Integration of Hybridization Strategies in Pyridine–Urea Scaffolds for Novel Anticancer Agents: Design, Synthesis, and Mechanistic Insights
Abstract
1. Introduction
2. Results and Discussions
2.1. Chemistry
2.2. Biological Evaluation
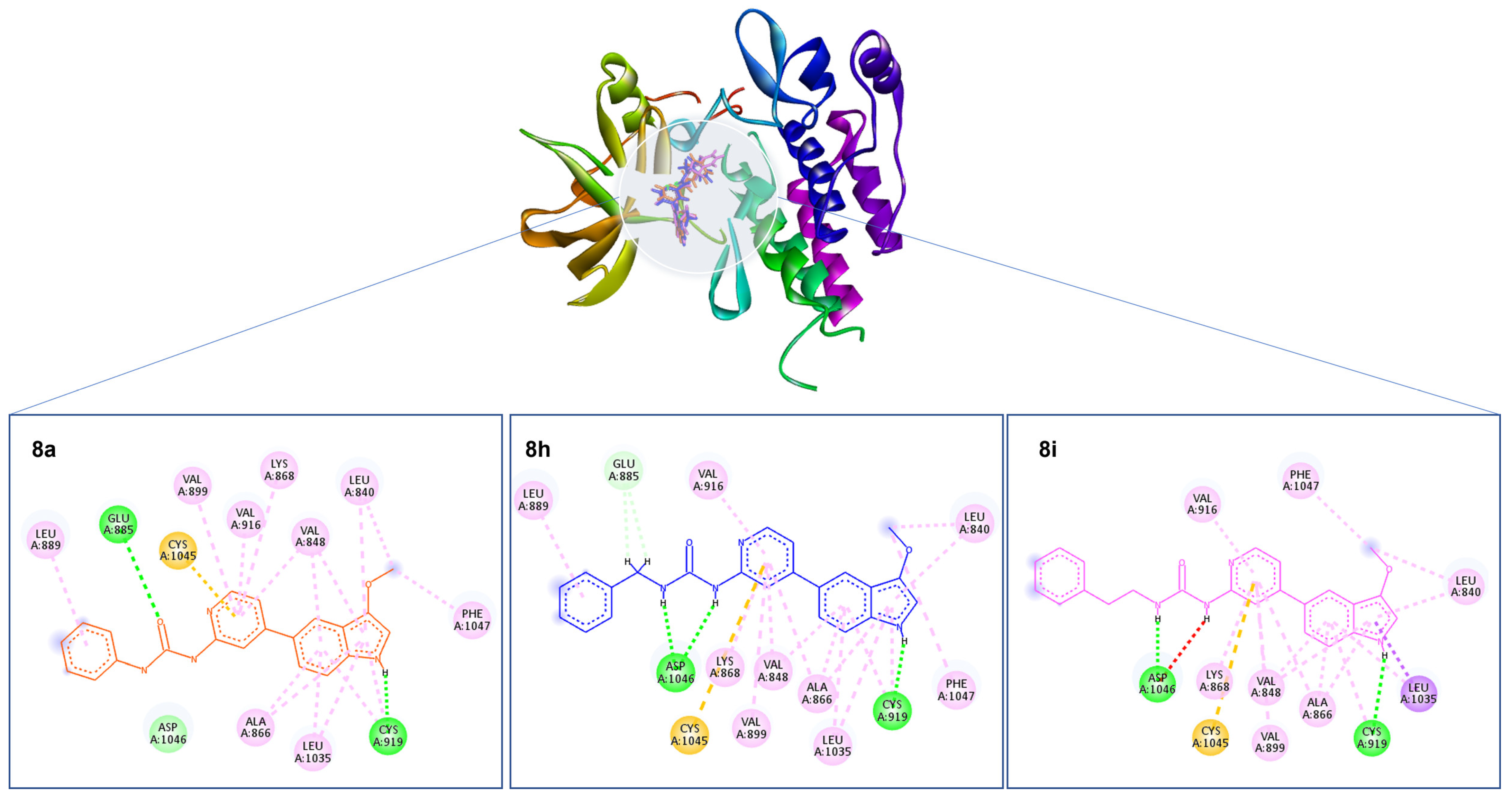
2.3. Molecular Docking
2.4. MD Simulations
2.5. MM–GBSA
2.6. In Silico Pharmacokinetic Study
3. Conclusions
4. Methodology
4.1. Chemistry
4.2. Biology
4.2.1. Cell Culture
4.2.2. SRB Assay of Antiproliferative Activity
4.2.3. SRB Assay of Antiproliferative Activity on Normal Cells
4.3. Molecular Docking
4.4. MD Calculations
4.5. Molecular Mechanics–Generalized Born Surface Area (MM–GBSA) Calculations
4.6. In Silico Pharmacokinetic Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Melo, A.P.S.; Dippenaar, I.N.; Johnson, S.C.; Weaver, N.D.; de Assis Acurcio, F.; Malta, D.C.; Ribeiro, A.L.P.; Júnior, A.A.G.; Wool, E.E.; Naghavi, M.; et al. All-cause and cause-specific mortality among people with severe mental illness in Brazil’s public health system, 2000–2015: A retrospective study. Lancet Psychiatry 2022, 9, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.H. Global challenges in breast cancer detection and treatment. Breast 2022, 62, S3–S6. [Google Scholar] [CrossRef]
- Fahrner, M.; Bronsert, P.; Fichtner-Feigl, S.; Jud, A.; Schilling, O. Proteome biology of primary colorectal carcinoma and corresponding liver metastases. Neoplasia 2021, 23, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Costarelli, L.; Cianchetti, E.; Corsi, F.; Friedman, D.; Ghilli, M.; Lacaria, M.; Menghini, L.; Murgo, R.; Ponti, A.; Rinaldi, S.; et al. Microinvasive breast carcinoma: An analysis from ten Senonetwork Italia breast centres. Eur. J. Surg. Oncol. 2019, 45, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Sharma, S.; Vora, J.; Shrivastava, N. Emerging role of sirtuins in breast cancer metastasis and multidrug resistance: Implication for novel therapeutic strategies targeting sirtuins. Pharmacol. Res. 2020, 158, 104880. [Google Scholar] [CrossRef]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef]
- Parmar, D.R.; Soni, J.Y.; Guduru, R.; Rayani, R.H.; Kusurkar, R.V.; Vala, A.G.; Talukdar, S.N.; Eissa, I.H.; Metwaly, A.M.; Khalil, A.; et al. Discovery of new anticancer thiourea-azetidine hybrids: Design, synthesis, in vitro antiproliferative, SAR, in silico molecular docking against VEGFR-2, ADMET, toxicity, and DFT studies. Bioorganic Chem. 2021, 115, 105206. [Google Scholar] [CrossRef]
- Bérubé, G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef]
- Helal, M.H.; El-Awdan, S.A.; Salem, M.A.; Abd-elaziz, T.A.; Moahamed, Y.A.; El-Sherif, A.A.; Mohamed, G.A. Synthesis, biological evaluation and molecular modeling of novel series of pyridine derivatives as anticancer, anti-inflammatory and analgesic agents. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2015, 135, 764–773. [Google Scholar] [CrossRef]
- Lu, T.; Goh, A.W.; Yu, M.; Adams, J.; Lam, F.; Teo, T.; Li, P.; Noll, B.; Zhong, L.; Diab, S.; et al. Discovery of (E)-3-((styrylsulfonyl)methyl)pyridine and (E)-2-((styrylsulfonyl)methyl)pyridine derivatives as anticancer agents: Synthesis, structure-activity relationships, and biological activities. J. Med. Chem. 2014, 57, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Nada, H.; Elkamhawy, A.; Lee, K. Structure Activity Relationship of Key Heterocyclic Anti-Angiogenic Leads of Promising Potential in the Fight against Cancer. Molecules 2021, 26, 553. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Kishbaugh, T.L. Pyridines and Imidazopyridines with Medicinal Significance. Curr. Top. Med. Chem. 2016, 16, 3274–3302. [Google Scholar] [CrossRef]
- Gould, S.E.; Low, J.A.; Marsters, J.C.; Robarge, K.; Rubin, L.L.; de Sauvage, F.J.; Sutherlin, D.P.; Wong, H.; Yauch, R.L. Discovery and preclinical development of vismodegib. Expert Opin. Drug Discov. 2014, 9, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Seufferlein, T. Regorafenib. In Small Molecules in Oncology; Martens, U.M., Ed.; Springer International Publishing: New York, NY, USA, 2018; pp. 45–56. [Google Scholar]
- Li, H.Q.; Lv, P.C.; Yan, T.; Zhu, H.L. Urea derivatives as anticancer agents. Anti Cancer Agents Med. Chem. 2009, 9, 471–480. [Google Scholar] [CrossRef]
- Eze, C.C.; Ezeokonkwo, A.M.; Ugwu, I.D.; Eze, U.F.; Onyeyilim, E.L.; Attah, I.S.; Okonkwo, I.V. Azole-Pyrimidine Hybrid Anticancer Agents: A Review of Molecular Structure, Structure Activity Relationship, and Molecular Docking. Anti Cancer Agents Med. Chem. 2022, 22, 2822–2851. [Google Scholar] [CrossRef]
- Kaur, K.; Utreja, D.; Dhillon, N.K.; Anupam; Buttar, H.S. Heterocyclic Moieties as Prospective Nematicides: An Overview. Curr. Org. Chem. 2022, 26, 1703–1724. [Google Scholar]
- Dungo, R.T.; Keating, G.M. Afatinib: First Global Approval. Drugs 2013, 73, 1503–1515. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, C.; Wang, C.; Zhou, X.; Li, W.; Zhang, J.-Y.; Wang, M.; Xu, Y.; Sun, L.-P. Design, synthesis and anticancer evaluation of 3-methyl-1H-indazole derivatives as novel selective bromodomain-containing protein 4 inhibitors. Bioorganic Med. Chem. 2022, 55, 116592. [Google Scholar] [CrossRef]
- Elkaeed, E.B.; Yousef, R.G.; Elkady, H.; Gobaara, I.M.M.; Alsfouk, B.A.; Husein, D.Z.; Ibrahim, I.M.; Metwaly, A.M.; Eissa, I.H. Design, Synthesis, Docking, DFT, MD Simulation Studies of a New Nicotinamide-Based Derivative: In Vitro Anticancer and VEGFR-2 Inhibitory Effects. Molecules 2022, 27, 4606. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.M.; El-Gaby, M.S.A.; El-Adl, K.; Abd El-Sattar, N.E.A. Design, green synthesis, molecular docking and anticancer evaluations of diazepam bearing sulfonamide moieties as VEGFR-2 inhibitors. Bioorganic Chem. 2020, 104, 104350. [Google Scholar] [CrossRef]
- Van Gool, M.L.M. Preparation of 6,7-Dihydropyrazolo[1,5-a]Pyrazin-4(5h)-one Compounds and Their Use as Negative Allosteric Modulators of mGluR2 Receptors; World Intellectual Property Organization: Geneva, Switzerland, 2014; WO2014195311 A1. [Google Scholar]
- Smith, N.D. Preparation of Cyclohexanecarboxamide Derivatives as Farnesoid X Receptor Agonists and Uses Thereof; World Intellectual Property Organization: Geneva, Switzerland, 2020; WO2020061116 A1. [Google Scholar]
- Allegretti, P.A.; Horton, T.M.; Abdolazimi, Y.; Moeller, H.P.; Yeh, B.; Caffet, M.; Michel, G.; Smith, M.; Annes, J.P. Generation of highly potent DYRK1A-dependent inducers of human β-Cell replication via Multi-Dimensional compound optimization. Bioorganic Med. Chem. 2020, 28, 115193. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Li, N.; Qiu, X.; Ye, Y.; Chen, H.; Hua, J.; Yin, P.; Zhuang, G. TIPE regulates VEGFR2 expression and promotes angiogenesis in colorectal cancer. Int. J. Biol. Sci. 2020, 16, 272–283. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, L.; Li, Y.; Zhao, X.; Sun, B. VEGFR2 regulates endothelial differentiation of colon cancer cells. BMC Cancer 2017, 17, 593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, X.; Zhu, Y.; Kadel, D.; Sun, H.; Chen, J.; Luo, Q.; Sun, H.; Yang, L.; Yang, J.; et al. The dual blockade of MET and VEGFR2 signaling demonstrates pronounced inhibition on tumor growth and metastasis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 93. [Google Scholar] [CrossRef]
- Goyal, L.; Muzumdar, M.D.; Zhu, A.X. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 2310–2318. [Google Scholar] [CrossRef]
- Zengin, M.; Unsal Tan, O.; Arafa, R.K.; Balkan, A. Design and synthesis of new 2-oxoquinoxalinyl-1,2,4-triazoles as antitumor VEGFR-2 inhibitors. Bioorganic Chem. 2022, 121, 105696. [Google Scholar] [CrossRef]
- Lee, A.; Lee, K.; Kim, D. Using reverse docking for target identification and its applications for drug discovery. Expert Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef]
- Nada, H.; Sivaraman, A.; Lu, Q.; Min, K.; Kim, S.; Goo, J.-I.; Choi, Y.; Lee, K. Perspective for Discovery of Small Molecule IL-6 Inhibitors through Study of Structure-Activity Relationships and Molecular Docking. J. Med. Chem. 2023, 66, 4417–4433. [Google Scholar] [CrossRef]
- Rampogu, S.; Baek, A.; Zeb, A.; Lee, K.W. Exploration for novel inhibitors showing back-to-front approach against VEGFR-2 kinase domain (4AG8) employing molecular docking mechanism and molecular dynamics simulations. BMC Cancer 2018, 18, 264. [Google Scholar] [CrossRef]
- Demapan, D.; Kussmann, J.; Ochsenfeld, C.; Cui, Q. Factors That Determine the Variation of Equilibrium and Kinetic Properties of QM/MM Enzyme Simulations: QM Region, Conformation, and Boundary Condition. J. Chem. Theory Comput. 2022, 18, 2530–2542. [Google Scholar] [CrossRef]
- Nowroozi, A.; Rad, O.R. A comparative study of cooperative effects between the intramolecular hydrogen bond and cation···π interaction in various complexes of ortho-aminobenzaldehyde with its thio and seleno analogous. Theor. Chem. Acc. 2017, 136, 23. [Google Scholar] [CrossRef]
- Guterres, H.; Im, W. Improving Protein-Ligand Docking Results with High-Throughput Molecular Dynamics Simulations. J. Chem. Inf. Model. 2020, 60, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Onufriev, A.; Bashford, D.; Case, D.A. Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 2004, 55, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Nada, H.; Lee, K.; Gotina, L.; Pae, A.N.; Elkamhawy, A. Identification of novel discoidin domain receptor 1 (DDR1) inhibitors using E-pharmacophore modeling, structure-based virtual screening, molecular dynamics simulation and MM-GBSA approaches. Comput. Biol. Med. 2022, 142, 105217. [Google Scholar] [CrossRef]
- Schwartz, P.A.; Murray, B.W. Protein kinase biochemistry and drug discovery. Bioorganic Chem. 2011, 39, 192–210. [Google Scholar] [CrossRef]
- Sanphanya, K.; Wattanapitayakul, S.K.; Phowichit, S.; Fokin, V.V.; Vajragupta, O. Novel VEGFR-2 kinase inhibitors identified by the back-to-front approach. Bioorganic Med. Chem. Lett. 2013, 23, 2962–2967. [Google Scholar] [CrossRef]
- Bauer, D.; Whittington, D.A.; Coxon, A.; Bready, J.; Harriman, S.P.; Patel, V.F.; Polverino, A.; Harmange, J.-C. Evaluation of indazole-based compounds as a new class of potent KDR/VEGFR-2 inhibitors. Bioorganic Med. Chem. Lett. 2008, 18, 4844–4848. [Google Scholar] [CrossRef]
- Honda, T.; Nagahara, H.; Mogi, H.; Ban, M.; Aono, H. KDR inhibitor with the intramolecular non-bonded interaction: Conformation–activity relationships of novel indole-3-carboxamide derivatives. Bioorganic Med. Chem. Lett. 2011, 21, 1782–1785. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Vascular Endothelial Growth Factor Receptor (VEGFR-2)/KDR Inhibitors: Medicinal Chemistry Perspective. Med. Drug Discov. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Machado, V.A.; Peixoto, D.; Costa, R.; Froufe, H.J.C.; Calhelha, R.C.; Abreu, R.M.V.; Ferreira, I.C.F.R.; Soares, R.; Queiroz, M.-J.R.P. Synthesis, antiangiogenesis evaluation and molecular docking studies of 1-aryl-3-[(thieno[3,2-b]pyridin-7-ylthio)phenyl]ureas: Discovery of a new substitution pattern for type II VEGFR-2 Tyr kinase inhibitors. Bioorganic Med. Chem. 2015, 23, 6497–6509. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecher, T.; Labahn, A. Towards Accurate Free Energy Calculations in Ligand Protein-Binding Studies. Curr. Med. Chem. 2010, 17, 767–785. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M. In silico docking and ADME study of deketene curcumin derivatives (DKC) as an aromatase inhibitor or antagonist to the estrogen-alpha positive receptor (Erα+): Potent application of breast cancer. Struct. Chem. 2022, 33, 571–600. [Google Scholar] [CrossRef] [PubMed]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Elsherbeny, M.H.; Elkamhawy, A.; Nada, H.; Abdellattif, M.H.; Lee, K.; Roh, E.J. Development of New Meridianin/Leucettine-Derived Hybrid Small Molecules as Nanomolar Multi-Kinase Inhibitors with Antitumor Activity. Biomedicines 2021, 9, 1131. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, N.; Kim, S.J.; Song, J.; Gong, Y.D.; Kim, S.Y. Anti-cancer effect of a quinoxaline derivative GK13 as a transglutaminase 2 inhibitor. J. Cancer Res. Clin. Oncol. 2013, 139, 1279–1294. [Google Scholar] [CrossRef]
- McTigue, M.; Murray, B.W.; Chen, J.H.; Deng, Y.-L.; Solowiej, J.; Kania, R.S. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc. Natl. Acad. Sci. USA 2012, 109, 18281–18289. [Google Scholar] [CrossRef]
- Nada, H.; Kim, S.; Godesi, S.; Lee, J.; Lee, K. Discovery and optimization of natural-based nanomolar c-Kit inhibitors via in silico and in vitro studies. J. Biomol. Struct. Dyn. 2023, 1–12. [Google Scholar] [CrossRef]
- Nada, H.; Elkamhawy, A.; Lee, K. Identification of 1H-purine-2,6-dione derivative as a potential SARS-CoV-2 main protease inhibitor: Molecular docking, dynamic simulations, and energy calculations. PeerJ 2022, 10, e14120. [Google Scholar] [CrossRef] [PubMed]
- Greenidge, P.A.; Kramer, C.; Mozziconacci, J.-C.; Wolf, R.M. MM/GBSA Binding Energy Prediction on the PDBbind Data Set: Successes, Failures, and Directions for Further Improvement. J. Chem. Inf. Model. 2013, 53, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, A.; Al-Mulla, F.; Wei, D.-Q.; Abubaker, J. Remdesivir MD Simulations Suggest a More Favourable Binding to SARS-CoV-2 RNA Dependent RNA Polymerase Mutant P323L Than Wild-Type. Biomolecules 2021, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Dominy, B.N. Molecular Recognition and Binding Free Energy Calculations in Drug Development. Curr. Pharm. Biotechnol. 2008, 9, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
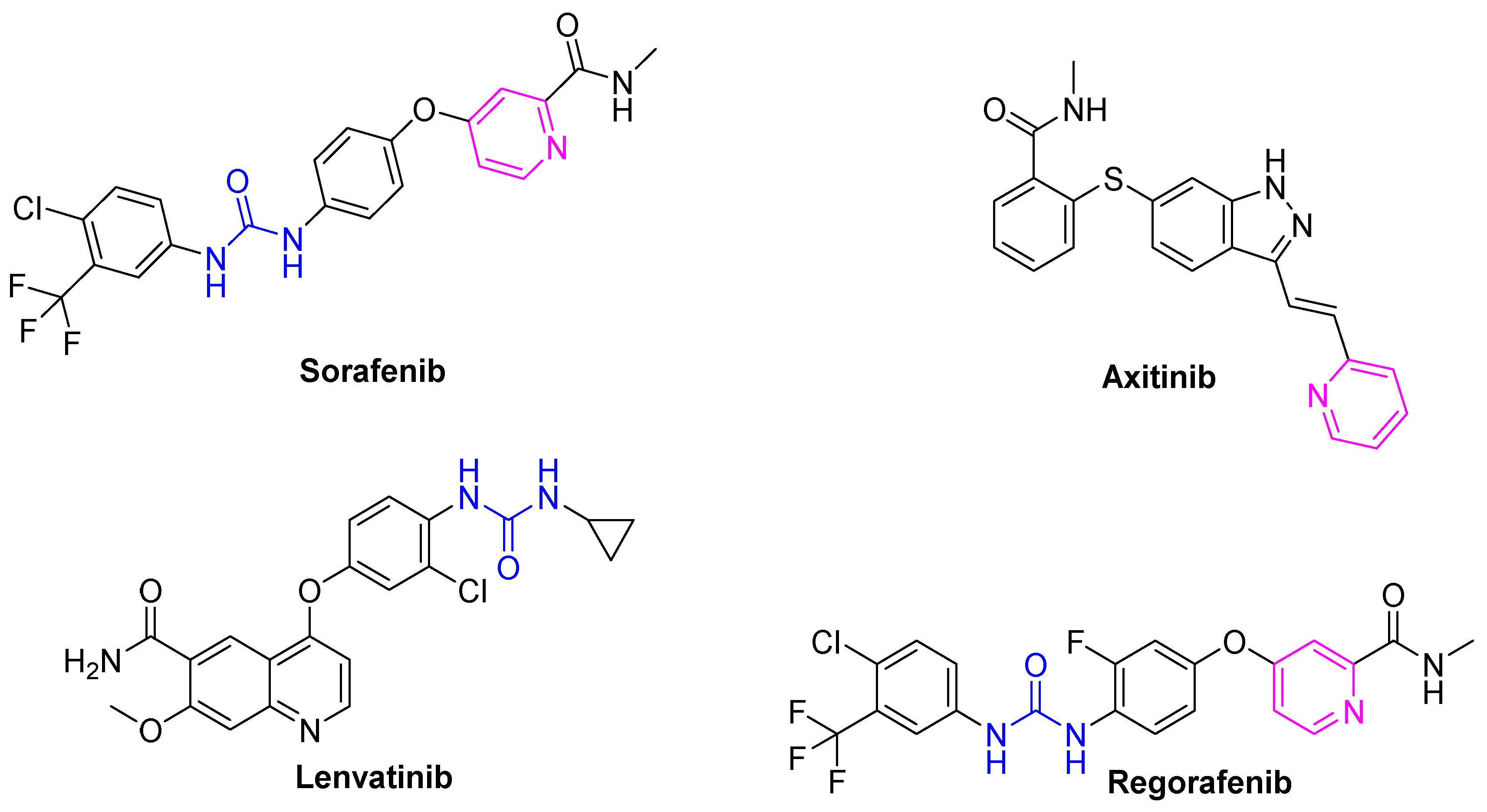
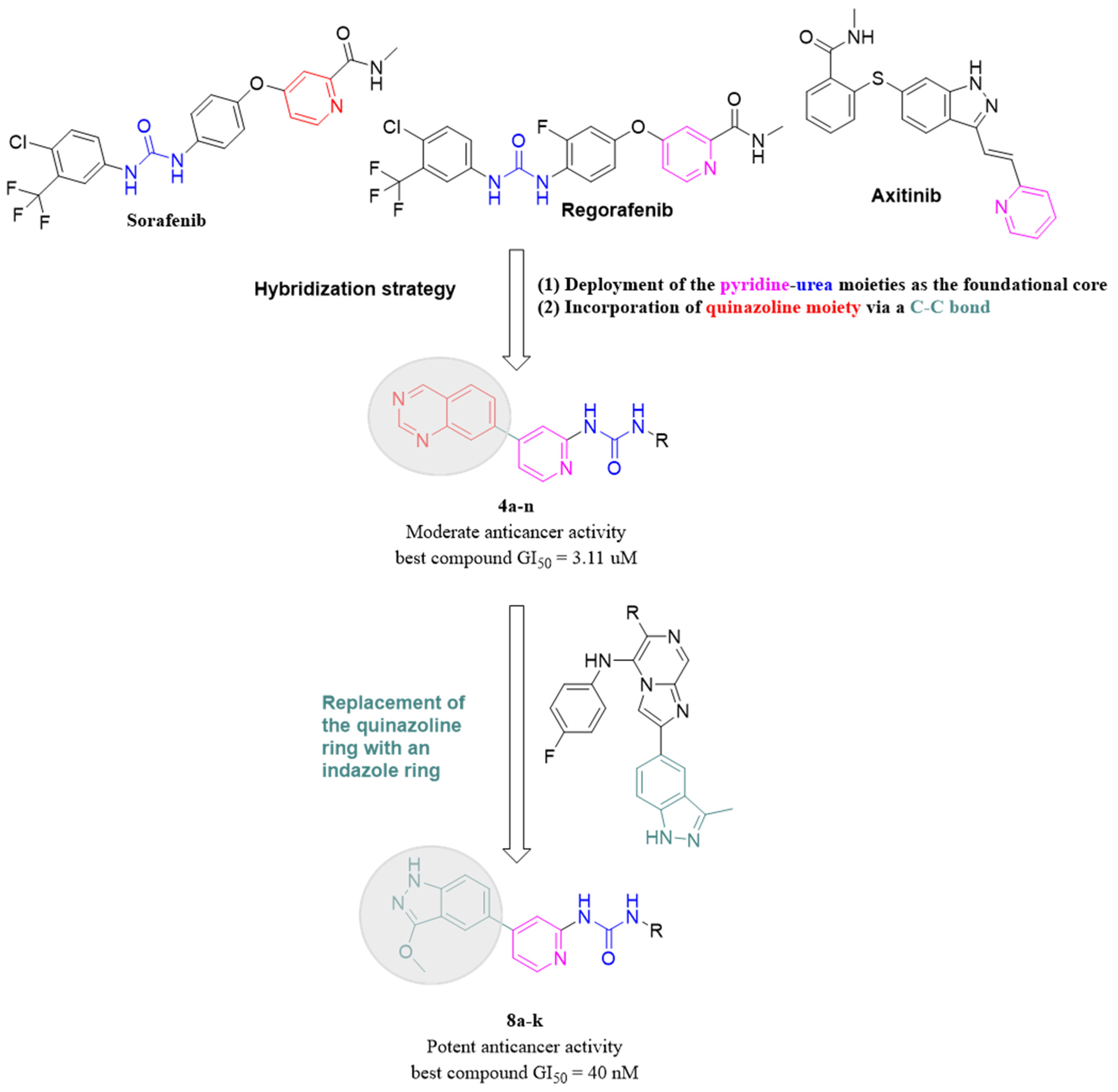
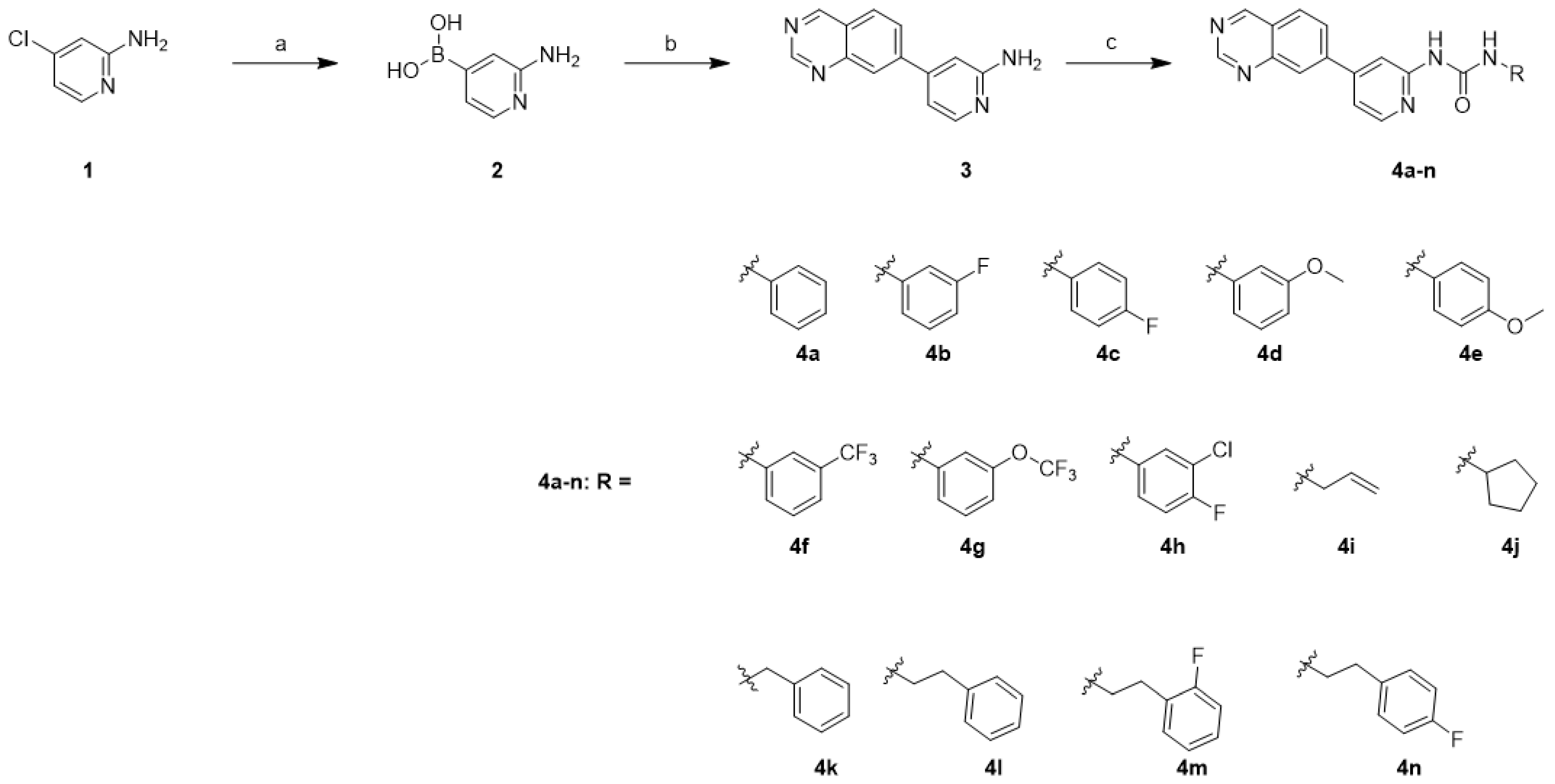
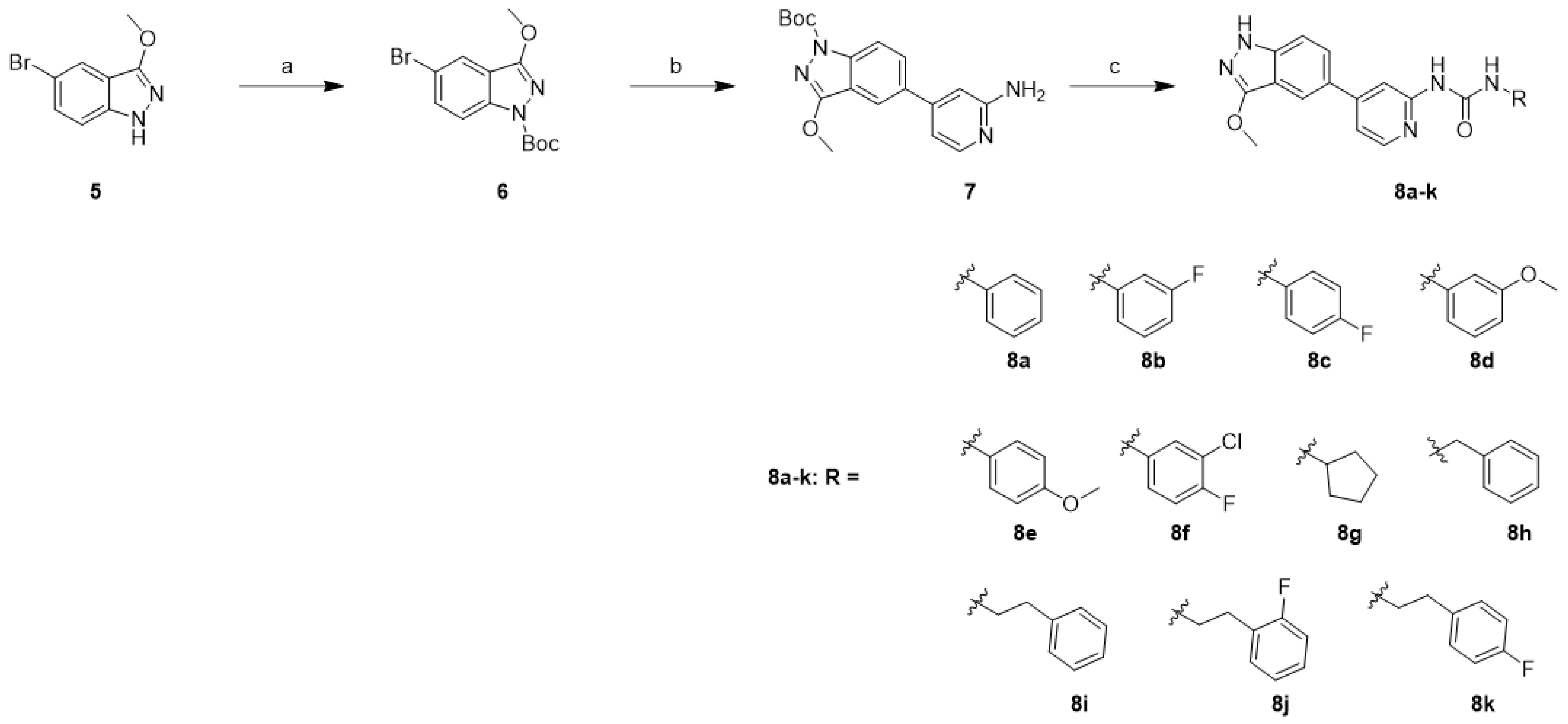

| Compd. | GI50 (μM) * | |
|---|---|---|
| MCF7 | HCT116 | |
| 4a | >10 | >10 |
| 4b | >10 | >10 |
| 4c | >10 | >10 |
| 4d | >10 | >10 |
| 4e | >10 | >10 |
| 4f | >10 | >10 |
| 4g | >10 | >10 |
| 4h | >10 | >10 |
| 4i | 3.35 ± 0.101 | >10 |
| 4j | >10 | >10 |
| 4k | 3.03 ± 0.061 | >10 |
| 4l | 9.00 ± 0.347 | >10 |
| 4m | >10 | >10 |
| 4n | >10 | >10 |
| 8a | 0.06 ± 0.014 | 1.19 ± 0.035 |
| 8b | >10 | >10 |
| 8c | 3.73 ± 0.075 | 1.22 ± 0.141 |
| 8d | 1.89 ± 0.26 | >10 |
| 8e | 4.37 ± 0.359 | >10 |
| 8f | 0.89 ± 0.209 | 0.92 ± 0.114 |
| 8g | 1.17 ± 0.059 | 1.25 ± 0.037 |
| 8h | 0.23 ± 0.015 | 0.33 ± 0.042 |
| 8i | 0.16 ± 0.013 | 1.08 ± 0.006 |
| 8j | 0.75 ± 0.142 | >10 |
| 8k | 1.13 ± 0.003 | 1.38 ± 0.086 |
| Sorafenib | 11.2 [31] | - |
| Irinotecan | 0.35 ± 0.069 | 0.62 ± 0.003 |
| Complex Name | MM-GBSA (kcal/mol) | ||||
|---|---|---|---|---|---|
| Average ΔGBind | Average ΔGCoulomb | Average ΔGH_bond | Average ΔGLipo | Average ΔGSolv_GB | |
| Compound 8a–VEGFR2 complex | −55.64 | −9.25 | −0.88 | −21.71 | 25.87 |
| Compound 8h–VEGFR2 complex | −48.78 | −9.65 | −0.84 | −20.40 | 27.55 |
| Compound 8i–VEGFR2 complex | −59.55 | −16.67 | −1.17 | −20.86 | 30.61 |
| Compound | Lipinski Violations | Solubility in Water | BBB Permeant | GI Absorption |
|---|---|---|---|---|
| 8a | 0 | Moderately soluble | No | High |
| 8h | 0 | Moderately soluble | No | High |
| 8i | 0 | Moderately soluble | No | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godesi, S.; Nada, H.; Lee, J.; Kang, J.-H.; Kim, S.-Y.; Choi, Y.; Lee, K. Integration of Hybridization Strategies in Pyridine–Urea Scaffolds for Novel Anticancer Agents: Design, Synthesis, and Mechanistic Insights. Molecules 2023, 28, 4952. https://doi.org/10.3390/molecules28134952
Godesi S, Nada H, Lee J, Kang J-H, Kim S-Y, Choi Y, Lee K. Integration of Hybridization Strategies in Pyridine–Urea Scaffolds for Novel Anticancer Agents: Design, Synthesis, and Mechanistic Insights. Molecules. 2023; 28(13):4952. https://doi.org/10.3390/molecules28134952
Chicago/Turabian StyleGodesi, Sreenivasulu, Hossam Nada, Joohan Lee, Joon-Hee Kang, Soo-Youl Kim, Yongseok Choi, and Kyeong Lee. 2023. "Integration of Hybridization Strategies in Pyridine–Urea Scaffolds for Novel Anticancer Agents: Design, Synthesis, and Mechanistic Insights" Molecules 28, no. 13: 4952. https://doi.org/10.3390/molecules28134952
APA StyleGodesi, S., Nada, H., Lee, J., Kang, J.-H., Kim, S.-Y., Choi, Y., & Lee, K. (2023). Integration of Hybridization Strategies in Pyridine–Urea Scaffolds for Novel Anticancer Agents: Design, Synthesis, and Mechanistic Insights. Molecules, 28(13), 4952. https://doi.org/10.3390/molecules28134952






