Synthesis, Characterization, and NH3-SCR Catalytic Performance of Fe-Modified MCM-36 Intercalated with Various Pillars
Abstract
1. Introduction
2. Results
2.1. Physicochemical Properties of the Materials
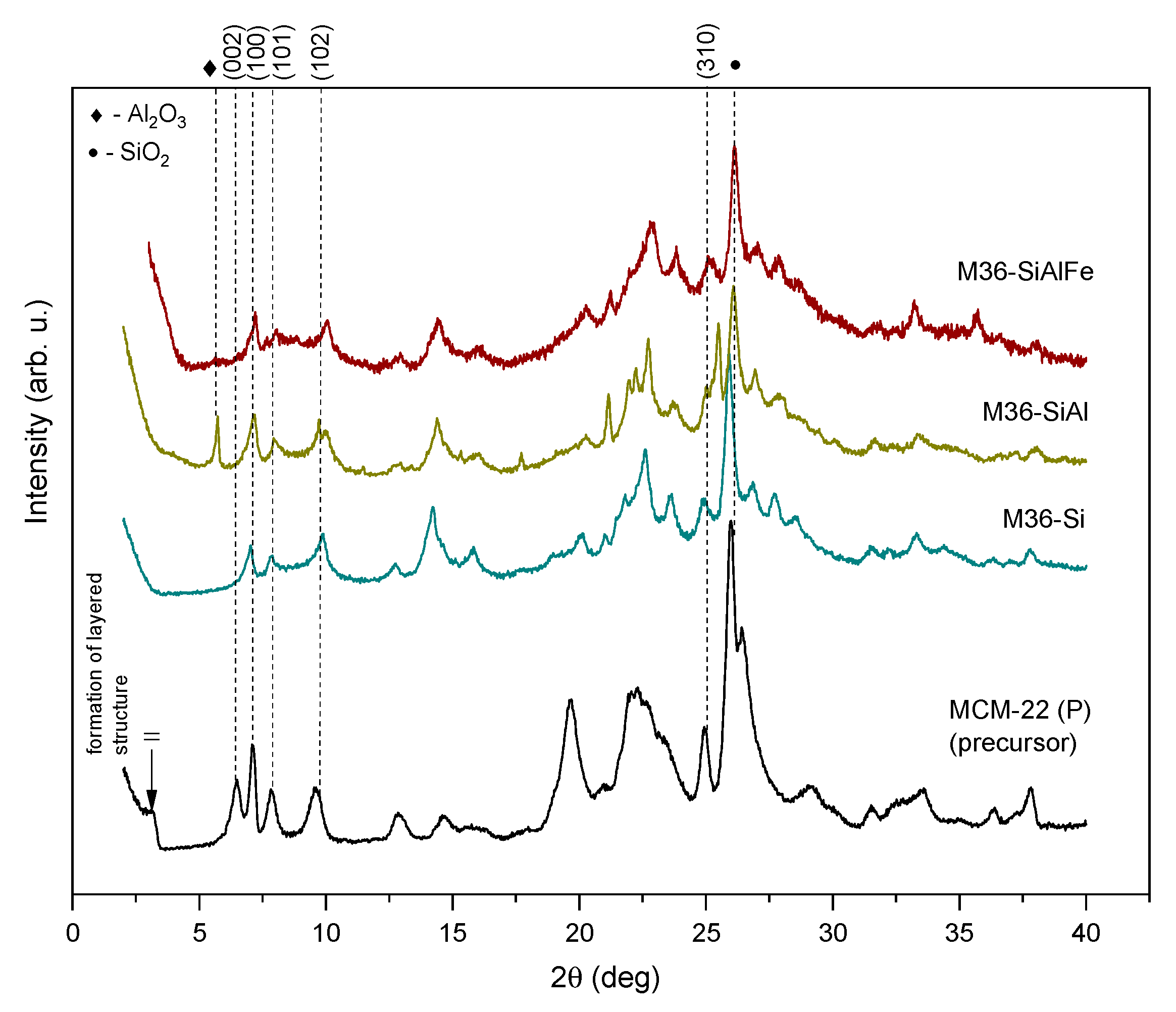
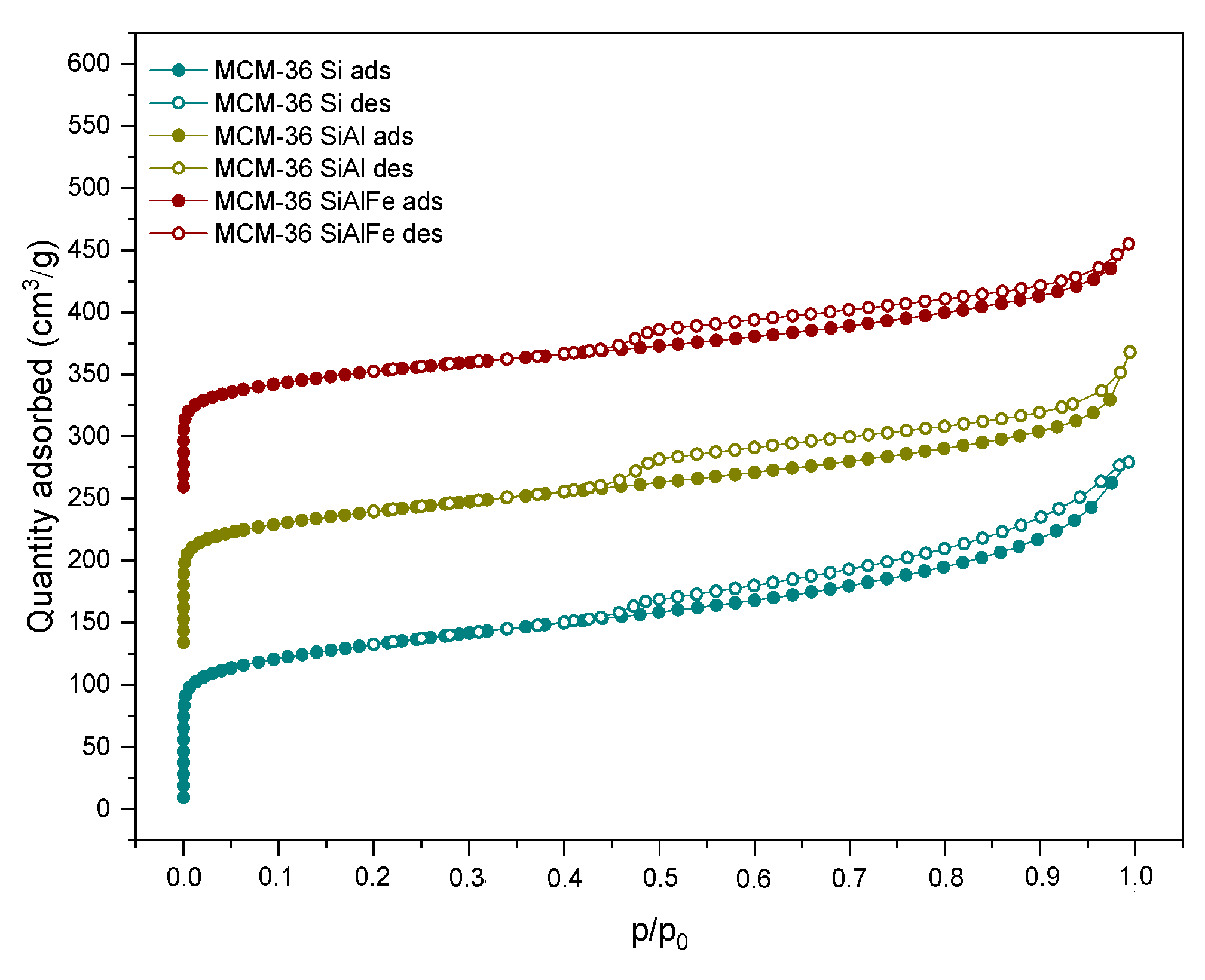
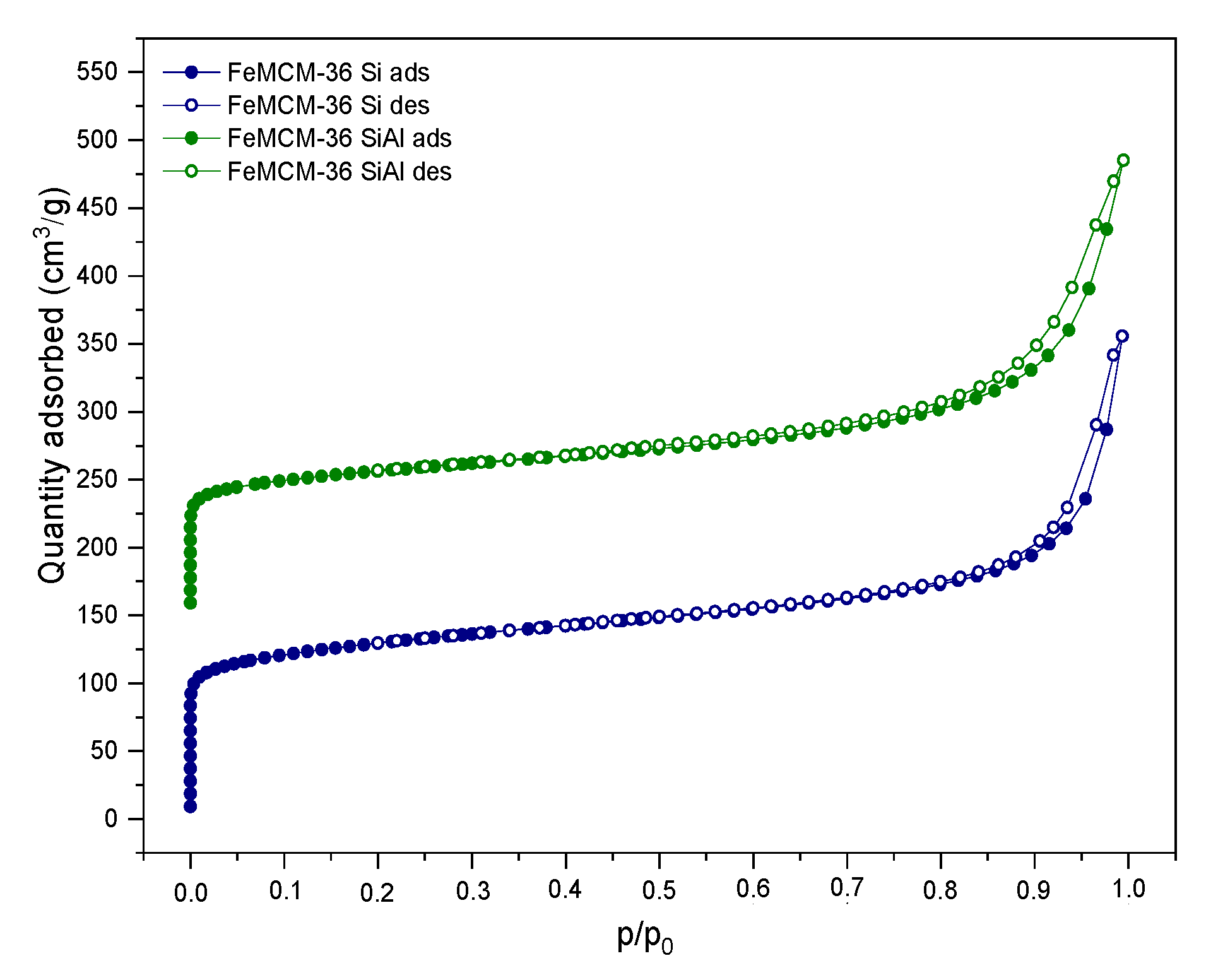
2.1.1. Chemical Composition, Crystallinity, and Textural Characterization
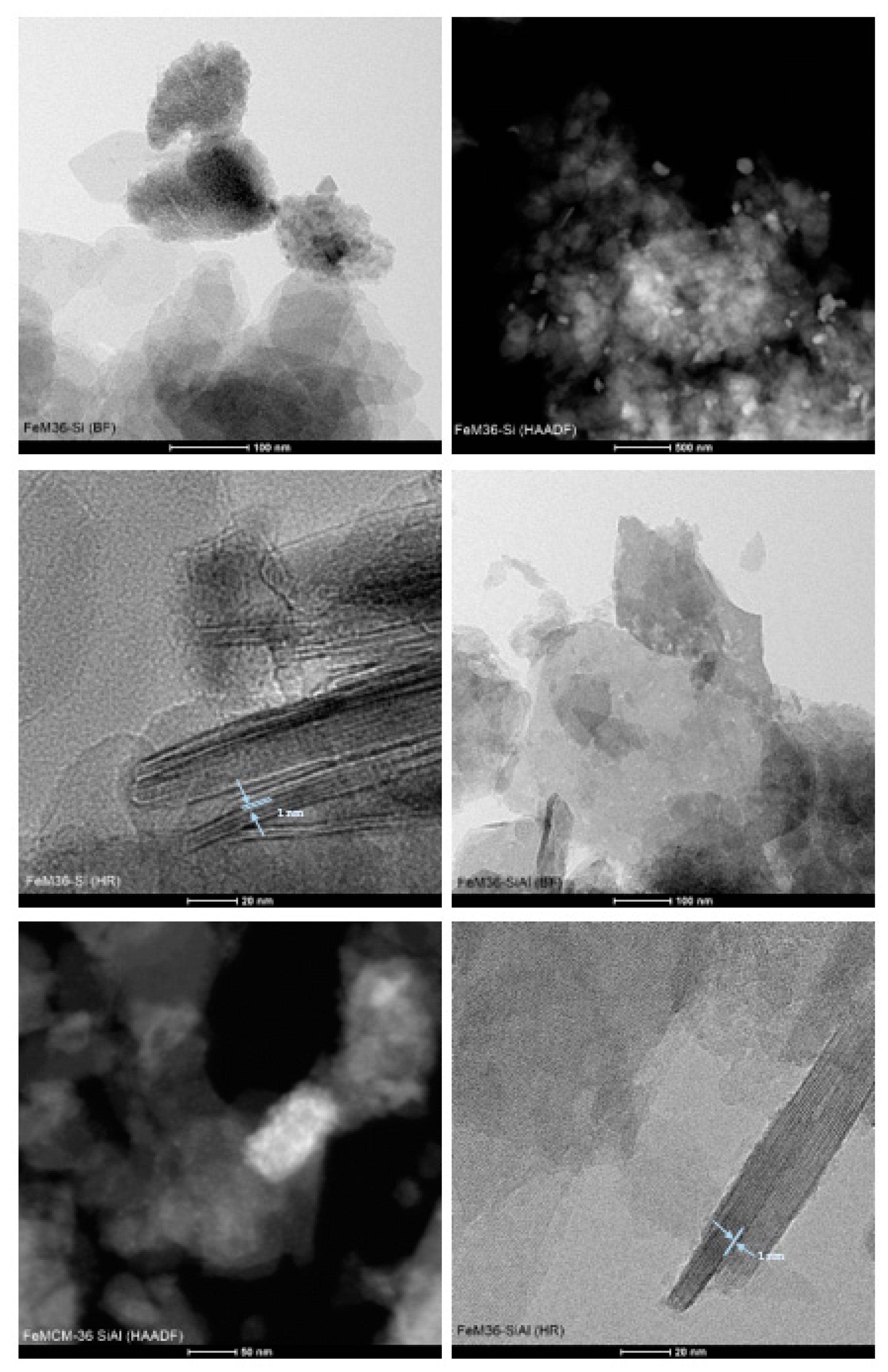
2.1.2. Morphology of the Materials
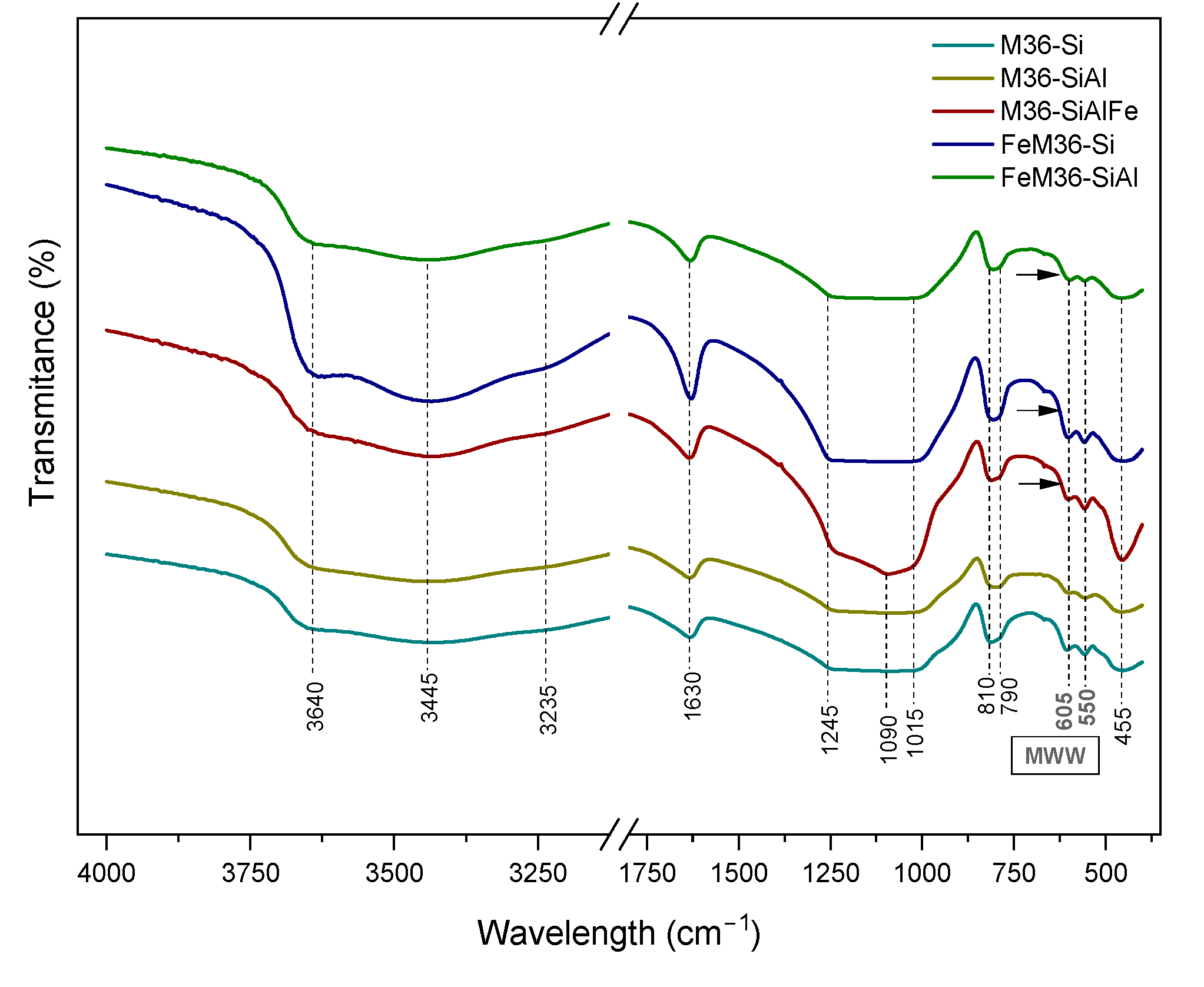
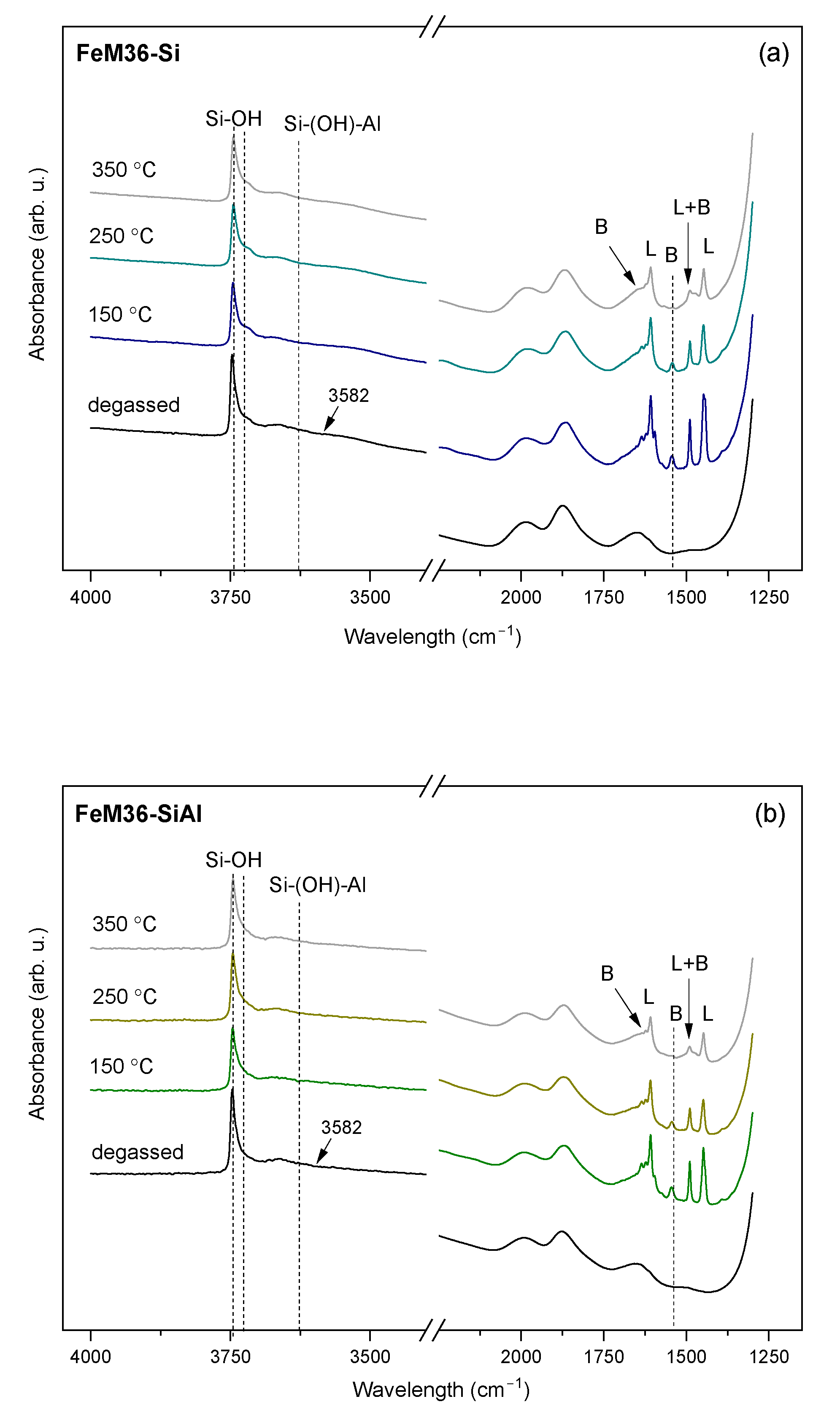
2.1.3. Characteristic Chemical Groups Present in the Materials
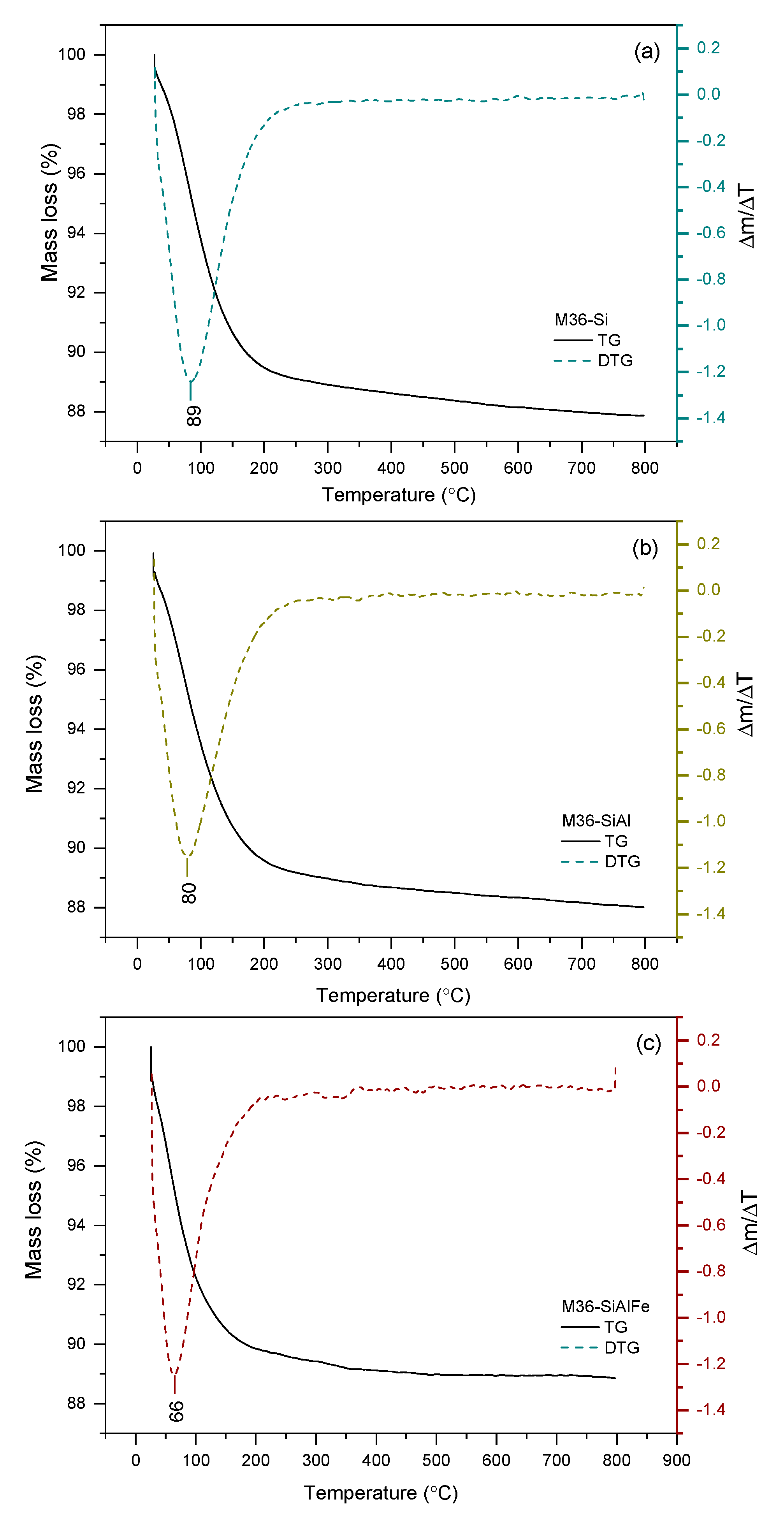
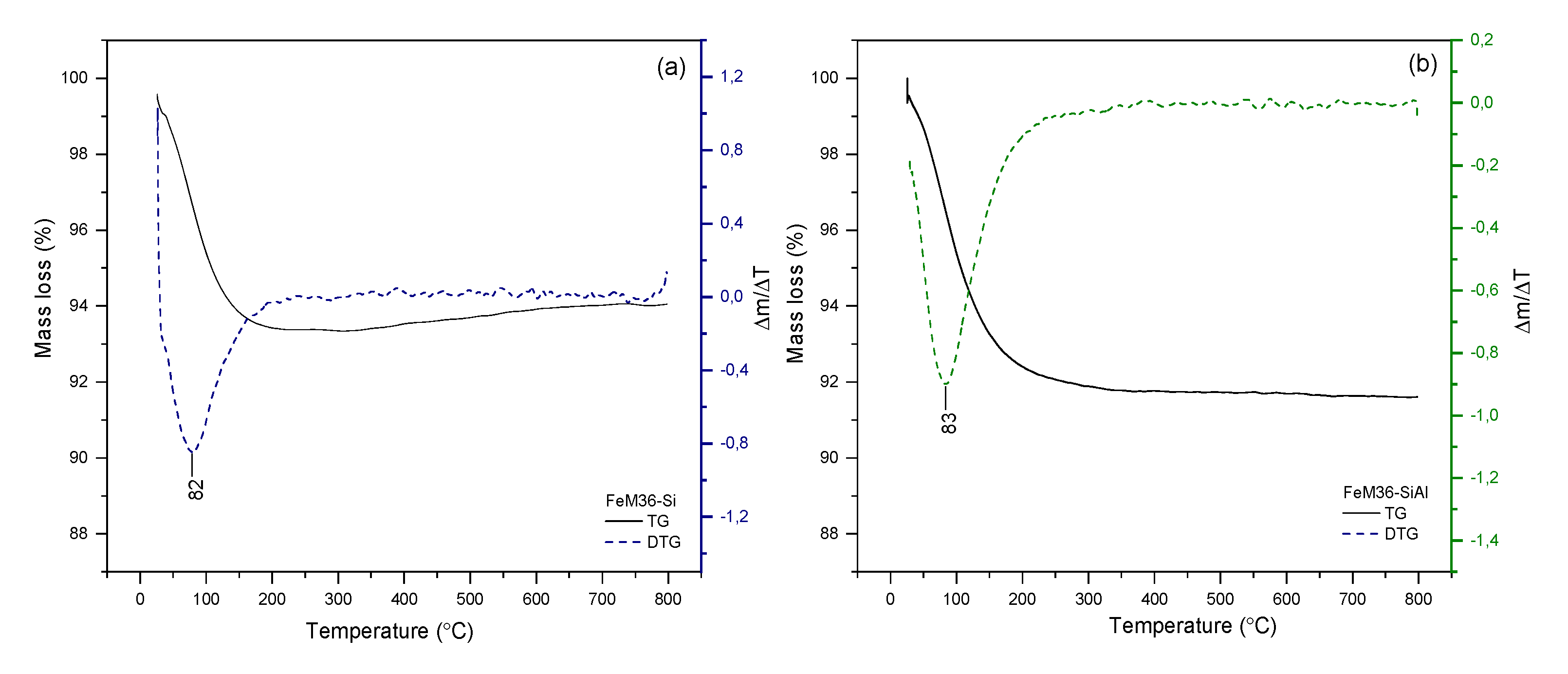
2.1.4. Thermal Stability of the Materials
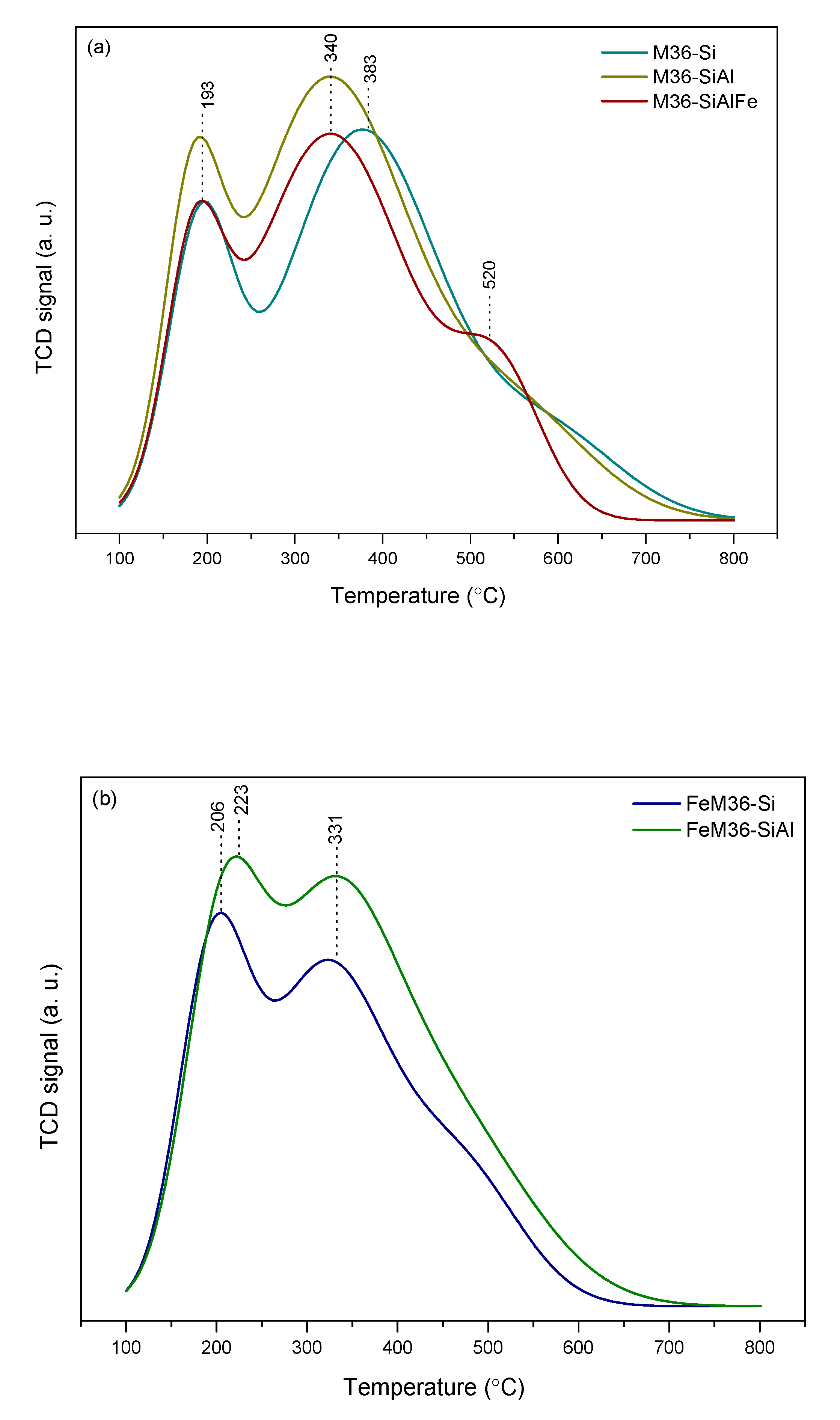
2.1.5. Acidic Properties of the Materials—NH3-TPD and Pyridine Adsorption Studies
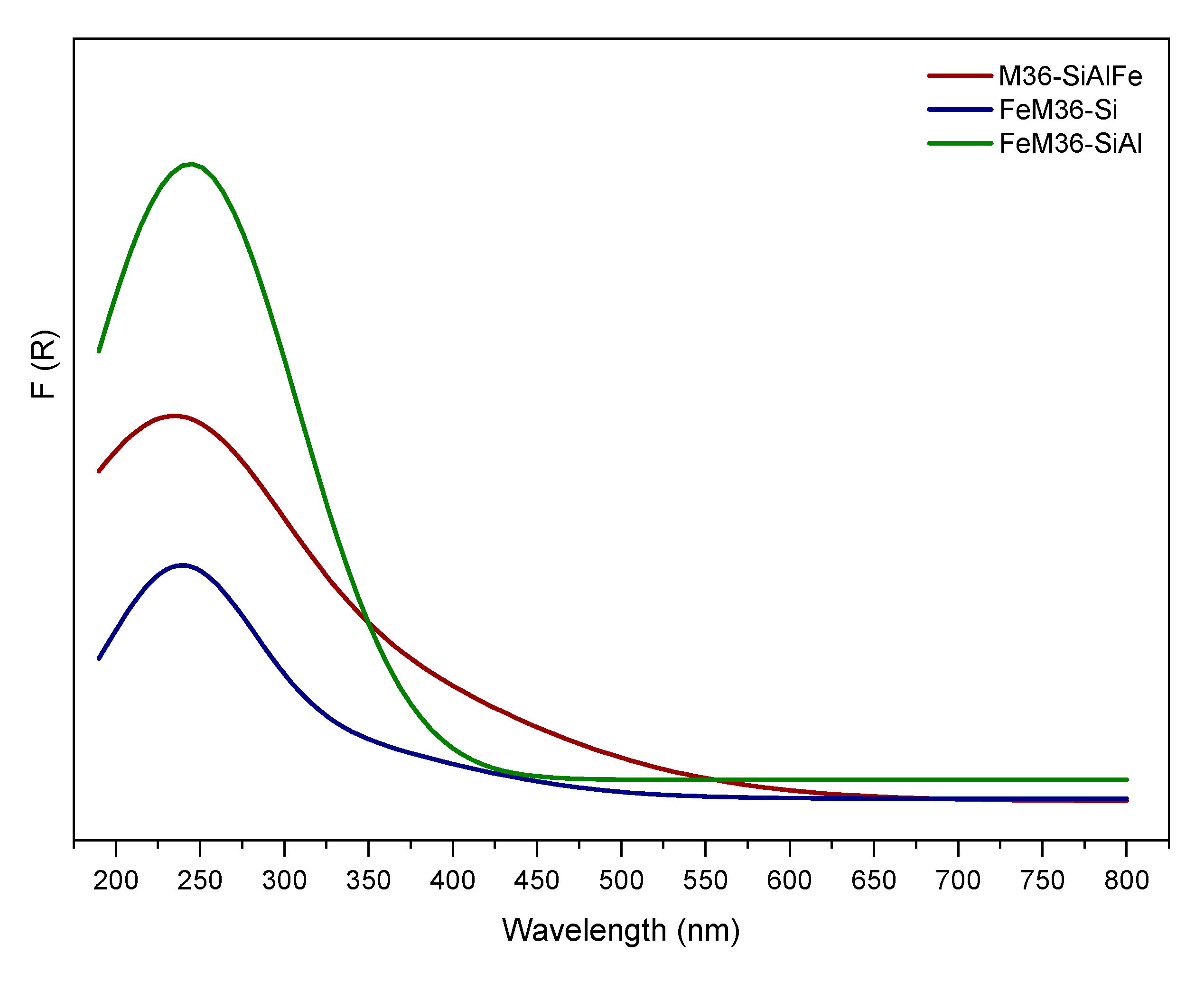
2.1.6. Distribution of Iron in the Materials
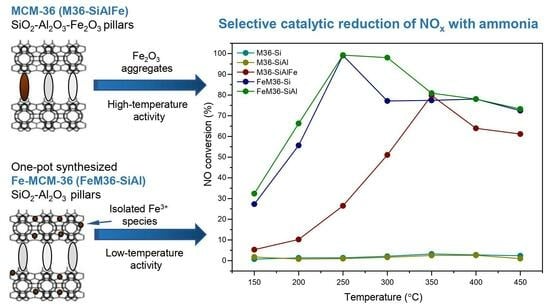
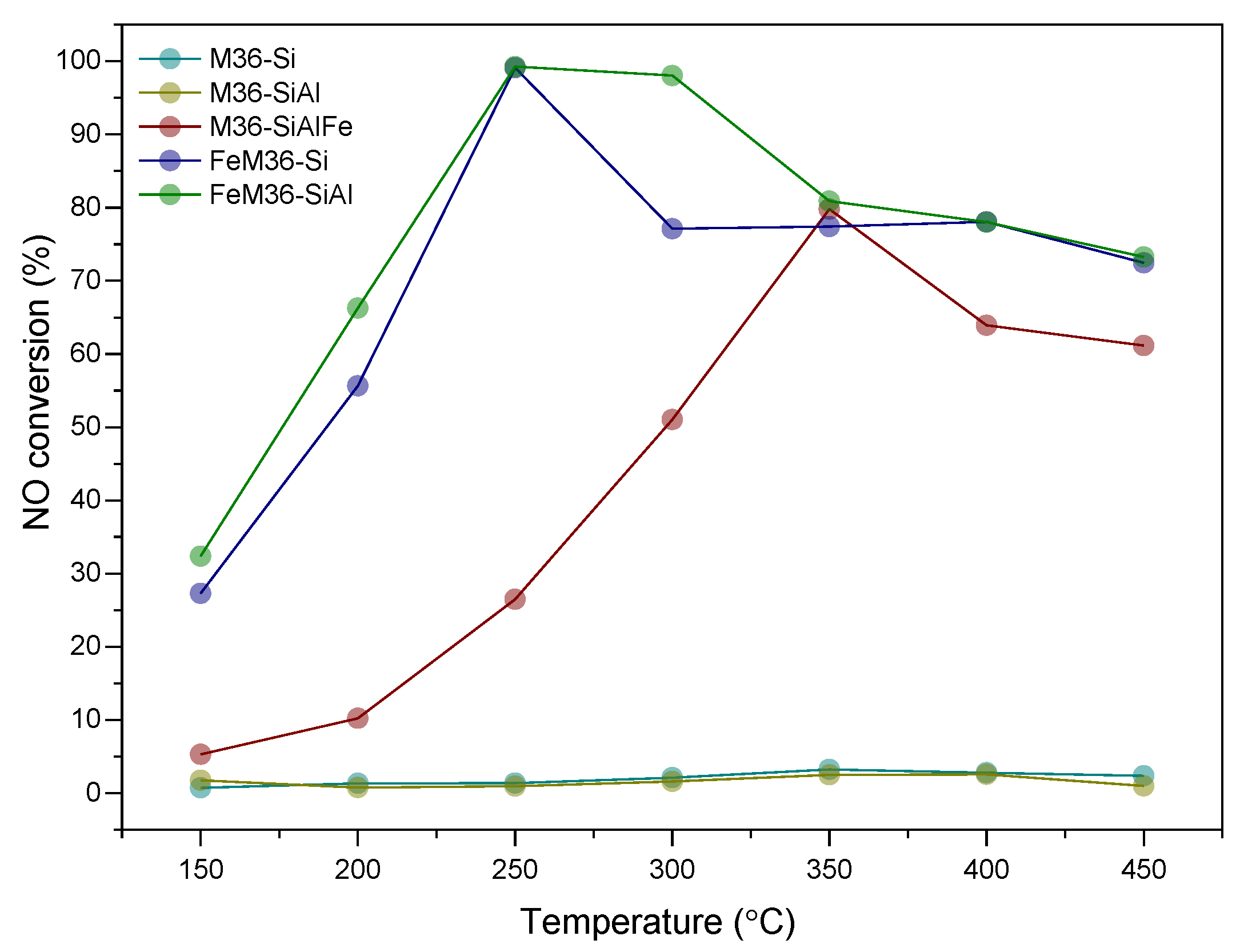
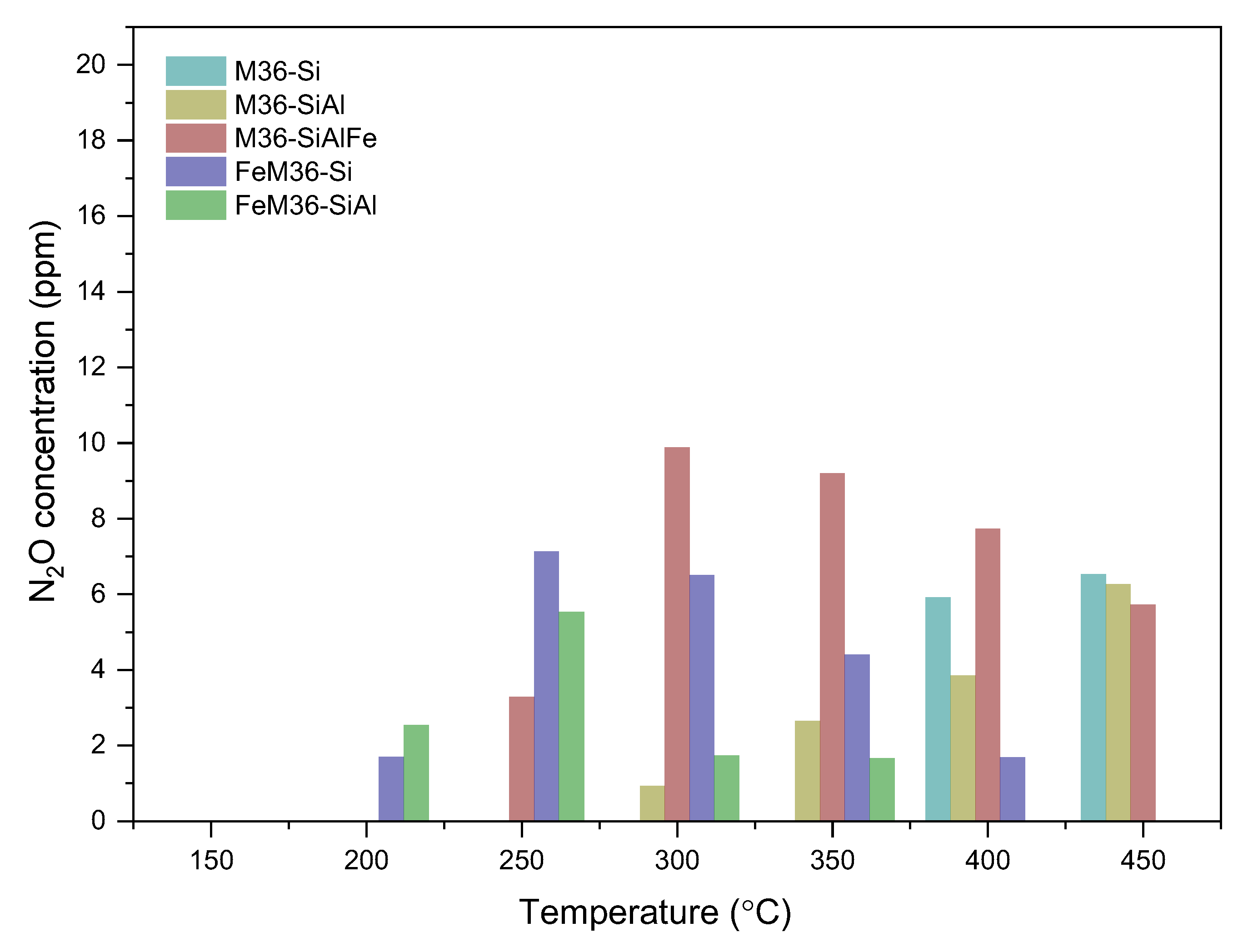
2.2. NH3-SCR Catalytic Performance of the Materials
3. Discussion
4. Materials and Methods
4.1. Preparation of the Materils
4.1.1. Synthesis of the Precursor MCM-22 (P)
4.1.2. Swelling of MCM-22 (P)
4.1.3. Intercalation with Various Pillars
4.1.4. One-Pot Synthesis of Fe-MCM-36 Intercalated with Silica or Silica-Alumina Pillars
4.2. Physicochemical Characterization of the Materials
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Díaz, U. Layered Materials with Catalytic Applications Pillared and Delaminated Zeolites. Int. Sch. Res. Netw. 2012, 1, 537164. [Google Scholar]
- Yang, W.; Wang, Z.; Sun, H.; Zhang, B. Advances in Development and Industrial Applications of Ethylbenzene Processes. Cuihua Xuebao Chin. J. Catal. 2016, 37, 16–26. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J. Recent Advances in the Synthesis and Application of Two-Dimensional Zeolites. Adv. Energy Mater. 2016, 6, 441. [Google Scholar] [CrossRef]
- Riaz, F.; Guarino, C.H.L. Mobil/Badger Cumene Process. Hydrocarb. Eng. 1999, 4, 39–43. [Google Scholar]
- Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Cu and Fe Modified Derivatives of 2D MWW-Type Zeolites (MCM-22, ITQ-2 and MCM-36) as New Catalysts for DeNOx Process. Appl. Catal. B Environ. 2015, 168–169, 531–539. [Google Scholar] [CrossRef]
- Bennett, J.M.; Chang, C.D.; Lawton, S.L.; Leonowicz, M.E.; Lissy, D.N.; Rubin, M.K. Synthetic Porous Crystalline MCM-49, Its Synthesis and Use. US5236575A, 17 August 1993. [Google Scholar]
- Ostroumova, V.A.; Maksimov, A.L. MWW-Type Zeolites: MCM-22, MCM-36, MCM-49, and MCM-56 (A Review). Pet. Chem. 2019, 59, 788–801. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Guil, J.M.; Pergher, S.; Maesen, T.L.M.; Buglass, J.G. Preparation, Characterisation and Catalytic Activity of ITQ-2, a Delaminated Zeolite. Microporous Mesoporous Mater. 2000, 38, 301–309. [Google Scholar] [CrossRef]
- Szymaszek-Wawryca, A.; Díaz, U.; Samojeden, B.; Motak, M. Catalytic Performance of One-Pot Synthesized Fe-MWW Layered Zeolites (MCM-22, MCM-36, and ITQ-2) in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Molecules 2022, 27, 2983. [Google Scholar] [CrossRef]
- Marosz, M.; Samojeden, B.; Kowalczyk, A.; Rutkowska, M.; Motak, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. MCM-22, MCM-36, and ITQ-2 Zeolites with Different Si/Al Molar Ratios as Effective Catalysts of Methanol and Ethanol Dehydration. Materials 2020, 13, 2399. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Chlubná, P.; Jindrova, T.; Kubicka, D. Peculiar Behavior of MWW Materials Aldol Condensation of Furfural and Acetone—MCM-22 MCM-36. Dalton Trans. 2014, 43, 10628. [Google Scholar] [CrossRef]
- Kaskow, I.; Wojtaszek-gurdak, A.; Sobczak, I. Methanol Oxidation on AuAg-Zn/MCM-36—The Effect of Catalyst Components and Pretreatment. Catal. Today 2020, 354, 123–132. [Google Scholar] [CrossRef]
- Jankowska, A.; Kowalczyk, A.; Rutkowska, M.; Mozgawa, W.; Gil, B.; Chmielarz, L. Silica and Silica-Titania Intercalated MCM-36 Modified with Iron as Catalysts for Selective Reduction of Nitrogen Oxides-The Role of Associated Reactions. Catal. Sci. Technol. 2020, 10, 7940–7954. [Google Scholar] [CrossRef]
- Kresge, C.T.; Roth, W.J.; Simmons, K.G.; Vartuli, J.C. Crystalline Oxide Material. US Patent 5,229,341, 20 July 1993. [Google Scholar]
- Zhang, Z.; Zhu, W.; Zai, S.; Jia, M.; Zhang, W.; Wang, Z. Synthesis, Characterization and Catalytic Properties of MCM-36 Pillared via the MCM-56 Precursor. J. Porous Mater. 2013, 20, 531–538. [Google Scholar] [CrossRef]
- Ahmad, N.; Hussain, S.T.; Muhammad, B.; Ali, N.; Abbas, S.M.; Ali, Z. Zr-Pillared Montmorillonite Supported Cobalt Nanoparticles for Fischer–Tropsch Synthesis. Prog. Nat. Sci. Mater. Int. 2013, 23, 374–381. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, P.; Dang, Z.; Zhu, N.; Li, P.; Wu, J.; Wang, X. Iron Pillared Vermiculite as a Heterogeneous Photo-Fenton Catalyst for Photocatalytic Degradation of Azo Dye Reactive Brilliant Orange X-GN. Sep. Purif. Technol. 2010, 71, 315–323. [Google Scholar] [CrossRef]
- Kornatowski, J.; Barth, J.O.; Erdmann, K.; Rozwadowski, M. Effect of Various Pillaring Oxides on Adsorption Behaviour of Novel MCM-36 Derivatives. Microporous Mesoporous Mater. 2006, 90, 251–258. [Google Scholar] [CrossRef]
- Barth, J.; Kornatowski, J.; Lercher, J.A. Synthesis of New MCM-36 Derivatives Pillared with Alumina or Magnesia—Alumina. J. Mater. Chem. 2002, 1, 369–373. [Google Scholar] [CrossRef]
- Barth, J.; Jentys, A.; Kornatowski, J.; Lercher, J.A.; Al, O.; Omar, M. Al Control of Acid—Base Properties of New Nanocomposite Derivatives of MCM-36 by Mixed Oxide Pillaring. Chem. Mater. 2004, 8, 724–730. [Google Scholar] [CrossRef]
- Wang, T.; Jin, F.; Yi, X.; Wu, G.; Zheng, A. Atom-Planting Synthesis of MCM-36 Catalyst to Investigate the Influence of Pore Structure and Titanium Coordination State on Epoxidation Activity. Microporous Mesoporous Mater. 2021, 310, 110645. [Google Scholar] [CrossRef]
- Jin, F.; Chen, S.Y.; Jang, L.Y.; Lee, J.F.; Cheng, S. New Ti-Incorporated MCM-36 as an Efficient Epoxidation Catalyst Prepared by Pillaring MCM-22 Layers with Titanosilicate. J. Catal. 2014, 319, 247–257. [Google Scholar] [CrossRef]
- Jin, F.; Huang, S.; Cheng, S.; Wu, Y.; Chang, C.C.; Huang, Y.W. The Influences of Al Species and Ti Species on the Catalytic Epoxidation over Si/Ti-Pillared MCM-36 Synthesized from MCM-22. Catal. Sci. Technol. 2015, 5, 3007–3016. [Google Scholar] [CrossRef]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Zyrkowski, M.; Motak, M.; Samojeden, B.; Szczepanek, K. Deactivation of V2O5-WO3/TiO2 DeNOx Catalyst under Commercial Conditions in Power Production Plant. Energies 2020, 13, 6200. [Google Scholar] [CrossRef]
- Liang, J.; Hu, W.F.; Song, B.; Mou, T.; Zhang, L.; Luo, Y.; Liu, Q.; Alshehri, A.A.; Hamdy, M.S.; Yang, L.M.; et al. Efficient Nitric Oxide Electroreduction toward Ambient Ammonia Synthesis Catalyzed by a CoP Nanoarray. Inorg. Chem. Front. 2022, 9, 1366–1372. [Google Scholar] [CrossRef]
- Liang, J.; Chen, H.; Mou, T.; Zhang, L.; Lin, Y.; Yue, L.; Luo, Y.; Liu, Q.; Li, N.; Alshehri, A.A.; et al. Coupling Denitrification and Ammonia Synthesis via Selective Electrochemical Reduction of Nitric Oxide over Fe2O3 Nanorods. J. Mater. Chem. A Mater. 2022, 10, 6454–6462. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Wang, Y.; Mou, T.; Lin, Y.; Yue, L.; Li, T.; Liu, Q.; Luo, Y.; Li, N.; et al. High-Performance Electrochemical NO Reduction into NH3 by MoS2 Nanosheet. Angew. Chem.—Int. Ed. 2021, 60, 25263–25268. [Google Scholar] [CrossRef]
- Szymaszek-Wawryca, A.; Diaz, U.; Duraczyńska, D.; Świerczek, K.; Samojeden, B.; Motak, M. Catalytic Performance and Sulfur Dioxide Resistance of One-Pot Synthesized Fe-MCM-22 in Selective Catalytic Reduction of Nitrogen Oxides with Ammonia (NH3-SCR)—The Effect of Iron Content. Int. J. Mol. Sci. 2022, 23, 10754. [Google Scholar] [CrossRef]
- Li, K.; Lei, J.; Yuan, G.; Weerachanchai, P.; Wang, J.Y.; Zhao, J.; Yang, Y. Fe-, Ti-, Zr- and Al-Pillared Clays for Efficient Catalytic Pyrolysis of Mixed Plastics. Chem. Eng. J. 2017, 317, 800–809. [Google Scholar] [CrossRef]
- Kizilduman, B.K.; Alkan, M.; Doğan, M.; Turhan, Y. Al-Pillared-Montmorillonite (AlPMt)/Poly(Methyl Methacrylate)(PMMA) Nanocomposites: The Effects of Solvent Types and Synthesis Methods. Adv. Mater. Sci. 2017, 17, 5–23. [Google Scholar] [CrossRef]
- Banković, P.; Milutinović-Nikolić, A.; Mojović, Z.; Jović-Jovičić, N.; Žunić, M.; Dondur, V.; Jovanović, D. Al,Fe-Pillared Clays in Catalytic Decolorization of Aqueous Tartrazine Solutions. Appl. Clay Sci. 2012, 58, 73–78. [Google Scholar] [CrossRef]
- Barrault, J.; Abdellaoui, M.; Bouchoule, C.; Majesté, A.; Tatibouët, J.M.; Louloudi, A.; Papayannakos, N.; Gangas, N.H. Catalytic Wet Peroxide Oxidation over Mixed (Al-Fe) Pillared Clays. Appl. Catal. B Environ. 2000, 27, 3373. [Google Scholar] [CrossRef]
- Boroń, P.; Chmielarz, L.; Gurgul, J.; Łątka, K.; Gil, B.; Marszałek, B.; Dzwigaj, S. Influence of Iron State and Acidity of Zeolites on the Catalytic Activity of FeHBEA, FeHZSM-5 and FeHMOR in SCR of NO with NH3 and N2O Decomposition. Microporous Mesoporous Mater. 2015, 203, 73–85. [Google Scholar] [CrossRef]
- Chen, J.; Peng, G.; Zheng, W.; Zhang, W.; Guo, L.; Wu, X. Excellent Performance of One-Pot Synthesized Fe-Containing MCM-22 Zeolites for the Selective Catalytic Reduction of NO: Xwith NH3. Catal. Sci. Technol. 2020, 10, 6583–6598. [Google Scholar] [CrossRef]
- Mauricio, B.; Andrade, H.M.C.; Mascarenhas, A.J.S. Oxidative Dehydration of Glycerol over Alternative H,Fe-MCM-22 Catalysts: Sustainable Production of Acrylic Acid. Microporous Mesoporous Mater. 2019, 278, 366–377. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Makowski, W.; Sławek, A.; Korzeniowska, A.; Grzybek, J.; Siwek, M.; Michorczyk, P. Framework-Substituted Cerium MCM-22 Zeolite and Its Interlayer Expanded Derivative MWW-IEZ. Catal. Sci. Technol. 2016, 6, 2742–2753. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Piwowarska, Z.; Michalik, M.; Dudek, B.; Dziembaj, R. Natural Micas Intercalated with Al2O3 and Modified with Transition Metals as Catalysts of the Selective Oxidation of Ammonia to Nitrogen. Top. Catal. 2009, 52, 1017–1022. [Google Scholar] [CrossRef]
- Kim, S.H.; Komarneni, S.; Heo, N.H. ZSM-5 and Ferrierite Single Crystals with Lower Si/Al Ratios: Synthesis and Single-Crystal Synchrotron X-Ray Diffraction Studies. Microporous Mesoporous Mater. 2011, 143, 243–248. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- He, Y.J.; Nivarthy, G.S.; Eder, F.; Seshan, K.; Lercher, J.A. Synthesis, Characterization and Catalytic Activity of the Pillared Molecular Sieve MCM-36. Microporous Mesoporous Mater. 1998, 25, 207–224. [Google Scholar] [CrossRef]
- Chlubná, P.; Roth, W.J.; Zukal, A.; Kubu, M.; Jules, J.P. Pillared MWW Zeolites MCM-36 Prepared by Swelling MCM-22P in Concentrated Surfactant Solitions. Catal. Today 2012, 179, 35–42. [Google Scholar] [CrossRef]
- Maheshwari, S.; Jordan, E.; Kumar, S.; Bates, F.S.; Penn, R.L.; Shantz, D.F.; Tsapatsis, M. Layer Structure Preservation during Swelling, Pillaring, and Exfoliation of a Zeolite Precursor. J. Am. Chem. Soc. 2008, 130, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, R.; Bandyopadhyay, R. Preparation, Characterization, and Post-Synthetic Modification of Layered MCM-22 Zeolite Precursor. J. Chem. Sci. 2017, 129, 1671–1676. [Google Scholar] [CrossRef]
- Sobhanardakani, S.; Jafari, A.; Zandipak, R.; Meidanchi, A. Removal of Heavy Metal (Hg(II) and Cr(VI)) Ions from Aqueous Solutions Using Fe2O3@SiO2 Thin Films as a Novel Adsorbent. Process. Saf. Environ. Prot. 2018, 120, 348–357. [Google Scholar] [CrossRef]
- Carriço, C.S.; Cruz, F.T.; Santos, M.B.; Pastore, H.O.; Andrade, H.M.C.; Mascarenhas, A.J.S. Efficiency of Zeolite MCM-22 with Different SiO2/Al2O3 Molar Ratios in Gas Phase Glycerol Dehydration to Acrolein. Microporous Mesoporous Mater. 2013, 181, 74–82. [Google Scholar] [CrossRef]
- Juybar, M.; Khanmohammadi Khorrami, M.; Bagheri Garmarudi, A.; Zandbaaf, S. Determination of Acidity in Metal Incorporated Zeolites by Infrared Spectrometry Using Artificial Neural Network as Chemometric Approach. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 228, 117539. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium Bentonite and Kaolin Clays: Comparative Study on Their FT-IR, XRF, and XRD. Mater. Today Proc. 2020, 22, 737–742. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Aiello, R.; Garrone, E. H-Bond Formation and Proton Transfer in H-MCM-22 Zeolite as Compared to H-ZSM-5 and H-MOR: An FTIR Study. J. Phys. Chem. B 2002, 106, 1684–1690. [Google Scholar] [CrossRef]
- Onida, B.; Geobaldo, F.; Testa, F.; Crea, F.; Garrone, E. FTIR Investigation of the Interaction at 77 K of Diatomic Molecular Probes on MCM-22 Zeolite. Microporous Mesoporous Mater. 1999, 30, 119–127. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Dziembaj, R.; Cool, P.; Vansant, E.F. SBA-15 Mesoporous Silica Modified with Metal Oxides by MDD Method in the Role of DeNOx Catalysts. Microporous Mesoporous Mater. 2010, 127, 133–141. [Google Scholar] [CrossRef]
- Nawab, M.; Barot, S.; Bandyopadhyay, R. Solvent-Free Selective Oxidation of Toluene over Metal-Doped MCM-22. New J. Chem. 2019, 43, 4406–4412. [Google Scholar] [CrossRef]
- Gil, B.; Marszałek, B.; Micek-Ilnicka, A.; Olejniczak, Z. The Influence of Si/Al Ratio on the Distribution of OH Groups in Zeolites with MWW Topology. Top. Catal. 2010, 53, 1340–1348. [Google Scholar] [CrossRef]
- Mihályi, R.M.; Lázár, K.; Kollár, M.; Lónyi, F.; Pál-Borbély, G.; Szegedi, Á. Structure, Acidity and Redox Properties of MCM-22 Ferrisilicate. Microporous Mesoporous Mater. 2008, 110, 51–63. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C.; Kolodziejski, W.; Prez-pariente, J.; Quimica, I.D.T.; Polit, U.; Valencia, D. Infrared Spectroscopy, Acidity, Structure, and Stability Of Zeolites. Microporous Mesoporous Mater. 1995, 2449, 576–582. [Google Scholar]
- Grijndling, C.; Gründling, L.; Eder-Mirth, P. Infrared Studies of the Surface Acidity of Oxides and Zeolites Using Adsorbed Probe Molecules.Pdf. Catal. Today 1996, 27, 353–376. [Google Scholar]
- Góra-Marek, K.; Datka, J. IR Studies of OH Groups in Mesoporous Aluminosilicates. Appl. Catal. A Gen. 2006, 302, 104–109. [Google Scholar] [CrossRef]
- Usman, M.; Li, D.; Li, C.; Zhang, S. Highly Selective and Stable Hydrogenation of Heavy Aromatic-Naphthalene over Transition Metal Phosphides. Sci. China Chem. 2015, 58, 738–746. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure-Activity Relationship of VOx/CeO2 Nanorod for NO Removal with Ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Chen, J.; Liang, T.; Li, J.; Wang, S.; Qin, Z.; Wang, P.; Huang, L.; Fan, W.; Wang, J. Regulation of Framework Aluminum Siting and Acid Distribution in H–MCM-22 by Boron Incorporation and Its Effect on the Catalytic Performance in Methanol to Hydrocarbons. ACS Catal. 2016, 2, 2862. [Google Scholar] [CrossRef]
- Jenness, G.R.; Christiansen, M.A.; Caratzoulas, S.; Vlachos, D.G.; Gorte, R.J. Site-Dependent Lewis Acidity of γ-Al2O3 and Its Impact on Ethanol Dehydration and Etherification. J. Phys. Chem. C 2014, 118, 12899–12907. [Google Scholar] [CrossRef]
- Stephenson, N.A.; Bell, A.T. Mechanistic Insights into Iron Porphyrin-Catalyzed Olefin Epoxidation by Hydrogen Peroxide: Factors Controlling Activity and Selectivity. J. Mol. Catal. A Chem. 2007, 275, 54–62. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, X.; Cui, Y.; Luo, T.; Tang, X.; Wang, T.; Qian, W.; Wei, F. High Yield Production of C2-C3 Olefins and: Para- Xylene from Methanol Using a SiO2-Coated FeOx/ZSM-5 Catalyst. RSC Adv. 2017, 7, 28940–28944. [Google Scholar] [CrossRef]
- Guisnet, M.; De Poitiers, U.; Pineau, R.; Juin, M. Chapter 1. Introduction to Zeolite Science and Technology. In Zeolites for Cleaner Technologies; Imperial College Press: London, UK, 1974. [Google Scholar]
- Palčić, A.; Valtchev, V. Analysis and Control of Acid Sites in Zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Macina, D.; Piwowarska, Z.; Góra-Marek, K.; Tarach, K.; Rutkowska, M.; Girman, V.; Błachowski, A.; Chmielarz, L. SBA-15 Loaded with Iron by Various Methods as Catalyst for DeNOx Process. Mater. Res. Bull. 2016, 78, 72–82. [Google Scholar] [CrossRef]
- Rutkowska, M.; Jankowska, A.; Różycka-Dudek, E.; Dubiel, W.; Kowalczyk, A.; Piwowarska, Z.; Llopis, S.; Díaz, U.; Chmielarz, L. Modification of Mcm-22 Zeolite and Its Derivatives with Iron for the Application in N2o Decomposition. Catalysts 2020, 10, 1139. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The Opportunities and Challenges of Iron-Zeolite as NH3-SCR Catalyst in Purification of Vehicle Exhaust. Appl. Catal. A Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Ryu, T.; Hong, S.B. Iron-Exchanged UZM-35: An Active NH3-SCR Catalyst at Low Temperatures. Appl. Catal. B Environ. 2020, 266, 118622. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Chang, H.; Ma, L.; Qu, Z.; Yan, N.; Wang, C.; Li, J. Improvement of the Activity of γ-Fe2O3 for the Selective Catalytic Reduction of NO with NH3 at High Temperatures: NO Reduction versus NH3 Oxidization. Ind. Eng. Chem. Res. 2013, 52, 5601–5610. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Wojciechowska, M.; Boroń, P.; Dudek, B.; Michalik, M. Montmorillonite Intercalated with SiO2, SiO2-Al2O3 or SiO2-TiO2 Pillars by Surfactant-Directed Method as Catalytic Supports for DeNOx Process. Chem. Pap. 2014, 68, 1219–1227. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The Determination of the Activities of Different Iron Species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and Mechanistic Aspects of the Selective Catalytic Reduction of NO(x) by Ammonia over Oxide Catalysts: A Review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Koebel, M.; Madia, G.; Elsener, M. Selective Catalytic Reduction of NO and N2O at Low Temperatures. Catal. Today 2002, 73, 239–247. [Google Scholar] [CrossRef]
- Delahay, G.; Mauvezin, M.; Coq, B.; Kieger, S. Selective Catalytic Reduction of Nitrous Oxide by Ammonia on Iron Zeolite Beta Catalysts in an Oxygen Rich Atmosphere: Effect of Iron Contents. J. Catal. 2001, 202, 156–162. [Google Scholar] [CrossRef]
- Ben Younes, N.; Ortigosa, J.M.; Marie, O.; Blasco, T.; Mhamdi, M. Effect of Zeolite Structure on the Selective Catalytic Reduction of NO with Ammonia over Mn-Fe Supported on ZSM-5, BEA, MOR and FER. Res. Chem. Intermed. 2021, 2, e04382. [Google Scholar] [CrossRef]
- Giakoumelou, I.; Fountzoula, C.; Kordulis, C.; Boghosian, S. Molecular Structure and Catalytic Activity of V2O5/TiO2 Catalysts for the SCR of NO by NH3: In Situ Raman Spectra in the Presence of O2, NH3, NO, H2, H2O, and SO2. J. Catal. 2006, 239, 1–12. [Google Scholar] [CrossRef]
- Topsoe, N.-Y.; Dumesic, J.A.; Topsøe, H. Vanadia/Titania Catalysts for Selective Catalytic Reduction of NItric Oxide by Ammonia II. Stud. Act. Sites Formul. Catal. Cycles 1995, 1, 241–252. [Google Scholar]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-Shaped Fe2O3 as an Efficient Catalyst for the Selective Reduction of Nitrogen Oxide by Ammonia. Angew. Chem.—Int. Ed. 2012, 51, 2989–2993. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Z.; Chen, B.; Guo, Q.; Liu, N. Adsorption of NO and N 2 O on Fe-BEA and H-BEA Zeolites. Appl. Surf. Sci. 2010, 256, 4042–4047. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Characterization of Fe-ZSM-5 Catalyst for Selective Catalytic Reduction of Nitric Oxide by Ammonia. J. Catal. 2000, 194, 80–90. [Google Scholar] [CrossRef]
- Schwidder, M.; Santhosh Kumar, M.; Bentrup, U.; Pérez-Ramírez, J.; Brückner, A.; Grünert, W. The Role of Brønsted Acidity in the SCR of NO over Fe-MFI Catalysts. Microporous Mesoporous Mater. 2008, 111, 124–133. [Google Scholar] [CrossRef]
- Amores, J.M.G.; Sanchez Escribano, V.; Ramis, G.; Busca, G. An FT-IR Study of Ammonia Adsorption and Oxidation over Anatase-Supported Metal Oxides. Appl. Catal. B Environ. 1997, 13, 45–58. [Google Scholar] [CrossRef]
- Brandenberger, S.; Kröcher, O.; Wokaun, A.; Tissler, A.; Althoff, R. The Role of Brønsted Acidity in the Selective Catalytic Reduction of NO with Ammonia over Fe-ZSM-5. J. Catal. 2009, 268, 297–306. [Google Scholar] [CrossRef]
- Liu, Z.; Millington, P.J.; Bailie, J.E.; Rajaram, R.R.; Anderson, J.A. A Comparative Study of the Role of the Support on the Behaviour of Iron Based Ammonia SCR Catalysts. Microporous Mesoporous Mater. 2007, 104, 159–170. [Google Scholar] [CrossRef]
- Qu, W.; Chen, Y.; Huang, Z.; Gao, J.; Zhou, M.; Chen, J.; Li, C.; Ma, Z.; Chen, J.; Tang, X. Active Tetrahedral Iron Sites of γ-Fe2O3 Catalyzing NO Reduction by NH3. Environ. Sci. Technol. Lett. 2017, 4, 246–250. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Shan, W.; Shi, X. Mechanism of the Selective Catalytic Reduction of NOx with NH3 over Environmental-Friendly Iron Titanate Catalyst. Catal. Today 2011, 175, 18–25. [Google Scholar] [CrossRef]
- Apostolescu, N.; Geiger, B.; Hizbullah, K.; Jan, M.T.; Kureti, S.; Reichert, D.; Schott, F.; Weisweiler, W. Selective Catalytic Reduction of Nitrogen Oxides by Ammonia on Iron Oxide Catalysts. Appl. Catal. B Environ. 2006, 62, 104–114. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, R.T. N2O Formation Pathways over Zeolite-Supported Cu and Fe Catalysts in NH3-SCR. Energy Fuels 2018, 32, 2170–2182. [Google Scholar] [CrossRef]
- Corma, A.; Corell, C. Synthesis and Characterization of the MCM-22 Zeolite. Zeolites 1995, 15, 2–8. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Blanco, C.; Gil, A.; Vicente, M.Á.; Galeano, L.A. Preparation of Al/Fe-Pillared Clays: Effect of the Starting Mineral. Materials 2017, 10, 1364. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET Equation Applicable to Microporous Adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]



| Sample Code | Si (wt%) | Al (wt%) | Fe (wt%) | Si/Al |
|---|---|---|---|---|
| MCM-22 (P) | 33.23 | 1.41 | 0 | 23.0 |
| M36-Si | 39.47 | 2.08 | 0 | 29.0 |
| M36-SiAl | 36.58 | 4.74 | 0 | 7.5 |
| M36-SiAlFe | 34.01 | 6.09 | 5.21 | 5.4 |
| FeM22 (P) | 32.72 | 1.91 | 4.96 | 21.0 |
| FeM36-Si | 34.02 | 1.16 | 5.02 | 28.0 |
| FeM36-SiAl | 37.59 | 3.54 | 4.84 | 10.0 |
| Sample Code | SBET a (m2·g−1) | External Surface Area b (m2·g−1) | Micropore Area b (m2·g−1) | Total Pore Volume c (m3·g−1) | Micropore Volume b (m3·g−1) | Meso + Macropore Volume d (m3·g−1) |
|---|---|---|---|---|---|---|
| MCM-22 (P) | 569 | 141 | 434 | 0.480 | 0.172 | 0.308 |
| M36-Si | 410 | 213 | 197 | 0.315 | 0.084 | 0.231 |
| M36-SiAl | 363 | 203 | 160 | 0.285 | 0.069 | 0.216 |
| M36-SiAlFe | 716 | 507 | 209 | 0.740 | 0.162 | 0.578 |
| FeM22 (P) | 392 | 147 | 245 | 0.438 | 0.100 | 0.338 |
| FeM36-Si | 569 | 141 | 434 | 0.480 | 0.172 | 0.308 |
| FeM36-SiAl | 410 | 213 | 197 | 0.315 | 0.084 | 0.231 |
| Sample Code | Weight Loss (%) |
|---|---|
| M36-Si | 11.57 |
| M36-SiAl | 11.17 |
| M36-SiAlFe | 10.03 |
| FeM36-Si | 5.66 |
| FeM36-SiAl | 7.91 |
| Sample Code | Acid Site Density from NH3-TPD (µmol·g−1) a | Acid Site Density from Py-IR (µmol·g−1) | Accessibility c (%) | |||
|---|---|---|---|---|---|---|
| Total b | BASs b | LASs b | %BAS/%LAS | |||
| M36-Si | 1511 | 912 | 485 | 426 | 53/47 | 60 |
| M36-SiAl | 1609 | 878 | 386 | 492 | 44/56 | 55 |
| M36-SiAlFe | 1225 | 794 | 338 | 456 | 43/57 | 65 |
| FeM36-Si | 1253 | 570 | 112 | 458 | 20/80 | 45 |
| FeM36-SiAl | 1326 | 651 | 164 | 488 | 25/75 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymaszek-Wawryca, A.; Díaz, U.; Samojeden, B.; Motak, M. Synthesis, Characterization, and NH3-SCR Catalytic Performance of Fe-Modified MCM-36 Intercalated with Various Pillars. Molecules 2023, 28, 4960. https://doi.org/10.3390/molecules28134960
Szymaszek-Wawryca A, Díaz U, Samojeden B, Motak M. Synthesis, Characterization, and NH3-SCR Catalytic Performance of Fe-Modified MCM-36 Intercalated with Various Pillars. Molecules. 2023; 28(13):4960. https://doi.org/10.3390/molecules28134960
Chicago/Turabian StyleSzymaszek-Wawryca, Agnieszka, Urbano Díaz, Bogdan Samojeden, and Monika Motak. 2023. "Synthesis, Characterization, and NH3-SCR Catalytic Performance of Fe-Modified MCM-36 Intercalated with Various Pillars" Molecules 28, no. 13: 4960. https://doi.org/10.3390/molecules28134960
APA StyleSzymaszek-Wawryca, A., Díaz, U., Samojeden, B., & Motak, M. (2023). Synthesis, Characterization, and NH3-SCR Catalytic Performance of Fe-Modified MCM-36 Intercalated with Various Pillars. Molecules, 28(13), 4960. https://doi.org/10.3390/molecules28134960