LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Oil-Specific Metabolomic Markers
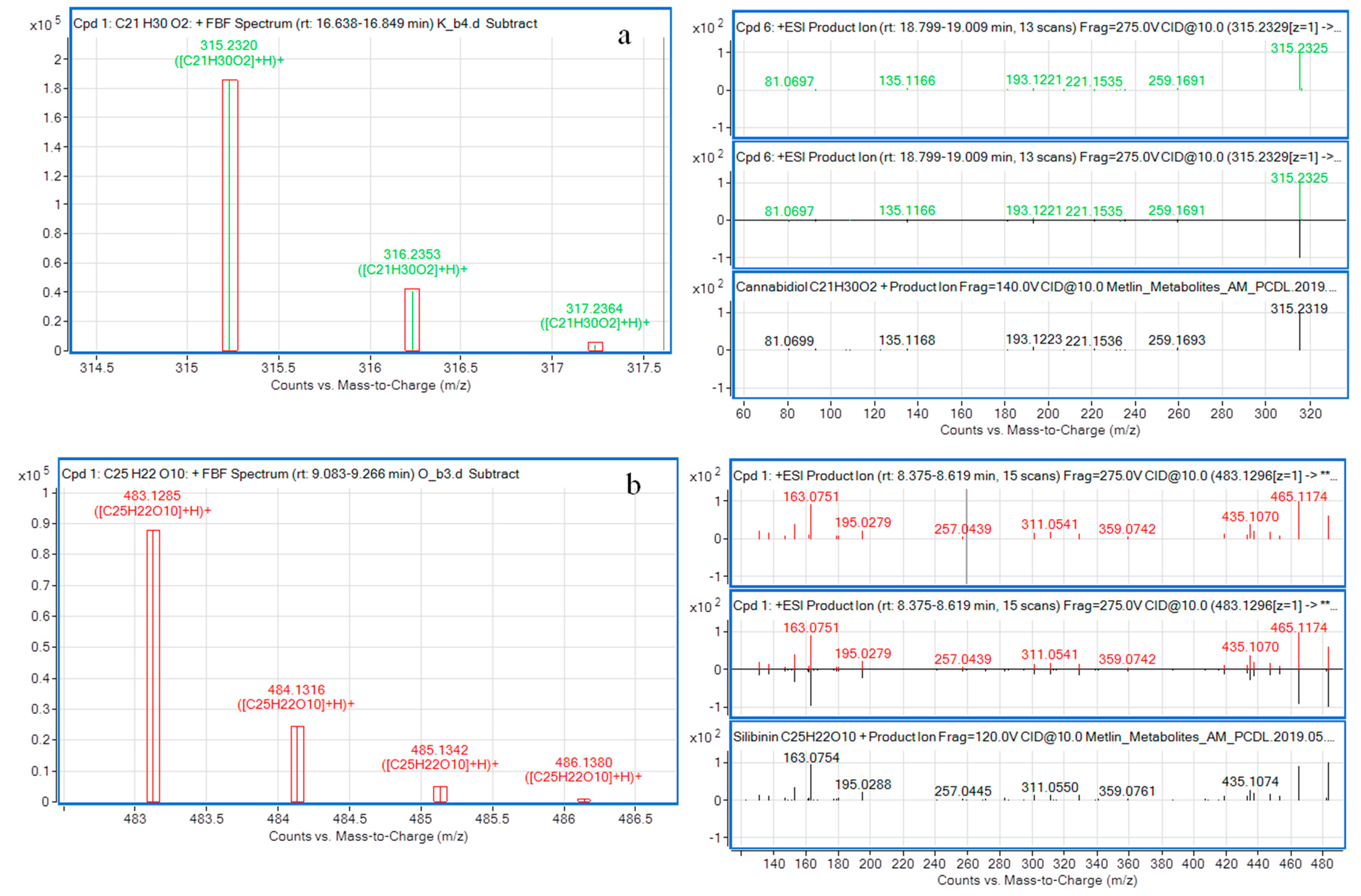
2.2. Fragment Spectra Matching
2.3. Molecular Formula Assignment
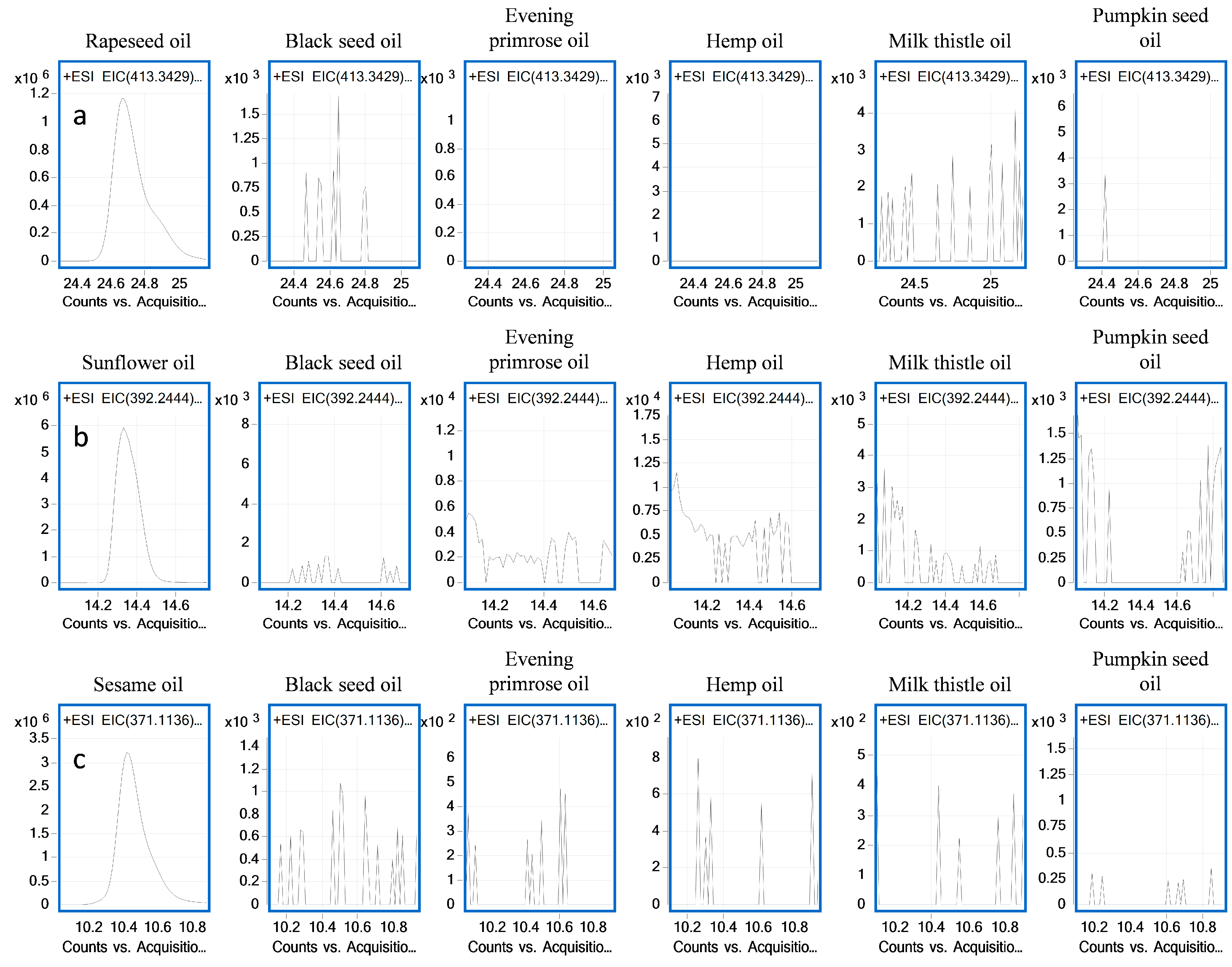
2.4. Effect of Matrix Variability on Marker Signal
2.5. Variability in Marker Intensity
2.6. Detection of Sunflower, Sesame and Rapeseed Oils in Binary Mixtures
2.7. The Detection of Specific Metabolite Markers in Commercial Unrefined, Cold-Pressed Oil Blends
3. Materials and Methods
3.1. Materials
3.2. Samples
3.3. Preparation of the Oil Mixtures
3.4. Extraction
3.5. LC–QTOF Analysis
3.6. LC–QTOF Data Processing
3.7. Evaluation of the Effect of Matrix Variability on Marker Signal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abe, M.; Abe, H. Lifestyle Medicine—An Evidence Based Approach to Nutrition, Sleep, Physical Activity, and Stress Management on Health and Chronic Illness. Pers. Med. Univ. 2019, 8, 3–9. [Google Scholar] [CrossRef]
- Reyes, L.I.; Constantinides, S.V.; Bhandari, S.; Frongillo, E.A.; Schreinemachers, P.; Wertheim-Heck, S.; Walls, H.; Holdsworth, M.; Laar, A.; Nguyen, T.; et al. Actions in Global Nutrition Initiatives to Promote Sustainable Healthy Diets. Glob. Food Secur. 2021, 31, 100585. [Google Scholar] [CrossRef]
- Munir, R.; Semmar, N.; Farman, M.; Ahmad, N.S. An Updated Review on Pharmacological Activities and Phytochemical Constituents of Evening Primrose (Genus oenothera). Asian Pac. J. Trop. Biomed. 2017, 7, 1046–1054. [Google Scholar] [CrossRef]
- Herculano, L.S.; Lukasievicz, G.V.B.; Sehn, E.; Torquato, A.S.; Belançon, M.P.; Savi, E.; Kimura, N.M.; Malacarne, L.C.; Baesso, M.L.; Astrath, N.G.C. The Correlation of Physicochemical Properties of Edible Vegetable Oils by Chemometric Analysis of Spectroscopic Data. Spectrochim. Acta Part A 2021, 245, 118877. [Google Scholar] [CrossRef] [PubMed]
- Aisa, H.A.; Xin, X.; Tang, D. Nigella Sativa: A Medicinal and Edible Plant That Ameliorates Diabetes, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128138229. [Google Scholar]
- Moustafa, H.A.M.; El Wakeel, L.M.; Halawa, M.R.; Sabri, N.A.; El-Bahy, A.Z.; Singab, A.N. Effect of Nigella Sativa Oil versus Metformin on Glycemic Control and Biochemical Parameters of Newly Diagnosed Type 2 Diabetes Mellitus Patients. Endocrine 2019, 65, 286–294. [Google Scholar] [CrossRef]
- Nigam, A.; Goenka, A.; Shrivastava, N. A Comparative Study of Effect of Ormeloxifene and Evening Primrose Oil in Treatment of Mastalgia. Indian J. Surg. 2019, 81, 259–264. [Google Scholar] [CrossRef]
- Nasri, K.; Akrami, S.; Rahimi, M.; Taghizadeh, M.; Behfar, M.; Mazandaranian, M.R.; Kheiry, A.; Memarzadeh, M.R.; Asemi, Z. The Effects of Vitamin D and Evening Primrose Oil Co-Supplementation on Lipid Profiles and Biomarkers of Oxidative Stress in Vitamin D-Deficient Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Endocr. Res. 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, H.; Takeda, H.; Nishihira, J. Pumpkin Seed Oil Extracted from Cucurbita Maxima Improves Urinary Disorder in Human Overactive Bladder. J. Tradit. Complement. Med. 2014, 4, 72–74. [Google Scholar] [CrossRef]
- Tantawy, S.A.; Elgohary, H.M.I.; Kamel, D.M. Trans-Perineal Pumpkin Seed Oil Phonophoresis as an Adjunctive Treatment for Chronic Nonbacterial Prostatitis. Res. Rep. Urol. 2018, 10, 95–101. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Jiang, N.; Yang, J.; Tu, J.; Zhou, Y.; Xiao, X.; Dong, Y. Silybum Marianum Oil Attenuates Hepatic Steatosis and Oxidative Stress in High Fat Diet-Fed Mice. Biomed. Pharmacother. 2018, 100, 191–197. [Google Scholar] [CrossRef]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of Dietary Hempseed Oil in Patients with Atopic Dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, Q.; Wu, W.; Zhang, D.; Mo, L.; Liu, J.; Yang, X. Food Fraud Vulnerability Assessment in the Edible Vegetable Oil Supply Chain: A Perspective of Chinese Enterprises. Food Control 2022, 138, 109005. [Google Scholar] [CrossRef]
- Kotecka-Majchrzak, K.; Sumara, A.; Fornal, E.; Montowska, M. Identification of Species-Specific Peptide Markers in Cold-Pressed Oils. Sci. Rep. 2020, 10, 19971. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Sun, C.; Jiang, W.; Zhang, X.; Hong, Y.; Jin, Q.; Tao, G.; Wang, X.; Yang, Z. Triacylglycerols Fingerprint of Edible Vegetable Oils by Ultra-Performance Liquid Chromatography-Q-ToF-MS. LWT 2019, 112, 108261. [Google Scholar] [CrossRef]
- Zhang, L.; Shuai, Q.; Li, P.; Zhang, Q.; Ma, F.; Zhang, W.; Ding, X. Ion Mobility Spectrometry Fingerprints: A Rapid Detection Technology for Adulteration of Sesame Oil. Food Chem. 2016, 192, 60–66. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Z.; Tian, R.; Niu, Y.; Gao, S.; Liu, H. Total Synchronous Fluorescence Spectroscopy Coupled with Deep Learning to Rapidly Identify the Authenticity of Sesame Oil. Spectrochim. Acta Part A 2021, 244, 118841. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Zhao, B.; Chen, X.; Zhang, Q. Discriminant Analysis of Vegetable Oils by Thermogravimetric-Gas Chromatography/Mass Spectrometry Combined with Data Fusion and Chemometrics without Sample Pretreatment. LWT 2022, 161, 113403. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging Applications of Metabolomics in Food Science and Future Trends. Food Chem. 2022, 16, 100500. [Google Scholar] [CrossRef]
- Dou, X.; Mao, J.; Zhang, L.; Xie, H.; Chen, L.; Yu, L.; Ma, F.; Wang, X.; Zhang, Q.; Li, P. Multispecies Adulteration Detection of Camellia Oil by Chemical Markers. Molecules 2018, 23, 241. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, J.; Xing, R.; Yu, N.; Chen, Y. Integration of Lipidomics and Metabolomics for the Authentication of Camellia Oil by Ultra-Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry Coupled with Chemometrics. Food Chem. 2022, 373, 131534. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; Elsohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Green, R.; Khalil, R.; Mohapatra, S.S.; Mohapatra, S. Role of Cannabidiol for Improvement of the Quality of Life in Cancer Patients: Potential and Challenges. Int. J. Mol. Sci. 2022, 23, 12956. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of Cannabinoids in Commercial Hemp Seed Oil and Decarboxylation Kinetics Studies of Cannabidiolic Acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, A.; Schmidt, H.H. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther. 2020, 37, 1279–1301. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Cavanna, D.; Hurkova, K.; Džuman, Z.; Serani, A.; Serani, M.; Dall’Asta, C.; Tomaniova, M.; Hajslova, J.; Suman, M. A Non-Targeted High-Resolution Mass Spectrometry Study for Extra Virgin Olive Oil Adulteration with Soft Refined Oils: Preliminary Findings from Two Different Laboratories. ACS Omega 2020, 5, 24169–24178. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Han, Q.; Zhang, J.; Zhang, R.; Wang, J.; Gu, W.; Kang, W.; Lian, K.; Ai, L. An Untargeted and Pseudotargeted Metabolomic Combination Approach to Identify Differential Markers to Distinguish Live from Dead Pork Meat by Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2020, 1610, 460553. [Google Scholar] [CrossRef]
- García, C.J.; Gil, M.I.; Tomas-Barberan, F.A. LC–MS Untargeted Metabolomics Reveals Early Biomarkers to Predict Browning of Fresh-Cut Lettuce. Postharvest Biol. Technol. 2018, 146, 9–17. [Google Scholar] [CrossRef]
- Przykaza, K.; Nikolaichuk, H.; Kozub, A.; Tomaszewska-Gras, J.; Peršurić, Ž.; Pavelić, S.K.; Fornal, E. Newly Marketed Seed Oils. What We Can Learn from the Current Status of Authentication of Edible Oils. Food Control 2021, 130, 108349. [Google Scholar] [CrossRef]
- Ben Rahal, N.; Barba, F.J.; Barth, D.; Chevalot, I. Supercritical CO2 Extraction of Oil, Fatty Acids and Flavonolignans from Milk Thistle Seeds: Evaluation of Their Antioxidant and Cytotoxic Activities in CaCO2 Cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sumara, A.; Stachniuk, A.; Olech, M.; Nowak, R.; Montowska, M.; Fornal, E. Identification of Sunflower, Rapeseed, Flaxseed and Sesame Seed Oil Metabolomic Markers as a Potential Tool for Oil Authentication and Detecting Adulterations. PLoS ONE 2023, 18, e0284599. [Google Scholar] [CrossRef] [PubMed]

| Oil | No | Observed m/z | tR [min] | MS/MS [m/z] | CE [eV] | Generated Formula | Theoretical (m/z) | Score | Diff (ppm) |
|---|---|---|---|---|---|---|---|---|---|
| Black seed | M1 | 166.1226 | 2.6 | 124.0754, 123.0677, 106.0650, 109.0520, 91.0540 | 20 | C10H15NO | 166.1226 | 98.18 | - |
| M2 | 328.1551 | 4.2 | 297.1116, 265.0856, 237.0909, 266.0900 | 20 | C17H19N4O3 | 328.1530 | 99.17 | −1.06 | |
| M3 | 342.1710 | 5.1 | 311.1279, 280.1095, 296.1042, 312.1306 | 10 | C20H23NO4 | 342.1700 | 99.77 | −0.52 | |
| M4 | 313.1802 | 7.3 | 135.0803, 99.0440, 255.1347, 295.1639,71.0484 | 15 | C20H24O3 | 313.1798 | 98.89 | 1.62 | |
| M5 | 238.0901 | 7.9 | 220.0790, 205.0551, 192.0474, 187.0988,179.0310 | 15 | C12H15NO2S | 238.0896 | 99.2 | −1.31 | |
| M6 | 131.0862 | 9.7 | 91.0544, 115.0539, 65.0387, 128.0620, 77.0389 | 10 | * | * | * | * | |
| M7 | 385.2376 | 11.4 | 231.1014, 367.2270, 273.1118, 203.1068, 229.1216 | 10 | C22H30N3O3 | 385.236 | 99.86 | −0.33 | |
| M8 | 404.2641 | 13.1 | 207.1744, 85.0650, 133.1012, 225.1849, 189.1635 | 10 | C20H37NO7 | 404.2643 | 99.63 | 0.71 | |
| M9 | 677.3231 | 19.1 | 554.2903, 283.2053, 124.0393, 265.1937, 255.2086 | 10 | C41H44N2O7 | 677.3221 | 99.52 | 0.61 | |
| M10 | 635.3118 | 20.9 | 512.2796, 267.2105, 124.0391, 390.2424, 258.2207 | 10 | C37H40N5O5 | 635.3102 | 99.22 | −0.98 | |
| Evening Primrose | M1 | 469.3317 | 10.9 | 405.3150, 187.1477, 201.1632, 199.1480, 451.3206 | 20 | C30H44O4 | 469.3312 | 96.56 | 0.74 |
| M2 | 455.3161 | 11.8 | 391.2988, 187.1471, 161.1317, 189.1269,191.1784 | 20 | C29H42O4 | 455.3156 | 98.27 | 0.22 | |
| M3 | 409.2207 | 14.4 | 163.0723, 55.0540, 269.1347, 69.0699, 112.9887 | 40 | C18H28N6O5 | 409.2194 | 98.42 | −1.79 | |
| M4 | 492.3174 | 16.5 | 97.0647, 343.2116, 229.1432, 475.2904, 361.2220 | 40 | C25H41N5O5 | 942.318 | 98.7 | 0.74 | |
| M5 | 634.4167 | 20.9 | 617.3896, 503.3217, 97.0646, 211.1325, 485.3110 | 10 | C33H55N5O7 | 634.4174 | 99.17 | −0.03 | |
| M6 | 720.4547 | 21.1 | 703.4265, 211.1325, 457.2794, 589.3585, 97.0647 | 10 | * | * | * | * | |
| M7 | 577.39 | 22.9 | 409.3461, 203.1790, 437.3413, 205.1578, 425.3411 | 20 | C37H52O5 | 577.3888 | 96.91 | −0.31 | |
| M8 | 429.3741 | 24.2 | 97.3727, 109.0646, 411.3619, 83.0852, 69.0699 | 20 | C29H48O2 | 429.3727 | 97.88 | −2.08 | |
| Hemp | M1 | 375.2178 | 13.3 | 357.2059, 339.1949, 275.1273, 358.2087, 340.1989 | 10 | C22H30O5 | 375.2166 | 98.81 | −1.57 |
| M2 | 369.1343 | 14.4 | 313.0703, 314.0739, 298.0458, 81.0692, 109.1004 | 20 | C21H20O6 | 369.1333 | 97.56 | −1.61 | |
| M3 | 437.1965 | 18.3 | 313.0708, 350.1531, 89.0592, 396.1594, 320.1490 | 10 | C26H28O6 | 437.1959 | 99.76 | −0.24 | |
| M4 | 341.2123 | 18.6 | 219.1014, 2 61.1483, 285.1481, 233.1168, 109.1009 | 20 | C22H28O3 | 341.2111 | 98.03 | −1.91 | |
| M5 | 315.2329 | 19.3 | 193.1221, 135.1166, 259.1689, 93.0695, 81.0696 | 20 | C21H30O2 | 315.2319 | 98.71 | −1.75 | |
| M6 | 498.3586 | 22.6 | 147.0439, 236.1272, 352.3204, 218.1185, 100.1115 | 10 | * | * | * | * | |
| M7 | 460.4163 | 28.2 | 121.0646, 138.0912, 138.0912, 57.0699, 71.0854 | 20 | C30H53NO2 | 460.4149 | 98.56 | −0.79 | |
| Milk Thistle | M1 | 145.0764 | 2.1 | 78.0336, 117.0569, 51.0230, 91.0540, 65.0385 | 40 | C9H8N2 | 145.076 | 99.52 | −1.04 |
| M2 | 483.1296 | 8.8 | 153.0178, 195.0286, 131.0488, 163.0751, 437.1226 | 20 | C25H22O10 | 483.1286 | 99.63 | 0.36 | |
| M3 | 233.1545 | 11.6 | 95.0491, 135.0803, 175.1113, 215.1427, 107.0852 | 10 | C15H20O2 | 233.1536 | 97.89 | −1.71 | |
| M4 | 239.2013 | 12.1 | 221.1902, 69.0701, 111.1167, 149.1321, 203.1792 | 10 | C15H26O2 | 239.2006 | 99.44 | −1.28 | |
| Pumpkin seed | M1 | 269.1904 | 8.7 | 195.1163, 185.1318, 145.0851, 199.1471, 227.1795 | 20 | C19H24O | 269.19 | 99.69 | −0.37 |
| M2 | 181.1231 | 17.9 | 125.0593, 98.9609, 93.0695, 135.1165, 107.0852 | 20 | C11H16O2 | 181.1223 | 98.57 | −2.43 | |
| M3 | 358.2389 | 18.4 | 137.0956, 312.2321, 218.1538, 93.0693, 44.0497 | 40 | C22H31NO3 | 358.2377 | 98.74 | −1.42 | |
| M4 | 578.4219 | 19.6 | 405.3522, 423.3628, 120.0444, 231.1745, 138.0548 | 20 | C38H51N5 | 578.4217 | 98.35 | 0.4 | |
| M5 | 697.4592 | 22.1 | 405.3517, 423.3620, 542.3993, 120.0443, 560.4096 | 10 | C45H56N6O | 697.4588 | 98.84 | −0.87 | |
| M6 | 682.4482 | 25.1 | 527.3866, 405.3510, 423.3615, 189.1636, 59.00597 | 10 | C45H55N5O | 682.4479 | 99.05 | 0.27 | |
| M7 | 578.5152 | 28.6 | 73.0464, 355.0709, 211.0850, 405.3460, 133.0843 | 20 | C36H67NO4 | 578.5143 | 98.84 | −0.87 |
| No. | m/z [M + H]+ | Regression Equation | R2 | LOD [%, v/v] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SO | RO | SeO | SO | RO | SeO | SO | RO | SeO | ||
| Black seed oil | ||||||||||
| M1 | 166.1226 | y = 789,870x + 72,825 | y = 765,452x + 59,749 | y = 753,902x + 43,066 | 0.9787 | 0.9707 | 0.9964 | 0.94 | 1.24 | 1.53 |
| M2 | 328.1551 | y = 40,413x + 1019 | y = 31,236x − 122 | y = 26,190x + 638 | 0.9972 | 0.993 | 0.999 | 8.82 | 20 | 12.5 |
| M3 | 342.1710 | y = 81,215x + 2180 | y = 75,462x + 988 | y = 66,784x + 861 | 0.9964 | 0.9982 | 0.9956 | 5.56 | 8.82 | 9.38 |
| M4 | 313.1802 | y = 5,478,074x + 285,848 | y = 5,130,947x + 378,506 | y = 5,125,274x + 302,426 | 0.9916 | 0.9855 | 0.9863 | 0.69 | 0.58 | 0.84 |
| M5 | 238.0901 | y = 14,043,686x + 157,530 | y = 12,914,264x + 380,895 | y = 12,737,396x + 225,908 | 0.999 | 0.9975 | 0.9983 | 0.38 | 0.29 | 0.36 |
| M6 | 131.0862 | y = 3,604,491x + 304,169 | y = 1,540,440x + 422,467 | y = 2,133,072x + 450,678 | 0.9756 | 0.9092 | 0.8801 | 6.52 | * | * |
| M7 | 385.2376 | y = 10,479,323x − 32,847 | y = 10,368,286x + 64,006 | y = 9,301,967x − 53,713 | 0.9961 | 0.9991 | 0.9951 | 0.29 | 0.08 | 0.07 |
| M8 | 404.2641 | y = 12,090,222x + 632,055 | y = 11,022,281x + 629,354 | y = 10,433,967x + 431,133 | 0.9938 | 0.9893 | 0.9952 | 0.14 | 0.07 | 0.16 |
| M9 | 677.3231 | y = 464,7476x − 461,806 | y = 1,417,653x − 114,829 | y = 1,819,364x − 269,027 | 0.9817 | 0.7397 | 0.8573 | * | * | * |
| M10 | 635.3118 | y = 6,161,239x − 457,510 | y = 2,523,982x − 208,112 | y = 2,934,313x − 392,763 | 0.9912 | 0.8349 | 0.9008 | * | * | * |
| Evening primrose oil | ||||||||||
| M1 | 469.3317 | y = 3,699,312x + 58,459 | y = 4,493,887x + 71,465 | y = 3,495,036x + 79,104 | 0.9996 | 0.9966 | 0.996 | 1.40 | 0.60 | 0.49 |
| M2 | 455.3161 | y = 297,109x + 9990 | y = 348,873x + 3114 | y = 302,159x + 2860 | 0.9982 | 0.9981 | 0.9937 | 3.95 | 1.67 | 1.22 |
| M3 | 409.2207 | y = 1,281,896x + 11305 | y = 1,311,303x − 27,405 | y = 1,499,518x + 21,161 | 0.9994 | 0.997 | 0.9959 | 0.7 | 0.74 | 0.75 |
| M4 | 492.3174 | y = 3,156,330x − 10739 | y = 3,195,110x – 31,464 | y = 3,224,260x + 19,978 | 0.9989 | 0.9996 | 0.9976 | 0.21 | 0.13 | 0.15 |
| M5 | 634.4167 | y = 2,937,485x + 53544 | y = 3,762,151x + 23,698 | y = 3,787,857x + 20,847 | 0.9922 | 0.9928 | 0.991 | 0.31 | 0.29 | 0.14 |
| M6 | 720.4547 | y = 2,677,027x − 15,907 | y = 3,927,701x − 65,507 | y = 3,951,119x − 21,469 | 0.9933 | 0.999 | 0.9897 | 0.19 | 0.18 | 0.21 |
| M7 | 577.39 | y = 221,915x + 11,190 | y = 217,440x + 19,556 | y = 261,795x + 2944 | 0.9958 | 0.9811 | 0.9951 | 4.05 | 3.85 | 4.19 |
| M8 | 429.3741 | y = 1,953,980x + 125,896 | y = 2,251,678x + 81,880 | y = 2,432,167x + 30,767 | 0.9885 | 0.9969 | 0.997 | 0.68 | 0.74 | 0.68 |
| Hemp oil | ||||||||||
| M1 | 375.2178 | y = 11,502,419x + 862,052 | y = 12,203,972x + 4083 | y = 1,170,168x + 1028.54 | 0.9851 | 0.993 | 0.9989 | 0.21 | 0.32 | 0.32 |
| M2 | 369.1343 | y = 833,660x + 34,874 | y = 1,346,765x + 424 | y = 1,155,917x − 50,071 | 0.9935 | 0.9921 | 0.947 | 0.5 | 2.5 | * |
| M3 | 437.1965 | y = 320,534x + 21,664 | y = 757,857x + 35704 | y = 562,753x + 27,474 | 0.0954 | 0.9939 | 0.8857 | 0.49 | 0.9 | * |
| M4 | 341.2123 | y = 3,134,659x + 3,220,487 | y = 5,694,353x + 5699612 | y = 5,155,590x + 7,664,520 | 0.9398 | 0.9502 | 0.8821 | * | 0.04 | * |
| M5 | 315.2329 | y = 5,627,335x + 238,675 | y = 14,455,766x + 38848 | y = 11,885,033x − 564,670 | 0.9368 | 0.9946 | 0.8898 | * | 0.1 | * |
| M6 | 498.3586 | y = 404,963x + 23,137 | y = 1,264,650x + 33514 | y = 833,971x − 13,357 | 0.9345 | 0.9938 | 0.8757 | * | 0.4 | * |
| M7 | 460.4163 | y = 1,214,547x + 25,860 | y = 2,336,849x + 2926 | y = 1,558,213x − 20893 | 0.9879 | 0.9988 | 0.892 | 0.23 | 0.80 | * |
| Milk thistle oil | ||||||||||
| M1 | 145.0764 | y = 630,817x − 7184 | y = 441,295x + 16,516 | y = 304,454x + 37,802 | 0.9342 | 0.9892 | 0.9399 | * | 2.27 | * |
| M2 | 483.1296 | y = 217,321x − 2053 | y = 203,452x + 3123 | y = 195,280x + 2726 | 0.9931 | 0.9959 | 0.9860 | 2.22 | 2.16 | 2.11 |
| M3 | 233.1545 | y = 4,130,535x + 175,405 | y = 3,660,480x + 120,505 | y = 3,229,384x + 123,499 | 0.997 | 0.9987 | 0.9942 | 0.8 | 0.24 | 0.35 |
| M4 | 239.2013 | y = 2,117,856x + 316,00 | y = 1,848,032x + 34,660 | y = 1,710,753x + 51,351 | 0.9979 | 0.9989 | 0.9917 | 0.67 | 0.31 | 0.5 |
| Pumpkin seed oil | ||||||||||
| M1 | 269.1904 | y = 690,664x − 3950 | y = 627,498x − 5341 | y = 676,594x − 8551 | 0.9985 | 0.997 | 0.9958 | 1.1 | 1.42 | 0.64 |
| M2 | 181.1231 | y = 2,516,933x − 46,852 | y = 2,998,961x + 109,634 | y = 2,628,907x − 34,395 | 0.9973 | 0.9973 | 0.9985 | 0.7 | 0.19 | 0.70 |
| M3 | 358.2389 | y = 1,112,206x − 19,675 | y = 1,665,518x − 9513 | y = 1,130,042x − 61,697 | 0.9986 | 0.9974 | 0.9922 | 0.34 | 0.22 | 0.22 |
| M4 | 578.4219 | y = 26,872,797x − 123,014 | y = 30,525,005x − 233,735 | y = 24,765,411x − 166,352 | 0.9931 | 0.978 | 0.9852 | 0.03 | 0.1 | 0.12 |
| M5 | 697.4592 | y = 655,508x − 2198 | y = 939,291x − 66,379 | y = 741,243x − 23,304 | 0.9864 | 0.969 | 0.9835 | 0.57 | 2.94 | 2.8 |
| M6 | 682.4482 | y = 13,396,606x + 350,014 | y = 23,961,874x − 122,911 | y = 14,079,951x − 586,077 | 0.996 | 0.9918 | 0.9952 | 0.08 | 0.06 | 0.1 |
| M7 | 578.5152 | y = 339,792x + 21,795 | y = 516,253x + 73,837 | y = 423,550x − 1278 | 0.9901 | 0.9937 | 0.9985 | 0.82 | 0.71 | 1.25 |
| No. | m/z | Mix1 | Mix2 | Mix3 | Mix4 | Mix5 | Mix6 | Mix7 |
|---|---|---|---|---|---|---|---|---|
| Black seed oil compliance with labeling | ||||||||
| 2% | 20% | 0% | 0% | 0% | 0% | 4% | ||
| M1 | 166.1226 | + | + | − | − | − | − | + |
| M2 | 328.1551 | + | + | − | − | − | − | + |
| M3 | 342.1710 | + | + | − | − | − | − | + |
| M4 | 313.1802 | + | + | − | − | − | − | + |
| M5 | 238.0901 | + | + | − | − | − | − | + |
| M6 | 131.0862 | + | + | − | − | − | − | + |
| M7 | 385.2376 | + | + | − | − | − | − | + |
| M8 | 404.2641 | + | + | − | − | − | − | + |
| M9 | 677.3231 | + | + | − | − | − | − | + |
| M10 | 635.3118 | + | + | − | − | − | − | + |
| Evening primrose oil compliance with labeling | ||||||||
| 0% | 0% | 3% | 20% | 0% | 0% | 5% | ||
| M1 | 469.3317 | − | − | + | + | − | − | + |
| M2 | 455.3161 | − | − | − | + | − | − | − |
| M3 | 409.2207 | − | − | − | + | − | − | − |
| M4 | 492.3174 | − | − | − | + | − | − | + |
| M5 | 634.4167 | − | − | − | − | − | − | + |
| M6 | 720.4547 | − | − | − | + | − | − | + |
| M7 | 577.39 | − | − | + | + | − | − | + |
| M8 | 429.3741 | − | − | − | + | − | − | + |
| Hemp oil compliance with labeling | ||||||||
| 0% | 0% | 0% | 0% | 0% | 5% | 0% | ||
| M1 | 375.2178 | − | − | − | − | − | + | − |
| M2 | 369.1343 | − | − | − | − | − | − | − |
| M3 | 437.1965 | − | − | − | − | − | − | − |
| M4 | 341.2123 | − | − | − | − | − | + | − |
| M5 | 315.2329 | − | − | − | − | − | + | − |
| M6 | 498.3586 | − | − | − | − | − | + | − |
| M7 | 460.4163 | − | − | − | − | − | − | − |
| Milk thistle oil compliance with labeling | ||||||||
| 0% | 0% | 27% | 0% | 20% | 0% | 0% | ||
| M1 | 145.0764 | − | − | + | − | + | − | − |
| M2 | 483.1296 | − | − | + | − | + | − | − |
| M3 | 233.1545 | − | − | + | − | + | − | − |
| M4 | 239.2013 | − | − | + | − | + | − | − |
| Pumpkin seed oil compliance with labeling | ||||||||
| 0% | 0% | 0% | 0% | 0% | 10% | 4% | ||
| M1 | 269.1904 | − | − | − | − | − | + | + |
| M2 | 181.1231 | − | − | − | − | − | + | − |
| M3 | 358.2389 | − | − | − | − | − | + | + |
| M4 | 578.4219 | − | − | − | − | − | + | + |
| M5 | 697.4592 | − | − | − | − | − | + | + |
| M6 | 682.4482 | − | − | − | − | − | + | + |
| M7 | 578.5152 | − | − | − | − | − | − | − |
| Sunflower oil compliance with labeling | ||||||||
| 0% | 0% | 0% | 40% | 0% | 0% | 0% | ||
| M1 | 231.1386 | − | − | − | + | − | − | − |
| M2 | 217.0865 | − | − | − | + | − | − | − |
| M3 | 345.0975 | − | − | − | + | − | − | − |
| M4 | 203.1076 | − | − | − | + | − | − | − |
| M5 | 301.2172 | − | − | − | + | − | − | − |
| M6 | 392.2444 | − | − | − | + | − | − | − |
| M7 | 420.2758 | − | − | − | + | − | − | − |
| M8 | 432.2758 | − | − | − | + | − | − | − |
| M9 | 434.2913 | − | − | − | + | − | − | − |
| M10 | 576.3394 | − | − | − | + | − | − | − |
| M11 | 590.3552 | − | − | − | + | − | − | − |
| M12 | 604.3699 | − | − | − | + | − | − | − |
| M13 | 618.3865 | − | − | − | + | − | − | − |
| Flax seed oil compliance with labeling | ||||||||
| 98% | 80% | 70% | 40% | 40% | 85% | 0% | ||
| M1 | 212.0823 | + | + | + | + | + | + | − |
| M2 | 287.2016 | + | + | + | + | + | + | − |
| M3 | 285.1853 | + | + | + | + | + | + | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumara, A.; Stachniuk, A.; Trzpil, A.; Bartoszek, A.; Montowska, M.; Fornal, E. LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration. Molecules 2023, 28, 4754. https://doi.org/10.3390/molecules28124754
Sumara A, Stachniuk A, Trzpil A, Bartoszek A, Montowska M, Fornal E. LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration. Molecules. 2023; 28(12):4754. https://doi.org/10.3390/molecules28124754
Chicago/Turabian StyleSumara, Agata, Anna Stachniuk, Alicja Trzpil, Adrian Bartoszek, Magdalena Montowska, and Emilia Fornal. 2023. "LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration" Molecules 28, no. 12: 4754. https://doi.org/10.3390/molecules28124754
APA StyleSumara, A., Stachniuk, A., Trzpil, A., Bartoszek, A., Montowska, M., & Fornal, E. (2023). LC–MS Metabolomic Profiling of Five Types of Unrefined, Cold-Pressed Seed Oils to Identify Markers to Determine Oil Authenticity and to Test for Oil Adulteration. Molecules, 28(12), 4754. https://doi.org/10.3390/molecules28124754







