Four New Unusual Pentacyclic Triterpenoids from the Roots of Jasminum sambac (L.) Ait
Abstract
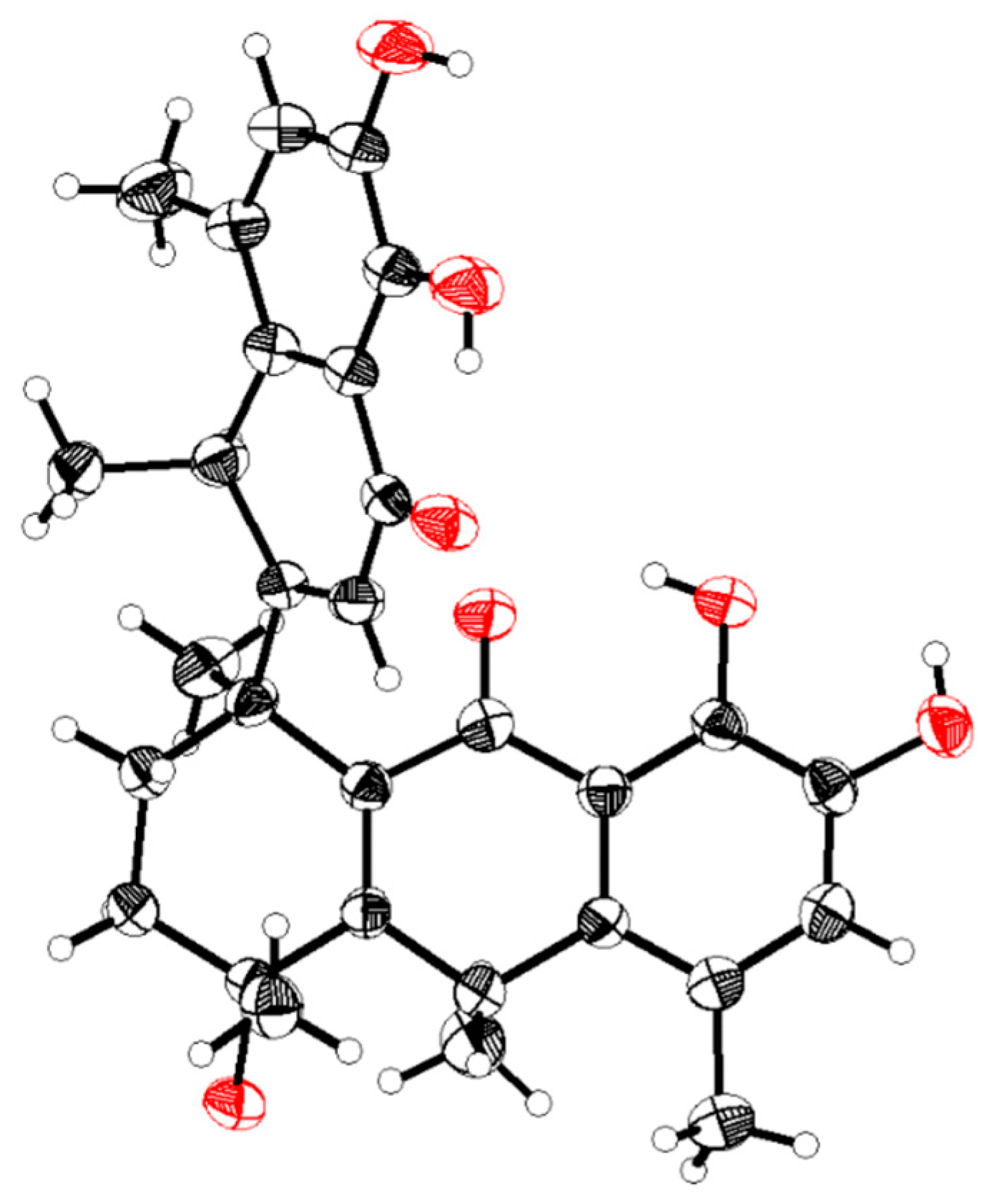
1. Introduction and Structural Elucidation
2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Guo, Z.Y.; Li, P.; Huang, W.; Wang, J.J.; Liu, Y.J.; Liu, B.; Wang, Y.L.; Wu, S.B.; Kennelly, E.J.; Long, C.L. Antioxidant and anti-inflammatory caffeoyl phenylpropanoid and secoiridoid glycosides from Jasminum nervosum stems, a Chinese folk medicine. Phytochemistry 2014, 106, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.; Perez, J.A.; Hernandez, J.M.; Trujillo, J. Secoiridoids from Jasminum odoratissimum. J. Nat. Prod. 1997, 60, 1334–1337. [Google Scholar] [CrossRef]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Monache, F.D.; Chifundera, K.; Galeffi, C. Oligomeric secoiridoid glucosides from Jasminum abyssinicum. Phytochemistry 2006, 67, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.H.; Hu, M.; Yan, Y.M.; Lu, Q.; Cheng, Y.X. Compounds from the roots of Jasminum sambac. J. Asian Nat. Prod. Res. 2012, 14, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.L.; Han, L.F.; Meng, D.L.; Li, N.; Li, X. Janceolaroside A and Janceoside A, Two New Compounds from the Stems and Roots of Jasminum lanceolarium. Nat. Prod. Commun. 2011, 6, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Han, Z.Z.; Zhang, C.G.; Ye, Z.; Wu, L.L.; Xu, H. Four new sesquiterpenoids with anti-inflammatory activity from the stems of Jasminum officinale. Fitoterapia 2019, 135, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Qin, H.; Li, Y.H.; Sun, Y.; Wang, Z.P.; Yang, T.H.; Liu, L.; Wang, M.C.; Feng, F.; Mei, Q.B. Chemical Constituents of the Root of Jasminum giraldii. Molecules 2013, 18, 4766–4775. [Google Scholar] [CrossRef]
- Sun, J.M.; Yang, J.S.; Zhang, H. Two New Flavanone Glycosides of Jasminum lanceolarium and Their Anti-oxidant Activities. Chem. Pharm. Bull. 2007, 55, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Tomassinia, L.; Ventronea, A.; Frezzaa, C.; Serafinib, I.; Biancob, A.; Cometa, M.F. Lignans and secoiridoid glycosides from the stem barks of Jasminum tortuosum. Nat. Prod. Res. 2018, 32, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.Z.; Yong, J.P.; Lu, C.Z. Isolation, Structural Elucidation of a New Triterpenoid from the Roots of Jasminum sambac (L.) Ait. with Potent Cytotoxicity against MCF 7 Cell Lines. ACS Omega 2023, 8, 14662–14664. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.Z.; Yong, J.P.; Lu, C.Z. Chemical Constituents from the roots of Jasminum sambac (L.) Ait. and cytotoxicity to the cancer cell lines. Anticancer Agents Med. Chem. 2023. [Google Scholar] [CrossRef]
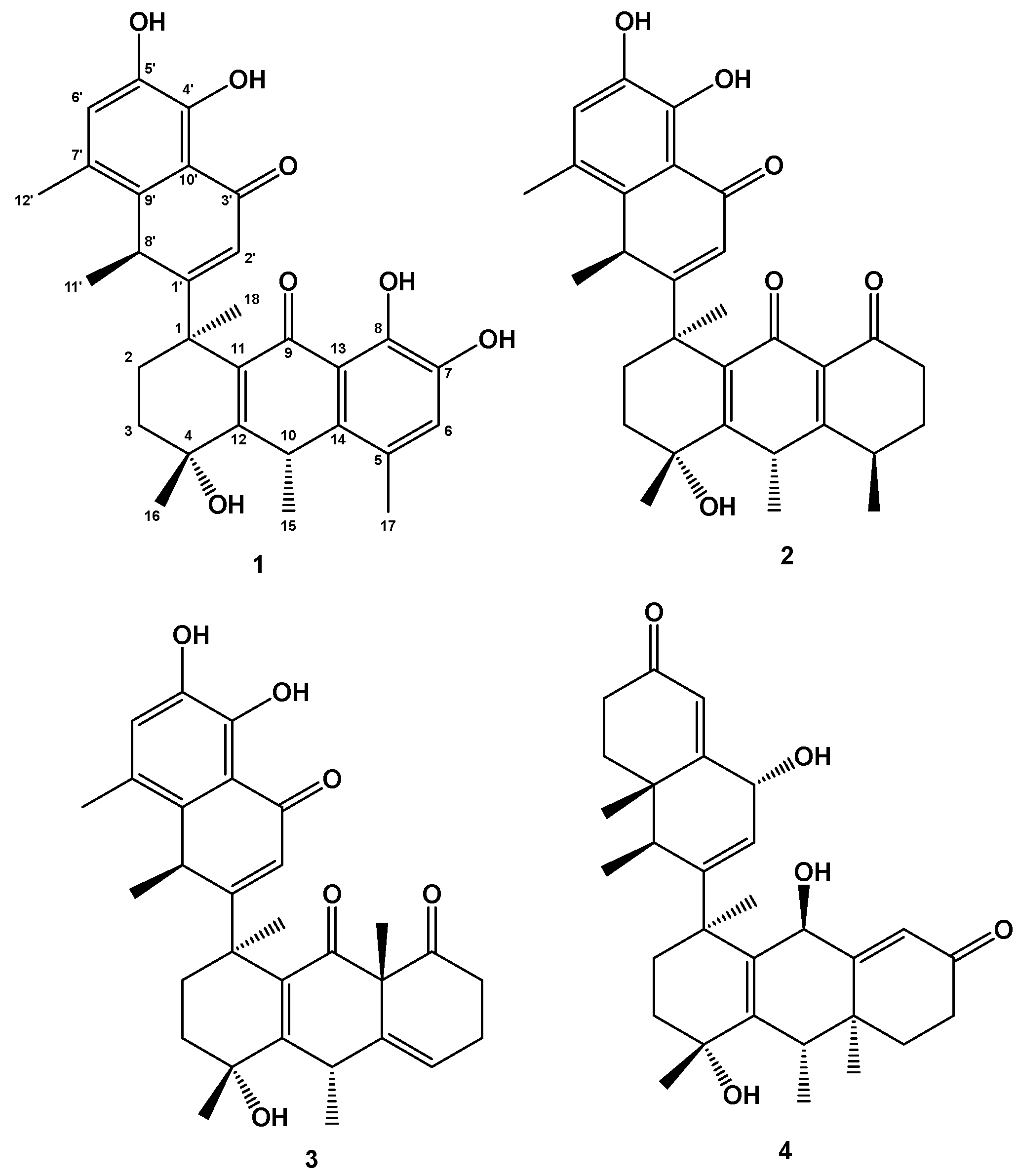
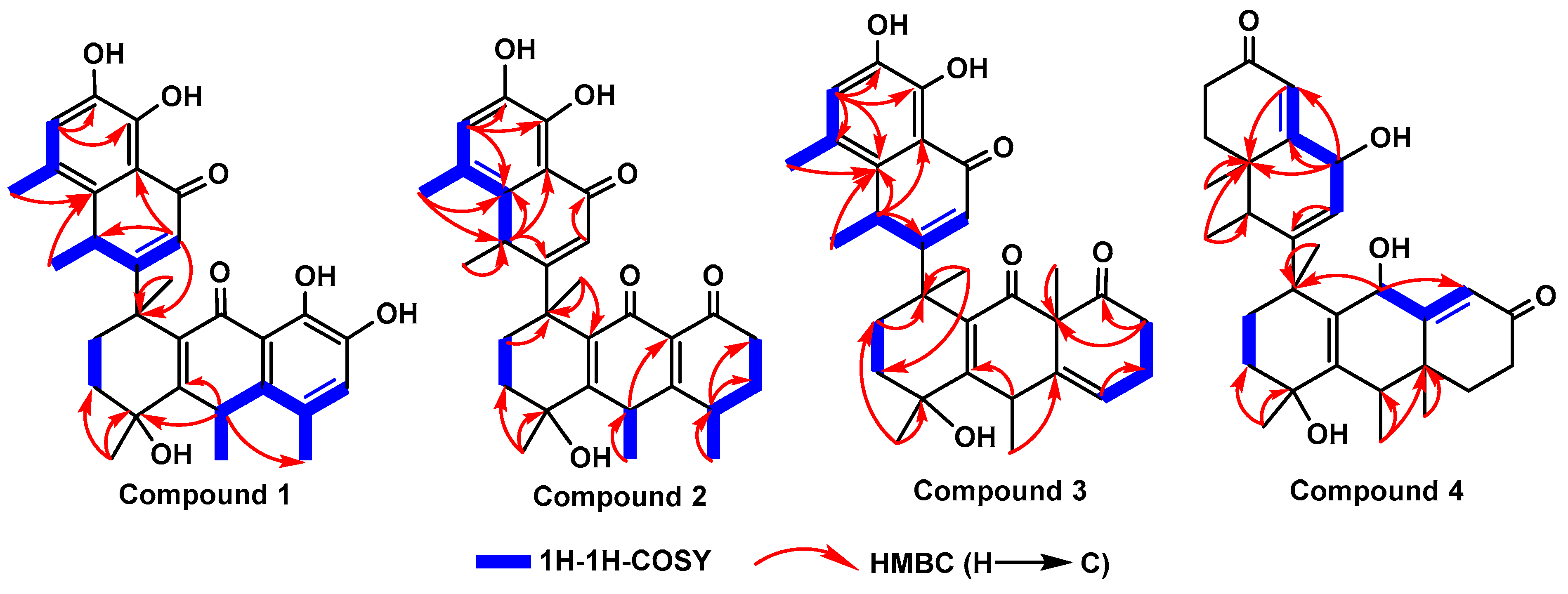
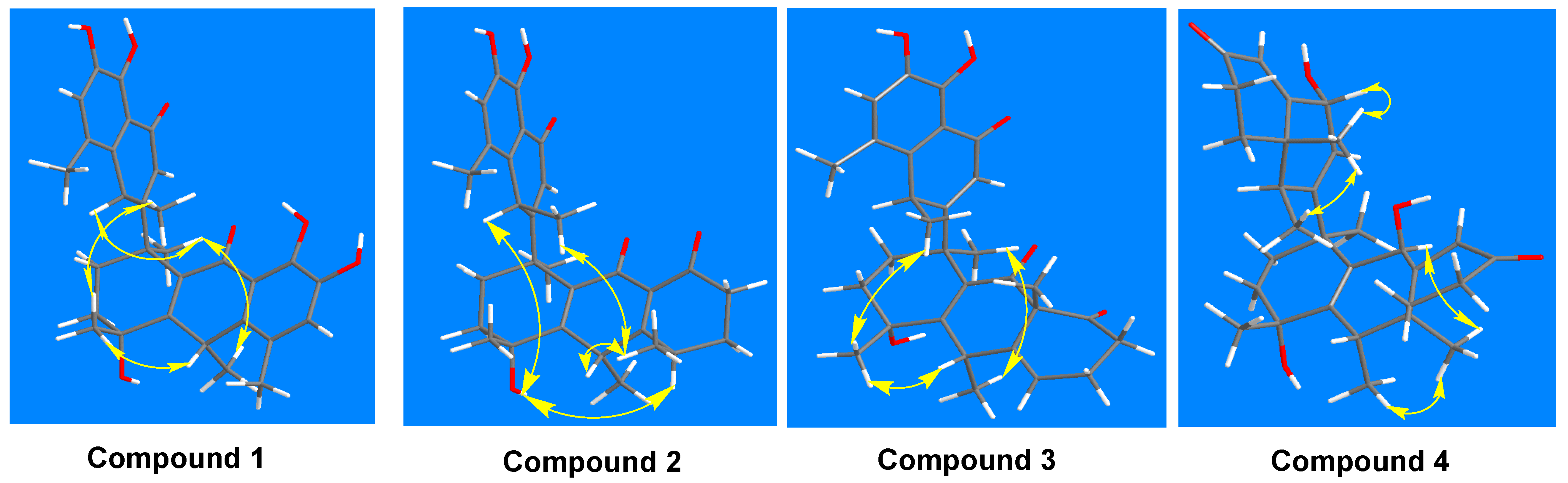
| No. | Compound 1 | Compound 2 | Compound 3 | Compound 4 | ||||
|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 43.9 C | 43.8 | 44.4 C | 41.9 C | ||||
| 2α | 1.98, m | 39.2 CH2 | 1.90, m | 33.1 CH2 | 1.85, dt(3.4, 2.5) | 34.0 CH2 | 1.24, m | 29.4 CH2 |
| 2β | 2.12, m | 1.53, m | 2.18, m | |||||
| 3α | 1.80, m | 34.8 CH2 | 1.61, m | 35.0 CH2 | 2.02, m | 35.5 CH2 | 1.76, m | 33.7 CH2 |
| 3β | 2.15, m | |||||||
| 4 | 71.1 C | 70.1 C | 71.3 C | 68.8 C | ||||
| 5 | 123.8 C | 1.22, m | 29.2 CH2 | 6.47, s | 126.4 CH | 2.30, m | 25.6 CH2 | |
| 6α | 6.84 | 123.9 CH | 2.56, m | 38.3 CH2 | 2.58, d(11.8) | 38.5 CH2 | 1.60, m | 26.0 CH2 |
| 6β | 2.46 | |||||||
| 7α | 143.4 C | 2.43, d(1.8) | 42.4 CH2 | 2.39, d(2.1) | 41.7 CH2 | 188.7 C | ||
| 7β | 2.21, m | 2.27, m | ||||||
| 8 | 148.3 C | 199.6 C | 200.4 C | 7.01, s | 139.9 CH | |||
| 9 | 191.9 C | 191.9 C | 187.8 C | 4.17, s | 67.9 CH | |||
| 10 | 4.01, q(4.4) | 33.1 CH | 3.26, m | 39.6 CH | 3.76, m | 39.9 CH | 2.24 | 30.7 CH |
| 11 | 137.1 C | 135.5 C | 136.7 C | |||||
| 12 | 148.34 | 148.8 C | 142.9 C | |||||
| 13 | 116.1 C | 135.6 C | 39.6 C | 139.1 C | ||||
| 14 | 136.9 C | 157.7 C | 158.1 C | 42.8 C | ||||
| 15 | 2.24, s | 17.7 CH3 | 0.99, s | 17.6 CH3 | 1.04, s | 16.8 CH3 | 0.97, s | 14.0 CH3 |
| 16 | 1.45, s | 28.9 CH3 | 1.51, s | 27.8 CH3 | 1.45, s | 27.2 CH3 | 1.49, s | 26.3 CH3 |
| 17 | 1.32, d(4.4) | 28.1 CH3 | 0.94, d(4.4) | 15.3 CH3 | 0.84, d(4.8) | 13.7 CH3 | 0.86, s | 18.1 CH3 |
| 18 | 1.72, s | 23.2 CH3 | 1.58, s | 25.6 CH3 | 1.73, s | 23.4 CH3 | 1.44, s | 23.1 CH3 |
| 1′ | 168.9 C | 167.5 C | 168.8 C | 138.5 C | ||||
| 2′ | 5.69, s | 121.9 CH | 6.27, d(0.7) | 126.8 CH | 6.89, s | 122.9 CH | 5.79, s | 125.7 CH |
| 3′ | 191.8 C | 186.9 C | 191.2 C | 4.51, s | 66.2 CH | |||
| 4′ | 148.4 C | 148.7 C | 147.9 C | 6.96, s | 139.2 CH | |||
| 5′ | 143.6 C | 143.5 C | 142.9 C | 187.2 C | ||||
| 6′ | 6.93, s | 124.0 CH | 6.89, s | 123.9 CH | 6.90, s | 122.4 CH | 2.28, m | 25.9 CH2 |
| 7′ | 124.5 C | 123.6 C | 123.7 C | 1.58, m | 25.4 CH2 | |||
| 8′ | 4.17, q(4.8) | 35.5 CH | 4.29, q(4.5) | 31.7 CH | 4.09, q(4.4) | 33.1 CH | 2.30 | 30.6 CH |
| 9′ | 137.2 C | 137.4 C | 136.8 C | 41.6 C | ||||
| 10′ | 114.8 C | 115.8 C | 115.2 C | 136.6 C | ||||
| 11′ | 2.31, s | 18.1 CH3 | 2.25, s | 17.7 CH3 | 2.32, s | 16.3 CH3 | 0.99, d(2.9) | 13.9 CH3 |
| 12′ | 1.26, d(4.4) | 24.4 CH3 | 1.26, d(4.5) | 27.9 CH3 | 1.42, d(4.4) | 26.7 CH3 | 0.93, s | 18.4 CH3 |
| 4-OH | 5.35, s | 5.40, s | ||||||
| 7-OH | 11.78, | |||||||
| 8-OH | 9.10, s | |||||||
| 4′-OH | 8.99, s | 9.07, s | ||||||
| 5′-OH | 12.53, s | 12.23, s | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunde, O.Z.; Yong, J.; Lu, C. Four New Unusual Pentacyclic Triterpenoids from the Roots of Jasminum sambac (L.) Ait. Molecules 2023, 28, 5097. https://doi.org/10.3390/molecules28135097
Olatunde OZ, Yong J, Lu C. Four New Unusual Pentacyclic Triterpenoids from the Roots of Jasminum sambac (L.) Ait. Molecules. 2023; 28(13):5097. https://doi.org/10.3390/molecules28135097
Chicago/Turabian StyleOlatunde, Olagoke Zacchaeus, Jianping Yong, and Canzhong Lu. 2023. "Four New Unusual Pentacyclic Triterpenoids from the Roots of Jasminum sambac (L.) Ait" Molecules 28, no. 13: 5097. https://doi.org/10.3390/molecules28135097
APA StyleOlatunde, O. Z., Yong, J., & Lu, C. (2023). Four New Unusual Pentacyclic Triterpenoids from the Roots of Jasminum sambac (L.) Ait. Molecules, 28(13), 5097. https://doi.org/10.3390/molecules28135097





