Fungal Biotransformation of Hazardous Organic Compounds in Wood Waste
Abstract
1. Introduction
2. Characteristics of Selected Components of Wood Protection
2.1. Pentachlorophenol
2.2. Lindane
2.3. Creosote
3. Characteristics of Biological Decomposition
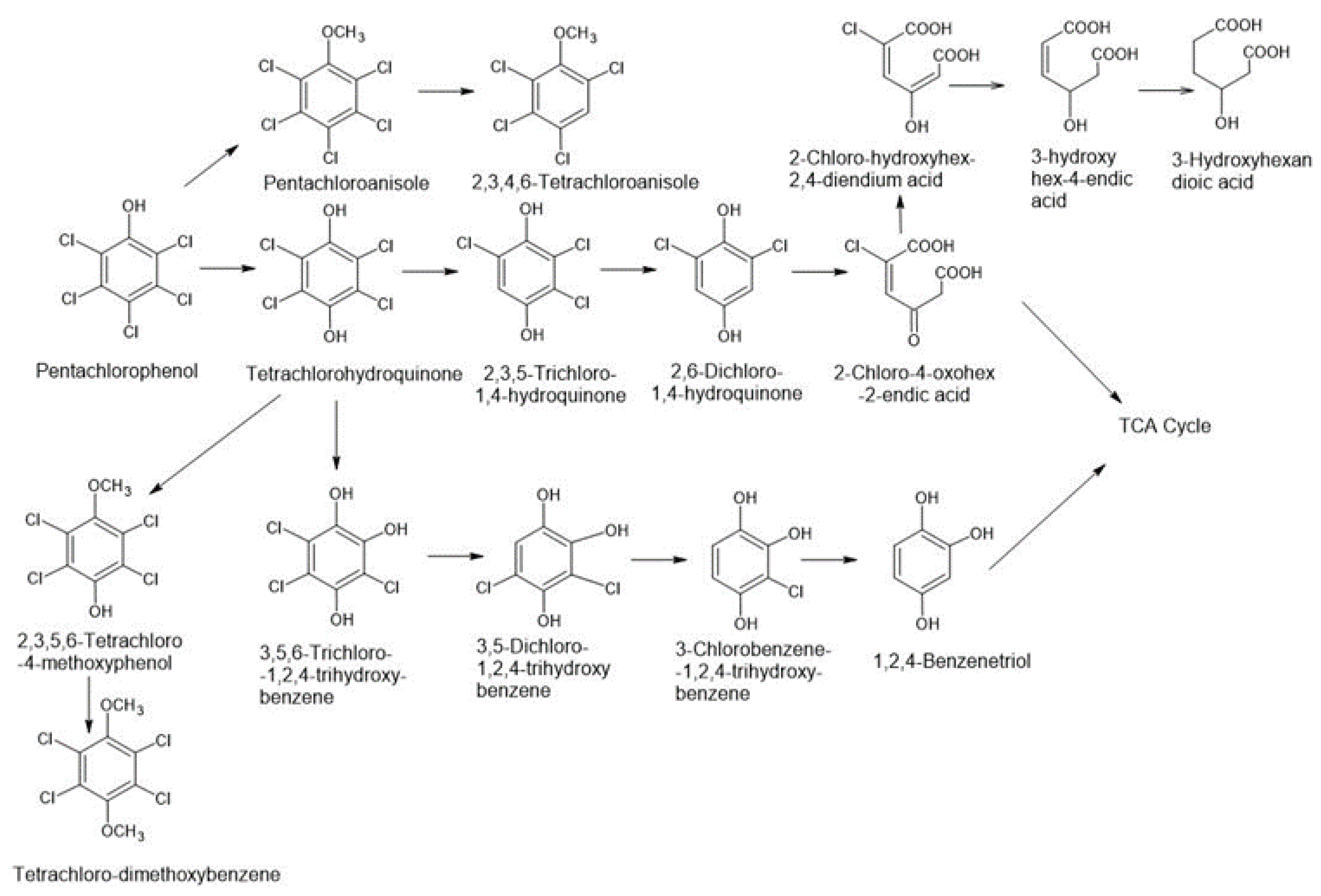
3.1. Fungal Degradation of PCP
3.2. Fungal Degradation of Lindane
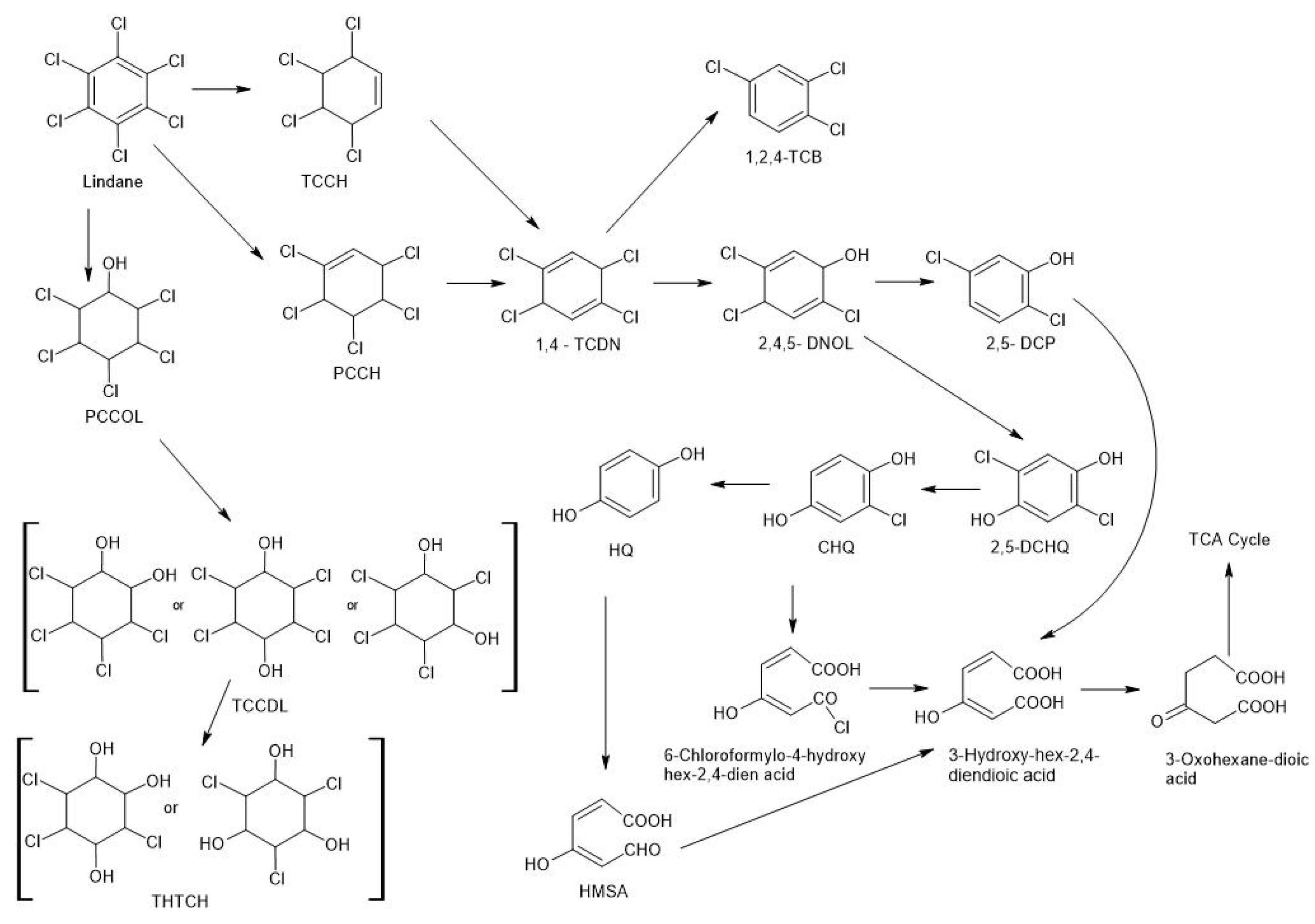
| Organisms/Lindane Transforming Fungi | Conversion Into Chemical Compounds | Research Object/Lindane Concentration | Bibliography |
|---|---|---|---|
| Phlebia brevispora Phlebia lindtneri | Several unreported hydroxylation metabolites, including monohydroxylated, dehydroxylated, and trihydroxylated products | Degradation 87.2 and 73.3% of lindane in low nitrogen medium and 75.8 and 64.9% of lindane in high nitrogen medium, respectively. | [43] |
| Ganoderma lucidum GL-2, Pleurotus ostreatus, Fusarium verticilliodes AT-100, Rhodotorula sp. VIT JzN03, Fusarium poae, Fusarium solani, Conidiobolous 03-1-56, Bjerkandera adusta, Cyathus bulleri | dehydrochlorinase (LinA), dehalogenase (LinB), dehydrogenase (LinC), and reductive dechlorinase (LinD) | Soil, leaves, rotten wood, mean degradation of lindane in %: 59.2–100 in 5–28 days. | [4] |
| Ganoderma lucidum GL-2 strain | dehydrochlorinase (LinA), dehalogenase (LinB), dehydrogenase (LinC) | grown on rice bran substrate for ligninolytic enzyme induction and 40 ppm lindane in liquid as well as solid-state fermentation. The maximum of 75.50% lindane degradation on the 28th day of incubation period, whereas under the solid-state fermentation system, 156.82 U/g laccase, 80.11 U/g manganese peroxidase and 18.61 U/g lignin peroxidase enzyme activities with 37.50% lindane degradation were obtained | [44] |
| Trametes versicolor, Pleurotus ostreatus, Gloeophyllum trabeum | adsorption onto fungal biomass, organic lindane derivatives | Liquid degradation started between 6 h and 1 day of exposure, and both fungi degraded lindane significantly after 3 days. After 21 days, less than 10% of initial lindane was determined. | [45] |
| Trametes versicolor, Hypoxylon fragiforme, Chondrostereum purpureum, Pleurotus ostreatus, Gloeophyllum trabeum | organic lindane derivatives | Amount of lindane was lower in solutions over 70% | [46] |
| Fusarium poae F. solani | organic lindane derivatives | Contaminated soil, 0–600 μg mL−1 10th day of incubation degradation by the two fungal strains demonstrated that the biodegradation of lindane by F. solani (59.4%) was slightly higher than that by the F. poae (56.7%) | [47] |
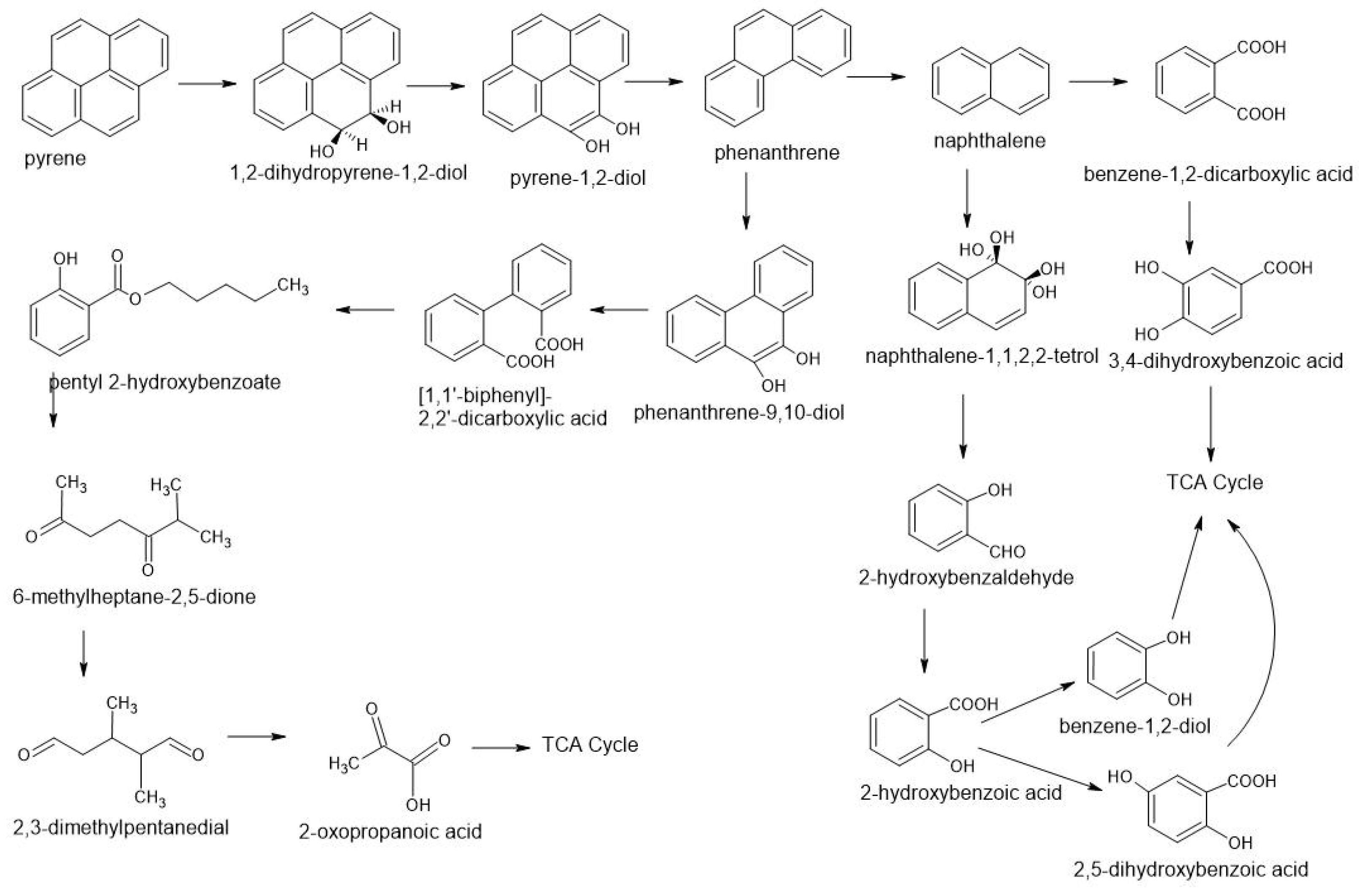
3.3. Fungal Degradation of PAHs
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; De Gruyter: Berlin, Germany; New York, NY, USA, 1983. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Rogoziński, T.; Stuper-Szablewska, K. Phenolic Compounds in Trees and Shrubs of Central Europe. Appl. Sci. 2020, 10, 6907. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, Z.; Pang, S.; Bhatt, P.; Chen, S. Insights Into the Biodegradation of Lindane (γ-Hexachlorocyclohexane) Using a Microbial System. In Frontiers in Microbiology; Frontiers Media SA: Lausanne, Switzerland, 2020; Volume 11. [Google Scholar] [CrossRef]
- EPA. Available online: https://www.epa.gov/ingredients-used-pesticide-products/creosote (accessed on 15 May 2023).
- Regulation of the European Parliament and of the Council (EU) nr 528/2012 date: 22 May 2012. (Dz.U. L 167 z 27 June 2012). Available online: https://eur-lex.europa.eu/eli/reg/2012/528/oj (accessed on 14 June 2023).
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Mair, C.; Stern, T. Cascading Utilization of Wood: A Matter of Circular Economy? Curr For. Rep. 2017, 3, 281–295. [Google Scholar] [CrossRef]
- Shanmugam, S.; Hari, A.; Pandey, A.; Mathimani, T.; Felix, L.O.; Pugazhendhi, A. Comprehensive review on the application of inorganic and organic nanoparticles for enhancing biohydrogen production. Fuel 2020, 270, 117453. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Małozięć, D. Hazards Resulting from the Burning Wood Impregnated with Selected Chemical Compounds. Appl. Sci. 2020, 10, 6093. [Google Scholar] [CrossRef]
- Berger, F.; Gauvin, F.; Brouwers, H.J.H. The recycling potential of wood waste into wood-wool/cement composite. Constr. Build. Mater. 2020, 260, 119786. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef] [PubMed]
- Bosso, L.; Cristinzio, G. A comprehensive overview of bacteria and fungi used for pentachlorophenol biodegradation. In Reviews in Environmental Science and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 13. [Google Scholar]
- Agrawal, N.; Barapatre, A.; Shahi, M.P.; Shahi, S.K. Biodegradation Pathway of Polycyclic Aromatic Hydrocarbons by Ligninolytic Fungus Podoscypha elegans Strain FTG4 and Phytotoxicity Evaluation of their Metabolites. Environ. Process. 2021, 8, 1307–1335. [Google Scholar] [CrossRef]
- Kumar, D.; Pannu, R. Perspectives of lindane (γ-hexachlorocyclohexane) biodegradation from the environment: A review. Bioresour. Bioprocess. 2018, 5, 29. [Google Scholar] [CrossRef]
- McAllister, K.A.; Lee, H.; Trevors, J.T. Microbial degradation of pentachlorophenol. In Biodegradation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 7. [Google Scholar]
- Szewczyk, R.; Długoński, J. Pentachlorophenol and spent engine oil degradation by Mucor ramosissimus. Int. Biodeterior. Biodegrad. 2009, 63, 123–129. [Google Scholar] [CrossRef]
- EPA. Pentachlorophenol 87-86-5. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/pentachlorophenol.pdf (accessed on 15 May 2023).
- IRIS on PCP–Integrated Risk Information System (IRIS) on Pentachlorophenol. National Center for Environmental Assessment, Office of Research and Development; U.S. Environmental Protection Agency: Washington, DC, USA, 1999.
- ILO; WHO. Pentachlorofenol, ICSC: 0069. Available online: https://www.ilo.org/dyn/icsc/showcard.listcards3?p_lang=pl (accessed on 15 May 2023).
- ILO; WHO. Lindan, ICSC: 0053. Available online: https://www.ilo.org/dyn/icsc/showcard.listcards3?p_lang=pl (accessed on 19 May 2023).
- Kubiak, M. Wielopierścieniowe węglowodory aromatyczne (WWA)–ich występowanie w środowisku i w żywności. Polycyclic Aromatic Hydrocarbons (PAHs)–their occurrence in the environment and food. Probl. Hig. Epidemiol. 2013, 94, 31–36. [Google Scholar]
- ILO; WHO. Kreozot, ISCS: 0572. Available online: https://www.ilo.org/dyn/icsc/showcard.listcards3?p_lang=pl (accessed on 19 May 2023).
- Toxicological Profile for Alpha-, Beta-, Gamma-, and Delta-Hexachlorocyclohexane. 2005. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp43.pdf (accessed on 15 May 2023).
- Struciński, P. 1,2,3,4,5,6-Heksachlorocykloheksan (techniczny). Dokumentacja dopuszczalnych wielkości narażenia zawodowego. Podstawy I Metod. Oceny Sr. Pr. 2009, 3, 75–126. [Google Scholar]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Berenguer, M.J. VOCs and PAHs emissions from creosote-treated wood in a field storage area. Sci. Total Environ. 2008, 402, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Commission Dir. 2001/90/EC. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:32001L0090&from=PL (accessed on 15 May 2023).
- Włodarczyk-Makuła, M.; Wierzbicka, M. Warunki biodegradacji WWA w środowisku wodnym. Lab Lab. Apar. Bad. 2013, 18, 28–32. [Google Scholar]
- Sing, N.N.; Zulkharnain, A.; Roslan, H.A.; Assim, Z.; Husaini, A. Bioremediation of PCP by Trichoderma and Cunninghamella strains isolated from sawdust. Braz. Arch. Biol. Technol. 2014, 57, 811–820. [Google Scholar] [CrossRef]
- Vacondio, B.; Birolli, W.G.; Ferreira, I.M.; Seleghim, M.H.R.; Gonçalves, S.; Vasconcellos, S.P.; Porto, A.L.M. Biodegradation of pentachlorophenol by marine-derived fungus Trichoderma harzianum CBMAI 1677 isolated from ascidian Didemnun ligulum. Biocatal. Agric. Biotechnol. 2015, 4, 266–275. [Google Scholar] [CrossRef]
- Xiao, P.; Kondo, R. Biodegradation and biotransformation of pentachlorophenol by wood-decaying white rot fungus Phlebia acanthocystis TMIC34875. J. Wood Sci. 2020, 66, 2. [Google Scholar] [CrossRef]
- Lopez-Echartea, E.; Macek, T.; Demnerova, K.; Uhlik, O. Bacterial biotransformation of pentachlorophenol and micropollutants formed during its production process. Int. J. Environ. Res. Public Health 2016, 13, 1146. [Google Scholar] [CrossRef]
- Okeke, B.C.; Paterson, A.; Smith, J.E.; Watson-Craik, I.A. Comparative biotransformation of pentachlorophenol in soils by solid substrate cultures of Lentinula edodes. Appl. Microbiol. Biotechnol. 1997, 48, 563–569. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Martins, I.; Leitão, M.C.; Garcia, H.; Rodrigues, C.; San Romão, V.; McLellan, I.; Hursthouse, A.; Silva Pereira, C. Screening pentachlorophenol degradation ability by environmental fungal strains belonging to the phyla Ascomycota and Zygomycota. J. Ind. Microbiol. Biotechnol. 2009, 36, 1249–1256. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Tavares, S.; Medeiros, J.; Núñez, O.; Gallart-Ayala, H.; Leitão, M.C.; Galceran, M.T.; Hursthouse, A.; Silva Pereira, C. Degradation pathway of pentachlorophenol by Mucor plumbeus involves phase II conjugation and oxidation–reduction reactions. J. Hazard. Mater. 2011, 198, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, M.; Lyytikäinen, M.; Oivanen, P.; Hatakka, A. Mineralization and conversion of pentachlorophenol (PCP) in soil inoculated with the white-rot fungus Trametes versicolor. Soil Biol. Biochem. 1998, 31, 65–74. [Google Scholar] [CrossRef]
- Carvalho, M.B.; Martins, I.; Medeiros, J.; Tavares, S.; Planchon, S.; Renaut, J.; Núñez, O.; Gallart-Ayala, H.; Galceran, M.T.; Hursthouse, A.; et al. The response of Mucor plumbeus to pentachlorophenol: A toxicoproteomics study. J. Proteom. 2013, 78, 159–171. [Google Scholar] [CrossRef] [PubMed]
- van Pée, K.-H.; Unversucht, S. Biological dehalogenation and halogenation reactions. Chemosphere 2003, 52, 299–312. [Google Scholar] [CrossRef] [PubMed]
- De Lipthay, J.R.; Barkay, T.; Sorensen, S.J. Enhanced degradation of phenoxyacetic acid in soil by horizontal transfer of the tfdA gene encoding a 2,4-dichlorophenoxyacetic acid dioxygenase. FEMS Microbiol. Ecol. 2003, 35, 75–84. [Google Scholar] [CrossRef]
- Andersson, B.E.; Lundstedt, S.; Tornberg, K.; Schnürer, Y.; Schnürer, S.; ÖBerg, L.G.; Mattiasson, B.O. Incomplete degradation of polycyclic aromatic hydrocarbons in soil inoculated with wood-rotting fungi and their effect on the indigenous soil bacteria. Environ. Toxicol. Chem. 2003, 22, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Gramss, G.; Voigt, K.D.; Kirsche, B. Degradation of polycyclic aromatic hydrocarbons with three to seven aromatic rings by higher fungi in sterile and unsterile soils. Biodegradation 1999, 10, 51–62. [Google Scholar] [CrossRef]
- Winquist, E.; Björklöf, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.T.; Jørgensen, K.S.; Tuomela, M. Bioremediation of PAH-contaminated soil with fungi–From laboratory to field scale. Int. Biodeterior. Biodegrad. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Xiao, P.; Kondo, R. Potency of Phlebia species of white rot fungi for the aerobic degradation, transformation and mineralization of lindane. J. Microbiol. 2020, 58, 395–404. [Google Scholar] [CrossRef]
- Kaur, H.; Kapoor, S.; Kaur, G. Application of ligninolytic potentials of a white-rot fungus Ganoderma lucidum for degradation of lindane. Environ. Monit. Assess. 2016, 188, 588. [Google Scholar] [CrossRef]
- Ulčnik, A.; Kralj Cigić, I.; Pohleven, F. Degradation of lindane and endosulfan by fungi, fungal and bacterial laccases. World J. Microbiol. Biotechnol. 2013, 29, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Ulčnik, A.; Cigić, I.K.; Zupančič-Kralj, L.; Tavzes, Č.; Pohleven, F. Bioremediation of Lindane by wood-decaying fungi | Biorazgradnja lindana pomoću gljiva uzročnika truljenja drva. Drv. Ind. 2012, 63, 271–276. [Google Scholar] [CrossRef]
- Sagar, V.; Singh, D.P. Biodegradation of lindane pesticide by non white-rots soil fungus Fusarium sp. World J. Microbiol. Biotechnol. 2011, 27, 1747–1754. [Google Scholar] [CrossRef]
- Sack, U.; Hofrichter, M.; Fritsche, W. Degradation of polycyclic aromatic hydrocarbons by manganese peroxidase of Nematoloma frowardii. FEMS Microbiol. Lett. 1997, 152, 227–234. [Google Scholar] [CrossRef]
- Pozdnyakova, N.; Dubrovskaya, E.; Chernyshova, M.; Makarov, O.; Golubev, S.; Balandina, S.; Turkovskaya, O. The degradation of three-ringed polycyclic aromatic hydrocarbons by wood-inhabiting fungus Pleurotus ostreatus and soil-inhabiting fungus Agaricus bisporus. Fungal Biol. 2018, 122, 363–372. [Google Scholar] [CrossRef]
- Mao, J.; Guan, W. Fungal degradation of polycyclic aromatic hydrocarbons (PAHs) by Scopulariopsis brevicaulis and its application in bioremediation of PAH-contaminated soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 399–405. [Google Scholar] [CrossRef]
- Canet, R.; Birnstingl, J.G.; Malcolm, D.G.; Lopez-Real, J.M.; Beck, A.J. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by native microflora and combinations of white-rot fungi in a coal-tar contaminated soil. Bioresour. Technol. 2001, 76, 113–117. [Google Scholar] [CrossRef] [PubMed]
| Compound (Cas Number and Names) | Occurrence/Application | Hazard/Toxicity | Bibliography |
|---|---|---|---|
| Pentachlorophenol CAS #: 87-86-5 | pesticide herbicide, insecticide, fungicide, algicide, disinfectant component of anti-fouling paints | da Non-flammable substance. Practically insoluble in water Chemical formula: C6Cl5OH Structural formula:  Molecular weight: 266.4 It decomposes at 309 °C Melting point: 191 °C Density: 1.98 g/cm3 Water solubility, g/100 mL at 20 °C: 0.001 Vapor pressure, Pa at 20 °C: 0.02 Relative vapor density (air = 1): 9.2 Relative density of the vapor/air-mixture at 20 °C (air = 1): 1.00 Octanol/water partition coefficient as log Pow: 5.01. Hygienic Standards: TLV: 0.5 mg/m3, as TWA. TLVSTEL1 1 mg/m3. (skin); A3 (agents proven to be carcinogenic in animals and not known to be carcinogenic in humans); DSB. MAK: skin absorption (H); carcinogen category: 2. | [16,18,19,20] |
| Lindane (γ-hexachlorophenol) Hexachlorocyclohexane 1,2,3,4,5,6-Hexachloro-cyclohexane gamma-1,2,3,4,5,6-Heksachlorocykloheksan gamma-BHC gamma-HCH CAS #: 58-89-9 | Pesticide Insecticide (on fruits, vegetables, forest crops, animals, and on animal premises) | Non-flammable substance. Practically insoluble in water. Chemical formula: C6H6Cl6 Structural formula: 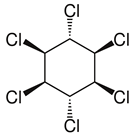 Molecular weight: 290.8 Boiling point: 323 °C Melting point: 113 °C Density: 1.9 g/cm3 Solubility in water, g/100 mL at 20 °C: 0.0007 (very poor) Vapor pressure, Pa at 20 °C: 0.0012 Relative density of the vapor/air-mixture at 20 °C (air = 1): 1 Octanol/water partition coefficient as log Pow: 3.61–3.72 Hygienic standards TLV: 0.5 mg/m3, as TWA. TLVSTEL1 1 mg/m3. (skin); A3 (agents proven to be carcinogenic in animals and not known to be carcinogenic in humans); DSB. MAK: skin absorption (H); carcinogen category: 2 | [4,21] |
| Creosote oil Coal creosote Example of CAS # acenaphthene: 83-32-9; acenaphthylene: 208-96-8; anthracene:120-12-7; benzo[a]pyrene: 50-32-8 | Pesticide, Fungicide, Insecticide, Miticide, Sporicide Products for wood used outdoors, e.g., railroad ties and utility poles, crossarms, fences, fence posts, foundation timbers, timbers, lumber, and pilings. Treated wood intended for exterior/outdoor uses only. | Black to brown, oily liquid with a characteristic odor. The formulas of the most important PAHs(Examples): Naphthalene 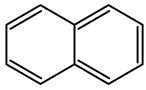 Anthracene 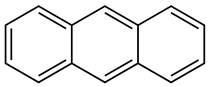 Pyrene 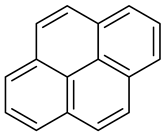 Benzo(a)pyrene 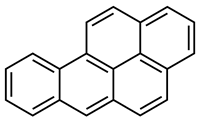 Boiling point: 200–400 °C Melting point: ~20 °C Density: 1.0–1.17 g/cm3 Water solubility: very poor Vapor pressure, kPa at 20 °C: ~6 Flash point: >66 °C c.c. Auto-ignition temperature: 335 °C Hygienic Standards ILO and WHO 2018: not given | [22,23] |
| Organisms/PCP Transforming Fungi | Conversion Into Chemical Compounds | Research Object/PCP Concentration | Bibliography |
|---|---|---|---|
| Anthracophyllum discolor | PCA; TCHQ | Soil 28 days 100–300 mg/kg | [13] |
| Agrocybe perfecta CCB161 | PCA, Chloride ions | Soil 90 days 1180–1278 mg/kg | [13] |
| Armillaria gallica 1057 | 2-methyl-1,3 benzenediol; 6-phenyl-dodecane | Liquid 7 days 25 mg mg/L | [13] |
| Armillaria mellea M51 | 2-methyl-1,3 benzenediol | Liquid 7 days 25 mg/L | [13] |
| Bjerkandera adusta ATCC 62023 | PCA; 2,3,4,6-tetrachloroanisole | Soil 4 weeks 100 µg/g | [13] |
| Bjerkandera adusta ATTC 90940 | PCA; TCHQ | Soil 28 days 100–300 mg/kg | [13] |
| Chrysonilia sitophila DSM 16514, Trichoderma longibrachiatum, Mucor plumbeus, Penicillium janczewskii P. glandicola | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Ganoderma lucidum HK-1 | 2-methyl-1,3 benzenediol; 1-octyl-benzene | Liquid 7 days 25 mg/L | [13] |
| Irpex lacteus ATCC 11245 | PCA; 2,3,4,6-tetrachloroanisole | Soil 4 weeks 100 µg/g | [13] |
| Lentinula edodes LE2 | PCA, 2,3,4,6-tetrachloroanisole (TCA), Tetrachlorophenol | Soil 10 weeks 200 mg/kg | [13,33] |
| Lentinula edodes L68 | 1-chloro-3-methoxy-benzene | Liquid 7 days 25 mg/L | [13] |
| Mucor ramonissimus IM 6203 6203 | 2,3,5,6-TCHQ; pentachloromethoxybenzene | Liquid 240 h 10 mg/L | [13,17] |
| Mucor plumbeus DSM16513 16513 | TriCHQ; TCHQ | Liquid 4 days 15–18.8 µM | [13,35] |
| Mucor ramosissimus IM 6203 | 2,3,5,6-TCHQ | Liquid 7 days 10 mg/L | [13,17] |
| Penicillium adametzii | TeCBQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium corylophilum | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium decumbens | DCBQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium glabrum DSM 16516 | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium glandicola | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium janczewskii | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Penicillium variabile | CHQ | Liquid 50–60 days 1–20 mg/L | [13,34] |
| Peniophora cinerea CCB204 | PCA; Chloride ions | Soil 90 days 1180–1278 mg/kg | [13] |
| Phanerochaete chrysosporium ATCC 42725 | PCA; 2,3,4,6-tetrachloroanisole | Soil 4 weeks 100 µg/kg | [13] |
| Phanerochaete chrysosporium BMK-F-1767 | Chloride ions | Liquid 3 days 250 mg/L | [13] |
| Phanerochaete chrysosporium M1 | 3,3-dimethyl-cyclohexanol | Liquid 7 days 25 mg/L | [13] |
| Phanerochaete sordida HHB-8922-Sp | PCA; 2,3,4,6-tetrachloroanisole | Soil 4 weeks 100 µg/kg | [13] |
| Phanerochaete sordida | PCA | Soil 56 days 175 ppm | [13] |
| Phlebia acanthocystis TMIC34875 Phlebia tremellosa TMIC30511 Phlebia aurea TMIC33908 | pentachloroanisole (PCA) p-tetrachlorohydroquinone (TCHQ) TCHQ transformed into TCMP and TCDB | Liquid 10 days | [31] |
| Pleurotus pulmonarius | TCHQ; TCP | Liquid 2 days 2–100 ppm | [13] |
| Polyporus sp. Cv-1 | 1-chloro-3-methoxy-benzene | Liquid 7 days 25 mg/L | [13] |
| Psilocybe castanella CCB444 | Chloride ions | Soil 90 days 1180–1278 mg/kg | [13] |
| Trametes versicolor HR131 | PCA | Soil 1–2 years 1000 mg/kg | [13] |
| Trametes versicolor MD-277 | PCA; 2,3,4,6-tetrachloroanisole | Soil 4 weeks 100 µg/kg | [13] |
| Trametes versicolor PRL 572 | PCA; 2,3,4,6-tetrachloroanisole | Soil 42 days 996 µg/g | [13,36] |
| Trametes villosa CCB176 | Chloride ions | Soil 90 days 1180–1278 mg/kg | [13] |
| Trametes villosa CCB213 | PCA; Chloride ions | Soil 90 days 1180–1278 mg/kg | [13] |
| Trichoderma harzianum CBMAI 1677 | pentachloroanisole (PCA) 2,3,4,6-tetrachloroanisole (2,3,4,6-TeCA) | Liquid 7 days 10–50 mg/L | [30] |
| Trichoderma longibrachiatum DSM 16517 | DCBQ | Liquid 50–60 days 1–20 mg/L | [13] |
| Phlebia acanthocystis | pentachloroanisole and p-tetrachlorohydroquinone, tetrachloro-4-methoxyphenol, tetrachloro-1,4-dimethoxybenzene, p-tetrachlorohydroquinone | potato dextrose agar medium 10 days of incubation remove 100% and 76% of PCP (25 μM) in low-nitrogen and potato dextrose broth media, respectively | [31] |
| Aspergillus sydowii DL6A; Apergillus versicolor DL5A; Cladosporium oxysporum DL5G; Fusarium proliferatum DL11A; Trichoderma harzianum CBMAI 1677 | pentachloroanisole (PCA); 2,3,4,6-tetrachloroanisole (2,3,4,6-TeCA) | Solid culture medium (3% malt), initial concentration of 20 mgL−1 of PCP) using a validated method. | [30] |
| Trametes sp. Phanerochaete sp., Anthracophyllum sp., Armillaria sp., Bjerkandera sp., Ganoderma sp., Lentinula sp., Penicillium sp, Trichoderma sp., Rhizopus sp. Plerotus sp. | p-tetrachlorohydroquinone, tetrachloro-4-methoxyphenol, tetrachloro-1,4-dimethoxybenzene, 2,3,4,6-tetrachloroanisole (2,3,4,6-TeCA), non-chlorinated or chlorinated phenol derivatives | Soils, starting concentration (μM): 54.2–100.00; PCP degradation yield (%): 33–76. | [13] |
| Cunninghamella sp. UMAS SD12 | - | Liquid, preliminary PCP biodegradation trial performed in minimal liquid medium supplemented with 20 mg/L of PCP, the degradation up to 51.7% of PCP in 15 days. | [29] |
| Mucor plumbeus | Tetrachlorohydroquinone and several phase II conjugates | Soil, degradation up to 60% of PCP in 20 days od incubation. | [37] |
| Mucor plumbeus | glucose, sulfate and ribose conjugates, and identified for the first time in fungi sulfate–glucose conjugates, tetra- and tri-chlorohydroquinones, sulfate–glucose conjugates | Liquid environmental pollution, percentage of PCP biodegradation ranged from 29% to 69%. | [35] |
| Organisms/Pahs Transforming Fungi | Conversion Into Chemical Compounds | Research Object/ Pahs Concentration | Bibliography |
|---|---|---|---|
| Pleurotus ostreatus Antrodia vaillantii | 9-fluorenone, benz[a]anthracene-7,12-dione, 4-hydroxy-9-fluorenone 4-oxapyrene-5-one | Soil artificially contaminated 12 weeks | [40] |
| Phanerochaete velutina | Full degradation | starting concentration 3500 mg·kg−1 sum of 16 PAH laboratory scale: starting concentration 3500 mg kg−1, sum of 16 PAH 96% of 4-ring PAHs and 39% of 5- and 6-ring PAHs were removed in three months In the uninoculated microcosms, 55% of 4-ring PAHs and only 7% of 5- and 6-ring PAHs were degraded. | [42] |
| Bjerkandera adusta (Willd.: Fr.)Karst.; Gymnopilus sapineus (Fr.)Mre.; Hypholoma fasciculare (Huds.: Fr.)Kumm.; Kuehneromyces mutabilis (Schaeff.: Fr.)Sing. & Smith; Lenzites betulina (L.: Fr.)Fr.; Pleurotus sp. (Argentina); Pleurotus ostreatus (Jacq.: Fr.)Kumm. | phenanthrene (PHEN, 3 R), anthracene (ANTH, 3 R), fluoranthene (FLUA, 4 R), pyrene (PYR, 4 R), perylene (PER, 5 R), benzo[g,h,i]perylene (BENZ, 6 R), and coronene (COR, 7 R) | Three to 12 days after spiking, 22 to 38% of the PAH could no longer be recovered from the soils. At 287 days, 88.5 to 92.7%, 83.4 to 87.4%, and 22.0 to 42.1% of the 3-, 4-, and 5- to 7-R PAH, respectively, had disappeared from the unsterile, uninoculated soils. | [41] |
| Phanerochaete chrysosporium IMI 232175 Pleurotus ostreatus IMI 341687, Coriolus versicolor IMI 210866 Wye isolate #7 | organic derivatives nontoxic | Wheat straw and non-sterile coal-tar contaminated soil to determine their potential to degrade polycyclic aromatic hydrocarbons (PAHs) | [51] |
| Podoscypha elegans FTG4 | phenanthrene (PHE) and pyrene (PYR) | - in-vitro and in-vivo conditions 99% of PHE and 98.9% of PYR from the degradation medium (20 mg L−1 concentration individually), although in the in-vivo condition, it reached up to 50.6% of PHE and 48% of PYR (50 mg kg−1 concentration individually). | [14] |
| Pleurotus ostreatus D1, Agaricus bisporus F-8 | two isomeric three-ringed polycyclic aromatic hydrocarbons, phenanthrene and anthracene | liquid medium, mean degradation 48% | [49] |
| Scopulariopsis brevicaulis | phenanthrene, fluoranthene, Pyrene, benzo[a]pyrene | liquid medium phenanthrene (60% removed), fluoranthene (62%), pyrene (64%), benzo[a]pyrene (82%) 30 days of incubation; a PAH-contaminated soil removed 77% of total PAHs from the soil with the addition of the PZ-4 suspension, phenanthrene (89% removed) and benzo[a]pyrene (75%) Incubation for 28 days | [50] |
| Selected Fungi Organism | PCP | Lindane | PAHs |
|---|---|---|---|
| Aspergillus terreus | + | ||
| Aspergillus flavus | + | ||
| Bjerkandera adusta | + | + | + |
| Chrysosporium lignorum | + | ||
| Cunninghamella elegans | + | ||
| Fusarium solani | + | + | |
| Ganoderma lucidum | + | + | |
| Irpex lacteus | + | + | |
| Penicilium trodum | + | ||
| Phanerochaete chrysosporium, | + | + | + |
| Pleurotus ostreatus | + | + | |
| Podoscypha elegans | + | ||
| Rhizoctonia solani, | + | ||
| Trametes versicolor | + | + | + |
| Scerotium rolfsii | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komorowicz, M.; Janiszewska-Latterini, D.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Fungal Biotransformation of Hazardous Organic Compounds in Wood Waste. Molecules 2023, 28, 4823. https://doi.org/10.3390/molecules28124823
Komorowicz M, Janiszewska-Latterini D, Przybylska-Balcerek A, Stuper-Szablewska K. Fungal Biotransformation of Hazardous Organic Compounds in Wood Waste. Molecules. 2023; 28(12):4823. https://doi.org/10.3390/molecules28124823
Chicago/Turabian StyleKomorowicz, Magdalena, Dominika Janiszewska-Latterini, Anna Przybylska-Balcerek, and Kinga Stuper-Szablewska. 2023. "Fungal Biotransformation of Hazardous Organic Compounds in Wood Waste" Molecules 28, no. 12: 4823. https://doi.org/10.3390/molecules28124823
APA StyleKomorowicz, M., Janiszewska-Latterini, D., Przybylska-Balcerek, A., & Stuper-Szablewska, K. (2023). Fungal Biotransformation of Hazardous Organic Compounds in Wood Waste. Molecules, 28(12), 4823. https://doi.org/10.3390/molecules28124823








