A DFT and Matrix–Isolation IR/UV-Visible Study of High-Coordinated Lanthanide-CO Complexes
Abstract
1. Introduction
2. Results and Discussion
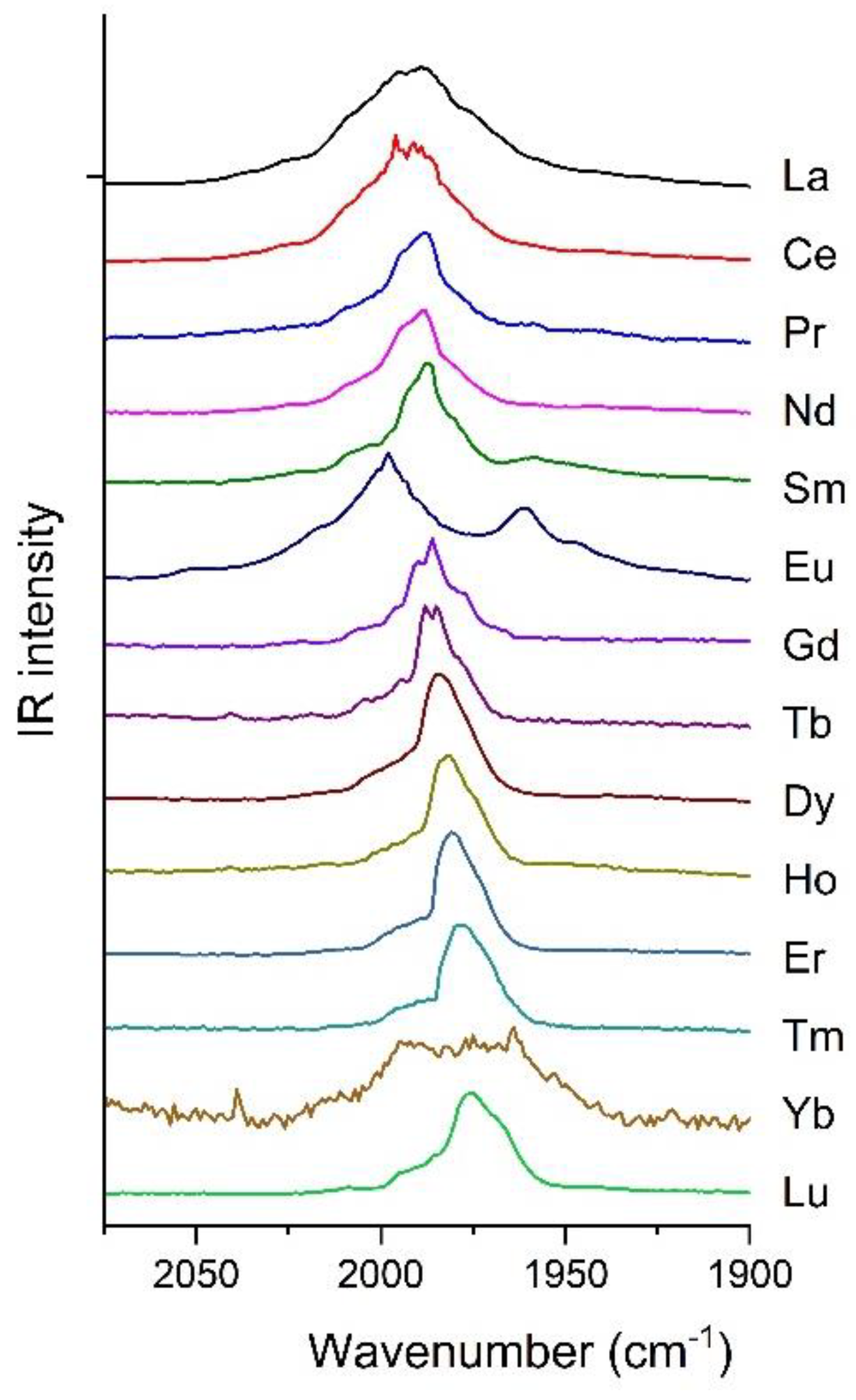
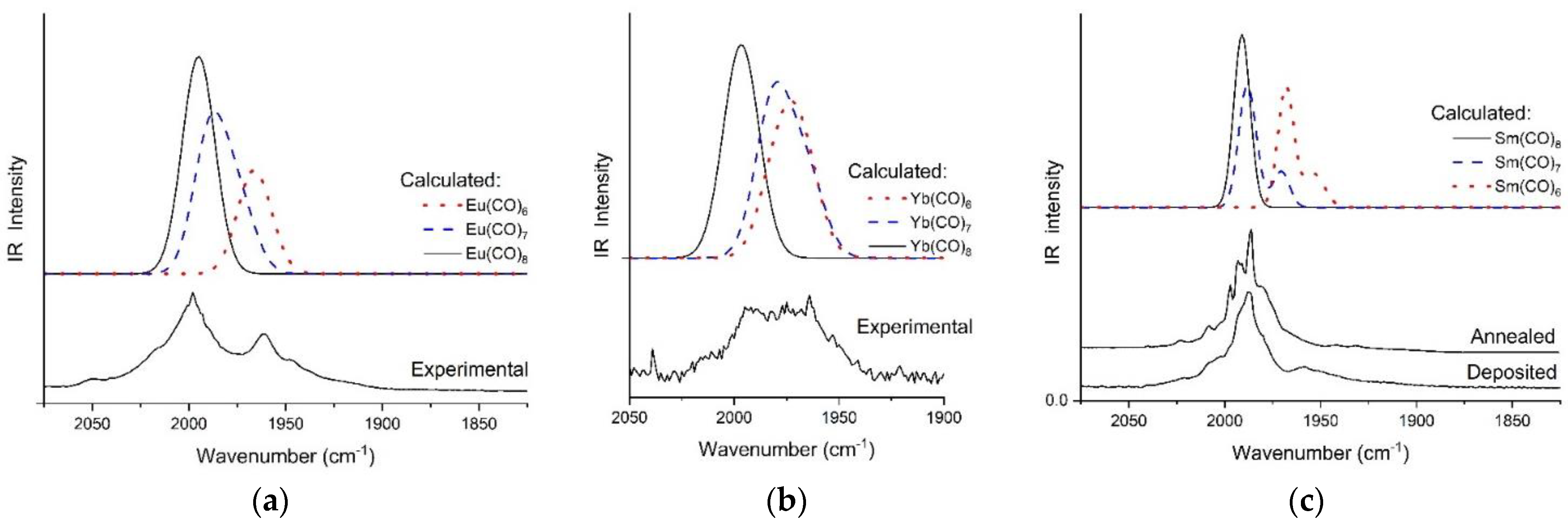
2.1. Experimental Results
2.2. Structures and Electronic Properties of Ln(CO)8 Complexes
| Character | La | Eu | Yb | ||
|---|---|---|---|---|---|
| α | β | α | β | ||
| Ln→(CO)8 | −922.2 | −883.7 | −818.4 | - | −2022.9 |
| −343.3 | −303.0 | ||||
| (CO)8→Ln | −88.6 | −88.3 | −86.2 | −76.5 | −130.4 |
| −29.2 | −22.1 | ||||
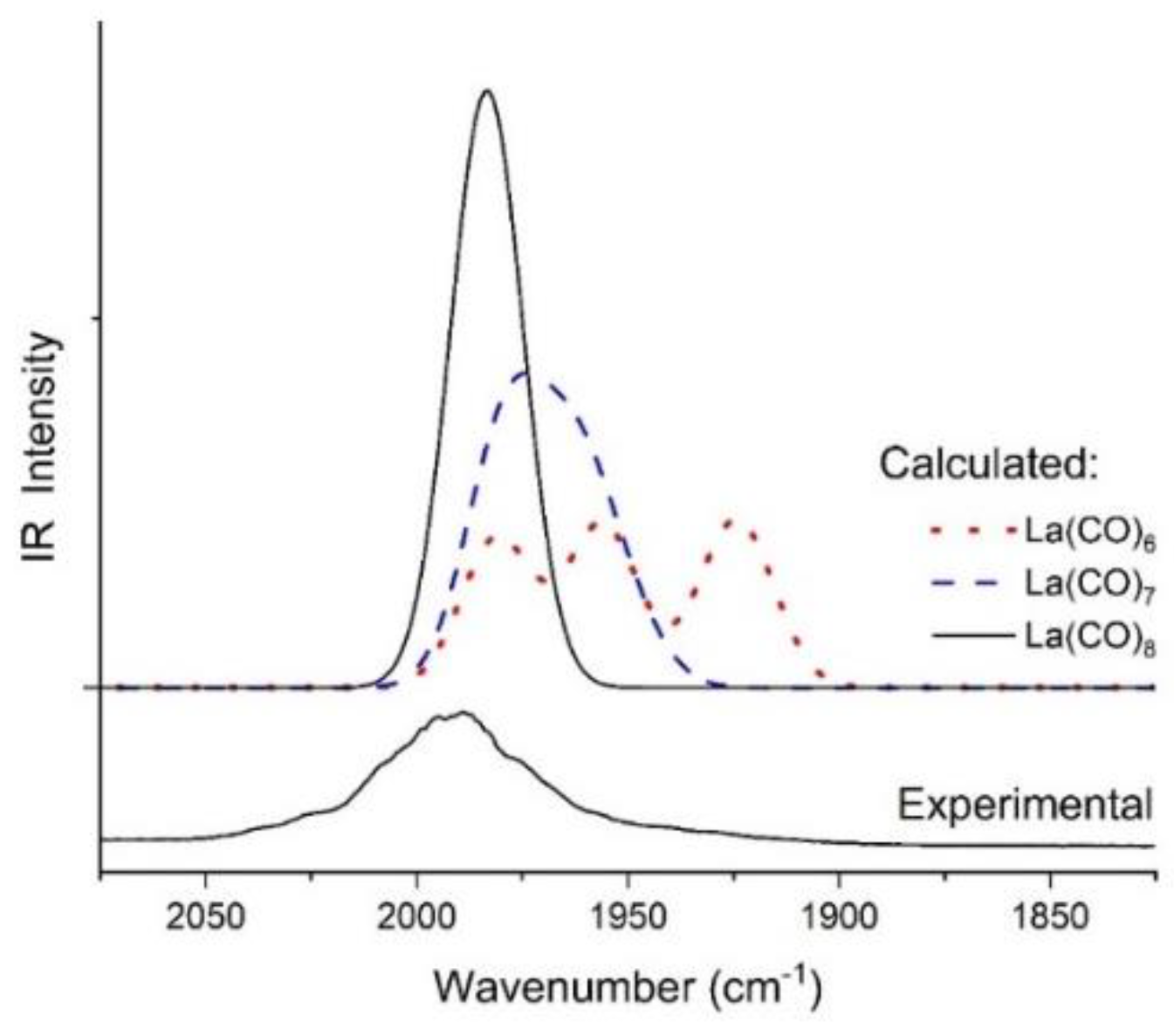
2.3. Identification of La(CO)8 in the MI-IR Spectra Assisted by DFT Calculations
3. Materials and Methods
3.1. Matrix–Isolation Spectroscopy
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yorimitsu, H.; Kotora, M.; Patil, N.T. Special Issue: Recent Advances in Transition-Metal Catalysis. Chem. Rec. 2021, 21, 3335–3337. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, Z.; Wang, Z.; Wang, H.; Xiong, W.; Song, B.; Qin, H.; Xu, P.; Zeng, G. Recent progress on mixed transition metal nanomaterials based on metal–organic frameworks for energy-related applications. J. Mater. Chem. A 2022, 10, 9788–9820. [Google Scholar] [CrossRef]
- Moskovits, M.; Ozin, G.A. Cryochemistry; Wiley: Hoboken, NJ, USA, 1976. [Google Scholar]
- Burdett, J.K. Matrix isolation studies on transition metal carbonyls and related species. Coord. Chem. Rev. 1978, 27, 1–58. [Google Scholar] [CrossRef]
- Perutz, R.N. Matrix Photochemistry Of Transition Metal Complexes: Principles, Applications and Links to Other Methods. In Low Temperature Molecular Spectroscopy; NATO ASI Series 483; Fausto, R., Ed.; Springer: Dordrecht, The Netherlands, 1996; pp. 95–124. [Google Scholar] [CrossRef]
- Turner, J.J.; George, M.W.; Poliakoff, M.; Perutz, R.N. Photochemistry of transition metal carbonyls. Chem. Soc. Rev. 2022, 51, 5300–5329. [Google Scholar] [CrossRef] [PubMed]
- DeKock, R.L. Preparation and identification of intermediate carbonyls of nickel and tantalum by matrix isolation. Inorg. Chem. 1971, 10, 1205–1211. [Google Scholar] [CrossRef]
- Bellard, S.; Rubinson, K.A.; Sheldrick, G.M. Crystal and molecular structure of vanadium hexacarbonyl. Acta Cryst. Sect. B 1979, 35, 271–274. [Google Scholar] [CrossRef]
- Elschenbroich, C.; Salzer, A. Organometallics: A Concise Introduction, 2nd ed.; Wiley-VCH: Weinheim, Germany, 1992. [Google Scholar]
- Busby, R.; Klotzbuecher, W.; Ozin, G.A. Titanium hexacarbonyl, Ti(CO)6, and titanium hexadinitrogen, Ti(N2)6. 1. Synthesis using titanium atoms and characterization by matrix infrared and ultraviolet-visible spectroscopy. Inorg. Chem. 1977, 16, 822–828. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Q.-S.; Yu, Z.H.; Xie, Y.; King, R.B.; Schaefer, H.F., III. Bonding of Seven Carbonyl Groups to a Single Metal Atom: Theoretical Study of M(CO)n (M = Ti, Zr, Hf; n = 7, 6, 5, 4). J. Am. Chem. Soc. 2008, 130, 7756–7765. [Google Scholar] [CrossRef]
- Frenking, G.; Fernández, I.; Holzmann, N.; Pan, S.; Krossing, I.; Zhou, M. Metal–CO Bonding in Mononuclear Transition Metal Carbonyl Complexes. JACS Au 2021, 1, 623–645. [Google Scholar] [CrossRef]
- Slater, J.L.; Sheline, R.K.; Lin, K.C.; Weltner, W. Synthesis of Uranium Carbonyls Using Matrix Isolation. J. Chem. Phys. 1971, 55, 5129–5130. [Google Scholar] [CrossRef]
- Slater, J.L.; De Vore, T.C.; Calder, V. Detection of neodymium and ytterbium carbonyls using matrix isolation. Inorg. Chem. 1973, 12, 1918–1921. [Google Scholar] [CrossRef]
- Slater, J.L.; DeVore, T.C.; Calder, V. Detection of praseodymium, europium, gadolinium, and holmium carbonyls using matrix isolation. Inorg. Chem. 1974, 13, 1808–1812. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Suleimanov, G.Z.; Shifrina, R.R.; Mekhdiev, R.Y.; Agdamskii, T.A.; Khandozhko, V.N.; Kolobova, N.E. Synthesis and structure of metal carbonyl derivatives of lanthanium, samarium and ytterbium. J. Organomet. Chem. 1986, 299, 239–244. [Google Scholar] [CrossRef]
- Klotzbücher, W.E.; Petrukhina, M.A.; Sergeev, G.B. Reactions of Samarium Atoms in Inert and Reactive Matrices. Mendeleev Commun. 1994, 4, 5–7. [Google Scholar] [CrossRef]
- Zhou, M.; Andrews, L.; Li, J.; Bursten, B.E. Reactions of Th Atoms with CO: The First Thorium Carbonyl Complex and an Unprecedented Bent Triplet Insertion Product. J. Am. Chem. Soc. 1999, 121, 12188–12189. [Google Scholar] [CrossRef]
- Baker, A.B.; Andrews, L. Reactions of Group 3 Transition Metal Atoms with CS2 and OCS: Matrix Isolation Infrared Spectra and Density-Functional Calculations of SMCS, SM-(η2-CS), SMCO, and SM-(η2-CO) in Solid Argon. J. Phys. Chem. A 2006, 110, 10419–10426. [Google Scholar] [CrossRef]
- Zhou, M.; Jin, X.; Li, J. Reactions of Cerium Atoms and Dicerium Molecules with CO: Formation of Cerium Carbonyls and Photoconversion to CO-Activated Insertion Molecules. J. Phys. Chem. A 2006, 110, 10206–10211. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Q. Reactions of Laser-Ablated La and Y Atoms with CO: Matrix Infrared Spectra and DFT Calculations of the M(CO)x and MCO+ (M = La, Y; x = 1−4) Molecules. J. Phys. Chem. A 2007, 111, 3271–3277. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Qin, Z.; Shi, L.; Tang, Z.; Xing, X. Octacoordinate Metal Carbonyls of Lanthanum and Cerium: Experimental Observation and Theoretical Calculation. J. Phys. Chem. A 2014, 118, 9380–9385. [Google Scholar] [CrossRef]
- Jia, Y.Q. Some possible structures of lanthanoid carbonyl compounds. Inorg. Chim. Acta 1987, 132, 289–292. [Google Scholar] [CrossRef]
- Lin, X.; Hong, G.; Li, L. Study on the geometry, electronic structure and bonding of LaCO. Wuji Huaxue Xuebao 1996, 12, 197–199. [Google Scholar]
- Boudreaux, E.A. SCMEH-MO calculations on lanthanide systems. III. Ln(CO)6, Ln(OC)6 (Ln = Nd, Sm). Int. J. Quantum Chem. 1996, 60, 1673–1677. [Google Scholar] [CrossRef]
- Hong, G.; Lin, X.; Li, L.; Xu, G. Linkage Isomerism and the Relativistic Effect in Interaction of Lanthanoid and Carbon Monoxide. J. Phys. Chem. A 1997, 101, 9314–9317. [Google Scholar] [CrossRef]
- Ermilov, A.Y.; Nemukhin, A.V.; Kovba, V.M. Characterization of structures and spectra of holmium complexes formed in the CO and N2 matrices. Mendeleev Commun. 1999, 9, 88–89. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, L.; Zou, R.-Q. Infrared-Spectroscopic and Density-Functional-Theory Investigations of the LaCO, La2[η2(μ2-C,O)], and c-La2(μ-C)(μ-O) Molecules in Solid Argon. Chem. Eur. J. 2006, 12, 3226–3232. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, L.; Xu, Q.; Zhou, M. Reactions of Gadolinium Atoms and Dimers with CO: Formation of Gadolinium Carbonyls and Photoconversion to CO Activated Molecules. J. Phys. Chem. A 2006, 110, 12585–12591. [Google Scholar] [CrossRef]
- Xu, W.; Jin, X.; Chen, M.; Pyykkö, P.; Zhou, M.; Li, J. Rare-earth monocarbonyls MCO: Comprehensive infrared observations and a transparent theoretical interpretation for M = Sc; Y; La–Lu. Chem. Sci. 2012, 3, 1548–1554. [Google Scholar] [CrossRef]
- Ricks, A.M.; Gagliardi, L.; Duncan, M.A. Infrared Spectroscopy of Extreme Coordination: The Carbonyls of U+ and UO2+. J. Am. Chem. Soc. 2010, 132, 15905–15907. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, L.; Jin, J.; Pan, S.; Li, W.; Jin, X.; Wang, G.; Zhou, M.; Frenking, G. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 2018, 361, 912–916. [Google Scholar] [CrossRef]
- Bettens, T.; Pan, S.; De Proft, F.; Frenking, G.; Geerlings, P. Alkaline Earth Metals Activate N2 and CO in Cubic Complexes Just Like Transition Metals: A Conceptual Density Functional Theory and Energy Decomposition Analysis Study. Chem. Eur. J. 2020, 26, 12785–12793. [Google Scholar] [CrossRef]
- Yang, X. The 18-electron rule for main-group alkaline earth octacarbonyl complexes. Nat. Sci. Rev. 2019, 6, 8–9. [Google Scholar] [CrossRef]
- Deng, G.; Lei, S.; Pan, S.; Jin, J.; Wang, G.; Zhao, L.; Zhou, M.; Frenking, G. Filling a Gap: The Coordinatively Saturated Group 4 Carbonyl Complexes TM(CO)8 (TM=Zr, Hf) and Ti(CO)7. Chem. Eur. J. 2020, 26, 10487–10500. [Google Scholar] [CrossRef] [PubMed]
- Brathwaite, A.D.; Maner, J.A.; Duncan, M.A. Testing the Limits of the 18-Electron Rule: The Gas-Phase Carbonyls of Sc+ and Y+. Inorg. Chem. 2014, 53, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yang, T.; Xin, K.; Wang, G.; Jin, X.; Zhou, M.; Frenking, G. Octacarbonyl Anion Complexes of Group Three Transition Metals [TM(CO)8]− (TM=Sc, Y, La) and the 18-Electron Rule. Angew. Chem. Int. Ed. 2018, 57, 6236–6241. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Pan, S.; Jin, X.; Lei, S.; Zhao, L.; Frenking, G.; Zhou, M. Octacarbonyl Anion Complexes of the Late Lanthanides Ln(CO)8− (Ln=Tm, Yb, Lu) and the 32-Electron Rule. Chem. Eur. J. 2019, 25, 3229–3234. [Google Scholar] [CrossRef]
- Chi, C.; Pan, S.; Jin, J.; Meng, L.; Luo, M.; Zhao, L.; Zhou, M.; Frenking, G. Octacarbonyl Ion Complexes of Actinides [An(CO)8]+/− (An=Th, U) and the Role of f Orbitals in Metal–Ligand Bonding. Chem. Eur. J. 2019, 25, 11772–11784. [Google Scholar] [CrossRef]
- Martin, W.C.; Zalubas, R.; Hagan, L. Atomic Energy Levels—The Rare-Earth Elements. The Spectra of Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and Lutetium; National Bureau of Standards, U.S. Department of Commerce: Washington, DC, USA, 1978.
- Sansonetti, J.E.; Martin, W.C. Handbook of Basic Atomic Spectroscopic Data. J. Phys. Chem. Ref. Data 2005, 34, 1559–2259. [Google Scholar] [CrossRef]
- Cotton, S. The Lanthanides—Principles and Energetics. In Lanthanide and Actinide Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 9–22. [Google Scholar] [CrossRef]
- Kovács, A.; Klotzbücher, W. Octa-coordination in complexes of lanthanides with N2 confirmed by matrix-isolation IR spectroscopy and DFT calculations. J. Mol. Struct. 2023, 1272, 134222. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, K.; Jin, J.; Li, W.; Wang, Q.; Wang, X.; Wang, G. Infrared photodissociation spectroscopic investigation of TMO(CO)n+ (TM = Sc, Y, La): Testing the 18-electron rule. Phys. Chem. Chem. Phys. 2019, 21, 6743–6749. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, UK, 2010. [Google Scholar]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the rare earth elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Cao, X.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struct. (Theochem) 2002, 581, 139–147. [Google Scholar] [CrossRef]
- Dunning Jr., T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Landis, C.R.; Weinhold, F. NBO 6.0; Theoretical Chemistry Institute, University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 6.0: Natural Bond Orbital Analysis Program. J. Comput. Chem. 2013, 34, 1429–1437. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theor. Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Edinburgh, UK, 2009. [Google Scholar]

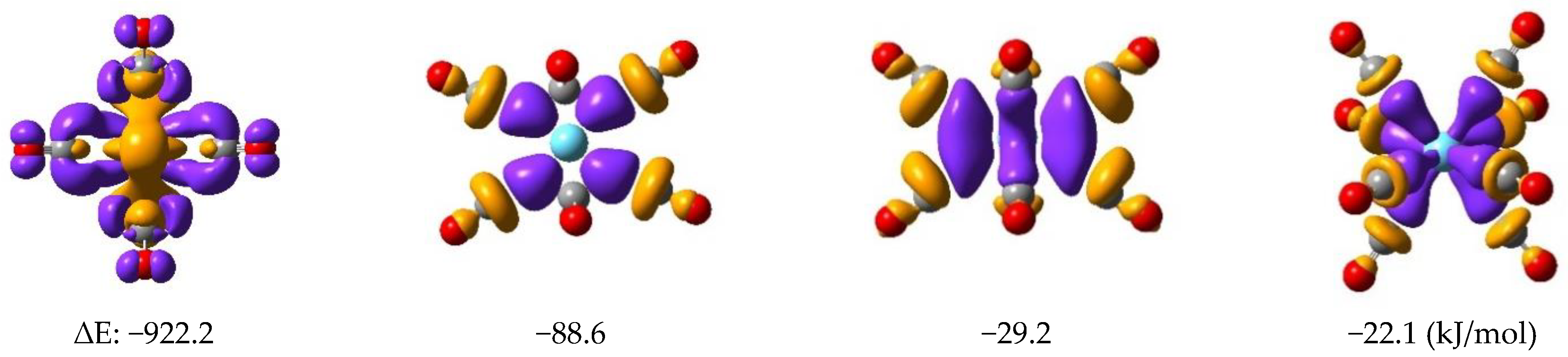
| Ln | m | PopLn | Sym | Ln-C | C≡O | ||
|---|---|---|---|---|---|---|---|
| 6s | 4f | 5d | |||||
| La | 2 | 0.38 | 0.09 | 2.98 | D4h | 2.703 | 1.136 |
| Ce | 3 | 0.40 | 1.08 | 3.07 | D4h | 2.651 | 1.136 |
| Pr | 4 | 0.40 | 2.08 | 3.10 | D4h | 2.627 | 1.136 |
| Nd | 5 | 0.41 | 3.06 | 3.10 | D4h | 2.606 | 1.136 |
| Sm | 9 | 0.39 | 5.83 | 1.99 | Oh | 2.692 | 1.135 |
| Eu | 10 | 0.39 | 6.86 | 1.95 | Oh | 2.704 | 1.135 |
| Gd | 9 | 0.72 | 7.00 | 2.75 | D4h | 2.607 | 1.136 |
| Tb | 8 | 1.00 | 7.42 | 3.16 | D2d | 2.543 | 1.137 |
| Dy | 7 | 0.93 | 8.49 | 3.16 | Cs | 2.511, 2.515 | 1.137 |
| Ho | 6 | 0.44 | 10.10 | 3.18 | D4h | 2.494 | 1.137 |
| Er | 5 | 0.44 | 11.01 | 3.15 | D4h | 2.479 | 1.138 |
| Tm | 4 | 0.44 | 12.02 | 3.14 | D4h | 2.464 | 1.137 |
| Yb | 1 | 0.43 | 13.77 | 2.37 | D4d | 2.528 | 1.136 |
| Lu | 2 | 0.44 | 14.00 | 2.95 | D2h | 2.447 | 1.138 |
| Ln | qLn | CT | |
|---|---|---|---|
| (CO)8→Ln | Ln→(CO)8 | ||
| La | −0.46 | 1.99 | 1.53 |
| Ce | −0.54 | 2.12 | 1.58 |
| Pr | −0.58 | 2.14 | 1.56 |
| Nd | −0.57 | 2.15 | 1.58 |
| Sm | −0.21 | 1.63 | 1.42 |
| Eu | −0.20 | 1.58 | 1.38 |
| Gd | −0.47 | 1.97 | 1.50 |
| Tb | −0.59 | 1.99 | 1.61 |
| Dy | −0.59 | 1.99 | 1.62 |
| Ho | −0.63 | 2.26 | 1.63 |
| Er | −0.61 | 2.26 | 1.66 |
| Tm | −0.60 | 2.28 | 1.68 |
| Yb | −0.57 | 2.00 | 1.43 |
| Lu | −0.39 | 2.12 | 1.72 |
| Ln(CO)x | BP86 | B3LYP | B3LYP Scaled 2 | Experimental 3 |
|---|---|---|---|---|
| La(CO)8 | 2 × 1984 (3025), 1982 (2605) | 2 × 2075 (3542), 2072 (3236) | 1991 | 1992; 1985 [28]; 1983.3 [21] |
| La(CO)8− | 3 × 1907 (3483) | 1914 [37] | ||
| La(CO)8Ar8 | 2 × 1984 (3144), 1982 (2654) | |||
| La(CO)8(CO)8 | 2 × 1985 (3156), 1984 (2618) | |||
| Ce(CO)8 | 2077 (3352), 2 × 2070 (3221) | 1990 | 1992; 1985.1 [20] | |
| Pr(CO)8 | 2 × 2075 (3212), 2068 (3158) | 1989 | 1988; 1989 [15] | |
| Nd(CO)8 | 2074 (3322), 2 × 2071 (3132) | 1989 | 1987; 1990 [14] | |
| Sm(CO)8 | 3 × 2079 (3425) | 1996 | 1992 4 | |
| Sm(CO)7 | 2 × 2072 (2972), 2068 (1914) | 1989, 1985 | 1986, 1981 | |
| Sm(CO)6 | 2 × 2050 (3593) | 1967 | 1959 | |
| Eu(CO)8 | 3 × 1993 (2589) | 3 × 2078 (3562) | 1995 | 1999; 2000 [15] |
| Eu(CO)6 | 2 × 1970 (2717), 1957 (1289) | 2 × 2049 (4400), 2043 (1771) | 1967 | 1960 |
| Gd(CO)8 | 2 × 1987 (2385), 1979 (2930) | 2 × 2076 (2957), 2066 (3493) | 1993, 1983 | 1989, 1985; 1986 [15] |
| Tb(CO)8 | 2 × 2071 (3105), 2069 (2965) | 1987 | 1985 | |
| Dy(CO)8 | 2 × 2070 (2642), 2068 (2912) | 1986 | 1983 | |
| Ho(CO)8 | 2067 (3170), 2 × 2065 (2982) | 1983 | 1980 5; 1982 [15] | |
| Er(CO)8 | 2066 (3167), 2 × 2064 (2986) | 1982 | 1980 | |
| Tm(CO)8 | 3 × 2063 (3100) | 1980 | 1977 | |
| Tm(CO)7 | 2070 (1505), 2059 (1461) 2054 (3196), 2048 (2595) | 1987, 1969 | 1991, 1971 | |
| Yb(CO)8 | 3 × 1992 (2310) | 2081 (3105), 2 × 2079 (3025) | 1997 | 1990; 1995 and 2008 [14] |
| Yb(CO)6 | 1975 (2941), 2 × 1964 (1675) | 2061 (3841), 2 × 2050 (2199) | 1973 | 1967 |
| Lu(CO)8 | 2063 (3104), 2 × 2062 (2960) | 1980 | 1975 | |
| Lu(CO)7 | 2074 (1290), 2059 (1391), 2054 (3216), 2047 (1459), 2047 (1182) | 1991, 1972 | 1990, 1965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.; Klotzbücher, W. A DFT and Matrix–Isolation IR/UV-Visible Study of High-Coordinated Lanthanide-CO Complexes. Molecules 2023, 28, 5043. https://doi.org/10.3390/molecules28135043
Kovács A, Klotzbücher W. A DFT and Matrix–Isolation IR/UV-Visible Study of High-Coordinated Lanthanide-CO Complexes. Molecules. 2023; 28(13):5043. https://doi.org/10.3390/molecules28135043
Chicago/Turabian StyleKovács, Attila, and Werner Klotzbücher. 2023. "A DFT and Matrix–Isolation IR/UV-Visible Study of High-Coordinated Lanthanide-CO Complexes" Molecules 28, no. 13: 5043. https://doi.org/10.3390/molecules28135043
APA StyleKovács, A., & Klotzbücher, W. (2023). A DFT and Matrix–Isolation IR/UV-Visible Study of High-Coordinated Lanthanide-CO Complexes. Molecules, 28(13), 5043. https://doi.org/10.3390/molecules28135043






