Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
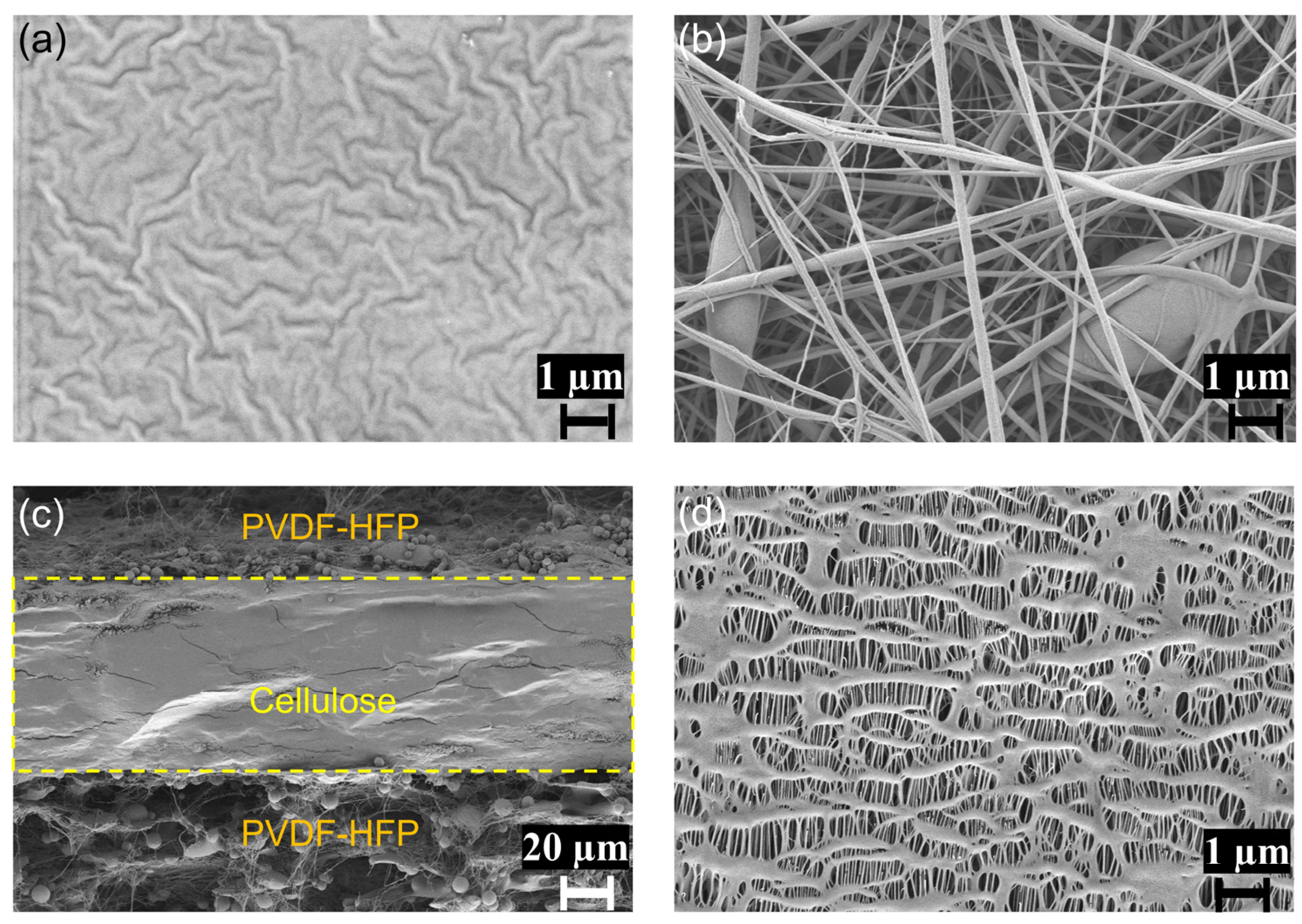
2.1. Surface Morphology of Membranes
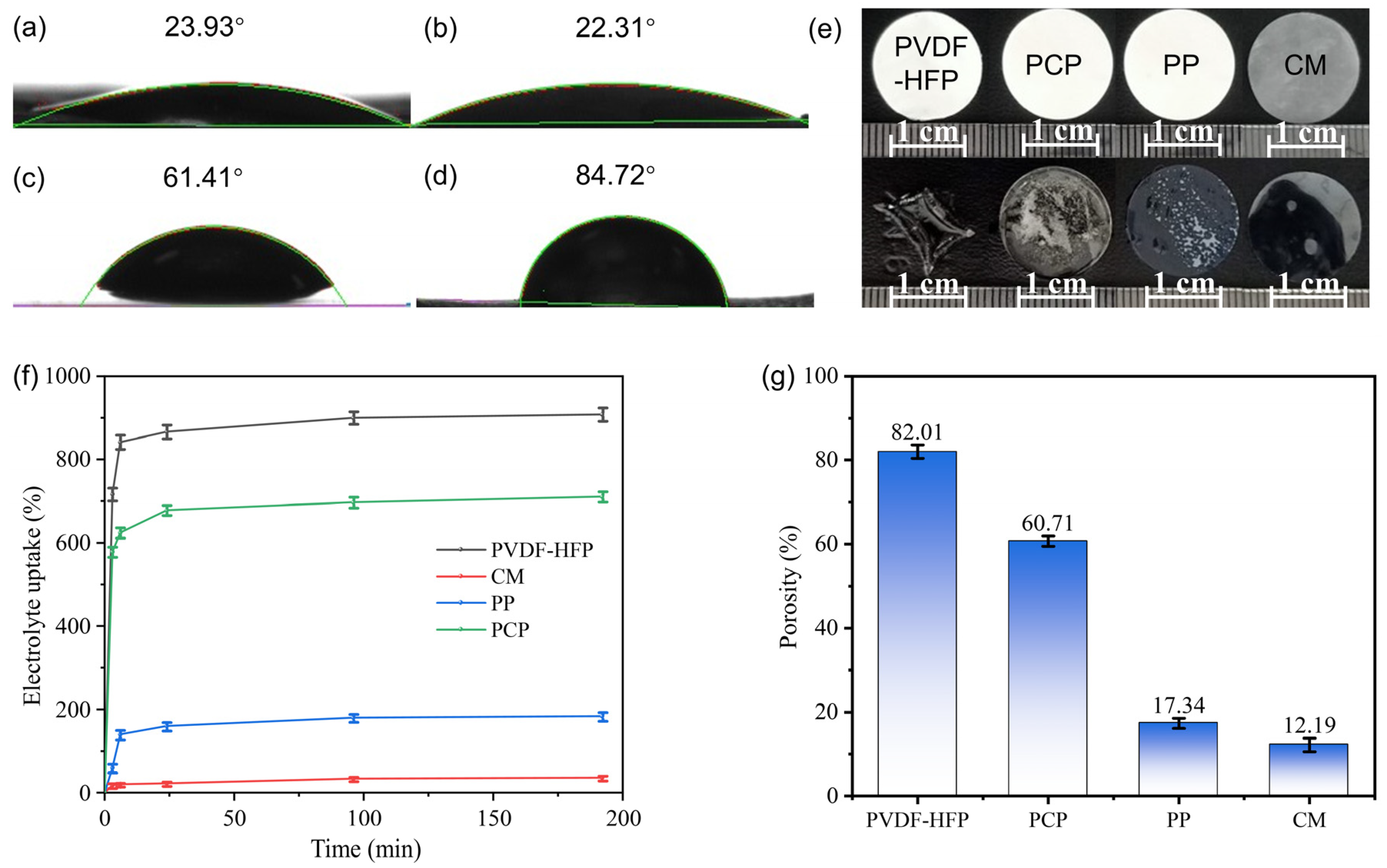
2.2. Electrolyte Uptake Performance
2.3. Surface Analysis
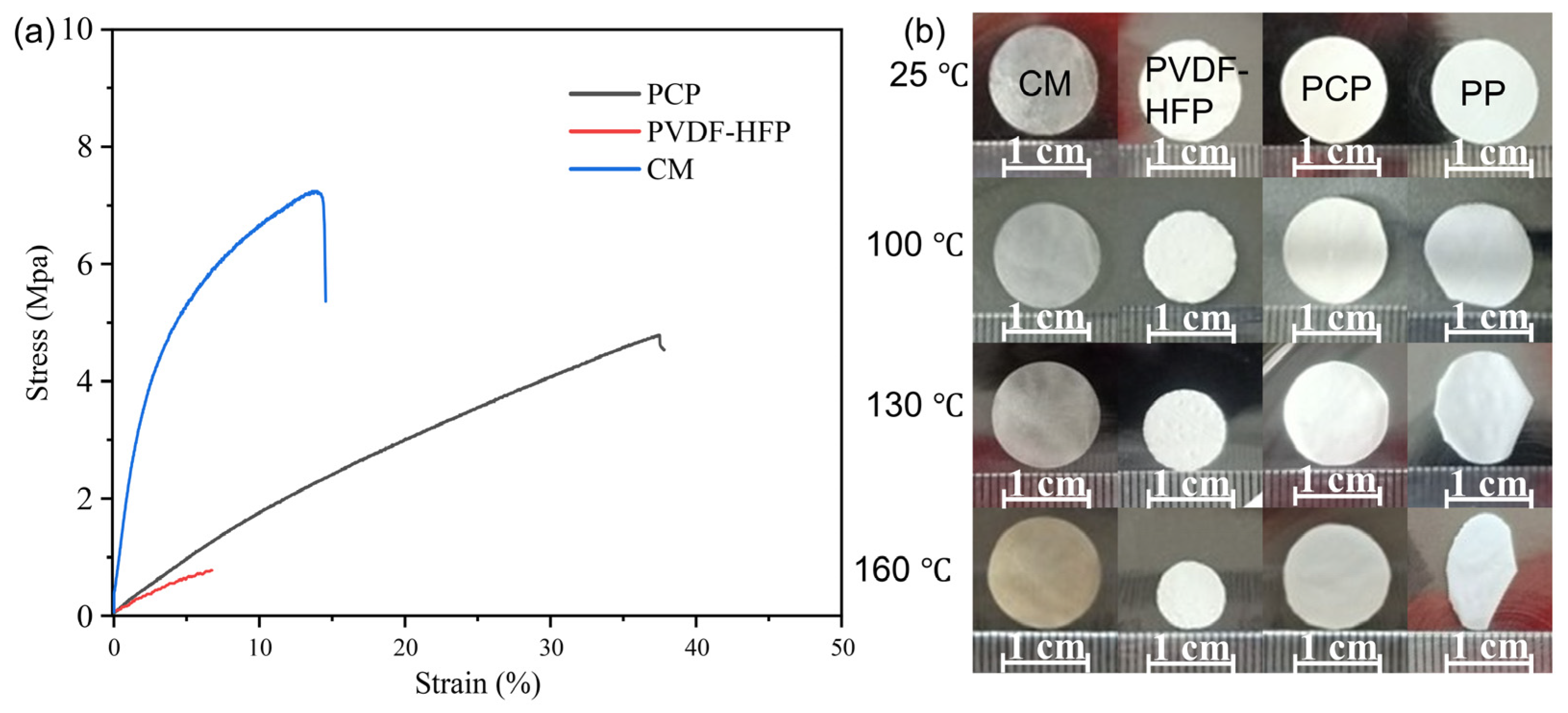
2.4. Mechanical Properties of Membranes
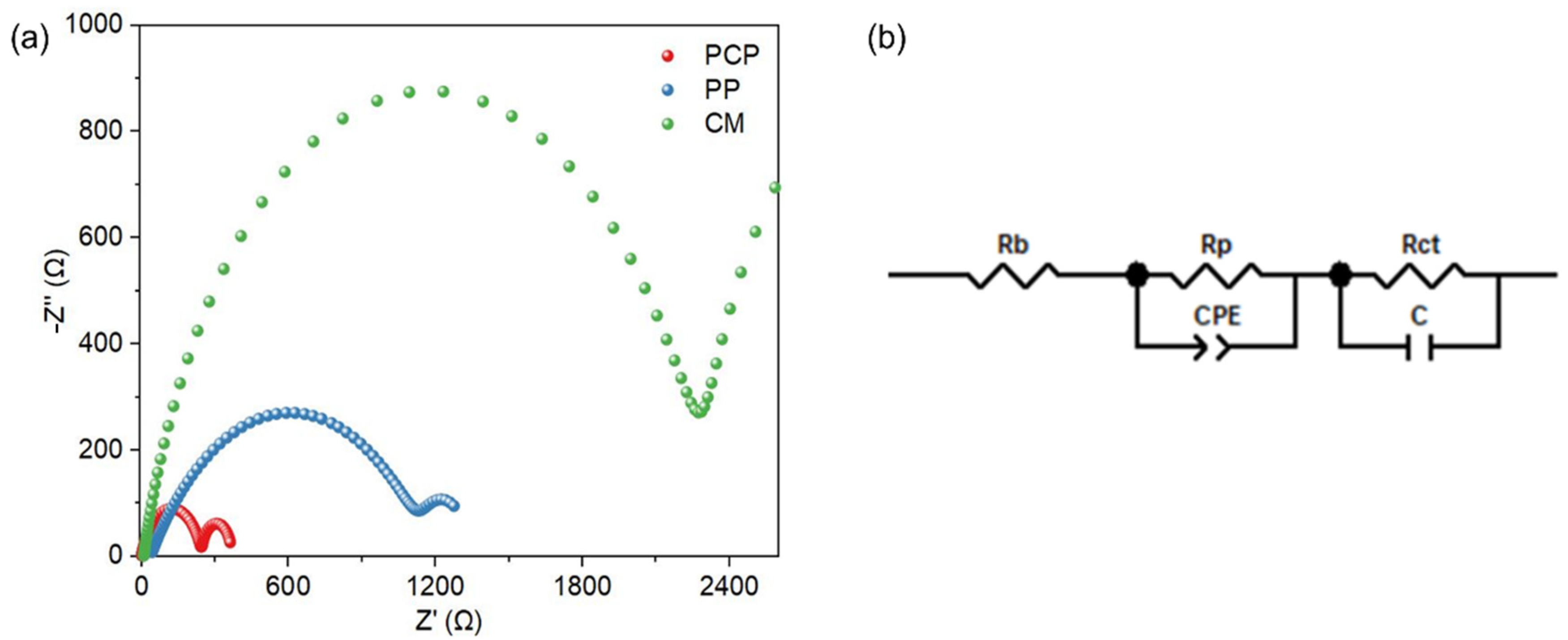
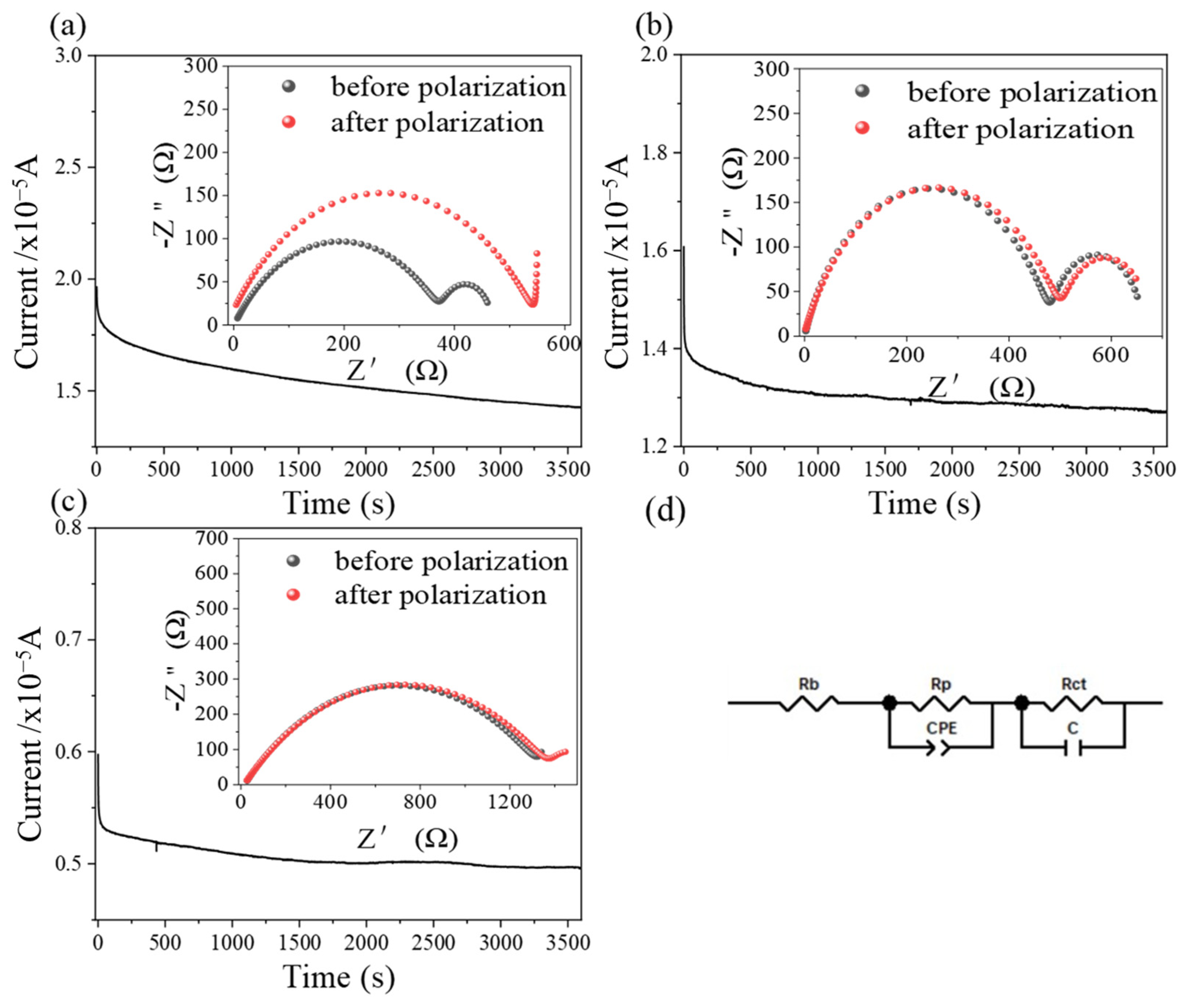
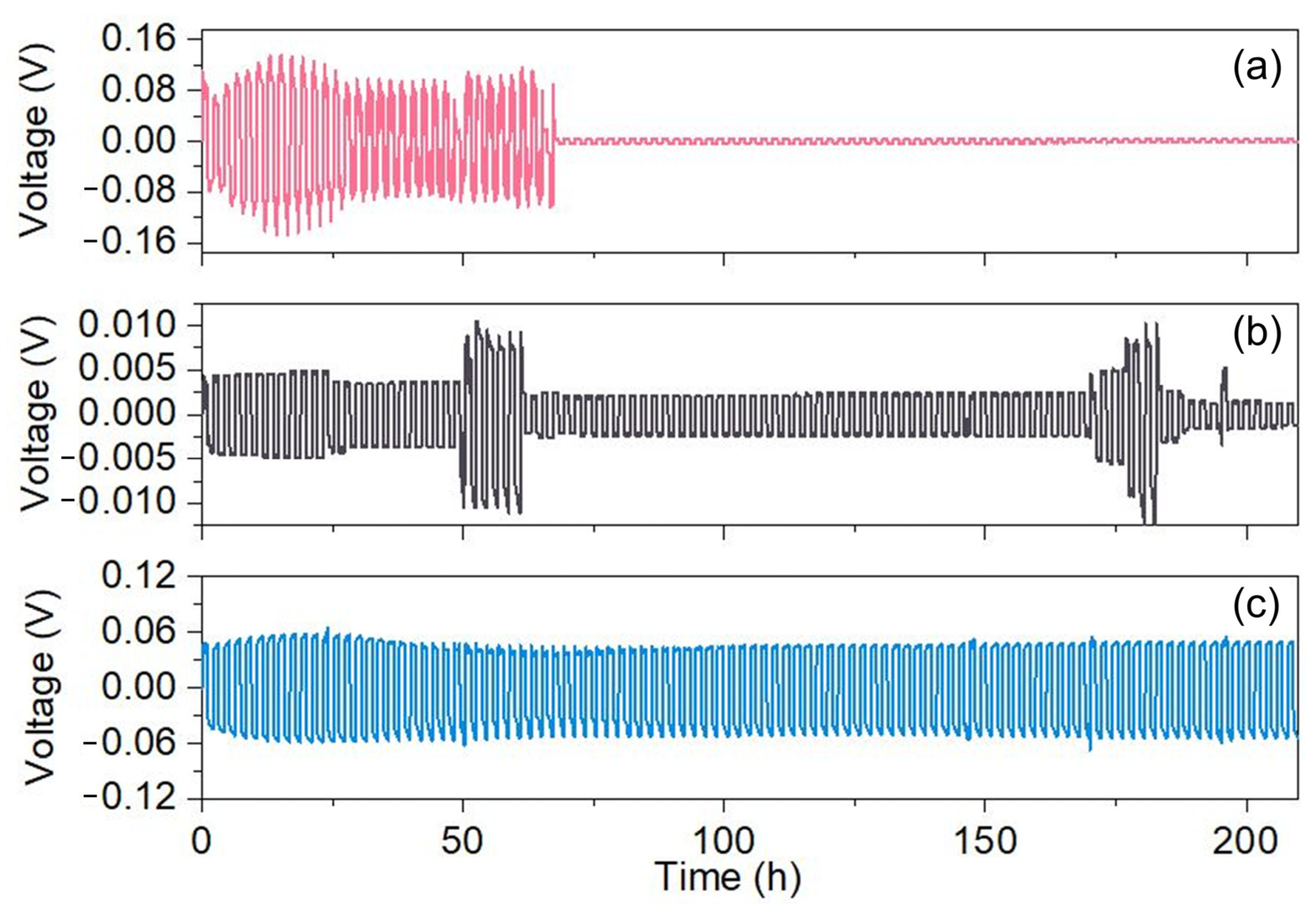
2.5. Electrochemistry Properties of Membranes
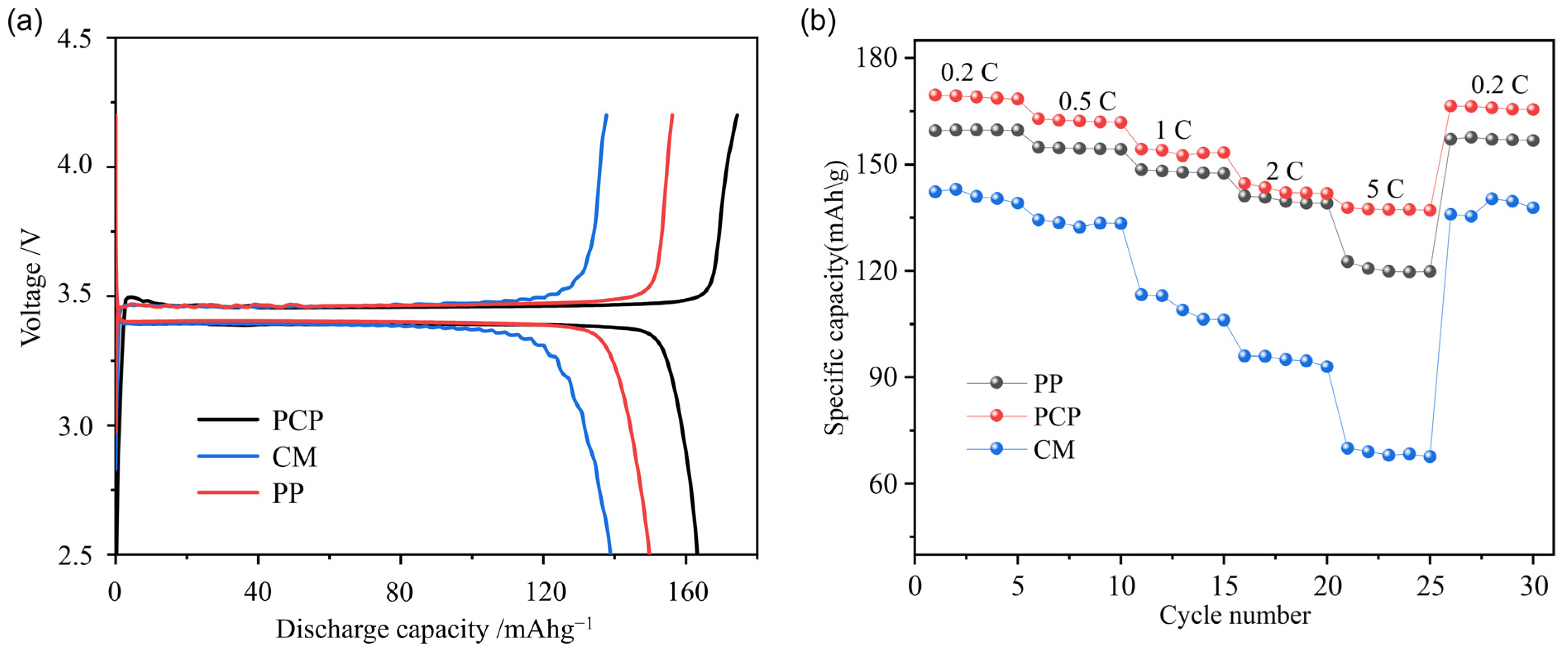
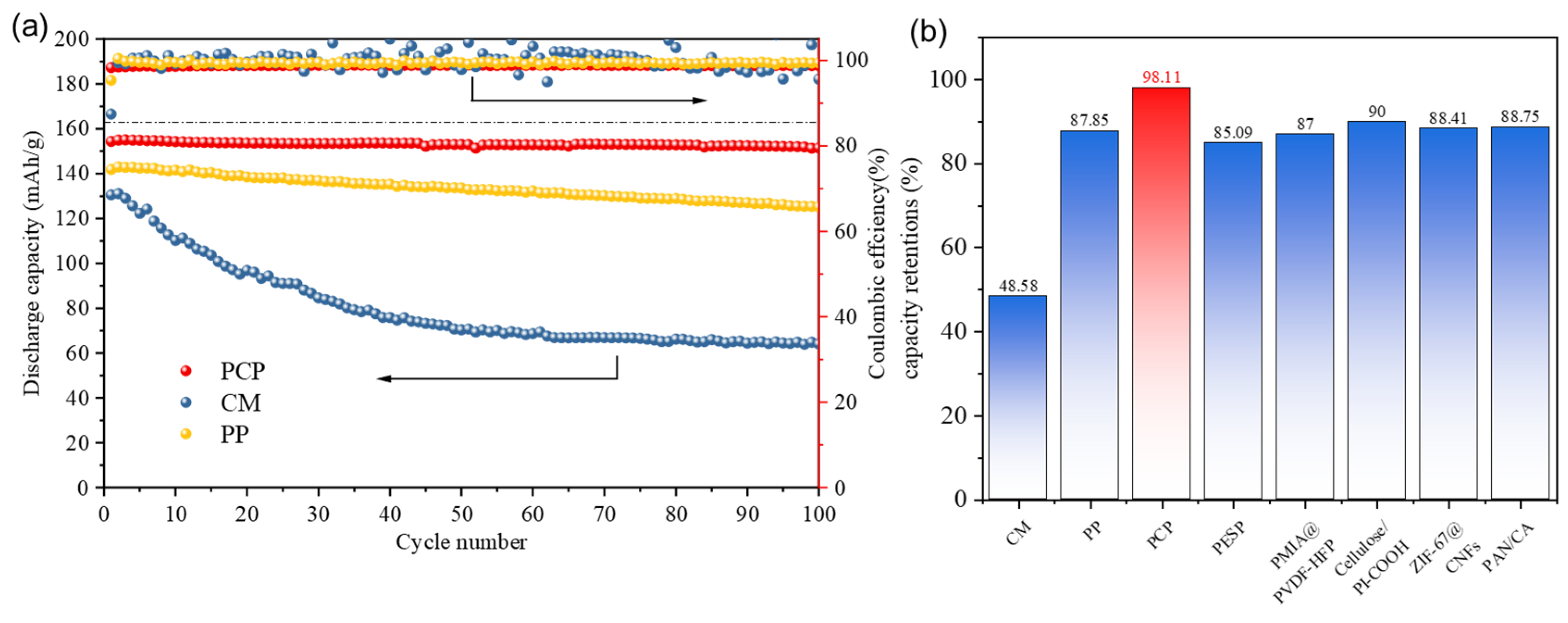
2.6. Battery Performance
3. Materials and Methods
3.1. Materials
3.2. Methods
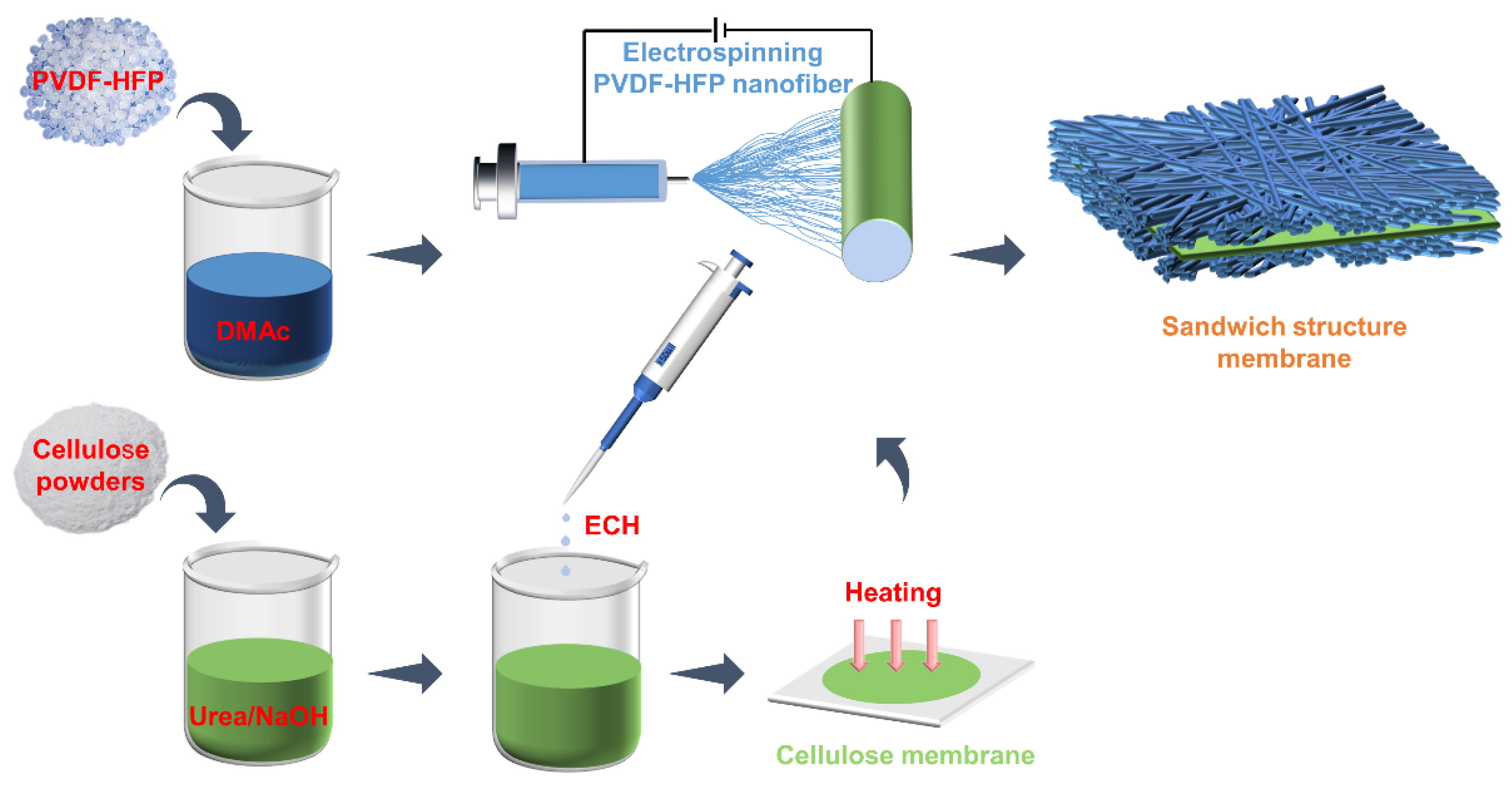
3.2.1. Preparation of Cellulose Membrane
3.2.2. Preparation of Sandwich-Like Structure of PCP Composite Membrane
3.3. Characterization
3.3.1. Microstructure and Morphology of Membranes
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.3.3. Thermal Property of Membranes
3.3.4. Electrolyte Uptake of Membrane
3.3.5. Contact Angle Measurement
3.3.6. Porosity
3.4. Electrochemical Characterization
3.4.1. Ionic Conductivity
3.4.2. Lithium-Ion Transference Number
3.4.3. Interfacial Compatibility
3.4.4. Electrochemical Stability
3.5. Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wen, X.Y.; Luo, J.H.; Xiang, K.X.; Zhou, W.; Zhang, C.F.; Chen, H. High-Performance Monoclinic WO3 Nanospheres with the Novel Nh4+Diffusion Behaviors for Aqueous Ammonium-Ion Batteries. Chem. Eng. J. 2023, 458, 141381. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, H.; Zhao, L.; Sun, Z.; Li, Y.; Mo, Y.; Chen, Y. A flexible Cellulose/Methylcellulose gel polymer electrolyte endowing superior Li+ conducting property for lithium-ion battery. Carbohyd. Polym. 2020, 246, 116622. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Xia, X.H.; Xie, D.; Xu, R.C.; Xu, Y.J.; Xia, Y.; Wu, J.B.; Yao, Z.J.; Wang, X.L.; Tu, J.P. Facile interfacial modification via in-situ ultraviolet solidified gel polymer electrolyte for high-performance solid-state lithium-ion batteries. J. Power Sources 2019, 409, 31–37. [Google Scholar] [CrossRef]
- Mu, X.J.; Song, Y.; Qin, Z.M.; Meng, J.M.; Wang, Z.H.; Liu, X.X. Core-shell structural vanadium oxide/polypyrrole anode for aqueous ammonium-ion batteries. Chem. Eng. J. 2023, 453, 139575. [Google Scholar] [CrossRef]
- Li, D.J.; Guo, H.T.; Jiang, S.H.; Zeng, G.L.; Zhou, W.; Li, Z.A. Microstructures and electrochemical performances of TiO2-coated Mg-Zr co-doped NCM as a cathode material for lithium-ion batteries with high power and long circular life. New J. Chem. 2021, 45, 19446–19455. [Google Scholar] [CrossRef]
- Yang, H.Q.; Lee, J.; Cheong, J.Y.; Wang, Y.F.; Duan, G.G.; Hou, H.Q.; Jiang, S.H.; Kim, I. Molecular engineering of carbonyl organic electrodes for rechargeable metal-ion batteries: Fundamentals, recent advances, and challenges. Energy Environ. Sci. 2021, 14, 4228–4267. [Google Scholar] [CrossRef]
- Gan, W.; Wang, Y.; Xiao, K.; Zhai, M.; Wang, H.; Xie, Y. Research review of energy storage and conversion materials based on wood cell wall functional modification. J. For. Eng. 2022, 6, 1–12. [Google Scholar]
- Zhao, H.; Kang, W.; Deng, N.; Liu, M.; Cheng, B. A fresh hierarchical-structure gel poly-m-phenyleneisophthalamide nanofiber separator assisted by electronegative nanoclay-filler towards high-performance and advanced-safety lithium-ion battery. Chem. Eng. J. 2020, 384, 123312. [Google Scholar] [CrossRef]
- Zhang, K.; Yin, J.X.; He, Y.Z. Acoustic emission detection and analysis method for health status of lithium ion batteries. Sensors 2021, 21, 712. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Lin, L.; You, H. A composite porous membrane based on derived cellulose for transient gel electrolyte in transient lithium-ion batteries. Materials 2022, 15, 1584. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Liu, K.X.; Wang, Z.Y.; Shi, L.Y.; Yuan, S. Multifunctional separators for high-performance lithium ion batteries. J. Power Sources 2021, 499, 12731. [Google Scholar] [CrossRef]
- Maier, J. Thermodynamics of Electrochemical Lithium Storage. Angew. Chem. Int. Ed. 2013, 52, 4998. [Google Scholar] [CrossRef]
- Li, A.; Yuen, A.; Wang, W.; Cordeiro, I.; Wang, C.; Chen, T.; Zhang, J.; Chan, Q.N.; Yeoh, G.H. A review on lithium-ion battery separators towards enhanced safety performances and modelling approaches. Molecules 2021, 26, 478. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Li, Y.Q.; Cui, X.J.; Guan, S.J.; Tu, L.; Tang, H.L.; Li, Z.H.; Li, J.S. Understanding the advantageous features of bacterial cellulose-based separator in li-s battery. Adv. Mater. Interfaces 2022, 10, 3321. [Google Scholar] [CrossRef]
- Lv, D.; Chai, J.; Wang, P.; Zhu, L.; Liu, C.; Nie, S.; Li, B.; Cui, G. Pure cellulose lithium-ion battery separator with tunable pore size and improved working stability by cellulose nanofibrils. Carbohyd. Polym. 2021, 251, 116975. [Google Scholar] [CrossRef]
- Sheng, J.; Tong, S.H.; He, Z.B.; Yang, R.D. Recent developments of cellulose materials for lithium-ion battery separators. Cellulose 2017, 24, 4103–4122. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Fu, Y. All-cellulose gel electrolyte with black phosphorus based lithium ion conductors toward advanced lithium-sulfurized polyacrylonitrile batteries. Carbohyd. Polym. 2022, 296, 119950. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, K.Y.; Cheng, W.K.; Zeng, S.Q.; Dou, S.; Chen, W.S.; Zhao, D.W.; Yu, H.P. A non-newtonian fluidic cellulose-modified glass microfiber separator for flexible lithium-ion batteries. Ecomat 2021, 3, 242. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, L.L.; Ma, X.Y.; Dong, L.K.; Jin, Z.F.; Xia, G.B.; Du, P.F.; Xiong, J. Electrospun cellulose polymer nanofiber membrane with flame resistance properties for lithium-ion batteries. Carbohyd. Polym. 2020, 234, 3583. [Google Scholar] [CrossRef]
- Jeong, S.S.; Bockenfeld, N.; Balducci, A.; Winter, M.; Passerini, S. Natural cellulose as binder for lithium battery electrodes. J. Power Sources 2012, 199, 331–335. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Gu, J.; Zhang, W.W.; Hu, C.S.; Lin, X.Y. Rational design of cellulose nanofibrils separator for sodium-ion batteries. Molecules 2021, 26, 5539. [Google Scholar] [CrossRef]
- Chen, L.; Cao, S.; Huang, L.; Wu, H.; Hu, H.; Liu, K.; Lin, S. Development of bamboo cellulose preparation and its functionalization. J. For. Eng. 2021, 6, 75. [Google Scholar]
- Xu, L.; Meng, T.T.; Zheng, X.Y.; Li, T.Y.; Brozena, A.H.; Mao, Y.M.; Zhang, Q.; Clifford, B.C.; Rao, J.C.; Hu, L.B. Nanocellulose-carboxymethylcellulose electrolyte for stable, high-rate zinc-ion batteries. Adv. Funct. Mater. 2023, 2, 32. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.A.; Lizundia, E.; Niederberger, M. Hierarchical nanocellulose-based gel polymer electrolytes for stable Na electrodeposition in sodium ion batteries. Small 2022, 18, 34. [Google Scholar] [CrossRef]
- Du, Z.; Su, Y.; Qu, Y.; Zhao, L.; Jia, X.; Mo, Y.; Yu, F.; Du, J.; Chen, Y. A mechanically robust, biodegradable and high performance cellulose gel membrane as gel polymer electrolyte of lithium-ion battery. Electrochim. Acta 2019, 299, 19–26. [Google Scholar] [CrossRef]
- Mi, Q.; Ma, S.; Yu, J.; He, J.; Zhang, J. Flexible and transparent cellulose aerogels with uniform nanoporous structure by a controlled regeneration process. ACS Sustain. Chem. Eng. 2015, 4, 656–660. [Google Scholar] [CrossRef]
- Gavillon, R.; Budtova, T. Aerocellulose: New highly porous cellulose prepared from cellulose-naoh aqueous solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Yu, J.; Zhang, J. Cellulose aerogel membranes with a tunable nanoporous network as a matrix of gel polymer electrolytes for safer lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 24591–24599. [Google Scholar] [CrossRef]
- Li, M.X.; Wang, X.W.; Yang, Y.Q.; Chang, Z.; Wu, Y.P.; Holze, R. A dense cellulose-based membrane as a renewable host for gel polymer electrolyte of lithium ion batteries. J. Membr. Sci. 2015, 476, 112–118. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Wang, F.X.; Yang, Y.Q.; Chang, Z.; Wu, Y.P. An environmentally friendly and economic membrane based on cellulose as a gel polymer electrolyte for lithium ion batteries. RSC Adv. 2014, 4, 76–81. [Google Scholar] [CrossRef]
- Gou, J.R.; Liu, W.Y.; Tang, A.M. To improve the interfacial compatibility of cellulose-based gel polymer electrolytes: A cellulose/pegda double network-based gel membrane designed for lithium ion batteries. Appl. Surf. Sci. 2021, 568, 45. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.S.; Lee, B.S.; Yu, W.R. Recent progress in coaxial electrospinning: New parameters, various structures, and wide applications. Adv. Mater. 2018, 30, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Deng, N.P.; Yan, J.; Kang, W.M.; Ju, J.G.; Wang, L.Y.; Li, Z.J.; Cheng, B.W. Effect of octaphenylpolyhedral oligomeric silsesquioxane on the electrospun poly-m-phenylene isophthalamid separators for lithium-ion batteries with high safety and excellent electrochemical performance. Chem. Eng. J. 2019, 356, 11–21. [Google Scholar] [CrossRef]
- Bicy, K.; Gueye, A.B.; Rouxel, D.; Kalarikkal, N.; Thomas, S. Lithium-ion battery separators based on electrospun PVDF: A review. Surf. Interfaces 2022, 31, 101977. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Q.Q.; Luo, N.; Chen, F.; Fu, Q. High safety and electrochemical performance electrospun para-aramid nanofiber composite separator for lithium-ion battery. Compos. Sci. Technol. 2022, 225, 67. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Zhou, R.X.; Zhao, H.H.; Ye, F.; Zhang, X.W.; Ge, Y.Q. Oriented pan/pvdf/pan laminated nanofiber separator for lithium-ion batteries. Text. Res. J. 2022, 92, 2635–2642. [Google Scholar] [CrossRef]
- Yang, K.C.; Liu, Z.L.; Chai, J.C.; Zheng, Y.; Fu, X.N.; Shen, Y.H.; Chen, J.; Liu, Z.H.; Shi, S.W. High performance polyimide-based separator for 4.5v high voltage licoo2 battery with superior safety. Mater. Chem. Phys. 2022, 282, 95. [Google Scholar] [CrossRef]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Chen, W.Y.; Liu, Y.B.; Ma, Y.; Liu, J.Z.; Liu, X.R. Improved performance of PVdF-HFP/PI nanofiber membrane for lithium ion battery separator prepared by a bicomponent cross-electrospinning method. Mater. Lett. 2014, 133, 67–70. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Shao, Z.; Liu, S. Cellulosic materials-enhanced sandwich structure-like separator via electrospinning towards safer lithium-ion battery. Carbohyd. Polym. 2019, 214, 328–336. [Google Scholar] [CrossRef]
- Raghaven, P.; Choi, J.W.; Ahn, J.H.; Cheruvally, G.; Chauhan, G.S.; Ahn, H.J.; Nah, C. Novel electrospun poly(vinylidene fluoride-co-hexafluoropropylene)-in situ sio2 composite membrane-based polymer electrolyte for lithium batteries. J. Power Sources 2008, 184, 437–443. [Google Scholar] [CrossRef]
- Wang, D.X.; Yang, W.Y.; Feng, S.Y.; Liu, H.Z. Amine post-functionalized poss-based porous polymers exhibiting simultaneously enhanced porosity and carbon dioxide adsorption properties. RSC Adv. 2016, 6, 13749–13756. [Google Scholar] [CrossRef]
- Liu, J.; Mo, Y.; Wang, S.; Ren, S.; Han, D.; Xiao, M.; Sun, L.; Meng, Y. Ultrastrong and heat-resistant poly(ether ether ketone) separator for dendrite-proof and heat-resistant lithium-ion batteries. ACS Appl. Energy Mater. 2019, 2, 3886–3895. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Y.L.; Liu, X.W.; Jiang, X.Y.; Zhong, F.P.; Yang, H.X.; Ai, X.P.; Cao, Y.L. A controllable thermal-sensitivity separator with an organic-inorganic hybrid interlayer for high-safety lithium-ion batteries. Mater. Chem. Front. 2021, 5, 2313–2319. [Google Scholar] [CrossRef]
- Yang, N.; Liang, Y.H.; Jia, S.J. Enhanced thermal stability and electrochemical performance of polyacrylonitrile/cellulose acetate-electrospun fiber membrane by boehmite nanoparticles: Application to high-performance lithium-ion batteries. Macromol. Mater. Eng. 2021, 306, 34. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, L.L.; Ma, X.Y.; Chu, Z.D.; Zhuang, Z.S.; Dong, L.K.; Du, P.F.; Xiong, J. Electrospun pmia and pvdf-hfp composite nanofibrous membranes with two different structures for improved lithium-ion battery separators. Solid State Ion. 2020, 347, 24. [Google Scholar] [CrossRef]
- Sun, X.X.; Xu, W.W.; Zhang, X.Q.; Lei, T.Z.; Lee, S.Y.; Wu, Q.L. Zif-67@cellulose nanofiber hybrid membrane with controlled porosity for use as li-ion battery separator. J. Energy Chem. 2021, 52, 170–180. [Google Scholar] [CrossRef]
- Deng, J.H.; Cao, D.Q.; Yang, X.Q.; Zhang, G.Q. Cross-linked cellulose/carboxylated polyimide nanofiber separator for lithium-ion battery application. Chem. Eng. J. 2022, 433, 33. [Google Scholar] [CrossRef]

| Element | wt.% | wt.% Sigma | At% |
|---|---|---|---|
| C | 23.23 | 0.19 | 31.15 |
| N | 4.22 | 0.19 | 4.86 |
| O | 44.05 | 0.16 | 44.34 |
| Na | 27.24 | 0.11 | 19.08 |
| Cl | 1.25 | 0.02 | 0.57 |
| Element | wt.% | wt.% Sigma | At% |
|---|---|---|---|
| C | 45.84 | 0.19 | 57.09 |
| O | 1.76 | 0.09 | 1.64 |
| F | 52.4 | 0.18 | 41.26 |
| Membrane | Thickness (μm) | σ at 25 °C (mS/cm) |
|---|---|---|
| PCP | 630 | 0.73 |
| CM | 100 | 0.21 |
| PP | 30 | 0.26 |
| Membranes | R0 | RS | I0 (10−5 A) | IS (10−5 A) | tLi+ |
|---|---|---|---|---|---|
| PCP | 363.36 | 540.97 | 1.97 | 1.43 | 0.91 |
| PP | 477.9 | 499.79 | 1.61 | 1.27 | 0.50 |
| CM | 1316 | 1369 | 0.60 | 0.50 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zi, X.; Wu, H.; Song, J.; He, W.; Xia, L.; Guo, J.; Luo, S.; Yan, W. Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries. Molecules 2023, 28, 4998. https://doi.org/10.3390/molecules28134998
Zi X, Wu H, Song J, He W, Xia L, Guo J, Luo S, Yan W. Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries. Molecules. 2023; 28(13):4998. https://doi.org/10.3390/molecules28134998
Chicago/Turabian StyleZi, Xingfu, Hongming Wu, Jiling Song, Weidi He, Lu Xia, Jianbing Guo, Sihai Luo, and Wei Yan. 2023. "Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries" Molecules 28, no. 13: 4998. https://doi.org/10.3390/molecules28134998
APA StyleZi, X., Wu, H., Song, J., He, W., Xia, L., Guo, J., Luo, S., & Yan, W. (2023). Electrospun Sandwich-like Structure of PVDF-HFP/Cellulose/PVDF-HFP Membrane for Lithium-Ion Batteries. Molecules, 28(13), 4998. https://doi.org/10.3390/molecules28134998







