2.1. Some Physicochemical Properties of Water, RL, ET and TX165
To understand the behaviour of the ternary mixture of RL, ET and TX165 at the water-air interface, some of its and water physicochemical properties must be known.
The analysis of the bond length between O and H, the angle between the -OH bonds and the average distance between the water molecules indicates that a water molecule at the temperature of 293 K can be inscribed in a regular cube with the edge length of 3.11 Å. Thus, the contactable area of water molecules with other molecules is 9.7 Å
2. This value is close to that of the contact area determined by Groszek [
31] based on the water vapour adsorption on the quartz surface, which is equal to 10 Å
2. This means that 1 × 16.6
−6 mole of water is needed to cover the given surface by its monolayer. The value of the water molecule contactable area equal to 10 Å
2 is very often used for the thermodynamic consideration of the surfactants adsorption at the water-air interface [
26,
32]. This adsorption decreases the water surface tension. According to van Oss et al. [
33,
34,
35] water is the bipolar liquid and its surface tension results from the Lifshitz-van der Waals and Lewis acid-base intermolecular interactions. The Lewis acid-base interactions lead to the hydrogen bonds formation between the water molecules. Thus, the water surface tension can be divided into the Lifshitz-van der Waals component (LW) and the acid-base (AB) one, which results from the electron-acceptor and electron-donor parameters. As a result of the surfactants adsorption at the water-air interface, the surface tension of water is reduced, especially its AB component [
26,
32].
It proved that the adhesion work of the aqueous solution of many hydrocarbon surfactants to the PTFE surface does not depend on their concentration and is equal to the water adhesion work [
36,
37]. From the Young-Dupre equation and Fowkes approach to the interface tension [
38], it follows that in this case the LW component of the surfactants solution and water is the same and its value is equal to 26.85 mN/m at 293 K (
Table 1). This value is considerably higher compared with that determined based on the water-
n-alkane interface tension [
38,
39]. As a matter of fact, for water it was assumed that the electron-acceptor and electron-donor parameters of the acid-base component of its surface tension are the same [
33,
34,
35]. It should be remembered that the parameters of other liquids or solids surface tension are consistent with this assumption. These parameters for the ethanol surface tension (
are not the same (
Table 1). However, the LW component of the ethanol surface tension is close to that of water determined based on the water-
n-alkane interface tension [
29,
30,
39].
The ET molecule volume calculated using the bonds length and the angle between them as well as the average distance of molecules in the bulk phase at 293 K is close to 97 Å
3 and to the value obtained from the ET density, which is equal to 97.3 Å
3. As follows from the calculations the ethanol molecule can be put in a regular cube with the edge equal to 4.6 Å [
30]. Thus, the contact area of the ET molecule with other one does not depend on its orientation and is about 21 Å
2 [
30]. This point out that one ET molecule can replace two water molecules in the interface monolayer.
Unlike ET and water, the surface tension of RL and TX165 depends on the orientation of their molecules towards the air phase (
Table 1). If their molecules are oriented with the hydrophobic group towards the air phase, then we treat the surface tension as the surface tension of the tail. However, with the orientation of the RL and TX165 molecules with the hydrophilic group towards the air, this tension is called the surface tension of the head (
Table 1).
The tail surface tension of TX165 and RL is close to LW of ET and water determined from the water-
n-alkane interface tension. However, these values are considerably smaller than LW for water determined from the contact angle of water on the hydrophobic solids [
40]. Based on the analysis of the chemical bonds length, the angle between them and the average distance between the molecules at 293 K, it appears that the RL and TX165 molecules cannot be entered into one cube, but into different ones, for the head and tails of their molecules, respectively [
26]. It follows from this analysis that the contactable area of RL and TX165 molecules at their perpendicular orientation towards the interface is equal to 69.09 Å
2 and 35.7 Å
2, respectively. At the parallel orientation of RL molecule towards the air phase the contactable area of its tail is equal to 87.3 Å
2 and that of the head to 72.1 Å
2 [
26]. In the case of TX165 at the parallel orientation of its molecule at the interface, the contactable area of the tail is equal to 52.12 Å
2 and that of the head to 101.4 Å
2. Taking into account the contactable area of water and ET it can be stated that in the bulk phase one molecule of ET can be surrounded by 12 molecules of water. The tail of the TX165 can be surrounded by about 20 water molecules, and the head can be bound by strong hydrogen bonds with about 40 molecules and weak ones also with 40 water molecules. In the case of RL its tail can be surrounded by about 30 molecules of water and the head by 28 ones.
The number of the water molecules contacted with the tail and the head of surfactant decide about its tendency to adsorb at the water-air interface. In turn the monolayer formed at the water-air interface reduces the water surface tension. In fact, in the case of ET the water surface tension is reduced to that of ET because the ET is infinitely miscible with water (
Figure S1). One would expect that the minimum surface tension of RL and ET aqueous solutions should be close to the surface tension of their tails. In the case of RL, the minimum surface tension of its aqueous solution is not much higher than LW for the water obtained from the contact angle on the surface of hydrophobic solids. However, in the case of TX165 there is a great difference between the minimal surface tension of its solution and LW for water (
Figure S1c) (
Table 1). This may be due to the fact that the number of water molecules surrounding the head of the TX165 molecule are much greater than that of water molecules surrounding its tail.
The isotherms of the aqueous solution of ET, RL and TX165 can be described by the exponential function of the second order which has the form:
where
,
,
,
and
are the constants,
is the concentration.
These constants are probably connected with particular intermolecular interactions between the water molecules and the surface active ones. It was earlier stated that the constant is related to the LW intermolecular interactions and the other constants to the acid-base ones.
The possibility to describe the isotherm of the surface tension is very useful for determination of the surface Gibbs excess concentration (
). The surface tension isotherms of the aqueous solution of RL, ET and TX165 can be also described by the Szyszkowski equation which has the form [
1]:
where
is the solvent surface tension,
R is the gas constant,
T is the absolute temperature,
is the maximal Gibbs surface excess concentration and
is the constant.
This equation can be applied for determination of the constant
related to the maximal Gibbs surface excess concentration. Additionally, the surface tension isotherm of the aqueous solution of ET can be described by the Connors equation [
30,
41], which is as follows:
where
is the surface tension of alcohol,
and
are the empirical constants and
is the mole fraction of ET in the bulk phase.
In the case of the ET aqueous solution, despite the possibility of describing the surface tension isotherm by the well-defined mathematical function, it is difficult to determine the real Gibbs surface excess concentration at the water-air interface, and more so the total concentration of ET in the surface layer. For this reason one can find conflicting opinions about this issue in the literature [
30]. ET is the surface active agent which, as mentioned above, is infinitely miscible with water. Similarly to the classical surfactants, it forms aggregates at a certain concentration in water, however, they cannot be treated as typical micelles [
29,
30]. Moreover, unlike typical surfactant, at the concentrations above the critical aggregation concentration (CAC), a decrease in the surface tension of the solution is still observed [
30]. The number of the ET moles in 1 dm
3 are also changed as a function of its concentration. Depending on the ET concentration it is treated in the practice as the co-surfactant and/or co-solvent [
1,
42]. This fact causes that the aqueous solution of ET must be treated thermodynamically in a different way from the aqueous solutions of typical surfactants.
In the case of non-ideal solution in which the solute concentration is small, the chemical potential (
) of the component of the solution is defined asymmetrically. For the solute it can be written:
In the case of the mixture of solvents the chemical potential is defined symmetrically and can be expressed:
where
and
are the standard chemical potentials, which depend only on temperature and pressure,
and
are chemical potentials of mixing,
and
are the activities,
and
are the activity coefficients and
is the mole fraction of the solute.
It is known that in the concentration range from zero to 0.01 mol/dm
3 most surfactants are present in the bulk phase in the monomeric form which decides about their concentration in the bulk phase [
1]. In this range of surfactants concentration it be can assumed that with a small error
(
is the concentration of surfactants and
is the number of the water moles in 1 dm
3) and
. Indeed, in the considered concentration range of the surface active agent ET fulfills such conditions if its chemical potential is defined asymmetrically. In such case it can written:
where
is the constant which depends on the type of the surface active agent and for ET is equal to 1.
The studies by Chodzińska et al. [
30] proved that in the ET concentration range from 0 to 0.01 mol/dm
3, the
values calculated using
and
do not differ much. However, the difference increases as the increasing ET concentration. It was concluded by them that the most reliable values of the Gibbs surface excess concentration of ET at the solution-air interface can be obtained from the following equation [
26,
32]:
Indeed, in the case of ET the values are not equal to its total concentration in the surface layer at the solution-air interface. Moreover, they do not tend to zero as tends to 1.
To determine the total concentration of ET at the solution-air interface (
) the values of
should be recalculated as the Guggenheim-Adam ones (
). For this purpose there was applied the following equation [
43]:
where
is the average molar volume of the ET aqueous solution and can be expressed as:
where
and
are the molar volumes of water and ET, respectively.
Taking into account the
values for ET which were going to zero if the ET molar fraction achieved unity it was possible to determine the
using the expression [
30]:
where
is the ET molecule length.
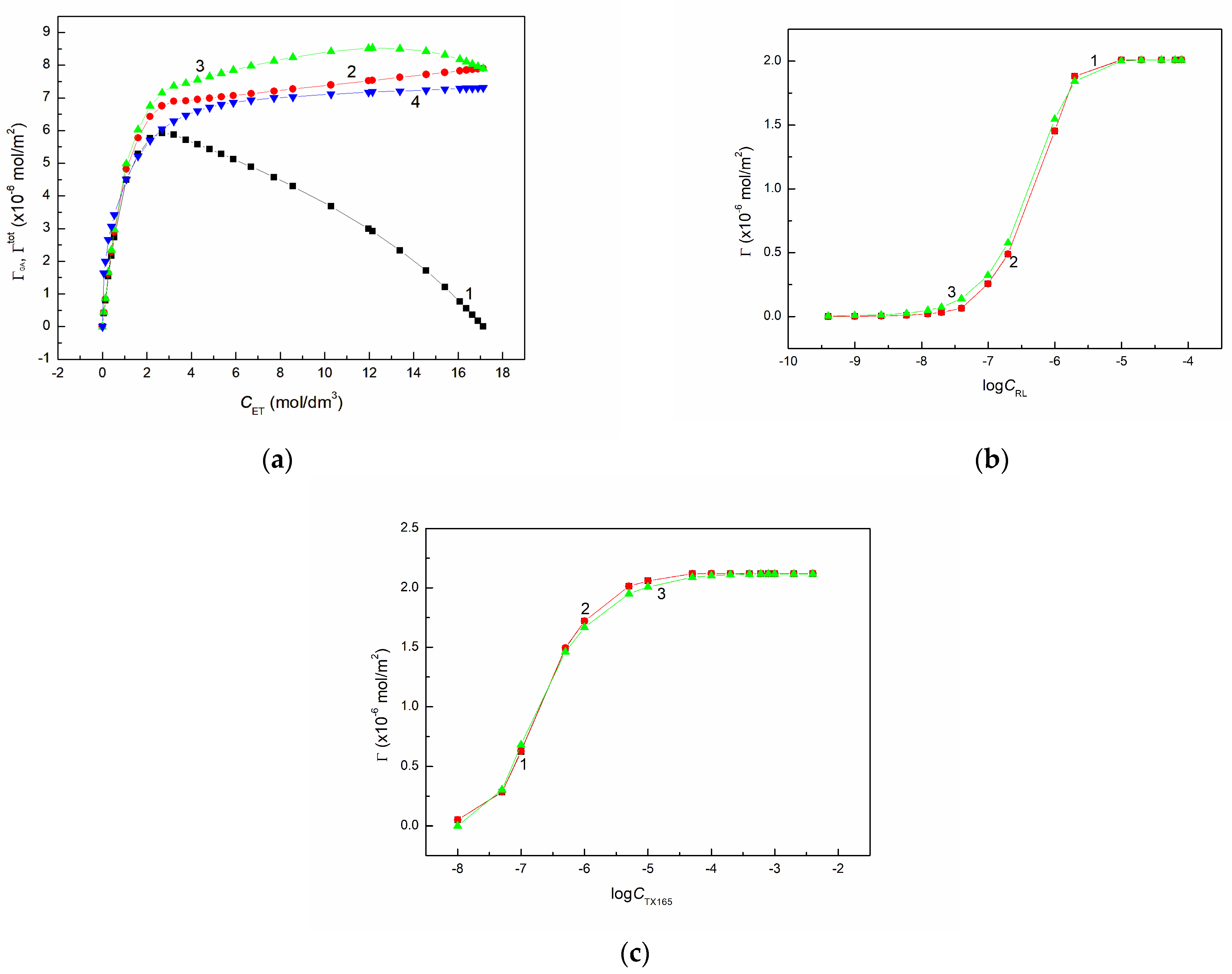
It appeared that the
values calculated in this way are not linear from
corresponding to the maximum of
of the pure ET (
Figure 1a). It is possible that due to the fact that not the total part of ET is in the air phase the
value for ET equal 4.6 Å should be slightly smaller.
The problem of
determination based on the
values can be solved in another way. Taking into account the limiting contactable area of water
and ET
molecules as well as the number of water
and ET
moles in 1 dm
3, it is possible to calculate the surface area occupied by the water and ET molecules covering the surface by monolayer. Next, it is possible to determine the two dimensional concentration of ET in the monolayer (
) corresponding to its concentration in the bulk phase based on the equation:
Introducing this equation to Equation (10) instead of
one obtains:
As follows from
Figure 1a the ET total concentration at the solution-air interface determined from Equation (12) is quite real. This equation can be also applied for the aqueous solution of surfactants if instead of
is used. Then:
where the index
S refers to the surfactants.
The values of
calculated for RL and TX165 are not much higher than those of
(
Figure 1b,c), which confirms that the Gibbs surface excess concentration of the surfactants is practically equal to the total one. The total concentration of the surfactant and also ET can be determined using the Frumkin equation which has the form:
where
is the water surface tension.
It appeared that the isotherm of the ethanol concentration in the surface layer calculated from Equation (14) is similar to that determined based on Equation (12) (
Figure 1a). There is also agreement between the isotherms of RL and ET concentrations in the surface region determined from Equations (13) and (14) (
Figure 1b,c). For RL and TX165 there is also agreement between the
values calculated from Equations (6) and (14).
Knowing the total two dimensional concentration of ET, RL and TX165 it is possible to determine the fraction of surface occupied by their molecules (
). The fraction of the surface area occupied by the ET, RL and TX165 molecules can be established from the following expression:
In fact,
differs from the surfactant molar fraction (
), which can be determined from the following equation:
The fractions
and
are applied for determination of the chemical potential in the surface region. In the Langmuir isotherm adsorption equation the value of
is associated with the constant
a [
1]. Using the
values the chemical potential can be defined as:
In the equilibrium state the chemical potential of a given compound in the surface region is equal to that in the bulk phase. Based on Equations (4) and (17) one can obtain:
where
is the standard Gibbs free energy of adsorption.
As mentioned above at small surfactants concentration Equation (18) assumes the form:
where
(
is the adsorption constant).
In order to examine in which concentration range of ET, RL and TX165 in their aqueous solution the constant has the same value, the values were calculated from Equation (15) using their total concentration determined from Equation (12) as well as from the Frumkin equation (Equation (14)). In the case of TX165 the contactable area of its molecules at the perpendicular and parallel orientations was calculated. The values of were also determined for ET.
It proved that for ET the values of
and
are not constant and depend on
(
Figure S2) regardless of whether they were calculated using the values
determined based on the Frumkin isotherm or the equation proposed by us (Equation (12)). This confirms that probably only in the range of ET concentration from 0 to 0.01 mol/dm
3 the values of
can be constant. Unfortunately, in this range of ET
it is difficult to measure reliable values of the surface tension. It should be mentioned that above
= 1 mol/dm
3 the changes of
and
a as a function of
are almost linear. From these dependences one can deduce that
is not equal to unity and increases with the increasing
. It is interesting that the ET concentration in the surface region determined from the Frumkin equation (Equation (14)) in the range of
C in the bulk phase from zero to the value corresponding to the maximal Gibbs surface excess concentration at the solution-air interface fulfils the linear form of the Langmuir equation [
1]:
The value of the constant
determined from Equation (20) is similar to that of
at the low ET concentration in the aqueous solution. As the matter of fact, in a such case the
of ET calculated from Equation (19) based on
and
is similar (
Table S1). It should be mentioned that the
values are close to those determined from Equation (2) in the whole range of ET concentration.
For the aqueous solution of RL and TX165 the values of
obtained both from the Frumkin equation as well as our equation fulfills Equation (20). The constants
obtained from Equation (20) are similar to
which are constant in the range of TX165 and RL concentration in which they are present in the bulk phase in the monomeric form (
Figure S2). In the case of TX165
was calculated using the limiting contactable area of TX165 molecule at its perpendicular and parallel orientations of TX165 molecule tail. From the calculation of
using the contactable area of TX165 at the perpendicular orientation it appeared that the
maximal value is smaller than 0.5 and the area occupied by the water molecules is larger than the contactable area of TX165 molecule tail.
There are different possibilities of TX165 molecule orientation at the water-air interface. As it was mentioned above the hydrophilic oxyethylene groups are strongly hydrated. Moreover, it is possible that H
3O
+ ions are joined with the oxyethylene group [
44,
45]. For this reason the hydrophilic long part of TX165 molecule cannot be oriented perpendicularly towards the water-air interface at the perpendicular orientation of tail. This way of orientation increases the area occupied by one TX165 molecule at the interface. This is also another way of TX165 molecule orientation. The tail is oriented parallel to the water-air interface and the head perpendicularly. This way of TX165 molecule orientation also increase its contactable area. However, the two ways of TX165 molecules orientation give almost the same values of
which are close to that of
determined from Equation (20) as well as from the Szyszkowski equation (
Table S1). The same dependences as for TX165 take place in the case of RL (
Figure S2) (
Table S1). However, the maximal fraction of the surface occupied by the RL molecules is close to its perpendicular orientation. Indeed, the values of
calculated from Equation (19) using the values of constant
determined from the linear form of the Langmuir equation, Szyszkowski equation and calculated from
are similar for a given surfactant.
2.2. Surface Behaviour of ET + RL + TX165 Mixtures
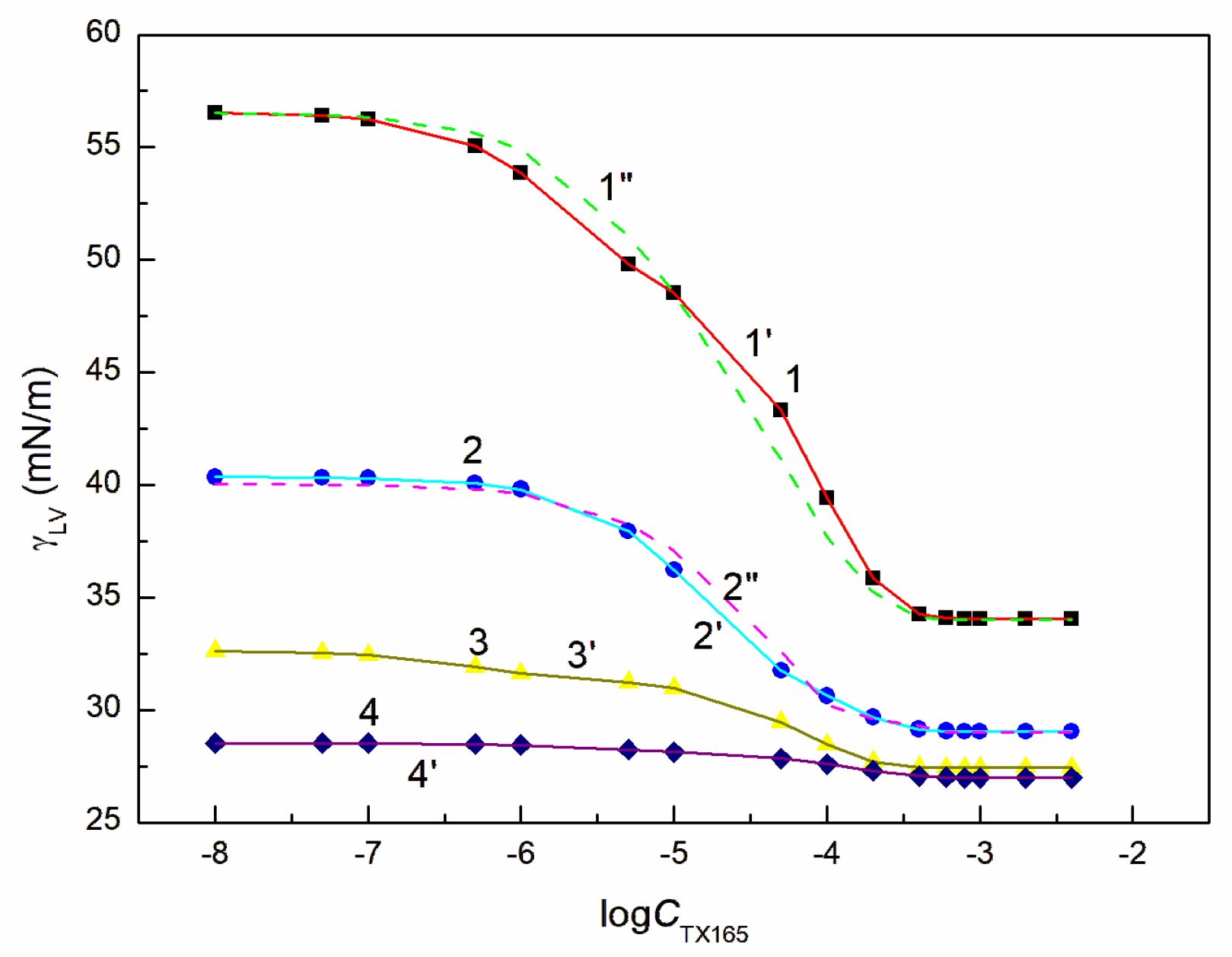
Behaviour of the RL + ET + TX165 mixture at the solution-air interface was considered based on the surface tension measurements of the aqueous solution of this mixture at the constant concentration of the RL mixture with ET and the variable TX165 concentration from zero to that higher than its CMC. For better understanding the behaviour of this mixture at the solution-air interface there were considered the surface tension isotherms of the binary mixtures of the components present in the ternary ones at this interface. For these considerations the surface tension isotherms of the aqueous solutions of TX165 mixtures with RL as well as RL with ET were taken from the literature [
26,
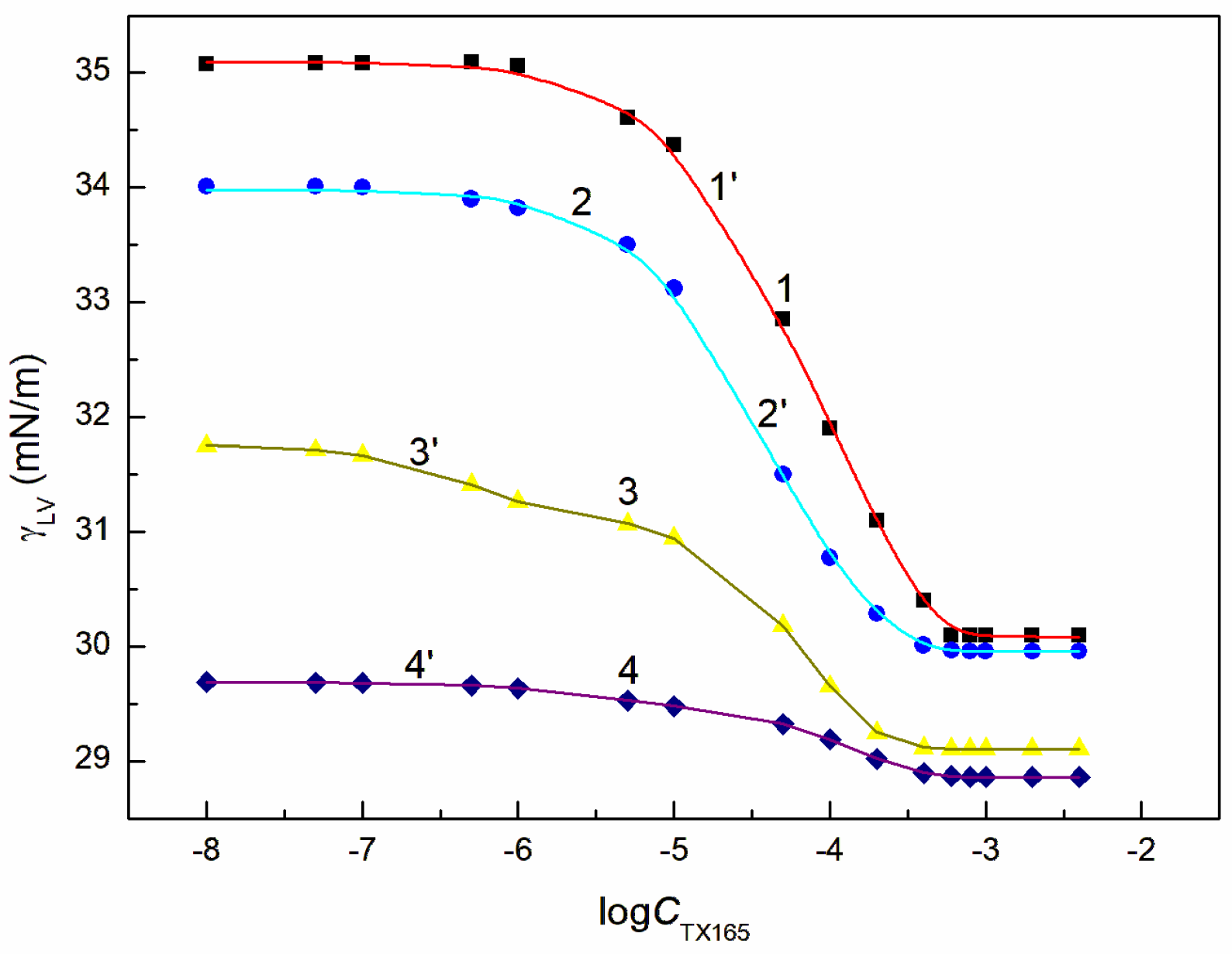
46]. The surface tension isotherms of the aqueous solution of the TX165 mixtures with ET were determined by the surface tension measurements as a function of TX165 concentration at the constant ET concentration (
Figure 2 and
Figure S3). The constant concentration of RL and ET mixture applied for measurements of the surface tension of solution of RL + ET + TX165 mixture at the TX165 variable concentration was selected based on the RL and ET individual concentration at the solution-air interface. The chosen ET concentrations were equal to 1.07, 3.74, 6.69 and 10.27 mol/dm
3. These concentrations in the bulk phase were close to the ET concentration corresponding to its unsaturated monolayer at the solution-air interface
the maximum Gibbs surface excess concentration
, critical aggregation concentration (CAC) and to that higher than CAC, respectively.
In the case of RL the constant concentration were equal to 0.01 (1.98 × 10
−8 mol/dm
3), 0.5 (9.92 × 10
−7 mol/dm
3), 5 (9.92 × 10
−6 mol/dm
3) and 20 mg/dm
3 (3.96 × 10
−5 mol/dm
3). The RL concentrations in the bulk phase equal to 0.01, 0.5 and 5 mg/dm
3 corresponded to the unsaturated monolayer at the water-air interface
, the first concentration at which the saturated RL layer was formed
and to that smaller than CMC but larger than
, respectively. The RL concentration equal 20 mg/dm
3 is close to its CMC [
47,
48].
As mentioned above, the surface tension of the TX165 and RL tails does not differ much from the LW component of the ET surface tension. However, their ability to reduce the water surface tension by the formed adsorption layer is different. Adsorption tendency of ET, RL and TX165 depends, among others, on the number of water molecules that can contact with them and the energy effect of this contact. The ET molecules in the aqueous environment can contact with each other as well as with water ones. Due to the small difference between the surfaces of water molecules and ET, the energy effect of their contact is not great, as evidenced by the small absolute value of the Gibbs adsorption free energy. It is different in the case of the behaviour of water molecules with those of RL and TX165.
The changes of energy of the aqueous solution of RL and TX165 result from the orientation of water molecules relative to the tail and head of surfactant molecules. The orientation of the water molecules around the head of the RL and TX165 molecules causes a decrease in the solution energy, which depends on the number and strength of hydrogen bonds. The number of water molecules hydrogenbound to the head of the RL molecule are much smaller than that of water molecules connected to the TX165 head as mentioned earlier. The number of water molecules that can contact with the tail of the RL molecule are also much smaller than in the case of the TX165 molecule. However, as mentioned above, the ratio of water molecules surrounding the head of the TX165 molecule to those surrounding the tail is much higher than in the case of RL Probably for this reason, the effect of the water surface tension reduction by adsorption of RL molecules at the water-air interface is greater than that of TX165. Moreover, RL is a weak organic acid and repulsive electrostatic interactions can play a role in the adsorption of its molecules at the water-air interface. However, due to the strong bond between the oxyethylene group and H3O+, the head of the TX165 molecule can become ionic. In this case weak repulsive intermolecular interactions can occur.
In the case of the RL and TX165 mixture synergism in the reduction of water surface tension was present but it was smaller than expected [
26]. The mutual influence of RL with ET as well as TX165 with ET mixtures on the reduction of the water surface tension is different from that of the mixture of RL with TX165 (
Figure 2 and
Figure S3a,b). If ET is treated as co-solvent, then there is formed the aqueous and ET solution of RL or TX165 and the RL mixture with TX165. In such case the RL or TX165 molecules adsorb at the water + ET—air interface and the mixture of RL with TX165 adsorbs at the water-air one.
The tendency to adsorb RL and TX165 molecules at the water + ET—air interface depends on the competition of water and ET molecules in the solution bulk phase for the contact with the tail and head of RL or TX165 molecules [
26]. If the ET molecules substitute for the water molecules surrounding only the head of RL or TX165 molecules, then the tendency to adsorb at the interface of RL or TX 165 should increase. In the case the water molecules surroundings tail of RL or TX165 ones are displaced by ET molecules it should decrease. Due to the fact that more water molecules surround the head of TX165 molecule than RL, the ET has a greater effect on the adsorption of TX165 than on RL. This conclusion is confirmed by the surface tension isotherms of the mixtures of RL with ET and TX165 with ET at the ET concentrations equal to
(
Figure 2 and
Figure S3b). However, at the concentration of ET higher than that of CAC [
30], the surface tension of the aqueous solutions of RL and ET as well as ET and TX165 mixtures is close to that of the ET aqueous solution (
Figure 2,
Figures S1a and S3b). In this case, it is difficult to assess the mutual influence of ET and RL as well as of ET and TX165 on the water surface tension reduction. However, the adsorption of RL and TX165 at the water + ET-air interface is not excluded due to the surface tension of RL and TX165 tail similar to that of ET [
26,
30]. In the case of the aqueous solutions of the RL + ET + TX165 mixture, with the constant concentration of RL + ET mixture equal
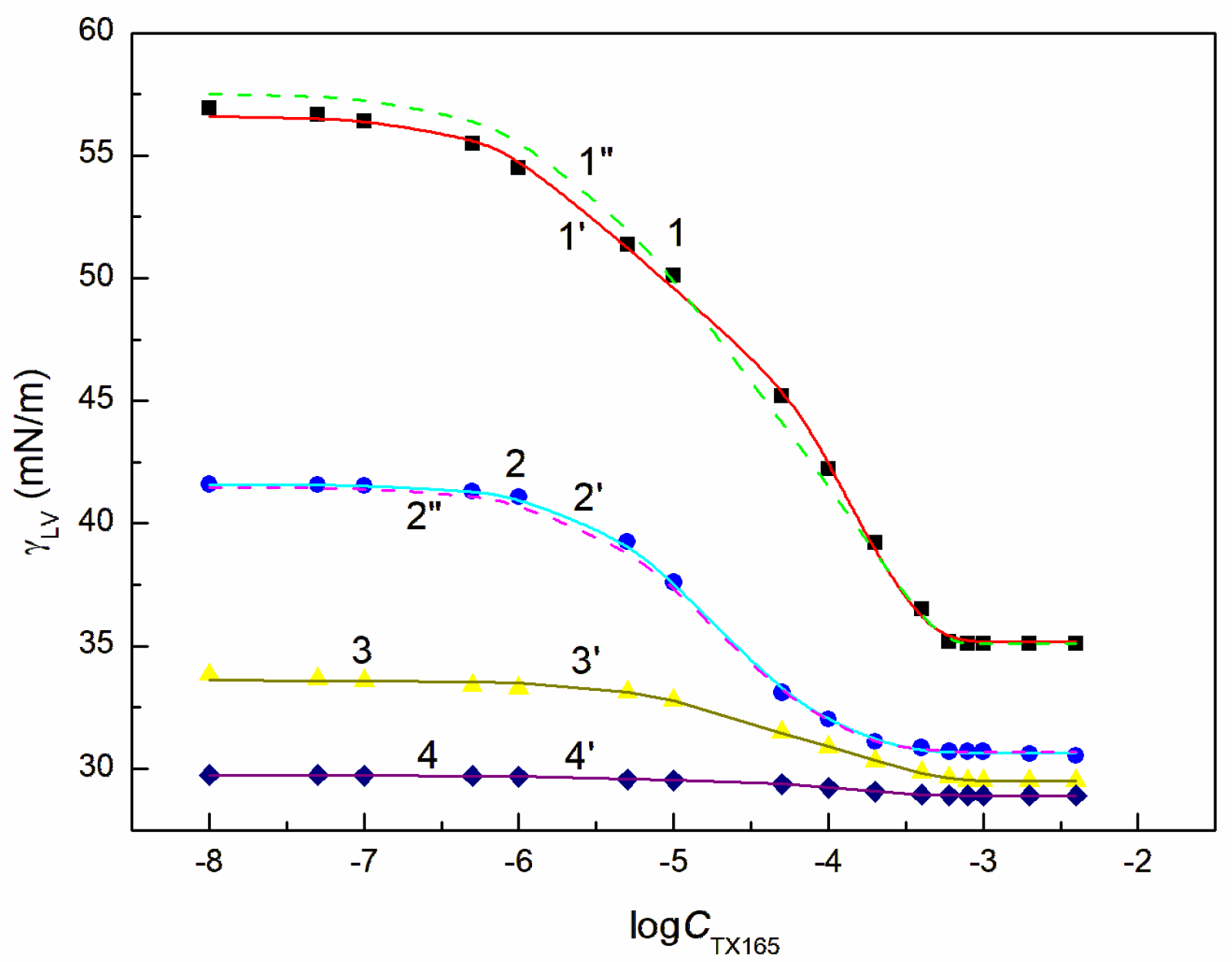
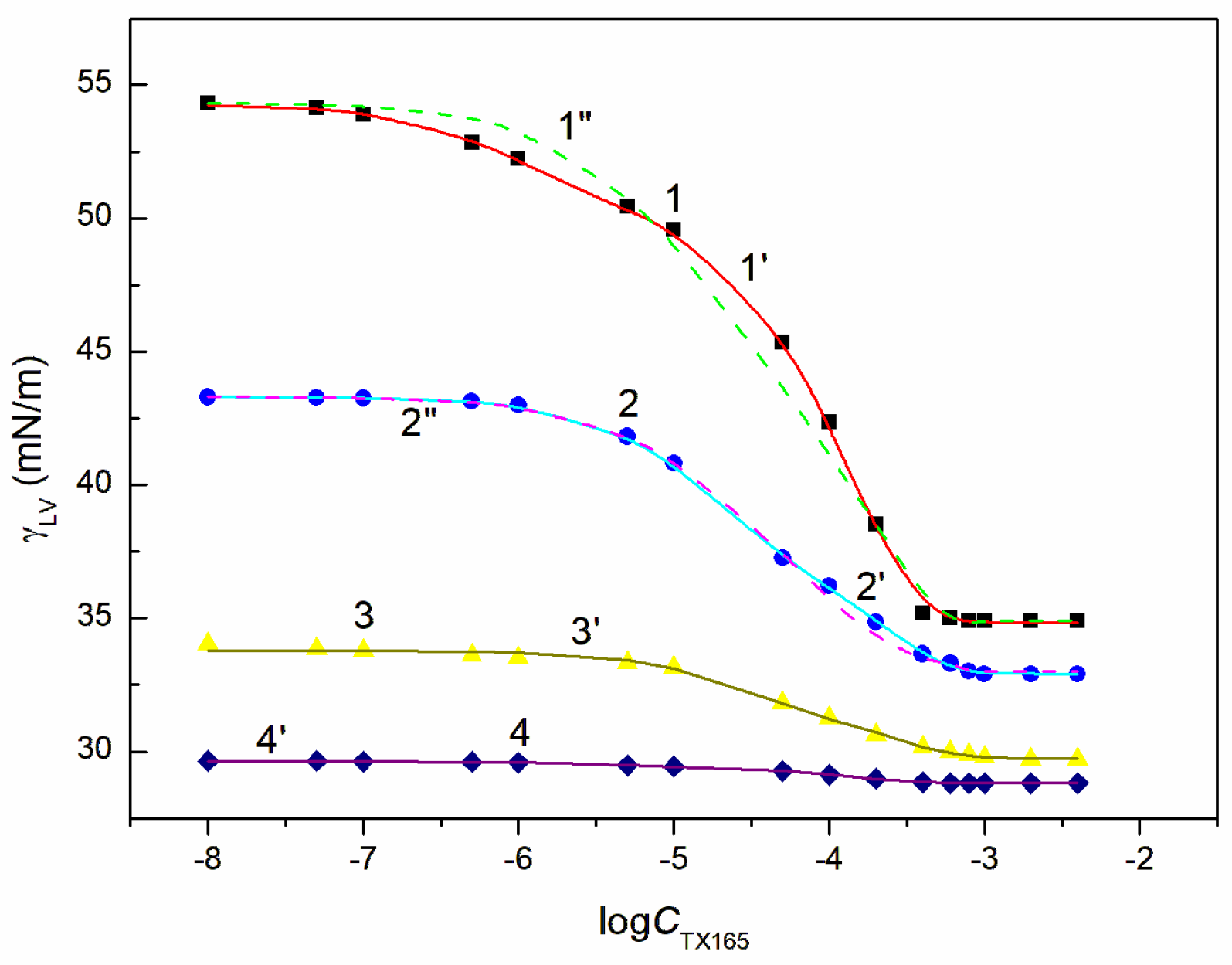
and
, respectively, and the variable TX165 concentration, ET has a greater effect on the water surface tension reduction than RL (
Figure 3,
Figure 4,
Figure 5 and
Figure 6). For this arrangement, the minimum surface tension is smaller than that of individual TX165 [
49].
The surface tension isotherms for this system can be described not only by the second-order exponential function but also by the Szyszkowski equation (
Figure 3,
Figure 4,
Figure 5 and
Figure 6).
With the increase of ET concentration to or above CAC and RL to the concentration close to its CMC, the surface tension of the RL + ET + TX165 aqueous solution does not change much as a function of the TX165 concentration and its values do not differ much from the surface tension of the individual ET aqueous solution (
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure S1a) [
30]. However, the values of the surface tension of aqueous solutions of the RL + ET + TX165 mixture at the constant ET concentration equal to 10.27 mol/dm
3 similar to the surface tension of an individual ET aqueous solution does not prove that there is no adsorption of RL and TX165 at the solution-air interface. As mentioned above the surface tension of RL and TX165 tails is close to that of ET. Therefore, it is difficult to determine directly the lack of adsorption of RL and TX165 at the high concentration of ET based only on the surface tension. Our previous studies [
26,
32] proved that the composition of the adsorption monolayer at the first approximation, can be predicted from the surface tension isotherms of aqueous solutions of the individual mixture components. Thus, it is possible to explain the presence in the adsorption monolayer of not only ET molecules but also RL as well as TX165 at the ET concentration at which the surface tension of the mixture solution is close to that of the ET itself.
According to this suggestion the relative molar fraction of particular components of the mixture can be expressed by , and , respectively.
Based on the relative molar fraction of ET, RL and TX165 in the mixed monolayer one can calculate their contribution to the water surface tension reduction from the following expression:
Taking into account the contribution of particular components to the reduction of the water surface tension it was possible to determine the concentration of all components of the binary and ternary mixtures in the mixed surface layer using the Frumkin equation (Equation (14) (
Figures S4a–S10d)). In the case of TX165 it was also possible to calculate its surface excess concentration from the Gibbs isotherm equation (Equation (6)). It proved that the Gibbs surface excess concentration of TX165 in the systems in which it was possible to determine is close to that calculated from the Frumkin equation. As follows Equation (21) is useful for determination of the concentration of all components of the binary and ternary mixtures at the solution-air interface. The calculations indicate that the same values of the surface tension of the aqueous solution of the binary and ternary mixtures as that of the aqueous solution of individual ET does not prove the absence of RL and TX165 in the surface region.
The sum of the Frumkin isotherms adsorption of the binary and ternary mixtures suggests that the packing of mixed monolayers is greater than that of single one of particular components of the mixture or that the TX165 or RL or RL + TX165 mixtures are adsorbed not at the water-air but at the water + ET-air interface (
Figures S4–S10). From the Frumkin isotherms of the surface concentration of the particular components of the mixed monolayer it was also possible to determine the adsorption constants for all studied systems as well as the standard Gibbs free energy of adsorption using Equations (15) and (19). Such calculations were made both for the binary and ternary systems (
Figures S11–S14). The adsorption constants were compared to those calculated from the Langmuir linear equation as well as from the Szyszkowski equation (
Figures S11 and S12).
The
values for TX165 both in the binary and ternary mixtures are constant only in the range of its low concentration and are almost the same independently of the constant concentration of ET and RL (
Figures S11a and S12a). However, the
values for RL and ET practically do not depend on the TX165 concentration but on their constant concentration values to a large extent and in the case of ET and RL mixture also on its composition (
Figures S11b,c and S12b,c). The values of
for TX165, RL and ET differ a little from the constant adsorption obtained from the Szyszkowski and linear Langmuir equations. Thus, in the case of TX165 it takes place only in the range of its low concentration. However, it was not possible to determine the constant adsorption for all studied systems using the linear Langmuir and Szyszkowski equations.
Moreover it should be emphasized that in some cases both for the binary and ternary mixtures the total values of are greater than unity. This confirms the above mentioned conclusion that the layer of RL or TX165 or RL + TX165 can be formed at the water + ET mixed solvent-air interface. Thus, the surface region can be treated as the sum of two monolayers, the ET and mixed RL + TX165 one. Such ET, RL and TX165 behaviour explains why the sum of the surface fraction occupied by particular components of the ternary system is greater than zero.
Taking into account the adsorption constants the standard Gibbs free energy of adsorption of ET, RL and TX165 was calculated from Equation (19) (
Figures S13 and S14) (
Table S1). As follows from the calculations both RL and ET influence on TX165 tendency to adsorb only in a small extent and the absolute value of
both for the binary and ternary systems is close to that of individual TX165 at its low concentration (
Figures S13a and S14a) (
Table S1). In the case of RL and ET the
values depend on their individual mutual constant concentrations as well as that of TX165 (
Figures S13b,c and S14b,c).