Abstract
The genus Thymus L., belonging to the Lamiaceae family, contains about 220 species with a distribution that mainly extends in Europe, northwest Africa, Ethiopia, Asia, and southern Greenland. Due to their excellent biological properties, fresh and/or dried leaves and aerial parts of several Thymus ssp. have been utilized in the traditional medicine of many countries. To evaluate not only the chemical aspects but also the biological properties, the essential oils (EOs), obtained from the pre-flowering and flowering aerial parts of Thymus richardii subsp. nitidus (Guss.) Jalas, endemic to Marettimo Island (Sicily, Italy), were investigated. The chemical composition of the EOs, obtained by classical hydrodistillation and GC-MS and GC-FID analyses, showed the occurrence of similar amounts of monoterpene hydrocarbons, oxygenated monoterpenes, and sesquiterpene hydrocarbons. The main constituents of the pre-flowering oil were β-bisabolene (28.54%), p-cymene (24.45%), and thymol methyl ether (15.90%). The EO obtained from the flowering aerial parts showed as principal metabolites β-bisabolene (17.91%), thymol (16.26%), and limonene (15.59%). The EO of the flowering aerial parts, and its main pure constituents, β-bisabolene, thymol, limonene, p-cymene, and thymol methyl ether were investigated for their antimicrobial activity against oral pathogens and for their antibiofilm and antioxidant properties.
1. Introduction
The genus Thymus L. (Lamiaceae) is considered one of the largest genus in the Lamiaceae family, comprising approximately 220 accepted species. Most of these species are chamaephytes, and they are distributed throughout Europe, northwest Africa, Ethiopia, Asia, and southern Greenland [1,2]. Due to its pleasant flavor and nutritional and medicinal values, Thymus ssp. has been largely employed in the food, pharmaceutical, cosmetic, and perfume industries [3,4]. Due to their excellent biological properties, the fresh or dried leaves and flowering parts of several Thymus ssp. have been utilized in the traditional medicine of many countries as antimicrobial, anti-spasmodic, and antioxidant treatments for different digestive and respiratory illnesses [5].
Several Thymus ssp. extracts have been investigated for their non-volatile organic compounds. The main metabolites occurring in them are flavonoids, phenylpropanoids, lignans, tannins, organic acids, and terpenoids. Furthermore, the antimicrobial, antioxidant, antitumor, anti-inflammatory, analgesic, antispasmodic, carminative, anti-hypertensive, anti-diabetic, etc., properties of many of them have been proven both in vitro and in vivo [5]. Thymus plants, such as T. vulgaris L., T. serpyllum L., T. pulegioides L., and T. zygis L., have a rich historical background and hold significant commercial value [6]. Traditionally, these plants have been utilized in various ways, such as tea, spices, and traditional remedies, due to their favorable effects on digestive and respiratory ailments [4,7]. Thymus plants are considered warming and pungent in traditional Chinese medicine, stimulating appetite and aiding digestion [8]. Furthermore, a comprehensive review has been conducted on the phytochemical and pharmacological investigations of T. daenensis Celak from Iran, used extensively in folk medicine [9]. In fact, the medicinal properties of Thymus plants are widely acknowledged, including their use for inflammatory conditions, cardiovascular diseases, kidney disorders, and women’s health concerns [10,11,12].
For instance, in Spain, T. vulgaris is used for postpartum cleansing [13]. In India, T. serpyllum is recognized for its effectiveness in treating menstrual disorders [14]. In Tunisia, T. algeriensis Boiss. and Reut. is believed to prevent abortion and complications during pregnancy [15]. Apart from their medicinal properties, Thymus plants are extensively utilized in culinary preparations for their aromatic qualities and taste. They are incorporated into various recipes, including baked goods, sauces, meats, vegetarian dishes, desserts, and fresh salads [16,17]. In Italy, dried Thymus leaves are also combined with other herbs for perfuming clothes or rooms [14].
Furthermore, most of the published papers concerning the essential oils (EOs) of Thymus ssp. were characterized, in many cases, by the occurrence of two main aromatic compounds, carvacrol and thymol, together with a minor quantity of p-cymene and γ-terpinene [18,19]. Other metabolites present in the EOs are linalool, borneol, and 1,8-cineole [20,21].
Furthermore, thanks to their antimicrobial and/or antioxidant compounds, EOs of the Thymus species have been used in recent years as alternatives to commercial synthetic chemicals. In fact, to prolong the shelf-life of fresh foods, they have been incorporated into packaging materials [22,23,24], used as corrosion inhibitors for different metals in various acids [25], and applied in the disinfection of historical art materials [26,27,28,29].
Based on current knowledge, there are 20 taxa present in Italy [30], of which five are found in Sicily. The Sicilian taxa consist of T. spinulosus Ten. and T. paronychioides Čelak., which belong to Thymus sect. Hypodromi, as well as T. richardii subsp. nitidus (Guss.) Jalas, T. longicaulis C. Presl, and T. praecox subsp. parvulus (Lojac.) Bartolucci, Peruzzi, and N.G.Passal, which belong to Th. sect. Serpyllum [31].
Thymus richardii s.l., according to Euro + Med PlantBase [32], has a distribution that extends beyond Sicily, Spain, and the Balkans. Within the species, four subspecies are distinguished: T. richardii Pers. subsp. richardii [syn. T. aureopunctatus (Beck) K. Malý] present in the Balkans and Baleares Islands; T. richardii subsp. ebusitanus (Font Quer) Jalas [syn. T. ebusitanus (Font Quer) Romo] exclusive to the Baleares Islands; T. richardii subsp. vigoi Riera, Güemes and Rosselló growing in Spain; and T. richardii subsp. nitidus (Guss.) Jalas [syn. T. nitidus Guss.; T. lucidus Guss.] endemic of the island of Marettimo (Sicily, Italy).
Thymus richardii subsp. nitidus (Guss.) Jalas, [≡ Thymus nitidus Guss. ≡ Thymus serpyllum var. nitidus (Guss.) Bég.] (Figure 1) is a chamaephyte that grows 8–15 cm tall. It has woody, ascending, or suberect stems with amphitrichous indumentum (hairs on two opposite sides).

Figure 1.
The habitus of Thymus richardii subsp. nitidus (a); inflorescence with purplish flowers (b); T. richardii subsp. nitidus in the collection of Palermo Botanical Garden (c); the rocky habitats at Punta Madonnuzza on Marettimo Island (d).
Its lanceolate leaves are 7–9 mm long and 3–4 mm wide, glabrous, and not ciliate on the margin. The subspherical inflorescence has purplish flowers, and the calyx is hirsute with glandular hairs, while the corolla is 7–9 mm long. Flowering occurs from May to June [33]. Morales [34] reported that the chromosome number for this plant is 2n = 28. This taxon is endemic to Marettimo Island (W. Sicily). It grows on habitats with rocky, calcareous substrates and occurs in five localities on the island: Mt. Lissandro, Semaforo, Punta Anzini, Libbano, and Punta Madonnuzza (200–600 m a.s.l.) [35,36].
Thymus richardii subsp. nitidus can be considered one of the rarest thyme species in Italy, although it is listed as Near Threatened (NT) [37]. This is because its habitat is not significantly threatened and the population is mostly stable [38].
The only previous report on the biological properties of T. richardii subsp. nitidus concerns the methanolic extract of the aerial parts that was screened for its inhibitory effect on the production of leukotriene B4 by 5-lipoxygenase in intact cells. It showed remarkable activity, inhibiting almost completely the LTB4 production in intact rat PMNL at 200 μg/mL. This effect was maintained even at a dose of 50 μg/mL, indicating its possible use as a source of potent 5-LOX inhibitors [39].
Essential oils are effective antioxidants, mostly because of their activity in food preservation [40], and they are known to possess anti-carcinogenic, antimicrobial, and anti-inflammatory properties due to over 200 constituents [41,42]. Essential oils are a mixture of volatile constituents produced by aromatic plants, serving as a protective mechanism against microorganisms [43]. Tea tree, thyme, cinnamon, citrus, bergamot, lavender, peppermint, and many other EOs were used in dentistry to counteract bacterial pathogen action [44]. To increase antibiotic resistance and for economic reasons, people still use natural products for primary healthcare [45].
Consequently, as a continuation of our research on plants of the Mediterranean area [46,47,48,49] and their EOs biological properties [50,51,52], in the present study, it is described the EO composition of the aerial parts of T. richardii subsp. nitidus, collected at two different vegetative stages, as well as the biological properties of the EO obtained from the full-flowering aerial parts. In addition, it is reported the antimicrobial, antibiofilm, and antioxidant effects of the EO of the flowering aerial parts and of its main pure constituents, β-bisabolene and thymol.
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
Hydrodistillation of T. richardii subsp. nitidus aerial parts collected at a pre-flowering stage (PF) gave a yellow EO. Overall, sixteen compounds were identified, representing 98.13% of total components, listed in Table 1 according to their retention indices on a DB-Wax column and classified into four classes based on their chemical structures. Monoterpene hydrocarbons formed the main class, representing 33.92% of the total, with p-cymene (24.45%) and limonene (6.23%) as the most abundant components. Sesquiterpene hydrocarbons occurred in similar amounts (33.76%), with β-bisabolene (28.54%) being the principal constituent of the class and of the EO. Oxygenated monoterpenes were also present in large amounts (30.17%), with thymol methyl ether (15.90%), thymol (4.56%), and carvacrol (4.49%) as the main components of this class.

Table 1.
Chemical composition (%) of Thymus richardii subsp. nitidus EO before and during flowering, collected in Sicily, Italy.
The EO obtained from the aerial parts collected at the full flowering stage (F) showed a similar chemical profile. In this case, twenty-one metabolites were identified, representing 96.72% of the total composition. In this case, the main class was represented by oxygenated monoterpenes (39.65%), showing a larger amount of thymol (16.26%) and carvacrol (7.82%) with respect to PF and a minor quantity of thymol methyl ether (8.87%). It is noteworthy for the presence of linalool (6.70%), which is practically absent in PF (0.18%). The main constituent of the EO was always β-bisabolene (17.91%), but it was present in a minor amount with respect to PF. Among the sesquiterpene hydrocarbons, it must also be mentioned the good occurrence of germacrene D (6.14%). In F, among monoterpene hydrocarbons (29.27%), limonene (15.59%) represented the main metabolite, while p-cymene occurred only for 9.04%.
In a previous report [53], the EO of Thymus richardii subsp. nitidus, always collected on Marettimo Island but at a post-flowering stage, was analyzed. Thirty-six compounds were identified, among which β-bisabolene (32.30%), carvacrol (13.10%), thymol methyl ether (12.40%), trans-dihydrocarvone (5.l0%), t-cadinol (4.00%), and β-caryophyllene (3.40%) were identified as the main constituents. Successively, Llorens et al. [54] investigated the chemical compositions of the EOs of several Thymus taxa belonging to T. richardii Pers., namely T. richardii subsp. richardii from Bosnia and Majorca (Spain), T. richardii subsp. ebusitanus (Font Quer) Jalas from Ibiza (Spain), T. richardii subsp. vigoi Riera, Güemes and Rosselló from Valencia (Spain), as well as T. richardii subsp. nitidus from Marettimo (Sicily, Italy). In this investigation, the main constituents of the EO of T. richardii subsp. nitidus, collected at the full flowering stage, were p-cymene (25.10–15.30%), β-bisabolene (17.70–16.60%), limonene (16.70–8.80%), thymol methyl ether (14.90–2.40%), carvacrol (15.20–0%), and thymol (13.90–1.50%). Our results are quite similar to the previously reported investigations; in fact, p-cymene (24.45% and 9.04%, for PF and F, respectively), β-bisabolene (28.54% and 17.91%, for PF and F, respectively), limonene (6.23% and 15.59%, for PF and F, respectively), thymol methyl ether (15.90% and 8.87%, for PF and F, respectively), thymol (4.56% and 16.24%, for PF and F, respectively), and carvacrol (4.49% and 7.82%, for PF and F, respectively) also occurred in good amounts.
From the comparison with the chemical compositions of EOs from other species of the Thymus genus belonging to the same section (Serpyllum) and subsection (Insulares), a clear difference in the composition of F was observed.
Among the main compounds obtained in this study, there was only the common presence of thymol in a similar percentage (20%) with T. dreatensis Bratt, whose EO was found to have the ability to remove hydroxyl radicals and prevent the degradation of deoxyribose [55], while with T. guyonii de Noè, that showed antioxidant activity, the presence of p-cymene (19%), thymol (11%), and thymol methyl ether (11%) was observed [56]. On the other hand, the composition of the EO of T. willkommii Ronniger was found to be completely different, with α-terpenyl acetate (36–69%) and linalool (0–57%) as the main secondary metabolites [20]. As the main constituent, β-bisabolene was not found in other plants of the Thymus genus except for another one belonging to the same section but a different subsection (Alternantes), namely T. pulegioides L. subsp. similialpestris Debray, but in a lesser amount (7%) [57]. As regards Thymus vulgaris EOs, these have been shown to have important antibacterial and anti-biofilm activity, exhibited in seven distinct chemotypes characterized by chemical variability for the presence of compounds like thymol, linalool, carvacrol, geraniol, thujanol-4, terpineol, and 1,8-cineole [58,59]. In these compositions, the only main metabolites in common were thymol, carvacrol, and linalool, which, however, were present in greater abundance than those of T. richardii subsp. nitidus.
2.2. Antimicrobial Activity of T. richardii EO (F)
The Thymus genus exhibited potent antimicrobial activity against a wide range of microorganisms, and its primary bioactive components were EOs, particularly thymol [3,60]. These EOs have demonstrated significant inhibitory effects on both susceptible and resistant bacterial strains, and they have also exhibited strong synergistic effects when combined with other antimicrobial drugs, such as norfloxacin, clotrimazole, nystatin, and ketoconazole [61,62,63]. For example, the EO fraction of T. magnus (Nakai) Nakai, along with its major constituents, effectively inhibited Salmonella typhimurium, Staphylococcus aureus, and Streptococcus pneumoniae strains, with minimum inhibitory concentrations (MICs) ranging from 0.125 to 8 mg/mL. Notably, a synergistic effect was observed when combined with norfloxacin against S. aureus strains [62]. Thymus capitatus (L.) Hoffmanns and Link EOs, incorporated into phospholipid vesicles, demonstrated efficacy against oral cavity bacteria, including cariogenic Lactobacillus acidophilus, Streptococcus mutans, and commensal Streptococcus sanguinis, suggesting their potential in oral cavity disease treatment. The primary metabolite found in T. capitatus EO was carvacrol, present at a concentration of approximately 817 mg/mL [64]. Thymus vulgaris EO exhibited bacteriostatic activity against two major foodborne pathogens, Listeria monocytogenes and S. aureus, thanks to its high levels of p-cymene (47.9%) and thymol (43.1%) [65].
Thymus EO (F) extracted from fully flowering plants was tested on Gram-positive and Gram-negative bacteria by a modified Kirby and Bauer assay. Since there is an inhibition halo, the bacterial growth decreases when the quantity of EO increases.
Figure 2 shows the inhibition halo formed by the antibiotic (positive control) and the absence of the DMSO halo in which the EO is resuspended (negative control). As also shown in Figure 2, the EO appears to be active on both Gram-negative E. coli and Gram-positive S. aureus model strains. This type of analysis is commonly used as a first approach, which represents a qualitative screening to understand if an EO or a compound has antimicrobial activity [66].
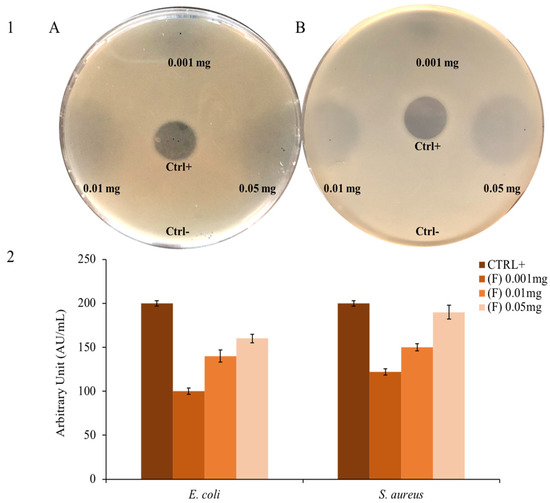
Figure 2.
Bacterial growth inhibition: panel (1) shows the inhibition halo of (F) against (A) E. coli and (B) S. aureus. The positive control is ampicillin; panel (2) shows the inhibition halo expressed in AU/mL.
To deepen the analysis of the antimicrobial activity, dose-response curves were carried out, increasing the concentration of EO and evaluating the survival of different bacteria. Dental researchers are developing and testing new therapeutic substances that are low- or non-toxic to prevent or eradicate dental plaque-related disorders. For this reason, three oral pathogenic strains were chosen as Gram-positive (S. mutans, S. oralis, and S. aureus) [67] and three oral and/or opportunistic pathogenic strains as Gram-negative bacteria (P. aeruginosa, S. Typhimurium, and E. coli) [68]. As can be seen in Figure 3, there is a proportionality between the increase in EO concentration and the decrease in bacterial survival. In general, the EO appears to be active at lower concentrations on Gram-positive bacteria than on negative ones.
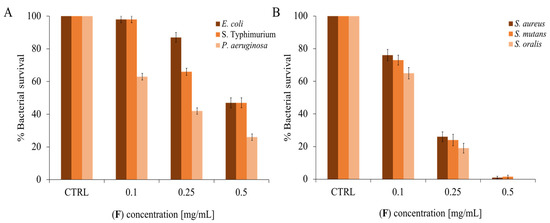
Figure 3.
(F) Antimicrobial activity against panel (A): Gram-negative strains; panel (B): Gram-positive strains. The assays were performed in three independent experiments. Standard deviations are always less than 10%.
A study of several Thymus species by Ballester-Costa et al. [69] suggests that organic EOs of T. mastichina L., T. zygis, T. capitatus, and T. vulgaris could be used as antibacterial agents in food preservation. These EOs can be accepted by consumers and authorized by regulatory agencies as natural preservative agents in organic foods.
Recent studies highlight the ability of Thymus to produce not only EOs but also methanolic extracts and volatile substances that have good antimicrobial activity. According to a study conducted by Vassiliou and collaborators [70], the EOs can be used in conjunction with conventional antibiotics. This approach may permit a reduction in the concentration of the synthetic antibiotic, in side effects, and in antibiotic resistance too. This strategy has been used for many years now with clavulanic acid and amoxicillin, for example.
Based on several studies and considering some EOs possible applications, it was decided to conduct further analysis on the antimicrobial effect of this interesting EO of T. richardii subsp. nitidus species.
To complete the analysis of the antimicrobial activity and make it quantitative, three independent experiments were performed to determine the MIC values through the microdilution method. As shown in Table 2, the lowest MIC values are observed against Gram-positive bacteria, with S. oralis CECT 8313 being the most sensitive. The MIC values found with (F) are very interesting because they are lower than most of the other EOs [71].

Table 2.
Determination of minimum concentration values (MIC) inhibiting bacterial growth. The MIC100 is expressed in mg/mL of (F) against Gram negative and Gram-positive bacteria. The values were obtained from a minimum of three independent experiments.
2.3. Antimicrobial Activity of the Components Present in T. richardii EO (F)
They have analyzed the antimicrobial activity of the single main compounds, which is more representative in terms of percentage amount within the EO of T. richardii (indicated in bold in the last column of Table 1). In order, p-cymene, thymol ether, limonene, thymol, and β-bisabolene were tested. Figure 4 shows the proportionality between the increase in compound concentration and the decrease in cell survival against E. coli (panel A) and S. aureus (panel B).
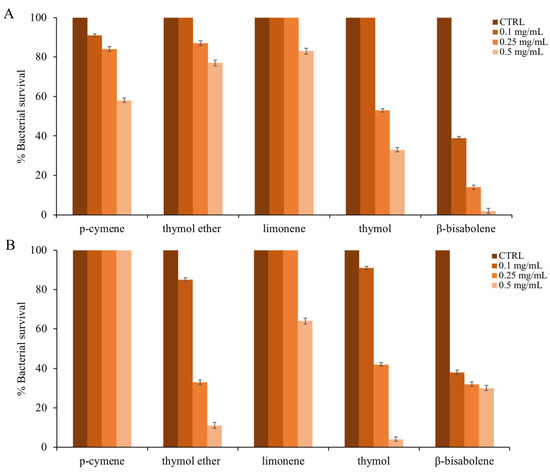
Figure 4.
Antimicrobial activity of the main components presented in (F) against panel (A): E. coli; panel (B): S. aureus. The assays were performed in three independent experiments. Standard deviations are always less than 10%.
The single compounds were used at different concentrations, which respected the quantities present in the EO at 0.5 mg/mL.
The effect of each single compound seems more directed against the Gram-positive bacterial model (thymol ether, thymol, and β-bisabolene). However, the exception is p-cymene, which works (at the used concentrations) only on the Escherichia coli strain.
In a study conducted by Gomori et al. [72], it was shown that in the EO of another species of Thymus, there is a high production of p-cymene, which retains good antimicrobial activity and is enhanced by the combined use of thymol. Thymol ether also has some antimicrobial activity, which is higher on S. aureus and lower on E. coli. In the literature, this compound is rarely analyzed for its antimicrobial activity, and this is another novelty of this study.
In general, thymol and especially β-bisabolene were the single compounds responsible for the antimicrobial activity of T. richardii EO [73]. According to Braga [74], thymol has excellent antimicrobial properties; it acts on bacterial and fungal adhesion to various types of eukaryotic cells as well as possessing strong antioxidant activity, for example, protecting the vaginal cells.
2.4. Fluorescence Microscopy Analysis
To study the action mechanism of (F) and its main compounds that are most involved in its antimicrobial activity, fluorescence microscopy experiments were performed. To verify the effect of EO and single compounds on bacterial membrane integrity, E. coli and S. aureus cells were used and stained with DAPI, a fluorescent stain for DNA that emits blue light, and propidium iodide, which emits red light.
The latter can enter cells only through damaged membranes and is therefore considered an indicator of cell membrane damage.
As shown in Figure 5 and Figure 6, panels 1, and A, untreated bacterial cells—used as a control—appear intact and blue because of DAPI fluorescence.
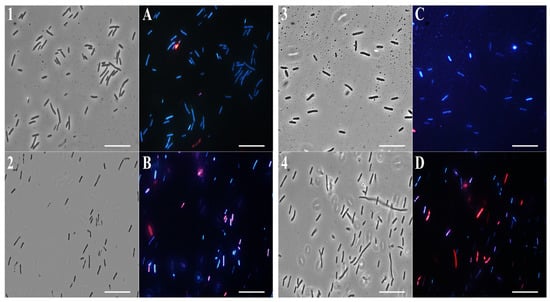
Figure 5.
Fluorescence microscopy images. Panels show E. coli bacterial cells. Panels (1–4) obtained from optical microscope images, and (A–D) from fluorescence microscope images. Untreated bacterial cells (1,A); cells treated with (F) (2,B); cells treated with β-bisabolene (3,C); cells treated with thymol (4,D).
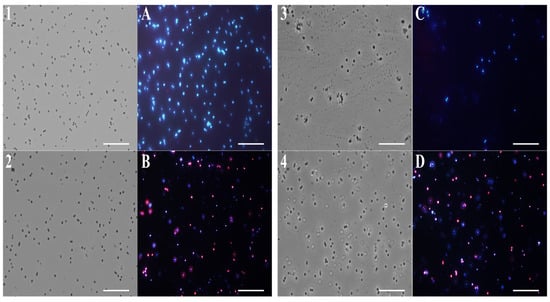
Figure 6.
Fluorescence microscopy images. Panels show S. aureus bacterial cells. Panels (1–4) obtained from optical microscope images, and (A–D) from fluorescence microscope images. Untreated bacterial cells (1,A); cells treated with (F) (2,B); cells treated with β-bisabolene (3,C); cells treated with thymol (4,D).
Notably, after 4 h of EO (0.5 mg/mL) treatment, a significant amount of E. coli (Figure 5B) and S. aureus (Figure 6B) cells developed a red fluorescence, suggesting breakdown of membranes. After the β-bisabolene treatment, E. coli and S. aureus membranes are intact, and bacterial cells appear blue for the entry of DAPI into the bacterial cell (Figure 5C and Figure 6C). As shown in Figure 5 and Figure 6, respectively, in panels D, treatment with thymol at the percentage contained in thyme EO causes damage to the membranes after 4 h of exposure to the compound itself. Results analysis suggests that EO biocide action towards Gram-negative and -positive strains might likely be exerted through membrane damage, in accordance with previous reported studies [75].
In fact, it is known in the literature that monoterpenes damage the biomembranes of both Gram-positive and Gram-negative bacteria. These compounds disturb the lipid fraction of the microorganism’s plasma membrane, causing alterations in the permeability and leakage of intracellular material [76]. This effect may be related to the physicochemical characteristics of the EO, the lipid composition, and the net surface charge of the microbial membranes.
T. richardii EO probably exerts its antimicrobial action through thymol. Its chemical structure is hydrophobic, which suggests a capacity to permeabilize the cell membrane. Several reports exploring the action mechanisms of phenolic compounds have indicated that they mainly disrupt bacterial cell membranes, resulting in a leakage of intracellular materials required for normal metabolism and survival directed against bacterial membranes [77]. The damage to the membrane probably favors the entry of β-bisabolene (which belongs to the polygodial class) into the bacterial cells.
Although these authors also showed that this class of compounds inhibited both respiration and the synthesis of cellular macromolecules, such as DNA, RNA, proteins, and polysaccharides, they concluded that these were secondary effects of the cell damage caused by polygodial since the inhibition of these macromolecules was not specific [78]. Probably, the simultaneous action of all the compounds contained in the EO is essential to exerting the antimicrobial activity.
2.5. Antibiofilm Activity of Essential oils (F), Thymol, and β-Bisabolene
Essential oil (F) used in low concentrations may have properties that prevent the formation of bacterial biofilms. As it is known from previous studies, different EOs [79], even at low concentrations, can have an antibiofilm effect. To validate this hypothesis, experiments on a biofilm-forming model strain (M. smegmatis) were performed.
The dose-response curves are shown in Figure 7A, and MIC values were also calculated to identify the concentrations of thymol, β-bisabolene, and EO that did not cause the bacterium’s death. Once the concentration that did not inhibit the growth of M. smegmatis was identified, lower concentrations were used, from 0.01 to 0.075 mg/mL (Figure 7B). As can be seen in panel B of the same figure (Figure 7), there is a biofilm inhibition of about 60% using both the (F) and thymol compounds, while the β-bisabolene percentage is slightly smaller (about 50%).
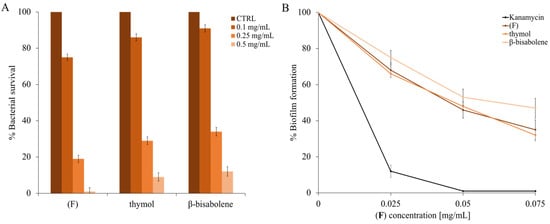
Figure 7.
Determination of the antibiofilm activity. Panel (A) shows the percentage of antimicrobial activity determined by (F), β-bisabolene and thymol against S. smegmatis. Panel (B) shows the percentage of M. smegmatis biofilm formation. Different concentrations of (F), β-bisabolene and thymol were tested (x-axis). DMSO is the negative control, and kanamycin is the positive control. The assays were performed in three independent experiments. Standard deviations are always less than 5%.
This aspect has a significant impact on the possible use of EO to preserve plants from pathogenic bacteria [80]. Indeed, the failure of conventional antibiotic treatments suggests that the eradication of microbial biofilms needs continuous updating [81]. Natural anti-biofilm substances target persistent biofilms and promote the diffusion of antimicrobials in the biofilm matrix. Usually, these natural agents are active at different stages of biofilm formation to degenerate the matrix and eventually kill the released cells. The goal of an antibiofilm agent is to destroy the biofilm and kill the bacterial cells contained in it; for this purpose, our thymus EO could be employed.
There are many new applications of thyme EO; for example, in a study by Arrais et al. [82], the inclusion of the EO in tablets allows a gradual and prolonged release, increasing the exposure time of the bacteria to the latter. This application is especially ideal for microbial biofilms of S. aureus and P. aeruginosa that are more difficult to eradicate than planktonic bacterial cells.
2.6. Cytotoxic Activity of (F) and Its Principal Components
To verify whether the EO of thyme and the compounds found within it could be toxic to eukaryotic cells, human keratinocytes were used to perform an assay using the MTT reagent, as reported in the methods. As shown in Figure 8A (4 h exposure to compounds), even at the maximum concentration (0.5 mg/mL), the compounds are not cytotoxic. Increasing the exposure time to 24 h at the maximum concentration (panel B), the compounds begin to exert a slight cytotoxic effect [49]. Thus, it is possible to conclude that under the experimental conditions used, (F) is non-toxic to this cell line. Similar results were observed in a study in which the EO of the thymus presented antimicrobial activity against several microorganisms, including Pseudomonas aeruginosa, Proteus vulgaris, Citrobacter koseri, and Klebsiella pneumoniae [83]. According to our observations, the EO of thyme does not affect HaCaT cell viability.
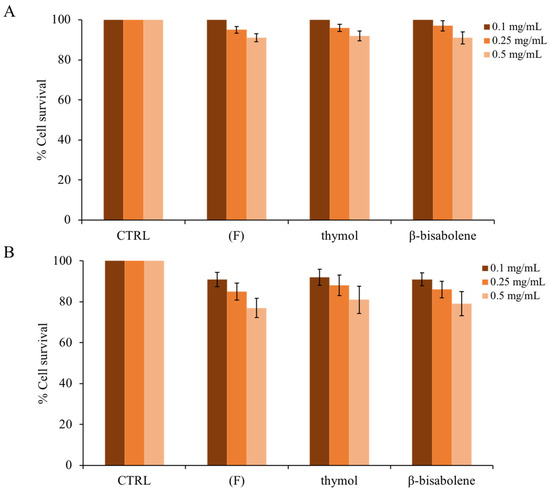
Figure 8.
Cytotoxic effect of (F) and of the individual components present on eukaryotic cells. HaCat cells were treated for 4 h (A) and for 24 h (B). Cellular cytotoxicity was determined by the MTT assay. The assays were performed in three independent experiments. Standard deviations are always less than 5%.
2.7. Antioxidant Activity of T. richardii EO (F)
With regard to the antioxidant activity, the main action was shown by the extracts, like that of T. laevigatus Vahl. (strong radical scavenging activity in the DPPH assay compared to the standard antioxidant) and T. vulgaris [dose-dependent DPPH-scavenging capability similar to standard antioxidants butylhydroxyanisol (BHA) and butylated hydroxytoluene (BHT)] [60,84]. The essential oil from T x citriodorus (Pers.) Schreb. leaves had relevant cytotoxic activity against HepG2 cells, inducing apoptosis with the expression of NF-κB [85]. Essential oils of Greek T. vulgaris instead attenuated the LPS-induced elevation in nuclear factor-kappa (NF-κB), cyclooxygenase-2 (COX-2), TNF-g, inducible nitric oxide synthase (iNOS), NO, and oxidative stress [86].
EO (F) is rich in oxygenated monoterpenes; these types of compounds possess various biological properties, including antioxidant ones [87]. Other studies on the antioxidant activity of EO have shown that the abatement ability of ABTS radicals is closely related to the concentration of EOs and has a strong connection with its chemical components, especially its main constituents [88]. The primary components of (F) are oxygenated terpenes, which have a great impact on the antioxidant activity of the EO. According to the analysis of the primary components of the EO, the antioxidant activity is positively correlated with the amount of oxygenated terpenoids (oxygenated monoterpenes and sesquiterpenes) [89,90]. Figure 9 shows the increasing percentage of scavenging activities of ABTS and H2O2 radicals as the concentration (0–0.2 mg/mL) of EO increases. The data shown in Figure 9 are expressed in Table 3 and Table 4 as IC50 values, representing the EO concentration that causes a 50% reduction in ABTS (Table 3) and H2O2 (Table 4) radicals. The EO shows anti-H2O2 activity with IC50 values of 0.2 mg/mL and the lowest anti-radical effect (IC50 value > 100 mg/mL) for ABTS.
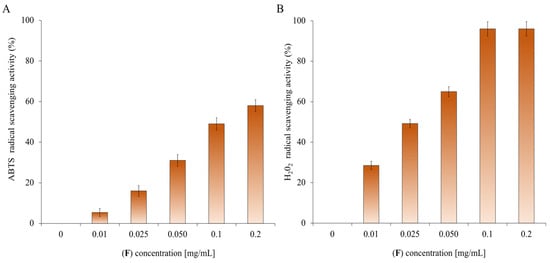
Figure 9.
Determination of the antioxidant activity of (F). Panel (A) shows the abatement activity of ABTS radicals reported as % of ABTS removed with respect to the control. Panel (B) shows the hydrogen peroxide scavenging activity reported as % of H2O2 removed relative to the control. The data are the mean of three independent experiments. Standard deviations are always less than 10%.

Table 3.
Concentration at 50% scavenging activity. ABTS: 2,20-azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid); H2O2: hydrogen peroxide. The positive control is ascorbic acid for ABTS and resveratrol for H2O2.

Table 4.
Concentration at 50% scavenging activity. ABTS: 2,20-azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid); H2O2: hydrogen peroxide. The positive control is ascorbic acid for ABTS and resveratrol for H2O2.
ROS-sensitive fluorescent dye was used to investigate whether the (F) prevents H2O2-induced ROS generation. HaCaT cells that had been exposed to H2O2 showed a significant increase in the accumulation of intracellular ROS, whereas this induction was significantly inhibited by the EO or thymol pretreatment (Figure 10). Accordingly, recent studies revealed an increased antioxidant effect of Thymus EOs in HaCat cells in a dose-dependent manner, as observed in this study [83].
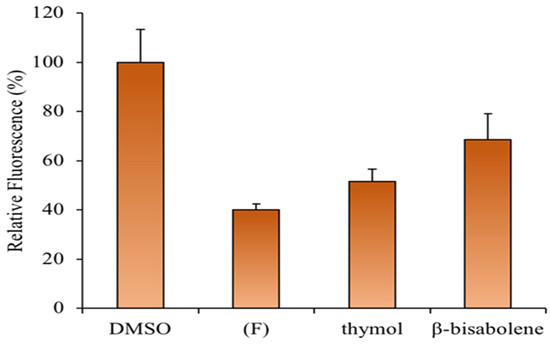
Figure 10.
Effects of (F), thymol, and β-bisabolene on intracellular ROS generation in HaCaT cells upon H2O2-treatment.
3. Materials and Methods
3.1. Plant Material
The pre-flowering aerial parts of T. richardii subsp. nitidus were collected at Punta Madonnuzza on Marettimo Island, Sicily, Italy (37°59′03″ N, 12°03′06″ E, 400 m a.s.l.), in April 2022, and a voucher specimen has been deposited in the STEBICEF Department, University of Palermo (PAL113474). The full flowering material was collected in the same location in June 2022.
3.2. Isolation of Essential Oil
The extraction of EOs was carried out according to Basile et al. [91]. Fresh samples were ground in a Waring blender and then subjected to hydrodistillation for 3 h, according to the standard procedure described in the European Pharmacopoeia (2020). The EOS were dried over anhydrous sodium sulfate and stored in sealed vials under N2 at −20 °C, ready for the GC-MS and GC-FID analyses; the samples yielded 0.05% and 0.07% of oils (w/w) for PF and F, respectively.
3.3. GC-MS Analysis
The analysis of EOs was performed according to the procedure reported by Badalamenti et al. [92]. GC-MS analysis was performed using a Shimadzu QP 2010 plus equipped with an AOC-20i autoinjector (Shimadzu, Kyoto, Japan) gas chromatograph equipped with a FID, a capillary column (DB-Wax) 30 m × 0.25 mm i.d., film thickness 0.25 μm, and a data processor. The oven program was as follows: temperature increase at 40 °C for 5 min, at a rate of 2 °C/min up to 260 °C, then isothermal for 20 min. Helium was used as a carrier gas (1 mL min−1). The injector and detector temperatures were set at 250 °C and 290 °C, respectively. One μL of EO solution (3% EO/Hexane v/v) was injected with split mode 1.0; MS range 40–600. The percentages in Table 1 are calculated with the TIC from MS. The settings were as follows: ionization voltage, 70 eV; electron multiplier energy, 2000 V; transfer line temperature, 295 °C; solvent delay, 4 min. Linear retention indices (LRI) were determined by using retention times of n-alkanes (C8–C40), and the peaks were identified by comparison with mass spectra and by comparison of their relative retention indices with WILEY275, NIST 17, ADAMS, and FFNSC2 libraries.
3.4. Pure Compounds
Limonene and β-bisabolene were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA), p-cymene and thymol from Tokyo Chemical Industry Co. (Chuo-Ku, Tokyo, Japan), while the thymol methyl ether (54 mg) was prepared from thymol (50 mg) using diazomethane under stirring for 30 min.
3.5. Bacterial Strains
Gram-negative bacteria: Escherichia coli DH5α, Pseudomonas aeruginosa PAOI ATCC 15692, and Salmonella typhimurium ATCC14028, and Gram-positive ones: Staphylococcus aureus ATCC6538P, Streptococcus oralis CECT 8313, Streptococcus mutans ATCC 35668, and Mycobacterium smegmatis mc2 155, were chosen to evaluate antibacterial activity.
3.6. Antimicrobial Activity Assay
The presence of antimicrobial molecules in (F) was detected using the Kirby-Bauer test with modifications [93]. Three volumes (10, 15, and 20 µL) of EO [5 mg/mL] were placed on Luria bertani agar plates and then coated with the indicator strains: E. coli and S. aureus. The negative control was dimethyl sulfoxide (15 µL) used to resuspend (F); the positive control was the antibiotic ampicillin (1 µL) concentrated at 5 mg/mL. Antimicrobial activity was calculated as reported in Pota et al. [94].
Another method to evaluate the antimicrobial activity involved the Gram-positive and Gram-negative strains cell viability counting. Microbial cells were treated with both EOs at 0.10, 0.25, and 0.50 mg/mL concentrations. Microbial cells without EOs were the positive control; instead, cells with DMSO were used as the negative control. The following day, the survival rate of bacterial cells was calculated by counting the colonies [95]. The same assay was carried out to evaluate the antimicrobial activity of compounds mostly present in (F). Each compound was tested at its EO maximum concentration, considering the percentage at which it is present in the EO. In this study, the main constituents of the EO collected at the full flowering stage were p-cymene ~9% (0.045 mg/mL), β-bisabolene ~18% (0.09 mg/mL), limonene ~16% (0.08 mg/mL), thymol methyl ether ~9% (0.045 mg/mL, and thymol ~16% (0.08 mg/mL). All experiments were carried out in triplicate, and the reported result was an average of three independent experiments (p value of < 0.05).
3.7. Determination of Minimal Inhibitory Concentration
Minimal Inhibitory Concentrations (MICs) of (F) against the Gram-positive and Gram-negative strains were determined according to the microdilution method established by the Clinical and Laboratory Standards Institute (CLSI) [96]. Five samples of 105 CFU/mL were added to 95 µL of Mueller-Hinton broth (CAM-HB; Difco), supplemented or not with various concentrations (0.1–0.5 mg/mL) of (F). After overnight incubation at 37 °C, MIC100 values were determined to be the lowest concentration responsible for the lack of bacterial growth.
3.8. Fluorescence Microscopy Experiments
E. coli DH5α and S. aureus ATCC6538P cells were incubated in the dark for 4 h at 37 °C with or without (F) 0.5 mg/mL, β-bisabolene 0.09 mg/mL, and thymol 0.08 mg/mL. Samples were observed as described in Di Napoli et al. [97].
3.9. Antibiofilm Inhibition Tests
The antibiofilm activity against M. smegmatis mc2 155 was evaluated by colorimetric testing. Microbial cells with DMSO were the negative control, and the antibiotic kanamycin (2 µg/mL) was the positive control. Treated prokaryotic cells contained EO (0.025, 0.05, and 0.075 mg/mL), 16% thymol, and 18% β-bisabolene at these EO concentrations. The plate was incubated at 37 °C for 36 h [79]. The percentage of biofilm formed was evaluated by comparing the optical density values of the treated and untreated samples.
3.10. Eukaryotic Cell Culture
HaCat cells (human keratinocytes) are immortal keratinocyte cell lines used in research laboratories [98]. These cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) at 37 °C with 5% CO2. EO (F), β-bisabolene, and thymol at concentrations of 0.10, 0.25, and 0.50 mg/mL, respectively, were used for the MTT assay [99].
3.11. ABTS and H2O2 Scavenging Capacity Assay
For this test, based on the scavenging of ABTS radicals, the protocol of Napolitano et al. was used [100]. The ABTS solution was added to 100 µL of EO and/or individual compounds (concentrations of 0.01, 0.025, 0.05, 0.1, and 0.2 mg/mL). Each test was performed at least three times.
The scavenging capacity of H2O2 was evaluated by the variation of the absorbance at 240 nm, as described in the literature [101]. Different concentrations (0.01, 0.025, 0.05, 0.1, and 0.2 mg/mL) of EO and individual compounds were mixed with the hydrogen peroxide. After half an hour, the concentration of hydrogen peroxide was calculated by measuring the absorbance. Each assay was performed at least three times.
3.12. Antioxidant Test on HaCat Cells
HaCaT cells were seeded in 12-well plates and then incubated at 37 °C with 5% CO2 for 24 h. Cells were treated with (F) (0.25 mg/mL), thymol (0.04 mg/mL), β-bisabolene (0.045 mg/mL), or DMSO as a control. After 1 h of treatment, cells were exposed to H2O2 (800 µM) for the next 3 h before intracellular ROS detection. ROS Assay Stain (88-5930, Invitrogen, Waltham, MA, USA) was added to cells in culture media ccording to the manufacturer protocol [102]. Cells were incubated for 1 h at 37 °C with 5% CO2. Fluorescence intensity (530 nm) was measured using a Synergy H4 Hybrid Microplate reader (Agilent, Santa Clara, CA, USA).
4. Conclusions
This study highlights the antimicrobial properties of Thymus richardii subsp. nitidus EO, which are especially active against Gram-positive bacteria, pathogens of the oral cavity. Very interesting are the properties of thymol and β-bisabolene, which become the major constituents of the EO antimicrobial activity; they have good activity at much lower concentrations than 0.5 mg/mL. Furthermore, the EO of this Thymus species inhibits the formation of model biofilms at low concentrations—nearly 50% at 0.75 mg/mL. The beneficial effect it has on eukaryotic cells is demonstrated by its low toxicity and the antioxidant action it is able to exert on human epithelial cells. The most common dental diseases are dental cavities, periodontitis, gingivitis, and oral cancer. EOs seem to have a beneficial role in each one of them. This study is based on an endemic Sicilian species of Thymus; the composition of its essential oil is very interesting and differs from many other species due to the abundant presence of β-bisabolene. Furthermore, the latter, together with thymol, constitutes a compound with high antimicrobial activity that is essential for the correct functioning of thyme as an antibacterial agent. This new oil has multiple properties and can potentially be used in various fields, ranging from food preservation to cosmetics or even in dentistry. In this case, Thymus richardii subsp. nitidus EO represents a valid ally for oral healthiness.
Author Contributions
Conceptualization, A.Z., G.B. and M.B.; methodology, A.V., M.D.N., D.A., and G.C.; formal analysis, M.D.N. and A.V.; investigation, G.C., D.A., A.V. and M.D.N.; data curation, N.B.; writing—original draft preparation, M.V., D.A., A.V. and A.Z.; writing—review and editing, A.Z., M.V., A.V., G.B. and N.B.; supervision, A.Z and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received external funding from National Biodiversity Future Center S.c.a.r.l., Piazza Marina 61 (c/o Palazzo Steri), Palermo, Italy, C.I. CN00000033—CUP UNIPA B73C22000790001.
Institutional Review Board Statement
HaCat (human keratinocyte) cells were purchased from Cell Lines Service (CLS catalog number 300493) and are spontaneously transformed into aneuploid immortal keratinocyte cell lines from adult human skin, widely used in scientific research.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- POWO. Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 22 January 2023).
- Vila, R. Flavonoids and further polyphenols in the genus Thymus. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 144–176. [Google Scholar]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus spp. plants—Food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Wang, L.; Wang, X.; Xu, X.; Sui, H.; et al. Review traditional uses, chemical constituents and biological activities of plants from the Genus Thymus. Chem. Biodivers. 2019, 16, e1900254. [Google Scholar] [CrossRef] [PubMed]
- Stahl-Biskup, E.; Venskutonis, R.P. Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2012; Volume 1, pp. 499–525. [Google Scholar]
- Alarcón, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, G.J. A Dictionary of Compendium of Materia Medica; Shandong Science and Technology Press: Shandong, China, 2007. [Google Scholar]
- Zarshenas, M.M.; Krenn, L. A critical overview on Thymus daenensis Celak.: Phytochemical and pharmacological investigations. J. Integr. Med. 2015, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Mossa, J.S.; Al-Said, M.S.; Al-Yahya, M.A. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia 2004, 75, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Gilca, M.; Tiplica, G.S.; Satavastru, C.M. Traditional and ethnobotanical dermatology practices in Romania and other eastern European countries. Clin. Dermatol. 2018, 36, 338–352. [Google Scholar] [CrossRef]
- Parada, M.; Carrió, E.; Bonet, M.À.; Vallè, J. Ethnobotany of the Alt Empordà region (Catalonia, Iberian Peninsula): Plants used in human traditional medicine. J. Ethnopharmacol. 2009, 124, 609–618. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Bidak, L.M.; Kamal, S.A.; Halmy, M.W.A.; Heneidy, S.Z. Goods and services provided by native plants in desert ecosystems: Examples from the northwestern coastal desert of Egypt. J. Global Ecol. Environ. 2015, 3, 433–447. [Google Scholar] [CrossRef]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus × citriodorus using a validated HPLC-UV and ESI-MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Poulose, A.J.; Croteau, R. γ-Terpinene synthetase: Akey enzyme in the biosynthesis of aromatic monoterpenes. Arch. Biochem. Biophys. 1978, 191, 400–411. [Google Scholar] [CrossRef]
- Stahl-Biskup, E. Essential oil chemistry of the genus Thymus—A global view. In Thyme: The Genus Thymus; Stahl-Bishup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 75–124. [Google Scholar]
- Stahl-Biskup, E. Thyme in Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2004; Volume 2, pp. 297–320. [Google Scholar]
- Zare, M.; Namratha, K.; Thakur, M.S.; Byrappa, K. Biocompatibility assessment and photo-catalytic activity of bio-hydrothermal synthesis of ZnO nanoparticles by Thymus vulgaris leaf extract. Mater. Res. Bull. 2019, 109, 49–59. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; D’Arrigo, M.; Lopresti, F.; Marino, A.; Bruno, M.; Nostro, A. Flexible mats as promising antimicrobial systems via integration of Thymus capitatus (L.) Essential oil into PLA. Future Microbiol. 2020, 15, 1379–1392. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Gagliano Candela, R.; Maggi, F.; Lazzara, G.; Rosselli, S.; Bruno, M. The essential oil of Thymbra capitata and its application as a biocide on stone and derived surfaces. Plants 2019, 8, 300. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arabian J. Chem. 2019, 12, 2704–2712. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oil as natural biocides in conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef]
- D’Agostino, G.; Badalamenti, N.; Giambra, B.; Palla, F.; Bruno, M. The application of the essential oils of Thymus vulgaris L. and Crithmum maritimum L. as biocidal on two Tholu Bommalu Indian leather puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef]
- Bartolucci, F. Thymus L. In Flora d’Italia, 2nd ed.; Pignatti, S., Ed.; Media Business–Edagricole: Milan, Italy, 2018; Volume 3, pp. 278–290. [Google Scholar]
- Bartolucci, F.; Domina, G. The genus Thymus (Lamiaceae) in Sicily. Plant Biosyst. 2015, 149, 710–719. [Google Scholar] [CrossRef]
- Euro+Med PlantBase. Available online: https://ww2.bgbm.org/EuroPlusMed/query.asp (accessed on 16 January 2023).
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Media Business–Edagricole: Milano/Bologna, Italy, 2017; Volume 4. [Google Scholar]
- Morales, R. Synopsis of the genus Thymus L. in the Mediterranean area. Lagascalia 1997, 19, 249–262. [Google Scholar]
- Gianguzzi, L.; Scuderi, L.; Pasta, S. The vascular flora of Marettimo Island (Egadian Archipelago, W Sicily): Updating and phytogeographic analysis. Webbia 2006, 61, 359–402. [Google Scholar] [CrossRef]
- Giardina, G.; Raimondo, F.M.; Spadaro, V. A catalogue of plants growing in Sicily. Bocconea 2007, 20, 5–582. [Google Scholar]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Gugliuzza, G.; Domina, G.; Giovino, A. Seed germination of Thymus richardii subsp. nitidus (Lamiaceae). Flora Mediterranea 2021, 31, 291–293. [Google Scholar]
- Prieto, J.M.; Bader, A.; Martini, F.; Ríos, J.L.; Morelli, I. Screening of some rare endemic Italian plants for inhibitory activity on 5-lipoxygenase. Fitoterapia 2005, 76, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential oils and health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microbial Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.; Denny, C.; Benso, B.; De Alencar, S.; Rosalen, P. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef] [PubMed]
- Toscano-Garibay, J.D.; Arriaga-Alba, M.; Sánchez-Navarrete, J.; Mendoza-García, M.; Flores-Estrada, J.J.; Moreno-Eutimio, M.A.; Espinosa-Aguirre, J.J.; González-Ávila, M.; Ruiz-Pérez, N.J. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Sci. Rep. 2017, 7, 11479. [Google Scholar] [CrossRef]
- Rahman, M.; Alam Tumpa, A.; Zehravi, M.; Sarker, T.; Yamin; Islam, R.; Harun-Or-Rashid; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An overview of antimicrobial stewardship optimization: The use of antibiotics in humans and animals to prevent resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Mocanu, R.C.; Martu, M.-A.; Luchian, I.; Sufaru, I.G.; Maftei, G.A.; Ioanid, N.; Martu, S.; Tatarciuc, M. Microbiologic profiles of patients with dental prosthetic treatment and periodontitis before and after photoactivation therapy—Randomized clinical trial. Microorganisms 2021, 9, 713. [Google Scholar] [CrossRef]
- De Feo, V.; Bruno, M.; Tahiri, B.; Napolitano, F.; Senatore, F. Chemical composition and anti-bacterial activity of the essential oils of Thymus spinulosus Ten. (Lamiaceae). J. Agr. Food Chem. 2003, 51, 3849–3853. [Google Scholar] [CrossRef]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of essential oil and evaluation of its bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Badalamenti, N.; Vaglica, A.; Maggio, A.; Bruno, M. A new ferulol derivative isolated from the aerial parts of Ferulago nodosa (L.) Boiss. growing in Sicily (Italy). Nat. Prod. Res. 2022, in press. [Google Scholar] [CrossRef]
- Maresca, V.; Badalamenti, N.; Ilardi, V.; Bruno, M.; Bontempo, P.; Basile, A. Chemical composition of Thymus leucotrichus var. creticus essential oil and its protective effects on both damage and oxidative stress in Leptodictyum riparium Hedw. induced by cadmium. Plants 2022, 11, 3529. [Google Scholar] [CrossRef]
- Di Napoli, M.; Maresca, V.; Varcamonti, M.; Bruno, M.; Badalamenti, N.; Basile, A.; Zanfardino, A. (+)-(E)-Chrysanthenyl acetate: A molecule with interesting biological properties contained in the Anthemis secundiramea (Asteraceae) flowers. Appl. Sci. 2020, 10, 6808. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Gagliano Candela, R.; Maggi, F. Chemical composition of the es-sential oil of Elaeoselinum asclepium (L.) Bertol subsp. meoides (Desf.) Fiori (Umbelliferae) collected wild in Central Sicily and its antimicrobial activity. Nat. Prod. Res. 2022, 36, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Maggio, A.; Badalamenti, N.; Bruno, M.; D’Angelo, G.D.; D’Anneo, A. Essential oil of Foeniculum vulgare subsp. piperitum fruits exerts an anti-tumor effect in triple-negative breast cancer cells. Mol. Med. Rep. 2022, 26, 243. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.; Flamini, G.; Cioni, P.L.; Morelli, I. Thymus richardii Pers. subsp. nitidus (Guss.) Jalas: A new thymus type rich in β-bisabolene. J. Essent. Oil Res. 2001, 13, 8–10. [Google Scholar] [CrossRef]
- Llorens, L.; Llorens-Molina, J.A.; Agnello, S.; Boira, H. Geographical and environment-related variations of essential oils in isolated populations of Thymus richardii Pers. in the Mediterranean basin. Biochem. System. Ecol. 2014, 56, 246–254. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Michet, A.; Chalchat, J.-C.; Figuéredo, G.; Thebaud, G.; Billy, F.; Petel, G. Chemotypes in the volatiles of wild thyme (Thymus pulegioides L.). J. Essent. Oil Res. 2008, 20, 101–103. [Google Scholar] [CrossRef]
- Torras, J.; Grau, M.D.; López, J.F.; de las Heras, F.X.C. Analysis of essential oils from chemotypes of Thymus vulgaris in Catalonia. J. Sci. Food Agric. 2007, 87, 2327–2333. [Google Scholar] [CrossRef]
- Adzet, T.; Granger, R.; Passet, J.; San Martin, R. Le polymorphisme chimique dans le genre Thymus: Sa signification taxonomique. Biochem. Syst. Ecol. 1977, 5, 269–272. [Google Scholar] [CrossRef]
- Arsenijević, J.; Drobac, M.; Šoštarić, I.; Ražić, S.; Milenković, M.; Couladis, M.; Maksimović, Z. Bioactivity of herbal tea of Hungarian thyme based on the composition of volatiles and polyphenolics. Ind. Crops Prod. 2016, 89, 14–20. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.H. Antifungal activities of essential oils from Thymus quinquecostatus and T. magnus. Planta Med. 2004, 70, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.H. In vitro inhibitory activities of essential oils from two Korean Thymus species against antibiotic-resistant pathogens. Arch. Pharm. Sci. Res. 2005, 28, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, S. In vivo antifungal activity of the essential oil fraction from Thymus species and in vitro synergism with clotrimazole. Nat. Prod. Sci. 2007, 13, 258–262. [Google Scholar]
- Manconi, M.; Petretto, G.; D’Hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus essential oil extraction, characterization and incorporation in phospholipid vesicles for the antioxidant/antibacterial treatment of oral cavity diseases. Colloids Surf. B 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial activity of Oregano, Rosmarinus and Thymus essential oils against Staphylococcus aureus and Listeria monocytogenes in beef meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Vaglica, A.; Ilardi, V.; Varcamonti, M.; Bruno, M.; Zanfardino, A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of italian Prangos trifida (Mill.) Herrnst. & Heyn. Nat. Prod. Res. 2022, in press. [Google Scholar] [CrossRef]
- Tanhaieian, A.; Pourgonabadi, S.; Akbari, M.; Mohammadipour, H. The effective and safe method for preventing and treating bacteria-induced dental diseases by herbal plants and a recombinant peptide. J. Clin. Exp. Dent. 2020, 12, e523–e532. [Google Scholar] [CrossRef]
- Rivas Caldas, R.; Le Gall, F.; Revert, K.; Rault, G.; Virmaux, M.; Gouriou, S.; Héry-Arnaud, G.; Barbier, G.; Boisramé, S. Pseudomonas aeruginosa and periodontal pathogens in the oral cavity and lungs of cystic fibrosis patients: A case-control study. J. Clin. Microbiol. 2015, 53, 1898–1907. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-inflammatory and antimicrobial properties of Thyme oil and its main constituents. IJMS 2023, 24, 6936. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Barreto, A.S.; Semedo-Lemsaddek, T. Antimicrobial effects of essential oils on oral microbiota biofilms: The toothbrush in vitro model. Antibiotics 2020, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Gömöri, C.; Vidács, A.; Kerekes, E.B.; Nacsa-Farkas, E.; Böszörményi, A.; Vágvölgyi, C.; Krisch, J. Altered antimicrobial and anti-biofilm forming effect of thyme essential oil due to changes in composition. Nat. Prod. Commun. 2018, 13, 1934578X1801300. [Google Scholar] [CrossRef]
- Nascimento, A.M.A.; Brandão, M.G.L.; Oliveira, G.B.; Fortes, I.C.P.; Chartone-Souza, E. Synergistic bactericidal activity of Eremanthus erythropappus oil or β-bisabolene with ampicillin against Staphylococcus aureus. Antonie Leeuwenhoek 2007, 92, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C. Thymol: Antibacterial, antifungal and antioxidant activities. G. Ital. Ostet. Ginecol. 2005, 27, 267–272. [Google Scholar]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Napoli, M.D.; Varcamonti, M.; Basile, A.; et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha x piperita var. mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol. 2014, 165, 559–565. [Google Scholar] [CrossRef]
- Kipanga, P.N.; Demuyser, L.; Vrijdag, J.; Eskes, E.; D’hooge, P.; Matasyoh, J.; Callewaert, G.; Winderickx, J.; Van Dijck, P.; Luyten, W. Investigating the antifungal mechanism of action of polygodial by phenotypic screening in Saccharomyces cerevisiae. IJMS 2021, 22, 5756. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Antimicrobial, antibiofilm, and antioxidant properties of essential oil of Foeniculum vulgare Mill. leaves. Plants 2022, 11, 3573. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; De Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural anti-biofilm agents: Strategies to control biofilm-forming pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, E.; Cheng, Y.; Mahmood, T.; Ge, F.; Zhou, K.; Bao, M.; Lv, L.; Li, L.; Yi, J.; et al. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection? Biomed. Pharmacother. 2020, 128, 110184. [Google Scholar] [CrossRef] [PubMed]
- Arrais, A.; Bona, E.; Todeschini, V.; Caramaschi, A.; Massa, N.; Roncoli, M.; Minervi, A.; Perin, E.; Gianotti, V. Thymus vulgaris essential oil in beta-cyclodextrin for solid-state pharmaceutical applications. Pharmaceutics 2023, 15, 914. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Bučková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. The effect of ten essential oils on several cutaneous drug-resistant microorganisms and their cyto/genotoxic and antioxidant properties. Molecules 2019, 24, 4570. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. In vitro antimicrobial, cytotoxic and radical scavenging activities and chemical constituents of the endemic Thymus laevigatus (Vahl). Rec. Nat. Prod. 2010, 4, 49–63. [Google Scholar]
- Wu, S.; Wei, F.X.; Li, H.Z.; Liu, X.G.; Zhang, J.H.; Liu, J.X. Chemical composition of essential oil from Thymus citriodorus and its toxic effect on liver cancer cells. Zhongyaocai 2013, 36, 756–759. [Google Scholar]
- Habashy, N.H.; Abu Serie, M.M.; Attia, W.E.; Abdelgaleil, S.A.M. Chemical characterization, antioxidant and anti-inflammatory properties of Greek Thymus vulgaris extracts and their possible synergism with Egyptian Chlorella vulgaris. J. Funct. Foods. 2018, 40, 317–328. [Google Scholar] [CrossRef]
- Ling, Q.; Zhang, B.; Wang, Y.; Xiao, Z.; Hou, J.; Xiao, C.; Liu, Y.; Jin, Z. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum Camphora and Cinnamomum Bodinieri. Molecules 2022, 27, 7356. [Google Scholar] [CrossRef]
- Lu, C.; Li, H.; Li, C.; Chen, B.; Shen, Y. Chemical composition and radical scavenging activity of Amygdalus pedunculata Pall leaves’ essential oil. Food Chem. Toxicol. 2018, 119, 368–374. [Google Scholar] [CrossRef]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2-diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Luo, Q.; Tian, Z.; Zheng, T.; Xu, S.; Ma, Y.; Zou, S.; Zuo, Z. Terpenoid composition and antioxidant activity of extracts from four chemotypes of Cinnamomum camphora and their main antioxidant agents. Biofuels Bioprod. Bioref. 2022, 16, 510–522. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Pota, G.; Zanfardino, A.; Di Napoli, M.; Cavasso, D.; Varcamonti, M.; D’Errico, G.; Pezzella, A.; Luciani, G.; Vitiello, G. Bioinspired antibacterial PVA/melanin-TiO2 hybrid nanoparticles: The role of poly-vinyl-alcohol on their self-assembly and biocide activity. Coll. Surf. B Biointerfaces 2021, 202, 111671. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.; Melone, P.; Silvestri, B.; Pezzella, A.; Di Donato, P.; D’Errico, G.; Di Napoli, M.; Zanfardino, A.; Varcamonti, M.; Luciani, G. Titanium based complexes with melanin precursors as a tool for directing melanogenic pathways. Pure Appl. Chem. 2019, 91, 1605–1616. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) N-heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. IJMS 2021, 22, 2497. [Google Scholar] [CrossRef]
- Di Napoli, M.; Maresca, V.; Sorbo, S.; Varcamonti, M.; Basile, A.; Zanfardino, A. proteins of the fruit pulp of Acca sellowiana have antimicrobial activity directed against the bacterial membranes. Nat. Prod. Res. 2021, 35, 2942–2946. [Google Scholar] [CrossRef]
- Vitiello, G.; Zanfardino, A.; Tammaro, O.; Di Napoli, M.; Caso, M.F.; Pezzella, A.; Varcamonti, M.; Silvestri, B.; D’Errico, G.; Costantini, A.; et al. Bioinspired hybrid eumelanin–TiO2 antimicrobial nanostructures: The key role of organo–inorganic frameworks in tuning eumelanin’s biocide action mechanism through membrane interaction. RSC Adv. 2018, 8, 28275–28283. [Google Scholar] [CrossRef]
- Napoli, M.D.; Luccia, B.D.; Vitiello, G.; D’Errico, G.; Carpentieri, A.; Pezzella, A.; Pizzo, E.; Notomista, E.; Varcamonti, M.; Zanfardino, A. Characterisation of EFV12 a bio-active small peptide produced by the human intestinal isolate Lactobacillus gasseri SF1109. Benef. Microbes 2020, 11, 815–824. [Google Scholar] [CrossRef]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Piacente, S.; Maresca, V.; Cianciullo, P.; Capasso, L.; et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Seunghee, B.; Eun-Jin, L.; Jae, H.L.; In-Chul, P.; Su-Jae, L.; Hyung, J.H.; Kyu, J.H.; Sungkwan, A.; In-Sook, A.; Hwa, J.C. Oridonin protects HaCaT keratinocytes against hydrogen peroxide-induced oxidative stress by altering microRNA expression. IJMS 2014, 33, 185–193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).