Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls
Abstract
1. Introduction
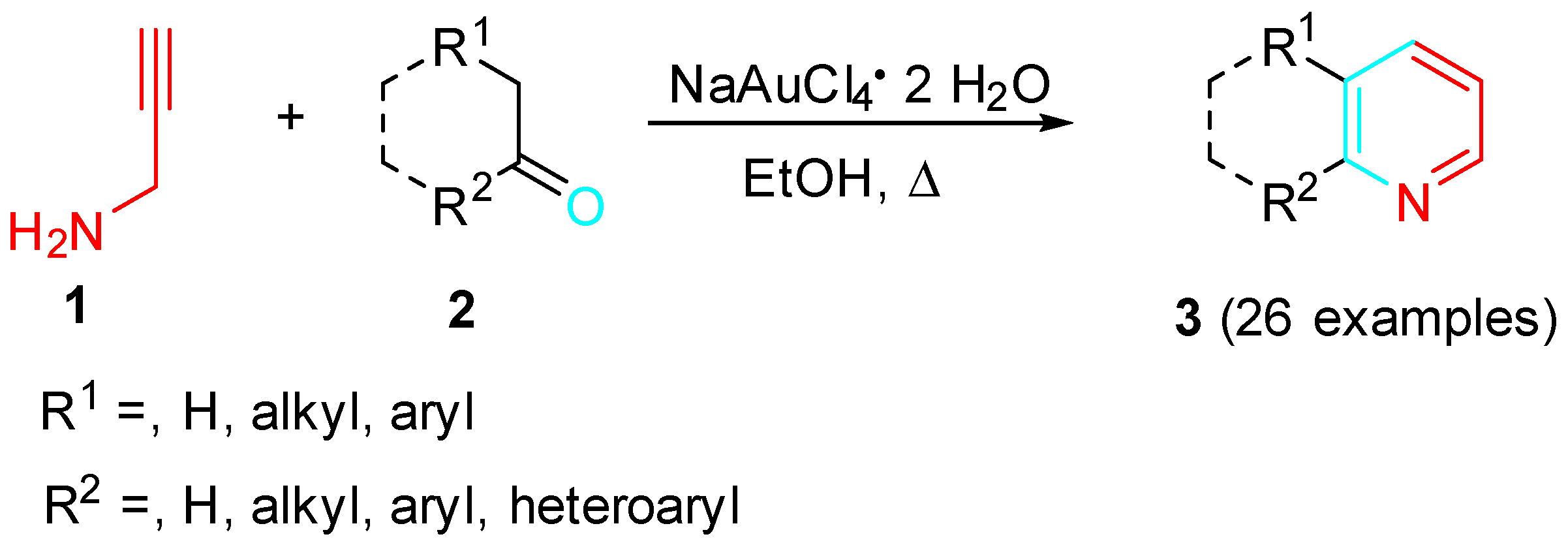
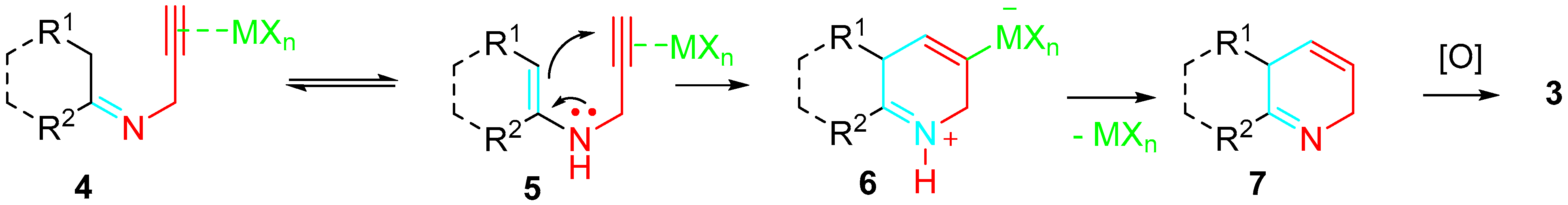
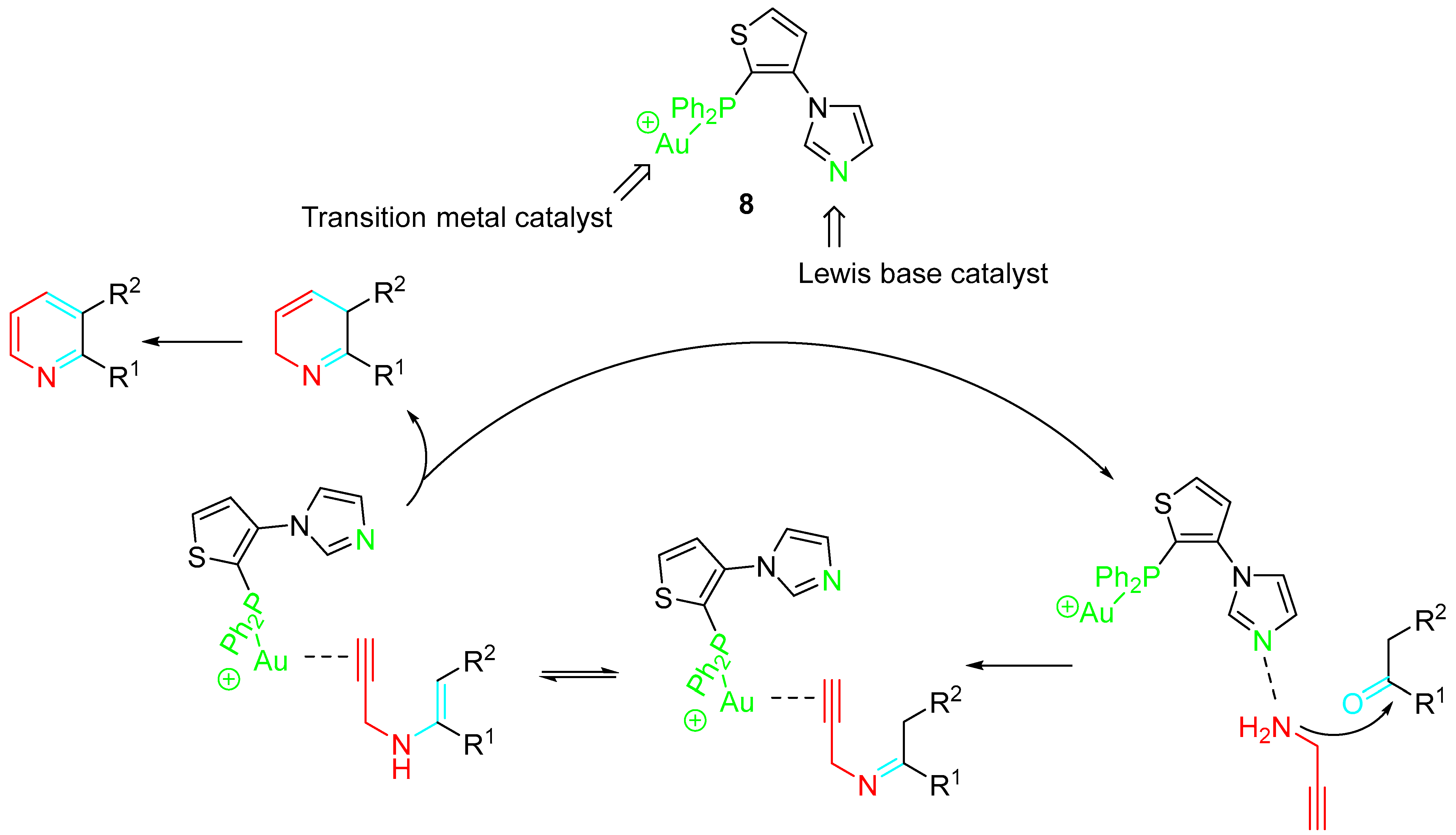
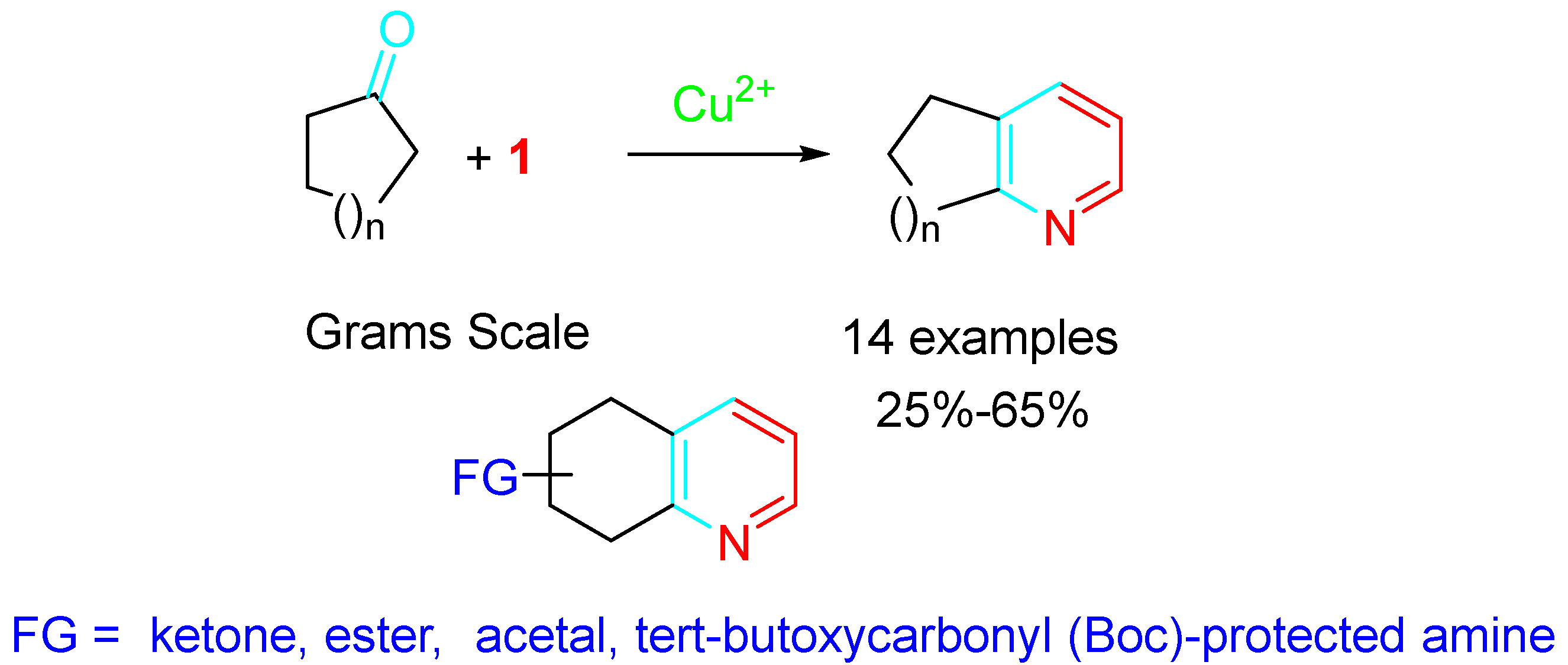
2. Sequential Reactions of α-Aminoalkynes (Propargylamines) with Carbonyls
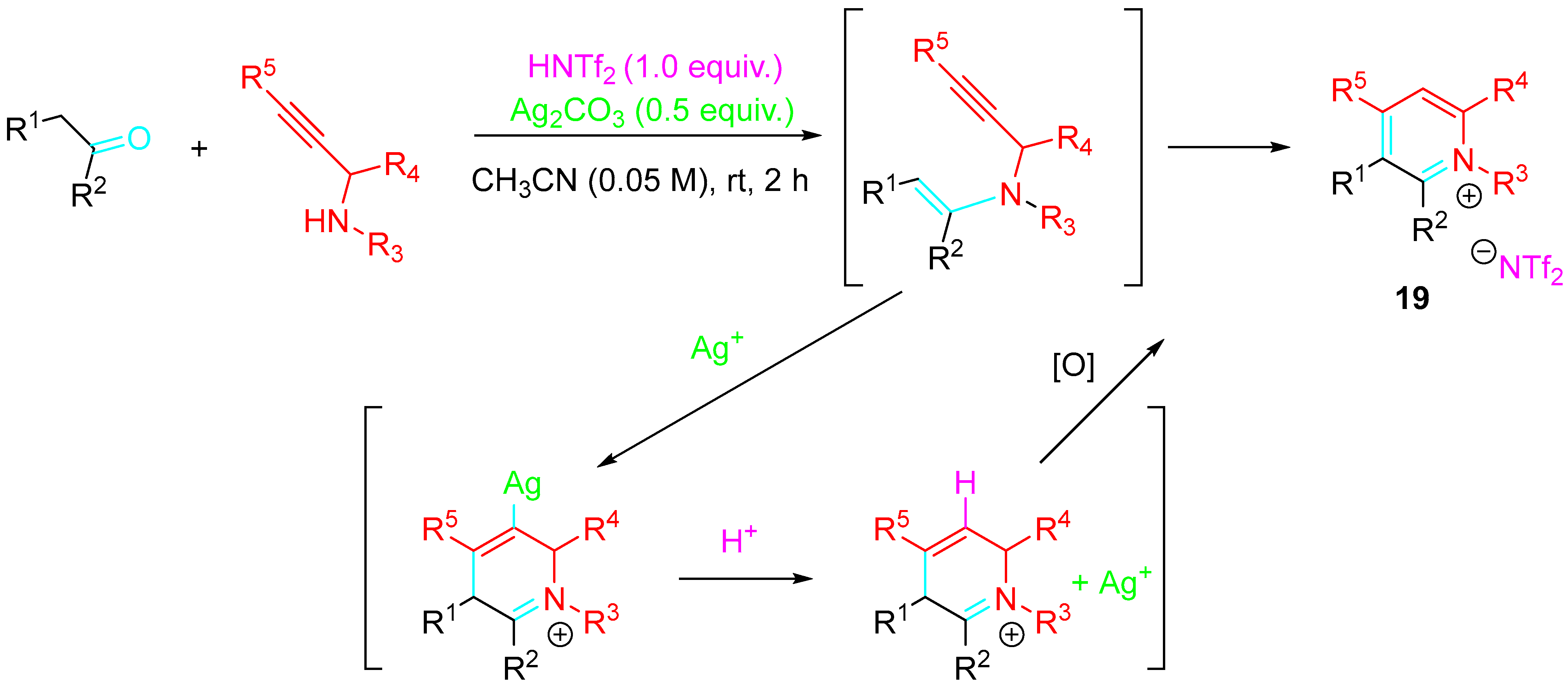
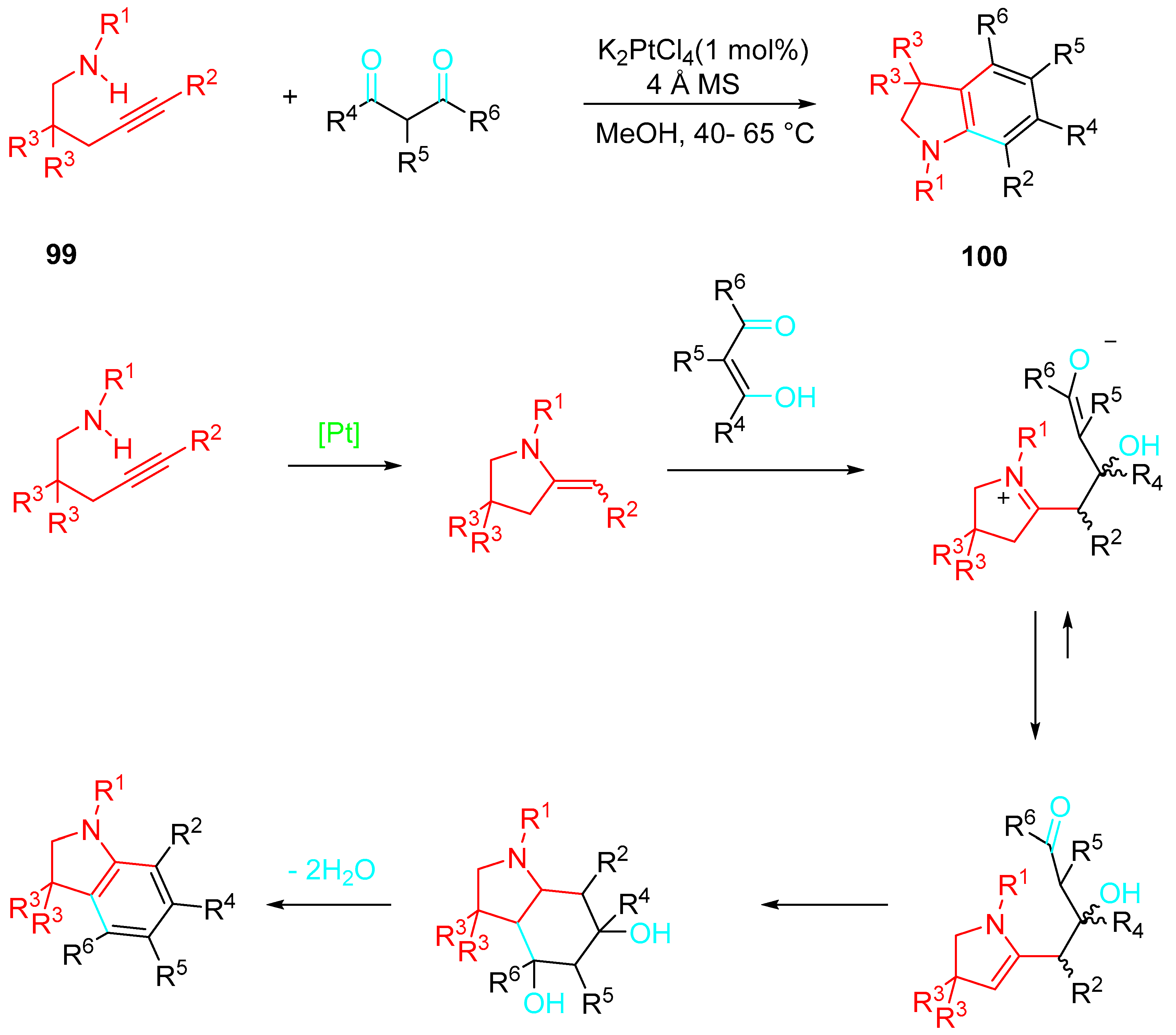
3. Sequential Reactions of β-Aminoalkynes with Carbonyls
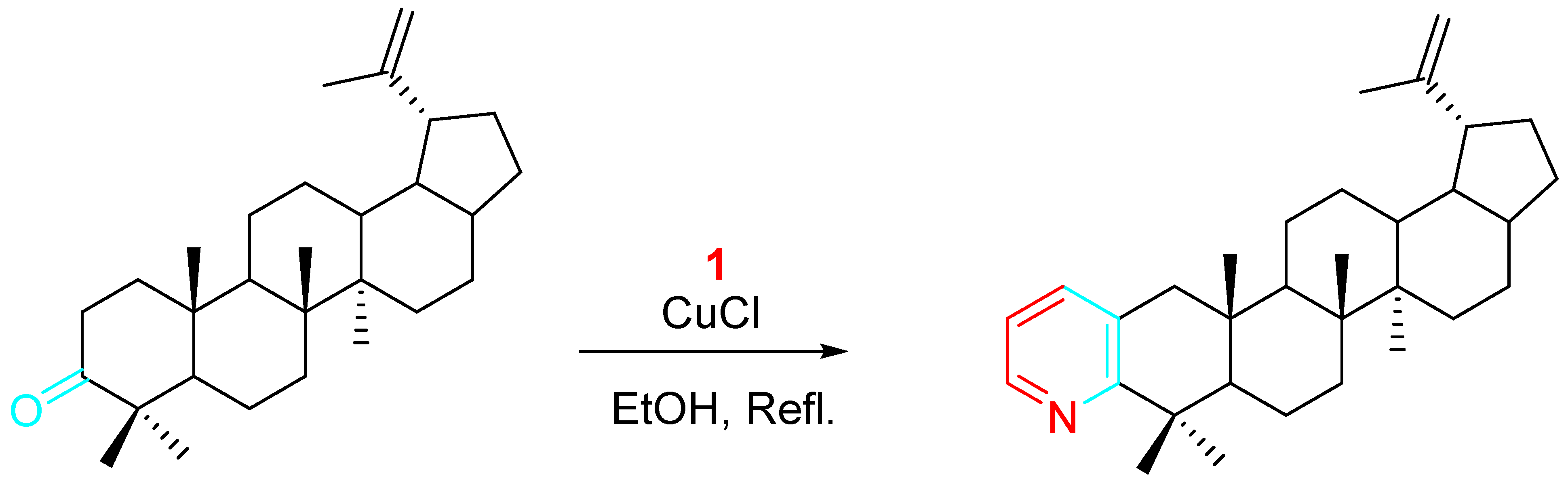
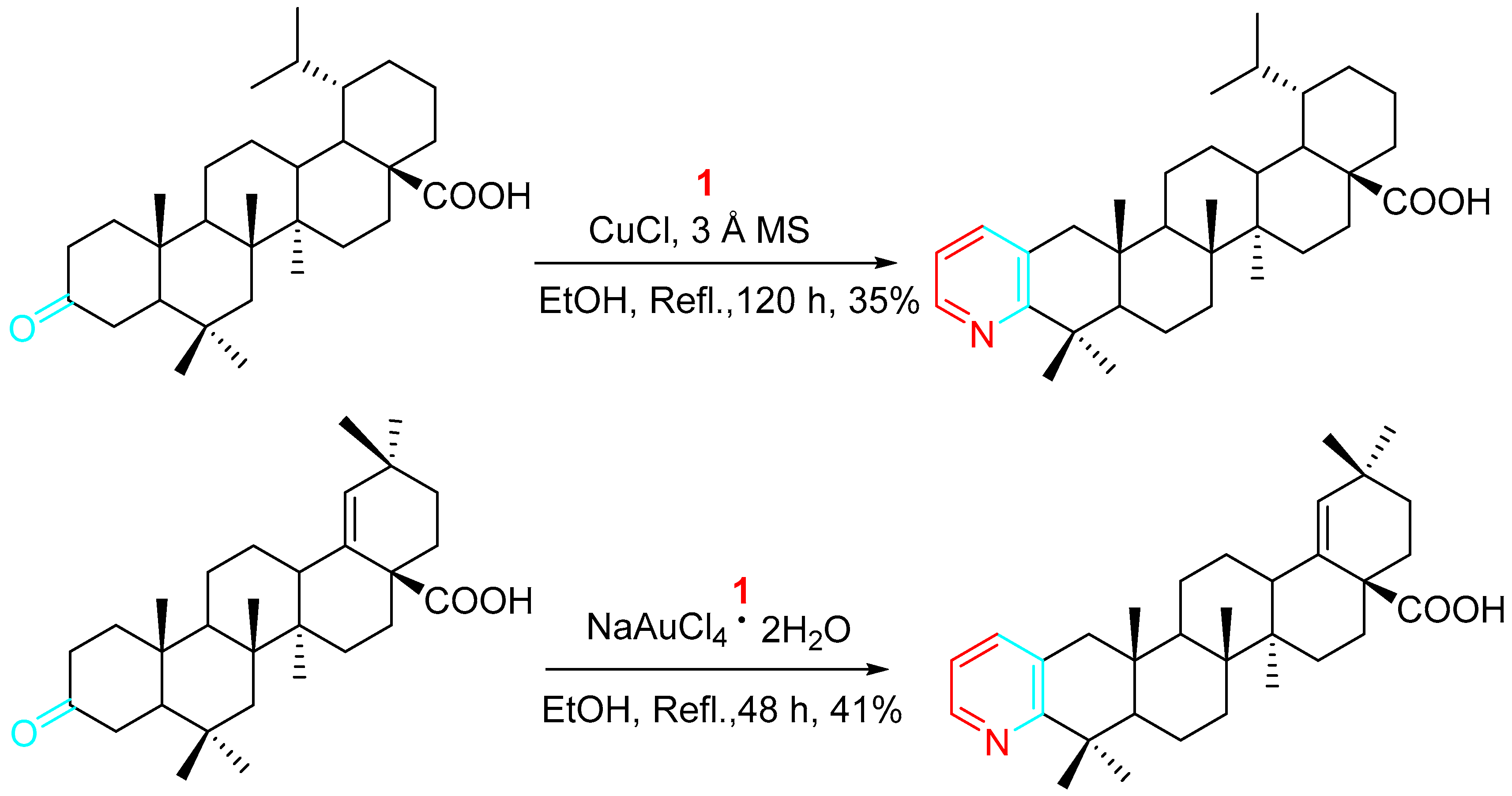
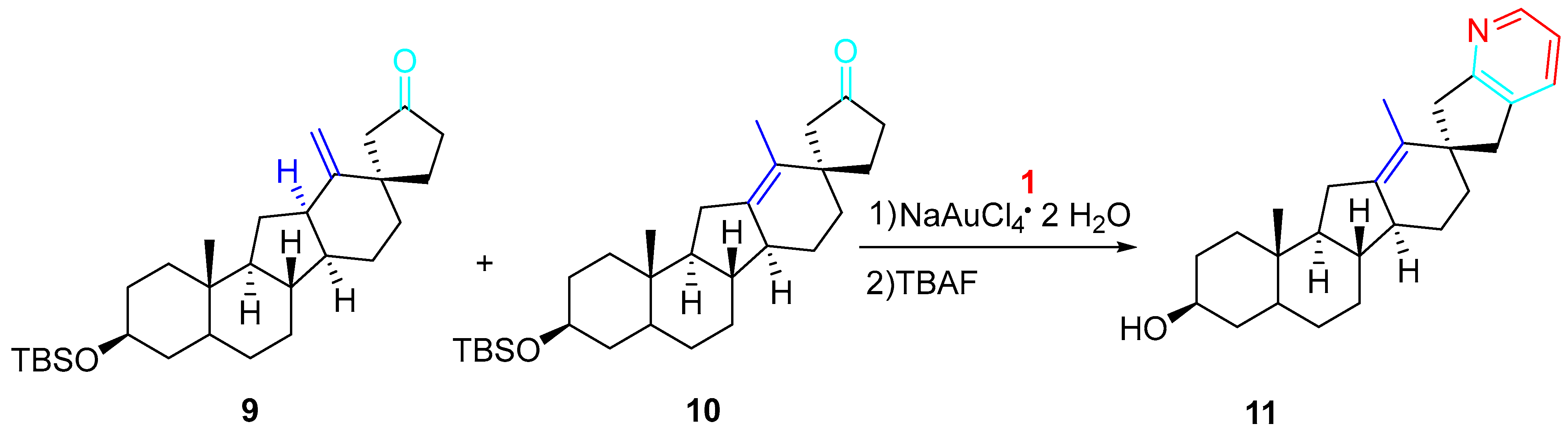
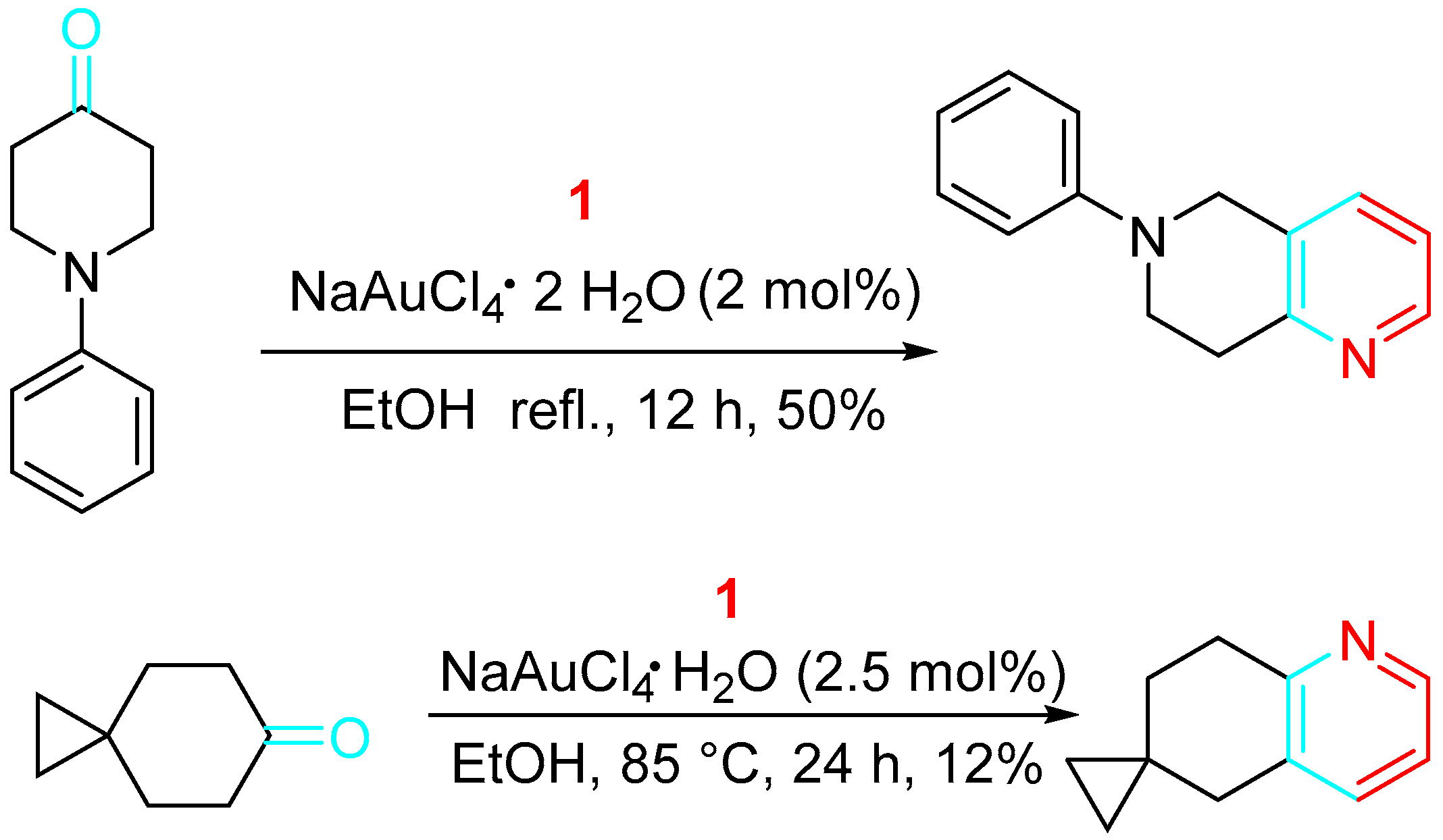
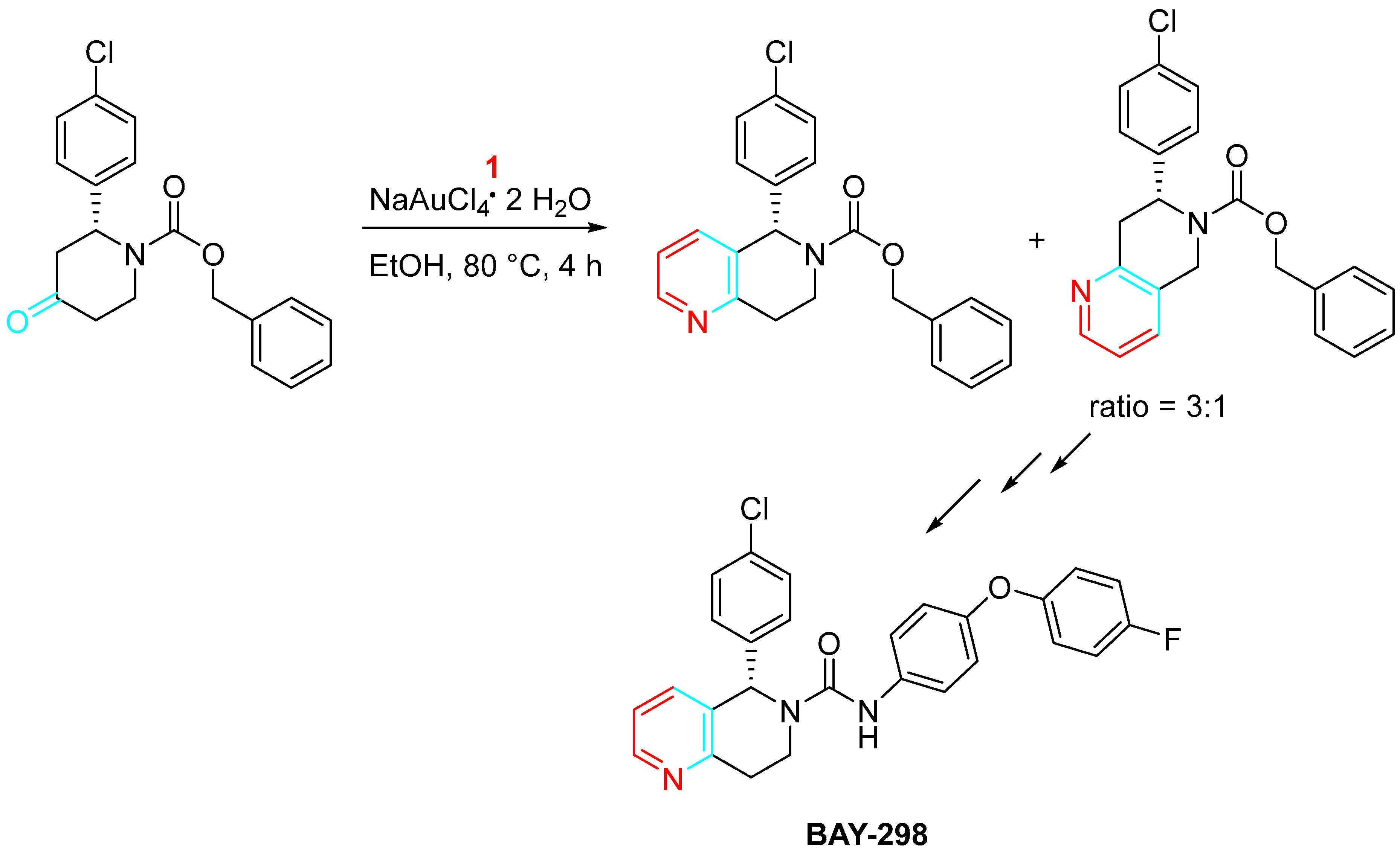
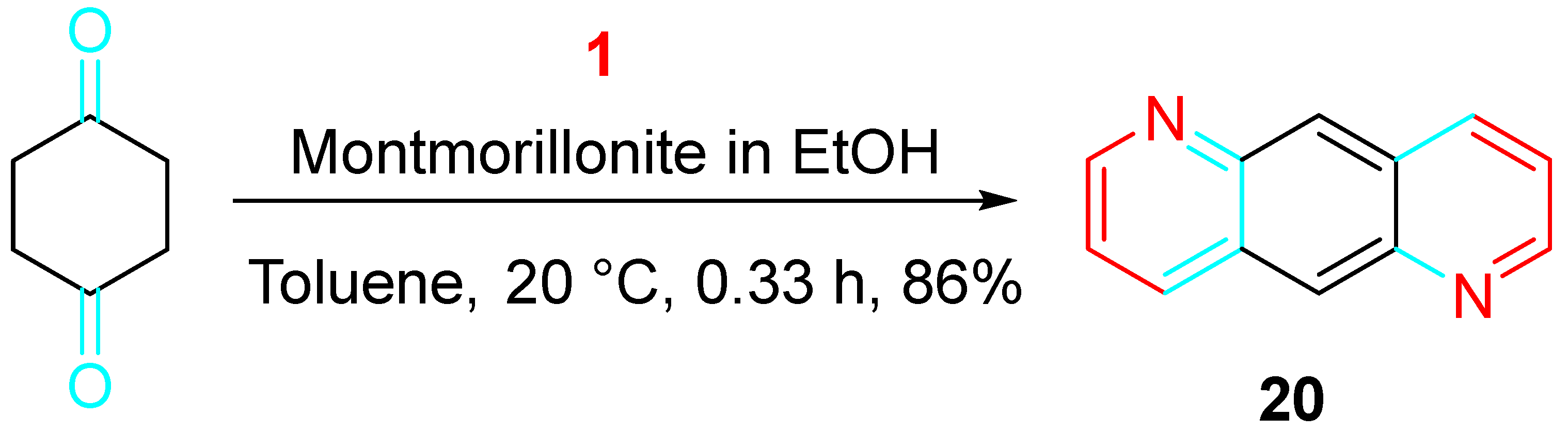
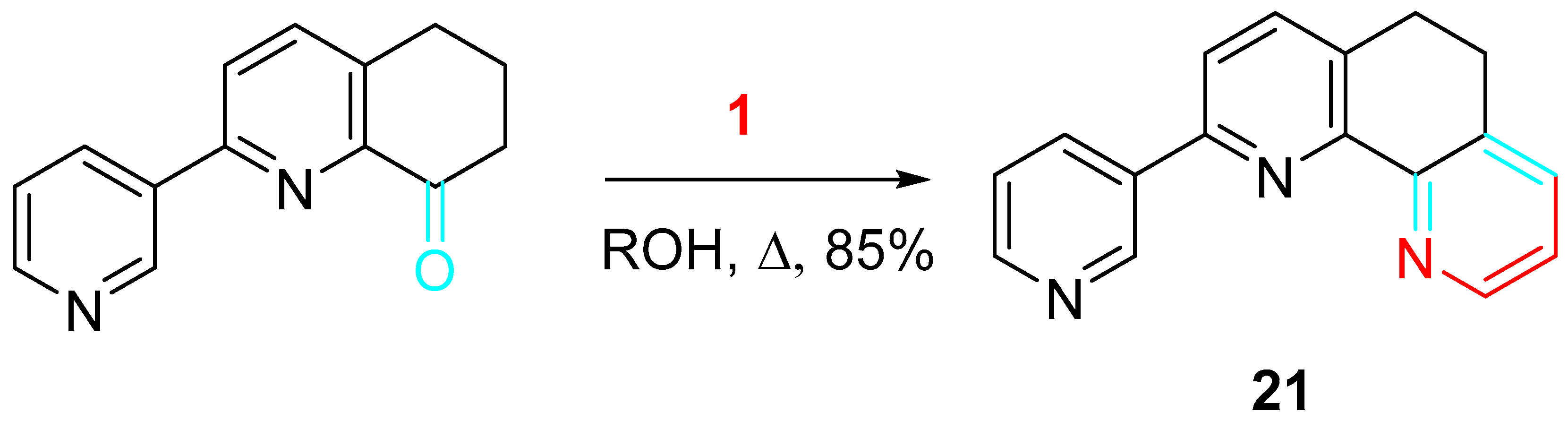
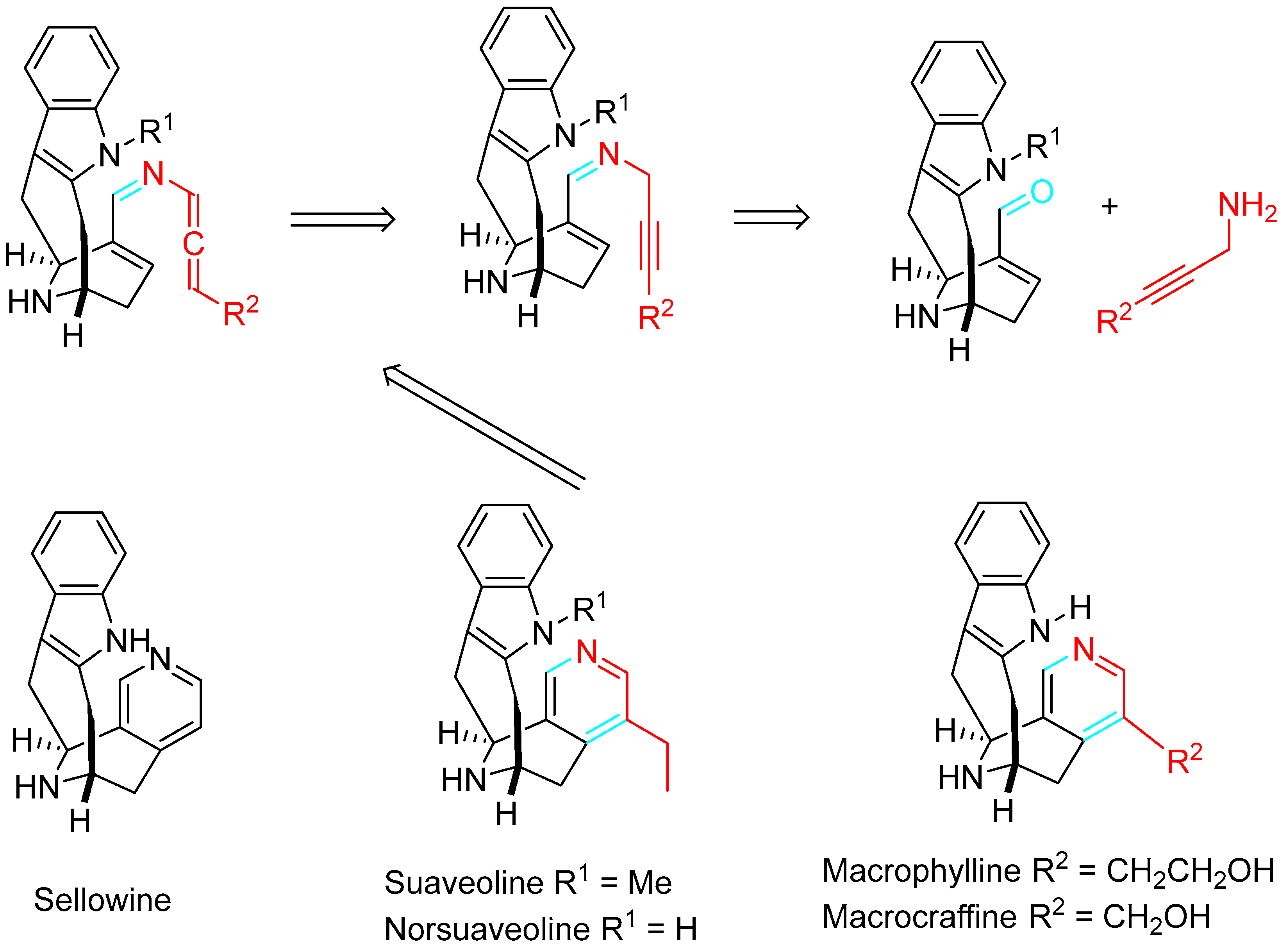
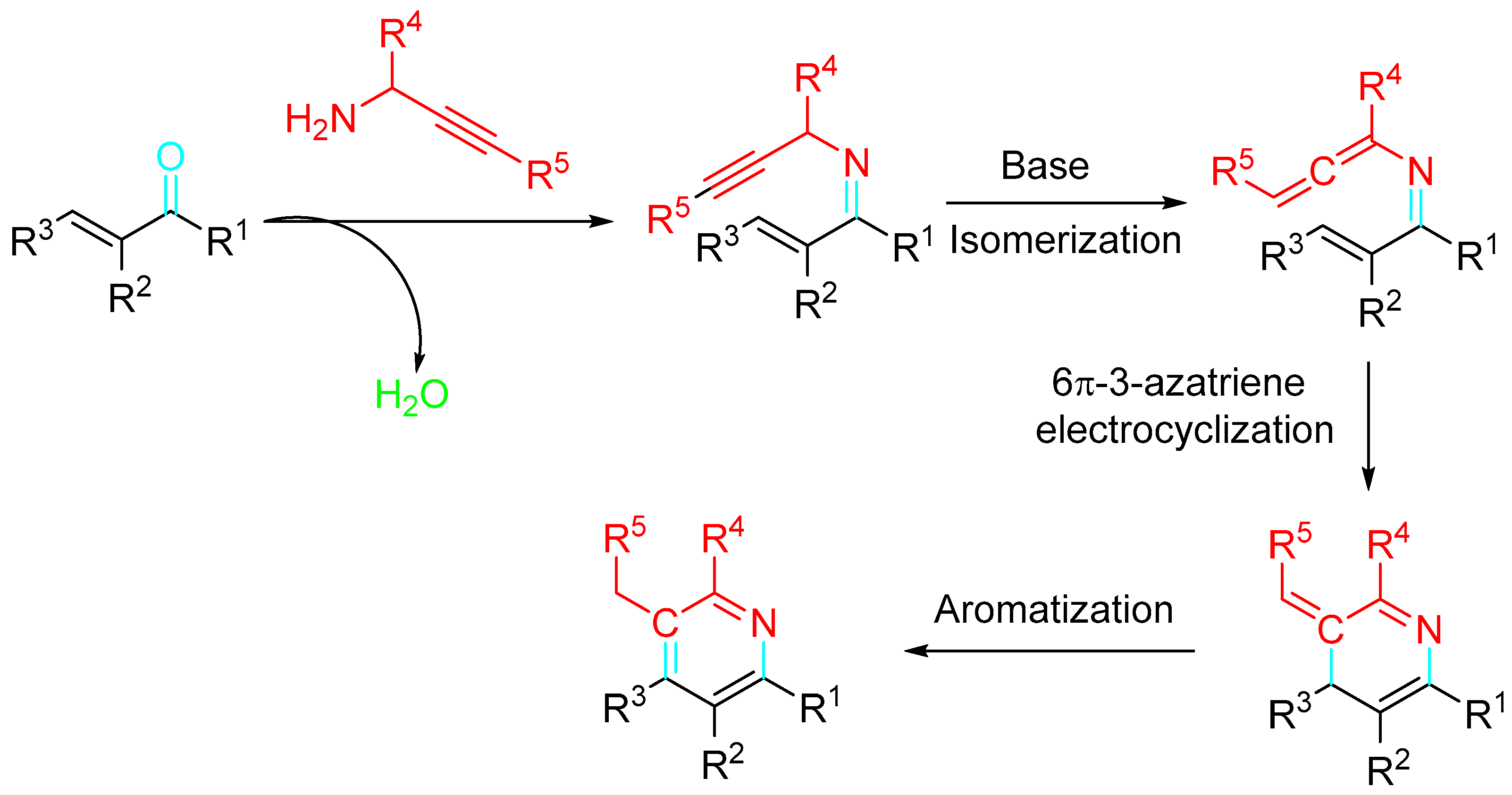
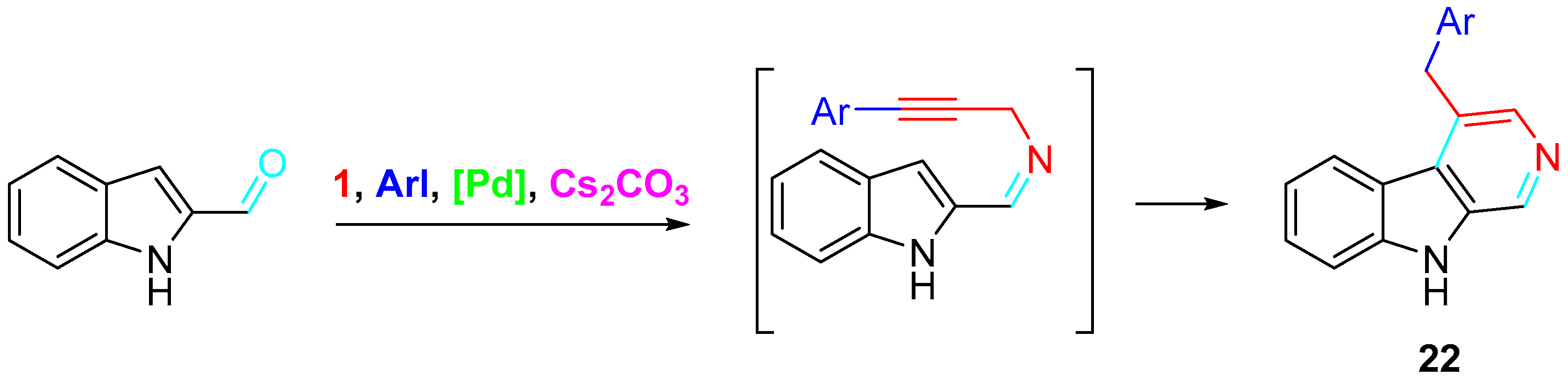
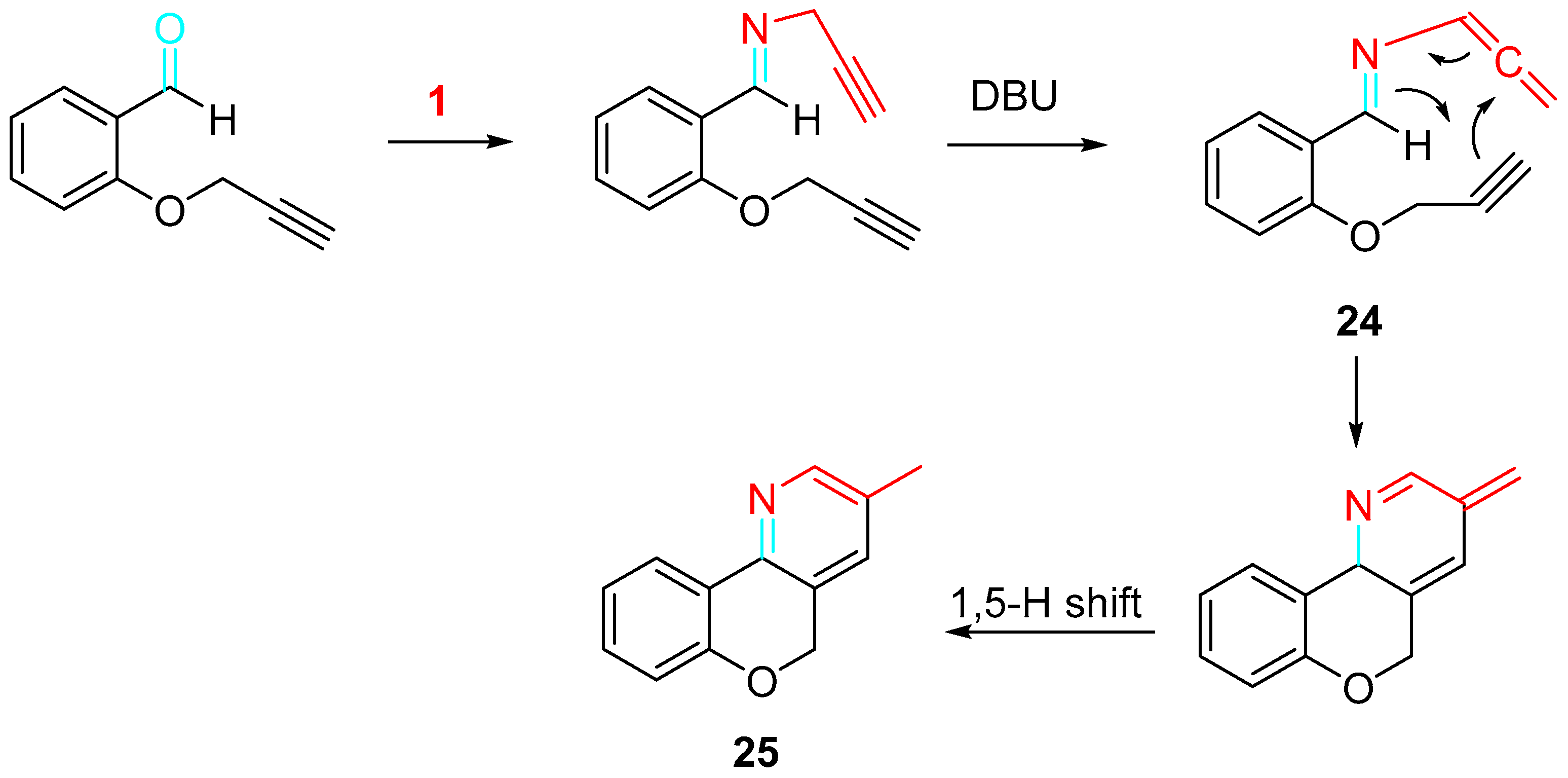
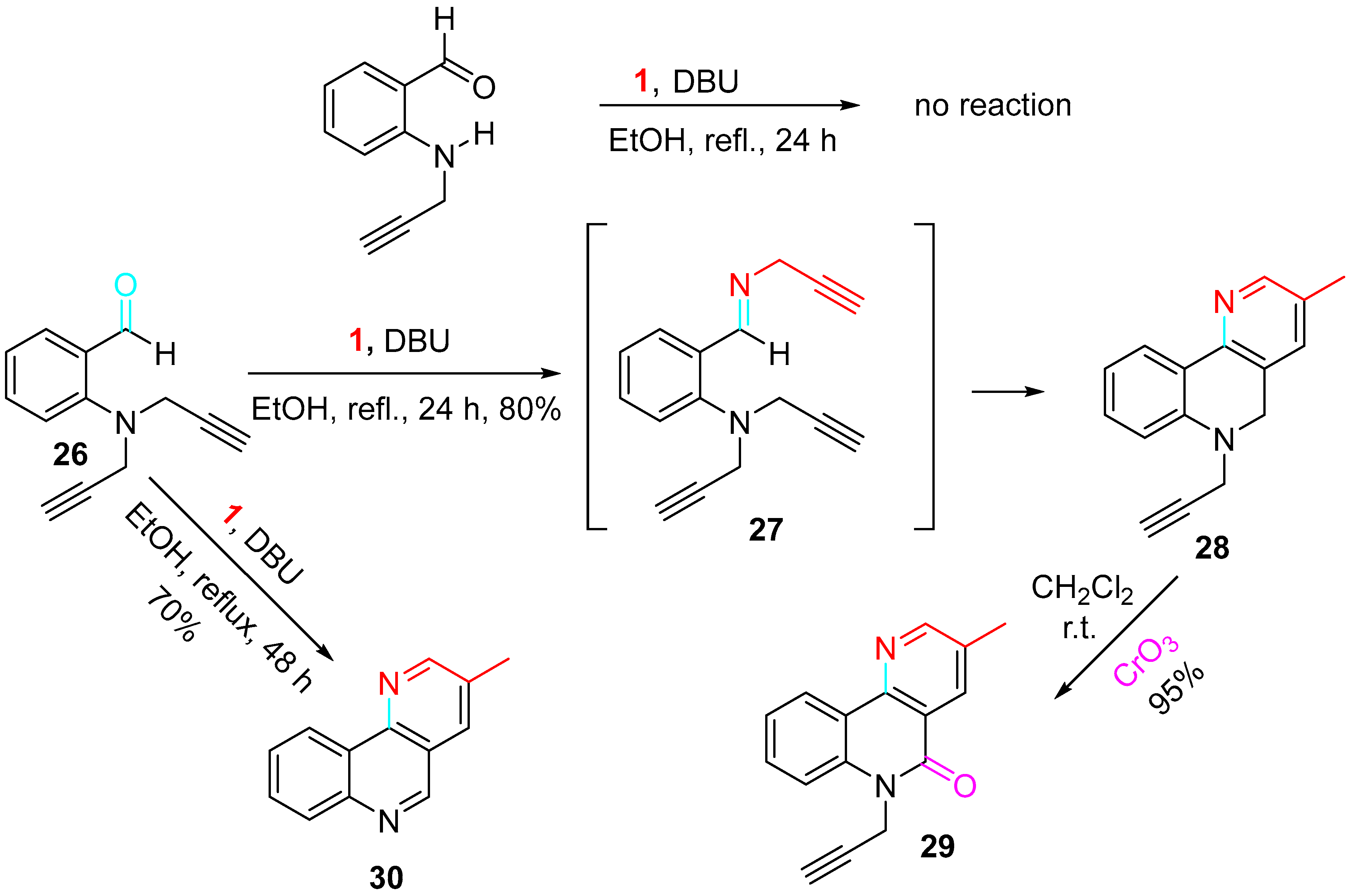
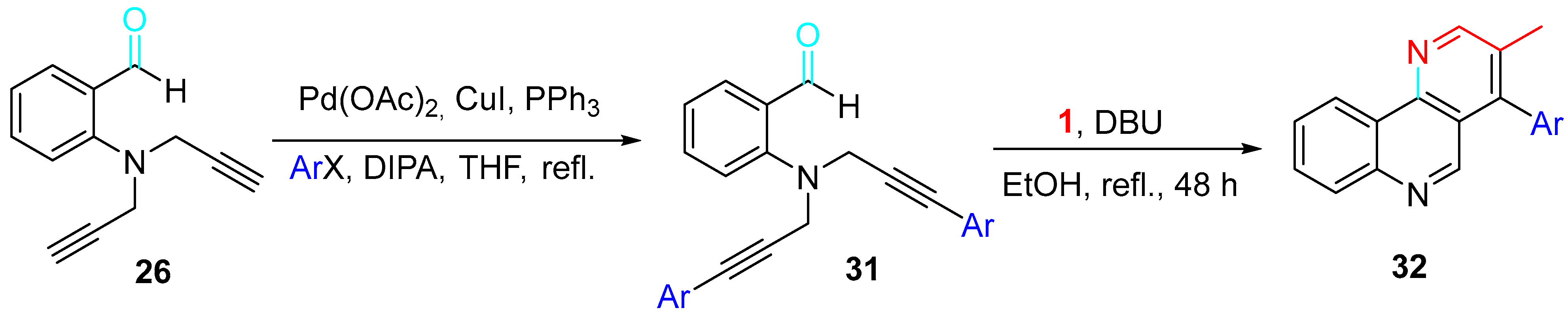
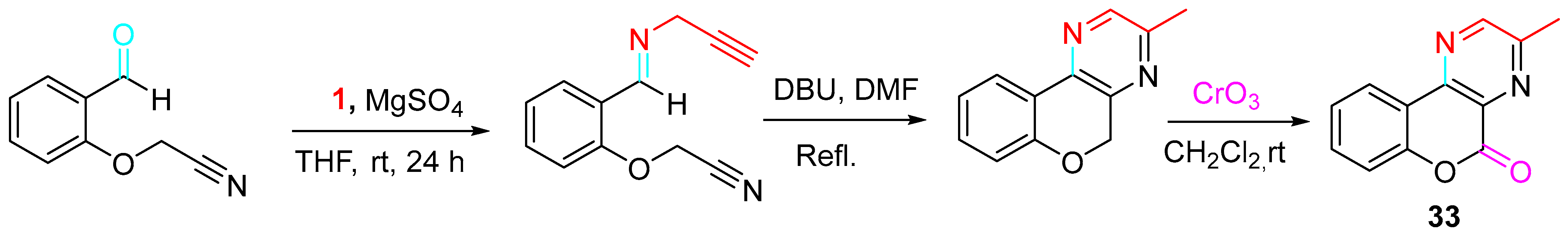
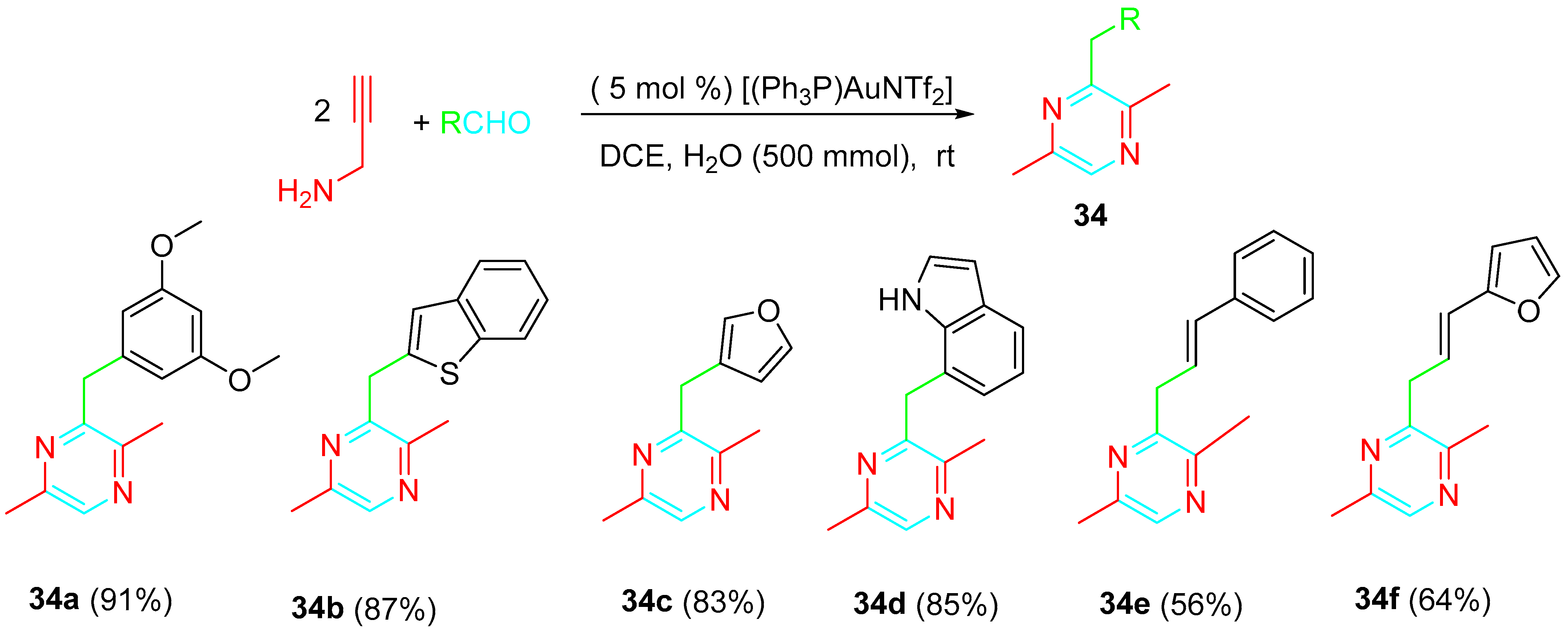
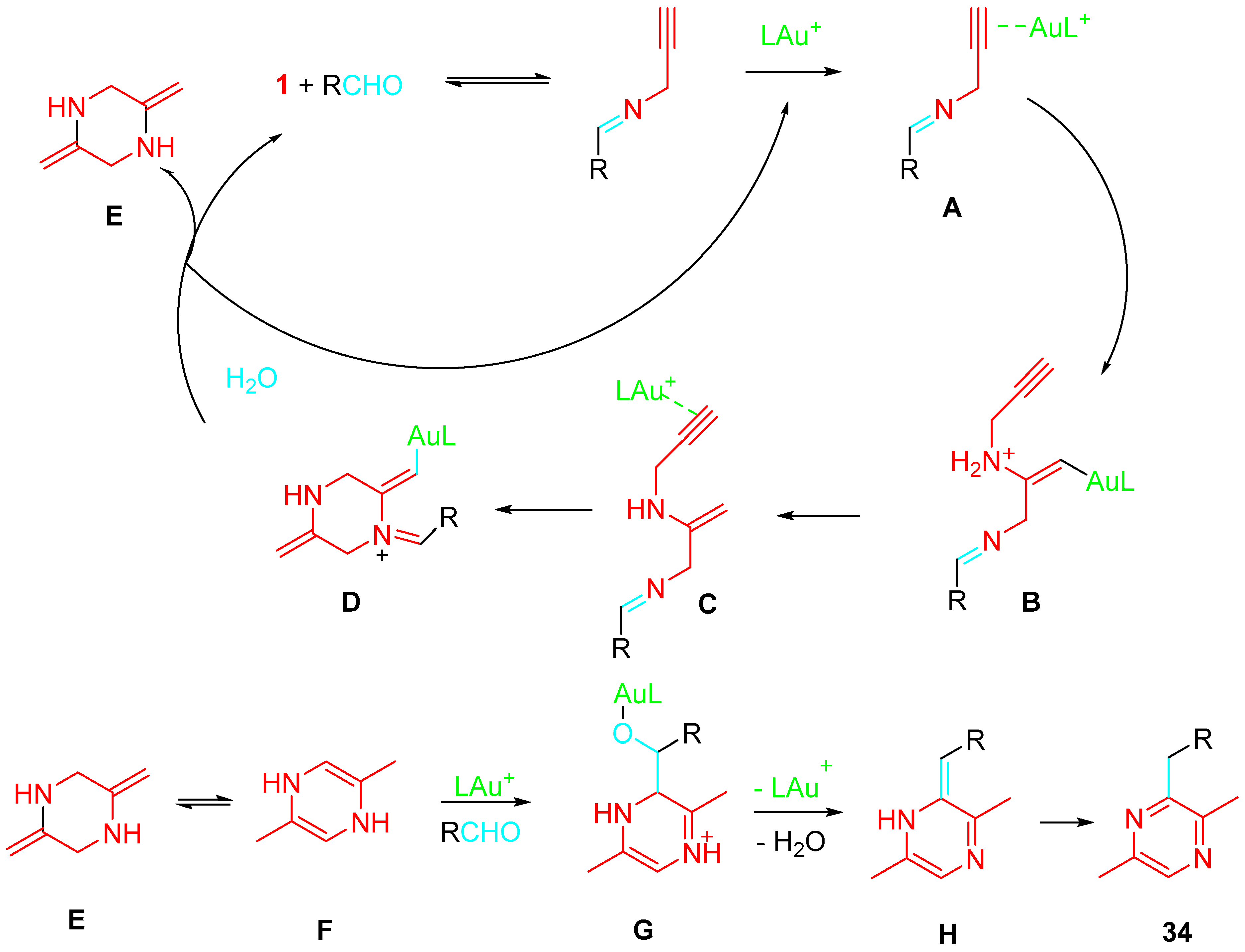
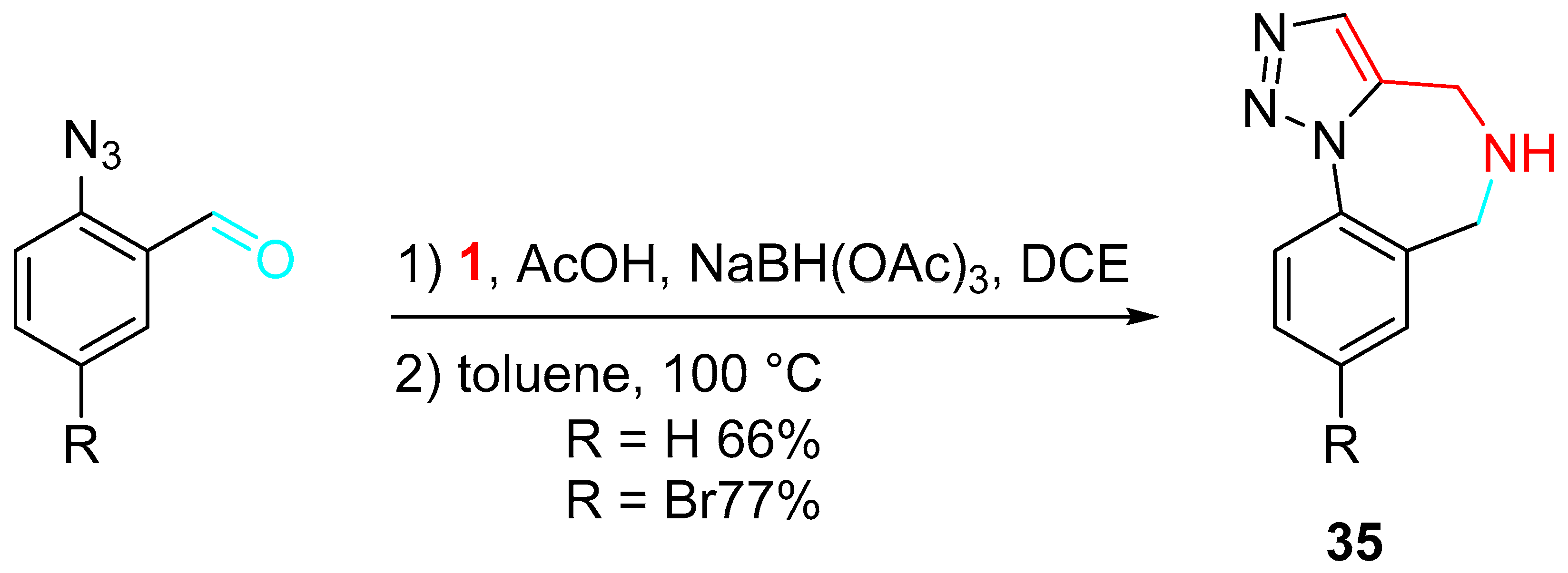
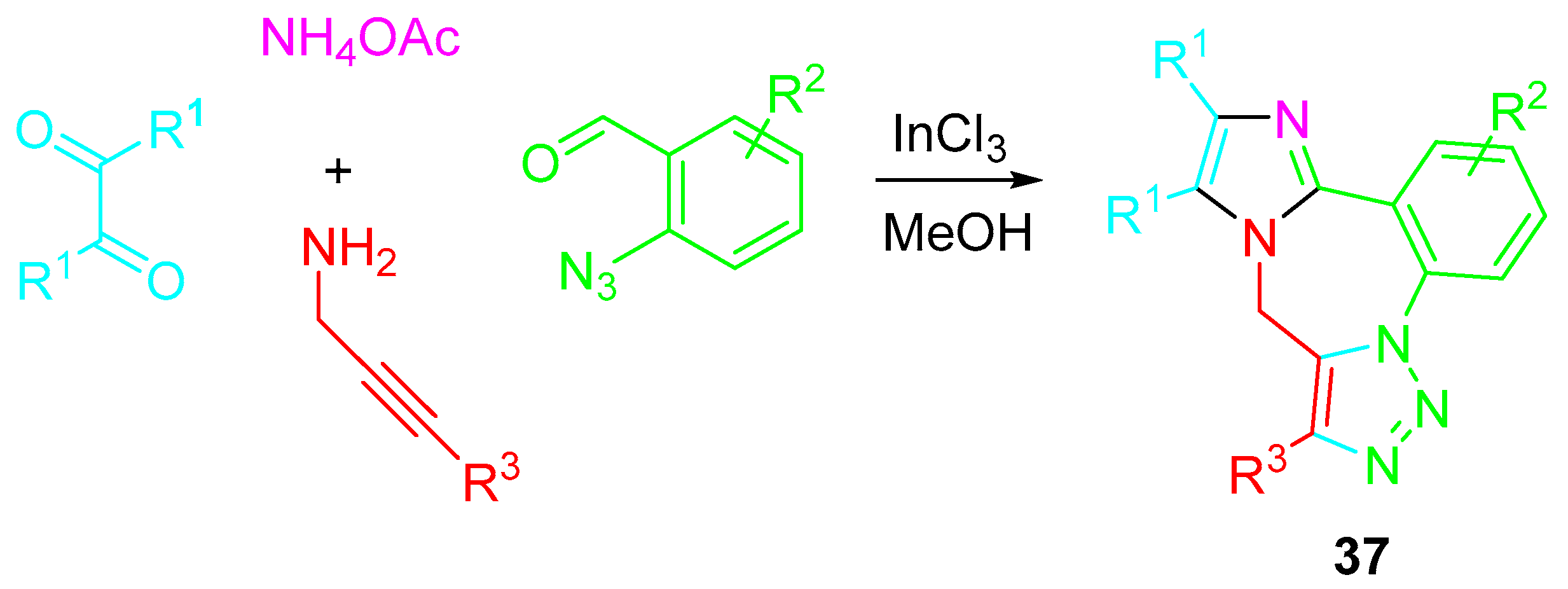
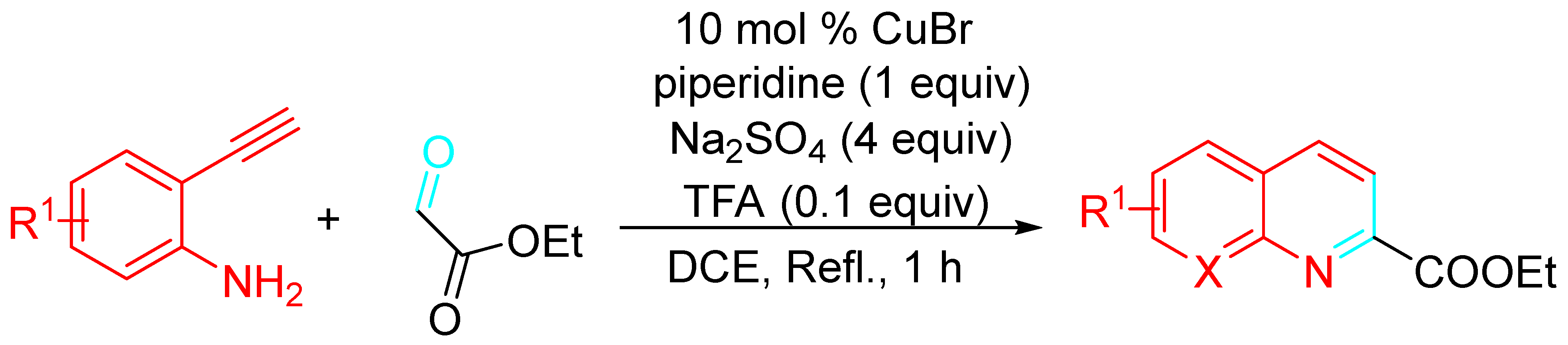
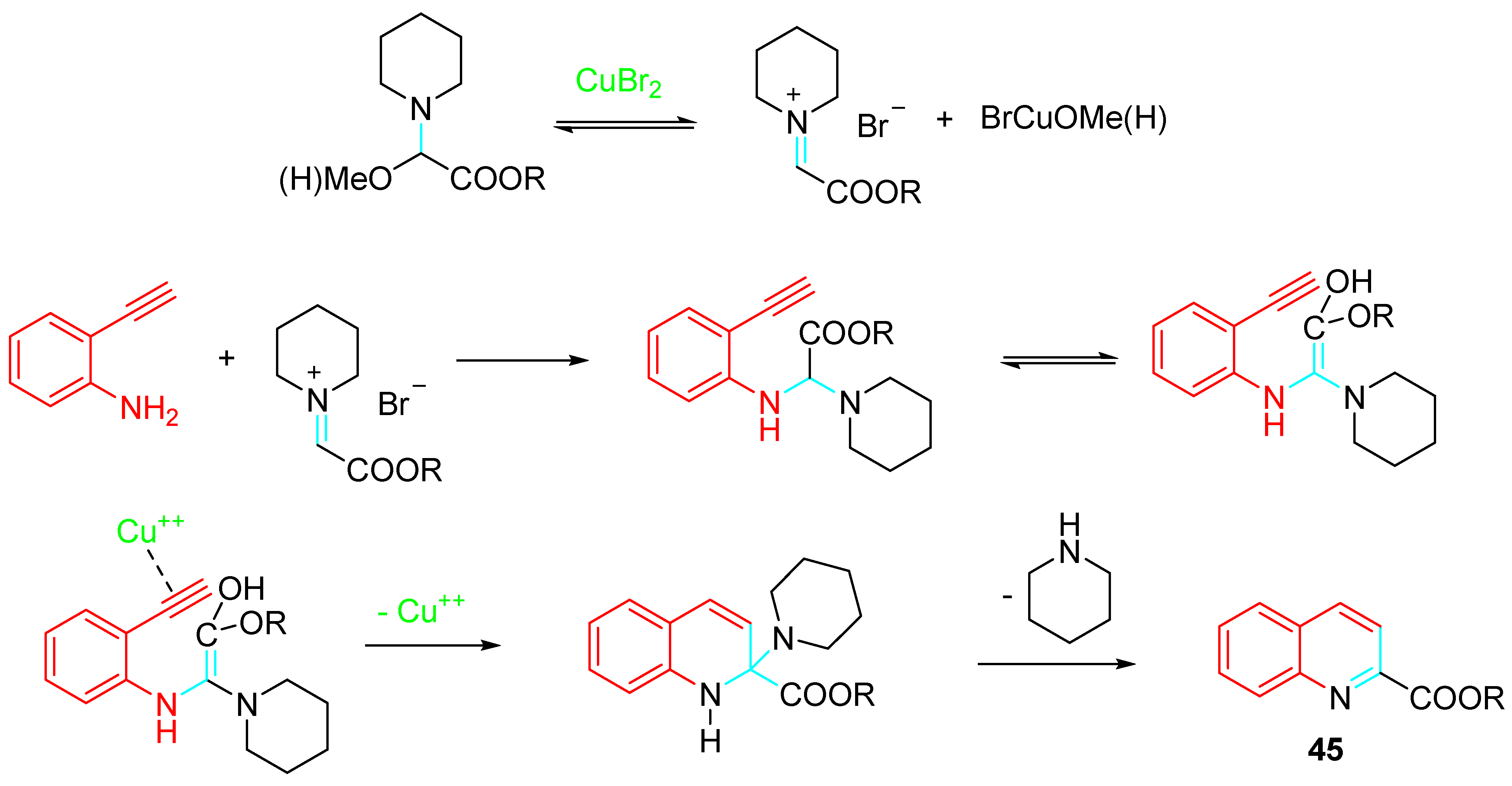
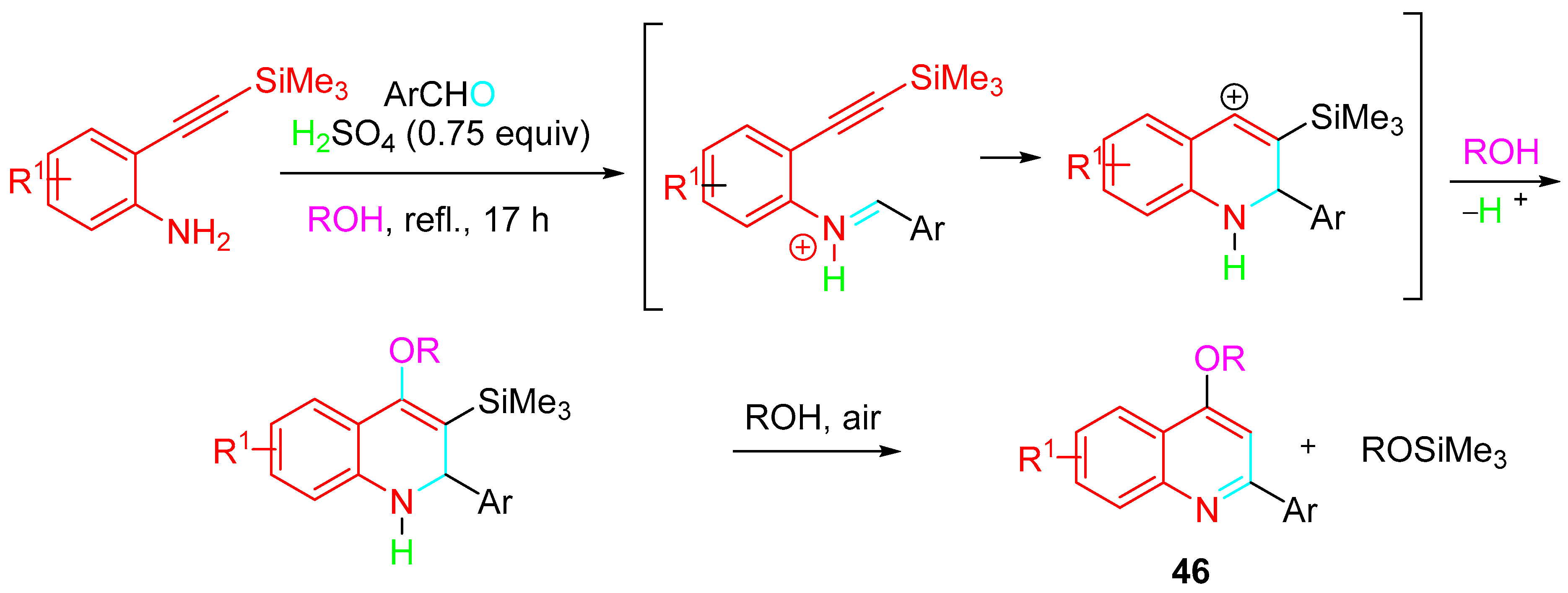
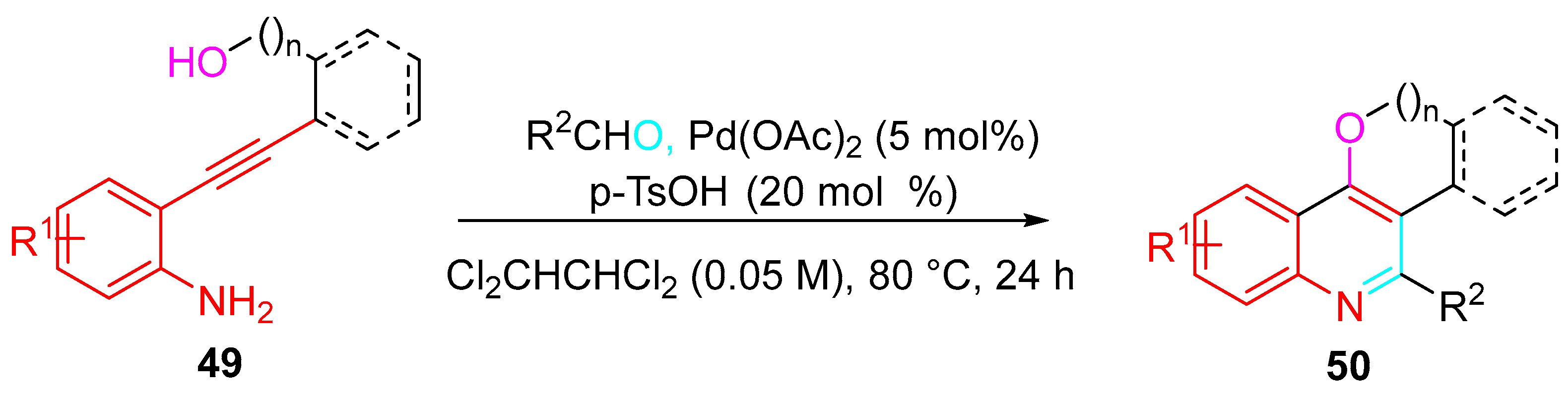
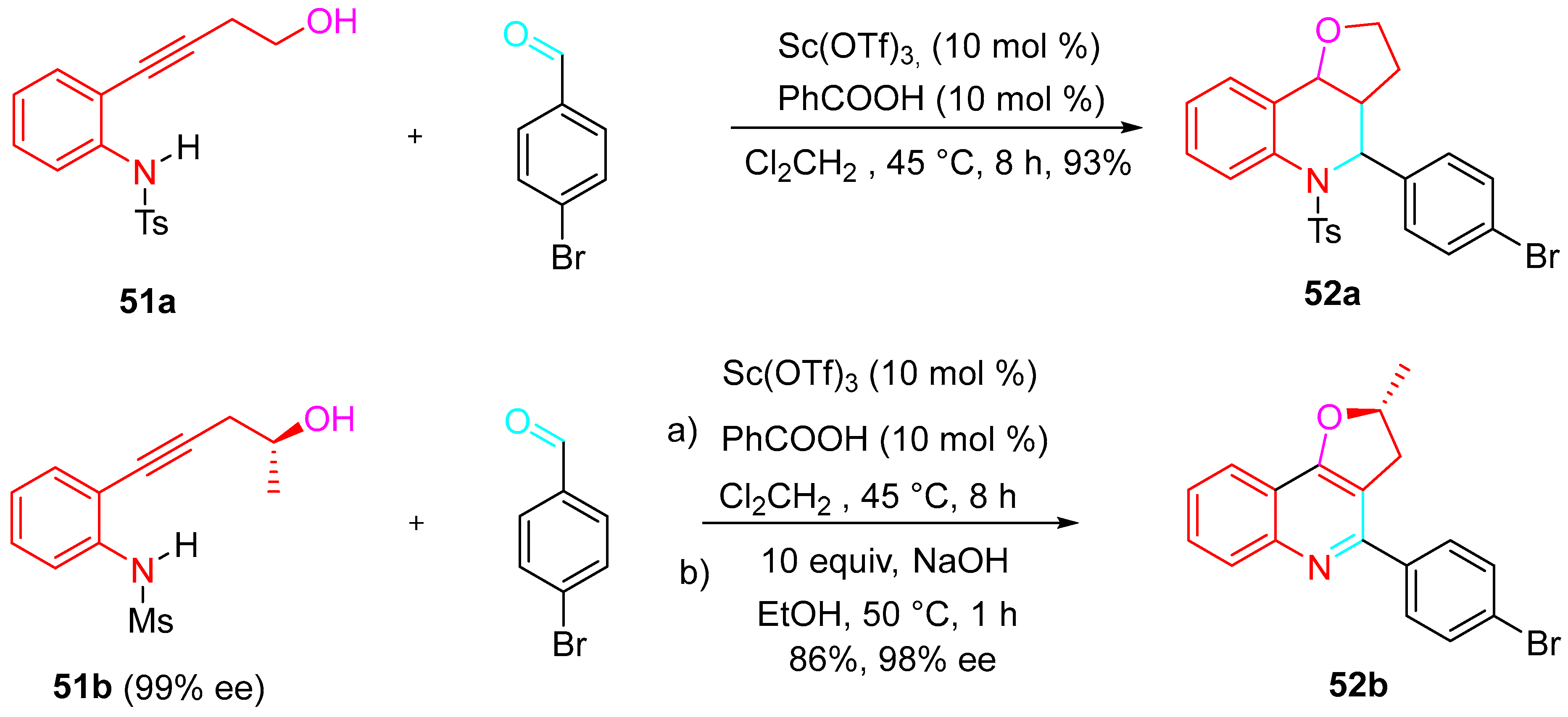
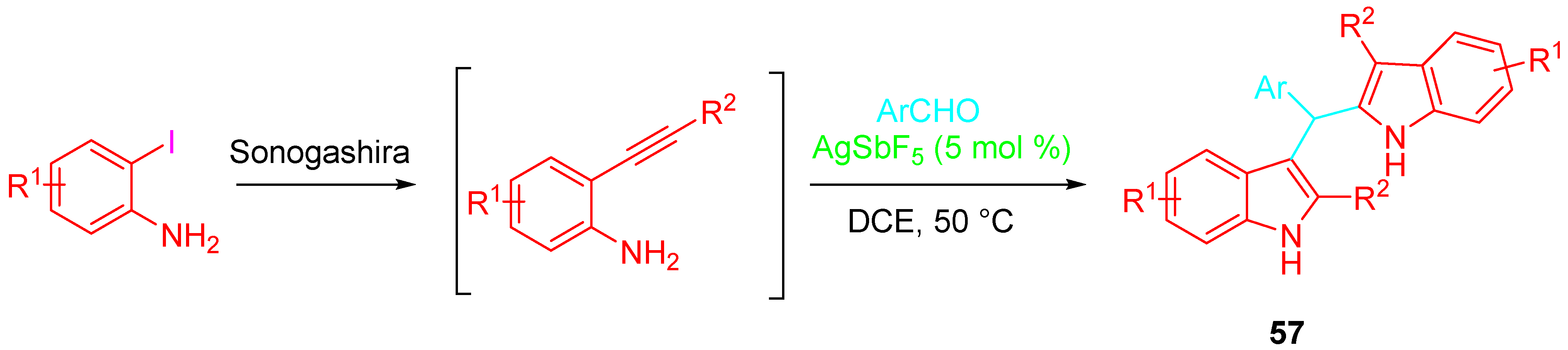
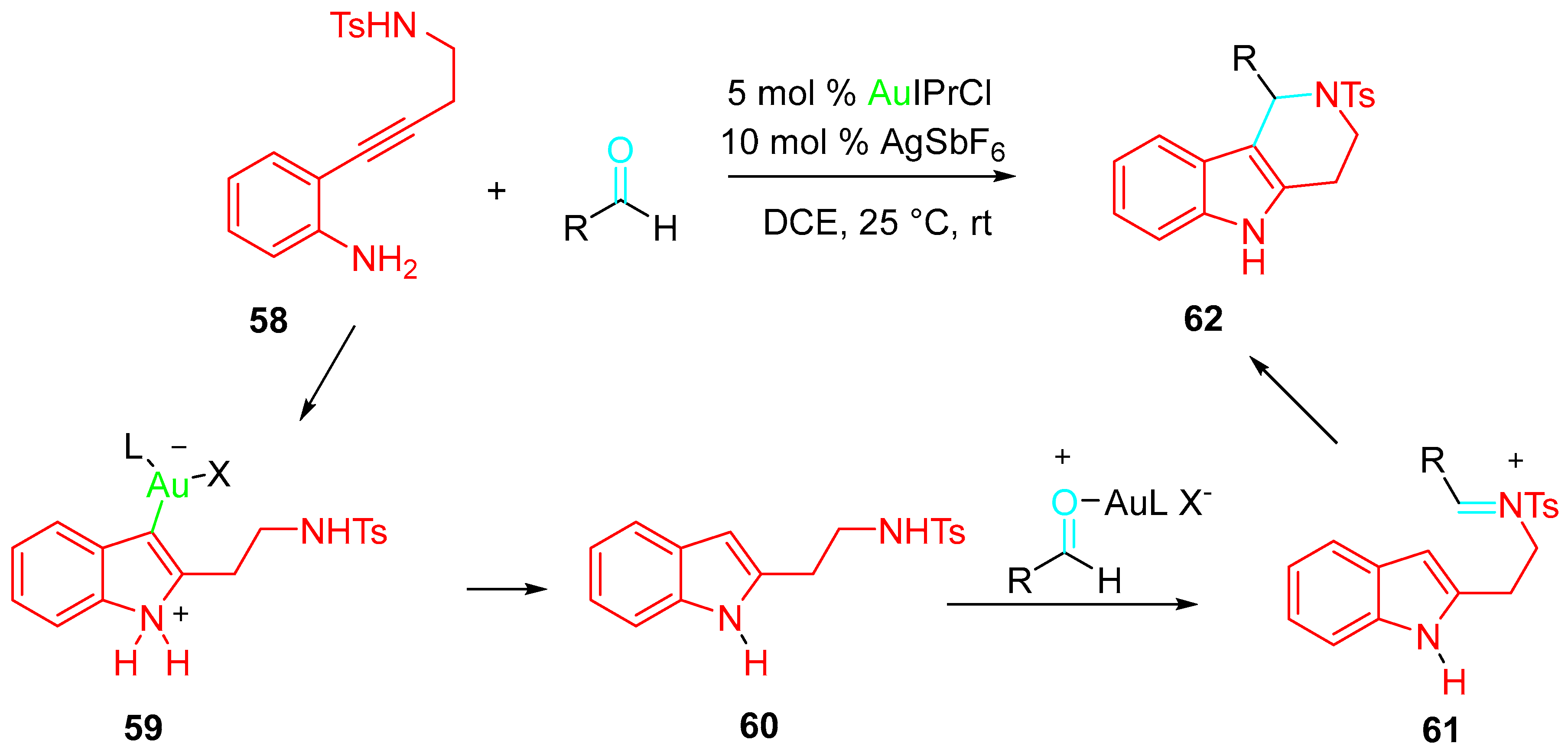
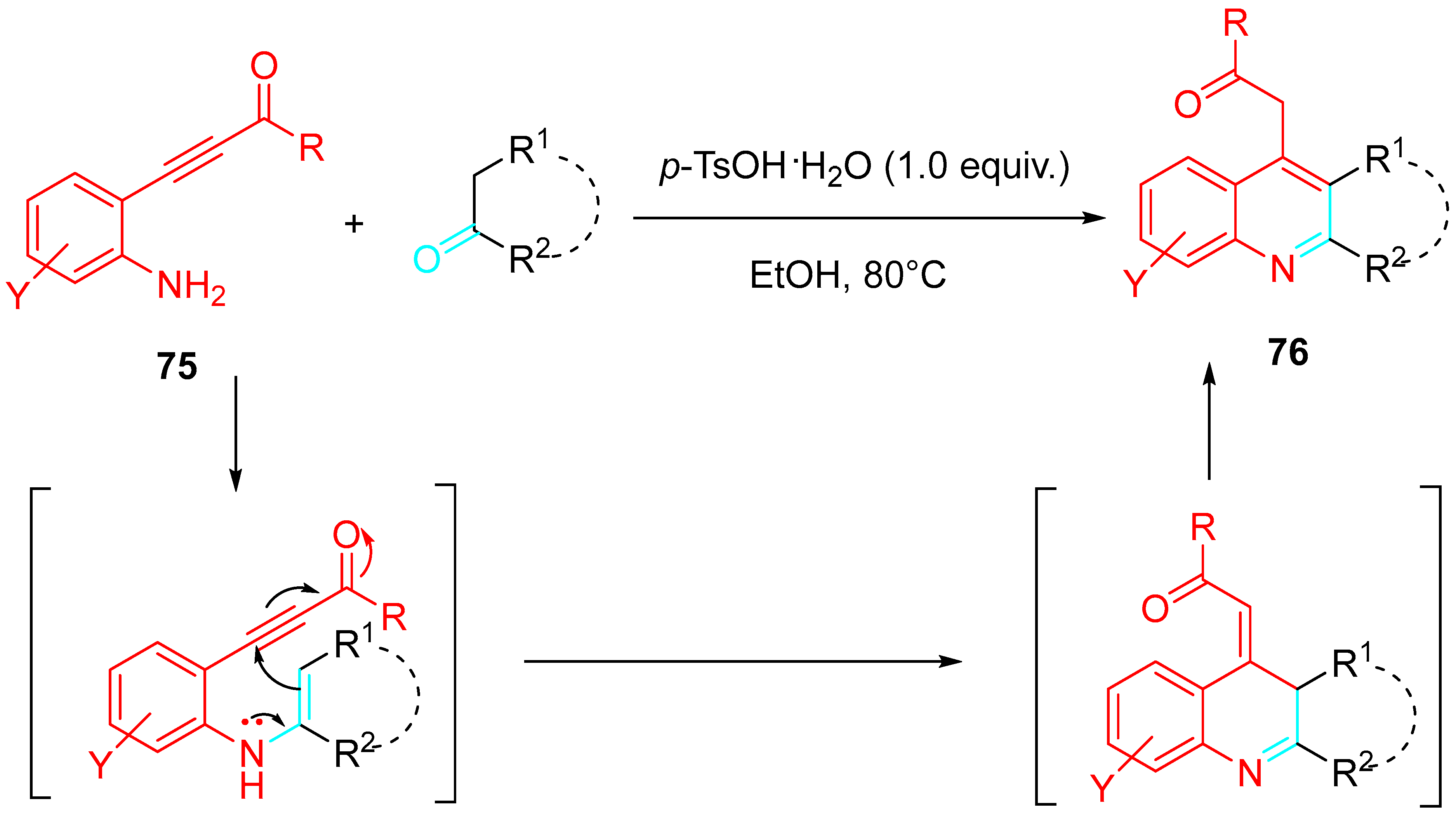
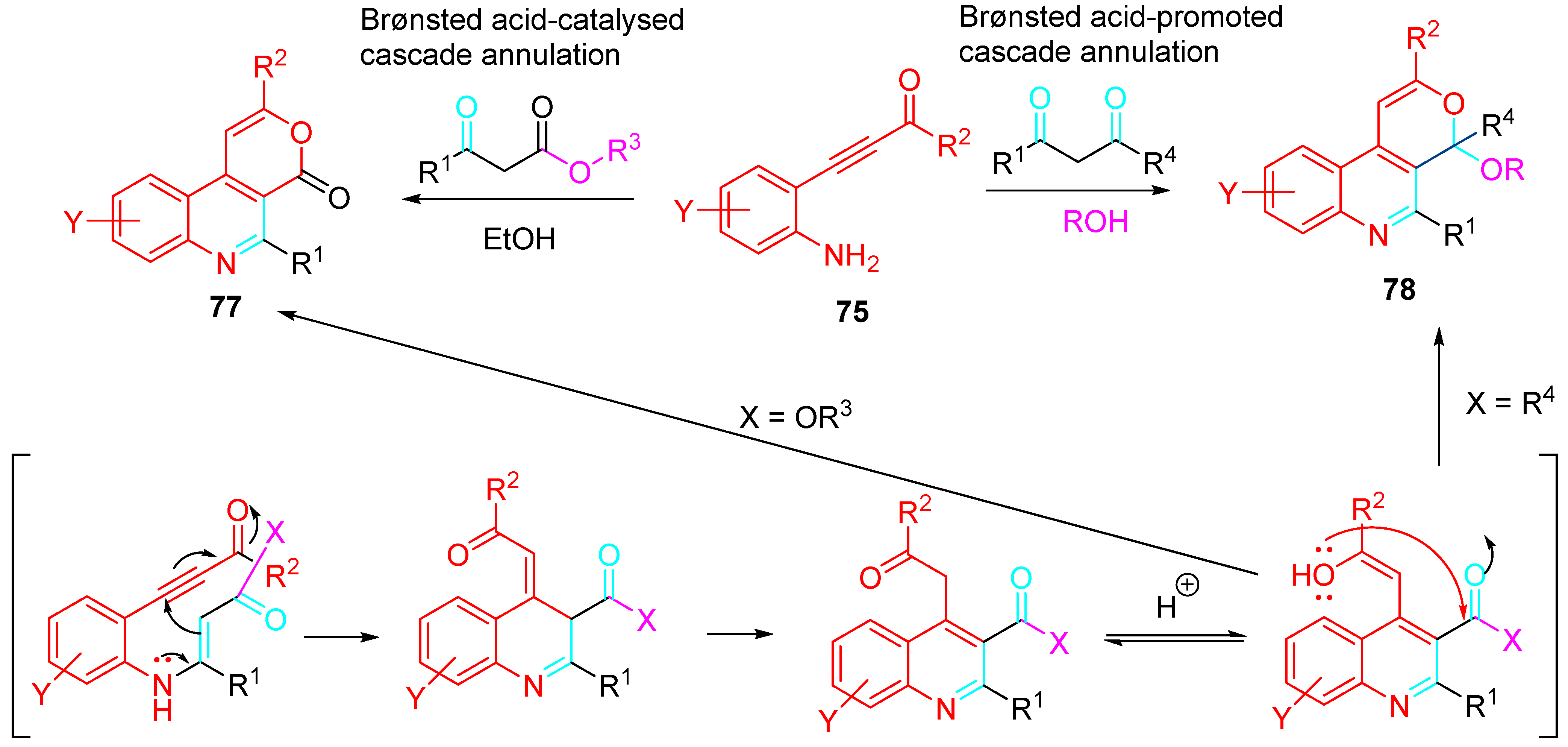
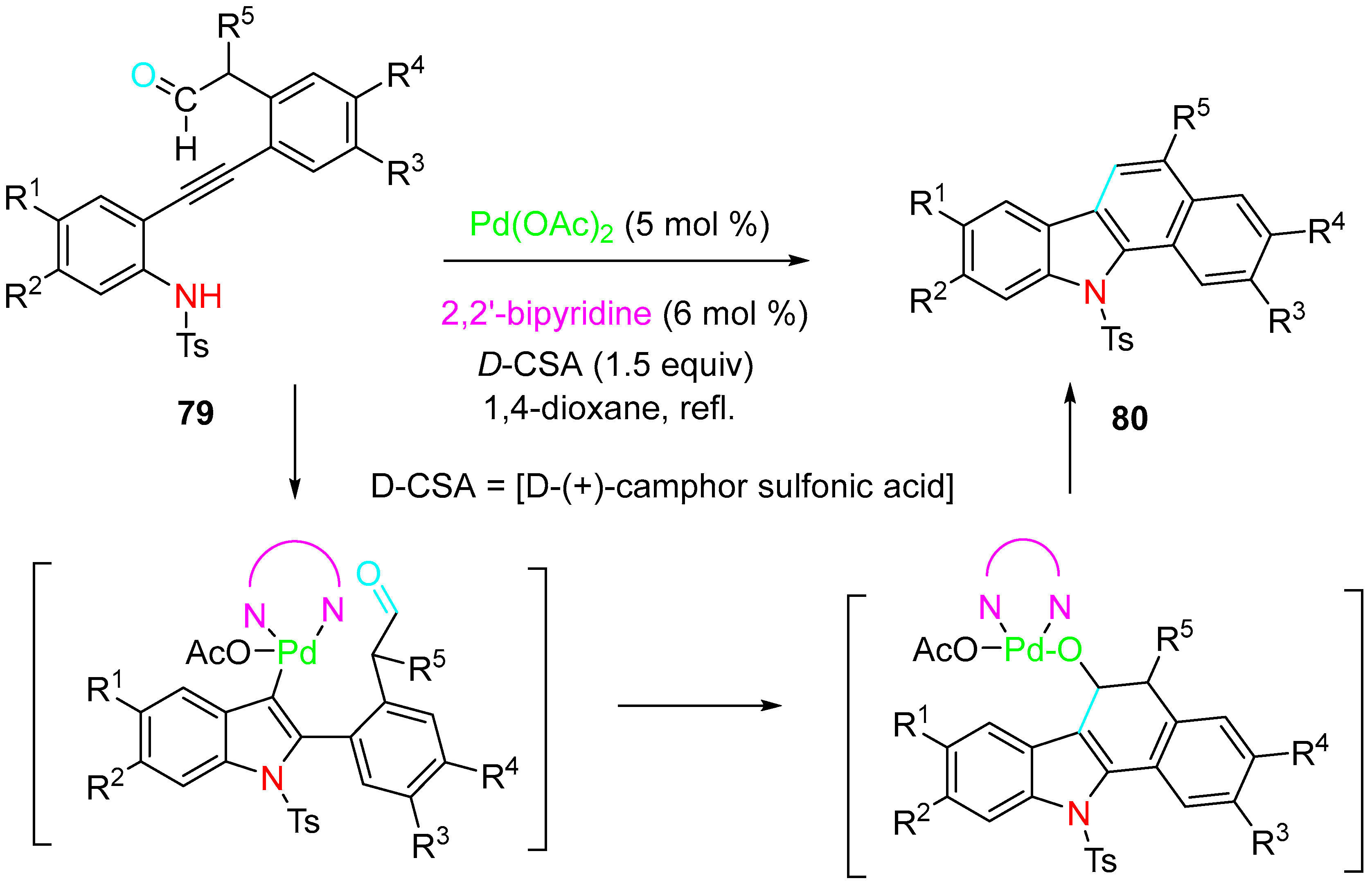
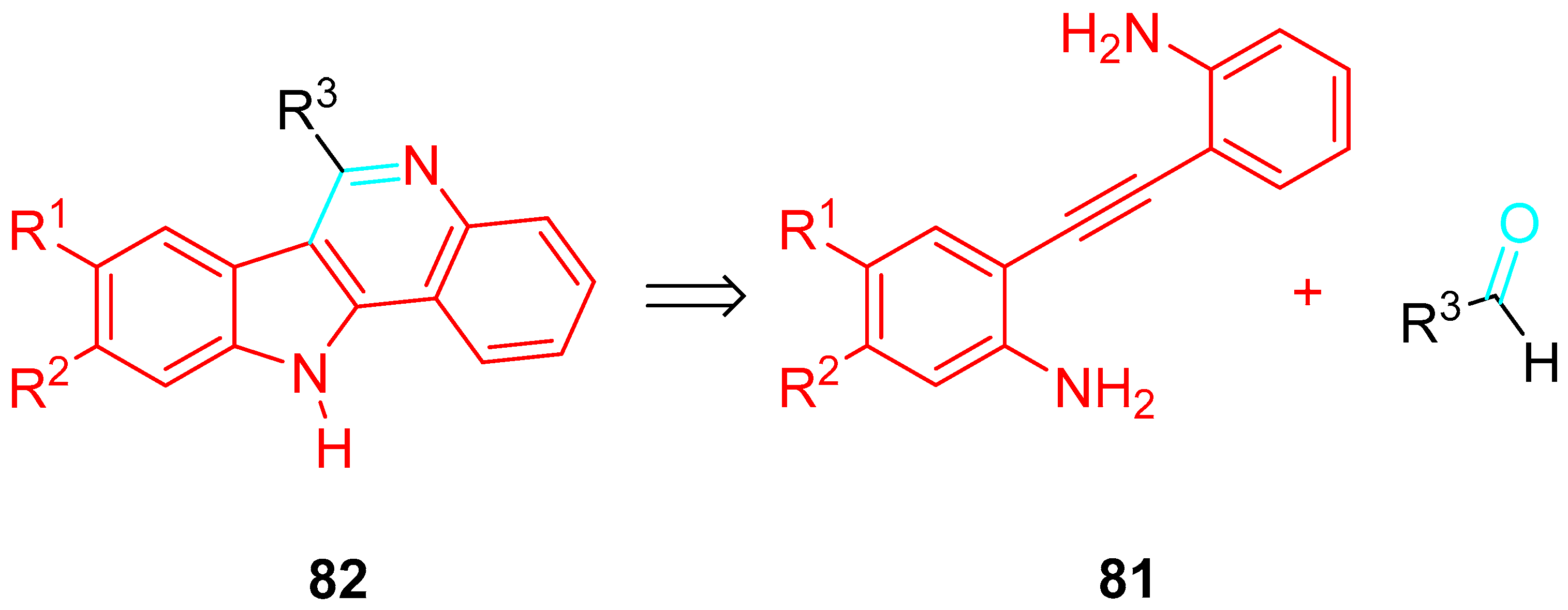
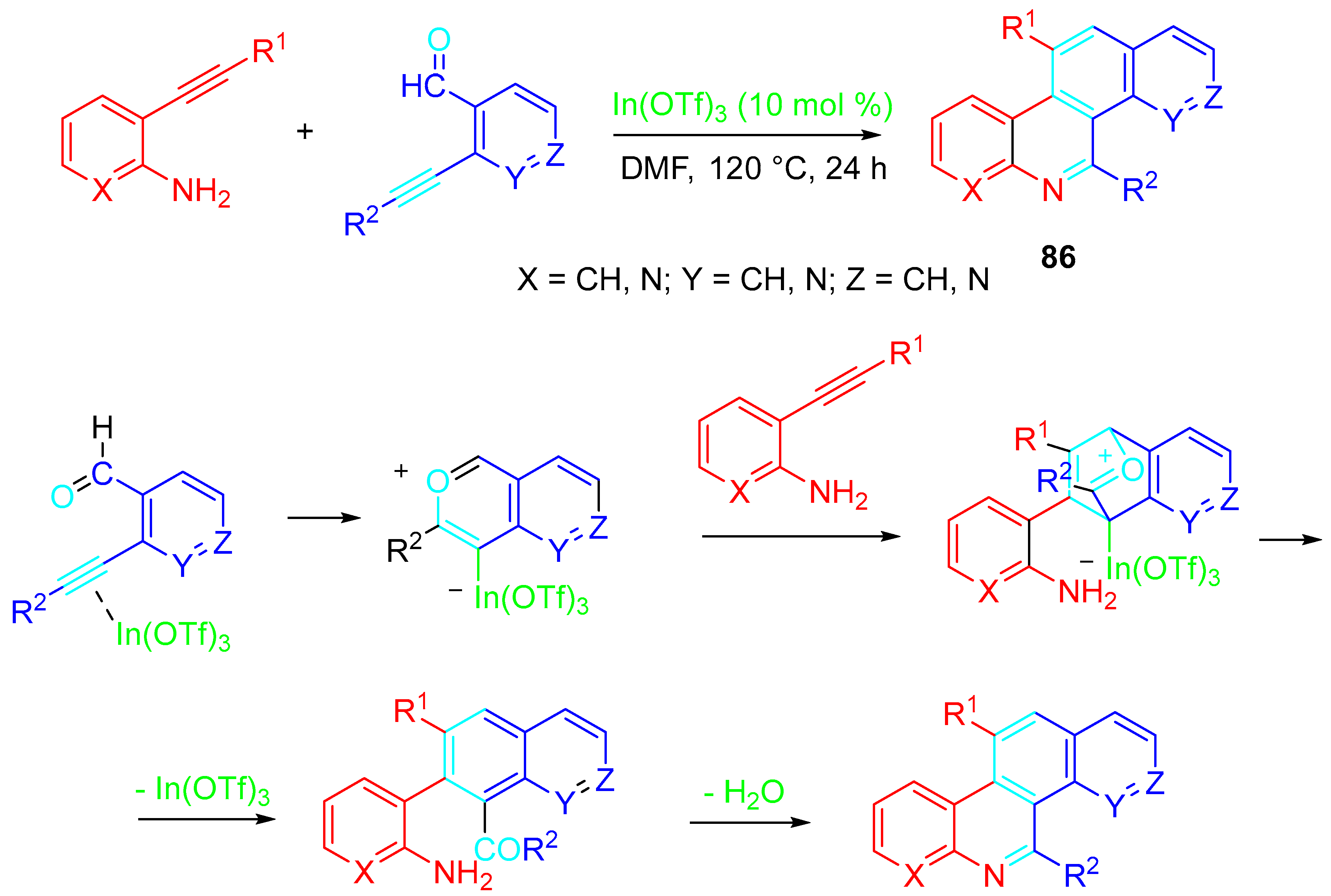
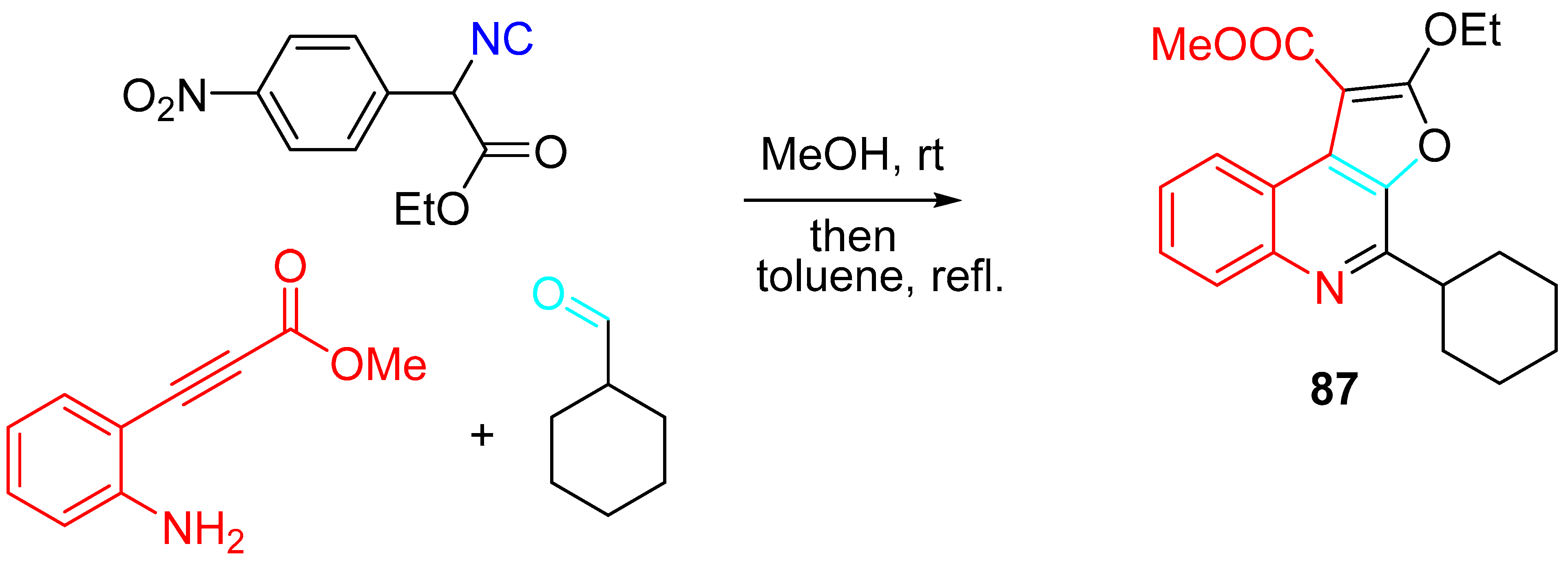
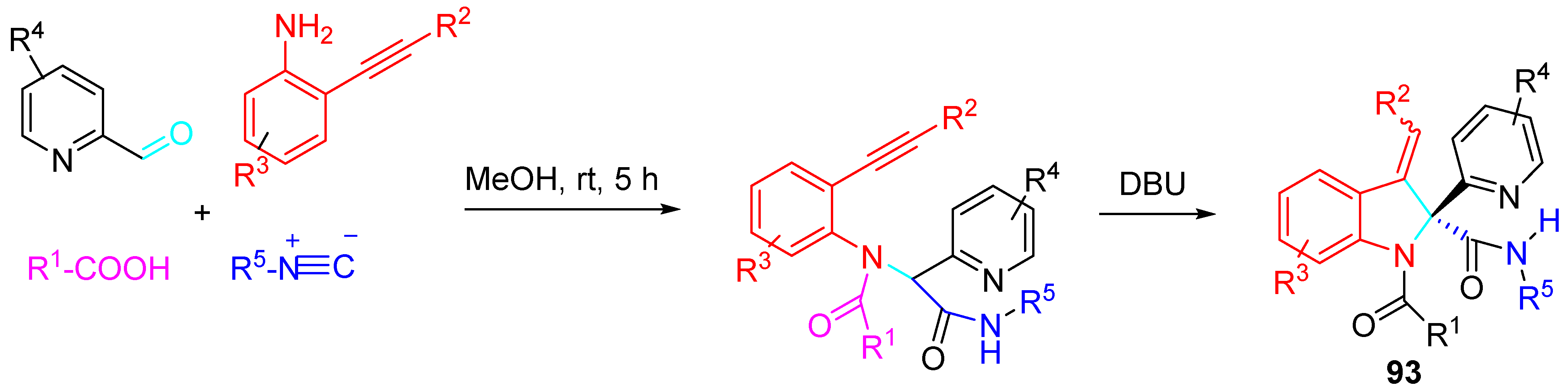
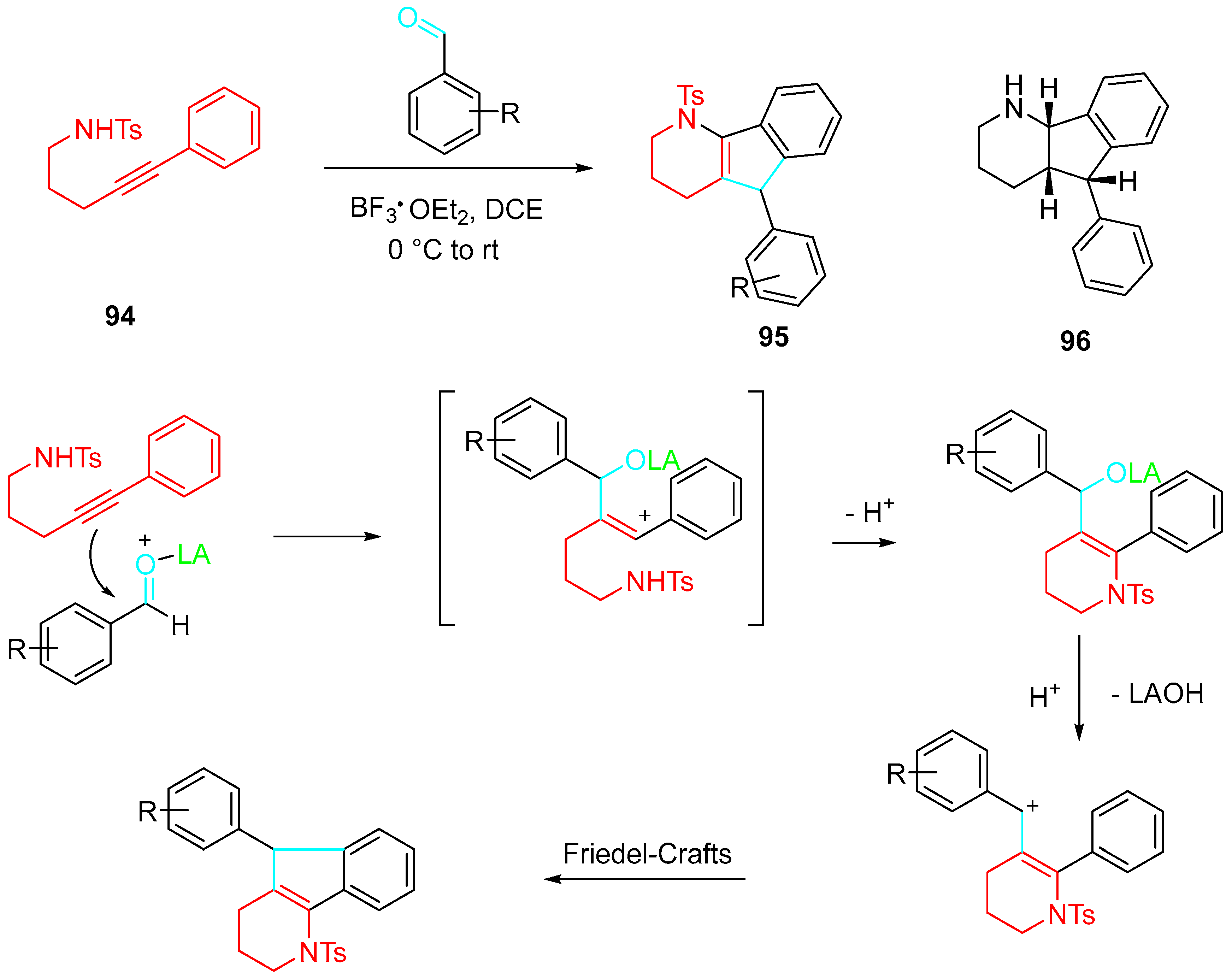
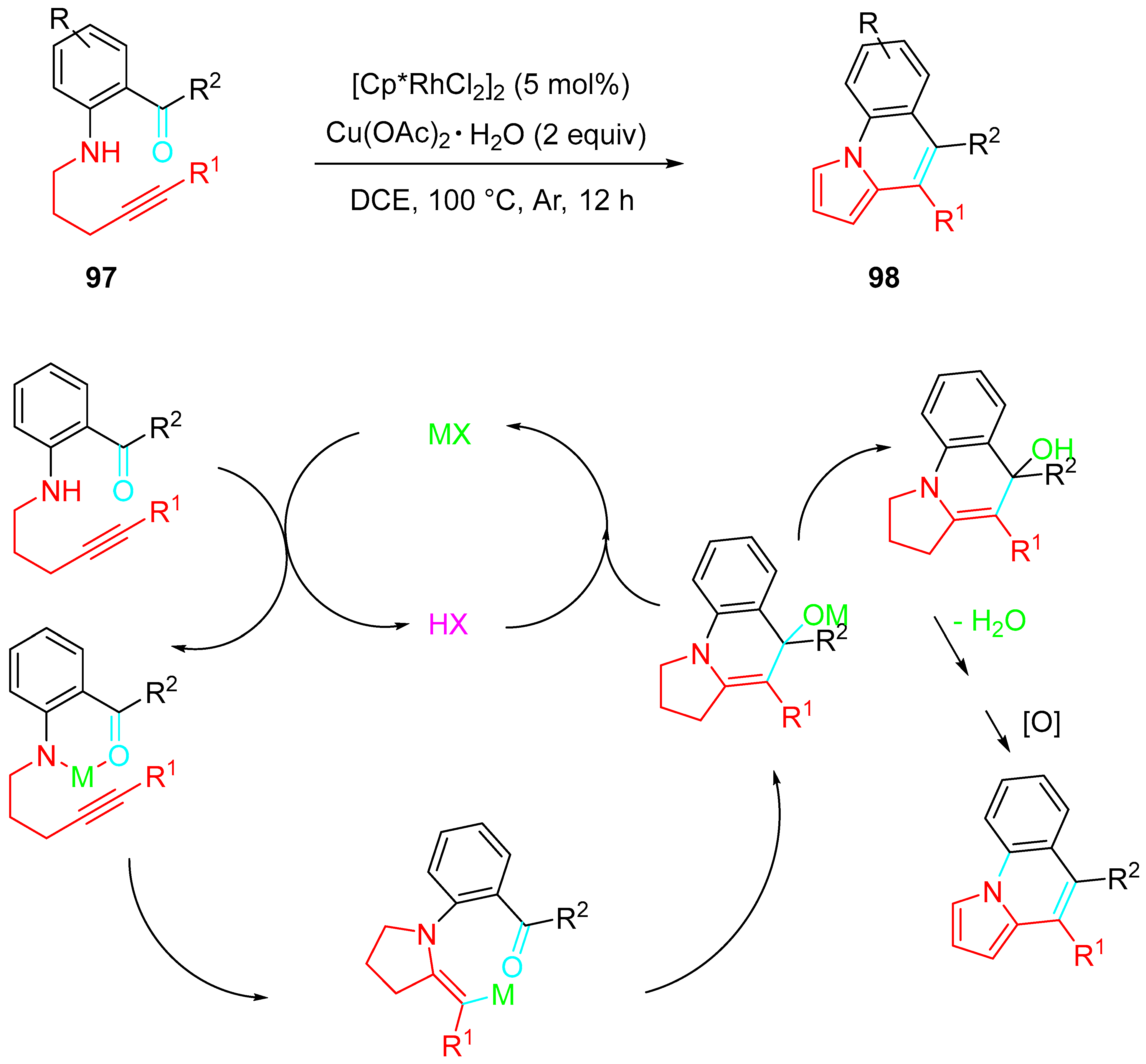
4. Sequential Reactions of γ- and δ-Aminoalkynes with Carbonyls
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade reactions in total synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Chen, J.S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 2009, 38, 2993–3009. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Nakanishi, Y.; Suzuki, H. Selective incorporation of primary amines into a trizirconium imido system and catalytic cyclization of aminoalkynes. Inorg. Chem. 2017, 56, 9802–9813. [Google Scholar] [CrossRef]
- Arcadi, A. Gold-catalyzed synthesis of nitrogen heterocyclic compounds via hydroamination reactions. In Au-Catalyzed Synthesis and Functionalization of Heterocycles; Topics in Heterocyclic Chemistry Book Series; Bandini, M., Ed.; Springer: Cham, Switzerland, 2016; Volume 46, pp. 53–86. [Google Scholar] [CrossRef]
- Arcadi, A.; Abbiati, G.; Rossi, E. Tandem imination/annulation of γ- and δ-ketoalkynes in the presence of ammonia/amines. J. Organomet. Chem. 2011, 696, 87–98. [Google Scholar] [CrossRef]
- Chang, S.; Lee, M.; Jung, D.Y.; Yoo, E.J.; Cho, S.H.; Han, S.K. Catalytic one-pot synthesis of cyclic amidines by virtue of tandem reactions involving intramolecular hydroamination under mild conditions. J. Am. Chem. Soc. 2006, 128, 12366–12367. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Che, C.-M. A highly efficient and selective AuI-catalyzed tandem synthesis of diversely substituted pyrrolo[1,2-a]quinolines in aqueous media. Angew. Chem. Int. Ed. 2008, 47, 3805–3810. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, E.; Liu, G.; Ye, D.; Li, J.; Jiang, H.; Liu, H. Gold-catalyzed one-pot cascade construction of highly functionalized pyrrolo[1,2-a]quinolin-1(2H)-ones. J. Org. Chem. 2009, 74, 7344–7348. [Google Scholar] [CrossRef]
- Ma, C.-L.; Zhao, J.-H.; Yang, Y.; Zhang, M.-K.; Shen, C.; Sheng, R.; Dong, X.-W.; Hu, Y.-Z. A copper-catalyzed tandem cyclization reaction of aminoalkynes with alkynes for the construction of tetrahydropyrrolo[1,2-a]quinolines scaffold. Sci. Rep. 2017, 7, 16640. [Google Scholar] [CrossRef]
- Ma, C.-L.; Li, X.-H.; Yu, X.-L.; Zhu, X.-L.; Hu, Y.-Z.; Dong, X.-W.; Tan, B.; Liu, X.-Y. Gold-catalyzed tandem synthesis of bioactive spiro-dipyrroloquinolines and its application in the one-step synthesis of incargranine B aglycone and seneciobipyrrolidine (I). Org. Chem. Front. 2016, 3, 324–329. [Google Scholar] [CrossRef]
- Galvàn, A.; Calleja, J.; Faňanás, F.J.; Rodríguez, F. Synthesis of pyrrolidine derivatives by a platinum/Brønsted acid relay catalytic cascade reaction. Chem. Eur. J. 2015, 21, 3409–3414. [Google Scholar] [CrossRef]
- Huple, D.B.; Liu, R.-S. One-pot stereocontrolled synthesis of bicyclic pyrrolidine derivatives by a platinum-Brønsted acid relay cascade reaction. ChemCatChem 2015, 7, 2824–2825. [Google Scholar] [CrossRef]
- Fujiwara, S.-I.; Shikano, Y.; Shin-ike, T.; Kambe, N.; Sonoda, N. Stereoselective synthesis of new selenium-containing heterocycles by cyclocarbonylation of aminoalkynes with carbon monoxide and selenium. J. Org. Chem. 2002, 67, 6275–6278. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, H. Highly selective construction of medium-sized lactams by palladium-catalyzed intramolecular hydroaminocarbonylation of aminoalkynes. Org. Lett. 2017, 19, 5070–5073. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Wang, H.; Wang, W.; Liu, L.; Chang, W.; Li, J. Synthesis of eight-membered nitrogen heterocycles via a heterogeneous PtI2-catalyzed cascade cycloaddition reaction of δ-aminoalkynes with electron-deficient alkynes. Adv. Synth. Catal. 2020, 362, 1525–1531. [Google Scholar] [CrossRef]
- Li, X.; Jiang, C.; Wang, X.; Ren, J.; Zeng, T.; Xu, X.; Li, J.; Liu, L. Platinum iodide-catalyzed formal three-component cascade cycloaddition reactions between γ-aminoalkynes and electron-deficient alkynes. J. Org. Chem. 2021, 86, 16614–16624. [Google Scholar] [CrossRef]
- Abbiati, G.; Arcadi, A.; Bianchi, G.; Di Giuseppe, S.; Marinelli, F.; Rossi, E. Sequential amination/annulation/aromatization reaction of carbonyl compounds and propargylamine: A new one-pot approach to functionalized pyridines. J. Org. Chem. 2003, 68, 6959–6966. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Wang, D.-L.; Lu, Y.; Zhao, X.-L.; Liu, Y. Au complex containing phosphino and imidazolyl moieties as a bifunctional catalyst for one-pot synthesis of pyridine derivatives. J. Mol. Catal. A Chem. 2016, 424, 323–330. [Google Scholar] [CrossRef]
- Sotnik, S.O.; Subota, A.I.; Kliuchynskyi, A.Y.; Yehorov, D.V.; Lytvynenko, A.S.; Rozhenko, A.B.; Kolotilov, S.V.; Ryabukhin, S.V.; Volochnyuk, D.M. Cu-catalyzed pyridine synthesis via oxidative annulation of cyclic ketones with propargylamine. J. Org. Chem. 2021, 86, 7315–7325. [Google Scholar] [CrossRef]
- Savić, M.P.; Ajduković, J.J.; Plavša, J.J.; Bekić, S.S.; Ćelić, A.S.; Klisurić, O.R.; Jakimov, D.S.; Petri, E.T.; Djurendić, E.A. Evaluation of A-ring fused pyridine D-modified androstane derivatives for antiproliferative and aldo-keto reductase 1C3 inhibitory activity. Med. Chem. Commun. 2018, 9, 969–981. [Google Scholar] [CrossRef]
- Yan, J.-Z.; Li, J.; Rao, G.-W. One-pot synthesis of new A-ring fused steroidal pyridines. Steroids 2007, 7, 736–739. [Google Scholar] [CrossRef]
- Laavola, M.; Haavikko, R.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Alakurtti, S.; Moreira, V.M.; Yli-Kauhaluoma, J.; Moilanen, E. Betulin derivatives effectively suppress inflammation in vitro and in vivo. J. Nat. Prod. 2016, 79, 274–280. [Google Scholar] [CrossRef]
- Haavikko, R.; Nasereddin, A.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. Med. Chem. Commun. 2014, 5, 445–451. [Google Scholar] [CrossRef]
- Hodoň, J.; Frydrych, I.; Trhlíková, Z.; Pokorný, J.; Borková, L.; Benická, S.; Vlk, M.; Lišková, B.; Kubíčková, A.; Medvedíková, M.; et al. Triterpenoid pyrazines and pyridines—Synthesis, cytotoxicity, mechanism of action, preparation of prodrugs. Eur. J. Med. Chem. 2022, 243, 114777. [Google Scholar] [CrossRef]
- Rabe, S.; Moschner, J.; Bantzi, M.; Heretsch, P.; Giannis, A. C-H-Functionalization logic guides the synthesis of a carbacyclopamine analog. Beilstein J. Org. Chem. 2014, 10, 1564–1569. [Google Scholar] [CrossRef]
- Simcere Pharmaceutical Group. Compound as Potassium Channel Modulating Agents. CN108250128 A, 6 July 2018. [Google Scholar]
- Suzhou Yunxuan Pharmaceutical Co Ltd. Heterocyclic Compound with Wnt Signal Path Inhibitory Activity and Application Thereof. CN105254613 A, 20 January 2016. [Google Scholar]
- Zhang, X.; Zheng, J.; Ma, H. Heteroaryl Compounds as cxcr4 Inhibitors, Composition and Method Using the Same. Patent WO2019/60860 A1, 28 March 2019. [Google Scholar]
- Wortmann, L.; Lindenthal, B.; Muhn, P.; Walter, A.; Nubbemeyer, R.; Heldmann, D.; Sobek, L.; Morandi, F.; Schrey, A.K.; Moosmayer, D.; et al. Discovery of BAY-298 and BAY-899: Tetrahydro-1,6-naphthyridine-based, potent, and selective antagonists of the luteinizing hormone receptor which reduce sex hormone levels in vivo. J. Med. Chem. 2019, 62, 10321–10341. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Chen, L.; Conway, C.M.; Denton, R.; Keavy, D.; Gulianello, M.; Huang, Y.; Kostich, W.; Lentz, K.A.; Mercer, S.E.; et al. Discovery of BMS-846372, a potent and orally active human CGRP receptor antagonist for the treatment of migraine. ACS Med. Chem. Lett. 2012, 3, 337–341. [Google Scholar] [CrossRef] [PubMed]
- C.H. Boehringer Sohn AG & Co. KG. Aryl- and Heroarylcarbonyl Derivatives of Benzomorphanes and Related Scaffolds, Medicaments Containing Such Compounds and Their Use. EP2220048 B1, 25 January 2017. [Google Scholar]
- Lowe, R.A.; Taylor, D.; Chibale, K.; Nelson, A.; Marsden, S.P. Synthesis and evaluation of the performance of a small molecule library based on diverse tropane-related scaffolds. Biorg. Med. Chem. 2020, 28, 115442. [Google Scholar] [CrossRef]
- Eckhardt, M.; Peters, S.; Nar, H.; Himmelsbach, F.; Zhuang, L. Boehringer Ingheleim Int, Aryl-and Heteroarylcarbonyl Derivatives of Hexahydroindenopyridine and Octahydrobenzoquinoline. U.S. Patent 2011/0136800 A1, 9 June 2011. [Google Scholar]
- Park, N.Y.; Lee, H.S.; Piao, L.H.; Park, S.Y.; Jeong, W. Transition Metal Compound for Olefin Polymerization Catalyst, and Olefin Polymerization Catalyst Including Same. U.S. Patent 11254759 B2, 22 February 2022. [Google Scholar]
- Faňanás, F.J.; Arto, T.; Mendoza, A.; Rodríguez, F. Synthesis of 2,5-dihydropyridine derivatives by gold-catalyzed reactions of β-ketoesters and propargylamines. Org. Lett. 2011, 13, 4184–4187. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoo, H.; Park, S.; Yoon, R.; Kim, S. Facile one-pot synthesis of polysubstituted pyridinium salts by annulation of enamines with alkynes. Chem. Eur. J. 2023, e202300059. [Google Scholar] [CrossRef] [PubMed]
- Changchun Haipurunsi Tech Co Ltd. A Kind of Miscellaneous Anthracene Derivant and Preparation Method Thereof and Organic Luminescent Device. CN108218860 A, 29 June 2018. [Google Scholar]
- University Zhejlang Technology. Method for Synthesizing Substituted Dihydrophenanthroline Compound. CN112480112 A, 15 October 2021. [Google Scholar]
- Watkins, E.B.; Uredi, D.; Motati, D.R. Novel Methods for Preparation of Substituted Pyridines and Related Novel Compounds. U.S. Patent 2020/0095245 A1, 10 August 2020. [Google Scholar]
- Uredi, D.; Motati, D.R.; Watkins, E.B. A simple, tandem approach to the construction of pyridine derivatives under metal-free conditions: A one-step synthesis of the monoterpene natural product, (-)-actinidine. Chem. Commun. 2019, 55, 3270–3273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wei, H.; Xiao, K.; Cheng, B.; Zhai, H.; Li, Y. Facile synthesis of pyridines from propargyl amines: Concise total synthesis of suaveoline alkaloids. Angew. Chem. Int. Ed. 2019, 58, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, Y. Quick access to pyridines through 6π-3-azatriene electrocyclization: Concise total synthesis of suaveoline alkaloids. Synlett 2019, 30, 1615–1620. [Google Scholar] [CrossRef]
- Uredi, D.; Motati, D.R.; Watkins, E.B. A unified strategy for the synthesis of β-carbolines, γ-carbolines, and other fused azaheteroaromatics under mild, metal-free conditions. Org. Lett. 2018, 20, 6336–6339. [Google Scholar] [CrossRef]
- Uredi, D.; Burra, A.G.; Watkins, E.B. Rapid access to 3-substituted pyridines and carbolines via a domino, copper-free, palladium-catalyzed Sonogashira cross-coupling/6π-aza cyclization sequence. J. Org. Chem. 2021, 86, 17748–17761. [Google Scholar] [CrossRef]
- Lanzhou University. A Kind of Polysubstituted Pyridine Derivative and Preparation Method Thereof. CN108358834 A, 3 August 2018. [Google Scholar]
- Chikayuki, Y.; Miyashige, T.; Yonekawa, S.; Kirita, A.; Matsuo, N.; Teramoto, H.; Sasaki, S.; Higashiyama, K.; Yamauchi, T. Transition-metal-free synthesis of pyridine derivatives by thermal cyclization of N-propargyl enamines. Synthesis 2020, 52, 1113–1121. [Google Scholar] [CrossRef]
- Keskin, S.; Balci, M. Intramolecular heterocyclization of O-propargylated aromatic hydroxyaldehydes as an expedient route to substituted chromenopyridines under metal-free conditions. Org. Lett. 2015, 17, 964–967. [Google Scholar] [CrossRef]
- Hoplamaz, E.; Keskin, S.; Balci, M. Regioselective synthesis of benzo[h][1,6]-naphthyridines and chromenopyrazinones through alkyne cyclization. Eur. J. Org. Chem. 2017, 1489–1497. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Alonso, J.M.; Fernández, I.; Gómez-Campillosa, G.; Torres, M.R. A gold-catalysed imine-propargylamine cascade sequence: Synthesis of 3-substituted-2,5-dimethylpyrazines and the reaction mechanism. Chem. Commun. 2014, 50, 4567–4570. [Google Scholar] [CrossRef]
- Nie, Q.; Yao, F.; Yi, F.; Cai, M. A heterogeneous gold(I)-catalyzed cascade annulation of aldehydes with propargylamine leading to 3-substituted 2,5-dimethylpyrazines. J. Organomet. Chem. 2017, 846, 343–350. [Google Scholar] [CrossRef]
- Donald, J.R.; Martin, S.F. Synthesis and diversification of 1,2,3-triazole-fused 1,4-benzodiazepine scaffolds. Org. Lett. 2011, 13, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Donald, J.R.; Wood, R.R.; Martin, S.F. Application of a sequential multicomponent assembly process/Huisgen cycloaddition strategy to the preparation of libraries of 1,2,3-triazole-fused 1,4-benzodiazepines. ACS Comb. Sci. 2012, 14, 135–143. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Palazzo, T.A.; Kurth, M.-J. Facile one-pot assembly of imidazotriazolobenzodiazepines via indium(III)-catalyzed multicomponent reactions. Org. Lett. 2013, 15, 4492–4495. [Google Scholar] [CrossRef]
- Festa, A.A.; Raspertov, P.V.; Voskressensky, L.G. 2-(Alkynyl)anilines and derivatives-versatile reagents for heterocyclic synthesis. Adv. Synth. Catal. 2022, 364, 466–486. [Google Scholar] [CrossRef]
- Vavsari, V.F.; Nikbakht, A.; Balalaie, S. Annulation of 2-alkynylanilines: The versatile chemical compounds. Asian J. Org. Chem. 2022, 11, e202100772. [Google Scholar] [CrossRef]
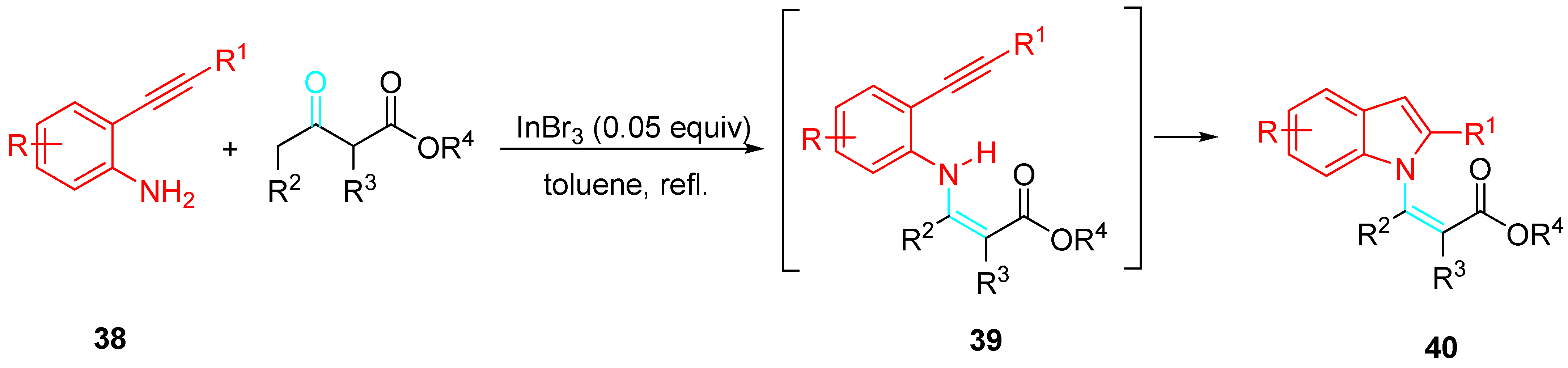
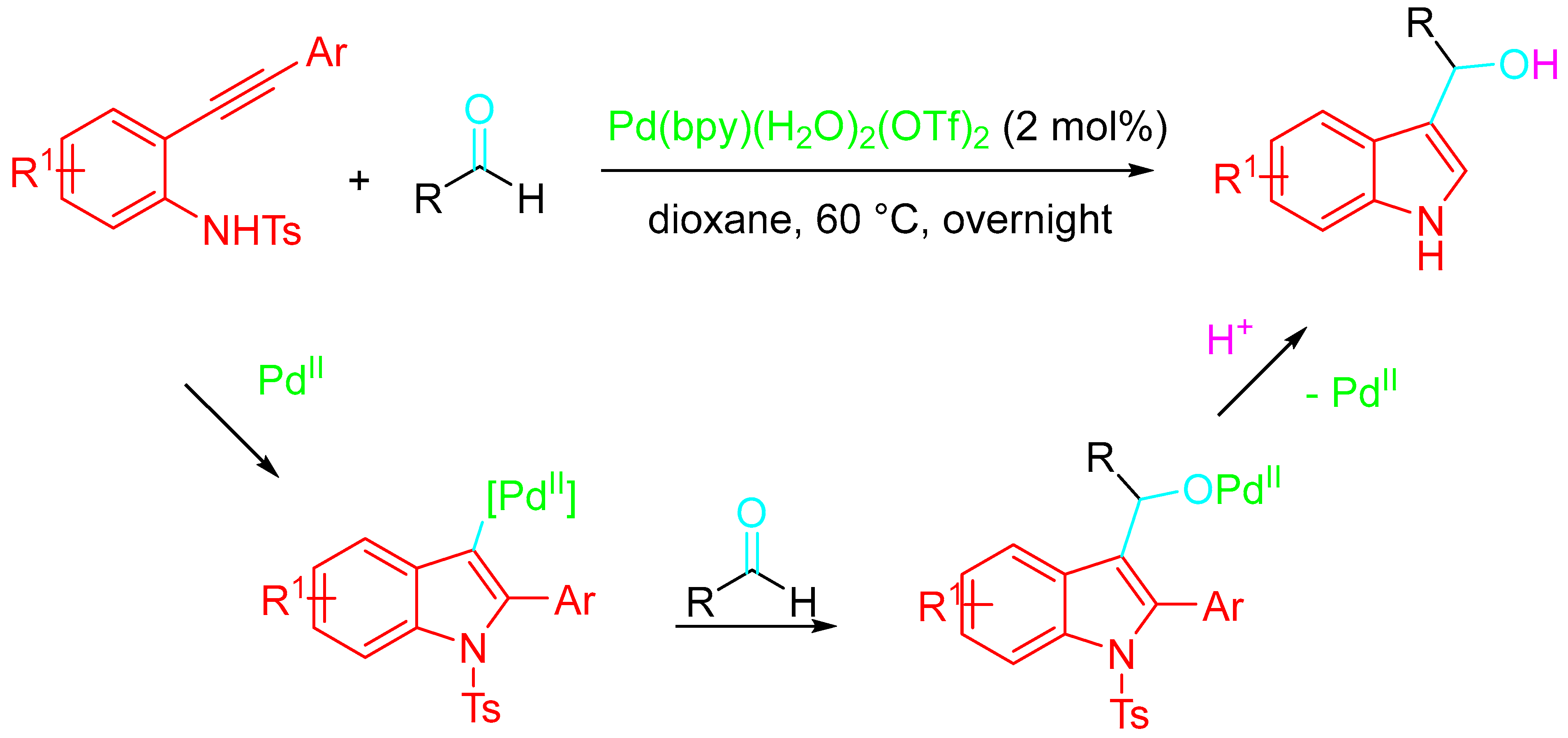
- Kamble, O.S.; Khatravath, M.; Dandela, R. Applications of ethynylanilines as substrates for construction of indoles and indole-substituted derivatives. ChemistrySelect 2021, 6, 7408–7427. [Google Scholar] [CrossRef]
- Murai, K.; Hayashi, S.; Takaichi, N.; Kita, Y.; Fujioka, H. Tandem β-enamino ester formation and cyclization with o-alkynyl anilines catalyzed by InBr3: Efficient synthesis of-β-(N-indolyl)-α,β-unsaturated esters. J. Org. Chem. 2009, 74, 1418–1421. [Google Scholar] [CrossRef]
- Arcadi, A.; Alfonsi, M.; Bianchi, G.; D’Anniballe, G.; Marinelli, F. Gold-catalysed direct couplings of indoles and pyrroles with 1,3-dicarbonyl compounds. Adv. Synth. Catal. 2006, 348, 331–338. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Y.; Liu, L.; Wang, H.; Zhao, J.; Zhu, Q. p-Toluenesulfonic acid promoted annulation of 2-alkynylanilines with activated ketones: Efficient synthesis of 4-alkyl-2,3-disubstituted quinolines. Eur. J. Org. Chem. 2010, 818–822. [Google Scholar] [CrossRef]
- Sivaraman, M.; Perumal, P.T. Synthesis of 4-methyl-2,3-disubstituted quinoline scaffolds via environmentally benign Fe(III) catalysed sequential condensation, cyclization and aromatization of 1,3-diketone and 2-ethynylaniline. RSC Adv. 2014, 4, 52060–52066. [Google Scholar] [CrossRef]
- Ortiz-Cervantes, C.; Flores-Alamo, M.; García, J.J. Synthesis of pyrrolidones and quinolines from the known biomass feedstock levulinic acid and amines. Tetrahedron Lett. 2016, 57, 766–771. [Google Scholar] [CrossRef]
- Wang, G.; Jia, J.; Liu, G.; Yu, M.; Chu, X.; Liu, X.; Zhao, X. Copper(I)-catalyzed tandem synthesis of 2-acylquinolines from 2-ethynylanilines and glyoxals. Chem. Commun. 2021, 57, 11811–11814. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Tamura, K.; Shimamura, K.; Ikeda, R.; Konakahara, T. Copper-catalyzed [5+1] annulation of 2-ethynylanilines with an N,O-acetal leading to construction of quinoline derivatives. Org. Lett. 2012, 14, 836–839. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, C.; Liu, L.; Zhao, J.; Su, L.; Zhu, Q. Sulfuric acid promoted condensation cyclization of 2-(2-(trimethylsilyl)ethynyl)anilines with arylaldehydes in alcoholic solvents: An efficient one-pot synthesis of 4-alkoxy-2-arylquinolines. Tetrahedron Lett. 2009, 50, 2261–2265. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Taher, A.; Ponra, S. Unusual product from condensative cyclization: Pyrano[3,2-f]quinolin-3,10-diones from 6-amino-5-[(trimethylsilyl)ethynyl]-2H-chromen-2-one and aryl aldehydes. Synlett 2010, 735–740. [Google Scholar] [CrossRef]
- Ock, S.K.; Youn, S.W. Synergistic effect of Pd(II) and acid catalysts on tandem annulation reaction for the regioselective synthesis of ring-fused quinolines. Bull. Korean Chem. Soc. 2010, 31, 704–707. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, S. Sc(OTf)3-catalyzed bicyclization of o-alkynylanilines with aldehydes: Ring-fused 1,2-dihydroquinolines. Angew. Chem. Int. Ed. 2014, 53, 13532–13535. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Xu, C.; Zou, G.; Hong, D.; Deng, D.; Wang, Z.; Ji, B. AuCl3-catalyzed tandem reaction of N-(o-alkynylphenyl)imines: A modular entry to polycyclic frameworks containing an indole unit. Synlett 2009, 5, 763–766. [Google Scholar] [CrossRef]
- Halim, R.; Scammells, P.J.; Flynn, B.L. Alternating iodonium-mediated reaction cascades giving indole- and quinoline-containing polycycles. Org. Lett. 2008, 10, 1967–1970. [Google Scholar] [CrossRef]
- Halim, R.; Aurelio, L.; Scammells, P.J.; Flynn, B.L. Scaffold-divergent synthesis of ring-fused indoles, quinolines, and quinolones via iodonium-induced reaction cascades. J. Org. Chem. 2013, 78, 4708–4718. [Google Scholar] [CrossRef]
- Kusama, H.; Takaya, J.; Iwasawa, N. A facile method for the synthesis of polycyclic indole derivatives: The generation and reaction of tungsten-containing azomethine ylides. J. Am. Chem Soc. 2002, 124, 11592–11593. [Google Scholar] [CrossRef] [PubMed]
- Kayeta, A.; Singh, V.K. A one-pot synthesis of 2,2’-disubstituted diindolylmethanes (DIMs) via a sequential Sonogashira coupling and cycloisomerization/C3-functionalization of 2-iodoanilines. Org. Biomol. Chem. 2017, 15, 6997–7007. [Google Scholar] [CrossRef] [PubMed]
- Subba Reddy, B.V.; Swain, M.; Madhusudana Reddy, S.; Yadav, J.S.; Sridhar, B. Gold-catalyzed domino cycloisomerization/Pictet-Spengler reaction of 2-(4-aminobut-1-yn-1-yl)anilines with aldehydes: Synthesis of tetrahydropyrido[4,3-b]indole scaffolds. J. Org. Chem. 2012, 77, 11355–11361. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, X. Cationic Pd(II)-catalyzed tandem reaction of 2-arylethynylanilines and aldehydes: An efficient synthesis of substituted 3-hydroxymethyl Indoles. Org. Lett. 2010, 12, 3336–3339. [Google Scholar] [CrossRef] [PubMed]
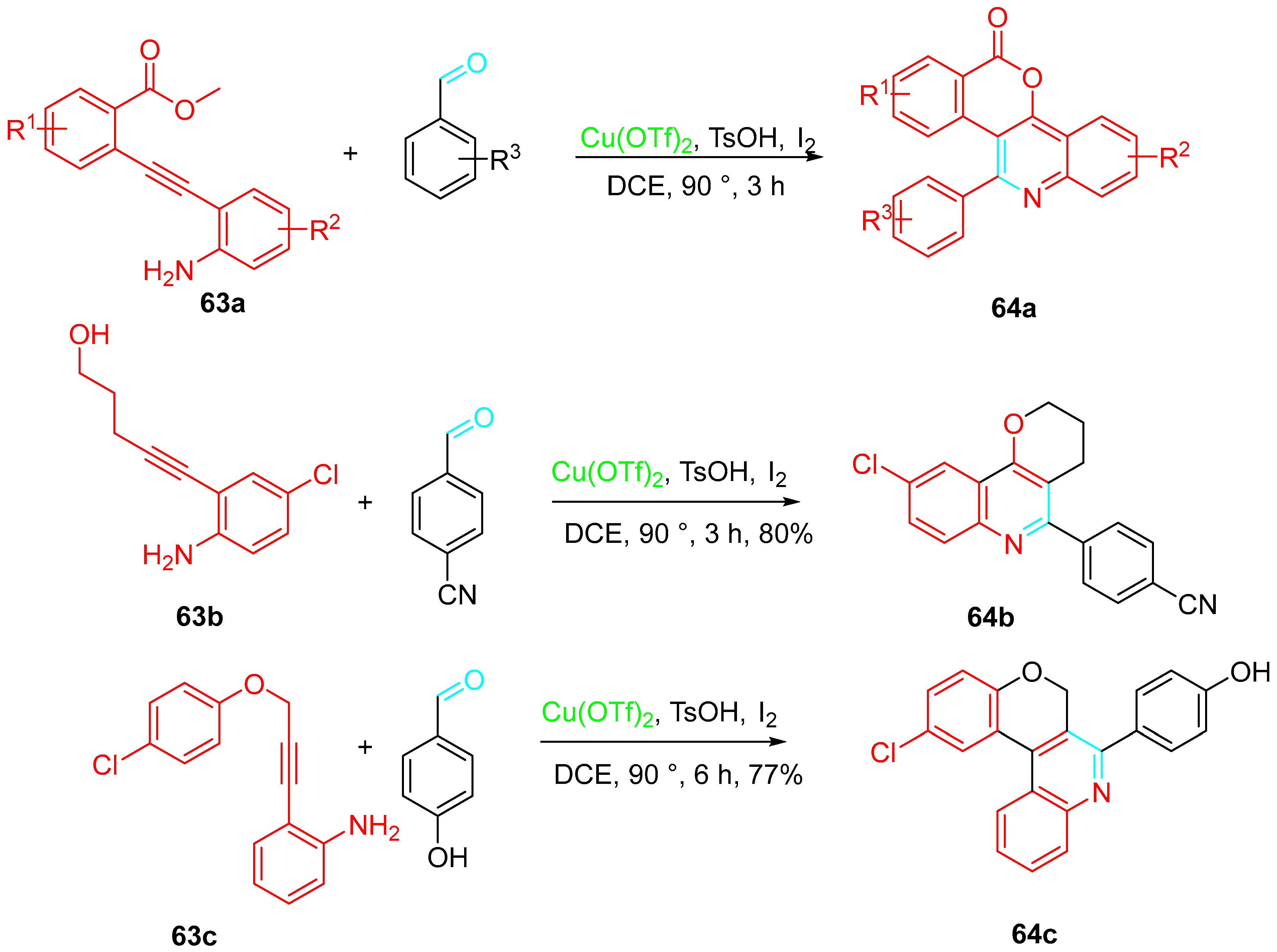
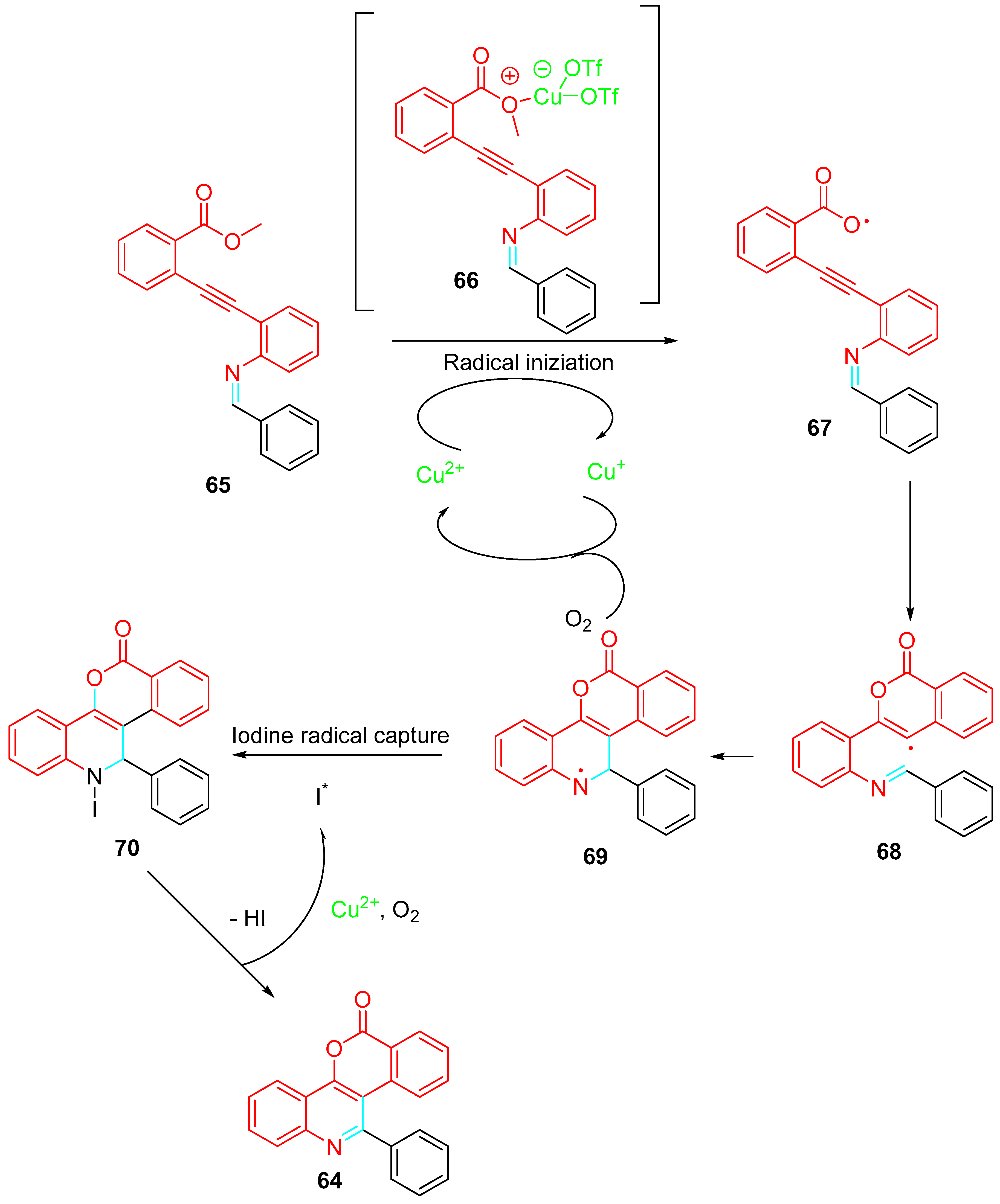
- Yuan, S.; Zhang, J.; Zhang, D.; Wei, D.; Zuo, J.; Song, J.; Yu, B.; Liu, H.-M. Cu(OTf)2-catalyzed intramolecular radical cascade reactions for the diversity-oriented synthesis of quinoline-annulated polyheterocyclic frameworks. Org. Lett. 2021, 23, 1445–1450. [Google Scholar] [CrossRef]
- Dhandabani, G.K.; Mutra, M.R.; Wang, J.-J. Palladium-catalyzed regioselective synthesis of 1-benzoazepine carbonitriles from o-alkynylanilines via 7-endo-dig annulation and cyanation. Adv. Synth. Catal. 2018, 360, 4754–4763. [Google Scholar] [CrossRef]
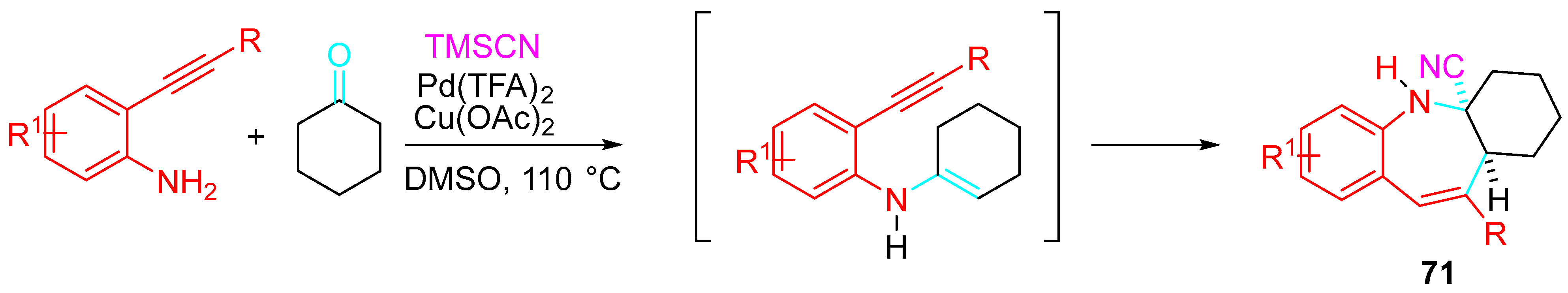
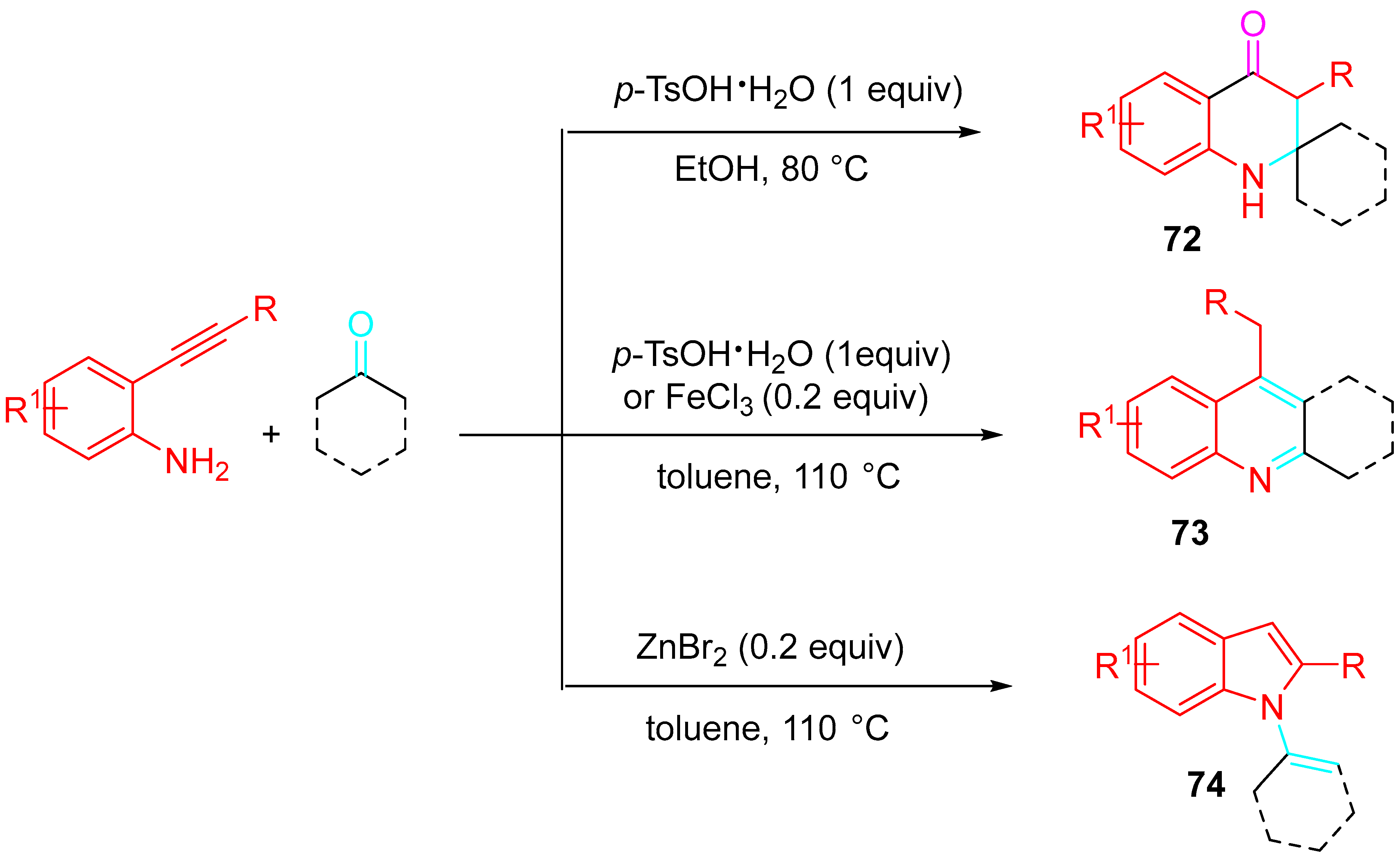
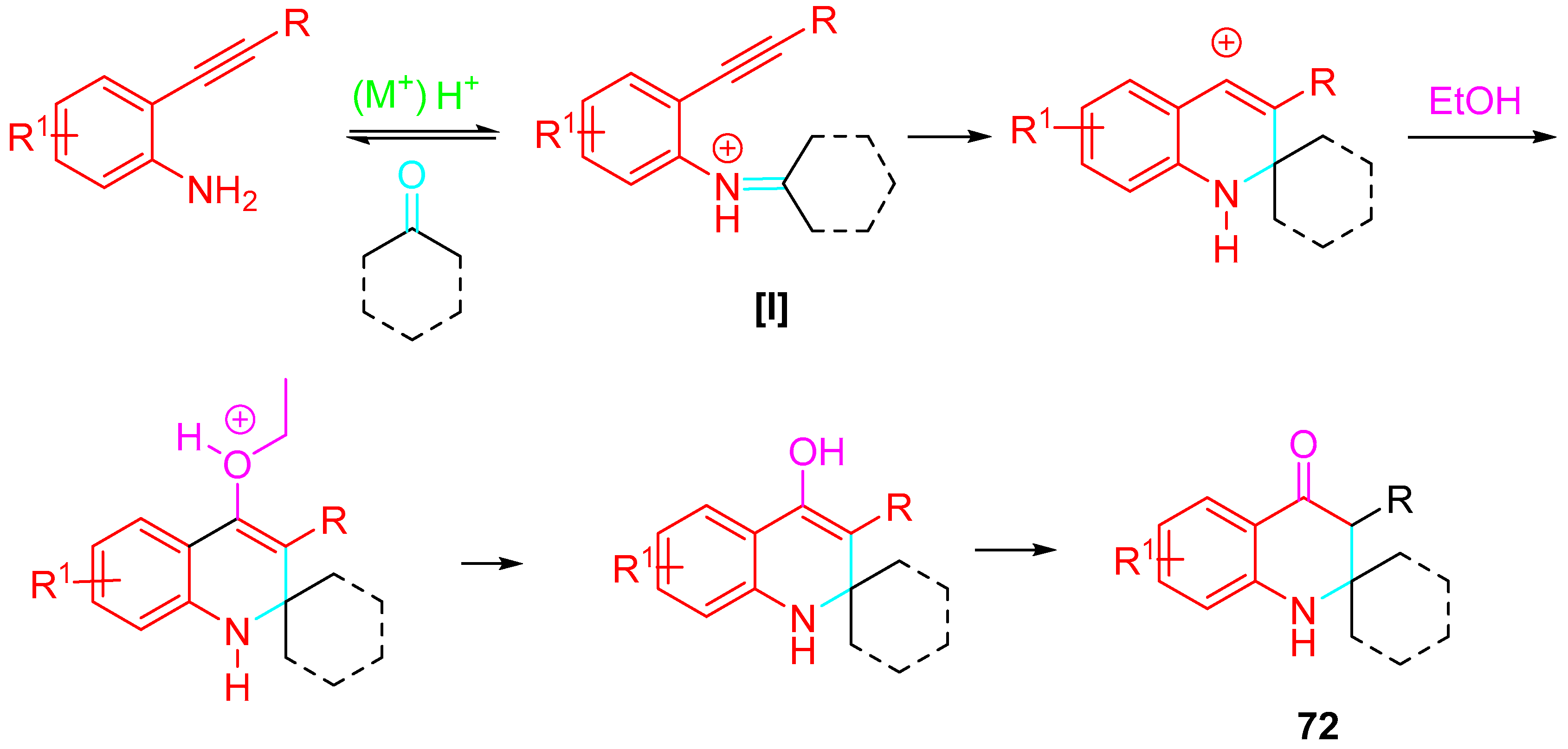
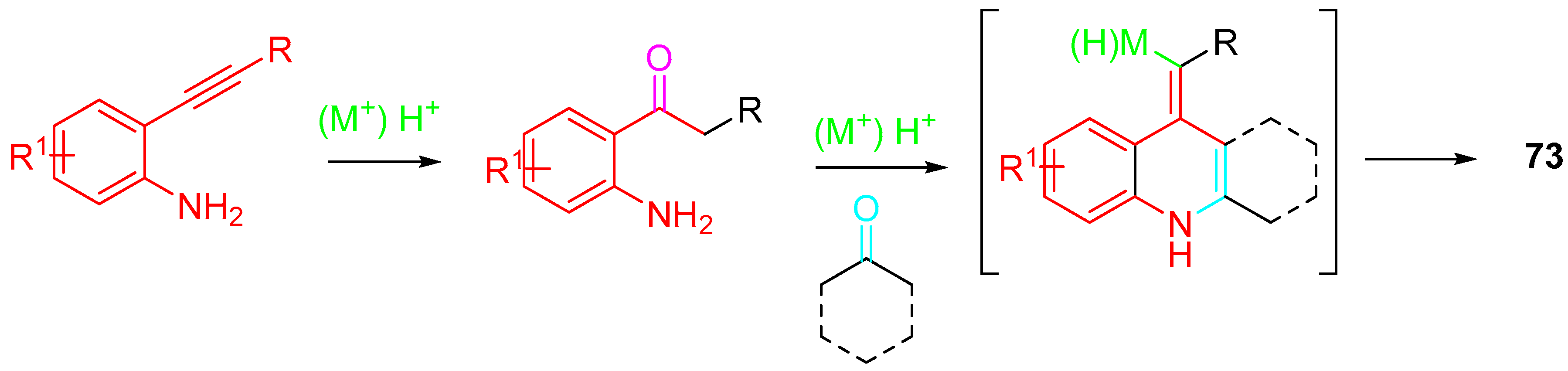
- Marsicano, V.; Arcadi, A.; Chiarini, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Synthesis of 2,2,3-substituted-2,3-dihydroquinolin-4(1H)-ones vs. quinoline or N-alkenylindole derivatives through sequential reactions of 2-alkynylanilines with ketones. Org. Biomol. Chem. 2021, 19, 421–438. [Google Scholar] [CrossRef]
- Dhandabani, G.K.; Shih, C.-L.; Wang, J.-J. Acid-promoted intramolecular decarbonylative coupling reactions of unstrained ketones: A modular approach to synthesis of acridines and diaryl ketones. Org. Lett. 2020, 22, 1955–1960. [Google Scholar] [CrossRef]
- Punjajom, K.; Ruengsangtongkul, S.; Tummatorn, J.; Paiboonsombat, P.; Ruchirawat, S.; Thongsornkleeb, C. Mn(OAc)3-mediated one-pot condensation-oxidative annulation of 2-alkynylanilines and 1,3-ketoesters: Synthesis of 2-substituted quinolines. J. Org. Chem. 2023, 88, 6736–6749. [Google Scholar] [CrossRef]
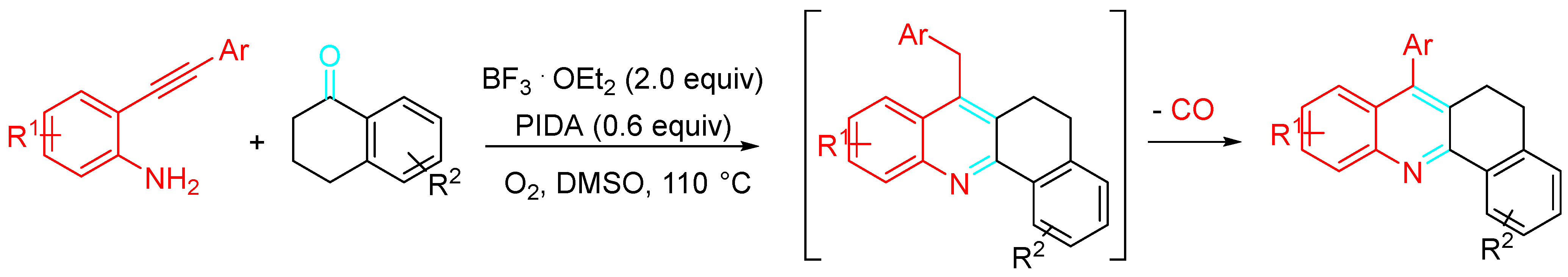
- Marsicano, V.; Chiarini, M.; Marinelli, F.; Arcadi, A. Synthesis of polycyclic quinolines by means of Brønsted acid mediated reaction of β-(2-aminophenyl)-α,β-ynones with ketones. Adv. Synth. Catal. 2019, 361, 2365–2370. [Google Scholar] [CrossRef]
- Gogoi, S.; Shekarrao, K.; Duarah, A.; Bora, T.C.; Gogoi, S.; Boruah, R.C. A microwave promoted solvent-free approach to steroidal quinolines and their in vitro evaluation for antimicrobial activities. Steroids 2012, 77, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, V.; Arcadi, A.; Chiarini, M.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A. Sequential condensation/biannulation reactions of β-(2-aminophenyl)-α,β-ynones with1,3-dicarbonyls. Org. Biomol. Chem. 2021, 19, 5177–5190. [Google Scholar] [CrossRef] [PubMed]
- Jash, M.; Das, B.; Chowdhury, C. One-pot access to benzo[a]carbazoles via palladium(II)-catalyzed hetero- and carboannulations. J. Org. Chem. 2016, 81, 10987–10999. [Google Scholar] [CrossRef]
- Abbiati, G.; Arcadi, A.; Chiarini, M.; Marinelli, F.; Pietropaolo, E.; Rossi, E. An alternative one-pot gold-catalyzed approach to the assembly of 11H-indolo-[3,2-c]quinolines. Org. Biomol. Chem. 2012, 10, 7801–7808. [Google Scholar] [CrossRef]
- Arcadi, A.; Chiarini, M.; D’Anniballe, G.; Marinelli, F.; Pietropaolo, E. Brønsted acid catalyzed cascade reactions of 2-[(2-aminophenyl)ethynyl]phenylamine derivatives with aldehydes: A new approach to the synthesis of 2,2′-disubstituted 1H,1′H-3,3′-biindoles. Org. Lett. 2014, 16, 1736–1739. [Google Scholar] [CrossRef]
- Qian, X.; Gao, H.-H.; Zhu, Y.-Z.; Lu, L.; Zheng, J.-Y. Biindole-based double D-π-A branched organic dyes for efficient dye-sensitized solar cells. RSC Adv. 2015, 5, 4368–4375. [Google Scholar] [CrossRef]
- Brambilla, E.; Gritti, A.; Pirovano, V.; Arcadi, A.; Germani, R.; Tiecco, M.; Abbiati, G. Acidic deep eutectics solvents as active media for a sustainable synthesis of biindoles starting from 2,2’-diaminotolanes and aldehydes. Eur. J. Org. Chem. 2023, e202300204. [Google Scholar] [CrossRef]
- Subba Reddy, B.V.; Swain, M.; Madhusudana Reddy, S.; Yadav, J.S.; Sridhar, B. Gold-catalyzed 5-endo-dig cyclization of 2-[(2-aminophenyl)-ethynyl]phenylamine with ketones for the synthesis of spiroindolone and indolo[3,2-c]quinolone scaffolds. Eur. J. Org. Chem. 2014, 3313–3318. [Google Scholar] [CrossRef]
- Yanada, R.; Hashimoto, K.; Tokizane, R.; Miwa, Y.; Minami, H.; Yanada, K.; Ishikura, M.; Takemoto, Y. Indium(III)-catalyzed tandem reaction with alkynylbenzaldehydes and alkynylanilines to heteroaromatic compounds. J. Org. Chem. 2008, 73, 5135–5138. [Google Scholar] [CrossRef]
- Bouma, M.J.; Masson, G.; Zhu, J. Exploiting the divergent reactivity of isocyanoacetates: One-pot three-component synthesis of functionalized angular furoquinolines. Eur. J. Org. Chem. 2012, 475–479. [Google Scholar] [CrossRef]
- Fayol, A.; Zhu, J. Synthesis of polysubstituted 4,5,6,7-tetrahydrofuro[2,3-c]pyridines by a novel multicomponent reaction. Org. Lett. 2004, 6, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; He, Y.; Gao, F.; Wei, Y.; Zhang, J.; Chen, M.; Gao, Y.; Zhang, Y.; Liu, J.-Y.; Guo, Z.; et al. Rapid access to C2-quaternary 3-methyleneindolines via base-mediated post-Ugi Conia-ene cyclization. Chem. Commun. 2023, 59, 3099–3102. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, U.; Borah, M.; Deka, M.J.; Saikia, A.K. Synthesis of tetrahydro-1H-indeno[1,2-b]pyridine via cascade cyclization and Friedel-Crafts reaction. J. Org. Chem. 2016, 81, 8736–8743. [Google Scholar] [CrossRef]
- Hu, Y.; Jia, Y.; Tuo, Z.; Zhou, W. Rhodium(III)-catalyzed intramolecular annulation and aromatization for the synthesis of pyrrolo[1,2-a]quinolines. Org. Lett. 2023, 25, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Che, C.-M. Highly efficient and regioselective platinum(II)-catalyzed tandem synthesis of multiply substituted indolines and tetrahydroquinolines. Angew. Chem. Int. Ed. 2009, 48, 2367–2371. [Google Scholar] [CrossRef]


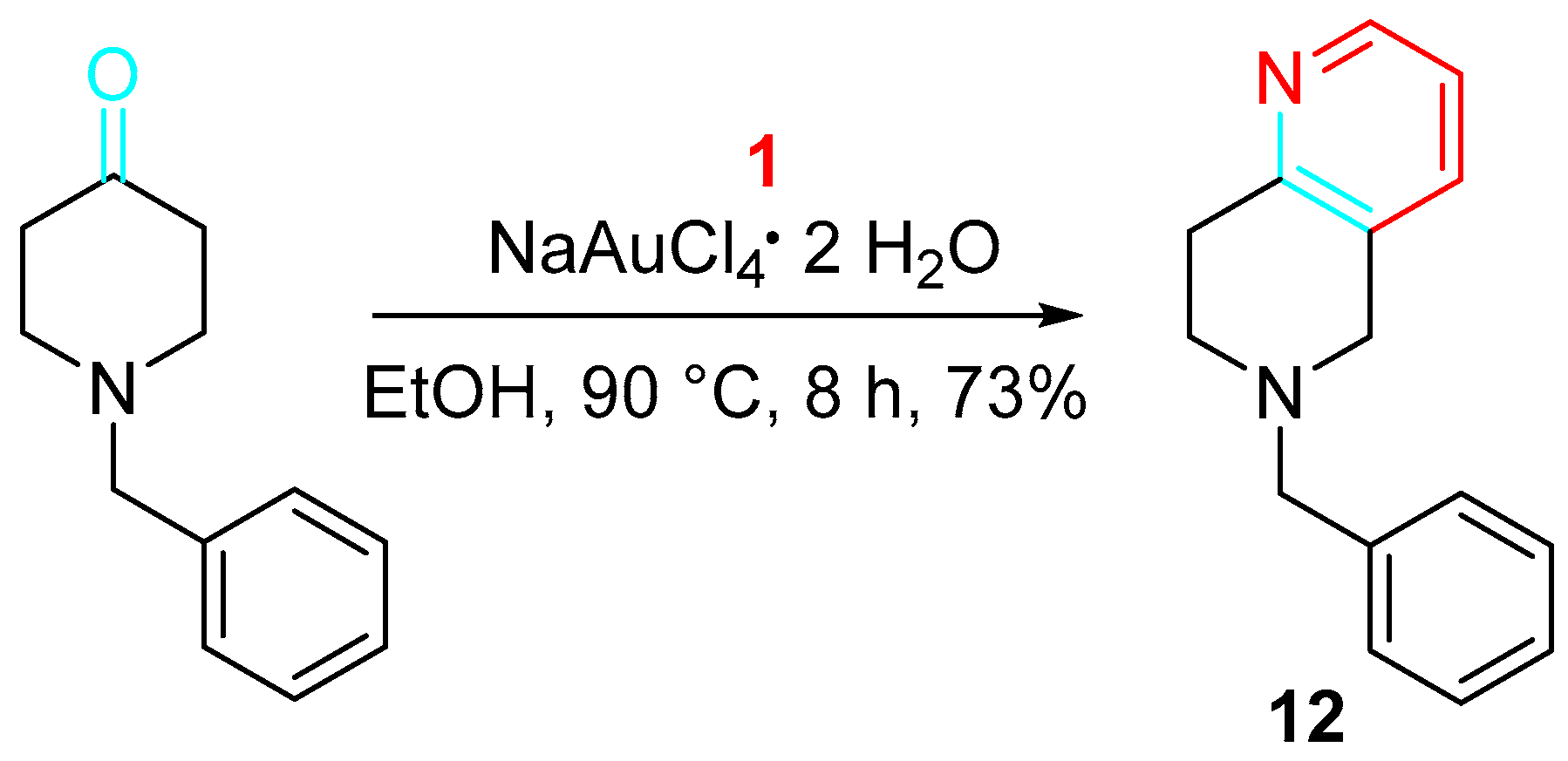
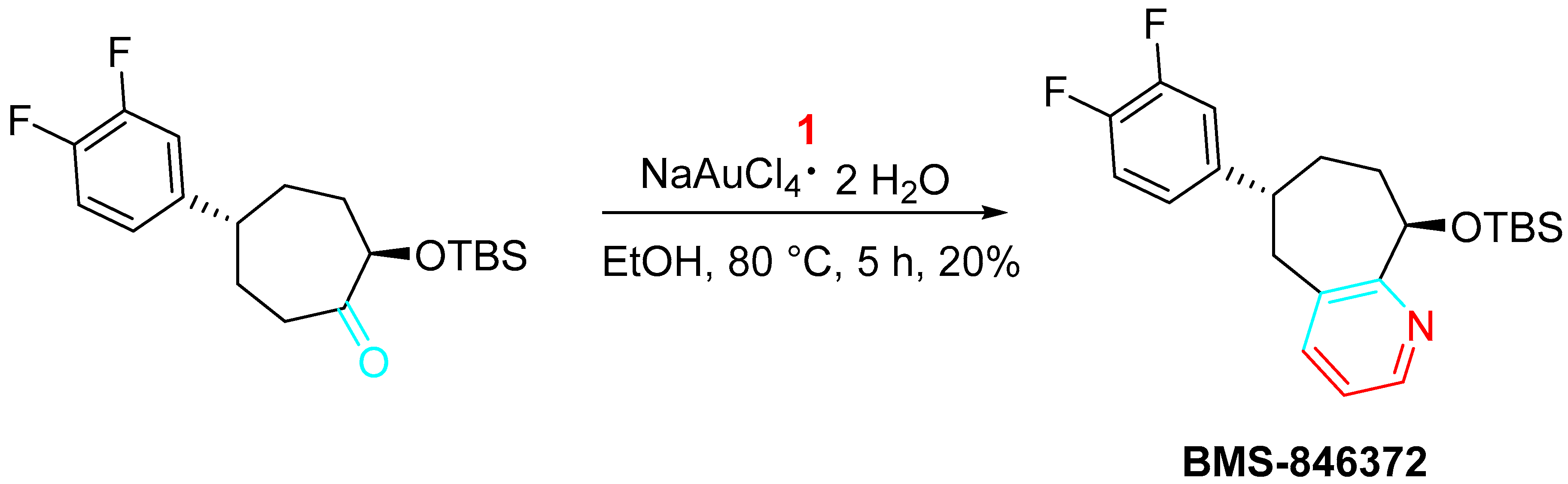

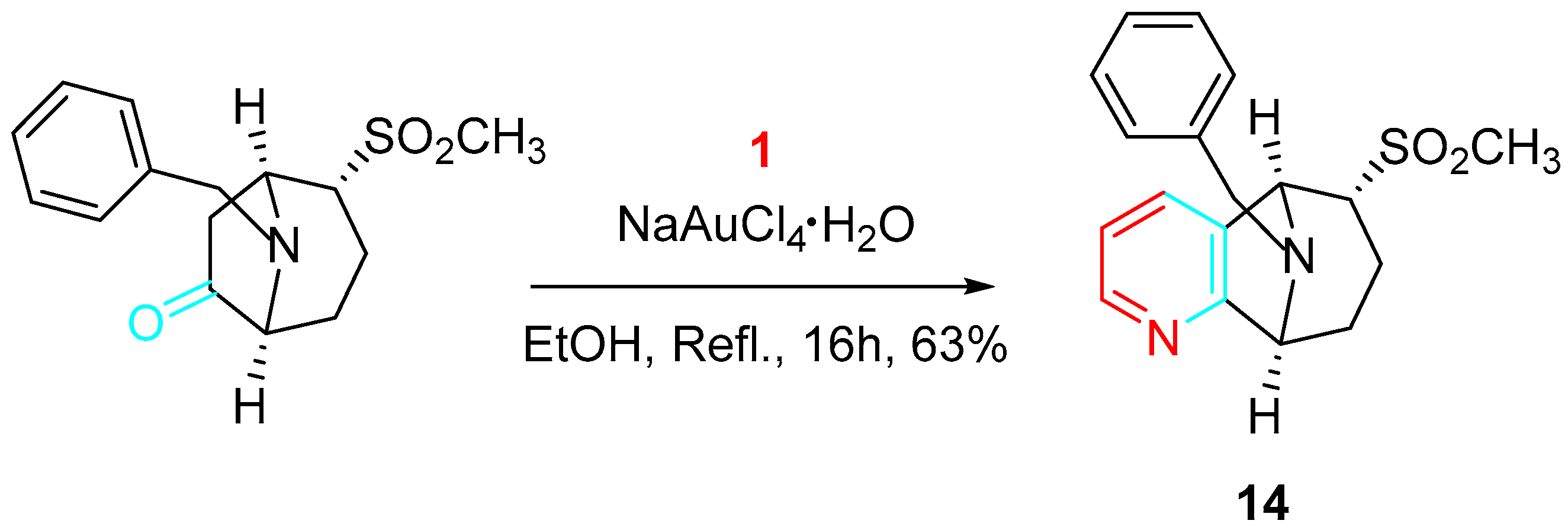

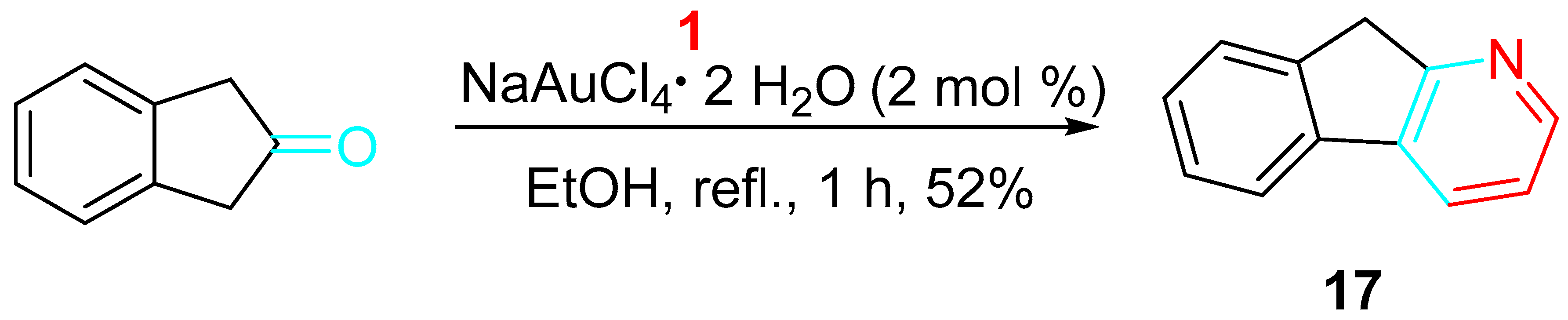






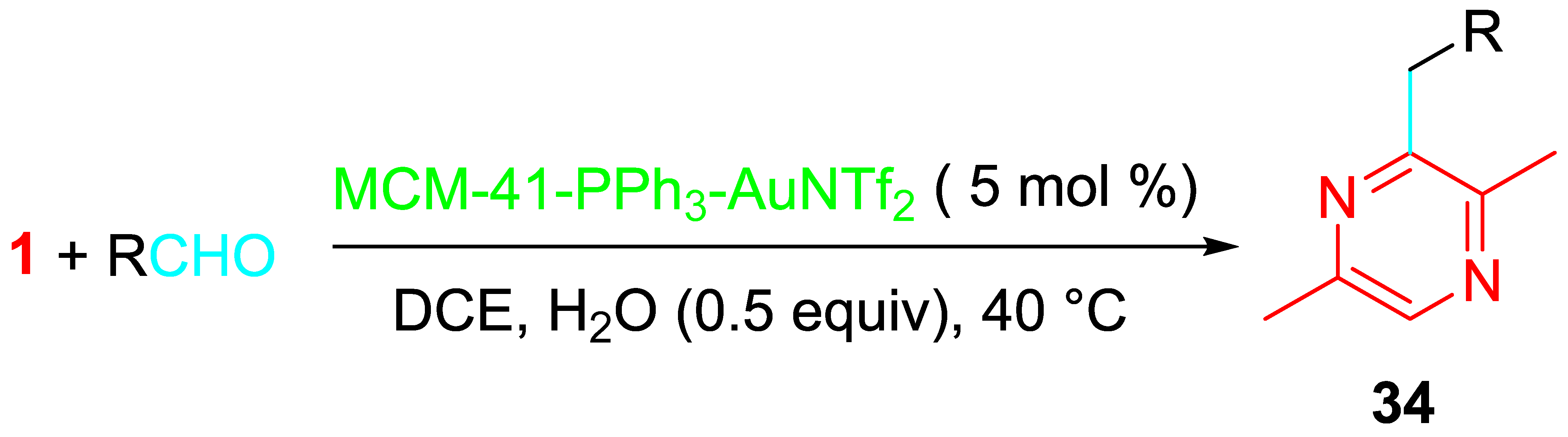

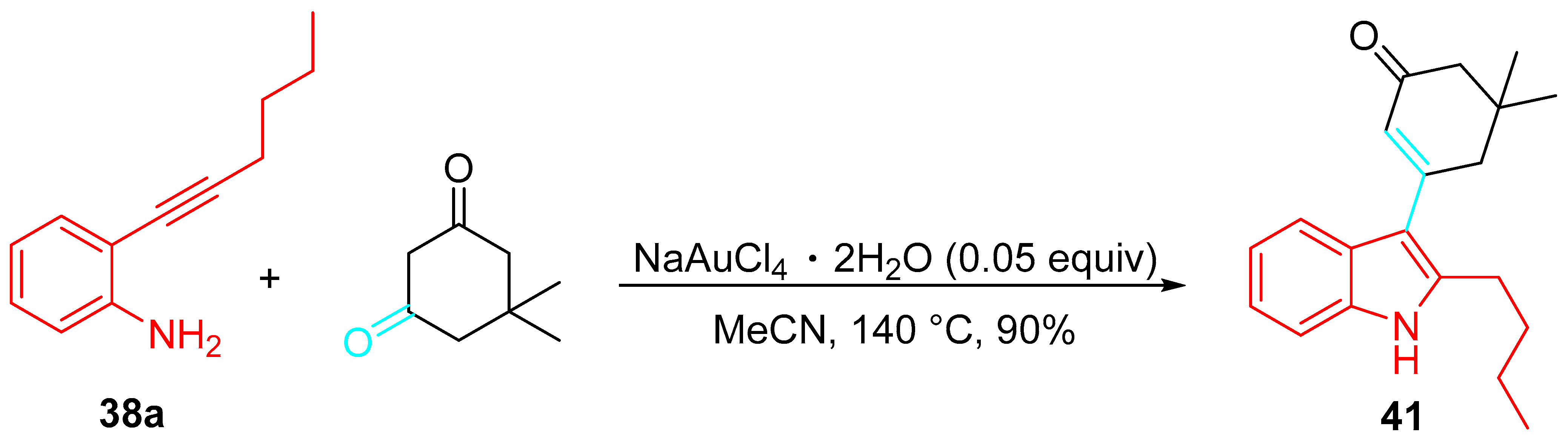
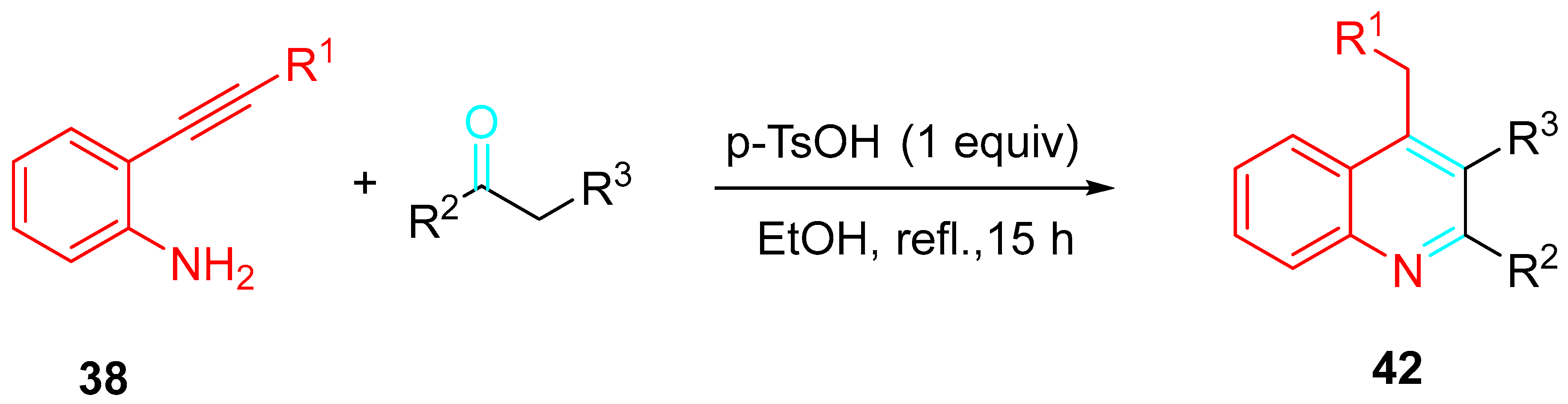

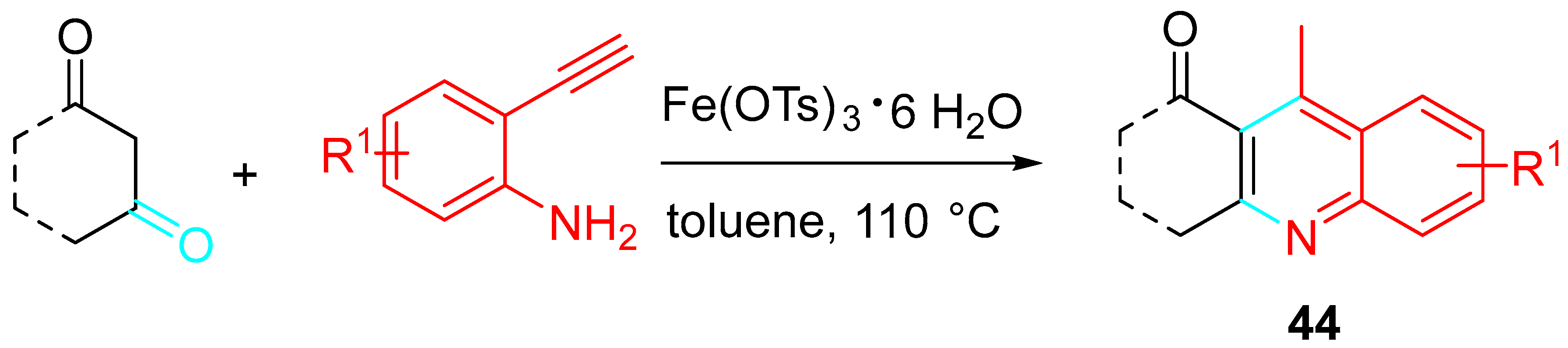

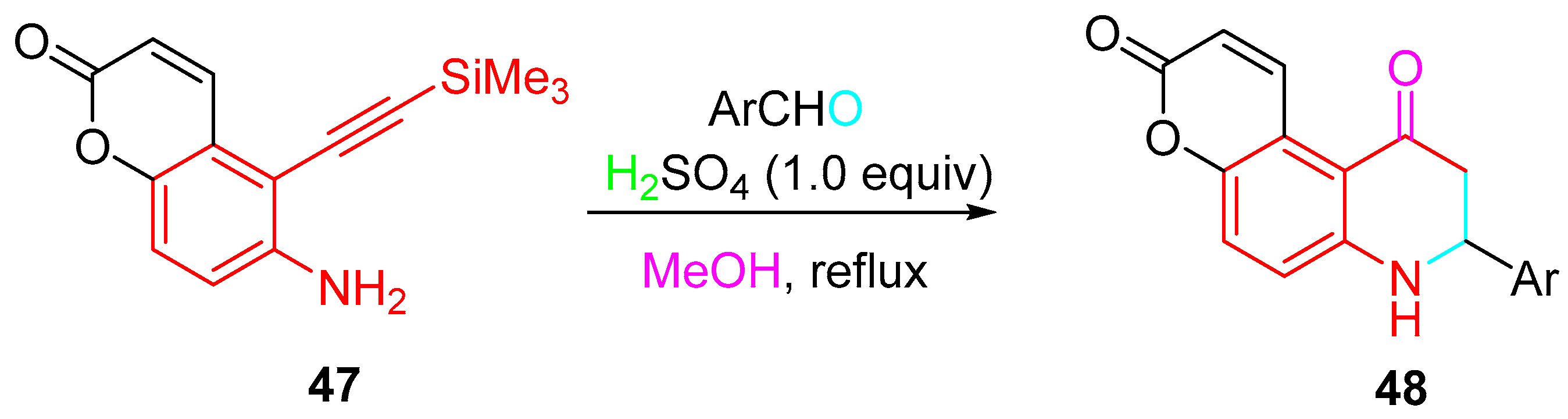











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcadi, A.; Morlacci, V.; Palombi, L. Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls. Molecules 2023, 28, 4725. https://doi.org/10.3390/molecules28124725
Arcadi A, Morlacci V, Palombi L. Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls. Molecules. 2023; 28(12):4725. https://doi.org/10.3390/molecules28124725
Chicago/Turabian StyleArcadi, Antonio, Valerio Morlacci, and Laura Palombi. 2023. "Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls" Molecules 28, no. 12: 4725. https://doi.org/10.3390/molecules28124725
APA StyleArcadi, A., Morlacci, V., & Palombi, L. (2023). Synthesis of Nitrogen-Containing Heterocyclic Scaffolds through Sequential Reactions of Aminoalkynes with Carbonyls. Molecules, 28(12), 4725. https://doi.org/10.3390/molecules28124725






