. Transparent and flexible ZnO nanorods induced by thermal dissipation annealing without polymer substrate deformation for next-generation wearable devices. RSC Adv. 2021, 11, 17538–17546. [Google Scholar] [CrossRef] [PubMed]
- Manikanika; Chopra, L. Photo-degradation of dyes and drugs using aloe vera synthesized zinc oxide nanoparticles—A review. Mater. Today-Proc. 2023, 72, 1613–1617. [Google Scholar] [CrossRef]
- Campos, A.C.; Paes, S.C.; Correa, B.S.; Cabrera-Pasca, G.A.; Costa, M.S.; Costa, C.S.; Otubo, L.; Carbonari, A.W. Growth of long ZnO nanowires with high density on the ZnO surface for gas sensors. ACS Appl. Nano Mater. 2020, 3, 175–185. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Song, W.W.; Li, P.; Ryou, J.H.; Dupuis, R.D.; Sunmmers, C.J.; Wang, Z.L. Growth of uniformly aligned ZnO nanowire heterojunction arrays on GaN, AlN, and Al0.5Ga0.5N substrates. J. Am. Chem. Soc. 2005, 127, 7920–7923. [Google Scholar]
- Chu, F.H.; Huang, C.W.; Hsin, C.L.; Wang, C.W.; Yu, S.Y.; Yeh, P.H.; Wu, W.W. Well-aligned ZnO nanowires with excellent field emission and photocatalytic properties. Nanoscale 2012, 4, 1471–1475. [Google Scholar] [CrossRef]
- Protasova, L.N.; Rebrov, E.V.; Choy, K.L.; Pung, S.Y.; Engels, V.; Cabaj, M.; Wheatley, A.E.H.; Schouten, J.C. ZnO based nanowires grown by chemical vapour deposition for selective hydrogenation of acetylene alcohols. Catal. Sci. Technol. 2011, 1, 768–777. [Google Scholar] [CrossRef]
- Lu, C.H.; Chi-Hsien Yeh, C.H. Influence of hydrothermal conditions on the morphology and particle size of zinc oxide powder. Ceram. Int. 2000, 26, 351–357. [Google Scholar] [CrossRef]
- Chen, D.R.; Jiao, X.L.; Cheng, G. Hydrothermal synthesis of zinc oxide powders with different morphologies. Solid State Commun. 2000, 113, 11363–11366. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Namgung, G.; Noh, J.S. Facile synthesis of porous metal-doped ZnO/g-C3N4 composites for highly efficient photocatalysts. J. Photoch. Photobiol. A 2019, 368, 110–119. [Google Scholar] [CrossRef]
- Ta, Q.T.H.; Cho, E.; Streedhar, A.; Noh, J.S. Mixed-dimensional, three-level hierarchical nanostructures of silver and zinc oxide for fast photocatalytic degradation of multiple dyes. J. Catal. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Shahi, S.K.; Sandhu, S.; Kaur, N.; Shahi, J.S.; Kaur, M.; Singh, V.; Singh, V. DES-mediated synthesis of ZnO nanostructures with exposed {0001} facets: Photoluminescence and photocatalytic properties. New J. Chem. 2022, 46, 18865–18873. [Google Scholar] [CrossRef]
- Benu, D.P.; Andriani, A.; Silmi, N.; Steky, F.V.; Failamani, F.; Yuliarto, B.; Mukti, R.R.; Suendo, V. Macroemulsion-mediated synthesis of fibrous ZnO microrods and their surface morphology contribution to the high photocatalytic degradation rate. New J. Chem. 2023, 47, 428–442. [Google Scholar] [CrossRef]
- Liu, S.T.; Zhou, J.S.; Song, H.H. 2D Zn-hexamine coordination frameworks and their derived N-rich porous carbon nanosheets for ultrafast sodium storage. Adv. Energy Mater. 2018, 8, 1800569. [Google Scholar] [CrossRef]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Darvishi, S.M.R. ZnO photocatalyst revisited: Effective photocatalytic degradation of emerging contaminants using S-doped ZnO nanoparticles under visible light radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Toupance, T.; Servant, L.; Müller, M.M.; Kleebe, H.J.; Ziegler, J.; Jaegermann, W. Nanostructured SnO2-ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg. Chem. 2012, 51, 7764–7773. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.X. Construction of highly ordered ZnO-TiO2 nanotube arrays (ZnO/TNTs) heterostructure for photocatalytic application. ACS Appl. Mater. Interfaces 2012, 4, 7055–7063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Wei, X.M.; Bai, X.; Liu, C.M.; Wang, B.Y.; Liu, J.W. Ethanol-water ambient precipitation of {111} facets exposed Ag3PO4 tetrahedra and its hybid with grapheme oxide for outstangding photoactivity and stability. Inorg. Chem. Front. 2018, 5, 951–961. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; Haq, A.U.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846. [Google Scholar] [CrossRef]
- Delekar, S.D.; Dhodamani, A.G.; More, K.V.; Dongale, T.D.; Kamat, R.K.; Acquah, S.F.A.; Dalal, N.S.; Panda, D.K. Structural and optical properties of nanocrystalline TiO2 with multiwalled carbon nanotubes and its photovoltaic studies using Ru(II) semsitizers. ACS Omega 2018, 3, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Georgekutty, R.; Seery, M.K.; Suresh, C.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Han, W.; Oh, S.; Lee, C.; Kim, J.; Park, H.H. ZnO nanocrystal thin films for quantum-dot light-emitting devices. ACS Appl. Nano Mater. 2020, 3, 7535–7542. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, Y.; Tay, Q.; Zhang, Y.; Malyi, O.I.; Wang, D.; Leng, J.; Lai, Y.; Zhou, H.; Chen, Z.; et al. Understanding the role of nanostructures for efficient hydrogen generation on immo-bilized photocatalysts. Adv. Energy Mater. 2013, 3, 1368–1380. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Shi, H.; Jin, B.; Qin, X.; Wang, G.; Li, K.; Zhang, T.; Zhang, H. In-site synthesis of an inorganic-framework molecular imprinted TiO2/CdS heterostructure for the photoelectrochemical sensing of bisphenol A. Anal. Methods 2021, 13, 2857–2864. [Google Scholar] [CrossRef]
- Wu, T.X.; Liu, G.M.; Zhao, J.C. Photoassisted degradation of dye pollutants. v. self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation inaqueous TiO2 dispersions. J. Phys. Chem. B 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Du, P.F.; Song, L.X.; Xiong, J. Photocatalytic degradation of Rhidamine B using electrospun TiO2 and ZnO nanofibers: A comparative study. J. Mater. Sci. 2013, 48, 8386–8392. [Google Scholar] [CrossRef]
- Du, Y.E.; Niu, X.J.; He, J.; Liu, L.; Liu, Y.F.; Chen, C.D.; Yang, X.J.; Qi Feng, Q. Hollow square rodLike microtubes composed of anatase nanocuboids with coexposed {100}, {010}, and {001} facets for improved photocatalytic performance. ACS Omega 2020, 5, 14147–14156. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Waclawik, E.R. Facet-controlled self-assembly of ZnO nanocrystals by non-hydrolytic aminolysis and their photodegradation activities. CrystEngComm 2012, 14, 4041–4048. [Google Scholar] [CrossRef]
- Carlucci, C.; Xu, H.; Scremin, B.F.; Giannini, C.; Altmura, D.; Carlino, E.; Videtta, V.; Conciauro, F.; Gihli, G.; Ciccarella, G. Selective synthesis of TiO2 nanocrystals with the microwave-solvothermal method. CrystEngComm 2014, 16, 1817–1824. [Google Scholar] [CrossRef]
- Udawatte, N.; Lee, M.; Kim, J.; Lee, D. Well-defined Au/ZnO nanoparticle composites exhibiting enhanced photocatalytic activities. ACS Appl. Mater. Interfaces 2011, 3, 4531–4538. [Google Scholar] [CrossRef]
- Du, Y.E.; Niu, X.J.; Li, W.X.; An, J.; Liu, Y.F.; Chen, Y.Q.; Wang, P.F.; Yang, X.J.; Feng, Q. Microwave-assisted synthesis of high-energy faceted TiO2 nanocrystals derived from exfoliated porous metatitanic acid nanosheets with improved photocatalytic and photovoltaic performance. Materials 2019, 12, 3614. [Google Scholar] [CrossRef]
- Wei, X.X.; Cui, B.Y.; Wang, X.X.; Cao, Y.Z.; Gao, L.P.; Guo, S.Q.; Chen, C.M. Tuning the physico-chemical properties of BiOBr via solvent adjustment: Towards an efficient photocatalyst for water treatment. CrystEngComm 2019, 21, 1750–1757. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, G.H.; Cheng, B.; Zhou, M.H. E_ects of hydrothermal temperature and time on the photocatalytic activity and microstructures of bimodal mesoporous TiO2 powders. Appl. Catal. B 2007, 69, 171–180. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, B.L.; Liu, Z.H.; Li, D.H.; Ge, C.Y.; Fang, Y.P. Controlled synthesis of ZnGa2O4 nanorod arrays from hexagonal ZnO microdishes and their photocatalytic activity on the degradation of RhB. RSC Adv. 2014, 4, 48590–48595. [Google Scholar] [CrossRef]
- Liu, X.S.; Wang, G.; Zhi, H.; Dong, J.; Hao, J.; Zhang, X.; Wang, J.; Danting Li, D.T.; Baosheng Liu, B.S. Synthesis of the porous ZnO nanosheets and TiO2/ZnO/FTO composite films by a low-temperature hydrothermal method and their applications in photocatalysis and electrochromism. Coatings 2022, 12, 695. [Google Scholar] [CrossRef]
- Tadesse, A.; Hagos, M.; Belachew, N.; Ananda Murthy, H.C.; Basavaiah, K. Enhanced photocatalytic degradation of Rhodamine B, antibacterial and antioxidant activities of green synthesised ZnO/N doped carbon quantum dot nanocomposites. New J. Chem. 2021, 45, 21852–21862. [Google Scholar] [CrossRef]
- Stefan, M.; Popa, A.; Toloman, D.; Leostean, C.; Barbu-Tudoran, L.; Falamas, A. Enhanced Plasmonic Photocatalysis of Au-decorated ZnO nanocomposites. Inorganics 2023, 11, 157. [Google Scholar] [CrossRef]
- Luque-Morales, P.Q.; Lopez-Peraza, A.; Nava-Olivas, O.J.; Amaya-Parra, G.; Baez-Lopez, Y.A.; Orozco-Carmona, V.M.; Garrafa-Galvez, H.E.; Chinchillas-Chinchillas, M.D.J. ZnO semiconductor nanoparticles and their application in photocatalytic degradation of various organic dyes. Materials 2021, 14, 7537. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, J.; Mohsennia, M.; Khayatkashani, M. Green synthesis and characterization of Ud-SnO2-ZnO using Urtica dioica leaf extract: A nanocomposite photocatalyst for degradation of Rhodamine B dye. Res. Chem. Intermed. 2021, 47, 4789–4802. [Google Scholar] [CrossRef]
- Rana, N.; Chand, S.; Gathania, A.K. Synthesis and characterization of flower-like ZnO structures and their applications in photocatalytic degradation of Rhodamine B dye. J. Mater. Sci. Mater. Electron. 2016, 27, 2504–2510. [Google Scholar] [CrossRef]



| Chemical Methods of Synthesis | Precursors | Synthesis Conditions | Morphologies | Reference |
|---|---|---|---|---|
| High-temperature solid-phase synthesis method | Zinc acetate dehydrate, sodium lauryl sulfate, sodium hydroxide | Calcined at 580 °C for 2 h | Nanoparticles | [1] |
| Reflux method | Anhydrous zinc acetate, benzylamine, dibenzyl ether | Refluxed at 220 °C for 5 h; refluxed at 210 °C, 2 h | Nanorods, nanobullets, nanolates | [2] |
| Hydrothermal method | Zinc nitrate hexahydrate, hexamethylenetetramine | Hydrothermal process at 140 °C for 4 h | Nanorods | [6] |
| Thermal oxidation procedure | Zn foils, isopropyl alcohol | Zinc foils were thermally oxidized at 520 °C for 60 min, subsequently, samples were heated at 620 °C for 60 min | Nanowires | [8] |
| Carbothermal reduction and vapor–liquid– solid method. | ZnO, graphite powder, | Calcined ZnO and graphite powder at 950 °C | Nanowires | [11] |
| Chemical vapor deposition method | Diethylzinc, Zn powder | Heated Zn powder and ZnO seed layer at 650 °C under a constant flow of argon gas | Nanowires | [12] |
| Hydrothermal method | Zinc nitrate, ammonia | Hydrothermal process at 100–200 °C for 0.5–2 h | Ellipsoidal shape and rod-like shape | [13] |
| Hydrothermal method | Zinc chloride, sodium hydroxide | Hydrothermal process at 100–220 °C for 5–10 h | Bullet-like, rod-like, sheet, polyhedron, crushed stone-like | [14] |
| Hydrothermal method | Zinc acetate, sodium hydroxide | Hydrothermal process at 140–170 °C for 4–15 h | Flower-like, cauliflower-like | [17] |
| Solvothermal method | Zinc nitrate hexahydrate, zinc acetate dehydrate, urea | Solvothermal process at 120 °C for 24 h, then calcined at 400 °C for 4 h | Fibrous microrods | [18] |
| Chemical Methods of Synthesis | Raw Materials | Test Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|
| Solvothermal treatment, 120 °C for 24 h, then calcined at 400 °C for 4 h | Zinc nitrate hexahydrate, zinc acetate dehydrate, urea | 0.0080 g fibrous ZnO microrods, 8 mL 5 ppm RhB aqueous, UV LED | 73.82% (60 min) | [18] |
| Hydrothermal method, 175 °C, 12 h | Stearic acid, Zinc stearate, distilled water | 0.100 g ZnO microdishes, 250 mL 1.04 × 10−5 mol/L RhB aqueous solution, 300 W mercury lamp | 57.1% (90 min) | [39] |
| Hydrothermal method, 150 °C, 12 h | Zn(NO3)2·6(H2O), Na2CO3, hexamethylenetetramine, | 0.100 g ZnO nanosheets, 40 mg/L RhB aqueous solution, unspecified volume of Rhodamine B solution, 500 W mercury lamp, | 96.8% (120 min) | [40] |
| Hydrothermal method, 200 °C, 8 h | Sesame oil, and urea, zinc acetate dehydrate, ammonia | 0.050 g ZnO nanoparticles, 100 mL 10 mg/L RhB aqueous solution, 100 μL 30% H2O2, unspecified intensity of ultraviolet light source | 44% (180 min) | [41] |
| Sonication, 80 °C, 2 h | zinc acetate dehydrate, sodium hydroxide, distilled water | 0.0060 g ZnO nanoparticles, 10 mL 1.0 × 10−5 mol/L RhB aqueous solution, 400 W halogen lamp | 43% (300 min) | [42] |
| Low-temperature solution method, 30 °C, 6 days | Zn(NO3)2·6(H2O), distilled water, AgNWs, NaOH, ethyl alcohol, EDA | 0.0200 g ZnO nanorods, 50 mL 1.0 × 10−5 mol/L RhB aqueous solution, an air mass 1.5 (AM 1.5) solar simulator, the distance from the solar simulator to the reactor was 20 cm, | 68.4% (40 min) | [16] |
| Green Synthesis | Zn(NO3)2·6(H2O) capsicum annuum var | 0.0500 g ZnO nanoparticles, 50 mL 15 mg/L RhB aqueous solution, 10 W UV light lamps | 92% (180 min) | [43] |
| Green Synthesis | Zinc acetate dihydrate, methanol, U. dioica leaf extract | 0.100 g ZnO, 100 mL 10 mg/L RhB aqueous solution, 250 W UV-A lamps | 85% (140 min) | [44] |
| Refluxed, 70 °C, 2.5 h | Zinc acetate dehydrate, methanol, Lithium hydroxide | 0.050 g flower-like ZnO, 100 mL 10 ppm RhB aqueous solution, 30 W UV lamp | 73% (5 h) | [45] |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T500-ZnO nanoparticles, 150 mL 5 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 97.96% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T500-ZnO nanoparticles, 150 mL 10 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 92.32% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T500-ZnO nanoparticles, 150 mL 15 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 70.84% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T500-ZnO nanoparticles, 150 mL 20 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 60.19% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T500-ZnO nanoparticles, 150 mL 10 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 92.32% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T700-ZnO nanoparticles, 150 mL 5 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 99.88% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T700-ZnO nanoparticles, 150 mL 10 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 99.12% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T700-ZnO nanoparticles, 150 mL 15 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 72.08% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T700-ZnO nanoparticles, 150 mL 20 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 60.12% (120 min) | This work |
| Precipitation method combined with high-temperature calcination process | Zn(NO3)2·6H2O,HMT, anhydrous ethanol | 0.100 g T800-ZnO nanoparticles, 150 mL 10 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 44.04% (120 min) | This work |
| - | - | 0.100 g CM-ZnO nanoparticles, 150 mL 10 mg/L RhB aqueous solution, distance 25 cm, 175 W low pressure mercury lamp | 88.38% (120 min) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Du, Y.; Qu, X.; Li, Y.; Yin, L.; Shen, K.; Zhang, J.; Liu, Y. Controllable Synthesis of ZnO Nanoparticles with Improved Photocatalytic Performance for the Degradation of Rhodamine B under Ultraviolet Light Irradiation. Molecules 2023, 28, 5135. https://doi.org/10.3390/molecules28135135
Ren X, Du Y, Qu X, Li Y, Yin L, Shen K, Zhang J, Liu Y. Controllable Synthesis of ZnO Nanoparticles with Improved Photocatalytic Performance for the Degradation of Rhodamine B under Ultraviolet Light Irradiation. Molecules. 2023; 28(13):5135. https://doi.org/10.3390/molecules28135135
Chicago/Turabian StyleRen, Xinyue, Yien Du, Xinji Qu, Yumei Li, Luxi Yin, Kaixin Shen, Jingwen Zhang, and Yufang Liu. 2023. "Controllable Synthesis of ZnO Nanoparticles with Improved Photocatalytic Performance for the Degradation of Rhodamine B under Ultraviolet Light Irradiation" Molecules 28, no. 13: 5135. https://doi.org/10.3390/molecules28135135
APA StyleRen, X., Du, Y., Qu, X., Li, Y., Yin, L., Shen, K., Zhang, J., & Liu, Y. (2023). Controllable Synthesis of ZnO Nanoparticles with Improved Photocatalytic Performance for the Degradation of Rhodamine B under Ultraviolet Light Irradiation. Molecules, 28(13), 5135. https://doi.org/10.3390/molecules28135135







