Phase-Controlled Cobalt Catalyst Boosting Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran
Abstract
1. Introduction
2. Results and Discussion
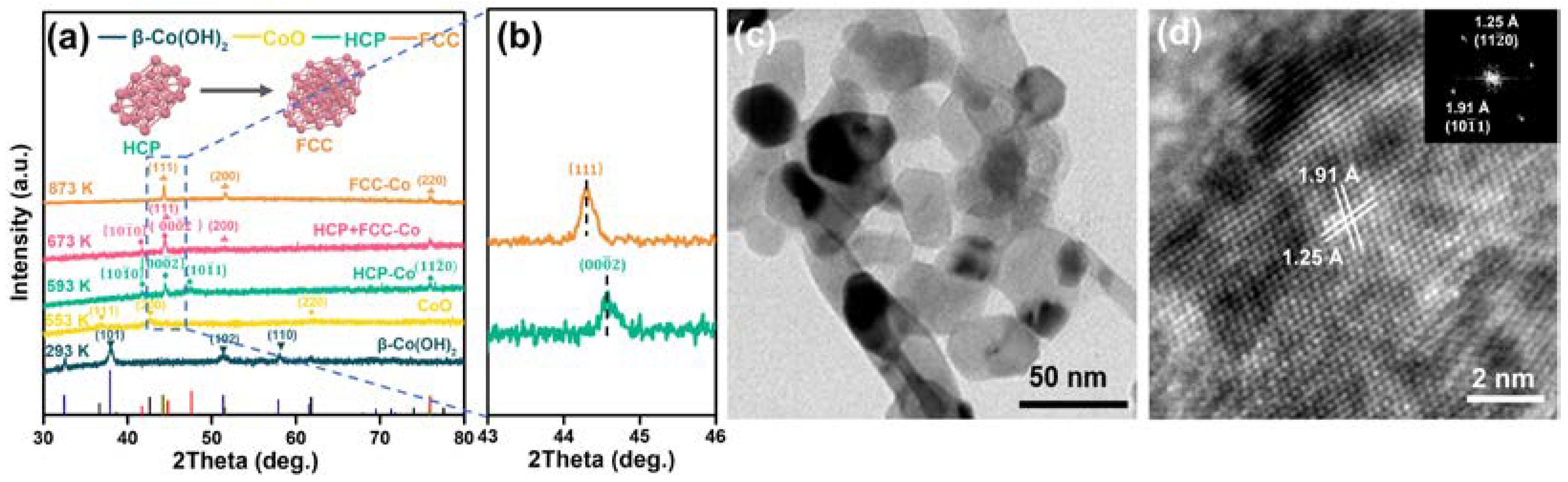
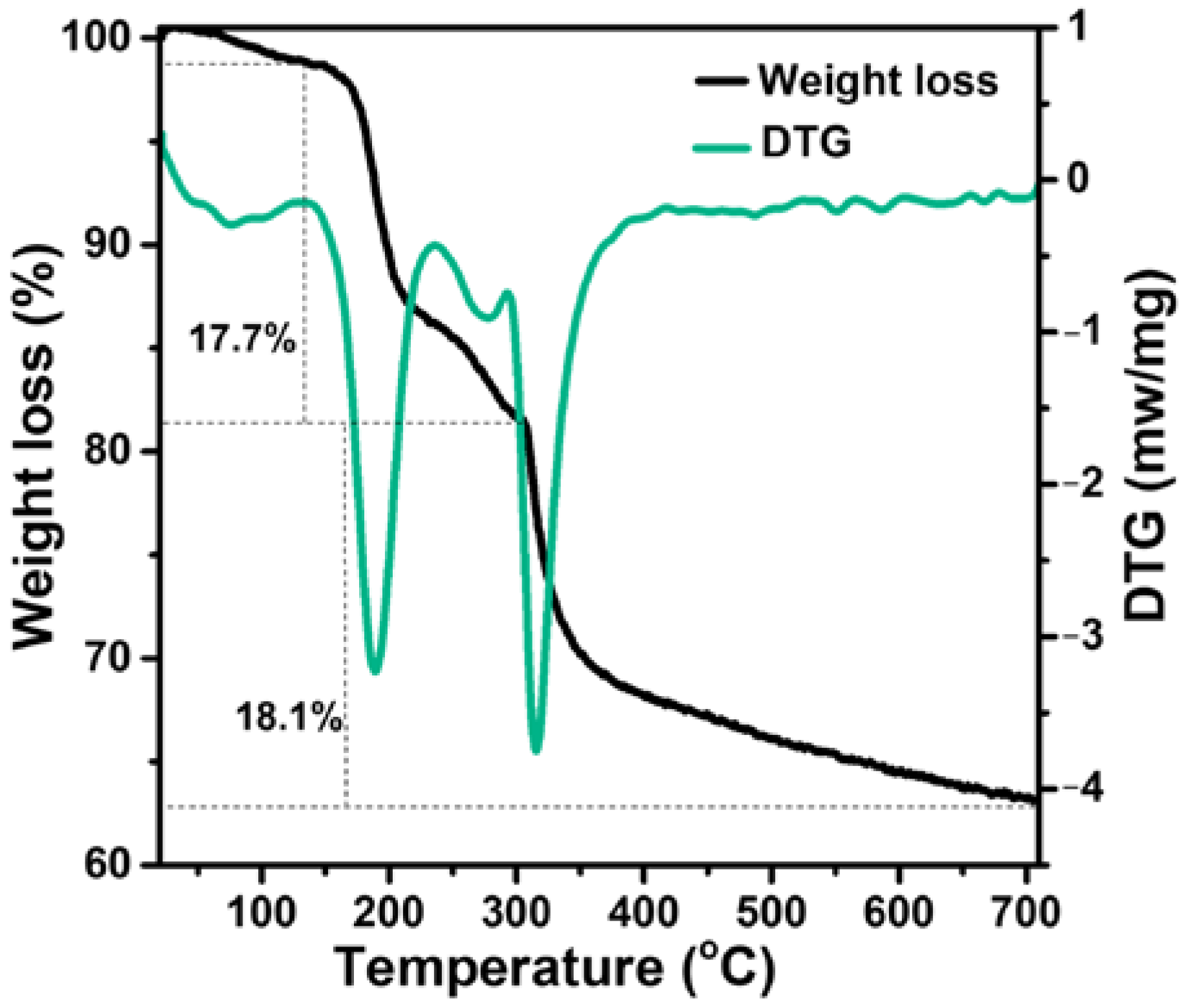
2.1. Characterization of Catalysts
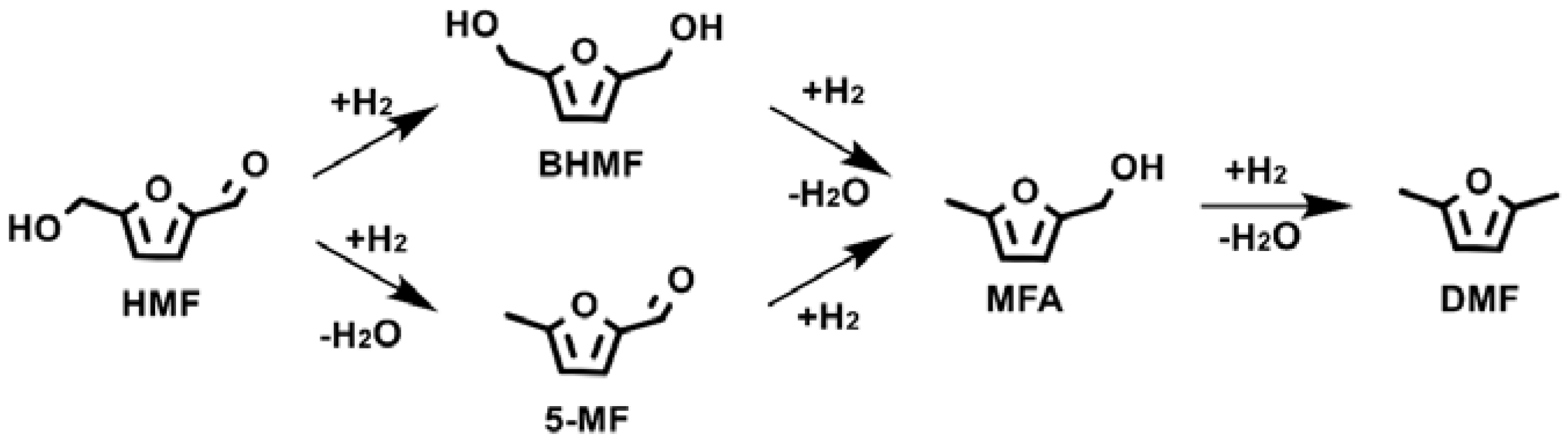
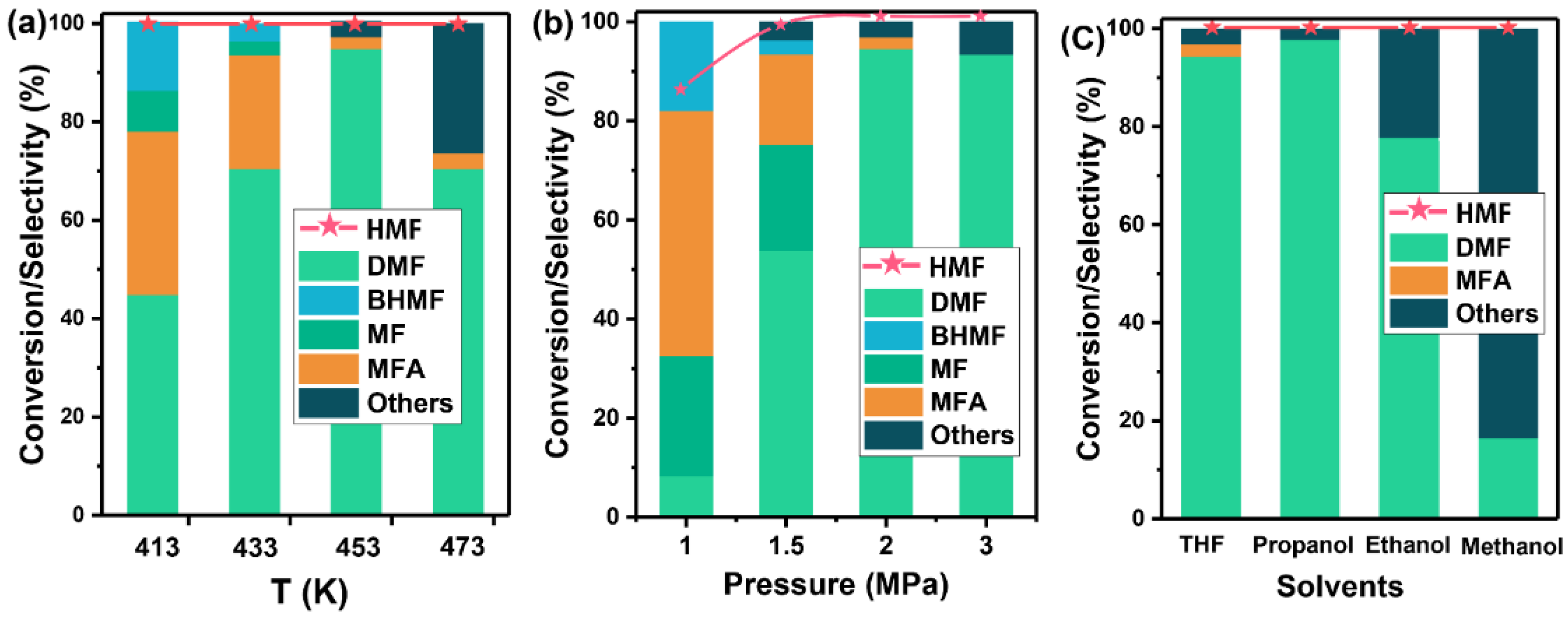
2.2. Hydrogenation Reaction over Cobalt Catalysts
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterizations
3.4. Catalytic Testing
3.5. Theoretical Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Van Ruijven, B.J.; De Cian, E.; Sue Wing, I. Amplification of future energy demand growth due to climate change. Nat. Commun. 2019, 10, 2762. [Google Scholar] [CrossRef]
- Favero, A.; Daigneault, A.; Sohngen, B. Forests: Carbon sequestration, biomass energy, or both? Sci. Adv. 2020, 6, eaay6792. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 192–200. [Google Scholar] [CrossRef]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Wang, R.; Li, Q.; Huang, B.; Guo, X.; Zhu, Z.; Su, T.; Lü, H. Synthesis of Dual-Active-Sites Ni-Ni2In catalysts for selective hydrogenation of furfural to furfuryl alcohol. Fuel 2022, 325, 124898. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, K.; Xu, H.; Zhu, L.; Wang, S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew. Sustain. Energy Rev. 2021, 139, 110706. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Zhang, Q.; Zhu, W. Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renew. Sustain. Energy Rev. 2020, 120, 109612. [Google Scholar] [CrossRef]
- Xia, H.; Xu, S.; Hu, H.; An, J.; Li, C. Efficient conversion of 5-hydroxymethylfurfural to high-value chemicals by chemo- and bio-catalysis. RSC Adv. 2018, 8, 30875–30886. [Google Scholar] [CrossRef]
- Endot, N.A.; Junid, R.; Jamil, M.S.S. Insight into Biomass Upgrade: A Review on Hydrogenation of 5-Hydroxymethylfurfural (HMF) to 2,5-Dimethylfuran (DMF). Molecules 2021, 26, 6848. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Fu, J.; Miao, C.; Jia, S.; Yan, P.; Huang, J. Highly selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan over metal-oxide supported Pt catalysts: The role of basic sites. Appl. Catal. A-Gen. 2022, 643, 118762. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2, 5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Huang, B.; Zhang, L.; Ma, X.; Zhang, S.; Zhu, Z.; Lü, H.; Yang, K. Hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran via synergistic catalysis of Ni2In and acid-base sites. Appl. Surf. Sci. 2022, 604, 154579. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1, 6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Liu, S. Efficient production of furan derivatives from a sugar mixture by catalytic process. Energy Fuels 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pandey, A.; Huang, Z.; Luque, R.; Ng, K.H.; Papadopoulos, A.M.; Chen, W.H.; Rajamohan, S.; Hadiyanto, H.; Nguyen, X.P.; et al. Catalyst-based synthesis of 2, 5-dimethylfuran from carbohydrates as a sustainable biofuel production route. ACS Sustain. Chem. Eng. 2022, 10, 3079–3115. [Google Scholar] [CrossRef]
- Talpade, A.D.; Tiwari, M.S.; Yadav, G.D. Selective hydrogenation of bio-based 5-hydroxymethyl furfural to 2,5-dimethylfuran over magnetically separable Fe-Pd/C bimetallic nanocatalyst. Mol. Catal. 2019, 465, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Liu, R.; Guo, Y.; Chen, L.; Gao, H. Selective Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural over Catalyst of Palladium Immobilized on Amine-Functionalized Metal–Organic Frameworks. ACS Catal. 2014, 5, 722–733. [Google Scholar] [CrossRef]
- Ledesma, B.; Juárez, J.; Mazarío, J.; Domine, M.; Beltramone, A. Bimetallic platinum/iridium modified mesoporous catalysts applied in the hydrogenation of HMF. Catal. Today 2021, 360, 147–156. [Google Scholar] [CrossRef]
- Mitra, J.; Zhou, X.; Rauchfuss, T. Pd/C-catalyzed reactions of HMF: Decarbonylation, hydrogenation, and hydrogenolysis. Green Chem. 2015, 17, 307–313. [Google Scholar] [CrossRef]
- Marafi, M.; Furimsky, E. Hydroprocessing catalysts containing noble metals: Deactivation, regeneration, metals reclamation, and environment and safety. Energy Fuels 2017, 31, 5711–5750. [Google Scholar] [CrossRef]
- Chirik, P.; Morris, R. Getting down to earth: The renaissance of catalysis with abundant metals. Acc. Chem. Res. 2015, 48, 2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, C. Transition metal silicides: Fundamentals, preparation and catalytic applications. Catal. Sci. Technol. 2019, 9, 4785–4820. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, Z.; Ma, H.; Liao, W.; Lü, H. Catalytic upgrading of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran over Beta zeolite supported non-noble Co catalyst. Mol. Catal. 2020, 486, 110882. [Google Scholar] [CrossRef]
- Kouachi, K.; Lafaye, G.; Especel, C.; Cherifi, O.; Marécot, P. Effects of support and metal loading on the characteristics of Co based catalysts for selective hydrogenation of citral. J. Mol. Catal. A-Chem. 2008, 280, 52–60. [Google Scholar] [CrossRef]
- Sun, X.; Olivos-Suarez, A.I.; Osadchii, D.; Romero, M.J.V.; Kapteijn, F.; Gascon, J. Single cobalt sites in mesoporous N-doped carbon matrix for selective catalytic hydrogenation of nitroarenes. J. Catal. 2018, 357, 20–28. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Chen, Y.; Peng, Z.; Liang, C. Acid-tolerant intermetallic cobalt–nickel silicides as noble metal-like catalysts for selective hydrogenation of phthalic anhydride to phthalide. Catal. Sci. Technol. 2019, 9, 1108–1116. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Zheng, X.; Chen, Y.; Wu, W.; Qiu, J.; Zhao, X.; Zhao, X.; Dai, Y.; Zeng, J. Incorporating nitrogen atoms into cobalt nanosheets as a strategy to boost catalytic activity toward CO2 hydrogenation. Nat. Energy 2017, 2, 869–876. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, B.; Liang, X. Controlled over-growth for nail-like and urchin-like cobalt with enhanced CO hydrogenation activity. Appl. Surf. Sci. 2021, 537, 147931. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Shen, W. Tuning the catalytic behavior of metal nanoparticles: The issue of the crystal phase. Chin. J. Catal. 2015, 9, 1409–1418. [Google Scholar] [CrossRef]
- Song, S.; Wang, D.; Di, L.; Wang, C.; Dai, W.; Wu, G.; Guan, N.; Li, L. Robust cobalt oxide catalysts for controllable hydrogenation of carboxylic acids to alcohols. Chin. J. Catal. 2018, 39, 250–257. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Yang, H.; Wang, X.; Polo-Garzon, F.; Wu, Z.; Zhu, J.; Wang, J.; Liu, Y.; Shi, C.; et al. Crystallographic dependence of CO2 hydrogenation pathways over HCP-Co and FCC-Co catalysts. Appl. Catal. B-Environ. 2022, 315, 121529. [Google Scholar] [CrossRef]
- Xie, Z.; An, H.; Zhao, X.; Wang, Y. Influence of different microstructures of cobalt on the catalytic activity for amination of ethylene glycol: Comparison of HCP cobalt and FCC cobalt. Catal. Sci. Technol. 2022, 12, 3148–3157. [Google Scholar] [CrossRef]
- Lyu, S.; Wang, L.; Zhang, J.; Liu, C.; Sun, J.; Peng, B.; Wang, Y.; Rappe, K.G.; Zhang, Y.; Li, J. Role of active phase in Fischer–Tropsch synthesis: Experimental evidence of CO activation over single-phase cobalt catalysts. ACS Catal. 2018, 8, 7787–7798. [Google Scholar] [CrossRef]
- Liu, J.-X.; Su, H.-Y.; Sun, D.-P.; Zhang, B.-Y.; Li, W.-X. Crystallographic dependence of CO activation on cobalt catalysts: HCP versus FCC. J. Am. Chem. Soc. 2013, 135, 16284–16287. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, J.X.; Fan, H.J.; Li, W.X. Morphology Evolution of FCC and HCP Cobalt Induced by a CO Atmosphere from Ab Initio Thermodynamics. J. Phys. Chem. C 2020, 124, 23200–23209. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Chen, P.; Liu, J.X.; Su, H.Y.; Li, W.X. Influence of Cobalt Crystal Structures on Activation of Nitrogen Molecule: A First–Principles Study. J. Phys. Chem. C 2019, 123, 10956–10966. [Google Scholar] [CrossRef]
- Yi, D.; Min, Y.; Muzzi, B.; Marty, A.; Romana, I.; Fazzini, P.-F.; Blon, T.; Viau, G.; Serp, P.; Soulantica, K. Epsilon Cobalt Nanoparticles as Highly Performant Catalysts in Cinnamaldehyde Selective Hydrogenation. ACS Appl. Nano Mater. 2022, 5, 5498–5507. [Google Scholar] [CrossRef]
- Okoye-Chine, C.G.; Moyo, M.; Liu, X.; Hildebrandt, D. A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol. 2019, 192, 105–129. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, B.; Zhang, Z.; Lin, J. Silver-exchanged heteropolyacid catalyst (Ag1H2PW): An efficient heterogeneous catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose. J. Ind. Eng. Chem. 2015, 21, 1127–1131. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Batch reactor coupled with water permselective membrane: Study of glycerol etherification reaction with butanol. Chem. Eng. J. 2015, 282, 187–193. [Google Scholar] [CrossRef]
- Li, Q.; Man, P.; Yuan, L.; Zhang, P.; Li, Y.; Ai, S. Ruthenium supported on CoFe layered double oxide for selective hydrogenation of 5-hydroxymethylfurfural. Mol. Catal. 2017, 431, 32–38. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Yuan, F.; Niu, X.; Zhu, Y. Efficient production of the liquid fuel 2,5-dimethylfuran from 5-hydroxymethylfurfural in the absence of acid additive over bimetallic PdAu supported on graphitized carbon. Energy Fuels 2017, 31, 6364–6373. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chen, M.Y.; Yan, L.; Guo, Q.X.; Fu, Y. Nickel-Tungsten Carbide Catalysts for the Production of 2,5-Dimethylfuran from Biomass- Derived Molecules. ChemSusChem 2014, 7, 1068–1072. [Google Scholar] [CrossRef]
- Yang, P.; Xia, Q.; Liu, X.; Wang, Y. High-yield production of 2,5-dimethylfuran from 5-hydroxymethylfurfural over carbon supported Ni-Co bimetallic catalyst. J. Energy Chem. 2016, 25, 1015–1020. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. Influence of supports for selective production of 2,5-dimethylfuran via bimetallic copper-cobalt catalyzed 5-hydroxymethylfurfural hydrogenolysis. Chin. J. Catal. 2017, 38, 699–709. [Google Scholar] [CrossRef]
- Gyngazova, M.S.; Negahdar, L.; Blumenthal, L.C.; Palkovits, R. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over carbon-supported nickel catalysts. Chem. Eng. Sci. 2017, 173, 455–464. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.; Parikh, J. Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation. J. Mol. Catal. A-Chem. 2017, 426, 244–256. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, J.; Zheng, L.; Wang, B.; Bi, R.; He, Y.; Liu, H.; Li, D. Interfacial structure-determined reaction pathway and selectivity for 5-(hydroxymethyl) furfural hydrogenation over Cu-based catalysts. ACS Catal. 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- Guo, W.; Liu, H.; Zhang, S.; Han, H.; Liu, H.; Jiang, T.; Han, B.; Wu, T. Efficient hydrogenolysis of 5-hydroxymethylfurfural to 2, 5-dimethylfuran over a cobalt and copper bimetallic catalyst on N-graphene-modified Al2O3. Green Chem. 2016, 18, 6222–6228. [Google Scholar] [CrossRef]
- Goyal, R.; Sarkar, B.; Bag, A.; Siddiqui, N.; Dumbre, D.; Lucas, N.; Bhargava, S.K.; Bordoloi, A. Studies of synergy between metal–support interfaces and selective hydrogenation of HMF to DMF in water. J. Catal. 2016, 340, 248–260. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J.; Hafner, J. Theory of the crystal structures of selenium and tellurium: The effect of generalized-gradient corrections to the local-density approximation. Phys. Rev. B 1994, 50, 13181. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- González, J.; Giménez, X.; Bofill, J.M. Algorithm to evaluate rate constants for polyatomic chemical reactions. II. Applications. J. Comput. Chem. 2007, 28, 2111–2121. [Google Scholar] [CrossRef]



| Catalysts | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| HCP-Co | 9.95 | 0.113 | 44.77 |
| HCP-FCC-Co | 5.27 | 0.018 | 47.54 |
| FCC-Co | 3.18 | 0.007 | 46.59 |
| Reactant | Functional Group | Reaction Conditions | Conversion (%) | Catalysis Efficiency (molReactant·molCo−1·h−1) a | CEHCP-Co/CEFCC-Co | ||
|---|---|---|---|---|---|---|---|
| HCP-Co | FCC-Co | HCP-Co | FCC-Co | ||||
| HMF | Furyl-C=O | 453 K, 2 MPa, 0.5 h, 3 mmol | 87.6 | 19.4 | 10.2 | 1.2 | 8.5 |
| Cinnamyl aldehyde | Alkylene-C=O | 373 K, 2 MPa, 8 h, 4 mmol | 91.6 | 10.8 | 0.8 | 0.09 | 8.9 |
| Acetophenone | Aryl-C=O | 393 K, 2 MPa, 1 h, 4 mmol | 51.2 | 6.5 | 0.8 | 0.3 | 2.7 |
| Styrene | Aryl-C=C | 373 K, 2 MPa, 2 h, 4 mmol | 93.8 | 23.5 | 3.4 | 0.7 | 4.9 |
| Phenol | Aryl-C=C | 453 K, 2 MPa, 2 h, 4 mmol | 77.3 | 39.4 | 5.2 | 2.1 | 2.5 |
| Nitrobenzene | Aryl-NO2 | 393 K, 2 MPa, 1 h, 4 mmol | 85.8 | 15.0 | 3.5 | 0.3 | 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Chen, N.; Guo, X.; Zhang, R.; Sheng, X.; Ge, H.; Zhu, Z.; Yang, H.; Lü, H. Phase-Controlled Cobalt Catalyst Boosting Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Molecules 2023, 28, 4918. https://doi.org/10.3390/molecules28134918
Yang K, Chen N, Guo X, Zhang R, Sheng X, Ge H, Zhu Z, Yang H, Lü H. Phase-Controlled Cobalt Catalyst Boosting Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Molecules. 2023; 28(13):4918. https://doi.org/10.3390/molecules28134918
Chicago/Turabian StyleYang, Kaixuan, Naimeng Chen, Xiaomiao Guo, Ruoqi Zhang, Xiaoyu Sheng, Hui Ge, Zhiguo Zhu, Hengquan Yang, and Hongying Lü. 2023. "Phase-Controlled Cobalt Catalyst Boosting Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran" Molecules 28, no. 13: 4918. https://doi.org/10.3390/molecules28134918
APA StyleYang, K., Chen, N., Guo, X., Zhang, R., Sheng, X., Ge, H., Zhu, Z., Yang, H., & Lü, H. (2023). Phase-Controlled Cobalt Catalyst Boosting Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Molecules, 28(13), 4918. https://doi.org/10.3390/molecules28134918





