Combination of Lycopene and Curcumin Synergistically Alleviates Testosterone-Propionate-Induced Benign Prostatic Hyperplasia in Sprague Dawley Rats via Modulating Inflammation and Proliferation
Abstract
1. Introduction
2. Results
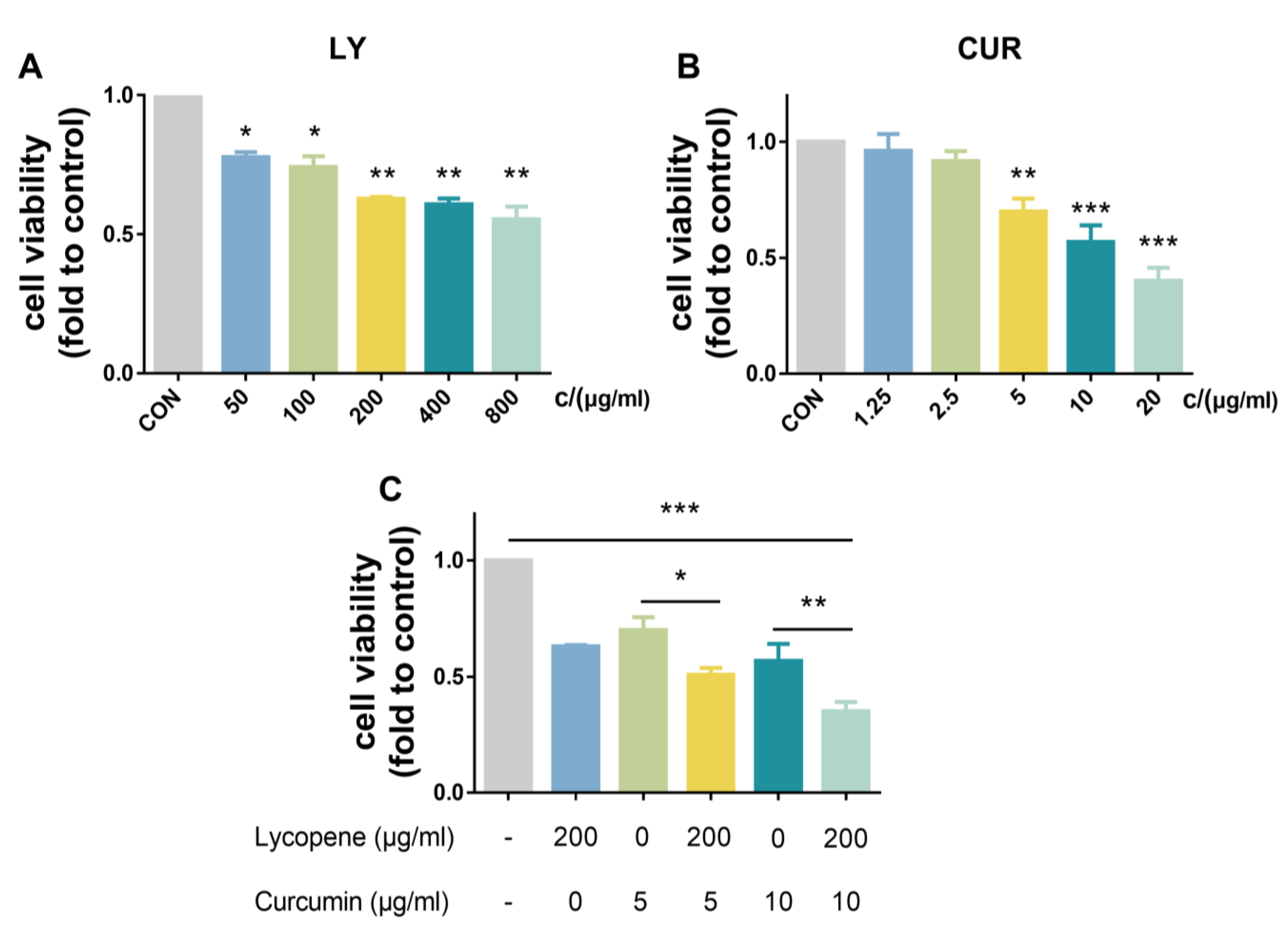
2.1. Effects of LY and CUR on the Viability of BPH-1 Cells In Vitro
2.2. Effects of LY and CUR Combination Treatments on BPH Development in Rats
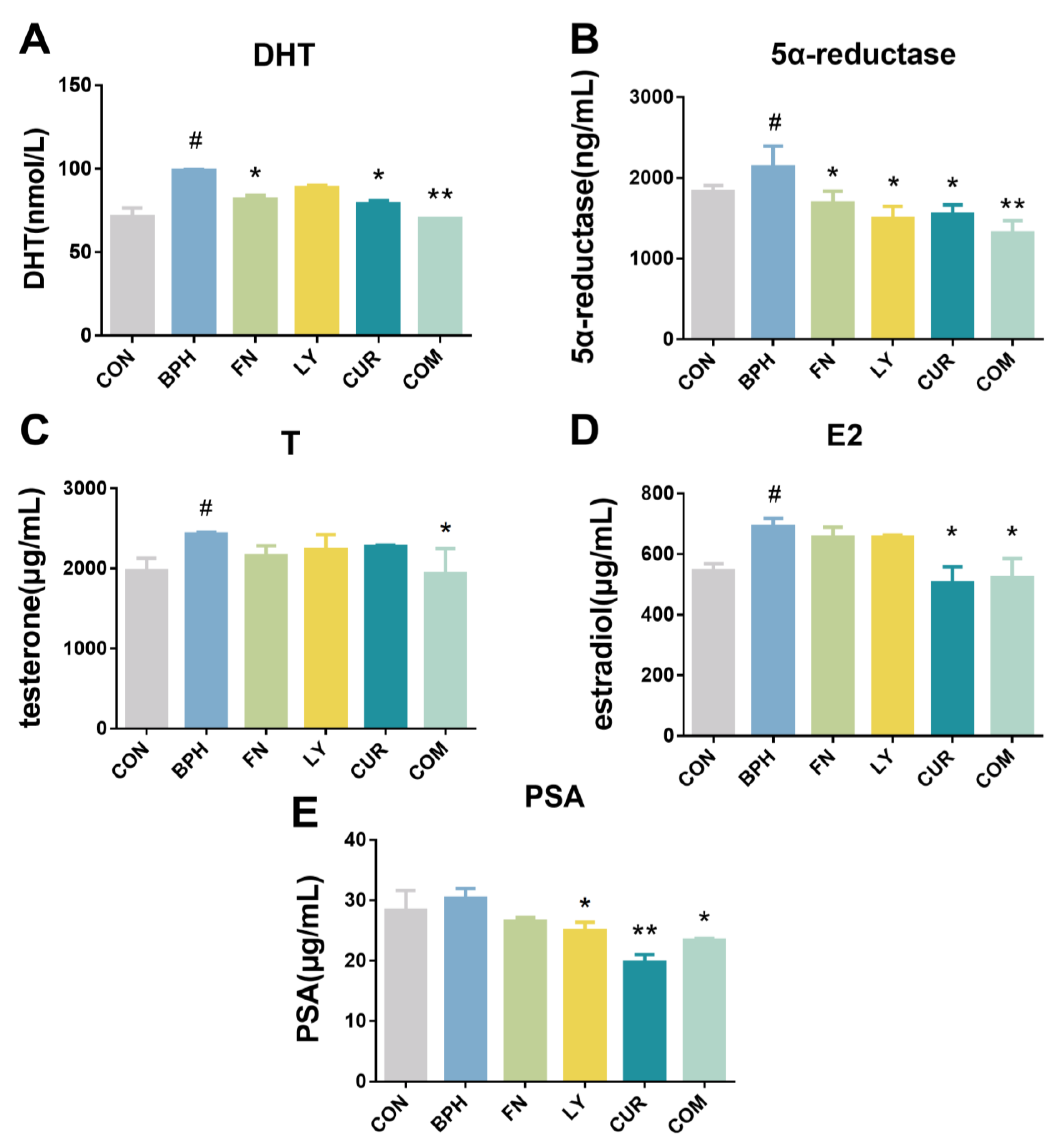
2.3. Effects of LY and CUR Combination Treatments on Serum Levels of Hormones in Rats
2.4. Effects of LY and CUR Combination Treatments on Inflammatory Responses in Rats
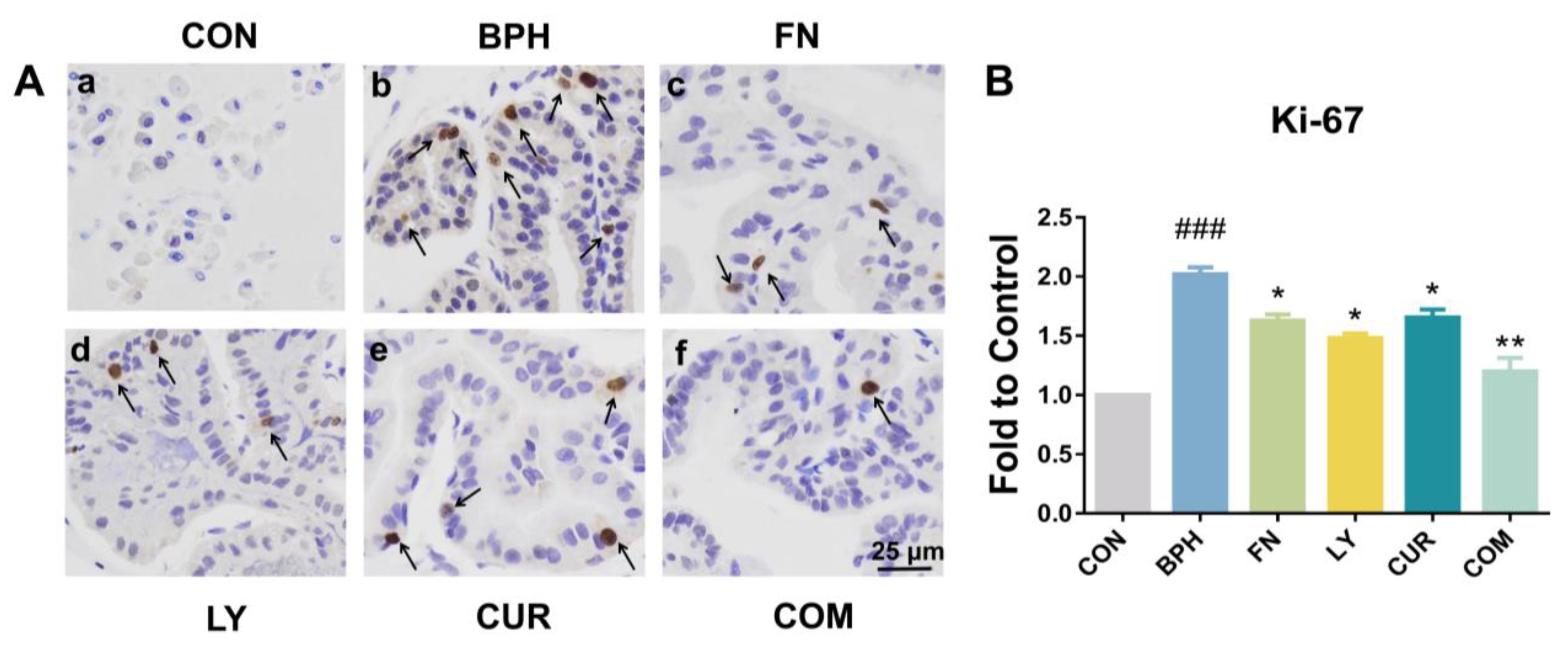
2.5. Effects of LY and CUR Combination Treatments on Prostate Cell Proliferation
2.6. Network Pharmacology Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture
3.3. Cell Viability Assay
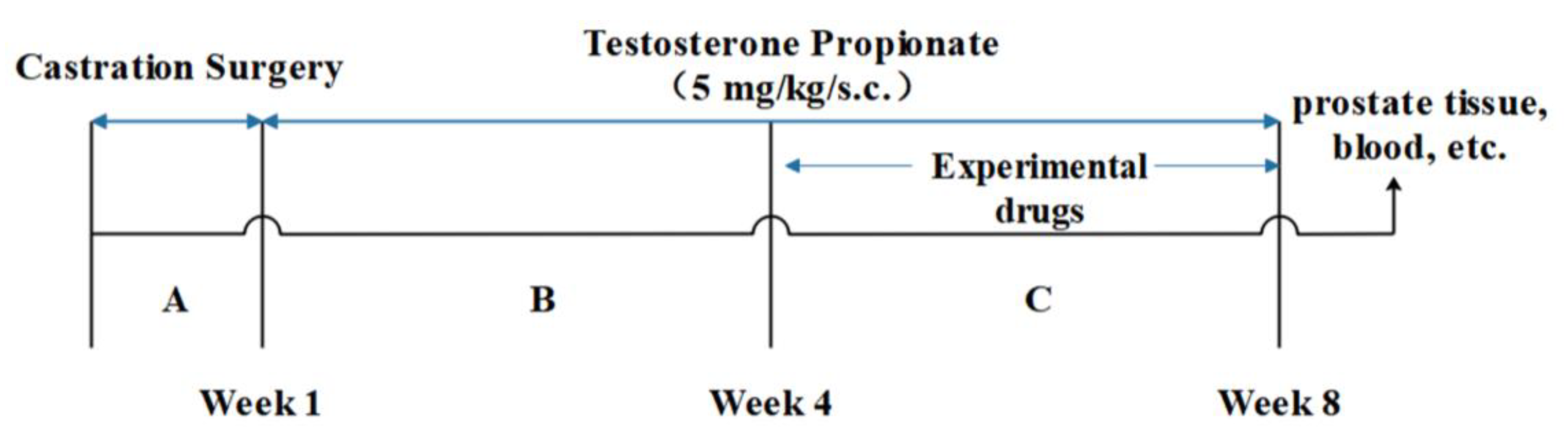
3.4. Animal Study
3.5. Histopathology and Immunohistochemistry (IHC)
3.6. Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Network Pharmacology
3.8. Statistical Analysis
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | benign prostatic hyperplasia |
| TP | testosterone propionate |
| LY | lycopene |
| CUR | curcumin |
| FN | finasteride |
| COM | combination |
| CON | control |
| H&E | hematoxylin and eosin |
| DHT | dihydrotestosterone |
| IL | interleukin |
| TNF-α | tumor necrosis factor-alpha |
| IHC | immunohistochemistry |
| ELISA | Enzyme-linked Immunosorbent Assay |
| E2 | estradiol |
| PSA | prostate-specific antigen |
| CCK-8 | Cell Counting Kit 8 |
| T | testosterone |
References
- El-Sherbiny, M.; El-Shafey, M.; El-Agawy, M.S.E.-d.; Mohamed, A.S.; Eisa, N.H.; Elsherbiny, N.M. Diacerein ameliorates testosterone-induced benign prostatic hyperplasia in rats: Effect on oxidative stress, inflammation and apoptosis. Int. Immunopharmacol. 2021, 100, 108082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, G.; Sun, Y.; Wu, F.; Wang, Q.; Xu, T.; Hu, H.; Xu, K. Depressive symptoms in individuals diagnosed with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) in middle-aged and older Chinese individuals: Results from the China Health and Retirement Longitudinal Study. J. Affect. Disord. 2022, 296, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Male Lower Urinary Tract Symptoms (LUTS) and Benign Prostatic Hyperplasia (BPH). Med. Clin. N. Am. 2011, 95, 87–100. [Google Scholar] [CrossRef]
- Rachael, N.; Dianna, M.E.B. Patient-Friendly Summary of the ACR Appropriateness Criteria: Lower Urinary Tract Symptoms-Suspicion of Benign Prostatic Hyperplasia. J. Am. Coll. Radiol. 2022, 19, e9. [Google Scholar]
- Abdel-Aziz, A.M.; El-Tahawy, N.F.G.; Abdel haleem, M.A.S.; Mohammed, M.M.; Ali, A.I.; Ibrahim, Y.F. Amelioration of testosterone-induced benign prostatic hyperplasia using febuxostat in rats: The role of VEGF/TGFβ and iNOS/COX-2. Eur. J. Pharmacol. 2020, 889, 173631. [Google Scholar] [CrossRef]
- Qian, S.; Sheng, X.; Xu, D.; Shen, H.; Qi, J.; Wu, Y. Variation of prostatic morphology in Chinese benign prostatic hyperplasia patients of different age decades. Aging Male 2019, 23, 457–463. [Google Scholar] [CrossRef]
- Foo, K.T. What is a disease? What is the disease clinical benign prostatic hyperplasia (BPH)? World J. Urol. 2019, 37, 1293–1296. [Google Scholar] [CrossRef]
- Wang, C.; Luo, F.; Zhou, Y.; Du, X.; Shi, J.; Zhao, X.; Xu, Y.; Zhu, Y.; Hong, W.; Zhang, J. The therapeutic effects of docosahexaenoic acid on oestrogen/androgen-induced benign prostatic hyperplasia in rats. Exp. Cell Res. 2016, 345, 125–133. [Google Scholar] [CrossRef]
- Abdel-Naim, A.B.; Neamatallah, T.; Eid, B.G.; Esmat, A.; Alamoudi, A.J.; El-Aziz, G.S.A.; Ashour, O.M. 2-Methoxyestradiol Attenuates Testosterone-Induced Benign Prostate Hyperplasia in Rats through Inhibition of HIF-1α/TGF-β/Smad2 Axis. Oxidative Med. Cell. Longev. 2018, 2018, 4389484. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Amin, H.A.A.; Kamel, A.S.; Ibrahim, S.M.; Helmy, H. Immunomodulatory effect of diallyl sulfide on experimentally-induced benign prostate hyperplasia via the suppression of CD4+T/IL-17 and TGF-β1/ERK pathways. Inflammopharmacology 2020, 28, 1407–1420. [Google Scholar] [CrossRef]
- Youn, D.-H.; Park, J.; Kim, H.-L.; Jung, Y.; Kang, J.; Lim, S.; Song, G.; Kwak, H.J.; Um, J.-Y. Corrigendum: Berberine Improves Benign Prostatic Hyperplasia via Suppression of 5 Alpha Reductase and Extracellular Signal-Regulated Kinase in vivo and in vitro. Front. Pharmacol. 2019, 10, 541. [Google Scholar] [CrossRef]
- Lloyd, G.L.; Marks, J.M.; Ricke, W.A. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: What Is the Role and Significance of Inflammation? Curr. Urol. Rep. 2019, 20, 54. [Google Scholar] [CrossRef]
- Tsunemori, H.; Sugimoto, M. Effects of inflammatory prostatitis on the development and progression of benign prostatic hyperplasia: A literature review. Int. J. Urol. 2021, 28, 1086–1092. [Google Scholar] [CrossRef]
- Vignera, S.L.; Condorelli, R.A.; Russo, G.I.; Morgia, G.; Calogero, A.E. Endocrine control of benign prostatic hyperplasia. Andrology 2016, 4, 404–411. [Google Scholar] [CrossRef]
- Kortam, M.A.; Alawady, A.S.; Sadik, N.A.H.; Fathy, N. Fenofibrate mitigates testosterone induced benign prostatic hyperplasia via regulation of Akt/FOXO3a pathway and modulation of apoptosis and proliferation in rats. Arch. Biochem. Biophys. 2022, 723, 109237. [Google Scholar] [CrossRef]
- Manov, J.J.; Mohan, P.P.; Kava, B.; Bhatia, S. Benign Prostatic Hyperplasia: A Brief Overview of Pathogenesis, Diagnosis, and Current State of Therapy. Tech. Vasc. Interv. Radiol. 2020, 23, 100687. [Google Scholar] [CrossRef]
- Cai, T.; Cui, Y.; Yu, S.; Li, Q.; Zhou, Z.; Gao, Z. Comparison of Serenoa Repens with Tamsulosin in the Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis. Am. J. Men’s Health 2020, 14, 1557988320905407. [Google Scholar] [CrossRef]
- Zhou, Z.; Cui, Y.; Wu, J.; Ding, R.; Cai, T.; Gao, Z. Meta-analysis of the efficacy and safety of combination of tamsulosin plus dutasteride compared with tamsulosin monotherapy in treating benign prostatic hyperplasia. BMC Urol. 2019, 19, 17. [Google Scholar] [CrossRef]
- Olta, A.; Annabella, V. What do we know about phytotherapy of benign prostatic hyperplasia? Life Sci. 2015, 126, 42–56. [Google Scholar]
- Loeb, S.; Fu, B.C.; Bauer, S.R.; Pernar, C.H.; Chan, J.M.; Van Blarigan, E.L.; Giovannucci, E.L.; Kenfield, S.A.; Mucci, L.A. Association of plant-based diet index with prostate cancer risk. Am. J. Clin. Nutr. 2022, 115, 662–670. [Google Scholar] [CrossRef]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef] [PubMed]
- Kristian, L.; Mariana, J.; Damayanthi, D.; Finelli, R.; Majzoub, A.; Henkel, R.; Agarwal, A. A systematic review of herbal medicine in the clinical treatment of benign prostatic hyperplasia. Phytomed. Plus 2022, 2, 100153. [Google Scholar]
- Zou, Y.; Aboshora, W.; Li, J.; Xiao, T.; Zhang, L. Protective Effects of Lepidium meyenii (Maca) Aqueous Extract and Lycopene on Testosterone Propionate-Induced Prostatic Hyperplasia in Mice. Phytother. Res. 2017, 31, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Iweala, E.J.; Uche, M.E.; Dike, E.D.; Etumnu, L.R.; Dokunmu, T.M.; Oluwapelumi, A.E.; Okoro, B.C.; Dania, O.E.; Adebayo, A.H.; Ugbogu, E.A. Curcuma longa (Turmeric): Ethnomedicinal uses, phytochemistry, pharmacological activities and toxicity profiles—A review. Pharmacol. Res. Mod. Chin. Med. 2023, 6, 100222. [Google Scholar] [CrossRef]
- Selvam, C.; Prabu, S.L.; Jordan, B.; Purushothaman, Y.; Umamaheswari, A.; Zare, M.S.H.; Thilagavathi, R. Molecular Mechanisms of Curcumin and Its Analogs in Colon Cancer Prevention and Treatment. Life Sci. 2019, 239, 117032. [Google Scholar] [CrossRef]
- Jordan, B.C.; Kumar, B.; Thilagavathi, R.; Yadhav, A.; Kumar, P.; Selvam, C. Synthesis, evaluation of cytotoxic properties of promising curcumin analogues and investigation of possible molecular mechanisms. Chem. Biol. Drug Des. 2017, 91, 332–337. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary polyphenols suppress chronic inflammation by modulation of multiple inflammation-associated cell signaling pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Abouzaid, O.A.R.; El-Sonbaty, S.M.; Elkasapy, A.H.; Deiab, N.S.E. The potential and inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia in male rats. Benha Vet. Med. J. 2019, 33, 6–14. [Google Scholar]
- Alexander, P.; Benjamin, K. Medical Treatment of Benign Prostatic Hyperplasia. Urol. Clin. N. Am. 2022, 49, 231–238. [Google Scholar]
- Liu, K.M.; Yang, J.; Deng, J.; Fan, X.H.; Hu, Y.J. Global Patent Landscape of Benign Prostatic Hyperplasia Drugs. Urology 2022, 166, 209–215. [Google Scholar] [CrossRef]
- Wick, M.R. The hematoxylin and eosin stain in anatomic pathology—An often-neglected focus of quality assurance in the laboratory. Semin. Diagn. Pathol. 2019, 36, 303–311. [Google Scholar] [CrossRef]
- Zhou, J.-R.; Yu, L.; Zhong, Y.; Blackburn, G.L. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J. Nutr. 2003, 133, 516–521. [Google Scholar] [CrossRef]
- Yokoyama, Y. Synergy between Angiostatin and Endostatin: Inhibition of Ovarian Cancer Growth. Cancer Res. 2000, 60, 2190–2196. [Google Scholar]
- Lee, K.L.; Peehl, D.M. Molecular and Cellular Pathogenesis of Benign Prostatic Hyperplasia. J. Urol. 2004, 172, 1784–1791. [Google Scholar] [CrossRef]
- Samarinas, M.; Gravas, S. Chapter 3—The Relationship Between Inflammation and LUTS/BPH. In Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia; Academic Press: Cambridge, MA, USA, 2018; pp. 31–50. [Google Scholar]
- Rastrelli, G.; Vignozzi, L.; Corona, G.; Maggi, M. Testosterone and Benign Prostatic Hyperplasia. Sex. Med. Rev. 2019, 7, 259–271. [Google Scholar] [CrossRef]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Griend, D.J.V.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- Ajayi, A.; Abraham, K. Understanding the role of estrogen in the development of benign prostatic hyperplasia. Afr. J. Urol. 2018, 24, 93–97. [Google Scholar] [CrossRef]
- Tao, R.; Liu, E.; Zhao, X.; Han, L.; Yu, B.; Mao, H.; Yang, W.; Gao, X. Combination of Ligustri Lucidi Fructus with Ecliptae Herba and their phytoestrogen or phytoandrogen like active pharmaceutical ingredients alleviate oestrogen/testosterone-induced benign prostatic hyperplasia through regulating steroid 5-α-reductase. Phytomedicine 2022, 102, 154169. [Google Scholar] [CrossRef]
- Bernichtein, S.; Pigat, N.; Camparo, P. Anti-inflammatory properties of Lipidosterolic extract of Serenoa repens (Permixon®) in a mouse model of prostate hyperplasia. Prostate 2015, 75, 706–722. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, W.Y.; Park, J.; Ahn, K.S.; Lee, J.H.; Kwak, H.J.; Um, J.-Y. Therapeutic role of Glycyrrhiza Uralensis fisher on benign prostatic hyperplasia through 5 alpha reductase regulation and apoptosis. Phytomedicine 2022, 105, 154371. [Google Scholar] [CrossRef]
- Nevenka, M.; Milanka, M.; Josip, G.; Ksenija, M. Expression of proinflammatory cytokine interleukin-6 in tissue samples of human prostate obtained by needle biopsy. Pathol. Res. Pract. 2015, 211, 865–870. [Google Scholar]
- Jemaa, A.B.; Bouraoui, Y.; Rais, N.B.; Nouira, Y.; Oueslati, R. Cytokine profiling identifies an interaction of IL-6 and IL-1α to drive PSMA-PSA prostate clones. Immunobiology 2016, 211, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, K.; Nandeesha, H.; Dorairajan, L.N.; Ganesh, R.N. Over expression of PI3K-AkT reduces apoptosis and increases prostate size in benign prostatic hyperplasia. Aging Male 2018, 23, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, F.; Mantawy, E.M.; Azab, S.S.; El-Demerdash, E. The angiotensin converting enzyme inhibitor captopril attenuates testosterone-induced benign prostatic hyperplasia in rats; a mechanistic approach. Eur. J. Pharmacol. 2019, 865, 172729. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Alipour, M.; Abdolahinia, E.D.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Saadat, Y.R.; Sharifi, S.; Vahed, S.Z. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]



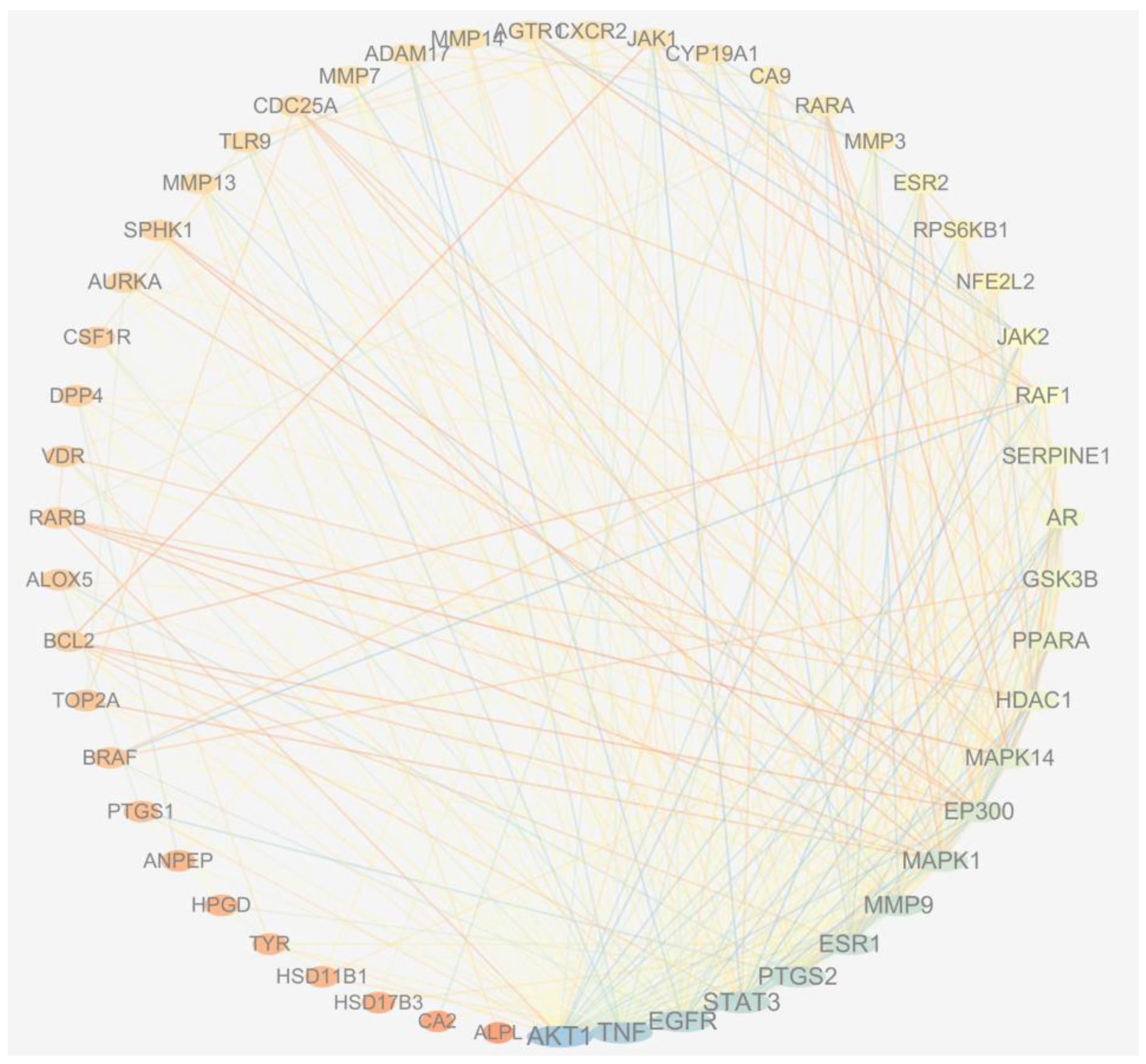


| No. | Target | Common Name | Uniprot ID |
|---|---|---|---|
| 1 | Androgen receptor | AR | P10275 |
| 2 | Estrogen receptor alpha | ESR1 | P03372 |
| 3 | Cytochrome P450 19A1 | CYP19A1 | P11511 |
| 4 | Estrogen receptor beta | ESR2 | Q92731 |
| 5 | Vitamin D receptor | VDR | P11473 |
| 6 | Retinoic acid receptor beta | RARB | P10826 |
| 7 | MAP kinase ERK2 | MAPK1 | P28482 |
| 8 | MAP kinase p38 alpha | MAPK14 | Q16539 |
| 9 | Histone deacetylase 1 | HDAC1 | Q13547 |
| 10 | Dual-specificity phosphatase Cdc25A | CDC25A | P30304 |
| 11 | Peroxisome proliferator-activated receptor alpha | PPARA | Q07869 |
| 12 | Retinoic acid receptor alpha | RARA | P10276 |
| 13 | Epidermal growth factor receptor erbB1 | EGFR | P00533 |
| 14 | Serine/threonine-protein kinase AKT | AKT1 | P31749 |
| 15 | Serine/threonine-protein kinase B-raf | BRAF | P15056 |
| 16 | Apoptosis regulator Bcl-2 | BCL2 | P10415 |
| 17 | Signal transducer and activator of transcription 3 | STAT3 | P40763 |
| 18 | Cyclooxygenase-2 | PTGS2 | P35354 |
| 19 | TNF-alpha | TNF | P01375 |
| 20 | Tyrosine-protein kinase JAK2 | JAK2 | O60674 |
| 21 | Matrix metalloproteinase 9 | MMP9 | P14780 |
| 22 | Histone acetyltransferase p300 | EP300 | Q09472 |
| 23 | Serine/threonine-protein kinase RAF | RAF1 | P04049 |
| 24 | Matrix metalloproteinase 14 | MMP14 | P50281 |
| 25 | Estradiol 17-beta-dehydrogenase 3 | HSD17B3 | P37058 |
| 26 | DNA topoisomerase II alpha | TOP2A | P11388 |
| 27 | Matrix metalloproteinase 7 | MMP7 | P09237 |
| 28 | Alkaline phosphatase, tissue-nonspecific isozyme | ALPL | P05186 |
| 29 | Serine/threonine-protein kinase Aurora-A | AURKA | O14965 |
| 30 | Glycogen synthase kinase-3 beta | GSK3B | P49841 |
| 31 | Ribosomal protein S6 kinase 1 | RPS6KB1 | P23443 |
| 32 | Nuclear factor erythroid 2-related factor 2 | NFE2L2 | Q16236 |
| 33 | Matrix metalloproteinase 13 | MMP13 | P45452 |
| 34 | Tyrosine-protein kinase JAK1 | JAK1 | P23458 |
| 35 | Arachidonate 5-lipoxygenase | ALOX5 | P09917 |
| 36 | Carbonic anhydrase IX | CA9 | Q16790 |
| 37 | Cyclooxygenase-1 | PTGS1 | P23219 |
| 38 | Toll-like receptor (TLR7/TLR9) | TLR9 | Q9NR96 |
| 39 | Plasminogen activator inhibitor-1 | SERPINE1 | P05121 |
| 40 | Interleukin-8 receptor B | CXCR2 | P25025 |
| 41 | Macrophage colony stimulating factor receptor | CSF1R | P07333 |
| 42 | Sphingosine kinase 1 | SPHK1 | Q9NYA1 |
| 43 | Type-1 angiotensin II receptor (by homology) | AGTR1 | P30556 |
| 44 | HERG | KCNH2 | Q12809 |
| 45 | ADAM17 | ADAM17 | P78536 |
| 46 | Matrix metalloproteinase 3 | MMP3 | P08254 |
| 47 | Dipeptidyl peptidase IV | DPP4 | P27487 |
| 48 | Tyrosinase | TYR | P14679 |
| 49 | Aminopeptidase N | ANPEP | P15144 |
| 50 | 11-beta-hydroxysteroid dehydrogenase 1 | HSD11B1 | P28845 |
| 51 | Carbonic anhydrase II | CA2 | P00918 |
| 52 | 15-hydroxyprostaglandin dehydrogenase [NAD+] | HPGD | P15428 |
| Group | LY mg/(kg.d) BW | CUR mg/(kg.d) BW | FN mg/(kg.d) BW | Oilve Oil (mL) |
|---|---|---|---|---|
| (i) CON | 0 | 0 | 0 | 1 |
| (ii) BPH | 0 | 0 | 0 | 1 |
| (iii) FN | 0 | 0 | 5 | 1 |
| (iv) LY | 12.5 | 0 | 0 | 1 |
| (v) CUR | 0 | 2.4 | 0 | 1 |
| (vi) COM | 12.5 | 2.4 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; He, W.; Li, W.; Zhou, J.-R.; Du, Z. Combination of Lycopene and Curcumin Synergistically Alleviates Testosterone-Propionate-Induced Benign Prostatic Hyperplasia in Sprague Dawley Rats via Modulating Inflammation and Proliferation. Molecules 2023, 28, 4900. https://doi.org/10.3390/molecules28134900
Wang S, He W, Li W, Zhou J-R, Du Z. Combination of Lycopene and Curcumin Synergistically Alleviates Testosterone-Propionate-Induced Benign Prostatic Hyperplasia in Sprague Dawley Rats via Modulating Inflammation and Proliferation. Molecules. 2023; 28(13):4900. https://doi.org/10.3390/molecules28134900
Chicago/Turabian StyleWang, Shanshan, Wenjiang He, Wenzhi Li, Jin-Rong Zhou, and Zhiyun Du. 2023. "Combination of Lycopene and Curcumin Synergistically Alleviates Testosterone-Propionate-Induced Benign Prostatic Hyperplasia in Sprague Dawley Rats via Modulating Inflammation and Proliferation" Molecules 28, no. 13: 4900. https://doi.org/10.3390/molecules28134900
APA StyleWang, S., He, W., Li, W., Zhou, J.-R., & Du, Z. (2023). Combination of Lycopene and Curcumin Synergistically Alleviates Testosterone-Propionate-Induced Benign Prostatic Hyperplasia in Sprague Dawley Rats via Modulating Inflammation and Proliferation. Molecules, 28(13), 4900. https://doi.org/10.3390/molecules28134900







