Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Components of Liqueur Wines
2.2. Aroma Quality of Liqueur Wines
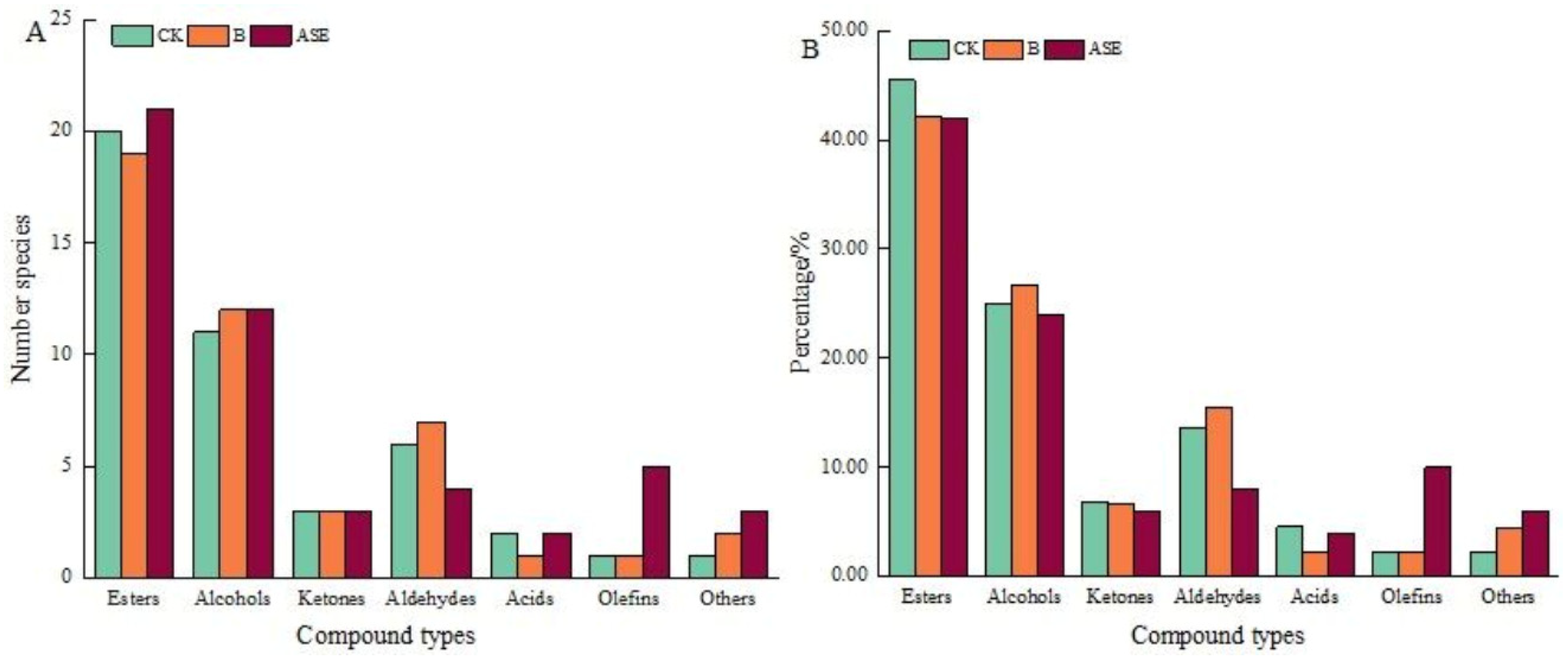
2.2.1. Volatile Compound Analysis
Esters
Alcohols
Carbonyl Compounds
Other Volatile Compounds
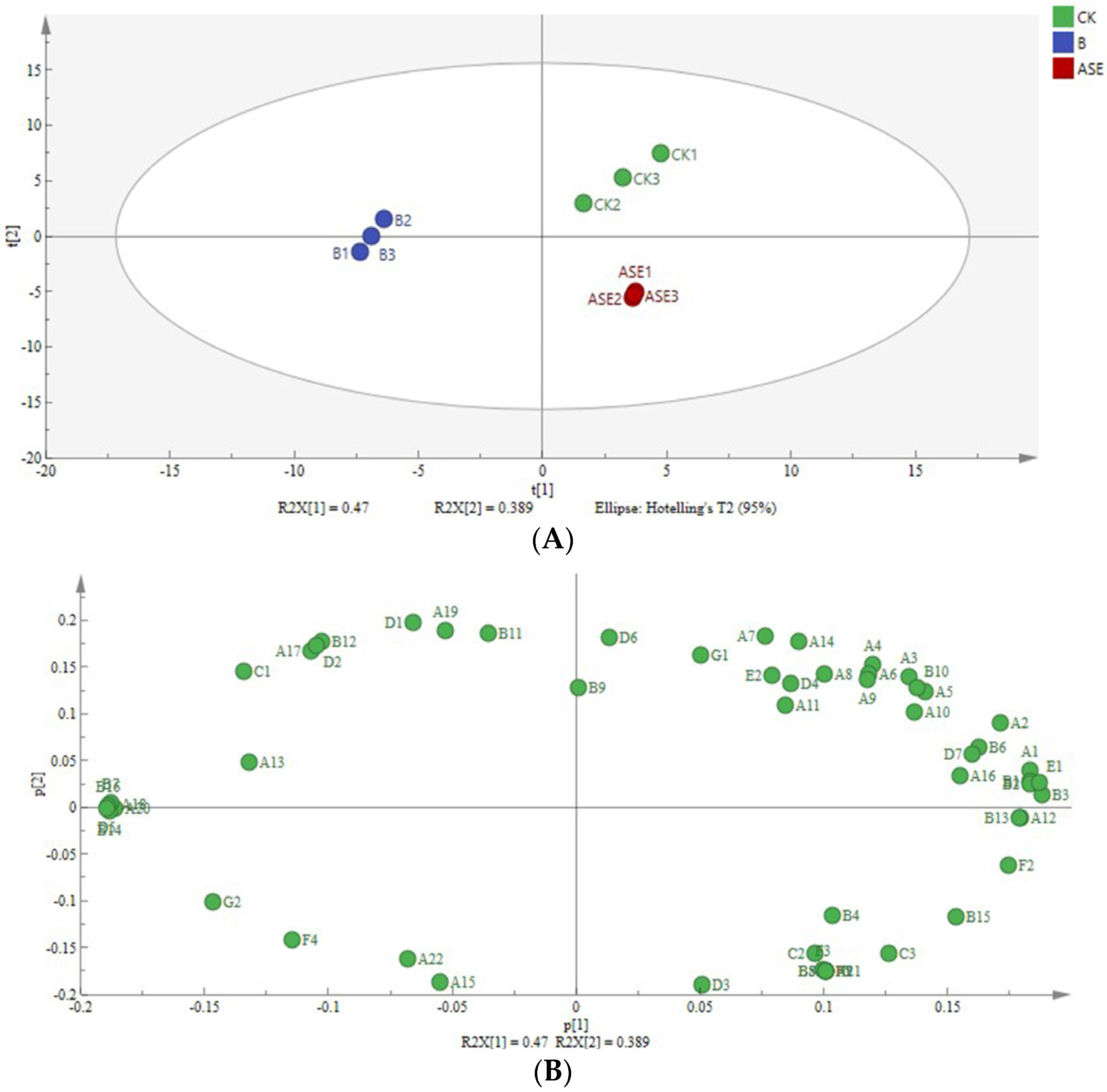
2.2.2. Principal Component Analysis
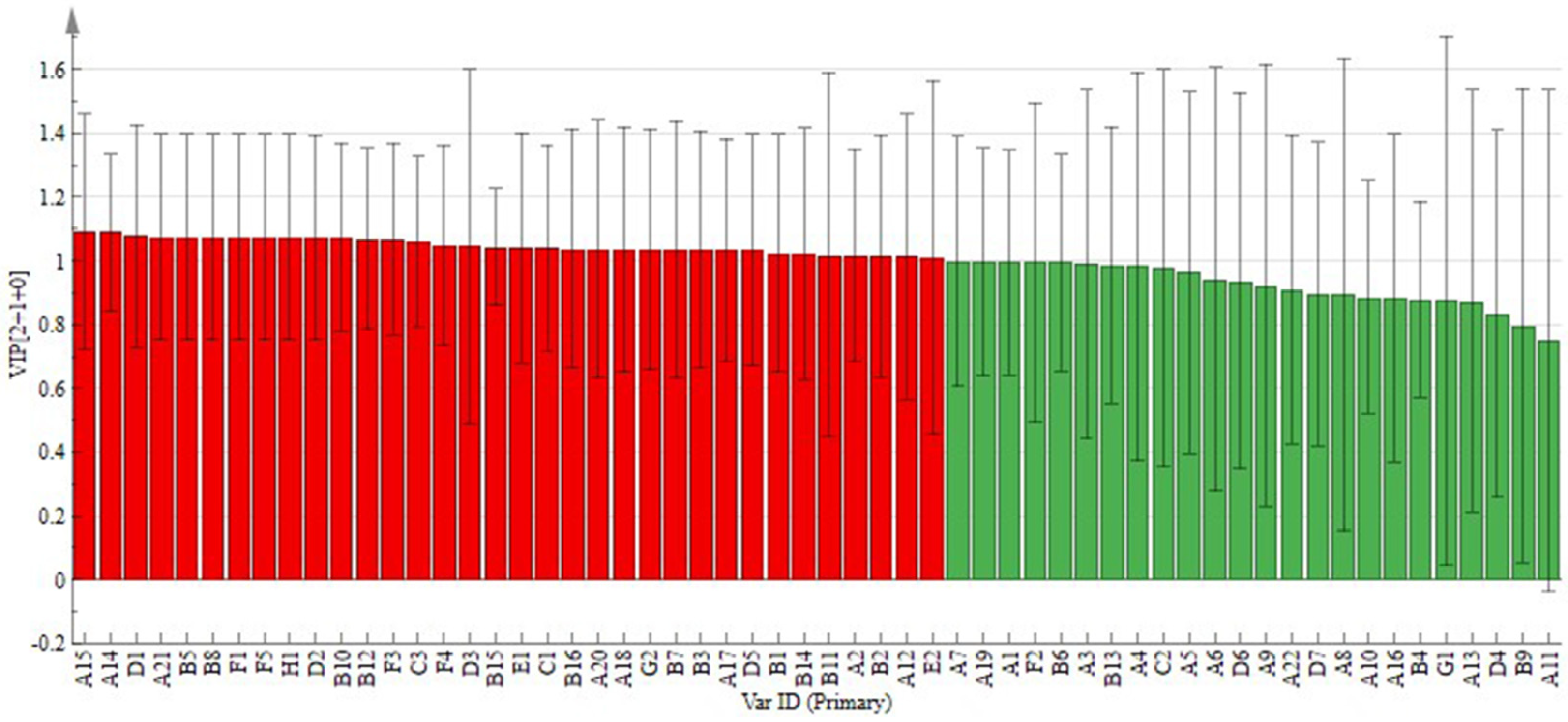
2.2.3. OPLS-DA Analysis
OPLS-DA Modeling and Model Evaluation
Potential Markers of Variability
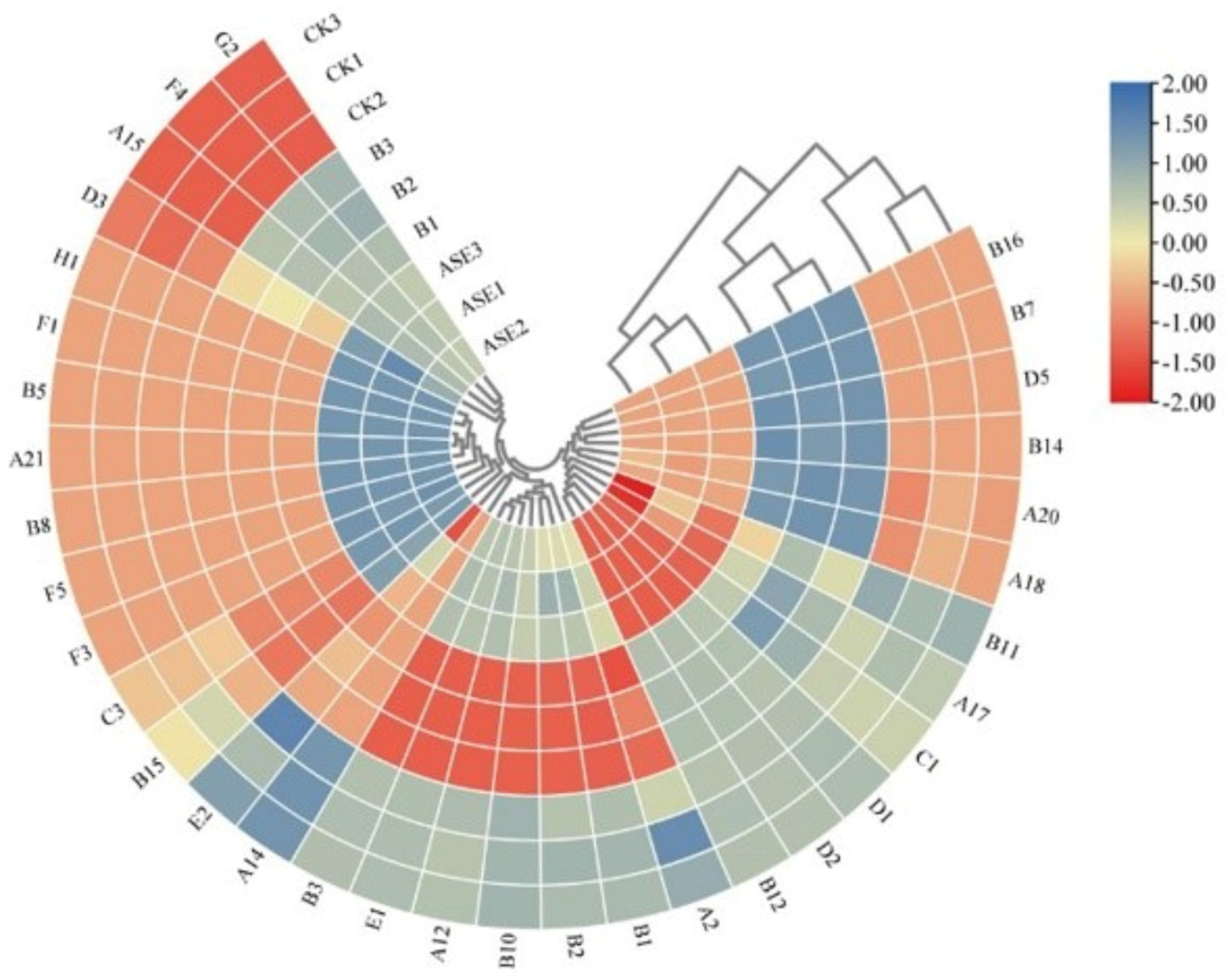
Hierarchical Clustering Analysis of Sign Variability Components Based on OPLS-DA Model VIP > 1
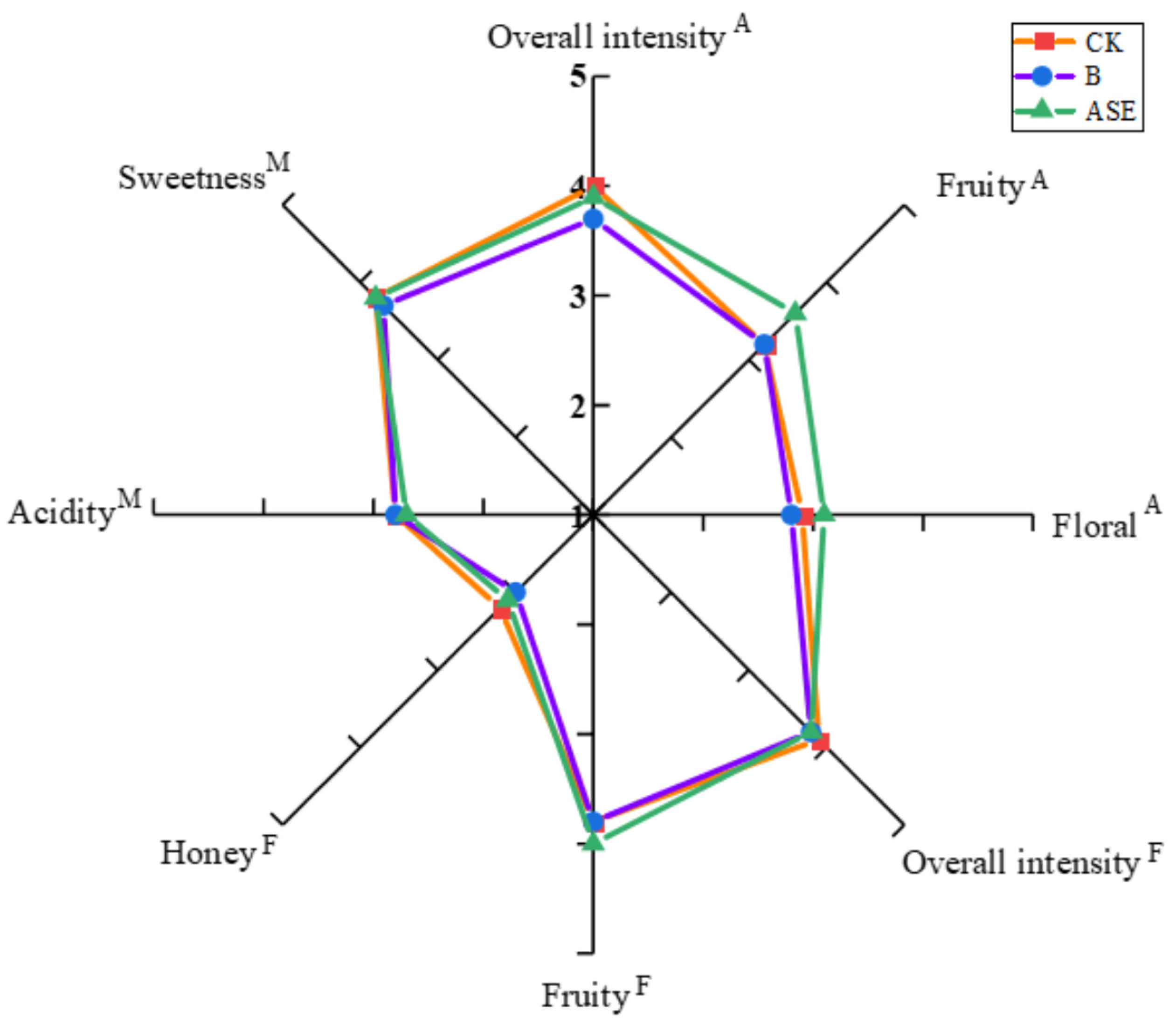
2.3. Sensory Evaluation
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Vinifications and Samples
3.2.1. Small-Scale Winemaking
3.2.2. Liqueur Preparation
3.2.3. Clarifying Treatment
3.3. Chemical Analysis
3.4. Volatile Analysis
3.4.1. Aroma Enrichment
3.4.2. GC-MS Conditions
3.4.3. Volatile Composition Analysis
3.5. Sensory Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milton, D.; Bernabé, D.C.L.; Maria, D.; Henriques, S.S.; Vidal, C.A.; Moreira, O.V.; Quintão, T.L.J. Banana liqueur: Optimization of the alcohol and sugar contents, sensory profile and analysis of volatile compounds. LWT Food Sci. Technol. 2018, 97, 31–38. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Toit, W.D. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. A current state-of-art review. Crit. Rev. Food Sci. 2021, 62, 11–17. [Google Scholar] [CrossRef]
- Li, S.Y.; Duan, C.Q.; Han, Z.H. Grape polysaccharides: Compositional changes in grapes and wines, possible effects on wine organoleptic properties, and practical control during winemaking. Crit. Rev. Food Sci. 2021, 63, 1–24. [Google Scholar] [CrossRef]
- Bora, F.D.; Rîpanu, O.; Donici, A.; Bunea, C.I.; Pop, N.; Lung, M.L.; Popescu, D. Influence of micro-, macroelements and heavy metals on wine quality. Annu. Food Sci. Technol. 2016, 17, 1–10. Available online: https://www.afst.valahia.ro (accessed on 5 April 2022).
- Ma, T.Z.; Gong, P.F.; Lu, R.R.; Zhang, B.; Morata, A.; Han, S.Y. Effect of different clarification treatments on the volatile composition and aromatic attributes of ‘Italian Riesling’ icewine. Molecules 2020, 25, 2657. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro, R. Novel analysis on aroma compounds of wine, vinegar and derived products. Foods 2021, 10, 1245. [Google Scholar] [CrossRef]
- Albuquerque, W.; Seidel, L.; Zorn, H.; Will, F.; Gand, M. Haze formation and the challenges for peptidases in wine protein fining. J. Agr. Food Chem. 2021, 69, 14402–14414. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Louazil, P.; Iturmendi, N.; Moine, V.; Cheynier, V.; Saucier, C. Effect of polyvinylpolypyrrolidone treatment on roses wines during fermentation: Impact on color, polyphenols and thiol aroma. Food Chem. 2019, 295, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Nándor, R.; Zoltán, K.; Béla, K.; Gabriella, A.; Szilárd, S.; Imre, J.H. Comparison and intercorrelation of various bentonite products for oenological properties, elemental compositions, volatile compounds and organoleptic attributes of white wine. Foods 2023, 12, 355. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite fining during fermentation reduces the dosage required and exhibits significant side-effects on phenols, free and bound aroma, and sensory quality of white wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef]
- Sommer, S.; Tondini, F. Sustainable replacement strategies for bentonite in wine using alternative protein fining agents. Sustainability 2021, 13, 1860. [Google Scholar] [CrossRef]
- Marangon, M.; Van Sluyter, S.C.; Robinson, E.M.C.; Muhlack, R.A.; Holt, H.E.; Haynes, P.A.; Godden, P.W.; Smith, P.A.; Waters, E.J. Degradation of white wine haze proteins by aspergillopepsin I and II during juice flash pasteurization. Food Chem. 2012, 135, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; McRae, J.M.; Wollan, D.; Muhlack, R.A.; Godden, P.; Wilkinson, K.L. Use of ultrafiltration and proteolytic enzymes as alternative approaches for protein stabilisation of white wine. Aust. J. Grape Wine Res. 2020, 27, 234–245. [Google Scholar] [CrossRef]
- Sui, Y.; Wollan, D.; McRae, J.M.; Muhlack, R.; Capone, D.L.; Godden, P.; Wilkinson, K.L. Chemical and sensory profiles of sauvignon blanc wine following protein stabilization using a combined ultrafiltration/heat/protease treatment. Front. Nutr. 2022, 9, 799809. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Delgado, J.A.; Ferrer, M.A.; Viñas, M.G. The aroma of la mancha chelva wines: Chemical and sensory characterization. Food Res. Int. 2019, 119, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.P.; Zhu, B.Q.; Song, R.R.; Zhang, B.; Lan, Y.B.; Zhu, X.; Duan, C.Q.; Han, S.Y. Volatile composition and aromatic attributes of wine made with, vitisvinifera l. cv cabernet sauvignon grapes in the Xinjiang region of China: Effect of different commercial yeasts. Int. J. Food Prop. 2018, 21, 1423–1441. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Vincenzi, S.; Panighel, A.; Gazzola, D.; Flamini, R.; Curioni, A. Study of combined effect of proteins and bentonite fining on the wine aroma loss. J. Agric. Food Chem. 2015, 63, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine R. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Chen, M.Y.; Zhang, S.T.; Ren, Y.X.; Le, Z.; Li, L.X.; Sun, B.S. Effects of different brewing technologies on polyphenols and aroma components of black chokeberry wine. Foods 2023, 12, 868. [Google Scholar] [CrossRef]
- He, Y.; Jiang, Y.; Li, J.; Mi, L.; Hu, Y. Effection of different yeasts and maceration enzymes on aromatic components of cabernet gernischt red wine. Trans. Chin. Soc. Agric. Eng. 2016, 32, 325–332. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Chen, K.; Ju, Y.; Fang, Y. Effect of Foliar Application of Fulvic Acid Antitranspirant on Sugar Accumulation, Phenolic Profiles and Aroma Qualities of Cabernet Sauvignon and Riesling Grapes and Wines. Food Chem. 2021, 351, 129308. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, K.; Xu, Y.; Li, J.M. Characterization of the key aroma compounds in chinese vidal icewine by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission Tests. J. Agr. Food Chem. 2017, 65, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.B.; Tao, Y.S. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chem. 2019, 284, 155–161. [Google Scholar] [CrossRef]
- Liu, R.C.; Li, R.; Wang, Y.; Jiang, Z.T. Analysis of volatile odor compounds and aroma properties of European vinegar by the ultra-fast gas chromatographic electronic nose. J. Food Compos. Anal. 2022, 112, 104673. [Google Scholar] [CrossRef]
- Yun, J.; Cui, C.J.; Zhang, S.H.; Zhu, J.; Hou, R. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Qian, Y.Y.; Shu, B.; Liu, Y.Y.; Tu, X.H.; Ouyang, H.J.; Li, Y.; Tan, G.; Yu, Z.W.; Chen, F. Effects of four drying methods on ganoderma lucidum volatile organic compounds analyzed via headspace solid-phase microextraction and comprehensive two-dimensional chromatography-time-of-flight mass spectrometry. Microchem. J. 2021, 166, 106258. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, H.; Su, Z.; Khoo, C.; Gu, L. Identifying cranberry juice consumers with predictive OPLS-DA models of plasma metabolome and validation of cranberry juice intake biomarkers in a double-blinded, randomized, placebo-controlled, cross-over study. Mol. Nutr. Food Res. 2020, 64, e1901242. [Google Scholar] [CrossRef]
- Huang, X.W.; Zhao, L.H.; Pang, S.; Liu, Y.J.; Liu, J.R.; Zhang, M.Q. Effects of varieties, cultivation methods, and origins of citrus sinensis ‘hongjiang’ on volatile organic compounds: HS-SPME-GC/MS analysis coupled with OPLS-DA. Agriculture 2022, 12, 1725. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Rong, Y.T.; Liu, F.Q.; Jiang, Y.W.; Deng, Y.L.; Dong, C.W.; Yuan, H.B. Rapid characterization of the volatile profiles in pu-erh tea by gas phase electronic nose and microchamber/thermal extractor combined with TD-GC-MS. J. Food Sci. 2021, 86, 2358–2373. [Google Scholar] [CrossRef]
- Dai, Q.; Jin, H.; Gao, J.; Ning, J.; Yang, X.; Xia, T. Investigating volatile compounds’ contributions to the stale odour of green tea. Int. J. Food Sci. Technol. 2020, 55, 1606–1616. [Google Scholar] [CrossRef]
- Comuzzo, P.; Voce, S.; Fabris, J.; Cavallaro, A.; Kallithraka, S. Effect of the combined application of heat treatment and proteases on protein stability and volatile composition of Greek white wines. OENO One 2020, 54, 175–188. [Google Scholar] [CrossRef]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Eduardo, C.C.; Juan, J.G.M.; Laila, Y.J.; Alberto, R.F.; Andrés, G.; Juan, M. An IoT barrel bung to monitor evolution wine elaborated under biological aging. Measurement 2022, 199, 111471. [Google Scholar] [CrossRef]
- Ding, K.; Chen, Y.X.; Li, W.; Wang, P.; Yang, X.S.; Han, S.Y.; Zhang, Z. Process optimization of white grape liqueur and analysis of ester aroma compounds. Food Ferment. Technol. 2021, 57, 49–56. [Google Scholar]
- OIV. Compendium of International Methods of Analysis of Wines and Musts. Available online: https://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 5 October 2020).
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Danner, L.; Crump, A.M.; Croker, A.; Gambetta, J.M.; Johnson, T.E.; Bastian, S.E. Comparison of rate-all-that-apply and descriptive analysis for the sensory profiling of wine. Am. J. Enol. Viticult 2018, 69, 12–21. [Google Scholar] [CrossRef]

| Residual Sugar/(g/L) | Total Acidity/(g/L) | Volatile Acidity/(g/L) | pH | Protein/(mg/L) | |
|---|---|---|---|---|---|
| CK | 98. 90 ± 0.75 bc | 4.24 ± 0.07 a | 0.57 ± 0.00 a | 4.11 ± 0.01 a | 59.86 ± 0.99 a |
| H | 99.23 ± 1.75 ab | 4.23 ± 0.06 a | 0.57 ± 0.02 a | 4.11 ± 0.00 a | 53.60 ± 0.25 b |
| B | 96.97 ± 0.06 c | 4.17 ± 0.01 a | 0.56 ± 0.01 a | 4.11 ± 0.00 a | 49.57 ± 0.98 c |
| ASE | 101.10 ± 0.90 a | 4.20 ± 0.04 a | 0.56 ± 0.02 a | 4.11 ± 0.00 a | 50.30 ± 0.25 c |
| Number | Compound Name | Average Content (µG·L−1) | Odor Description | ||
|---|---|---|---|---|---|
| CK | B | ASE | |||
| Esters | |||||
| A1 | Ethanedioic acid, diethyl ester | 142.90 ± 30.81 a | 88.55 ± 11.68 b | 137.46 ± 1.49 a | / |
| A2 | Ethyl acetate | 14,853.45 ± 3048.37 a | 8272.05 ± 753.22 b | 12,345.61 ± 505.73 a | Banana, strawberry fragrance, fruit flavor |
| A3 | Ethyl propionate | 323.53 ± 71.67 a | 225.61 ± 17.29 b | 259.05 ± 3.79 b | Fruity |
| A4 | Ethyl isobutyrate | 123.78 ± 22.57 a | 95.71 ± 11.03 b | 102.68 ± 1.57 b | Apple, floral, rubber, strawberry, sweet |
| A5 | Isobutyl acetate | 99.48 ± 22.09 a | 72.5 ± 10.49 b | 84.47 ± 0.73 ab | Sweet, banana, fruity, fruity aroma |
| A6 | Ethyl butanoate | 503.95 ± 114.94 a | 389.33 ± 55.90 b | 422.52 ± 9.31 ab | Papaya, creamy fragrance, pineapple, strawberry flavor |
| A7 | Ethyl 2-methylbutanoate | 47.03 ± 7.21 a | 37.06 ± 3.14 b | 35.96 ± 3.60 b | Fennel, apple, bubblegum, fruit, kiwi |
| A8 | Ethyl isovalerate | 56.51 ± 12.89 a | 46.45 ± 7.79 a | 48.62 ± 2.36 a | Fennel, apple, citrus, fruit, pineapple |
| A9 | Isoamyl acetate | 2143.51 ± 517.38 a | 1675.69 ± 230.34 a | 1823.19 ± 54.54 a | Sweet fruit, banana, green apple |
| A10 | Ethyl valerate | 117.48 ± 22.51 a | 82.75 ± 6.53 b | 100.31 ± 26.84 ab | Apple, dried fish, herbs, nuts, yeast |
| A11 | Ethyl hexanoate | 4385.23 ± 1064.73 a | 3828.77 ± 55.38 a | 3962.73 ± 585.16 a | Fruity, strawberry, pineapple, banana fragrance, green apple |
| A12 | Methyl 2,2-dimethoxyacetate | 110.88 ± 21.23 | nd | 114.01 ± 35.48 | / |
| A13 | Ethyl caprylate | 10,283.06 ± 2073.98 b | 12,903.69 ± 1777.82 a | 10,020.47 ± 1562.42 b | Fruity, oily, fruity, ripe fruit, pear, sweet aroma |
| A14 | Ethyl orthoformate | 311.89 ± 27.09 | nd | nd | / |
| A15 | Hex-(2 E)-enoate <ethyl-> | nd | 26.26 ± 2.84 | 32.63 ± 0.00 | / |
| A16 | Ethyl furoate | 143.31 ± 22.92 a | 121.86 ± 12.36 a | 143.41 ± 12.62 a | Floral fragrance |
| A17 | Diethyl succinate | 589.97 ± 28.38 a | 615.95 ± 67.93 a | 403.49 ± 63.70 b | Almond fragrance |
| A18 | Ethyl trans-4-decenoate | 181.54 ± 38.26 b | 1158.42 ± 43.22 a | 186.22 ± 4.99 b | / |
| A19 | Phenethyl acetate | 840.25 ± 142.26 a | 807.7 ± 114.43 a | 612.99 ± 36.36 b | Rose, jasmine aroma, floral, sweet |
| A20 | Dodecanoate <ethyl-> | 210.21 ± 34.08 b | 735.59 ± 40.59 a | 227.19 ± 27.57 b | Sweet, beeswax, floral and fruity |
| A21 | Benzoic acid, 2-methylpropyl ester | nd | nd | 40.35 ± 0.00 | / |
| A22 | Whiskey lactone | 101.54 ± 12.08 b | 295.35 ± 202.81 a | 307.58 ± 33.59 a | Citrus flavor, coconut flavor |
| Total | 35,569.51 ± 7335.47 | 31,479.28 ± 3424.82 | 31,410.94 ± 2971.84 | ||
| Alcohols | |||||
| B1 | 1-Propanol | 792.08 ± 37.30 a | 288.97 ± 5.14 b | 720.82 ± 150.20 a | Vegetable Aroma |
| B2 | Isobutyl alcohol | 3283.03 ± 231.54a | 1240.1 ± 8.25 b | 3051.51 ± 712.14 a | Solvent taste, raw green flavor |
| B3 | 1-Butanol | 71.81 ± 5.39 | nd | 67.25 ± 12.02 | Herbal, alcoholic odor |
| B4 | 3-Methyl-1-butanol | 47,175.56 ± 31,546.15 ab | 33,588.39 ± 904.38 b | 65,588.85 ± 12,669.62 a | Apple brandy, spicy |
| B5 | 1-Pentanol | nd | nd | 48.61 ± 0.00 | Spicy, grassy aroma |
| B6 | 1-Hexanol | 1648.18 ± 25.97 a | 1223.39 ± 50.94 b | 1502.62 ± 208.01 a | Herbaceous, grassy fragrance |
| B7 | 3-Hexen-1-ol | nd | 27.23 ± 3.20 | nd | Floral, botanical, fruity |
| B8 | 3-Hexen-1-ol, (Z)- | nd | nd | 42.42 ± 0.00 | Grass flavor |
| B9 | 1-Heptanol | 50.46 ± 0.66 a | 47.01 ± 2.35 a | 44.31 ± 8.17 a | Grape flavor |
| B10 | Butadienol <2,3-> | 469.36 ± 23.64 | nd | 158.26 ± 0.00 | Buttery, creamy, rubbery, fruity |
| B11 | Linalool | 272.86 ± 5.10 a | 252.08 ± 20.08 a | 212.94 ± 30.04 b | Floral, lavender fragrance |
| B12 | 1-Octanol | 68.52 ± 8.69 | 71.32 ± 5.34 | nd | Stimulating aromatic scent, jasmine, lemon |
| B13 | Phenylethyl alcohol | 4068.14 ± 82.68 a | 2050.14 ± 356.61 b | 4103.36 ± 788.08 a | Rose, floral, sweet fragrance |
| B14 | 2-Hexadecanol | nd | 31.95 ± 7.07 | nd | / |
| B15 | alpha-Terpineol | 147.02 ± 30.82 b | 101.32 ± 1.21 c | 229.78 ± 8.40 a | Fresh, woody fragrance |
| B16 | Triethylene glycol monododecyl ether | nd | 59.59 ± 2.12 | nd | / |
| Total | 58,047.00 ± 31,997.94 | 38,981.47 ± 1366.72 | 75,770.72 ± 14,586.69 | ||
| Ketones | |||||
| C1 | 2-methyl-3-Heptanone | 51.07 ± 0.75 b | 58.19 ± 8.05 a | 32.11 ± 0.00 c | / |
| C2 | Ionone | 335.52 ± 5.52 b | 324.53 ± 9.59 b | 496.47 ± 119.79 a | Violet fragrance |
| C3 | Damascenone <(E)-, beta-> | 36.67 ± 1.28 b | 29.26 ± 0.00 c | 69.82 ± 1.41 a | Poached apples, floral scents, fruits, honey |
| Total | 423.27 ± 7.55 | 411.99 ± 17.64 | 598.40 ± 121.20 | ||
| Aldehydes | |||||
| D1 | 1,1-Diethoxybutane | 521.59 ± 99.38 | 425.24 ± 58.79 | nd | / |
| D2 | Hexanal | 33.23 ± 1.07 | 34.16 ± 1.01 | nd | Grassy smell, apple fragrance |
| D3 | Isobutyraldehyde Diethyl Acetal | 153.69 ± 14.28 c | 226.07 ± 17.68 b | 408.07 ± 68.99 a | / |
| D4 | Furfural | 11,446.78 ± 2760.11 a | 9599.19 ± 1636.49 a | 9902.16 ± 1327.07 a | Herbal, tea, almond flavor, floral |
| D5 | Methylal | nd | 57.83 ± 5.78 | nd | / |
| D6 | Benzaldehyde | 438.71 ± 4.24 a | 387.21 ± 61.49 ab | 351.37 ± 31.49 b | Bitter almond flavor, oily flavor |
| D7 | 5-Methyl furfural | 1639.23 ± 251.23 a | 1276.68 ± 104.18 b | 1560.78 ± 226.86 ab | Caramel smell |
| Total | 14,233.23 ± 3130.31 | 12,006.38 ± 1885.42 | 12,222.38 ± 1654.41 | ||
| Acids | |||||
| E1 | Acetic acid | 82.07 ± 2.95 | nd | 70.27 ± 0.00 | Acetic acid smell |
| E2 | Octanoic acid | 109.19 ± 12.73 a | 76.45 ± 4.39 b | 78.3 ± 19.66 b | Fatty acids, dairy products |
| Total | 191.26 ± 15.68 | 76.45 ± 4.39 | 148.58 ± 19.66 | ||
| Olefins | |||||
| F1 | alpha-Pinene | nd | nd | 47.39 ± 0.00 | Pine wood, resin incense |
| F2 | γ-Terpinene | 39.44 ± 4.24 | nd | 60.03 ± 22.26 | Bitter, citrus, gasoline, resin, turpentine |
| F3 | Limonene | nd | nd | 60.26 ± 13.14 | Lemon scent |
| F4 | Styrene | nd | 56.1 ± 13.05 | 44.72 ± 7.31 | Special fragrance |
| F5 | Octene <alpha-> | nd | nd | 57.87 ± 0.00 | / |
| Total | 39.44 ± 4.24 | 56.10 ± 13.05 | 270.27 ± 42.71 | ||
| Others | |||||
| G1 | Bois de Rose oxide | 200.84 ± 48.69 a | 176.72 ± 27.46 a | 168.67 ± 7.62 a | / |
| G2 | Nerol oxide | nd | 44.96 ± 13.28 | 25.14 ± 0.00 | Flowers, oil |
| G3 | Butylated Hydroxytoluene | nd | nd | 40.29 ± 0.00 | Toasted bread slices |
| Total | 200.84 ± 48.69 | 211.68 ± 40.74 | 234.10 ± 7.62 | ||
| Aroma (A) | Flavor (F) | Mouthfeel (M) | Sensory Intensity Score |
|---|---|---|---|
| Overall intensity | Overall intensity | Acidity | 0 = “ not perceived “ 1 = “ extremely low “ 4 = “ moderate “ 7 = “ extremely high “ |
| Fruity | Fruity | Bitterness | |
| Floral | Floral | Sweetness | |
| Honey | Honey | Dryness | |
| Herbaceous | Herbaceous | Astringency | |
| Solvent | Solvent | Alcohol heat/warmth |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zhang, Z.; Zhao, Y.; Li, W.; Wang, L.; Shang, Q.; Du, J.; Jin, L. Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine. Molecules 2023, 28, 5129. https://doi.org/10.3390/molecules28135129
Li W, Zhang Z, Zhao Y, Li W, Wang L, Shang Q, Du J, Jin L. Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine. Molecules. 2023; 28(13):5129. https://doi.org/10.3390/molecules28135129
Chicago/Turabian StyleLi, Wen, Zhen Zhang, Yuanyuan Zhao, Wei Li, Li Wang, Qi Shang, Jianming Du, and Lina Jin. 2023. "Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine" Molecules 28, no. 13: 5129. https://doi.org/10.3390/molecules28135129
APA StyleLi, W., Zhang, Z., Zhao, Y., Li, W., Wang, L., Shang, Q., Du, J., & Jin, L. (2023). Effect of Protease Combined with Heat Treatment on the Volatile Composition and Aroma Quality in Liqueur Wine. Molecules, 28(13), 5129. https://doi.org/10.3390/molecules28135129





