Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods
Abstract
1. Introduction
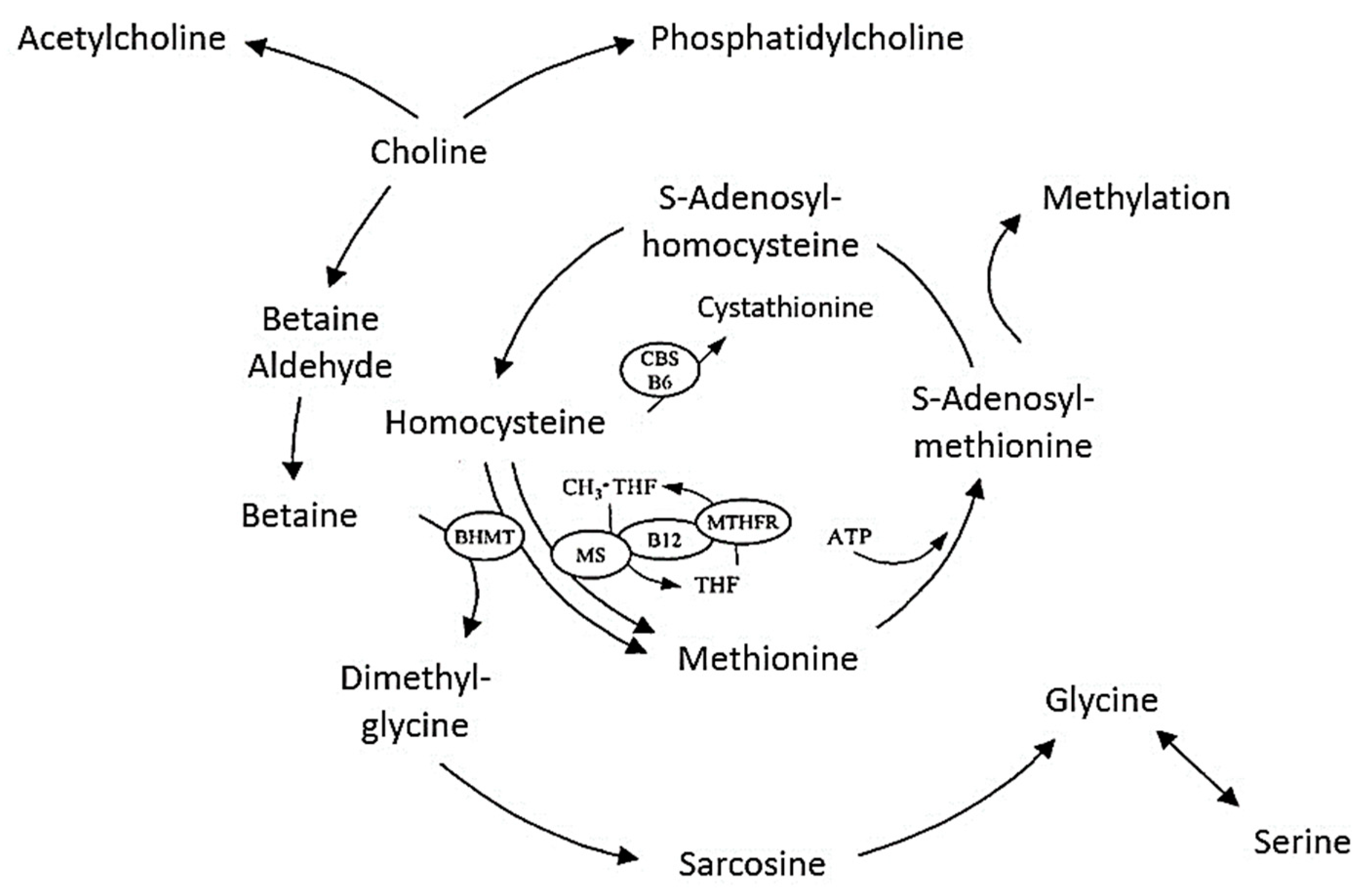
2. Metabolism and Physiology
3. Dietary Sources
4. Health-Promoting Properties
4.1. Redox Potential
4.2. Liver Diseases
4.2.1. Nonalcoholic Fatty Liver Disease (NAFLD)
4.2.2. Alcoholic Liver Disease (ALD)
4.2.3. Other Liver Diseases
4.3. Chronic Kidney Disease (CKD)
4.4. Cardiovascular Diseases
4.5. Carcinogenesis
4.6. Neuroprotective Properties
4.7. Body Composition and Sport Performance
5. Functional Applications
6. Extraction and Detection in Food Matrices
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine Supplementation May Improve Heat Tolerance: Potential Mechanisms in Humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Guimarães-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://chemdrawdirect.perkinelmer.cloud (accessed on 15 April 2023).
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 15 April 2023).
- European Commission. Commission Regulation (EU) No. 432/2012 of 16 May 2012 establishing a list of permitted health claims made on food, other than those referring to the reduction of disease risk and to children’s development and health. Off. J. Eur. Union 2012, 1, L136. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32012R0432 (accessed on 17 April 2023).
- Altinisik, S.; Zeidan, H.; Yilmaz, M.D.; Marti, M.E. Reactive Extraction of Betaine from Sugarbeet Processing Byproducts. ACS Omega 2023, 8, 11029–11038. [Google Scholar] [CrossRef]
- Pastor, K.; Pezo, L.; Vujić, D.; Jovanović, D.; Ačanski, M. Discriminating cereal and pseudocereal species using a binary system of GC–MS data—A pattern recognition approach. J. Serb. Chem. Soc. 2018, 83, 317–329. [Google Scholar] [CrossRef]
- Psodorov, Đ.; Ačanski, M.; Psodorov, D.; Vujić, Đ.; Pastor, K. Determination of the content of wheat and buckwheat flour in bread using GC-MS system and multivariate analysis. J. Food Nutr. Res. 2015, 54, 179–183. [Google Scholar]
- Pastor, K.; Ačanski, M.; Vujić, Đ. Chapter 3: A Review of Adulteration Versus Authentication of Flour. In Flour and Breads and Their Fortification in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 21–36. [Google Scholar] [CrossRef]
- McRae, M.P. Betaine supplementation decreases plasma homocysteine in healthy adult participants: A meta-analysis. J. Chiropr. Med. 2013, 12, 20–25. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Truitt, C.; Hoff, W.D.; Deole, R. Health functionalities of betaine in patients with homocystinuria. Front. Nutr. 2021, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Zawieja, E.E.; Zawieja, B.; Chmurzynska, A. Betaine supplementation moderately increases total cholesterol levels: A systematic review and meta-analysis. J. Diet. Suppl. 2021, 18, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Bagheri, R.; Ghanavati, M.; Asbaghi, O.; Tinsley, G.M.; Mombaini, D.; Wong, A. Effects of betaine supplementation on cardiovascular markers: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6516–6533. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Newmire, D.E.; Rossi, F.E.; Guimarães-Ferreira, L.; Zanchi, N.E. Chapter 60: An overview of betaine supplementation, sports performance, and body composition. In Nutrition and Enhanced Sports Performance, 2nd ed.; Bagchi, D., Nair, S., Sen, C.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 691–706. [Google Scholar] [CrossRef]
- Heidari, R.; Niknahad, H.; Sadeghi, A.; Mohammadi, H.; Ghanbarinejad, V.; Ommati, M.M.; Jamshidzadeh, A. Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed. Pharmacother. 2018, 103, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Verma, A.K.; Das, A.; Singh, P.; Bisht, P.; Wankar, A.K. Exploring the potentials of betaine supplementation in poultry and pig: A review. IJAN 2021, 38, 1–14. [Google Scholar] [CrossRef]
- Uyanga, V.A.; Oke, E.O.; Amevor, F.K.; Zhao, J.; Wang, X.; Jiao, H.; Lin, H. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J. Anim. Sci. Biotechnol. 2022, 13, 23. [Google Scholar] [CrossRef]
- Shah, A.M.; Ma, J.; Wang, Z.; Zou, H.; Hu, R.; Peng, Q. Betaine supplementation improves the production performance, rumen fermentation, and antioxidant profile of dairy cows in heat stress. Animals 2020, 10, 634. [Google Scholar] [CrossRef]
- Dunshea, F.R.; Oluboyede, K.; DiGiacomo, K.; Leury, B.J.; Cottrell, J.J. Betaine improves milk yield in grazing dairy cows supplemented with concentrates at high temperatures. Animals 2019, 9, 57. [Google Scholar] [CrossRef]
- Lakhani, P.; Kumar, P.; Alhussien, M.N.; Lakhani, N.; Grewal, S.; Vats, A. Effect of betaine supplementation on growth performance, nutrient intake and expression of IGF-1 in Karan Fries heifers during thermal stress. Theriogenology 2020, 142, 433–440. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Kelleppan, V.T.; King, J.P.; Butler, C.S.; Williams, A.P.; Tuck, K.L.; Tabor, R.F. Heads or tails? The synthesis, self-assembly, properties and uses of betaine and betaine-like surfactants. Adv. Colloid Interface Sci. 2021, 297, 102528. [Google Scholar] [CrossRef]
- Godek, E.; Grządka, E.; Maciołek, U. Influence of polysaccharides with different chemical character on stability of montmorillonite suspensions in the presence of pseudoamphoteric cocamidopropyl betaine. J. Mol. Liq. 2022, 357, 119097. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef]
- Burg, M. Molecular basis of osmotic regulation. Am. J. Physiol. 1995, 268, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Caldas, T.; Demont-Caulet, N.; Ghazi, A.; Richarme, G. Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, B.C.; Hafner, D.; Hohlfeld, T.; Balkenhol, N.; Laryea, M.D.; Wendel, U. Pharmacokinetics of oral betaine in healthy subjects and patients with homocystinuria. Br. J. Clin. Pharmacol. 2003, 55, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, M.; Weik, C.; Holneicher, C.; Haussinger, D. Betaine as an osmolyte in rat liver: Metabolism and cell-to-cell interactions. Hepatology 1998, 27, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kettunen, H.; Peuranen, S.; Tiihonen, K.; Saarinen, M. Intestinal uptake of betaine in vitro and the distribution of methyl groups from betaine, choline, and methionine in the body of broiler chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 269–278. [Google Scholar] [CrossRef]
- Lever, M.; Sizeland, P.C.; Bason, L.M.; Hayman, C.M.; Chambers, S.T. Glycine betaine and proline betaine in human blood and urine. Bioch. Biophys. Acta 1994, 1200, 259–264. [Google Scholar] [CrossRef]
- Frontiera, M.S.; Stabler, S.P.; Kolhouse, J.F.; Allen, R.H. Regulation of methionine metabolism: Effects of nitrous oxide and excess dietary methionine. J. Nutr. Biochem. 1994, 5, 28–38. [Google Scholar] [CrossRef]
- Virtanen, E. Piecing together the betaine puzzle. Feed Mix Mag. 1995, 3, 12–17. [Google Scholar]
- Burg, M.; Ferraris, J.; Dmitrieva, N. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Burg, M.B. Molecular basis for osmoregulation of organic osmolytes in renal medullary cells. J. Exp. Zool. 1994, 268, 171–175. [Google Scholar] [CrossRef]
- Petronini, P.G.; De Angelis, E.M.; Borghetti, P.; Borghetti, A.F.; Wheeler, K.P. Modulation by betaine of cellular responses to osmotic stress. Biochem. J. 1992, 282, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Söderlun, T.; Zhu, K.; Jutila, A.; Kinnunen, P.K.J. Effects of betaine on the structural dymanics of Thermomyces (Humicola) lanuginosa lipase. Coll. Surf. B Biointerfaces 2002, 26, 75–83. [Google Scholar] [CrossRef]
- Pícha, J.; Vaněk, V.; Buděšínský, M.; Mládková, J.; Garrow, T.A.; Jiráček, J. The development of a new class of inhibitors for betaine-homocysteine S-methyltransferase. Eur. J. Med. Chem. 2013, 65, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Price, R.K.; Keaveney, E.M.; Hamill, L.L.; Wallace, J.M.; Ward, M.; Ueland, P.M.; McNulty, H.; Strain, J.J.; Parker, M.J.; Welch, R.W. Consumption of wheat aleurone-rich foods increases fasting plasma betaine and modestly decreases fasting homocysteine and LDL-cholesterol in adults. J. Nutr. 2010, 140, 2153–2157. [Google Scholar] [CrossRef]
- Kettunen, H.; Peuranen, S.; Tiihonen, K. Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 129, 595–603. [Google Scholar] [CrossRef]
- Courtenay, E.S.; Capp, M.W.; Anderson, C.F.; Record, M.T., Jr. Vapor pressure osmometry studies of osmolyte-protein interactions: Implications for the action of osmoprotectants in vivo and for the interpretation of “osmotic stress” experiments in vitro. Biochemistry 2000, 39, 4455–4471. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- Spaggiari, M.; Calani, L.; Folloni, S.; Ranieri, R.; Dall’Asta, C.; Galaverna, G. The impact of processing on the phenolic acids, free betaine and choline in Triticum spp. L. whole grains milling by-products. Food Chem. 2020, 311, 125940. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.; Mansoor, M.; Pentieva, K.; Hamer, M.; Mishra, G. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: The national diet and nutrition survey of people aged 65 years and over. Brit. J. Nutr. 2010, 104, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Smith, S.; De Jager, C.; Whitbread, P.; Johnston, C.; Agacinski, G. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Lee, J.E.; Jacques, P.F.; Dougherty, L.; Selhub, J.; Giovannucci, E.; Zeisel, S.H.; Cho, E. Are dietary choline and betaine intakes determinants of total homocysteine concentration? Am. J. Clin. Nutr. 2010, 91, 1303–1310. [Google Scholar] [CrossRef]
- Wilken, D.E.; Wilken, B.; Dudman, N.P.; Tyrell, P.A. Homocystenuria-the effects of betaine in the treatment of patients not responsive to pyridoxine. N. Engl. J. Med. 1983, 309, 448–453. [Google Scholar] [CrossRef]
- Brouwer, I.A.; Verhoef, P.; Urgert, R. Betaine supplementation and plasma homocysteine in healthy volunteers. Arch. Intern. Med. 2000, 160, 2546–2547. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Giovannucci, E.L.; Hankinson, S.E.; Zeisel, S.H.; Dougherty, L.W.; Willett, W.C.; Rimm, E.B. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 2007, 86, 1073–1081. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Tuma, D.J. Betaine, ethanol, and the liver: A review. Alcohol 1996, 13, 395–398. [Google Scholar] [CrossRef]
- Parkhitko, A.A.; Jouandin, P.; Mohr, S.E.; Perrimon, N. Methionine metabolism and methyltransferases in the regulation of aging and lifespan extension across species. Aging Cell 2019, 18, e13034. [Google Scholar] [CrossRef]
- McFadden, J.W.; Myers, W.A. Trimethylamine N-Oxide in Humans and Dairy Cows: Should We Be Concerned? In Proceedings of the Virtual Cornell Nutrition Conference, Ithaca, NY, USA, 19–22 October 2020. [Google Scholar]
- Fu, B.C.; Hullar, M.A.J.; Randolph, T.W.; Franke, A.A.; Monroe, K.R.; Cheng, I.; Wilkens, L.R.; Shepherd, J.A.; Madeleine, M.M.; Le Marchand, L.; et al. Associations of plasma trimethylamine N-oxide, choline, carnitine, and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am. J. Clin. Nutr. 2020, 111, 1226–1234. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef]
- Filipčev, B.; Kojić, J.; Krulj, J.; Bodroža-Solarov, M.; Ilić, N. Betaine in Cereal Grains and Grain-Based Products. Foods 2018, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, K.K.; Riihinen, M.L.; Sarkkinen, E.; Craig, S.A.S.; Tenning, P. Chapter 12: Authorised EU health claims for betaine. In Foods, Nutrients and Food Ingredients with Authorised EU Health Claims; Sadler, M.J., Ed.; Woodhead Publishing Series in Food Science; Technology and Nutrition: Cambridge, UK, 2014; pp. 251–273. [Google Scholar] [CrossRef]
- De Zwart, F.J.; Slow, S.; Payne, R.J.; Lever, M.; George, P.M.; Gerrard, J.A.; Chambers, S.T. Glycine betaine and glycine betaine analogues in common foods. Food Chem. 2003, 83, 197–204. [Google Scholar] [CrossRef]
- Bruce, S.J.; Guy, P.A.; Rezzi, S.; Ross, A.B. Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J. Agric. Food Chem. 2010, 58, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Zangger, A.; Guiraud, S.P. Cereal foods are the major source of betaine in the Western diet–analysis of betaine and free choline in cereal foods and updated assessments of betaine intake. Food Chem. 2014, 145, 859–865. [Google Scholar] [CrossRef]
- Slow, S.; Donaggio, M.; Cressey, P.J.; Lever, M.; George, P.M.; Chambers, S.T. The betaine content of New Zealand foods and estimated intake in the New Zealand diet. J. Food Comp. Anal. 2005, 18, 473–485. [Google Scholar] [CrossRef]
- Corol, D.I.; Ravel, C.; Raksegi, M.; Bedo, Z.; Charmet, G.; Beale, M.H.; Shwery, P.R.; Ward, J.L. Effects of genotype and environment on the contents of betaine, choline, and trigonelline in cereal grains. J. Agric. Food Chem. 2012, 60, 5471–5481. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Li, H.; Lai, F.; Li, X.; Tang, Y.; Min, T.; Wu, H. Antioxidant Mechanism of Betaine without Free Radical Scavenging Ability. J. Agric. Food Chem. 2016, 64, 7921–7930. [Google Scholar] [CrossRef]
- Alirezaei, M.; Khoshdel, Z.; Dezfoulian, O.; Rashidipour, M.; Taghadosi, V. Beneficial antioxidant properties of betaine against oxidative stress mediated by levodopa/benserazide in the brain of rats. J. Physiol. Sci. 2015, 65, 243–252. [Google Scholar] [CrossRef]
- Hassanpour, S.; Rezaei, H.; Razavi, S.M. Anti-nociceptive and antioxidant activity of betaine on formalin- and writhing tests induced pain in mice. Behav. Brain Res. 2020, 390, 112699. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, M.; Xu, M.; Wang, Y.; Zou, Q.; Xie, S.; Wang, L. Effects of betaine on non-alcoholic liver disease. Nutr. Res. Rev. 2022, 35, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Vesković, M.; Labudović-Borović, M.; Mladenović, D.; Jadžić, J.; Jorgačević, B.; Vukićević, D.; Vučević, D.; Radosavljević, T. Effect of Betaine Supplementation on Liver Tissue and Ultrastructural Changes in Methionine–Choline-Deficient Diet-Induced NAFLD. Microsc. Microanal. 2020, 26, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; da Silva, R.P.; Lamarre, S.G.; Kelly, K.B.; Jacobs, R.L.; Brosnan, M.E.; Brosnan, J.T. Betaine supplementation prevents fatty liver induced by a high-fat diet: Effects on one-carbon metabolism. Amino Acids 2015, 47, 839–846. [Google Scholar] [CrossRef]
- Mukherjee, S. Role of betaine in liver disease-worth revisiting or has the die been cast? World J. Gastroenterol. 2020, 26, 5745–5758. [Google Scholar] [CrossRef]
- Rehman, A.; Mehta, K.J. Betaine in ameliorating alcohol-induced hepatic steatosis. Eur. J. Nutr. 2022, 61, 1167–1176. [Google Scholar] [CrossRef]
- Li, J.; Li, X.M.; Caudill, M.; Malysheva, O.; Bardag-Gorce, F.; Oliva, J.; French, B.; Gorce, E.; Morgan, K.; Kathirvel, E.; et al. Betaine feeding prevents the blood alcohol cycle in rats fed alcohol continuously for 1 month using the rat intragastric tube feeding model. Exp. Mol. Pathol. 2011, 91, 540–547. [Google Scholar] [CrossRef]
- Rajdl, D.; Racek, J.; Trefil, L.; Stehlik, P.; Dobra, J.; Babuska, V. Effect of Folic Acid, Betaine, Vitamin B6, and Vitamin B12 on Homocysteine and Dimethylglycine Levels in Middle-Aged Men Drinking White Wine. Nutrients 2016, 8, 34. [Google Scholar] [CrossRef]
- Shen, H.; French, B.A.; Tillman, B.C.; Li, J.; French, S.W. Increased DNA methylation in the livers of patients with alcoholic hepatitis. Exp. Mol. Pathol. 2015, 99, 326–329. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Dai, S.; Li, Y. Preventive and therapeutic role of betaine in liver disease: A review on molecular mechanisms. Eur. J. Pharmacol. 2021, 912, 174604. [Google Scholar] [CrossRef]
- Zhai, Y.; Tang, H.; Zhang, Q.; Peng, Y.; Zhao, L.; Zhang, B.; Yang, Y.; Ma, J.; Zhu, J.; Zhang, D. The Protective Effect of Lycium barbarum Betaine and Effervescent Tablet against Carbon Tetrachloride-Induced Acute Liver Injury in Rats. Nat. Prod. Commun. 2023, 18, 1934578X231161419. [Google Scholar] [CrossRef]
- Nezgoda, I.; Moroz, L.; Singh, S.; Singh, O. Modern approaches in management of children with chronic hepatitis b in remission of acute lymphoblastic leukemia. Georgian Med. News 2020, 308, 71–79. [Google Scholar]
- Alvarenga, L.; Ferreira, M.S.; Kemp, J.A.; Mafra, D. The Role of Betaine in Patients with Chronic Kidney Disease: A Narrative Review. Curr. Nutr. Rep. 2022, 11, 395–406. [Google Scholar] [CrossRef]
- Ephraim, E.; Jewell, D.E. Betaine and Soluble Fiber Improve Body Composition and Plasma Metabolites in Cats with Chronic Kidney Disease. Front. Biosci. 2023, 15, 8. [Google Scholar] [CrossRef]
- Ephraim, E.; Jewell, D.E. Effect of Added Dietary Betaine and Soluble Fiber on Metabolites and Fecal Microbiome in Dogs with Early Renal Disease. Metabolites 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, T.; Sharma, A.K.; Singh, B.; Pathak, D.; Yadav, H.N.; Singh, A.P. Betaine attenuates sodium arsenite-induced renal dysfunction in rats. Drug Chem. Toxicol. 2022, 45, 2488–2495. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.S.G.; Fanton, S.; Cardozo, L.; Borges, N.A.; Combet, E.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. Pink pressure: Beetroot (Beta vulgaris rubra) as a possible novel medical therapy for chronic kidney disease. Nutr. Rev. 2022, 80, 1041–1061. [Google Scholar] [CrossRef]
- Luo, M.; Wang, T.; Huang, P.; Zhang, S.; Song, X.; Sun, M.; Liu, Y.; Wei, J.; Shu, J.; Zhong, T.; et al. Association of Maternal Betaine-Homocysteine Methyltransferase (BHMT) and BHMT2 Genes Polymorphisms with Congenital Heart Disease in Offspring. Reprod. Sci. 2023, 30, 309–325. [Google Scholar] [CrossRef]
- Rosas-Rodríguez, J.A.; Valenzuela-Soto, E.M. The glycine betaine role in neurodegenerative, cardiovascular, hepatic, and renal diseases: Insights into disease and dysfunction networks. Life Sci. 2021, 285, 119943. [Google Scholar] [CrossRef]
- Van Puyvelde, H.; Dimou, N.; Katsikari, A.; Indave Ruiz, B.I.; Godderis, L.; Huybrechts, I.; De Bacquer, D. The association between dietary intakes of methionine, choline and betaine and breast cancer risk: A systematic review and meta-analysis. Cancer Epidemiol. 2023, 83, 102322. [Google Scholar] [CrossRef]
- Lu, M.S.; Fang, Y.J.; Pan, Z.Z.; Zhong, X.; Zheng, M.C.; Chen, Y.M.; Zhang, C.X. Choline and betaine intake and colorectal cancer risk in Chinese population: A case-control study. PLoS ONE 2015, 10, e0118661. [Google Scholar] [CrossRef]
- Guertin, K.A.; Li, X.S.; Graubard, B.I.; Albanes, D.; Weinstein, S.J.; Goedert, J.J.; Wang, Z.; Hazen, S.L.; Sinha, R. Serum Trimethylamine N-Oxide, Carnitine, Choline, and Betaine in Relation to Colorectal Cancer Risk in the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2017, 26, 945–952. [Google Scholar] [CrossRef]
- Seyyedsalehi, M.S.; Rossi, M.; Hadji, M.; Rashidian, H.; Marzban, M.; Parpinel, M.; Fiori, F.; Naghibzadeh-Tahami, A.; Hannun, Y.A.; Luberto, C.; et al. Dietary Choline and Betaine Intake and Risk of Colorectal Cancer in an Iranian Population. Cancers 2023, 15, 2557. [Google Scholar] [CrossRef] [PubMed]
- Kar, F.; Hacioglu, C.; Kacar, S.; Sahinturk, V.; Kanbak, G. Betaine suppresses cell proliferation by increasing oxidative stress–mediated apoptosis and inflammation in DU-145 human prostate cancer cell line. Cell Stress Chaperones 2019, 24, 871–881. [Google Scholar] [CrossRef]
- Han, P.; Bidulescu, A.; Barber, J.R.; Zeisel, S.H.; Joshu, C.E.; Prizment, A.E.; Vitolins, M.Z.; Platz, E.A. Dietary choline and betaine intakes and risk of total and lethal prostate cancer in the Atherosclerosis Risk in Communities (ARIC) Study. Cancer Causes Control 2019, 30, 343–354. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, Y.; Chen, Y.; Wang, L.; Zhao, L. Betaine Induced Autophagy to Protect Neurons Against Amyloid Beta in Alzheimer’s Disease. Res. Square 2021. [Google Scholar] [CrossRef]
- Huang, B.; Hu, X.; Hu, J.; Chen, Z.; Zhao, H. Betaine Alleviates Cognitive Deficits in Diabetic Rats via PI3K/Akt Signaling Pathway Regulation. Dement. Geriatr. Cogn. Disord. 2020, 49, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Sternbach, S.; Fleming, S.; Alkhayer, K.; Shelestak, J.; Popescu, D.; Weaver, A.; Clements, R.; Wasek, B.; Bottiglieri, T.; et al. Betaine restores epigenetic control and supports neuronal mitochondria in the cuprizone mouse model of multiple sclerosis. Epigenetics 2020, 15, 871–886. [Google Scholar] [CrossRef]
- Li, Q.; Qu, M.; Wang, N.; Wang, L.; Fan, G.; Yang, C. Betaine protects rats against ischemia/reperfusion injury-induced brain damage. J. Neurophysiol. 2022, 127, 444–451. [Google Scholar] [CrossRef]
- Cholewa, J.M.; Hudson, A.; Cicholski, T.; Cervenka, A.; Barreno, K.; Broom, K.; Barch, M.; Craig, S.A.S. The effects of chronic betaine supplementation on body composition and performance in collegiate females: A double-blind, randomized, placebo controlled trial. J. Int. Soc. Sport. Nutr. 2018, 15, 37. [Google Scholar] [CrossRef]
- Nobari, H.; Kargarfard, M.; Minasian, V.; Cholewa, J.M.; Pérez-Gómez, J. The effects of 14-week betaine supplementation on endocrine markers, body composition and anthropometrics in professional youth soccer players: A double blind, randomized, placebo-controlled trial. J. Int. Soc. Sport. Nutr. 2021, 18, 20. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Tinsley, G.M.; Asbaghi, O.; Salehpour, S.; Kashkooli, S.; Kooti, W.; Wong, A. Betaine supplementation fails to improve body composition: A systematic review and meta-analysis. Br. J. Nutr. 2022, 128, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-S.; Chor, W.-K.; Tuzan, A.D.; Shapawi, R.; Kawamura, G. Betaine is a feed enhancer for juvenile grouper (Epinephelus fuscoguttatus) as determined behaviourally. J. Appl. Anim. Res. 2016, 44, 415–418. [Google Scholar] [CrossRef]
- Zulkifli, I.; Mysahra, S.; Jin, L. Dietary Supplementation of Betaine (Betafin®) and Response to High Temperature Stress in Male Broiler Chickens. Anim. Biosci. 2004, 17, 244–249. [Google Scholar] [CrossRef]
- Filipčev, B.; Krulj, J.; Kojić, J.; Šimurina, O.; Solarov, M.B.; Pestorić, M. Quality attributes of cookies enriched with betaine. In Proceedings of the III International Congress Food Technology, Quality and Safety, Novi Sad, Serbia, 25–27 October 2016; pp. 47–52. [Google Scholar]
- Filipčev, B.; Krulj, J.; Brkljača, J.; Šimurina, O.; Jambrec, D.; Bodroža-Solarov, M. Fortification of gluten-free biscuits with betaine. In Proceedings of the 8th International Congress Flour-Bread’15, 10th Croatian Congress of Cereal Technologists, Opatija, Croatia, 29–30 October 2015; pp. 92–98. [Google Scholar]
- Filipčev, B.; Šimurina, O.; Brkljača, J.; Krulj, J.; Bodroža-Solarov, M.; Popov, S. Nutritional quality and baking performance of bread enriched with betaine. In Proceedings of the 11th Novel Technologies and Economic Development, Leskovac, Serbia, 23–24 October 2015; pp. 83–88. [Google Scholar]
- Kojić, J.S.; Ilić, N.M.; Kojić, P.S. Multiobjective process optimization for betaine enriched spelt flour based extrudates. J. Food Process. Eng. 2019, 42, 12942. [Google Scholar] [CrossRef]
- Perović, J.N.; Kojić, J.S.; Škrobot, D.J.; Krulj, J.A.; Peić Tukuljac, L.E.; Ilić, N.M.; Bodroža-Solarov, M.I. Betaine content in buckwheat enriched wholegrain wheat pasta. Acta Period. Technol. 2019, 50, 197–203. [Google Scholar] [CrossRef]
- Filipčev, B.; Šimurina, O.; Dapčević Hadnađev, T.; Jevtić-Mučibabić, R.; Filipović, V.; Lončar, B. Effects of liquid (native) and dry molasses originating from sugar beet on physical and textural properties of gluten-free biscuit dough. J. Texture Stud. 2015, 46, 353–364. [Google Scholar] [CrossRef]
- Filipčev, B.; Mišan, A.; Šarić, B.; Šimurina, O. Sugar beet molasses as an ingredient to enhance the nutritional and functional properties of gluten-free cookies. Int. J. Food Sci. Nutr. 2016, 67, 249–256. [Google Scholar] [CrossRef]
- Bota, M.; Chiș, S.; Pop, C.; Berbecea, A.; Alexa, E.; Muste, S. Spent malt rootlets—A new potential ingredient for functional foods. J. Agroaliment. Process. Technol. 2019, 25, 46–50. [Google Scholar]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a functional food with huge health benefits: Antioxidant, antitumor, physical function, and chronic metabolomics activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Bocanegra, A.; Macho-González, A.; Garcimartín, A.; Benedí, J.; Sánchez-Muniz, F.J. Whole Alga, Algal Extracts, and Compounds as Ingredients of Functional Foods: Composition and Action Mechanism Relationships in the Prevention and Treatment of Type-2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3816. [Google Scholar] [CrossRef]
- Musthafa, M.; Sandhu, D. Utilisation of dates for the formulation of functional food product. J. Pharm. Innov. 2022, SP-11, 519–526. [Google Scholar] [CrossRef]
- Rantanen, I.; Tenovuo, J.; Pienihäkkinen, K.; Söderling, E. Effects of a betaine-containing toothpaste on subjective symptoms of dry mouth: A randomized clinical trial. J. Contemp. Dent. Pract. 2003, 15, 11–23. [Google Scholar] [CrossRef]
- Duric, M.; Sivanesan, S.; Bakovic, M. Phosphatidylcholine functional foods and nutraceuticals: A potential approach to prevent non-alcoholic fatty liver disease. Eur. J. Lipid Sci. Technol. 2012, 114, 389–398. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Alkyl Betaines as Used in Cosmetics. Int. J. Toxicol. 2018, 37 (Suppl. S1), 28S–46S. [Google Scholar] [CrossRef]
- Fang, S.; Niu, Y.; Zhang, W.; Zhang, Y.; Yu, L.; Zhang, Y.; Li, X. Liposome-like nanocapsules of dual drug-tailed betaine for cancer therapy. Int. J. Pharm. 2015, 493, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, Y.; Xie, J.; Zhang, E.; Zhu, H.; Du, H.; Wang, K.; Song, B.; Yang, C.; Shi, Y.; et al. Zwitterionic Micelles Efficiently Deliver Oral Insulin without Opening Tight Junctions. Nat. Nanotechnol. 2020, 15, 605–614. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Qiblawey, H.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J. Mol. Liq. 2018, 256, 286–295. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Nava-Ocampo, M.F.; Al Fuhaid, L.; Santana, A.; Bucs, S.S.; Verpoorte, R.; Choi, Y.H.; Witkamp, G.J.; Vrouwenvelder, J.S.; Farinha, A.S.F. Structural properties and stability of the Betaine-Urea natural deep eutectic solvent. J. Mol. Liq. 2021, 343, 117655. [Google Scholar] [CrossRef]
- Fuad, F.M.; Nadzir, M.M. Ultrasound-assisted extraction of asiaticoside from Centella asiatica using betaine-based natural deep eutectic solvent. Ind. Crops Prod. 2023, 192, 116069. [Google Scholar] [CrossRef]
- Hefni, M.; McEntyre, C.; Lever, M.; Slow, S. Validation of HPLC-UV methods for the quantification of betaine in foods by comparison with LC-MS. Food Anal. Methods 2016, 9, 292–299. [Google Scholar] [CrossRef]
- MacKinnon, S.L.; Hiltz, D.; Ugarte, R.; Craft, C.A. Improved methods of analysis for betaines in Ascophyllum nodosum and its commercial seaweed extracts. J. Appl. Phycol. 2010, 22, 489–494. [Google Scholar] [CrossRef]
- Graham, S.; Hollis, J.H.; Miguard, M.; Browne, R.A. Analysis of betaine and choline contents of aleurone, bran, and flour fractions of wheat (Triticum aestivum L.) using 1H nuclear magnetic resonance (NMR) spectroscopy. J. Agric. Food Chem. 2009, 57, 1948–1951. [Google Scholar] [CrossRef]
- Chendrimada, T.P.; Neto, M.G.; Pesti, G.M.; Davis, A.J.; Bakalli, R.I. Determination of the betaine content of feed ingredients using high-performance liquid chromatography. J. Sci. Food Agric. 2002, 82, 1556–1563. [Google Scholar] [CrossRef]
- Gorham, J. Separation of plant betaines and their sulphur analogues by cation-exchange high-performance liquid chromatography. J. Chromatogr. A 1984, 287, 345–351. [Google Scholar] [CrossRef]
- Saarinen, M.T.; Kettunen, H.; Pulliainen, K.; Peuranen, S.; Tiihonen, K.; Remus, J. A novel method to analyze betaine in chicken liver: Effect of dietarymbetaine and choline supplementation on the hepatic betaine concentration in broiler chicks. J. Agric. Food Chem. 2001, 49, 559–563. [Google Scholar] [CrossRef]
- Huang, H.; Chen, X.S.; Liao, Q. Determination of the contents of betaine in Lycium barbarum L. by HPLC-ELSD. Chin. J. Exp. Trad. Med. Formulae 2010, 16, 20–27. [Google Scholar]
- Lee, S.M.; Park, C.K.; Cho, B.G.; Cho, K.S.; Min, B.S.; Bae, K.H. A convenient HPLC/ELSD method for the quantitative analysis of betaine in Lycium chinense. Nat. Prod. Sci. 2011, 17, 104–107. Available online: https://koreascience.kr/article/JAKO201121055567501.pdf (accessed on 18 May 2023).
- Shin, H.D.; Suh, J.H.; Kim, J.H.; Lee, H.Y.; Eom, H.Y.; Kim, U.Y.; Yang, D.H.; Han, S.B.; Youm, J.R. Determination of betaine in Fructus lycii using hydrophilic interaction liquid chromatography with evaporative light scattering detection. Bull. Korean Chem. Soc. 2012, 33, 553–558. [Google Scholar] [CrossRef]
- Zhao, B.T.; Jeong, S.Y.; Hwangbo, K.; Moon, D.C.; Seo, E.K.; Lee, D.; Min, B.S.; Ma, E.S.; Son, J.K.; Woo, M.H. Quantitative analysis of betaine in Lycii fructus by HILIC-ELSD. Arch. Pharm. Res. 2013, 36, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kojić, J.; Krulj, J.; Ilić, N.; Lončar, E.; Pezo, L.; Mandić, A.; Solarov, M.B. Analysis of betaine levels in cereals, pseudocereals and their products. J. Funct. Foods 2017, 37, 157–163. [Google Scholar] [CrossRef]
- Rivoira, L.; Studzińska, S.; Szultka-Młyńska, M.; Bruzzoniti, M.C.; Buszewski, B. New approaches for extraction and determination of betaine from Beta vulgaris samples by hydrophilic interaction liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 5133–5141. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Honarvar, M.; Zarei, A.R.; Mashhadi Akbar Boojar, M.; Bakhoda, H. A new approach for separation and recovery of betaine from beet molasses based on cloud point extraction technique. J. Food Sci. Technol. 2018, 55, 1215–1223. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Determination of Allura red in food samples after cloud point extraction using mixed micelles. Food Chem. 2011, 126, 1465–1469. [Google Scholar] [CrossRef]
- Abdollahzadeh, Z.; Honarvar, M.; Ghavami, M. Modeling the Release of Betaine Extracted from Sugar Beet Molasses in the Structure of Fast-Dissolving Electrospun Fibers of Plantago ovata Seed Gum. Food Biophys. 2022, 17, 448–459. [Google Scholar] [CrossRef]
| Food Source | µg/g DW | Ref. | Food Source | µg/g DW | Ref. |
|---|---|---|---|---|---|
| Cereals, Pseudocereals, and Products | Cereals, Pseudocereals, and Products | ||||
| Flour (wheat, refined) | 141–718 | [58,61] | Noodles (egg) | 190–1300 | [58,62] |
| Flour (wheat, wholegrain) | 604–1503 | [58,61] | Biscuits | 160–430 | [63] |
| Flour (spelt, refined) | 410–978 | [58] | Crackers (refined) | 401–460 | [61] |
| Flour (spelt, wholegrain) | 1296–1442 | [58] | Crackers (wholegrain) | 694–920 | [61] |
| Flour (rye, refined) | 310 | [58] | Crackers (rice) | 6 | [63] |
| Flour (rye, wholegrain) | 986–1500 | [58] | Bran (wheat) | 2300–7200 | [58,61,63] |
| Flour (barley, refined) | 250 | [58] | Bran (rye) | 1650–2135 | [61] |
| Flour (barley, wholegrain) | 776–1023 | [58] | Bran (oat) | 188 | [61] |
| Flour (corn, refined) | 2 | [58] | Grains (wheat) | 490–1320 | [58] |
| Flour (corn, wholegrain) | 120 | [58] | Grains (spelt wheat) | 565–2723 | [58] |
| Flour (rice, refined) | 8 | [58] | Grains (triticale) | 986–1030 | [58] |
| Flour (amaranth, wholegrain) | 871–1225 | [58] | Grains (rye) | 444–2213 | [58] |
| Flour (proso millet, refined) | 1320 | [58] | Grains (oat) | 200–1000 | [58,60] |
| Flour (buckwheat, wholegrain) | 7–108 | [58] | Grains (barley) | 460 | [58] |
| Flour (sorghum, refined) | 425 | [58] | Grains (corn) | 5–304 | [58,60] |
| Bread (white wheat) | 174–520 | [58,61,62,63] | Grains (proso millet, dehulled) | 281 | [58] |
| Bread (wholegrain wheat) | 499–1000 | [58,60,62,63] | Grains (quinoa) | 610–6300 | [58,62] |
| Bread (wholegrain rye) | 855–1666 | [58,61] | Grains (buckwheat) | 6–26 | [58,61] |
| Bread (wholegrain spelt) | 913 | [58] | Grains (amaranth) | 646–7420 | [58] |
| Pasta (white wheat) | 222–773 | [58,61] | Grains (white rice) | 2–5 | [61,62] |
| Pasta (wholegrain wheat) | 375–1327 | [58,61,62] | Grains (brown rice) | 3–9 | [61,62] |
| Pasta (barley) | 211 | [58] | Corn flakes | 6–120 | [58,63] |
| Pasta (cooked) | 228–352 | [60] | Flips (white, small grains) | 100–200 | [62] |
| Couscous (wholegrain) | 544–1299 | [61] | Biscuits | 160–430 | [63] |
| Tortilla (wheat) | 311 | [58] | Fruit and Vegetables | ||
| Breakfast cereal | 10–1041 | [61] | |||
| Noodles (white rice) | 3 | [61] | Apricot | Trace | [62] |
| Noodles (brown rice) | 6 | [61] | Apples | 1 | [57] |
| Grapes | 1 | [57,63] | Legumes and Products | ||
| Oranges | 1 | [57] | |||
| Blueberries | 2 | [57] | Peanuts | 6 | [57] |
| Strawberry | 2 | [57,63] | Soybean | 21 | [57,59] |
| Grapefruit | 2 | [57] | Tofu | 4 | [57] |
| Peaches | 3 | [57] | Lentils | <10 | [60] |
| Watermelon | 3 | [57] | Peas | <5 | [60] |
| Prune | 4 | [57,59,60] | Oils and Fats | ||
| Banana | <5 | [60] | |||
| Kiwifruit | <5 | [60] | Margarine (olive oil) | <5 | [60] |
| Pear | <10 | [60] | Olive oil | 1 | [57] |
| Avocado | 3–35 | [57,59,60,63] | Fungi | ||
| Raisins | 3 | [57,59] | |||
| Tomato | Trace | [63] | Mushrooms | 10–110 | [57,59,60] |
| Onion | Trace | [60,63] | Spices and Herbs | ||
| Celery | 1 | [57] | |||
| Potato | 26–350 | [59,63] | Pepper (red) | 12 | [63] |
| Asparagus | 33–45 | [63] | Pepper (green) | 24–31 | [63] |
| Cabbage | 3 | [57,59] | Mustard seed | 19 | [57] |
| Zucchini | 3 | [57] | Meat and Seafood | ||
| Carrot | 4 | [57,59] | |||
| Broccoli | <10 | [60] | Beef | 58–170 | [59,60,63] |
| Cauliflower | <10 | [60] | Mutton | 62–180 | [63] |
| Garlic | <10 | [60] | Lamb | 72 | [60] |
| Lettuce | <10 | [60] | Pork | 41 | [60] |
| Beetroot | 750–1290 | [57,60] | Chicken breast fillet | 180–200 | [59,60] |
| Beetroot (canned) | 2600–3337 | [57,59] | Chicken liver | 129 | [57,59] |
| Silverbeet | 910 | [60] | Bacon | 20–97 | [57,59,63] |
| Spinach | 675–1100 | [57,59,60] | Ham | 81–95 | [63] |
| Sausage (beef, raw) | 320 | [63] | Beverages | ||
| Sausage (pork) | 36 | [59] | |||
| Fish (fresh) | 120–150 | [63] | Orange juice | 3–20 | [57,59] |
| Fish (canned) | 20–45 | [63] | Apple juice | 1 | [57] |
| Fish sticks | 330 | [59] | Coffee (instant) | 62–68 | [63] |
| Clams | 2500 | [59,60] | Tea (black) | 10–120 | [59,60,63] |
| Cod | 25 | [60] | Wine (white) | Trace | [63] |
| Groper | 12 | [60] | Wine (red) | <5 | [60] |
| Monkfish | 500 | [60] | Beer | 56–81 | [59,63] |
| Mussel | 1120–11,600 | [60] | Ready-to-eat Food | ||
| Oyster | 2780–2810 | [60] | |||
| Scallops | 640–1180 | [60] | Pizza | 260 | [57,59] |
| Salmon | 19–43 | [59,60] | Hamburger | 333 | [57,59] |
| Perch | 26 | [59,60] | Hot dog and bun | 443 | [57,59] |
| Tuna | <10 | [60] | Fat-free salad dressing | 18 | [59] |
| Dairy and Eggs | Tacos/burritos | 150 | [57] | ||
| Lasagna | 61 | [57] | |||
| Milk | 7–28 | [57,59,60,63] | Falafel | 202 | [60] |
| Milk (chocolate) | 6 | [63] | Milk chocolate | 3–26 | [57,59,60] |
| Milk (soya) | 12 | [63] | Doughnuts | 270–380 | [58] |
| Yoghurt | 5–9 | [57,59,60] | Apple pie | 160 | [57,58] |
| Sour cream | 5–7 | [57,60] | Danish pastry | 140 | [57,58] |
| Cheese | 5–67 | [57,59,60,63] | Ready-to-eat pancakes | 690–720 | [58] |
| Ice cream | 6–11 | [57,59] | Fat-free salad dressing | 18 | [59] |
| Butter | 3 | [57] | Soy sauce | 396 | [57,59] |
| Eggs | 10–60 | [57,59,60] | Popcorn | 4 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrijević, D.; Pastor, K.; Nastić, N.; Özogul, F.; Krulj, J.; Kokić, B.; Bartkiene, E.; Rocha, J.M.; Kojić, J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules 2023, 28, 4824. https://doi.org/10.3390/molecules28124824
Dobrijević D, Pastor K, Nastić N, Özogul F, Krulj J, Kokić B, Bartkiene E, Rocha JM, Kojić J. Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules. 2023; 28(12):4824. https://doi.org/10.3390/molecules28124824
Chicago/Turabian StyleDobrijević, Dejan, Kristian Pastor, Nataša Nastić, Fatih Özogul, Jelena Krulj, Bojana Kokić, Elena Bartkiene, João Miguel Rocha, and Jovana Kojić. 2023. "Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods" Molecules 28, no. 12: 4824. https://doi.org/10.3390/molecules28124824
APA StyleDobrijević, D., Pastor, K., Nastić, N., Özogul, F., Krulj, J., Kokić, B., Bartkiene, E., Rocha, J. M., & Kojić, J. (2023). Betaine as a Functional Ingredient: Metabolism, Health-Promoting Attributes, Food Sources, Applications and Analysis Methods. Molecules, 28(12), 4824. https://doi.org/10.3390/molecules28124824