Rapid Construction of a Chloromethyl-Substituted Duocarmycin-like Prodrug
Abstract
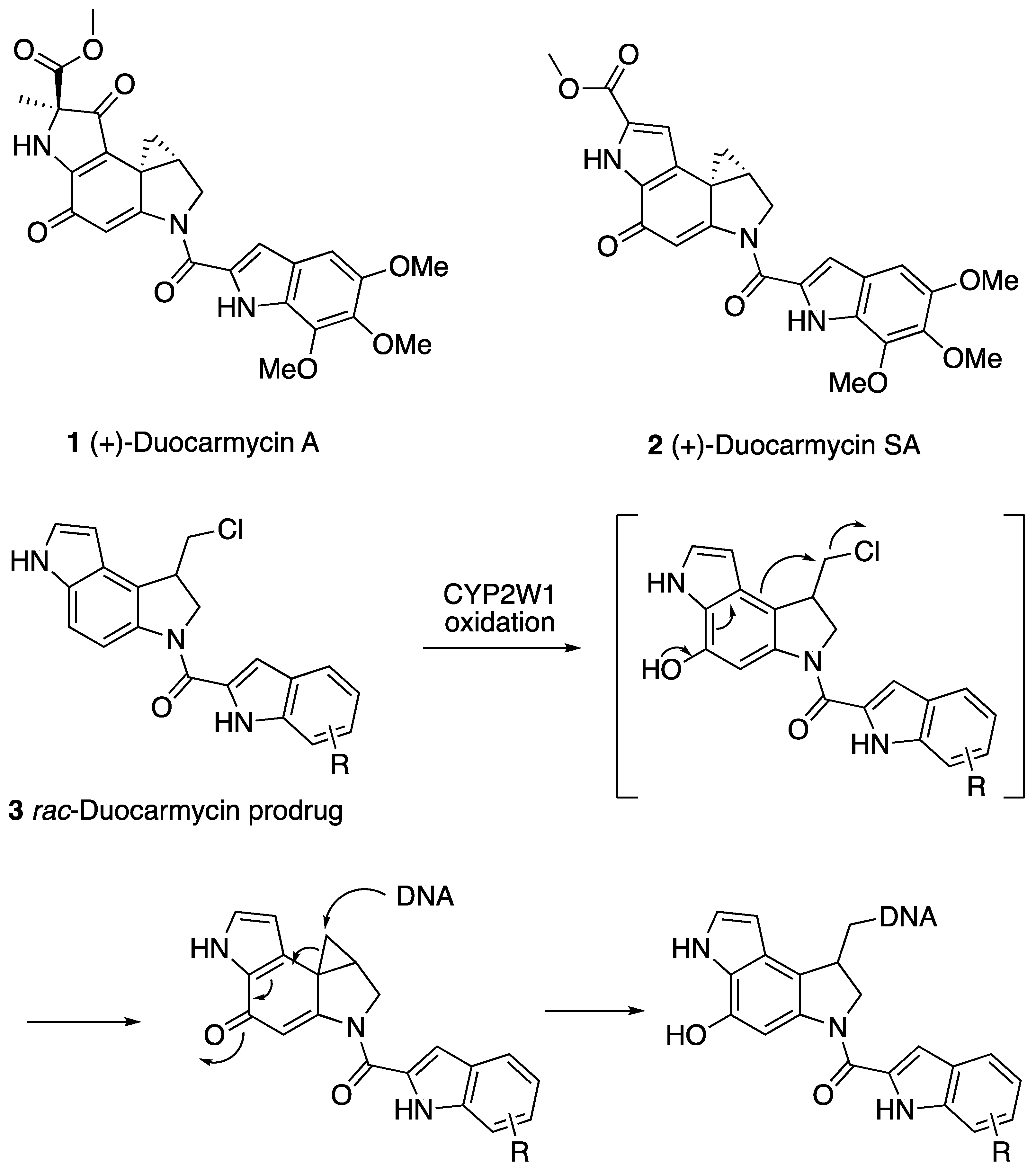
1. Introduction
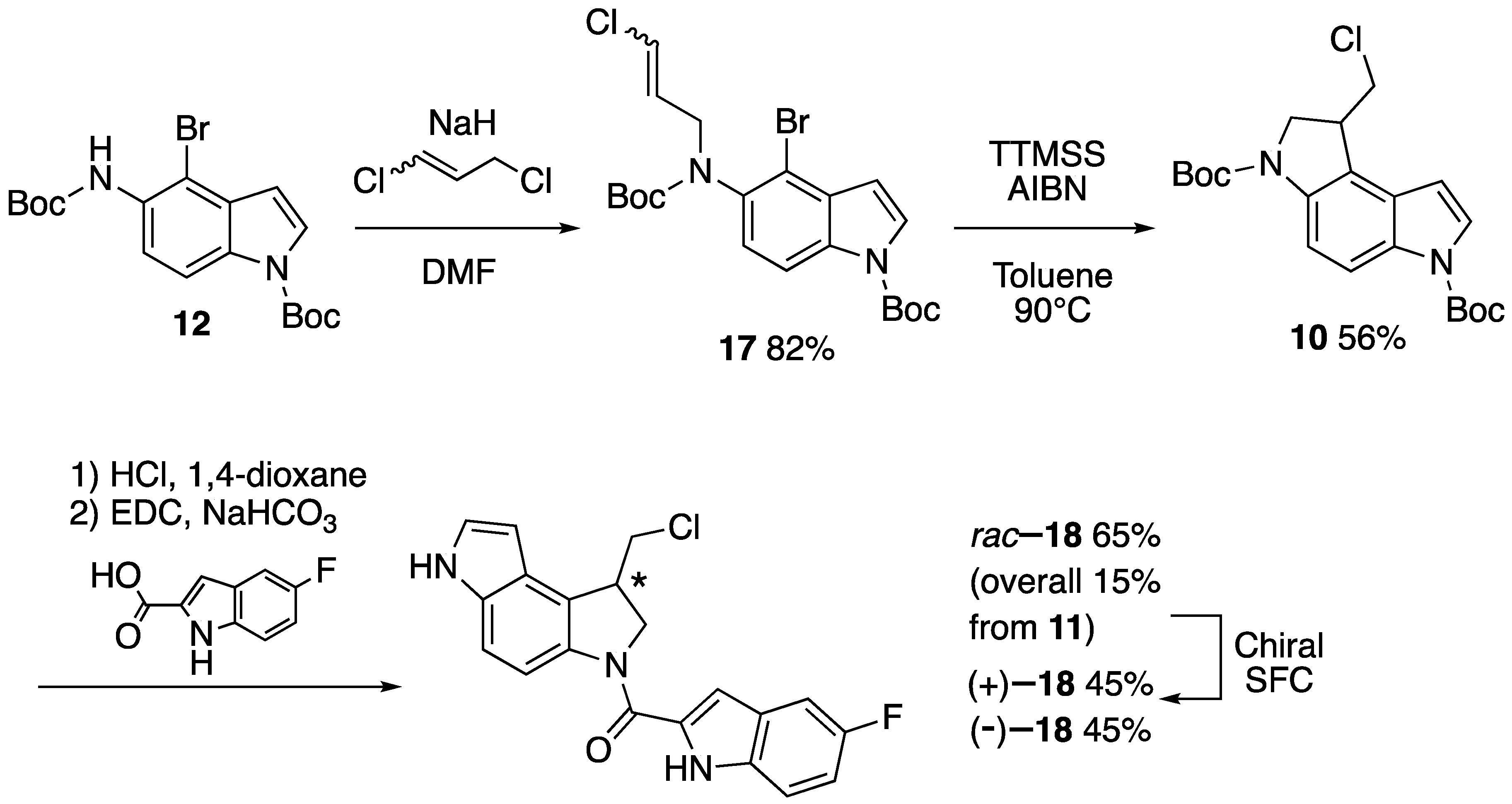
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Boger, D.L.; Johnson, D.S. CC-1065 and the Duocarmycins: Understanding their Biological Function through Mechanistic Studies. Angew. Chem. Int. Ed. Engl. 1996, 35, 1438–1474. [Google Scholar] [CrossRef]
- Boger, D.L.; Boyce, C.W.; Garbaccio, R.M.; Goldberg, J.A. CC-1065 and the Duocarmycins: Synthetic Studies. Chem. Rev. 1997, 97, 787–828. [Google Scholar] [CrossRef] [PubMed]
- Tercel, M.; Gieseg, M.A.; Denny, W.A.; Wilson, W.R. Synthesis and Cytotoxicity of Amino-seco-DSA: An Amino Analogue of the DNA Alkylating Agent Duocarmycin SA. J. Org. Chem. 1999, 64, 5946–5953. [Google Scholar] [CrossRef]
- Takahashi, I.; Takahashi, K.-I.; Ichimura, M.; Morimoto, M.; Asano, K.; Kawamoto, I.; Tomita, F.; Nakano, H. Duocarmycin A, a new antitumor antibiotic from Streptomyces. J. Antibiot. 1988, 41, 1915–1917. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, M.; Ogawa, T.; Takahashi, K.-I.; Kobayashi, E.; Kawamoto, I.; Yasuzawa, T.; Takahashi, I.; Nakano, H. Duocarmycin SA, a new antitumor antibiotic from Streptomyces sp. J. Antibiot. 1990, 43, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Nakatani, K.; Ito, Y.; Terashima, S. First total synthesis of dl-duocarmycin A. Tetrahedron Lett. 1990, 31, 6699–6702. [Google Scholar] [CrossRef]
- Boger, D.L.; McKie, J.A.; Nishi, T.; Ogiku, T. Enantioselective Total Synthesis of (+)-Duocarmycin A, epi-(+)-Duocarmycin A, and Their Unnatural Enantiomers. J. Am. Chem. Soc. 1996, 118, 2301–2302. [Google Scholar] [CrossRef]
- Boger, D.L.; Machiya, K. Total synthesis of (+)-duocarmycin SA. J. Am. Chem. Soc. 1992, 114, 10056–10058. [Google Scholar] [CrossRef]
- Tietze, L.F.; Schuster, H.J.; Schmuck, K.; Schuberth, I.; Alves, F. Duocarmycin-based prodrugs for cancer prodrug monotherapy. Bioorg. Med. Chem. 2008, 16, 6312–6318. [Google Scholar] [CrossRef]
- Li, L.-S.; Sinha, S.C. Studies toward the duocarmycin prodrugs for the antibody prodrug therapy approach. Tetrahedron Lett. 2009, 50, 2932–2935. [Google Scholar] [CrossRef]
- Schuster, H.J.; Krewer, B.; Von Hof, J.M.; Schmuck, K.; Schuberth, I.; Alves, F.; Tietze, L.F. Synthesis of the first spacer containing prodrug of a duocarmycin analogue and determination of its biological activity. Org. Biomol. Chem. 2010, 8, 1833–1842. [Google Scholar] [CrossRef]
- Lajiness, J.P.; Robertson, W.M.; Dunwiddie, I.; Broward, M.A.; Vielhauer, G.A.; Weir, S.J.; Boger, D.L. Design, Synthesis, and Evaluation of Duocarmycin O-Amino Phenol Prodrugs Subject to Tunable Reductive Activation. J. Med. Chem. 2010, 53, 7731–7738. [Google Scholar] [CrossRef]
- Tietze, L.E.; Schmuck, K.; Schuster, H.J.; Müller, M.; Schuberth, I. Synthesis and Biological Evaluation of Prodrugs Based on the Natural Antibiotic Duocarmycin for Use in ADEPT and PMT. Chem. Eur. J. 2011, 17, 1922–1929. [Google Scholar] [CrossRef]
- Pors, K.; Loadman, P.M.; Shnyder, S.D.; Sutherland, M.; Sheldrake, H.M.; Guino, M.; Kiakos, K.; Hartley, J.A.; Searcey, M.; Patterson, L.H. Modification of the duocarmycin pharmacophore enables CYP1A1 targeting for biological activity. Chem. Commun. 2011, 47, 12062–12064. [Google Scholar] [CrossRef]
- Wolfe, A.L.; Duncan, K.K.; Parelkar, N.K.; Weir, S.J.; Vielhauer, G.A.; Boger, D.L. A Novel, Unusually Efficacious Duocarmycin Carbamate Prodrug That Releases No Residual Byproduct. J. Med. Chem. 2012, 55, 5878–5886. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Denny, W.A.; Tercel, M.; Pruijn, F.B.; Ashoorzadeh, A. Nitro seco Analogues of the Duocarmycins Containing Sulfonate Leaving Groups as Hypoxia-Activated Prodrugs for Cancer Therapy. J. Med. Chem. 2012, 55, 2780–2802. [Google Scholar] [CrossRef]
- Sheldrake, H.M.; Travica, S.; Johansson, I.; Loadman, P.M.; Sutherland, M.; Elsalem, L.; Illingworth, N.; Cresswell, A.J.; Reuillon, T.; Shnyder, S.D.; et al. Re-engineering of the Duocarmycin Structural Architecture Enables Bioprecursor Development Targeting CYP1A1 and CYP2W1 for Biological Activity. J. Med. Chem. 2013, 56, 6273–6277. [Google Scholar] [CrossRef]
- Uematsu, M.; Brody, D.M.; Boger, D.L. A five-membered lactone prodrug of CBI-based analogs of the duocarmycins. Tetrahedron Lett. 2015, 56, 3101–3104. [Google Scholar] [CrossRef]
- Giddens, A.C.; Lee, H.H.; Lu, G.-L.; Miller, C.K.; Guo, J.; Phillips, G.D.L.; Pillow, T.H.; Tercel, M. Analogues of DNA minor groove cross-linking agents incorporating aminoCBI, an amino derivative of the duocarmycins: Synthesis, cytotoxicity, and potential as payloads for antibody–drug conjugates. Bioorg. Med. Chem. 2016, 24, 6075–6081. [Google Scholar] [CrossRef]
- Menderes, G.; Bonazzoli, E.; Bellone, S.; Black, J.; Altweger, G.; Masserdotti, A.; Pettinella, F.; Zammataro, L.; Buza, N.; Hui, P.; et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecol. Oncol. 2017, 146, 179–186. [Google Scholar] [CrossRef]
- Forbes, I.T.; Ham, P.; Booth, D.H.; Martin, R.T.; Thompson, M.; Baxter, G.S.; Blackburn, T.P.; Glen, A.; Kennett, G.A.; Wood, M.D. 5-Methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole: A Novel 5-HT2C/5-HT2B Receptor Antagonist with Improved Affinity, Selectivity, and Oral Activity. J. Med. Chem. 1995, 38, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Ganton, M.D.; Kerr, M.A. A Domino Amidation Route to Indolines and Indoles: Rapid Syntheses of Anhydrolycorinone, Hippadine, Oxoassoanine, and Pratosine. Org. Lett. 2005, 7, 4777–4779. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, D. N-Iodosaccharin—A New Reagent for Iodination of Alkenes and Activated Aromatics. Synlett 2000, 4, 544–546. [Google Scholar]
- Harrington, P.J.; Hegedus, L.S. Palladium-catalyzed reactions in the synthesis of 3- and 4-substituted indoles. Approaches to ergot alkaloids. J. Org. Chem. 1984, 49, 2657–2662. [Google Scholar] [CrossRef]
- Harrington, P.J.; Hegedus, L.S.; McDaniel, K.F. Palladium-catalyzed reactions in the synthesis of 3- and 4-substituted indoles. 2. Total synthesis of the N-acetyl methyl ester of (+/−)-clavicipitic acids. J. Am. Chem. Soc. 1987, 109, 4335–4338. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Toro, J.L.; Miles, W.H.; Harrington, P.J. Palladium-catalyzed reactions in the synthesis of 3- and 4-substituted indoles. 3. Total synthesis of (+/−)-aurantioclavine. J. Org. Chem. 1987, 52, 3319–3322. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Sestrick, M.R.; Michaelson, E.T.; Harrington, P.J. Palladium-catalyzed reactions in the synthesis of 3- and 4-substituted indoles. 4. J. Org. Chem. 1989, 54, 4141–4146. [Google Scholar] [CrossRef]
- Hellal, M.; Singh, S.; Cuny, G.D. Synthesis of Tetracyclic Indoles via Intramolecular α-Arylation of Ketones. J. Org. Chem. 2012, 77, 4123–4130. [Google Scholar] [CrossRef]
- Chen, K.X.; Vibulbhan, B.; Yang, W.; Sannigrahi, M.; Velazquez, F.; Chan, T.-Y.; Venkatraman, S.; Anilkumar, G.N.; Zeng, Q.; Bennet, F.; et al. Structure–Activity Relationship (SAR) Development and Discovery of Potent Indole-Based Inhibitors of the Hepatitis C Virus (HCV) NS5B Polymerase. J. Med. Chem. 2012, 55, 754–765. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bengtsson, C.; Gravenfors, Y. Rapid Construction of a Chloromethyl-Substituted Duocarmycin-like Prodrug. Molecules 2023, 28, 4818. https://doi.org/10.3390/molecules28124818
Bengtsson C, Gravenfors Y. Rapid Construction of a Chloromethyl-Substituted Duocarmycin-like Prodrug. Molecules. 2023; 28(12):4818. https://doi.org/10.3390/molecules28124818
Chicago/Turabian StyleBengtsson, Christoffer, and Ylva Gravenfors. 2023. "Rapid Construction of a Chloromethyl-Substituted Duocarmycin-like Prodrug" Molecules 28, no. 12: 4818. https://doi.org/10.3390/molecules28124818
APA StyleBengtsson, C., & Gravenfors, Y. (2023). Rapid Construction of a Chloromethyl-Substituted Duocarmycin-like Prodrug. Molecules, 28(12), 4818. https://doi.org/10.3390/molecules28124818






