In Situ Synthesis of 3D BiOCl–Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation
Abstract
1. Introduction
2. Results and Discussion
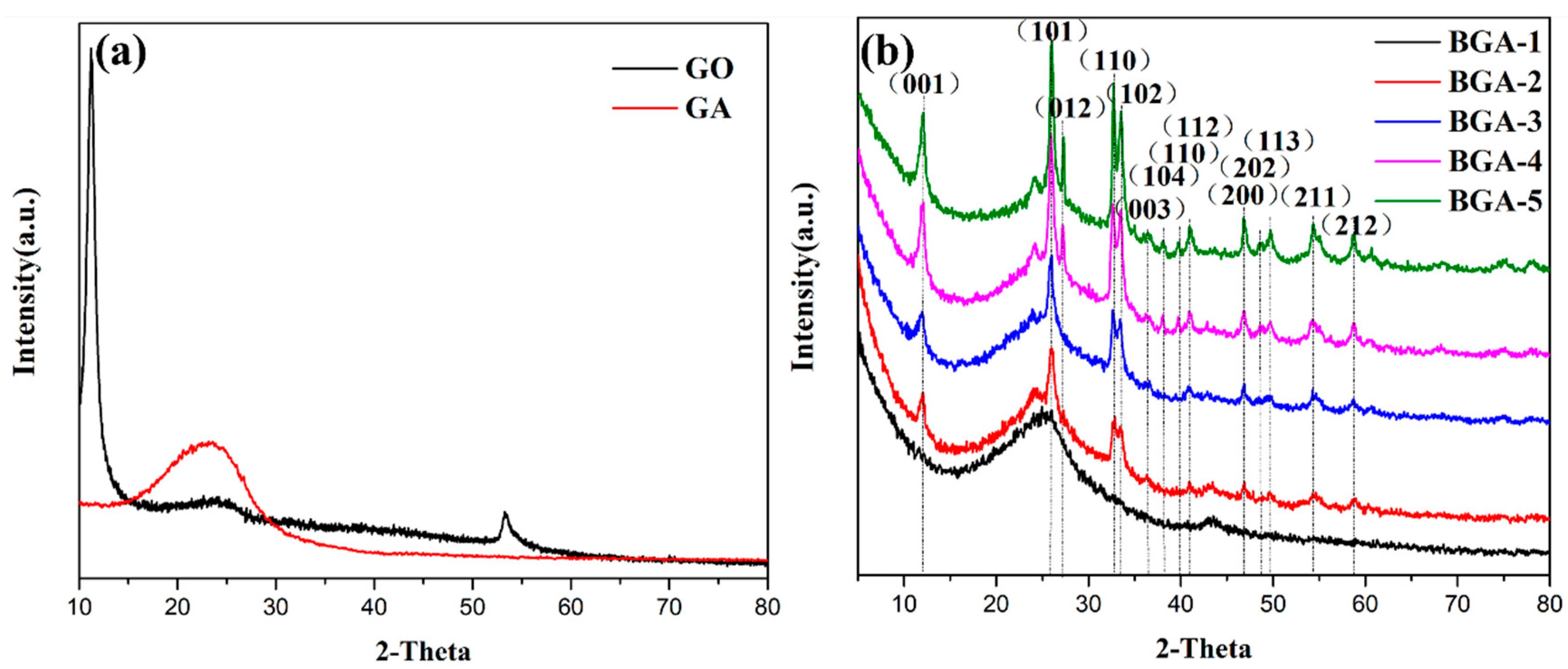
2.1. Crystal Structure of Fibers of BGA-4
2.2. Comparative Tests on MO Removal
2.3. Parameters Impacting MO Degradation by the BGA-4/SSL/PDS System
2.3.1. Effect of the Catalyst BGA-4 Concentration
2.3.2. Effect of PDS Concentration
2.3.3. Effect of BiOCl Doping Amount
2.3.4. Effect of pH
2.4. Effect of Co-Existing Components
2.5. Recycling Studies
2.6. Role of Reactive Oxidizing Species in the BGA-4/SSL/PDS System
| Process | Light Source | MO Concentration | Catalyst Dosage | PDS Concentration | Degradation Efficiency | Reaction Time | Ref. |
|---|---|---|---|---|---|---|---|
| GO/PDS/ES | Tungsten-halogen lamp (250 W) | 1.5 mM | 6 mg·L−1 | 0.05 mM | 77% | 240 min | [45] |
| P25-TiO2/ZnO/PDS | Tungsten lamp (100 W) | 20 mg/L | 0.5 g/L | 3 ppm | 82% | 240 min | [46] |
| ZnO/AgFeO2/PDS | Visible light (LED 50 W) | 1 × 10−5 M | 0.4 g/L | 1.48 mM | 70% | 300 min | [47] |
| TiO2/Fe3O4/PDS | UV light (300 W) | 20 mg/L | 0.4 g/L | 60 mM | 99.5% | 150 min | [48] |
| Fe0/PDS | solar | 10 mg/L | 100 mg/L | 360 mg/L | 95% | 120 min | [49] |
| BGA-4/PDS | simulated sunlight (Xe 300 W) | 15 mg/L | 0.4 g/L | 0.075 g/L | 89.9% | 60 min | This study |
3. Materials and Methods
3.1. Synthesis of GA and BiOCl/GA Composite
3.2. Characterization
3.3. MO Degradation Procedure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Lv, T.; Wang, L.; Xie, H.; Zhang, X.; Zhang, Y. Evolutionary overview of water resource management (1990–2019) based on a bibliometric analysis in Web of Science. Ecol. Inform. 2021, 61, 101218. [Google Scholar] [CrossRef]
- Wang, Y.; Danook, S.H.; Al-Bonsrulah, H.; Veeman, D.; Wang, F. A Recent and Systematic Review on Water Extraction from the Atmosphere for Arid Zones. Energies 2022, 15, 421. [Google Scholar] [CrossRef]
- Valavanidis, A. “Blue Planet” is Expected to Experience Severe Water Shortages? How Climate Change And Rising Temperatures Are Threatening The Global Water Cycle On Earth. 2019. Available online: http://chem-tox-ecotox.org/scientificreviews/ (accessed on 13 May 2023).
- Lu, X.; Yang, B.; Chen, J.; Sun, R. Treatment of wastewater containing azo dye reactive brilliant red X-3B using sequential ozonation and upflow biological aerated filter process. J. Hazard. Mater. 2009, 161, 241–245. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Xing, L.; Zhou, J.; Ren, J.; Ming, L.; Hua, Z.; Li, X.; Zhang, D. Ethanol as an efficient cosubstrate for the biodegradation of azo dyes by Providencia rettgeri: Mechanistic analysis based on kinetics, pathways and genomics. Bioresour. Technol. 2020, 319, 124117. [Google Scholar] [CrossRef]
- Matabola, K.P.; Mokhena, T.C.; Sikhwivhilu, K.; Mokhothu, T.H.; Mochane, M.J. Poly(vinyl alcohol) (PVA)-based nanofibers materials for azo dye adsorption: An overview. Int. J. Environ. Sci. Technol. 2022, 20, 7029–7054. [Google Scholar] [CrossRef]
- Quaff, A.R.; Venkatesh, S.; Venkatesh, K. Degradation of Azo Dye by Ozone Oxidation: Cost Analysis and Buffering Effects on Dye Decomposition. Natl. Acad. Sci. Lett. 2021, 44, 339–341. [Google Scholar] [CrossRef]
- Munoz, M.; Pedro, Z.D.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef]
- Nie, C.; Dai, Z.; Meng, H.; Duan, X.; Qin, Y.; Zhou, Y.; Ao, Z.; Wang, S.; An, T. Peroxydisulfate activation by positively polarized carbocatalyst for enhanced removal of aqueous organic pollutants. Water Res. 2019, 166, 115043. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, Y.; Liu, G.; Shi, J.; Wen, T.; Liu, M. A magnetic sludge carbon combined persulfate-based ISCO system for leachate-contaminated groundwater remediation. J. Water Process Eng. 2022, 50, 103331. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zou, J.; Chi, H. Spectrophotometric determination of persulfate anion via oxidative depolarization of methyl orange induced by ferrous ions. Desalination Water Treat. 2016, 57, 25235–25241. [Google Scholar] [CrossRef]
- Xu, N.; Hu, C.; Zhu, Z.; Wang, W.; Peng, H.; Liu, B. Establishment of a novel system for photothermal removal of ampicillin under near-infrared irradiation: Persulfate activation, mechanism, pathways and bio-toxicology. J. Colloid Interface Sci. 2023, 640, 472–486. [Google Scholar] [CrossRef]
- Liu, B.; Huang, B.; Wang, Z.; Tang, L.; Ji, C.; Zhao, C.; Feng, L.; Feng, Y. Homogeneous/Heterogeneous Metal-Catalyzed Persulfate Oxidation Technology for Organic Pollutants Elimination: A Review. J. Environ. Chem. Eng. 2023, 11, 109586. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhang, Z.; Dou, M.; Huo, K.; Ding, G.; Zhou, Y.; Qiao, C. Persulfate activation by sludge-derived biochar for efficient degradation of 2,4-dichlorophenol: Performance and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 45259–45273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Samad, Y.A.; Chan, V.; Liao, K. Cellular Graphene: Fabrication, Mechanical Properties, and Strain-Sensing Applications. Matter 2019, 1, 1148–1202. [Google Scholar] [CrossRef]
- Peng, W.; Liu, S.; Sun, H.; Yao, Y.; Zhi, L.; Wang, S. Synthesis of porous reduced graphene oxide as metal-free carbon for adsorption and catalytic oxidation of organics in water. J. Mater. Chem. A 2013, 1, 5854–5859. [Google Scholar] [CrossRef]
- Karbasi, S. Improving visible light photocatalytic inactivation of E. coli by inducing highly efficient radical pathways through peroxymonosulfate activation using 3-D, surface-enhanced, reduced graphene oxide (rGO) aerogels. Chem. Eng. J. 2020, 396, 125189. [Google Scholar] [CrossRef]
- Ma, D.; Li, J.; Liu, A.; Chen, C. Carbon Gels-Modified TiO2: Promising Materials for Photocatalysis Applications. Materials 2020, 13, 1734. [Google Scholar] [CrossRef]
- Cai, J.; Liu, W.; Li, Z. One-pot self-assembly of Cu2O/RGO composite aerogel for aqueous photocatalysis—ScienceDirect. Appl. Surf. Sci. 2015, 358, 146–151. [Google Scholar] [CrossRef]
- Chen, F.; Li, S.; Chen, Q.; Zheng, X.; Liu, P.; Fang, S. 3D graphene aerogels-supported Ag and Ag@Ag3PO4 heterostructure for the efficient adsorption-photocatalysis capture of different dye pollutants in water. Mater. Res. Bull. 2018, 105, 334–341. [Google Scholar] [CrossRef]
- Li, M.; Huang, S.; Li, H.; Feng, X.; Wang, Y.; Wang, C.; Ma, Y.; Guo, T.; Zhang, L.; He, Y. Unprecedented Eighteen-Faceted BiOCl with a Ternary Facet Junction Boosting Cascade Charge Flow and Photo-redox. Angew. Chem. 2019, 58, 9517–9521. [Google Scholar] [CrossRef] [PubMed]
- Xza, B.; Xx, A.; Jc, A.; Yi, W.A.; Hw, B. Understanding the effects of co-exposed facets on photocatalytic activities and fuel desulfurization performance in BiOCl singlet-crystalline sheets. J. Hazard. Mater. 2020, 391, 122198. [Google Scholar]
- Zhang, J.; Wang, Z.; Fan, M.; Tong, P.; Sun, J.; Dong, S.; Sun, J. Ultra-light and compressible 3D BiOCl/ RGO aerogel with enriched synergistic effect of adsorption and photocatalytic degradation of oxytetracycline—ScienceDirect. J. Mater. Res. Technol. 2019, 8, 4577–4587. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Chu, L.; Chen, R.; Fan, M. Unified photoelectrocatalytic microbial fuel cell harnessing 3D binder-free photocathode for simultaneous power generation and dual pollutant removal. J. Power Sources 2021, 481, 229133. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.; Zhu, Y.; Zhang, Y.; Zhu, Y. 3D-3D porous Bi2WO6/graphene hydrogel composite with excellent synergistic effect of adsorption-enrichment and photocatalytic degradation. Appl. Catal. B Environ. Int. J. Devoted Catal. Sci. Its Appl. 2017, 205, 228–237. [Google Scholar] [CrossRef]
- Xue, Y.; Shi, J.; Feng, L.; Li, C.; Liang, W. A three-dimensional BiOBr/RGO heterostructural aerogel with enhanced and selective photocatalytic properties under visible light. Appl. Surf. Sci. 2017, 396, 1775–1782. [Google Scholar]
- Dong, S.; Xia, L.; Chen, X.; Cui, L.; Fan, M. Interfacial and electronic band structure optimization for the adsorption and visible-light photocatalytic activity of macroscopic ZnSnO3/graphene aerogel. Compos. Part B Eng. 2021, 215, 108765. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Zhong, L.; Liu, D.; Cao, X.; Cui, F. Oxygen vacancy-rich 2D/2D BiOCl-g-C3N4 ultrathin heterostructure nanosheets for enhanced visible-light-driven photocatalytic activity in environmental remediation. Appl. Catal. B Environ. Int. J. Devoted Catal. Sci. Its Appl. 2018, 220, 290–302. [Google Scholar] [CrossRef]
- Shinde, N.M.; Xia, Q.X.; Yun, J.M.; Singh, S.; Mane, R.S.; Kim, K.H. A binder-free wet chemical synthesis approach to decorate nanoflowers of bismuth oxide on Ni-foam for fabricating laboratory scale potential pencil-type asymmetric supercapacitor device. Dalton. Trans. 2017, 46, 6601–6611. [Google Scholar] [CrossRef]
- Li, F.Q.; Sun, B.H.; Qin, Y.L.; Liu, X.Y.; Liu, Z.Y.; Ni, D.R.; Xiao, B.L.; Ma, Z.Y. Enhanced photocatalysis performance of BiOCl/graphene modified via polyvinylpyrrolidone. Vac. Technol. Appl. Ion Phys. Int. J. Abstr. Serv. Vac. Sci. Technol. 2021, 184, 109857. [Google Scholar] [CrossRef]
- Zz, A.; Hx, B.; Dl, A.; Js, A.; Dxa, B. Facile preparation and photocatalytic activity of oxygen vacancy rich BiOCl with {001} exposed reactive facets. Appl. Surf. Sci. 2019, 463, 1011–1018. [Google Scholar]
- Li, Y.; Li, D.; Qin, T.; Shi, Z.; Fu, P.; Xiong, D.; Dong, X. A comparative study of proton conduction between two new Cd(II) and Co(II) complexes and in vitro antibacterial study of the Cd(II) complex. Appl. Organomet. Chem. 2023, 37, e6920. [Google Scholar] [CrossRef]
- Kai, H.; Chen, G.; Zeng, G.; Chen, A.; Liang, H. Three-dimensional graphene supported catalysts for organic dyes degradation. Appl. Catal. B: Environ. 2018, 228, 19–28. [Google Scholar]
- Zhang, S.; Song, S.; Gu, P.; Ma, R.; Wei, D.; Zhao, G.; Wen, T.; Jehan, R.; Hu, B.; Wang, X. Visible-light-driven activation of persulfate over cyano and hydroxyl group co-modified mesoporous g-C3N4 for boosting bisphenol A degradation. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 5552–5560. [Google Scholar] [CrossRef]
- Xiao, C.; Wdo, C.; Phz, D.; Rdw, E.; Ttla, B. Surface construction of nitrogen-doped chitosan-derived carbon nanosheets with hierarchically porous structure for enhanced sulfacetamide degradation via peroxymonosulfate activation: Maneuverable porosity and active sites—ScienceDirect. Chem. Eng. J. 2020, 382, 122908. [Google Scholar]
- Hasanvandian, F.; Shokri, A.; Moradi, M.; Kakavandi, B.; Setayesh, S.R. Encapsulation of spinel CuCo2O4 hollow sphere in V2O5-decorated graphitic carbon nitride as high-efficiency double Z-type nanocomposite for levofloxacin photodegradation. J. Hazard. Mater. 2021, 423, 127090. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Kakavandi, B.; Bahadoran, A. Intensification of persulfate-mediated elimination of bisphenol A by a spinel cobalt ferrite-anchored g-C3N4 S-scheme photocatalyst; Catalytic synergies and mechanistic interpretation. Sep. Purif. Technol. 2022, 285, 120313. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, M.; Wang, Y.; Wang, L.; Gong, Y.; Guo, T.; Zhao, X. Persulfate enhanced photocatalytic degradation of bisphenol A by g-C3N4 nanosheets under visible light irradiation. Chemosphere 2017, 115, 115–122. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 2018, 53, 307–315. [Google Scholar] [CrossRef]
- Dong, S.; Cui, L.; Liu, C.; Zhang, F.; Li, K.; Xia, L.; Su, X.; Feng, J.; Zhu, Y.; Sun, J. Fabrication of 3D ultra-light graphene aerogel/Bi2WO6 composite with excellent photocatalytic performance: A promising photocatalysts for water purification. J. Taiwan Inst. Chem. Eng. 2019, 97, 288–296. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation. Appl. Catal. B Environ. 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Gu, M.; Farooq, U.; Lu, S.; Zhang, X.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by rGO supported nZVI catalyst under several oxic environments. J. Hazard. Mater. 2018, 349,, 35–44. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Liu, L.; Tian, X.; Liu, L.; Xia, Y.; Liang, X.; Wang, Y.; Song, Z.; Zhang, Y.; et al. Novel Carbon-based Fe-Co Oxides Derived from Prussian Blue Analogues Activating Peroxymonosulfate: Refractory Drugs Degradation without Metal Leaching. Chem. Eng. J. 2019, 379, 122274. [Google Scholar] [CrossRef]
- Govindan, K.; Suresh, A.K.; Sakthivel, T.; Murugesan, K.; Jang, A. Effect of peroxomonosulfate, peroxodisulfate and hydrogen peroxide on graphene oxide photocatalytic performances in methyl orange dye degradation. Chemosphere 2019, 237, 124479. [Google Scholar] [CrossRef] [PubMed]
- Joshaghani, M.; Yazdani, D.; Zinatizadeh, A.A. Statistical modeling of p-nitrophenol degradation using a response surface methodology (RSM) over nano zero-valent iron-modified Degussa P25-TiO2/ZnO photocatalyst with persulfate. J. Iran. Chem. Soc. 2017, 14, 2449–2456. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Chand, H.; Krishnan, V. Heterogeneous photocatalytic activation of persulfate ions with novel ZnO/AgFeO2 nanocomposite for contaminants degradation under visible light. J. Mater. Science. Mater. Electron. 2021, 32, 4272–4289. [Google Scholar] [CrossRef]
- Song, T.; Gao, Y.; Hu, R.; Li, G.; Yu, X. Degradation of Methyl Orange in Aqueous Solution via Magnetic TiO2/Fe3O4 Conjugated with Persulfate. Water Air Soil Pollut. 2023, 234, 508. [Google Scholar] [CrossRef]
- Munkoeva, V.A.; Sizykh, M.R.; Batoeva, A.A. Iop. Photo Degradation of Methyl Orange by Persulfate Activated with Zero Valent Iron. In Proceedings of the International Conference on Construction, Architecture and Technosphere Safety (ICCATS), Chelyabinsk, Russia, 21–22 September 2017. [Google Scholar]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, D.; Zhang, Y.; Chao, C.; Chen, Q.; Yao, S.; Liu, C. In Situ Synthesis of 3D BiOCl–Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation. Molecules 2023, 28, 4964. https://doi.org/10.3390/molecules28134964
Li Y, Zhang D, Zhang Y, Chao C, Chen Q, Yao S, Liu C. In Situ Synthesis of 3D BiOCl–Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation. Molecules. 2023; 28(13):4964. https://doi.org/10.3390/molecules28134964
Chicago/Turabian StyleLi, Yukun, Dan Zhang, Yongshu Zhang, Cong Chao, Qishi Chen, Sen Yao, and Cuixia Liu. 2023. "In Situ Synthesis of 3D BiOCl–Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation" Molecules 28, no. 13: 4964. https://doi.org/10.3390/molecules28134964
APA StyleLi, Y., Zhang, D., Zhang, Y., Chao, C., Chen, Q., Yao, S., & Liu, C. (2023). In Situ Synthesis of 3D BiOCl–Graphene Aerogel and Synergistic Effect by Photo-Assisted Activation of Persulfate for Methyl Orange Degradation. Molecules, 28(13), 4964. https://doi.org/10.3390/molecules28134964





