Essential Oils for the Conservation of Paper Items
Abstract
1. Plant Scented Arsenal Transferred into Archives
2. EO Mixtures and Their Different Degrees of Bioactivities
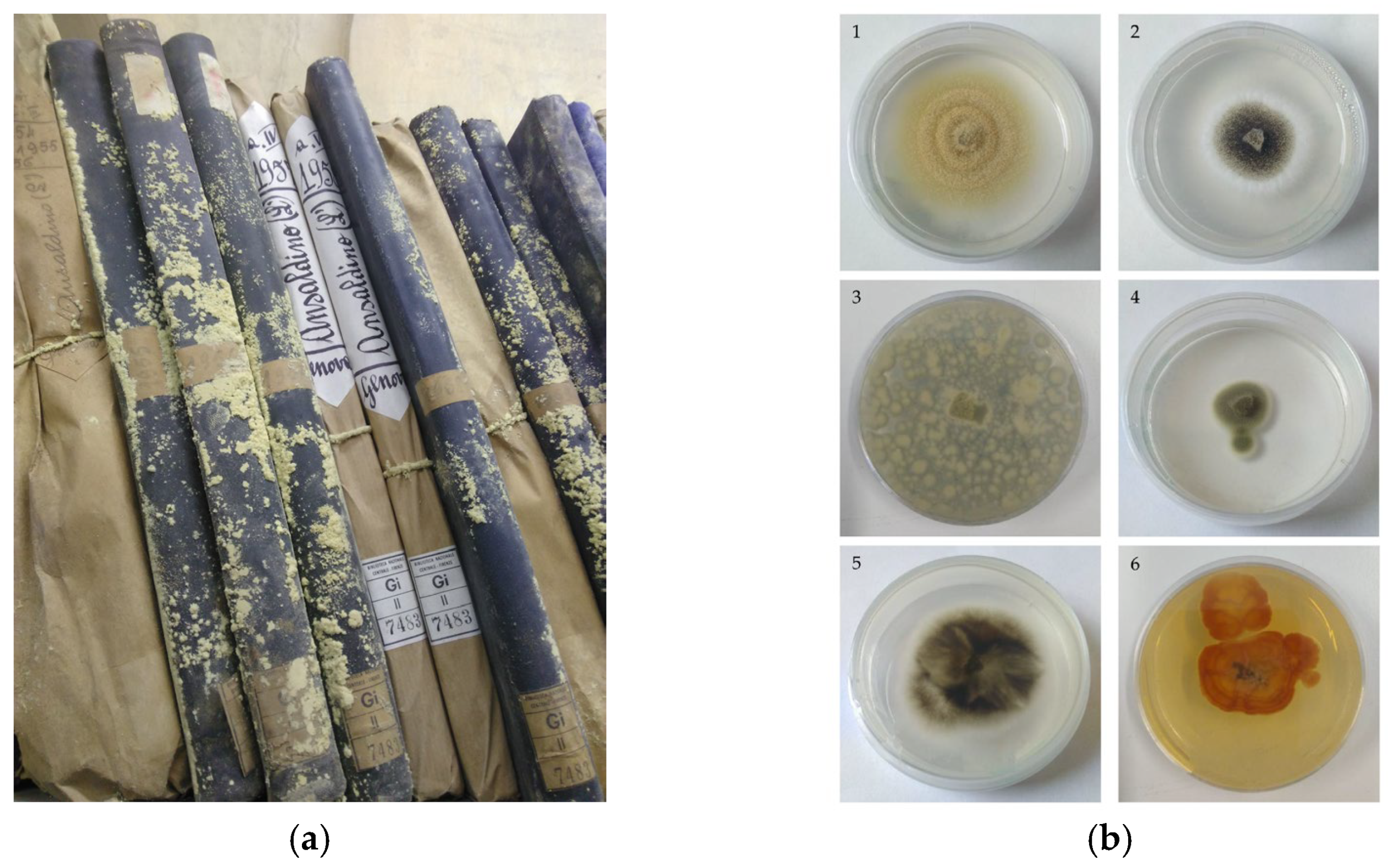
3. Paper Biodeteriogens, In Vitro Tests and Most Recurrent Substances
4. Book Disinfection with EOs
5. Structural Analysis of Paper
6. EO-Based Technologies
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Menicucci, F.; Palagano, E.; Raio, A.; Cencetti, G.; Luchi, N.; Ienco, A.; Michelozzi, M. Plant Sampling for Production of Essential Oil and Evaluation of Its Antimicrobial Activity In Vitro. In Plant Pathology: Method and Protocols; Springer: New York, NY, USA, 2022; Volume 2536, pp. 475–493. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher Plant Terpenoids: A Phytocentric Overview of Their Ecological Roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Bohlmann, J. Oleoresin Defenses in Conifers: Chemical Diversity, Terpene Synthases and Limitations of Oleoresin Defense under Climate Change. New Phytol. 2019, 224, 1444–1463. [Google Scholar] [CrossRef] [PubMed]
- Derbassi, N.B.; Pedrosa, M.C.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Plant volatiles: Using scented molecules as food additives. Trends Food. Sci. Technol. 2022, 122, 97–103. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Souza, A.G.; Quispe, Y.M.; Rosa, D.S. Essential Oils Loaded-Chitosan Nanocapsules Incorporation in Biodegradable Starch Films: A Strategy to Improve Fruits Shelf Life. Int. J. Biol. Macromol. 2021, 188, 628–638. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Rakotonirainy, M.S.; Lavédrine, B. Screening for Antifungal Activity of Essential Oils and Related Compounds to Control the Biocontamination in Libraries and Archives Storage Areas. Int. Biodeterior. Biodegrad. 2005, 55, 141–147. [Google Scholar] [CrossRef]
- Noshyutta, W.; Osman, E.; Mansour, M. An Investigation of the Biological Fungicidal Activity of Some Essential Oils Used as Preservatives for a 19th Century Egyptian Coptic Cellulosic Manuscript. Int. J. Conserv. Sci. 2016, 7, 41–56. [Google Scholar]
- Karbowska-Berent, J.; Górniak, B.; Czajkowska-Wagner, L.; Rafalska, K.; Jarmiłko, J.; Kozielec, T. The Initial Disinfection of Paper-Based Historic Items—Observations on Some Simple Suggested Methods. Int. Biodeterior. Biodegrad. 2018, 131, 60–66. [Google Scholar] [CrossRef]
- Benkovičová, M.; Kisová, Z.; Bučková, M.; Majková, E.; Šiffalovič, P.; Pangallo, D. The Antifungal Properties of Super-Hydrophobic Nanoparticles and Essential Oils on Different Material Surfaces. Coatings 2019, 9, 176. [Google Scholar] [CrossRef]
- Antonelli, F.; Bartolini, M.; Plissonnier, M.L.; Esposito, A.; Galotta, G.; Ricci, S.; Petriaggi, B.D.; Pedone, C.; Di Giovanni, A.; Piazza, S.; et al. Essential Oils as Alternative Biocides for the Preservation of Waterlogged Archaeological Wood. Microorganisms 2020, 8, 2015. [Google Scholar] [CrossRef]
- Ranaldi, R.; Rugnini, L.; Gabriele, F.; Spreti, N.; Casieri, C.; Di Marco, G.; Gismondi, A.; Bruno, L. Plant Essential Oils Suspended into Hydrogel: Development of an Easy-to-Use Protocol for the Restoration of Stone Cultural Heritage. Int. Biodeterior. Biodegrad. 2022, 172, 105436. [Google Scholar] [CrossRef]
- Zhu, C.; Li, Q.; Wang, X.; Hu, Y.; Zhang, B. Biocides for the Control of Mosses on Stone Cultural Relics. Stud. Conserv. 2022, 68, 502–511. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Zikeli, F.; Humar, M.; Biscontri, M.; Bergamasco, S.; Romagnoli, M. Essential Oils from Thymus spp. as Natural Biocide against Common Brown- and White-Rot Fungi in Degradation of Wood Products: Antifungal Activity Evaluation by in Vitro and FTIR Analysis. Eur. J. Wood Wood Prod. 2023, 81, 747–763. [Google Scholar] [CrossRef]
- Bloom, J.M. Paper before Print: The History and Impact of Paper in the Islamic World; Yale University Press: New Haven, CT, USA, 2001. [Google Scholar]
- Sequeira, S.; Cabrita, E.J.; Macedo, M.F. Antifungals on Paper Conservation: An Overview. Int. Biodeterior. Biodegrad. 2012, 74, 67–86. [Google Scholar] [CrossRef]
- Jinot, J.; Fritz, J.M.; Vulimiri, S.V.; Keshava, N. Carcinogenicity of Ethylene Oxide: Key Findings and Scientific Issues. Toxicol. Mech. Methods 2018, 28, 386–396. [Google Scholar] [CrossRef]
- Daniels, V.; Boyd, B. The Yellowing of Thymol in the Display of Prints. Stud. Conserv. 1986, 31, 156–158. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.; Borges, P. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. ISRN Microbiol. 2012, 2012, 826786. [Google Scholar] [CrossRef]
- Lavin, P.; de Saravia, S.G.; Guiamet, P. Scopulariopsis sp. and Fusarium sp. in the Documentary Heritage: Evaluation of Their Biodeterioration Ability and Antifungal Effect of Two Essential Oils. Microb. Ecol. 2016, 71, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, K.; Otlewska, A.; Danielewicz, D.; Dybka, K.; Pangallo, D.; Kraková, L.; Puškárová, A.; Bučková, M.; Scholtz, V.; Ďurovič, M.; et al. Disinfection of Archival Documents Using Thyme Essential Oil, Silver Nanoparticles Misting and Low Temperature Plasma. J. Cult. Herit. 2017, 24, 69–77. [Google Scholar] [CrossRef]
- Čabalová, I.; Češek, B.; Mikala, O.; Gojný, J.; Kačík, F.; Tribulová, T. The Influence of Selected Efficient Compounds of Essential Oils for Paper Protection. J. Cult. Herit. 2019, 37, 148–154. [Google Scholar] [CrossRef]
- Campanella, L.; Angeloni, R.; Cibin, F.; Dell’Aglio, E.; Grimaldi, F.; Reale, R.; Vitali, M. Capsulated Essential Oil in Gel Spheres for the Protection of Cellulosic Cultural Heritage. Nat. Prod. Res. 2021, 35, 116–123. [Google Scholar] [CrossRef]
- Bosco, F.; Mollea, C.; Demichela, M.; Fissore, D. Application of Essential Oils to Control the Biodeteriogenic Microorganisms in Archives and Libraries. Heritage 2022, 5, 2181–2195. [Google Scholar] [CrossRef]
- Menicucci, F.; Palagano, E.; Michelozzi, M.; Cencetti, G.; Raio, A.; Bacchi, A.; Mazzeo, P.P.; Cuzman, O.A.; Sidoti, A.; Guarino, S.; et al. Effects of Trapped-into-Solids Volatile Organic Compounds on Paper Biodeteriogens. Int. Biodeterior. Biodegrad. 2022, 174, 105469. [Google Scholar] [CrossRef]
- Tomić, A.; Šovljanski, O.; Nikolić, V.; Pezo, L.; Aćimović, M.; Cvetković, M.; Stanojev, J.; Kuzmanović, N.; Markov, S. Screening of Antifungal Activity of Essential Oils in Controlling Biocontamination of Historical Papers in Archives. Antibiotics 2023, 12, 103. [Google Scholar] [CrossRef]
- Lahlou, M. Methods to Study the Phytochemistry and Bioactivity of Essential Oils. Phyther. Res. 2004, 18, 435–448. [Google Scholar] [CrossRef]
- Nowicka, D.; Nawrot, U. Tinea Pedis—An Embarrassing Problem for Health and Beauty—A Narrative Review. Mycoses 2021, 64, 1140–1150. [Google Scholar] [CrossRef]
- Kale, R.A.; Zanwar, P.H. Isolation and screening of cellulolytic fungi. IOSR J. Biotechnol. Biochem. 2016, 2, 57–61. [Google Scholar]
- Rakotonirainy, M.S.; Juchauld, F.; Gillet, M.; Othman-Choulak, M.; Lavedrine, B. The effect of linalool vapour on silver-gelatine photographs and bookbinding leathers. Restaurator 2007, 28, 95–111. [Google Scholar] [CrossRef]
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225. [Google Scholar] [CrossRef]
- Cocca, M.; D’Arienzo, L.; D’Orazio, L. Effects of different artificial agings on structure and properties of Whatman paper samples. Int. Sch. Res. Not. 2011, 2011, 863083. [Google Scholar] [CrossRef]
- Grata, K. Determining Cellulolytic Activity of Microorganisms. Chem.-Didact.-Ecol.-Metrol. 2020, 25, 133–143. [Google Scholar] [CrossRef]
- Malešič, J.; Kraševec, I.; Cigić, I.K. Determination of Cellulose Degree of Polymerization in Historical Papers with High Lignin Content. Polymers 2021, 13, 1990. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential oils encapsulated in liposomes: A review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Wadhwa, G.; Kumar, S.; Chhabra, L.; Mahant, S.; Rao, R. Essential oil–cyclodextrin complexes: An updated review. J. Incl. Phenom. Macrocycl. Chem. 2017, 89, 39–58. [Google Scholar] [CrossRef]
| EO/VOC/EO-Based Product | GC-MS Analysis | Target Species (Bacterium/Fungus/Yeast/Insect) | Test with EO/VOC/EO-Based Product | Analysis and Examined Paper Parameters | Reference |
|---|---|---|---|---|---|
| Thymol | - | - | Direct contact treatment: thymol alcoholic solution (7% w/v) used to impregnate a blotting paper to be placed behind a pencil drawing | Visual inspection | [21] |
| Artemisia vulgaris (armoise), Peumus boldus (boldo), Eugenia caryophyllata (clove), Eucalyptus globulus (eucalyptus), Lavandula angustifola (lavender), Ravensara aromatica (ravensare), Malaleuca alternifola (tea tree), Thuja occidentalis (thuya), or Chenopodium ambrosioides (wormseed) | - | Aspergillus niger, A. fumigatus, A. repens, Cladosporium herbarum, Penicillium frequentans, Trichoderma viride, Chaetomium globosum, Paecilomyces variotii, Stachybotrys atra | Microatmosphere method (5, 35, 50, 60 µL in 90 mm Petri) | pH of the cold extract; diffuse reflectance factor (brightness); viscometric average degree of polymerisation (DPv) of cellulose | [10] |
| 1,8-cineole, eugenol, linalool, linalyl acetate,⍺+βthujone | Inoculation of a mix fungal suspension on paper supports inserted into books and exposed to vapors of linalool (295 and 415 ppm), at 25 °C for 21 days, in sealed chambers | ||||
| Pimpinella anisum L. (anice), Syzygium aromaticum L. (clove), Cuminum cyminum L. (cumin), Allium sativum L. (garlic), Laurus nobilis L. (laurel), Citrus sinensis (L.) Osbeck (orange sweet) or Origanum vulgare L. (oregano) | Yes | Bacillus sp., B. polymyxa, B. cereus, B. thuringiensis, Enterobacter agglomerans, Streptomyces sp. | Agar diffusion method | - | [22] |
| Aspergillus niger, A. clavatus, Penicillium sp., Fusarium sp. | |||||
| Origanum vulgare L. (oregano), Thymus vulgaris L. (thyme) | - | Fusarium sp., Scopulariopsis sp. | Microatmosphere method-10 µL of pure EOs | - | [23] |
| Lavender, tea tree, thyme | - | Bacillus subtilis | Disk-diffusion 0.031–0.063–0.125–0.25–0.5–1.0–2.5% (v/v) | Surface morphology (SEM) of colonized and non-colonized paper items | [11] |
| Aspergillus flavus, Eurotium chevalieri, Penicillium roqueforti, Trichoderma viride | Fumigation of EOs on mimic paper samples—concentrations used: 0.125–0.25–0.5% | Total color difference (ΔE), whiteness index (W), yellowness index (Y) | |||
| Application of tea tree (0.25% v/v) in the leaf casting stage of a manuscript | tensile strength, elongation at break | ||||
| FTIR-ATR | |||||
| Thymus vulgaris (thyme) | - | Bacillus cereus, B. licheniformis, Microbacterium aerolatum, Psychrobacillus psychrodurans, Staphylococcus epidermis, S. pasteuri, S. saprophyticus, S. succinus | Tests on paper-Thyme essential oil microatmosphere (conc. 10% in DMSO) to treat two books | Dimensional and structural parameters (weight; thickness; bulk; air resistance; ash; pH; Kappa number; intrinsic viscosity) | [24] |
| Aspergillus niger, Chaetomium elatum, C. globosum, C. murorum, Myxotrichum deflexum, Penicillium spinulosum, Rhodotorula mucilaginosa | Mechanical parameters (Stretch; tensile index; TEA; burst factor; tear factor; folding endurance; ZSFS) | ||||
| Optical parameters (R457; yellowness; L *; a *; b *; ΔE *) | |||||
| Tea tree | - | Cladosporium cladosporioides, Penicillium spinulosum *, Trichoderma pseudokoningii | paper exposure to tea tree vapors (1–3 mL/l) after infection with P. spinulosum | Surface pH | [12] |
| Total color difference (ΔE), difference in yellowing ΔRz | |||||
| Tear resistance | |||||
| super-hydrophobic nanoparticles loaded with Thuja plicata (arborvitae), Origanum vulgare L. (oregano) or Thymus vulgaris (thyme) EO | - | Aspergillus fumigatus *, Exophiala xenobiotica | Disk-diffusion (EO mix with SHNPs at conc. 10–30–50% in EtOH) | Rr (ratio of reflectivity) spectral reflectivity measurements | [13] |
| Test on Whatman paper (100 µL of EO/EO mix with SHNPs) | |||||
| Mix of linalyl acetate and citral | - | - | Aged and unaged paper samples exposed to vapors of linalyl acetate mixed with citral (1:1), RH = 75%, in a desiccator | Chemical parameters (Cellulose degree of depolymerization; content of saccharides and lignin) | [25] |
| Mechanical parameters (tensile index) | |||||
| Physical parameters (fiber length determination) | |||||
| Cinnamon EO-gel spheres | yes | Saccharomyces cerevisiae | Respirometric test | - | [26] |
| Origanum vulgare (oregano) or Thymus vulgaris (thyme) | - | Staphylococcus epidermidis | Thyme EO (0.75% v/v) nebulized immediately after the inoculation on agar plates or paper sheets | - | [27] |
| Alternaria alternata | Application of thyme EO (0.75% v/v) on a contaminated book cover by means of EO impregnated contact sheets | ||||
| Rhodotorula mucilaginosa | |||||
| β-cyclodextrins and cocrystals entrapping carvacrol, thymol or eugenol | Yes | Bacillus sp. | Micro-atmosphere method | - | [28] |
| Alternaria alternata, Aspergillus sp. (section Nigri), Cladosporium sp., Trichoderma orientale | Whatman paper exposed to vapors of carvacrol-based cocrystal (30 mg) after fungal infection | ||||
| Metschnikowia sp. | Olfactometer bioassay | ||||
| Lasioderma serricorne | |||||
| Eucalyptus globulus (eucalyptus), Cymbopogon citratus (lemongrass), Origanum vulgare (oregano), Mentha piperita (peppermint), or Rosmarinus officinalis (rosemary) | Yes | Aspergillus fumigatus, Cladosporium cladosporoides, Penicillium chrysogenum | Disk diffusion (15 µL of pure EOs) | - | [29] |
| Vapor phase (15 µL of pure EOs) | |||||
| Mixture of oregano, lemongrass and pepper mint EOs (1:1:1) in vapor phase (15 µL) in Petri dishes used to treat historical paper samples (1 cm2) inoculated with fungal suspension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menicucci, F.; Palagano, E.; Michelozzi, M.; Ienco, A. Essential Oils for the Conservation of Paper Items. Molecules 2023, 28, 5003. https://doi.org/10.3390/molecules28135003
Menicucci F, Palagano E, Michelozzi M, Ienco A. Essential Oils for the Conservation of Paper Items. Molecules. 2023; 28(13):5003. https://doi.org/10.3390/molecules28135003
Chicago/Turabian StyleMenicucci, Felicia, Eleonora Palagano, Marco Michelozzi, and Andrea Ienco. 2023. "Essential Oils for the Conservation of Paper Items" Molecules 28, no. 13: 5003. https://doi.org/10.3390/molecules28135003
APA StyleMenicucci, F., Palagano, E., Michelozzi, M., & Ienco, A. (2023). Essential Oils for the Conservation of Paper Items. Molecules, 28(13), 5003. https://doi.org/10.3390/molecules28135003








