Peripheralization Strategies Applied to Morphinans and Implications for Improved Treatment of Pain
Abstract
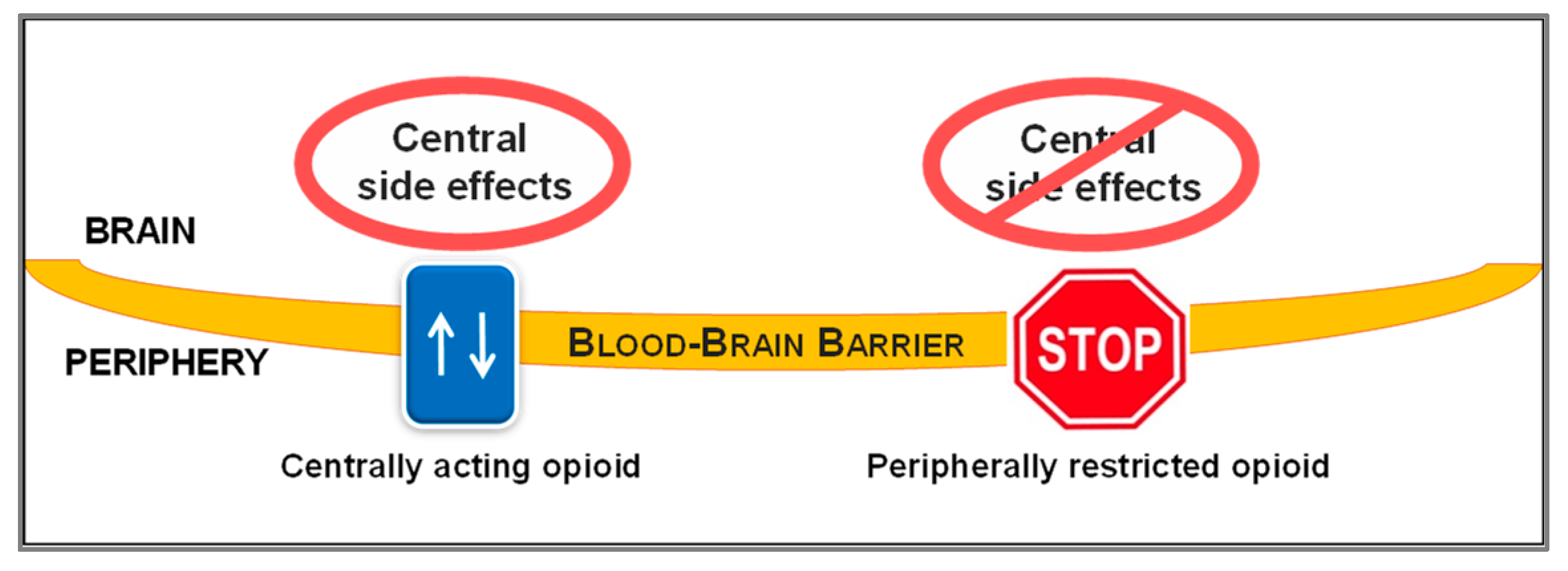
1. Introduction
2. Peripheralization Strategies Applied to Morphinans
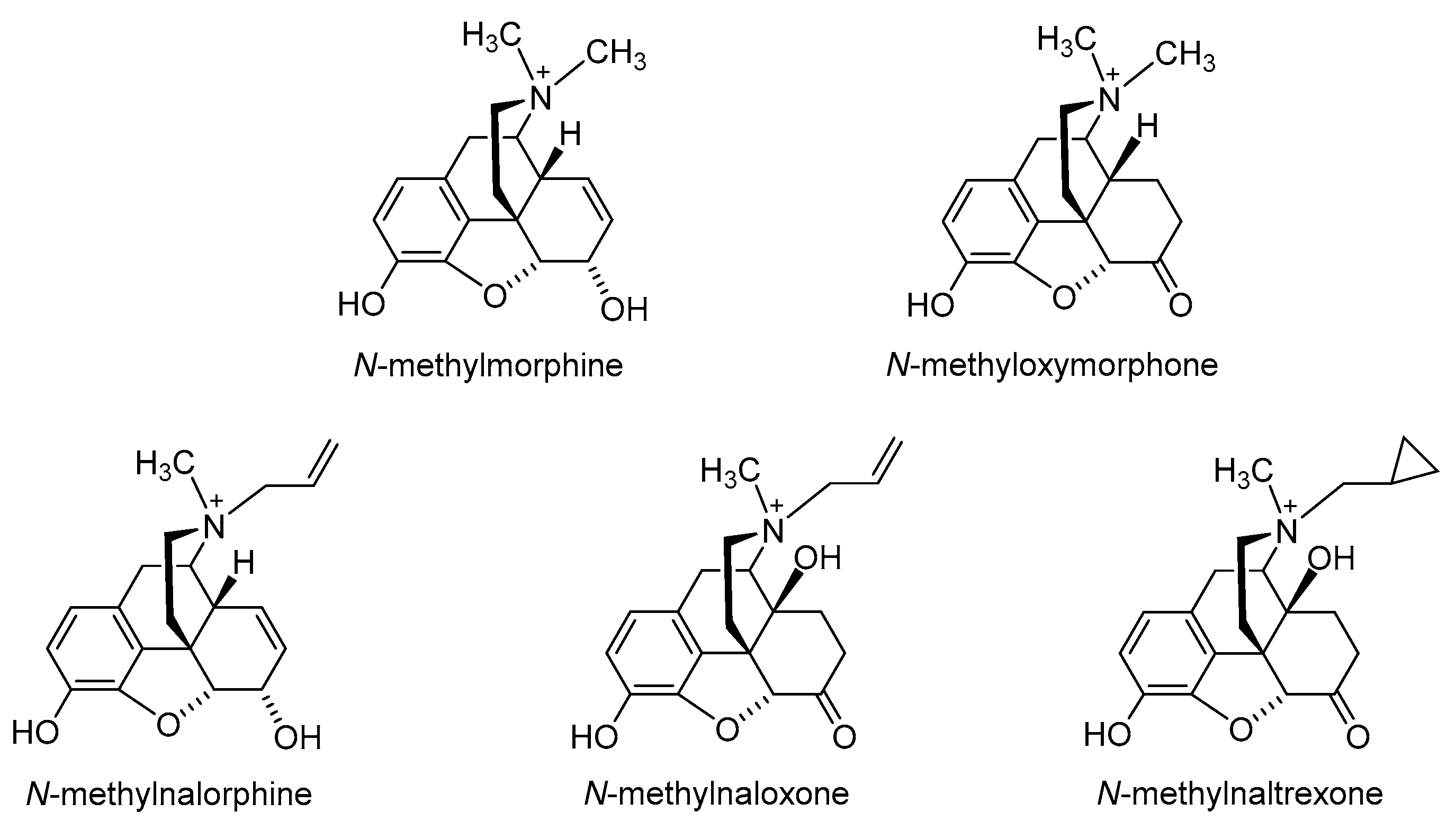
2.1. Quaternization of the Morphinan Nitrogen
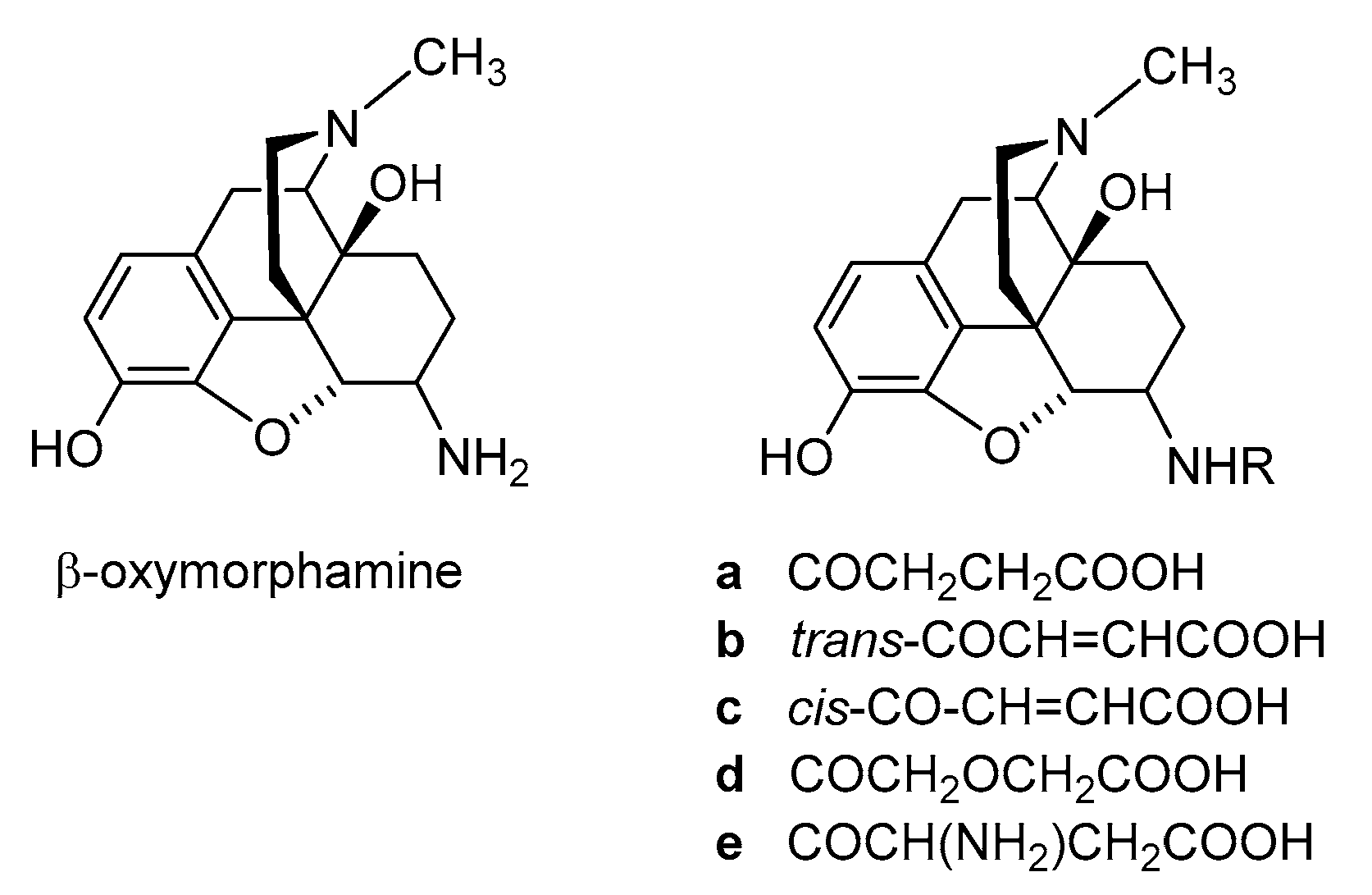
2.2. Introduction of Hydrophilic Substituents at Position 6
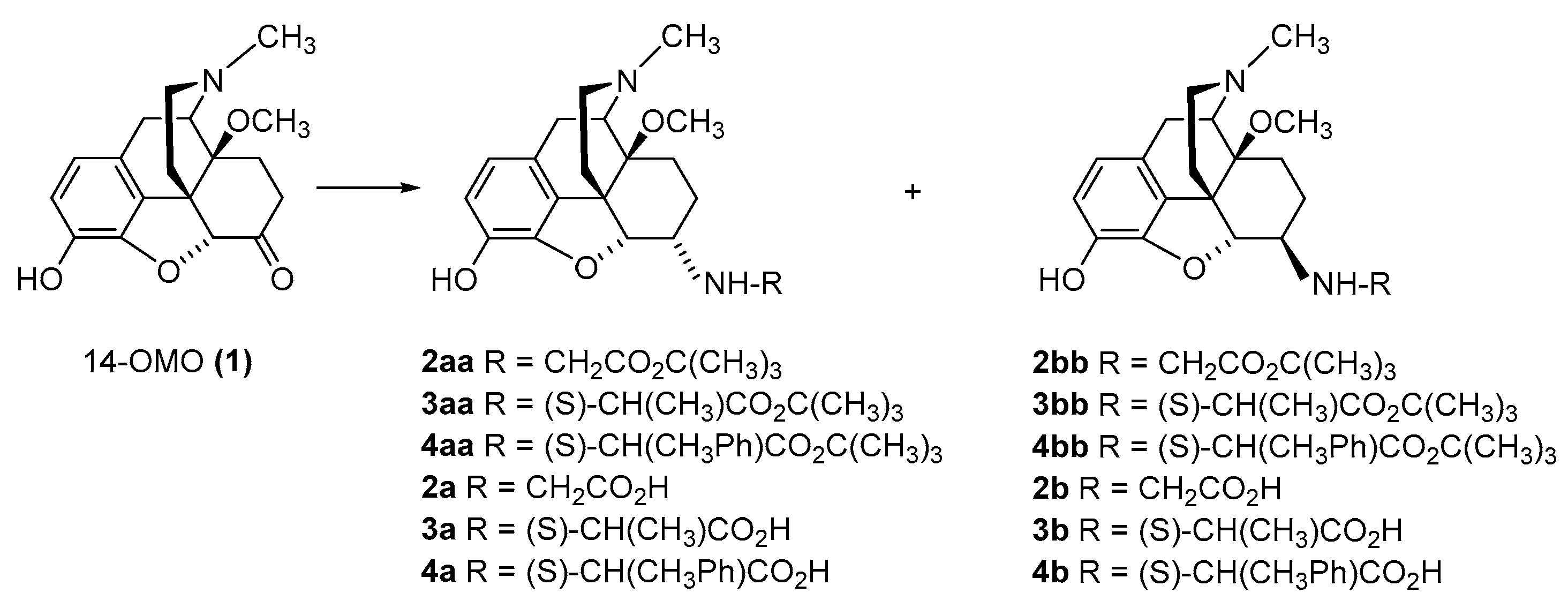
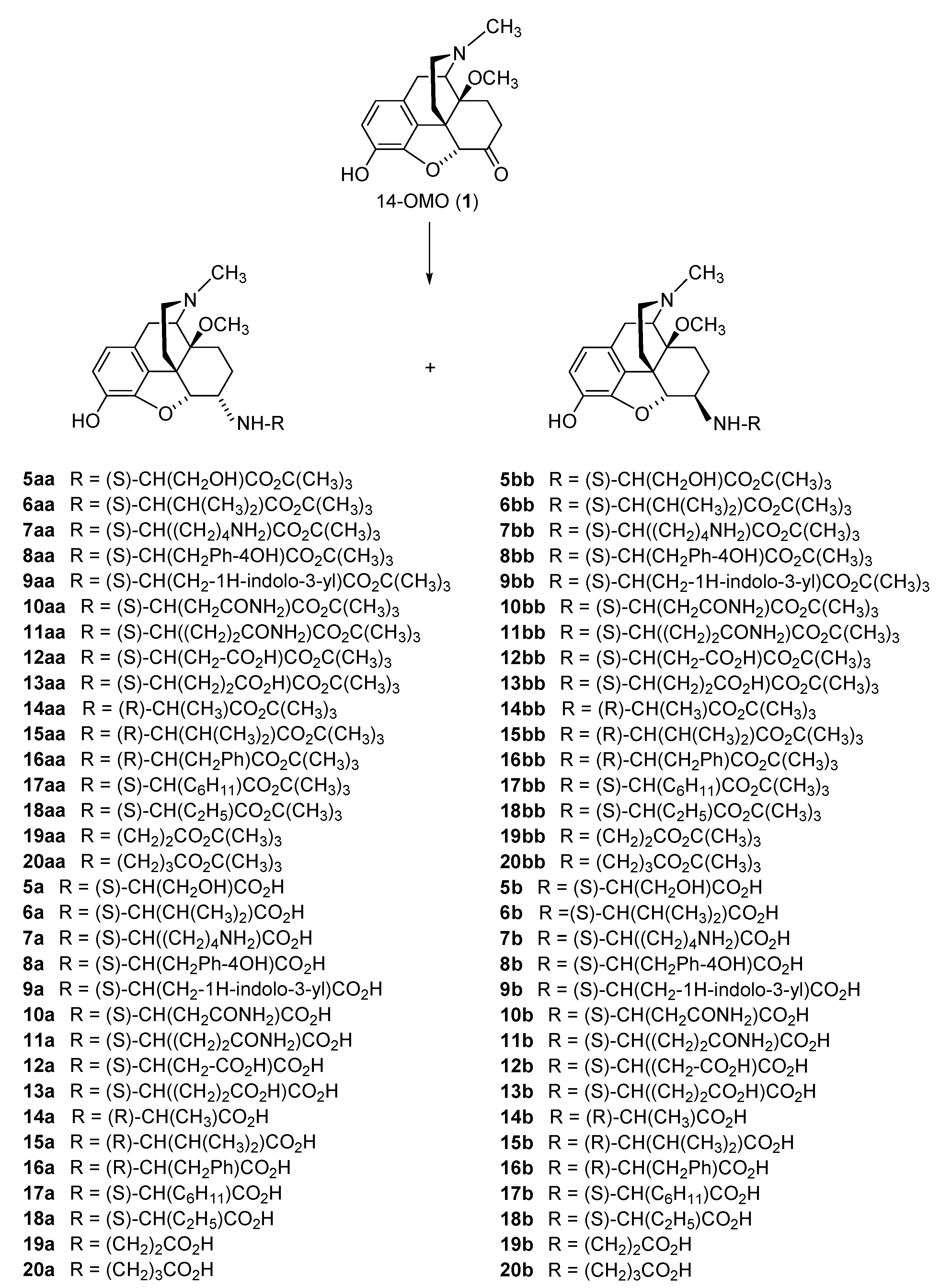
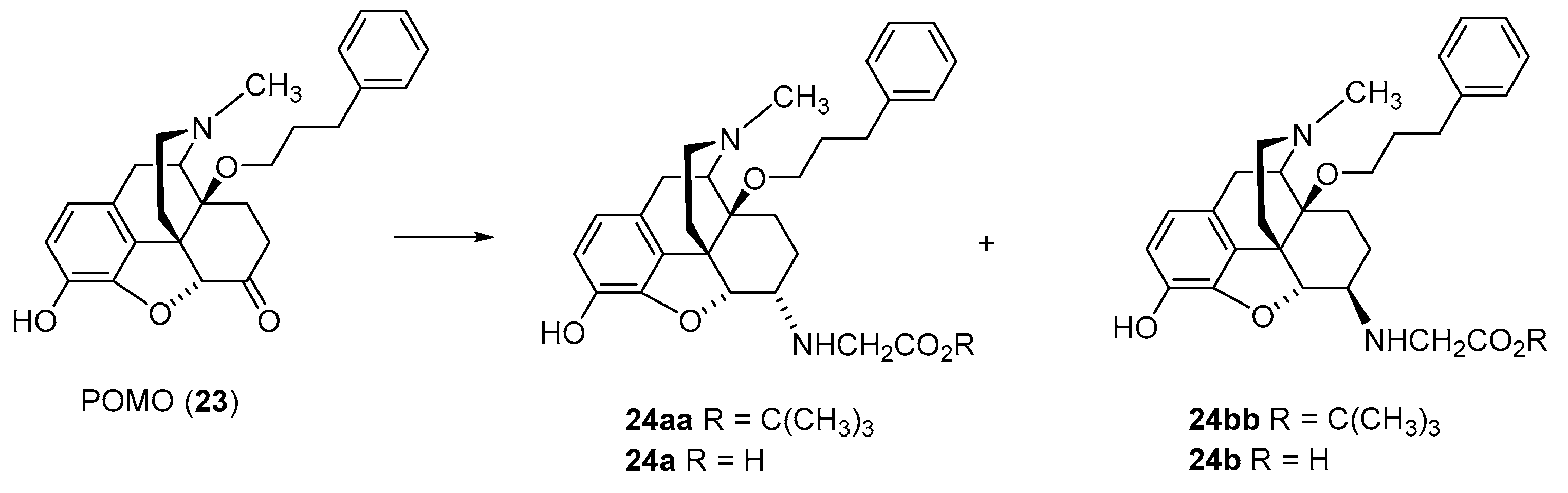
2.2.1. 6-Amino-acid-substituted 14-Alkoxymorphinans
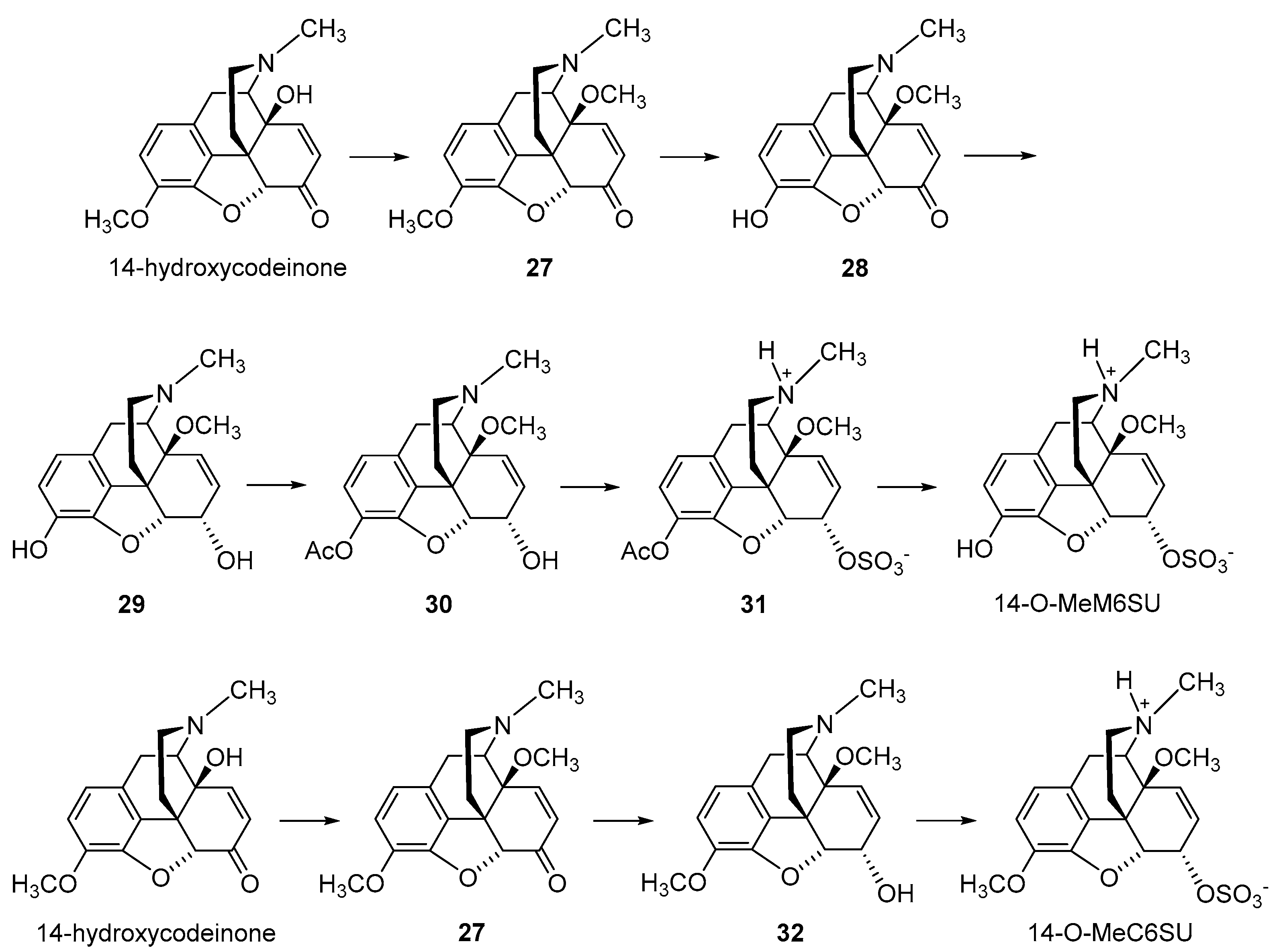
2.2.2. 6-O-Sulfate Esters of Morphine and Codeine
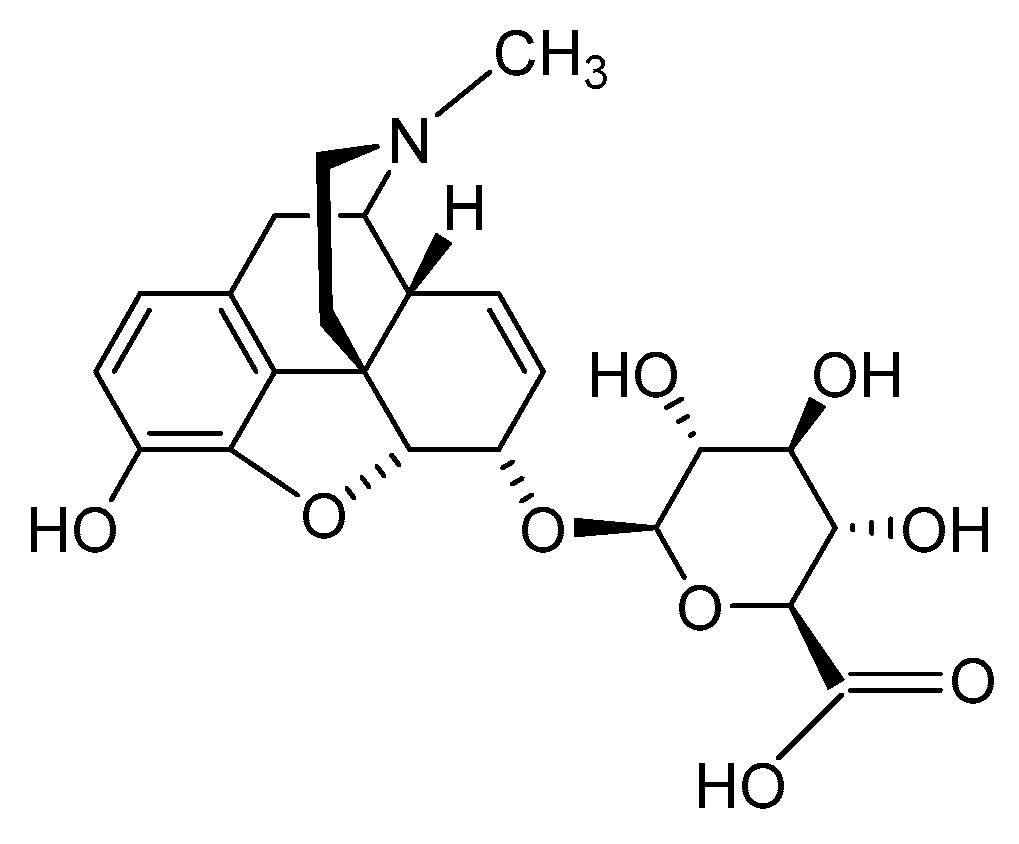
2.2.3. Morphine-6-glucuronide
2.3. Nanocarrier-Based Approaches of Drug Delivery
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid analgesia and opioid-induced adverse effects: A review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.; Benveniste, H.; McLellan, T.A. Use and misuse of opioids in chronic pain. Annu. Rev. Med. 2018, 69, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, Ł.; Goryński, K. Pharmacological aspects of over-the-counter opioid drugs misuse. Molecules 2020, 25, 3905. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). Available online: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed on 28 April 2023).
- Spetea, M. Opioid receptors and their ligands in the musculoskeletal system and relevance for pain control. Curr. Pharm. Des. 2013, 19, 7382–7390. [Google Scholar] [CrossRef]
- Sein, C. Opioid receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar]
- Che, T.; Roth, B.L. Structural insights accelerate the discovery of opioid alternatives. Annu. Rev. Biochem. 2021, 90, 739–761. [Google Scholar] [CrossRef]
- Spetea, M.; Asim, M.F.; Wolber, G.; Schmidhammer, H. The opioid receptor and ligands acting at the µ opioid receptor, as therapeutics and potential therapeutics. Curr. Pharm. Des. 2013, 19, 7415–7434. [Google Scholar] [CrossRef]
- Busserolles, J.; Lolignier, S.; Kerckhove, N.; Bertin, C.; Authier, N.; Eschalier, A. Replacement of current opioid drugs focusing on MOR-related strategies. Pharmacol. Ther. 2020, 210, 107519. [Google Scholar] [CrossRef]
- Darcq, E.; Kieffer, B.L. Opioid receptors: Drivers to addiction? Nat. Rev. Neurosci. 2018, 19, 499–514. [Google Scholar] [CrossRef]
- Imam, M.Z.; Kuo, A.; Ghassabian, S.; Smith, M.T. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 2018, 131, 238–255. [Google Scholar] [CrossRef]
- Fürst, S.; Hosztafi, S. The chemical and pharmacological importance of morphine analogues. Acta Physiol. Hung. 2008, 95, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.W.; Pan, Y.X. Mu opioids and their receptors: Evolution of a concept. Pharmacol. Rev. 2013, 65, 1257–1317. [Google Scholar] [CrossRef] [PubMed]
- Spetea, M.; Schmidhammer, H. Recent advances in the development of 14-alkoxy substituted morphinans as potent and safer opioid analgesics. Curr. Med. Chem. 2012, 19, 2442–2457. [Google Scholar] [CrossRef]
- Lewis, J.W.; Husbands, S.M. 14-Amino-4,5-epoxymorphinan derivatives and their pharmacological actions. Top. Curr. Chem. 2011, 299, 93–119. [Google Scholar] [PubMed]
- Stavitskaya, L.; Coop, A. Most recent developments and modifications of 14-alkylamino and 14-alkoxy-4,5-epoxymorphinan derivatives. Mini. Rev. Med. Chem. 2011, 11, 1002–1008. [Google Scholar] [CrossRef]
- Schmidhammer, H.; Spetea, M.; Windisch, P.; Schütz, J.; Riba, P.; Al-Khrasani, M.; Fürst, S. Functionalization of the carbonyl group in position 6 of morphinan-6-ones. Development of novel 6-amino and 6-guanidino substituted 14-alkoxymorphinans. Curr. Pharm. Des. 2013, 19, 7391–7399. [Google Scholar] [CrossRef]
- Devereaux, A.L.; Mercer, S.L.; Cunningham, C.W. DARK classics in chemical neuroscience: Morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407. [Google Scholar] [CrossRef]
- Spetea, M.; Schmidhammer, H. Recent chemical and pharmacological developments on 14-oxygenated-N-methylmorphinan-6-ones. Molecules 2021, 26, 5677. [Google Scholar] [CrossRef]
- Kalso, E.; Smith, L.; McQuay, H.J.; Moore, R.A. No pain, no gain: Clinical excellence and scientific rigour—Lessons learned from IA morphine. Pain 2002, 98, 269–275. [Google Scholar] [CrossRef]
- Stein, C.; Schäfer, M.; Machelska, H. Attaching pain at its source: New perspectives on opioids. Nat. Med. 2003, 9, 1003–1008. [Google Scholar] [CrossRef]
- Kapitzke, D.; Vetter, I.; Cabot, P.J. Endogenous opioid analgesia in peripheral tissues and the clinical implications for pain control. Ther. Clin. Risk Manag. 2005, 1, 279–297. [Google Scholar]
- Martinez, V.; Abalo, R. Peripherally acting opioid analgesics and peripherally-induced analgesia. Behav. Pharmacol. 2020, 31, 136–158. [Google Scholar] [CrossRef]
- Stein, C.; Comisel, K.; Haimaeri, E.; Yassouridis, A.; Lehrberger, K. Analgesic effects of intraarticular morphine after arthroscopic knee surgery. N. Engl. J. Med. 1991, 325, 1123–1126. [Google Scholar] [CrossRef]
- Likar, R.; Kapral, S.; Steinkellner, H.; Stein, C.; Schäfer, M. Dose-dependency of intra-articular morphine analgesia. Br. J. Anaesth. 1999, 83, 241–244. [Google Scholar] [CrossRef]
- Iorio, M.A.; Frigeni, V. Narcotic agonist/antagonist properties of quaternary diastereoisomers derived from oxymorphone and naloxone. Eur. J. Med. Chem. 1984, 19, 301–303. [Google Scholar]
- Brown, D.R.; Goldberg, L.I. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology 1985, 24, 181–191. [Google Scholar] [CrossRef]
- Foster, R.S.; Jenden, D.J.; Lomax, P. A comparison of the pharmacologic effects of morphine and N-methyl morphine. J. Pharmacol. Exp. Ther. 1967, 157, 185–195. [Google Scholar] [PubMed]
- Smith, T.W.; Buchan, P.; Parsons, D.N.; Wilkinson, S. Peripheral antinociceptive effects of N-methyl morphine. Life Sci. 1982, 31, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Oluyomi, A.O.; Hart, S.L.; Smith, T.W. Differential antinociceptive effects of morphine and methylmorphine in the formalin test. Pain 1992, 49, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Kosterlitz, H.W.; Waterfield, A.A. In vitro models in the study of structure-activity relationships of narcotic analgesics. Annu. Rev. Pharmacol. 1975, 15, 29–47. [Google Scholar] [CrossRef]
- Botros, S.; Lipkowski, A.; Larson, D.; Stark, P.; Takemori, A.; Portoghese, P.S. Opioid agonist and antagonist activities of peripherally selective derivatives of naltrexamine and oxymorphamine. J. Med. Chem. 1989, 32, 2068–2071. [Google Scholar] [CrossRef]
- Larson, D.L.; Hua, M.; Takemori, A.K.; Portoghese, P.S. Possible contribution of a glutathione conjugate to the long-duration action of funaltrexamine. J. Med. Chem. 1993, 36, 3669–3673. [Google Scholar] [CrossRef]
- Schütz, J.; Brandt, W.; Spetea, M.; Wurst, K.; Wunder, G.; Schmidhammer, H. Synthesis of 6-amino acid substituted derivatives of the highly potent analgesic 14-O-methyloxymorphone. Helv. Chim. Acta 2003, 86, 2142–2148. [Google Scholar] [CrossRef]
- Spetea, M.; Rief, S.B.; Haddou, T.B.; Fink, M.; Kristeva, E.; Mittendorfer, H.; Haas, S.; Hummer, N.; Follia, V.; Guerrieri, E.; et al. Synthesis, biological, and structural explorations of new zwitterionic derivatives of 14-O-methyloxymorphone, as potent μ/δ opioid agonists and peripherally selective antinociceptives. J. Med. Chem. 2019, 62, 641–653. [Google Scholar] [CrossRef]
- Spetea, M.; Windisch, P.; Guo, Y.; Bileviciute-Ljungar, I.; Schütz, J.; Asim, M.F.; Berzetei-Gurske, I.P.; Riba, P.; Kiraly, K.; Fürst, S.; et al. Synthesis and pharmacological activities of 6-glycine substituted 14-phenylpropoxymorphinans, a novel class of opioids with high opioid receptor affinities and antinociceptive potencies. J. Med. Chem. 2011, 54, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Spetea, M.; Friedmann, T.; Riba, P.; Schütz, J.; Wunder, G.; Langer, T.; Schmidhammer, H.; Fürst, S. In vitro opioid activity profiles of 6-amino acid substituted derivatives of 14-O-methyloxymorphone. Eur. J. Pharmacol. 2004, 483, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Rief, S.; Schmidhammer, H.; Negri, L.; Spetea, M. In vitro and in vivo pharmacological activities of 14-O-phenylpropyloxymorphone, a potent mixed mu/delta/kappa-opioid receptor agonist with reduced constipation in mice. Front. Pharmacol. 2018, 9, 1002. [Google Scholar] [CrossRef]
- Fürst, S.; Riba, P.; Friedmann, T.; Tímar, J.; Al-Khrasani, M.; Obara, I.; Makuch, W.; Spetea, M.; Schütz, J.; Przewlocki, R.; et al. Peripheral versus central antinociceptive actions of 6-amino acid-substituted derivatives of 14-O-methyloxymorphone in acute and inflammatory pain in the rat. J. Pharmacol. Exp. Ther. 2005, 312, 609–618. [Google Scholar] [CrossRef]
- Bileviciute-Ljungar, I.; Spetea, M.; Guo, Y.; Schütz, J.; Windisch, P.; Schmidhammer, H. Peripherally mediated antinociception of the mu-opioid receptor agonist 2-(4,5alpha-epoxy-3-hydroxy-14beta-methoxy-17-methylmorphinan-6beta-yl)aminoacetic acid (HS-731) after subcutaneous and oral administration in rats with carrageenan-induced hindpaw inflammation. J. Pharmacol. Exp. Ther. 2006, 317, 220–227. [Google Scholar]
- Al-Khrasani, M.; Spetea, M.; Friedmann, T.; Riba, P.; Király, K.; Schmidhammer, H.; Fürst, S. DAMGO and 6β-glycine substituted 14-O-methyloxymorphone but not morphine show peripheral, preemptive antinociception after systemic administration in a mouse visceral pain model and high intrinsic efficacy in the isolated rat vas deferens. Brain Res. Bull. 2007, 74, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Baillie, L.D.; Schmidhammer, H.; Mulligan, S.J. Peripheral μ-opioid receptor mediated inhibition of calcium signaling and action potential-evoked calcium fluorescent transients in primary afferent CGRP nociceptive terminals. Neuropharmacology 2015, 93, 267–273. [Google Scholar] [CrossRef]
- Obara, I.; Makuch, W.; Spetea, M.; Schütz, J.; Schmidhammer, H.; Przewlocki, R.; Przewlocka, B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur. J. Pharmacol. 2007, 558, 60–67. [Google Scholar] [CrossRef]
- Puls, K.; Schmidhammer, H.; Wolber, G.; Spetea, M. Mechanistic characterization of the pharmacological profile of HS-731, a peripherally acting opioid analgesic, at the µ-, δ-, κ-opioid and nociceptin receptors. Molecules 2022, 27, 919. [Google Scholar] [CrossRef] [PubMed]
- Fürst, S.; Zádori, Z.S.; Zádor, F.; Király, K.; Balogh, M.; László, S.B.; Hutka, B.; Mohammadzadeh, A.; Calabrese, C.; Galambos, A.R.; et al. On the role of peripheral sensory and gut mu opioid receptors: Peripheral analgesia and tolerance. Molecules 2020, 25, 2473. [Google Scholar] [CrossRef] [PubMed]
- Herz, A.; Teschemacher, H.J. Activities and sites of antinociceptive action of morphine-like analgesics and kinetics of distribution following intravenous, intracerebral and intraventricular application. In Advances in Drug Research; Harper, N., Simmonds, A.B., Eds.; Academic Press Limited: London, UK, 1971; Volume 6, pp. 79–118. [Google Scholar]
- Frances, B.; Gout, R.; Monsarrat, B.; Cros, J.; Zajac, J.M. Further evidence that morphine-6β-glucuronide is a more potent opioid agonist than morphine. J. Pharmacol. Exp. Ther. 1992, 262, 2531. [Google Scholar]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef]
- Mori, M.; Oguri, K.; Yoshimura, H.; Shimomura, K.; Kamata, O.; Ueki, S. Chemical synthesis and analgesic effect of morphine ethereal sulfates. Life Sci. 1972, 11, 525–533. [Google Scholar] [CrossRef]
- Váradi, A.; Gergely, A.; Béni, S.; Jankovics, P.; Noszál, B.; Hosztafi, S. Sulfate esters of morphine derivatives: Synthesis and characterization. Eur. J. Pharm. Sci. 2011, 42, 65–72. [Google Scholar] [CrossRef]
- Zuckerman, A.; Bolan, E.; de Paulis, T.; Schmidt, D.; Spector, S.; Pasternak, G.W. Pharmacological characterization of morphine-6-sulfate and codeine-6-sulfate. Brain Res. 1999, 842, 1–5. [Google Scholar] [CrossRef]
- Zádor, F.; Mohammadzadeh, A.; Balogh, M.; Zádori, Z.S.; Király, K.; Barsi, S.; Galambos, A.R.; László, S.B.; Hutka, B.; Váradi, A.; et al. Comparisons of in vivo and in vitro opioid effects of newly synthesized 14-methoxycodeine-6-O-sulfate and codeine-6-O-sulfate. Molecules 2020, 25, 1370. [Google Scholar] [CrossRef] [PubMed]
- Preechagoon, D.; Brereton, I.; Staatz, C.; Prankerd, R. Ester prodrugs of a potent analgesic, morphine-6-sulfate: Syntheses, spectroscopic and physicochemical properties. Int. J. Pharm. 1998, 163, 177–190. [Google Scholar] [CrossRef]
- Crooks, P.A.; Kottayil, S.G.; Al-Ghananeem, A.M.; Byrn, S.R.; Butterfield, A.D. Opiate receptor binding properties of morphine-, dihydromorphine-, and codeine 6-O-sulfate ester congeners. Bioorg. Med. Chem. Lett. 2006, 16, 4291–4295. [Google Scholar] [CrossRef]
- Kaspersen, F.M.; Van Boeckel, C.A.A. A review of the methods of chemical synthesis of sulphate and glucuronide conjugates. Xenobiotica 1987, 17, 1451–1471. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Desai, U.R. Chemical sulfation of small molecules—Advances and challenges. Tetrahedron 2010, 66, 2907–2918. [Google Scholar] [CrossRef]
- Lacko, E.; Varadi, A.; Rapavi, R.; Zador, F.; Riba, P.; Benyhe, S.; Borsodi, A.; Hosztafi, S.; Timar, J.; Noszal, B.; et al. A novel µ-opioid receptor ligand with high in vitro and in vivo agonist efficacy. Curr. Med. Chem. 2012, 54, 4699–4707. [Google Scholar] [CrossRef]
- Brown, C.E.; Roerig, S.C.; Burger, V.T. Analgesic potencies of morphine 3- and 6-sulfates, after intracerebroventricular administration in mice: Relationship to structural characteristics defined by mass spectrometry and nuclear magnetic resonance. J. Pharm. Sci. 1985, 74, 821–824. [Google Scholar] [CrossRef]
- Holtman, J.R.; Crooks, P.A.; Johnson-Hardy, J.; Wala, E.P. Antinociceptive effects and toxicity of morphine-6-O-sulfate sodium salt in rat models of pain. Eur. J. Pharmacol. 2010, 648, 87–94. [Google Scholar] [CrossRef]
- Al-Khrasani, M.; Lackó, E.; Riba, P.; Király, K.; Sobor, M.; Timár, J.; Mousa, S.; Schäfer, M.; Fürst, S. The central versus peripheral antinociceptive effects of µ-opioid receptor agonists in the new model of rat visceral pain. Brain Res. Bull. 2012, 87, 238–243. [Google Scholar] [CrossRef]
- Lackó, E.; Riba, P.; Giricz, Z.; Váradi, A.; Cornic, L.; Balogh, M.; Király, K.; Cseko, K.; Mousa, S.A.; Hosztafi, S.; et al. New morphine analogs produce peripheral antinociception within a certain dose range of their systemic administration. J. Pharmacol. Exp. Ther. 2016, 359, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Yadlapalli, J.S.K.; Ford, B.M.; Ketkar, A.; Wan, A.; Penthala, N.R.; Eoff, R.L.; Prather, P.L.; Dobretsov, M.; Crooks, P.A. Antinociceptive effects of the 6-O-sulfate ester of morphine in normal and diabetic rats: Comparative role of mu- and delta-opioid receptors. Pharmacol. Res. 2016, 13, 335–347. [Google Scholar] [CrossRef]
- Yadlapalli, J.S.K.; Jai Shankar, K.; Dogra, N.; Walbaum, A.W.; Wessinger, W.D.; Prather, P.L.; Crooks, P.A.; Dobretsov, M. Evaluation of analgesia, tolerance, and the mechanism of action of morphine-6-O-sulfate across multiple pain modalities in Sprague-Dawley Rats. Anesth. Analg. 2017, 125, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Balogh, M.; Zádori, Z.S.; Lázár, B.; Karádi, D.; László, S.; Mousa, S.A.; Hosztafi, S.; Zádor, F.; Riba, P.; Schäfer, M.; et al. The peripheral versus central antinociception of a novel opioid agonist: Acute inflammatory pain in rats. Neurochem. Res. 2018, 43, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Balogh, M.; Zádor, F.; Zádori, Z.S.; Shaqura, M.; Király, K.; Mohammadzadeh, A.; Varga, B.; Lázár, B.; Mousa, S.A.; Hosztafi, S.; et al. Efficacy-based perspective to overcome reduced opioid analgesia of advanced painful diabetic neuropathy in rats. Front. Pharmacol. 2019, 10, 347. [Google Scholar] [CrossRef]
- Khalefa, B.I.; Mousa, S.A.; Shaqura, M.; Lackó, E.; Hosztafi, S.; Riba, P.; Schäfer, M.; Ferdinandy, P.; Fürst, S.; Al-Khrasani, M. Peripheral antinociceptive efficacy and potency of a novel opioid compound 14-O-MeM6SU in comparison to known peptide and non-peptide opioid agonists in a rat model of inflammatory pain. Eur.J. Pharmacol. 2013, 713, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Rosow, C.E. Anesthetic drug interaction: An overview. J. Clin. Anesthn. 1997, 9, 27S–32S. [Google Scholar] [CrossRef] [PubMed]
- Yadlapalli, J.S.K.; Albayati, Z.A.F.; Penthala, N.R.; Hendrickson, H.P.; Crooks, H.A. Stability studies of potent opioid analgesic, morphine-6-O-sulfate in various buffers and biological matrices by HPLC-DAD analysis. Biomed. Chromatogr. 2017, 31, e3957. [Google Scholar] [CrossRef]
- Kiraly, K.; Caputi, F.F.; Hanuska, A.; Kató, E.; Balogh, M.; Köles, L.; Palmisano, M.; Riba, P.; Hosztafi, S.; Romualdi, P.; et al. A new potent analgesic agent with reduced liability to produce morphine tolerance. Brain Res. Bull. 2015, 117, 32–38. [Google Scholar] [CrossRef]
- Paul, D.; Standifer, K.M.; Inturrisi, C.E.; Pasternak, G.W. Pharmacological characterization of morphine-6 beta-glucuronide, a very potent morphine metabolite. J. Pharmacol. Exp. Ther. 1989, 251, 477–483. [Google Scholar]
- Osborne, R.; Joel, S.; Trew, D.; Slevin, M. Morphine and metabolite behavior after different routes of morphine administration: Demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin. Pharmacol. Ther. 1990, 47, 12–19. [Google Scholar] [CrossRef]
- Tegeder, I.; Meier, S.; Burian, M.; Schmidt, H.; Geisslinger, G.; Lötsch, J. Peripheral opioid analgesia in experimental human pain models. Brain 2003, 126, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.H.; Elliott, K.M.; Fung, M. Randomized, double-blind study of the analgesic efficacy of morphine-6-glucuronide versus morphine sulfate for postoperative pain in major surgery. Anesthesiology 2005, 102, 815–821. [Google Scholar] [CrossRef]
- Binning, A.R.; Przesmycki, K.; Sowinski, P.; Morrison, L.M.M.; Smith, T.W.; Marcus, P.; Lees, J.P.; Dahan, A. A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. Eur. J. Pain 2011, 15, 402–408. [Google Scholar] [CrossRef]
- Sverrisdóttir, E.; Lund, T.M.; Olesen, A.E.; Drewes, A.M.; Christrup, L.L.; Kreilgaard, M. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur. J. Pharm. Sci. 2015, 74, 45–62. [Google Scholar] [CrossRef]
- Kilpatrick, G.J.; Smith, T.W. Morphine-6-glucuronide: Actions and mechanisms. Med. Res. Rev. 2005, 25, 521–544. [Google Scholar] [CrossRef]
- Lötsch, J.; Geisslinger, G. Morphine-6-glucuronide: An analgesic of the future? Clin. Pharmacokinet. 2001, 40, 485–499. [Google Scholar] [CrossRef]
- Bickel, U.; Schumacher, O.P.; Kang, Y.S.; Voigt, K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood barrier in the rat. J. Pharmacol. Exp. Ther. 1996, 278, 107–113. [Google Scholar] [PubMed]
- Huwyler, J.; Drewe, J.; Klusemann, C.; Fricker, G. Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br. J. Pharmacol. 1996, 118, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Sattari, M.; Routledge, P.; Mashayekhi, S. The influence of active transport systems on morphine -6-glucuronide transport in MDCKII and MDCK-PGP cells. Daru 2011, 19, 412–416. [Google Scholar] [PubMed]
- Bourasset, F.; Cisternino, S.; Temsamani, J.; Scherrmann, J.-M. Evidence for an active transport of morphine-6-β-d-glucuronide but not P-glycoprotein-mediated at the blood–brain barrier. J. Neurochem. 2003, 86, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Cashman, J.R.; MacDougall, J.M. Dynamic medicinal chemistry in the elaboration of morphine-6-glucuronide analogs. Curr. Top. Med. Chem. 2005, 5, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Klimas, R.; Mikus, G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: A quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br. J. Anaesth. 2014, 113, 935–944. [Google Scholar] [CrossRef]
- Koning, G.A.; Schiffelers, R.M.; Storm, G. Endothelial cells at inflammatory sites as target for therapeutic intervention. Endothelium 2002, 9, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef]
- Nehoff, H.; Parayath, N.N.; Domanovitch, L.; Taurin, S.; Greish, K. Nanomedicine for drug targeting: Strategies beyond the enhanced permeability and retention effect. Int. J. Nanomed. 2014, 22, 2539–2555. [Google Scholar]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Moradkhani, M.R.; Karimi, A.; Negahdari, B. Nanotechnology application for pain therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 368–373. [Google Scholar] [CrossRef]
- Chakravarthy, K.V.; Boehm, F.J.; Christo, P.J. Nanotechnology: A promising new paradigm for the control of pain. Pain Med. 2018, 19, 232–243. [Google Scholar] [CrossRef]
- González-Rodríguez, S.; Quadir, M.A.; Gupta, S.; Walker, K.A.; Zhang, X.; Spahn, V. Polyglycerol-opioid conjugate produces analgesia devoid of side effects. Elife 2017, 6, e27081. [Google Scholar] [CrossRef]
- Martin, C.; Oyen, E.; Mangelschots, J.; Bibian, M.; Ben Haddou, T.; Andrade, J.; Gardiner, J.; Van Mele, B.; Madder, A.; Hoogenboom, R.; et al. Injectable peptide hydrogels for controlled-release of opioids. MedChemComm 2016, 7, 542–549. [Google Scholar] [CrossRef]
- Martin, C.; Oyen, E.; Van Wanseele, Y.; Haddou, T.B.; Schmidhammer, H.; Andrade, J.; Waddington, L.; Van Eeckhaut, A.; Van Mele, B.; Gardiner, J.; et al. Injectable peptide-based hydrogel formulations for the extended in vivo release of opioids. Mater. Today Chem. 2017, 3, 49–59. [Google Scholar] [CrossRef]



| Compound | Agonist Potencies IC50 × 108 (M) a | Antinociceptive Potencies ED50 b |
|---|---|---|
| a | 14.4 | 0.27 nmol/mouse, i.c.v. 9.8 µmol/kg, i.v. 30.3 µmol/kg, p.o. |
| b | 13.0 | - c |
| c | 3.9 | 0.17 nmol/mouse, i.c.v. 11 µmol/kg, i.v. ~20 µmol/kg, p.o. |
| d | 2.7 | 0.25 nmol/mouse, i.c.v. <20 µmol/kg, i.v. >25 µmol/kg, p.o. |
| e | 3.1 | 0.44 nmol/mouse, i.c.v. <0 µmol/kg, i.v. |
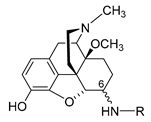
| Compound | R | Opioid Receptor Binding a | Agonist Activity b | clogD7.4 c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOR Ki (nM) | DOR Ki (nM) | KOR Ki (nM) | Ki Ratio MOR/DOR/KOR | MOR EC50 (nM) | MOR % stim. | DOR EC50 (nM) | DOR % stim. | KOR EC50 (nM) | KOR % stim. | |||
| 14-OMO (1) | 0.10 | 4.80 | 10.2 | 1/48/102 | 3.83 | 97 | 37.3 | 106 | 116 | 77 | 0.48 | |
| HS-730 (2a) | α-Gly | 0.89 | 15.4 | 43.2 | 1/7/49 | 1.16 | 99 | 9.61 | 103 | 399 | 87 | −3.35 |
| HS-731 (2b) | β-Gly | 0.83 | 7.86 | 44.8 | 1/9.5/54 | 3.78 | 98 | 7.92 | 103 | 361 | 82 | −3.35 |
| HS-935 (3a) | α-L-Ala | 0.77 | 26.9 | 142 | 1/35/184 | 1.34 | 97 | 9.55 | 93 | 214 | 51 | −2.81 |
| HS-936 (3b) | β-L-Ala | 1.90 | 7.71 | 63.7 | 1/4.1/34 | 6.24 | 87 | 5.20 | 104 | 392 | 64 | −2.81 |
| HS-937 (4a) | α-L-Phe | 0.95 | 3.67 | 28.5 | 1/3.9/30 | 0.38 | 93 | 0.39 | 102 | 219 | 39 | −1.13 |
| HS-938 (4b) | β-L-Phe | 2.58 | 1.03 | 151 | 1/0.4/59 | 6.76 | 99 | 0.48 | 94 | 1172 | 81 | −1.13 |
| 5a | α-L-Ser | 2.21 | 5.32 | 196 | 1/2.4/89 | 1.60 | 87 | 13.9 | 101 | 1213 | 44 | −3.89 |
| 5b | β-L-Ser | 2.14 | 5.29 | 152 | 1/2.5/71 | 3.56 | 101 | 6.98 | 98 | 201 | 88 | −3.89 |
| 6a | α-L-Val | 3.16 | 3.91 | 325 | 1/1.2/103 | 10.5 | 95 | 33.8 | 91 | 462 | 51 | −1.94 |
| 6b | β-L-Val | 3.04 | 3.52 | 305 | 1/1.2/100 | 11.7 | 84 | 5.73 | 96 | 1117 | 68 | −1.94 |
| 7a | α-L-Lys | 0.19 | 1.27 | 12.6 | 1/6.7/66 | 2.25 | 9 | 152 | 106 | 118 | 79 | −5.57 |
| 7b | β-L-Lys | 0.53 | 3.34 | 33.7 | 1/6.3/64 | 6.85 | 90 | 45.1 | 93 | 525 | 62 | −5.57 |
| 8a | α-L-Tyr | 0.83 | 2.18 | 39.5 | 1/2.6/48 | 1.87 | 92 | 1.76 | 88 | 100 | 62 | −1.41 |
| 8b | β-L-Tyr | 3.20 | 3.89 | 186 | 1/1.2/58 | 24.7 | 93 | 6.23 | 95 | 774 | 60 | −1.41 |
| 9a | α-L-Trp | 0.36 | 1.02 | 25.1 | 1/2.8/70 | 0.51 | 93 | 2.52 | 102 | 70.1 | 61 | −1.03 |
| 9b | β-L-Trp | 0.65 | 1.19 | 8.66 | 1/1.8/13 | 1.64 | 101 | 2.18 | 96 | 181 | 87 | −1.03 |
| 10a | α-L-Asn | 1.17 | 3.37 | 74.0 | 1/2.9/63 | 0.83 | 99 | 9.78 | 106 | 81.7 | 67 | −4.29 |
| 10b | β-L-Asn | 1.26 | 2.25 | 103 | 1/1.8/82 | 2.04 | 96 | 3.18 | 88 | 923 | 71 | −4.29 |
| 11a | α-L-Gln | 3.24 | 5.13 | 351 | 1/1.6/108 | 2.27 | 90 | 7.80 | 104 | 185 | 70 | −4.04 |
| 11b | β-L-Gln | 2.48 | 4.87 | 290 | 1/2.0/117 | 9.54 | 98 | 3.96 | 103 | 1410 | 63 | −4.04 |
| 12a | α-L-Asp | 1.36 | 14.6 | 50.2 | 1/11/37 | 4.10 | 90 | 10.1 | 97 | 2991 | 83 | −5.64 |
| 12b | β-L-Asp | 3.42 | 22.6 | 351 | 1/6.6/103 | 1.45 | 74 | 11.8 | 101 | 753 | 49 | −5.64 |
| 13a | α-L-Glu | 1.45 | 9.03 | 87.2 | 1/6.2/60 | 3.11 | 105 | 10.8 | 98 | 1167 | 68 | −5.39 |
| 13b | β-L-Glu | 11.6 | 7.64 | 1252 | 1/0.7/108 | 12.7 | 98 | 4.60 | 101 | 2233 | 76 | −5.39 |
| 14a | α-D-Ala | 0.69 | 10.4 | 71.5 | 1/15/104 | 1.44 | 100 | 24.3 | 106 | 254 | 67 | −2.81 |
| 14b | β-D-Ala | 1.48 | 11.3 | 142 | 1/7.6/96 | 15.4 | 102 | 5.46 | 106 | 1001 | 86 | −2.81 |
| 15a | α-D-Val | 1.70 | 1.93 | 202 | 1/1.1/119 | 4.51 | 105 | 1.12 | 93 | 2218 | 96 | −1.94 |
| 15b | β-D-Val | 1.02 | 1.68 | 159 | 1/1.6/156 | 2.38 | 101 | 1.30 | 99 | 1278 | 98 | −1.94 |
| 16a | α-D-Phe | 0.61 | 3.69 | 76.4 | 1/6.0/125 | 0.77 | 96 | 8.36 | 95 | 215 | 80 | −1.13 |
| 16b | β-D-Phe | 1.28 | 1.19 | 139 | 1/0.9/109 | 0.68 | 78 | 1.71 | 96 | 611 | 92 | −1.13 |
| 17a | α-L-Chg | 1.23 | 14.3 | 177 | 1/12/144 | 2.88 | 86 | 20.5 | 101 | 250 | 52 | −1.26 |
| 17b | β-L-Chg | 1.66 | 1.30 | 118 | 1/0.8/71 | 5.33 | 86 | 3.57 | 96 | 282 | 59 | −1.26 |
| 18a | α-L-Abu | 0.76 | 37.5 | 144 | 1/49/189 | 5.12 | 88 | 98.2 | 104 | 942 | 61 | −2.34 |
| 18b | β-L-Abu | 1.83 | 1.30 | 201 | 1/0.7/110 | 5.47 | 83 | 2.22 | 100 | 572 | 72 | −2.34 |
| 19a | α-β-Ala | 1.30 | 60.0 | 182 | 1/46/140 | 3.52 | 99 | 96.4 | 97 | 186 | 78 | −3.18 |
| 19b | β-β-Ala | 1.04 | 13.9 | 71.4 | 1/13/69 | 5.74 | 78 | 20.1 | 99 | 622 | 67 | −3.18 |
| 20a | α-GABA | 0.77 | 12.5 | 45.6 | 1/16/59 | 2.88 | 103 | 34.4 | 103 | 2034 | 104 | −2.93 |
| 20b | β-GABA | 1.41 | 6.61 | 147 | 1/4.7/104 | 12.3 | 86 | 6.06 | 103 | 3396 | 74 | −2.93 |
| 21a | α-L-Val-L-Tyr | 0.82 | 1.19 | 69.0 | 1/1.5/84 | 0.89 | 84 | 1.16 | 88 | 330 | 50 | −1.02 |
| 21b | β-L-Val-L-Tyr | 0.44 | 1.38 | 390 | 1/3.1/886 | 0.16 | 73 | 1.56 | 89 | 1884 | 63 | −1.02 |
| 22a | β-Gly-Gly | 4.62 | 7.52 | 203 | 1/1.6/44 | 4.39 | 85 | 2.84 | 102 | 885 | 75 | −4.27 |

| Compound | R | Opioid Receptor Binding, Ki (nM) a | clogD7.4 b | |||
|---|---|---|---|---|---|---|
| MOR | DOR | KOR | Ki Ratio MOR/DOR/KOR | |||
| POMO (23) | 0.073 | 0.13 | 0.30 | 1/1.8/4.1 | 2.89 | |
| 24a | α-Gly | 0.19 | 0.22 | 0.73 | 1/1.2/3.8 | −0.85 |
| 24b | β-Gly | 0.16 | 0.19 | 0.81 | 1/1.2/5.1 | −0.85 |
| Compound | Amino Acid Substitution at Position 6 | Radiant Heat Tail-Flick Test (Rat) ED50 (nmol/kg, s.c.) | Writhing Assay (Mouse) ED50 (µg/kg, s.c.) | Formalin Test (Rat) ED50 (nmol/kg, s.c.) | |
|---|---|---|---|---|---|
| Phase I | Phase II | ||||
| Morphine | 6053 | 437 | 1613 | 1472 | |
| Fentanyl | 38.6 | ||||
| 14-OMO (1) | 14.9 | 3.26 | |||
| HS-730 (2a) | α-Gly | 58.5 | 35.7 | 72 | 110 |
| HS-731 (2b) | β-Gly | 29.0 | 27.5 | 125 | 204 |
| HS-935 (3a) | α-L-Ala | 68.9 | 16.0 | ||
| HS-936 (3b) | β-L-Ala | 53.4 | 86.3 | ||
| HS-937 (4a) | α-L-Phe | 315 | 31.1 | 171 | 292 |
| HS-938 (4b) | β-L-Phe | >3600 | 579 | 79 | 107 |
| 5a | α-L-Ser | 32.1 | |||
| 6b | β-L-Val | 117 | |||
| 7a | α-L-Lys | 20.6 | |||
| 8a | α-L-Tyr | 14.6 | |||
| 9b | β-L-Trp | 92.7 | |||
| 10a | α-L-Asn | 15.2 | |||
| 12a | α-L-Asp | 38.2 | |||
| 13a | α-L-Glu | 36.1 | |||
| 15b | β-D-Val | 14.0 | |||
| 16a | α-D-Phe | 18.1 | |||
| 16b | β-D-Phe | 250 | |||
| 17a | α-L-Chg | 20.6 | |||
| 18a | α-L-Abu | 17.5 | |||
| 19a | α-β-Ala | 31.2 | |||
| 20a | α-GABA | 41.9 | |||
| 21a | β-L-Val-L-Tyr | 178 | |||
| 22a | β-Gly-Gly | 104 | |||
| 24a | α-Gly | 81.1 | |||
| 24b | β-Gly | 130 | |||
| Pain Model | Route | ED50 | Reference |
|---|---|---|---|
| Acute nociception Radiant heat tail-flick test (rat) | i.c.v. s.c. | 0.030 nmol/rat 29.0 nmol/kg | [40] [40] |
| Trigeminal nociception Eye wiping test (mouse) | i.p. | 50 µg/kg a | [43] |
| Visceral pain | |||
| Acetic-acid-induced writhing assay (mouse) | i.c.v. s.c. | 0.49 pmol/mouse 51 nmol/kg | [42] [42] |
| s.c. | 27.5 µg/kg | [36] | |
| Inflammatory pain Formalin test (rat) Carrageenan-induced thermal and mechanical hyperalgesia (rat) | i.pl. s.c. s.c. p.o. | Phase I: 0.2 nmol; Phase II: 0.4 nmol Phase I: 125 nmol/kg; Phase II: 204 nmol/kg 20 µg/kg a 10 mg/kg a | [44] [40] [41] [41] |
| Neuropathic pain, sciatic nerve ligation—Mechanical hyperalgesia (rat) | i.pl. | 441 nmol | [44] |
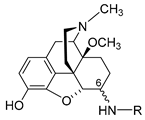
| Compound | R, Amino Acid Substitution at Position 6 | Radiant Heat Tail-Flick Test (Rat), ED50 | Ratio ED50 (nmol/kg, s.c.)/ ED50 (nmol/rat, i.c.v.). | |
|---|---|---|---|---|
| s.c. (nmol/kg) | i.c.v. (nmol/rat) | |||
| Morphine | 6053 | 35.1 | 172 | |
| Fentanyl | 38.6 | 1.66 | 23 | |
| 14-OMO (1) | 14.9 | 0.172 | 87 | |
| HS-730 (2a) | α-Gly | 58.5 | 0.031 | 1887 |
| HS-731 (2b) | β-Gly | 29.0 | 0.030 | 967 |
| HS-935 (3a) | α-L-Ala | 68.9 | 0.121 | 569 |
| HS-936 (3b) | β-L-Ala | 53.4 | 0.082 | 651 |
| HS-937 (4a) | α-L-Phe | 315 | 0.063 | 5000 |
| HS-938 (4b) | β-L-Phe | >3600 | 0.776 | >4600 |
| Compound | Opioid Receptor Binding, Ki (nM) a | |||
|---|---|---|---|---|
| MOR | DOR | KOR | Ki Ratio MOR/DOR/KOR | |
| Morphine | 4.37 | 2951 | 113 | 1/675/26 |
| Codeine | 737 | - b | - | - |
| M6SU | 11.5 | 525 | 275 | 1/46/24 |
| C6SU | 96.9 | 968 b | - | 1/10/- |
| 14-O-MeM6SU | 1.12 | 10.2 | 295 | 1/9/263 |
| 14-O-MeC6SU | 3.37 | 346 | 246 | 1/103/73 |
| Compound | MVD Bioassay | [35S]GTPγS Binding Assay | ||
|---|---|---|---|---|
| EC50 (nM) | Tissue | EC50 (nM) | Emax (%) | |
| Morphine | 347 | Rat brain Guinea-pig brain | 250 462 | 129 119 |
| Codeine | >1000 | Rat brain Guinea-pig brain | - a - | 110 104 |
| M6SU | 103 | Rat brain Guinea-pig brain | 105 - | 133 - |
| C6SU | >1000 | Rat brain Guinea-pig brain | >10,000 - | 121 102 |
| 14-O-MeM6SU | 4.38 | Rat brain Guinea-pig brain | 19.1 - | 201 - |
| 14-O-MeC6SU | 238 | Rat brain Guinea-pig brain | 301 >1000 | 128 130 |
| Compound | Pain Model (Species) | ED50 (Systemic Administration) | ED50 (Central Administration) | Reference |
|---|---|---|---|---|
| Morphine | hot-plate test (mouse) | 4.5 mg/kg, s.c. | [50] | |
| hot water tail-flick test (rat) | 3.41 mg/kg, i.p. | [64] | ||
| radiant heat tail-flick test (mouse) | 5.5 nmol/mouse, i.c.v. | [59] | ||
| radiant heat tail-flick test (mouse) | 314 ng/mouse, i.c.v. | [52] | ||
| radiant heat tail-flick test (rat) | 6221 nmol/kg, s.c. | 38.6 nmol/rat, i.c.v | [58] | |
| radiant heat tail-flick test (rat) | 2.68 mg/kg, i.p. | 3.51 µg/rat, i.t. | [60] | |
| radiant heat tail-flick test (rat) | 9.1 mg/kg, p.o. | [60] | ||
| acetic-acid-induced writhing assay (mouse) | 238.6 nmol/kg, i.p. | 2.02 nmol/mouse, i.c.v. | [61] | |
| formalin-induced inflammatory pain (rat) | Phase II: 0.259 mg/kg, i.p. | [61] | ||
| formalin-induced inflammatory pain (rat) | Phase I and II: 3884, 7769, 15,538, 31,075 nmol/kg, s.c. a | [65] | ||
| neuropathic pain, CCI (rat) hyperalgesia allodynia | 2.65 mg/kg, i.p. 1.45 mg/kg, i.p. | [60] | ||
| STZ-induced diabetic neuropathic pain— tail withdrawal test (rat) | 6.47 mg/kg, i.p. | [63] | ||
| STZ-induced diabetic neuropathic pain— paw withdrawal test (rat) | 40,000 nmol/kg, s.c. a | [66] | ||
| Codeine | radiant heat tail-flick test (rat) | 54.01 µmol/kg | [53] | |
| M6SU | hot-plate test (mouse) | 1.7 mg/kg, s.c. | [50] | |
| hot water tail-flick (rat) | 0.82 mg/kg, i.p. | [63] | ||
| radiant heat tail-flick test (mouse) | 0.19 nmol/mouse, i.c.v. | [59] | ||
| radiant heat tail-flick test (mouse) | 10.6 ng/mouse, i.c.v. | [52] | ||
| radiant heat tail-flick test (rat) | 9305 nmol/kg, s.c. | 0.356 nmol/rat, i.c.v. | [58] | |
| radiant heat tail-flick test (rat) | 0.54 mg/kg i.p. | 0.29 μg/rat, i.t. | [60] | |
| radiant heat tail-flick test (rat) | 4.97 mg/kg, p.o. | [60] | ||
| paw pressure threshold test (rat) | 2.3 mg/kg, i.p. | [64] | ||
| acetic-acid-induced writhing assay (mouse) | 1993 nmol/kg, s.c. | 9 pmol/mouse, i.c.v. | [62] | |
| formalin-induced inflammatory pain (rat) | Phase II: 0.094 mg/kg, i.p. | [60] | ||
| CFA-induced inflammatory pain—paw pressure test (rat) | 292 nmol/kg, s.c. | [62] | ||
| neuropathic pain, CCI (rat) hyperalgesia allodynia | 0.40 mg/kg, i.p. 0.19 mg/kg, i.p. | [60] | ||
| STZ-induced diabetic neuropathic pain—tail withdrawal test (rat) | 0.35 mg/kg, i.p. | [63] | ||
| C6SU | radiant heat tail-flick test (mouse) | 200 ng, i.c.v b | [52] | |
| radiant heat tail-flick test (rat) | weak effect (<20%), s.c. | [53] | ||
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.6 and 13.2 µmol/kg, s.c. a | [53] | ||
| 14-O-MeM6SU | radiant heat tail-flick test (rat) | 182.4 nmol/kg, s.c. | 0.0157 nmol/rat, i.c.v. | [58] |
| acetic-acid-induced writhing assay (mouse) | 87 nmol/kg, s.c. | 1.7 nmol/mouse, i.c.v. | [62] | |
| formalin-induced inflammatory pain (rat) | Phase I: 3506 and 1012 nmol/kg, s.c. a Phase II: 253, 506 and 1012 nmol/kg, s.c. a | [65] | ||
| CFA-induced inflammatory pain—paw pressure test (rat) | 45 nmol/kg, s.c. | [62] | ||
| STZ-induced diabetic neuropathic pain—paw withdrawal test (rat) | 253, 506 and 1012 nmol/kg, s.c. a | [66] | ||
| 14-O-MeC6SU | radiant heat tail-flick test (rat) | 5.34 µmol/kg, s.c. | 0.017 µmol/animal, i.c.v. | [53] |
| CFA-induced inflammatory pain—paw pressure test (rat) | 6.1 and 12.2 µmol/kg, s.c. a | [53] |
| Compound | Pain Model (Species) | ED50 Ratio Peripheral/Central Administration | Reference |
|---|---|---|---|
| Fentanyl | radiant heat tail-flick test (rat) | 23 | [50] |
| Morphine | radiant heat tail-flick test (rat) | 172 | [40] |
| 159 | [58] | ||
| 125 | [53] | ||
| acetic-acid-induced writhing assay (rat) | 118 | [61] | |
| M6SU | radiant heat tail-flick test (rat) | 25,493 | [58] |
| acetic-acid-induced writhing assay (mouse) | 221,444 | [62] | |
| 14-O-MeM6SU | radiant heat tail-flick test (rat) | 11,615 | [58] |
| acetic-acid-induced writhing assay (mouse) | 51,177 | [62] | |
| 14-O-MeC6SU | radiant heat tail-flick test (rat) | 314 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidhammer, H.; Al-Khrasani, M.; Fürst, S.; Spetea, M. Peripheralization Strategies Applied to Morphinans and Implications for Improved Treatment of Pain. Molecules 2023, 28, 4761. https://doi.org/10.3390/molecules28124761
Schmidhammer H, Al-Khrasani M, Fürst S, Spetea M. Peripheralization Strategies Applied to Morphinans and Implications for Improved Treatment of Pain. Molecules. 2023; 28(12):4761. https://doi.org/10.3390/molecules28124761
Chicago/Turabian StyleSchmidhammer, Helmut, Mahmoud Al-Khrasani, Susanna Fürst, and Mariana Spetea. 2023. "Peripheralization Strategies Applied to Morphinans and Implications for Improved Treatment of Pain" Molecules 28, no. 12: 4761. https://doi.org/10.3390/molecules28124761
APA StyleSchmidhammer, H., Al-Khrasani, M., Fürst, S., & Spetea, M. (2023). Peripheralization Strategies Applied to Morphinans and Implications for Improved Treatment of Pain. Molecules, 28(12), 4761. https://doi.org/10.3390/molecules28124761







