Effect of Boric Acid on the Ionization Equilibrium of α-Hydroxy Carboxylic Acids and the Study of Its Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Boric Acid on pH of Hydroxy Carboxylic Acid (HCA) Aqueous Solution
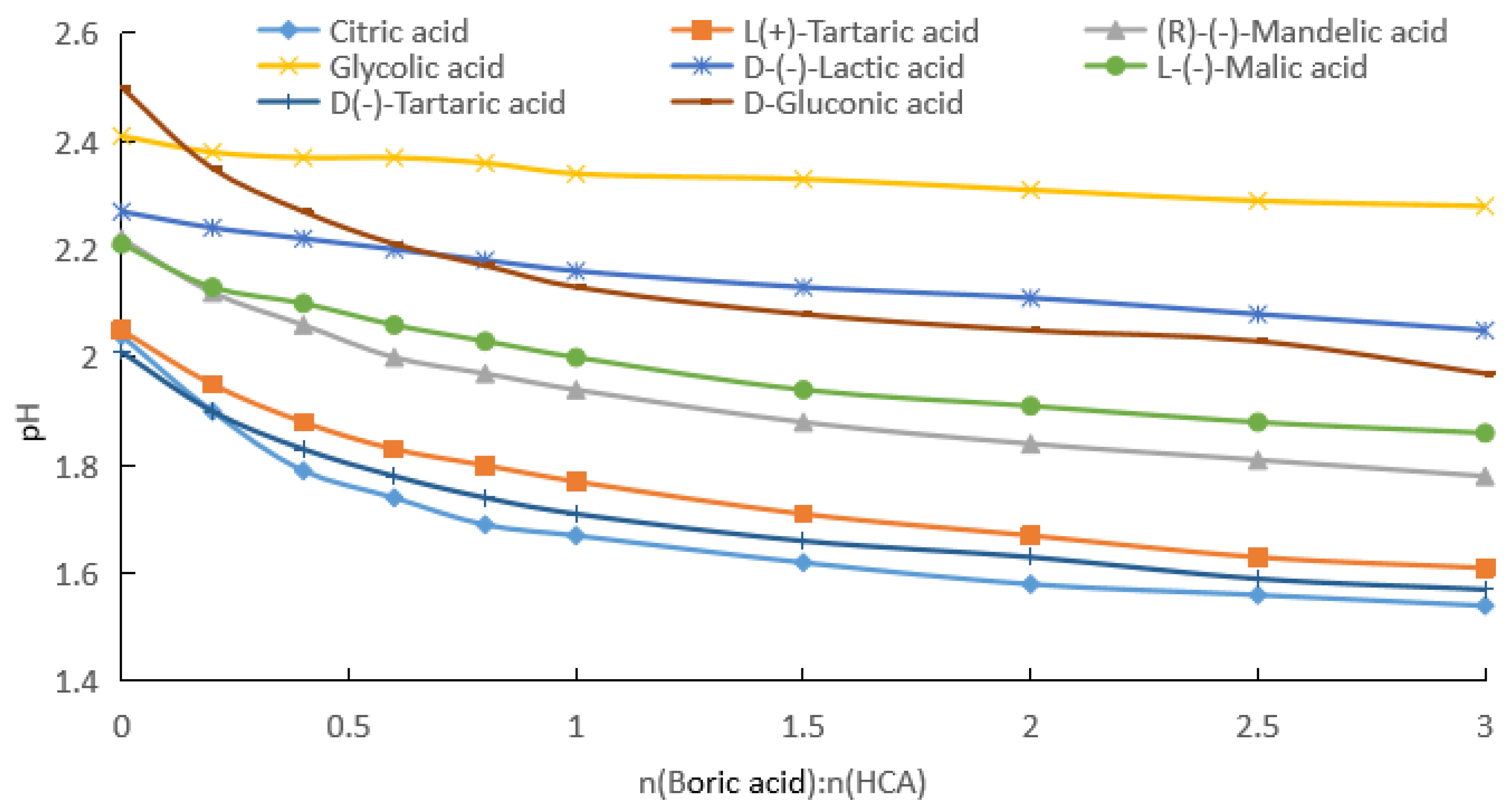
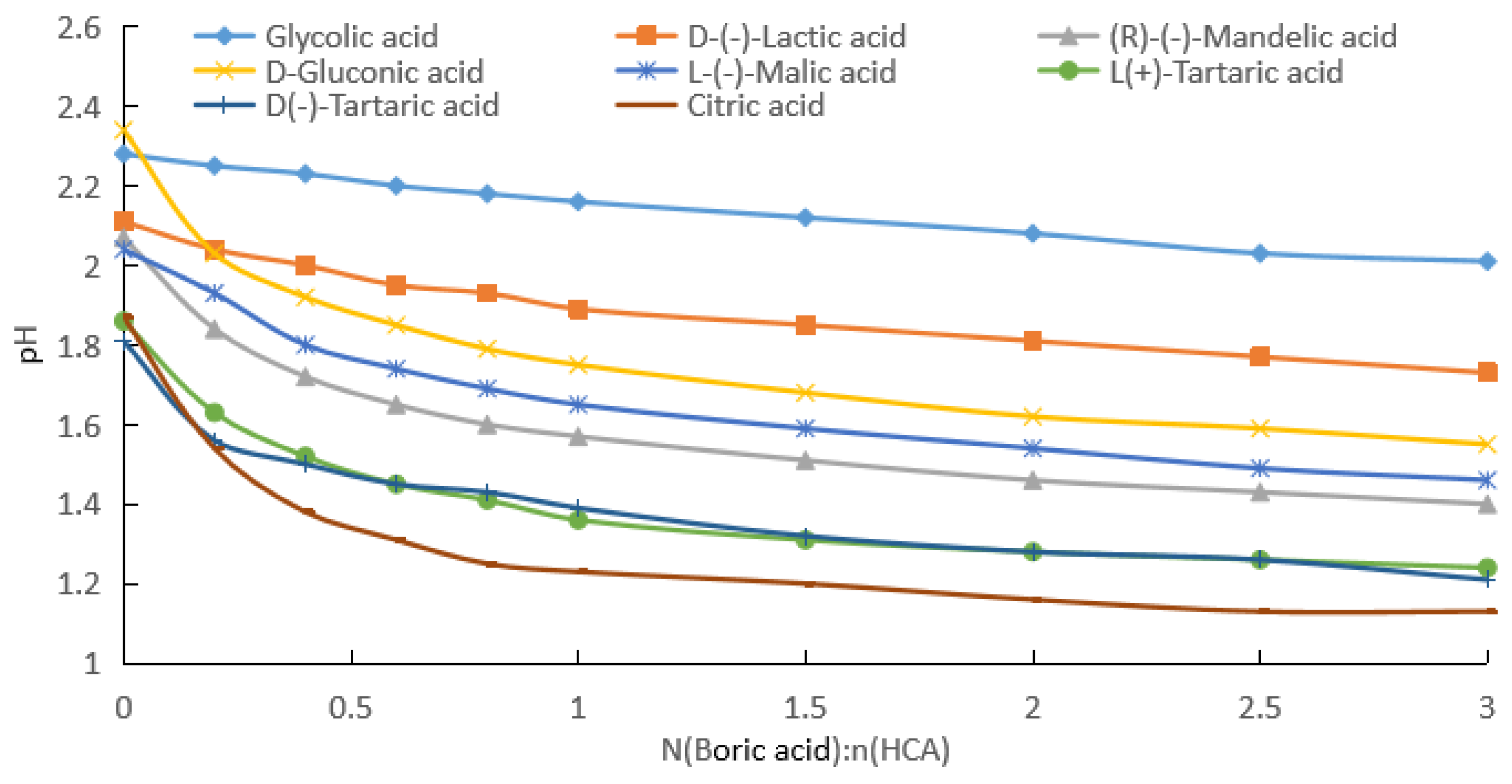
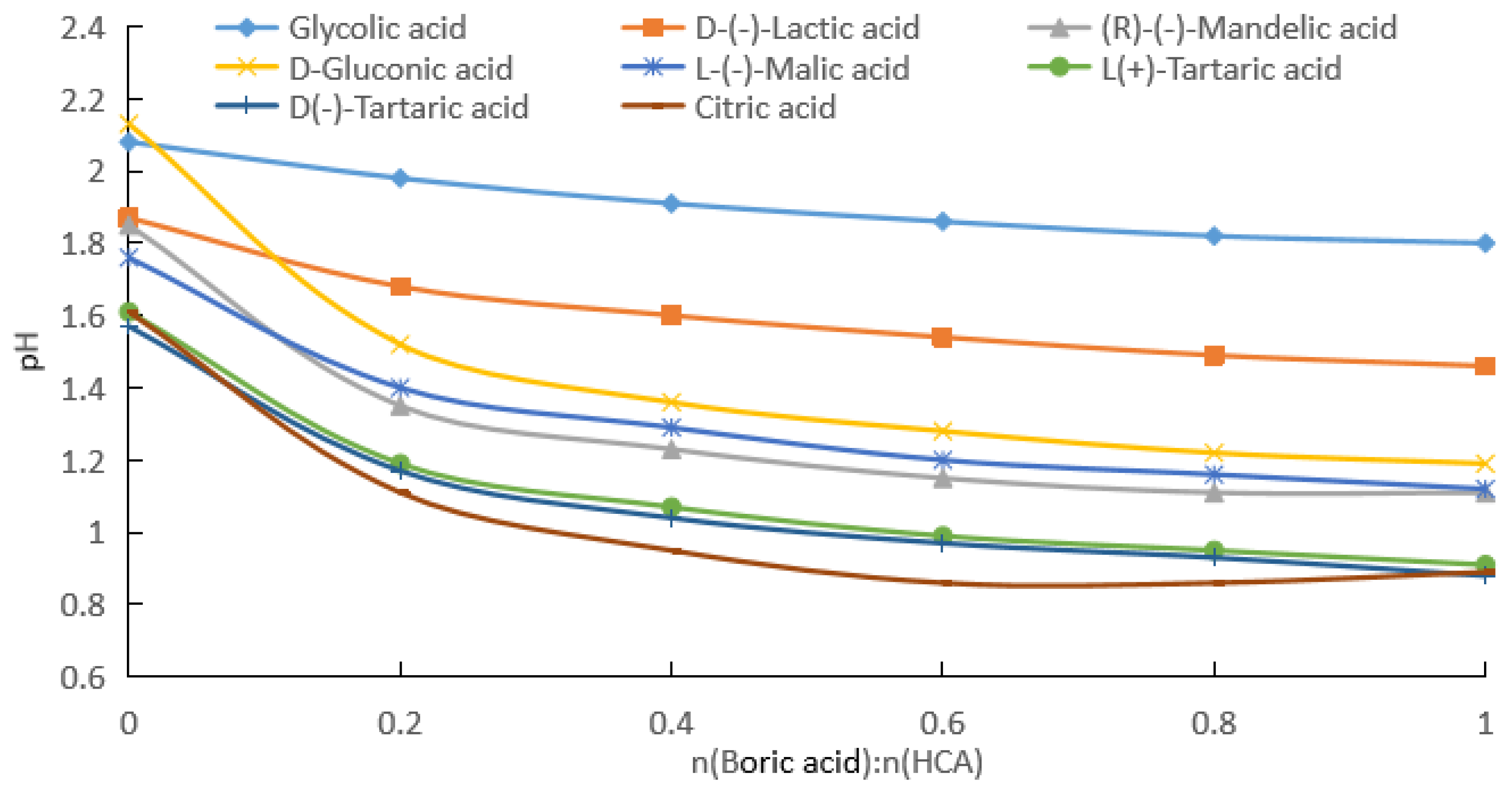
2.1.1. Measurement of pH after Addition of Boric Acid to Aqueous HCA Solution
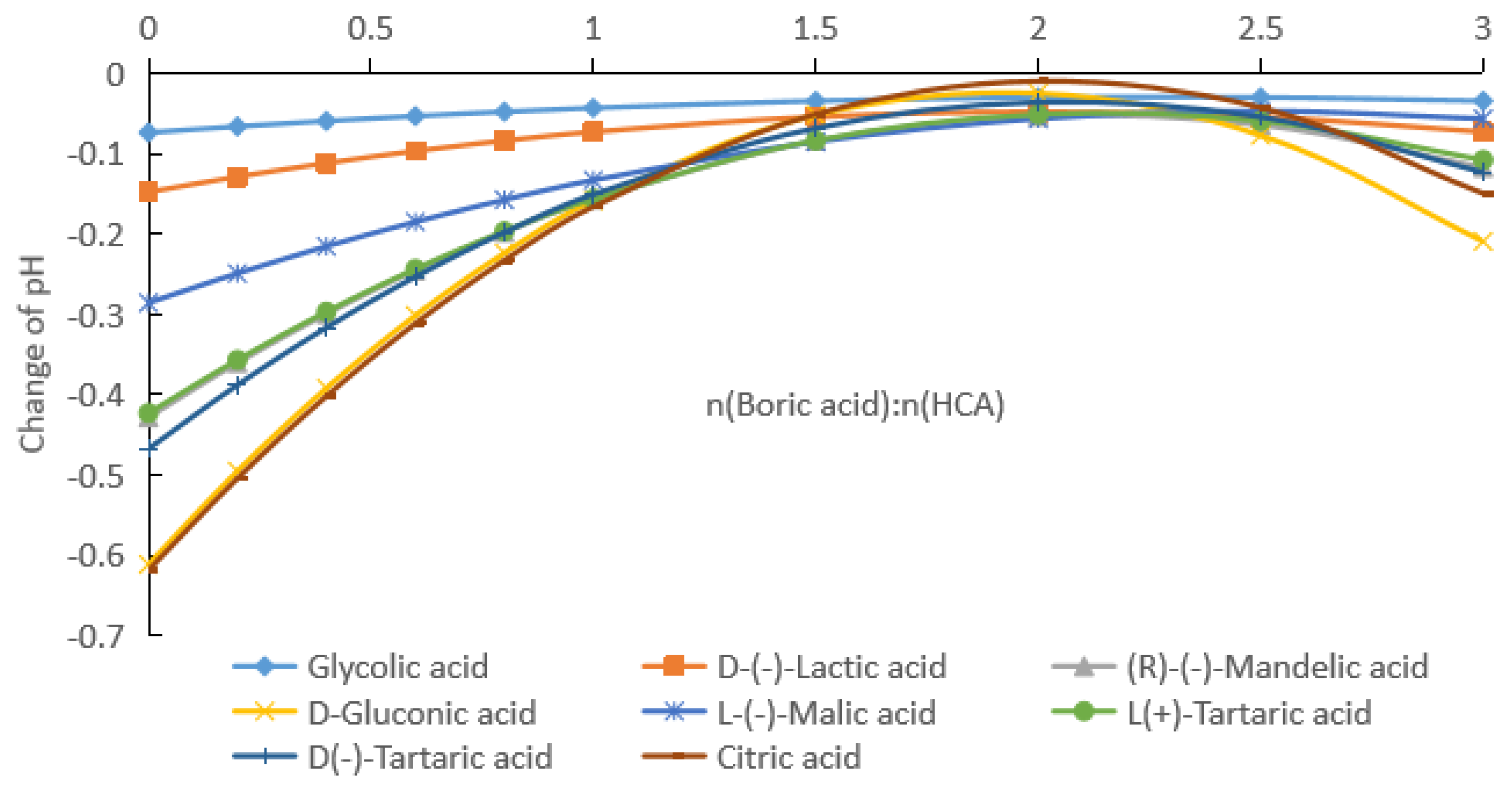
2.1.2. Rates of pH Change after Addition of Boric Acid to Aqueous HCA Solution
2.2. Comparison of Carboxylic Acids with Different Substituents
2.2.1. Comparative Experiments with Different Substituent Carboxylic Acids
2.2.2. Influence of HCA Molecular Structure
2.3. Complexation Reaction of Boric Acid with HCA
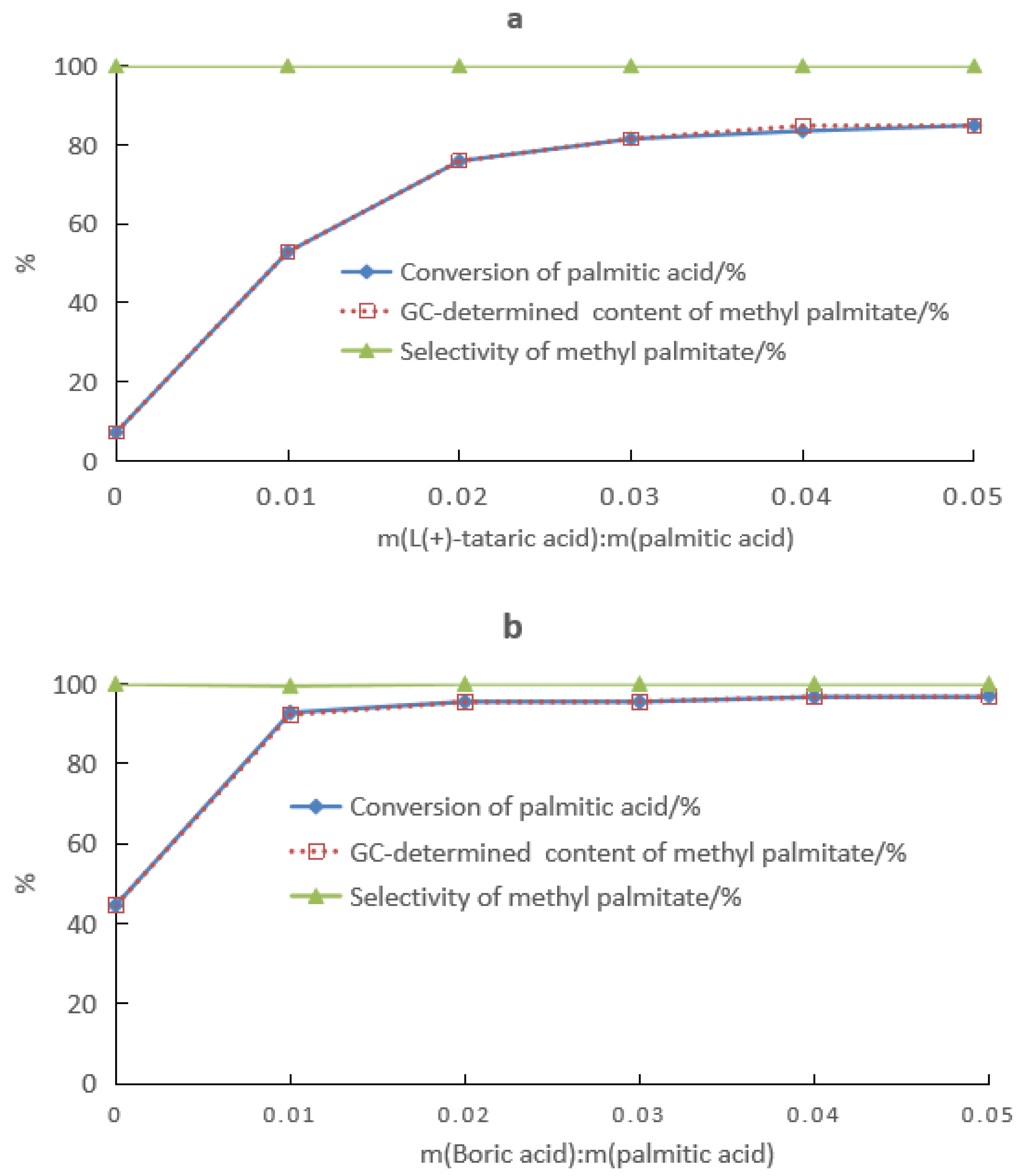
2.4. Fatty Acid Esterification Catalyzed by Boric Acid–HCA Complexes
3. Experimental Section
3.1. Materials and Apparatus
3.2. Experimental Methods
3.2.1. Determination of the pH of Boric Acid/HCA Mixtures
3.2.2. Fatty Acid Ester Synthesis
3.3. Analytical Methods
4. Conclusions
- (1)
- The effect of boric acid on the ionization balance of HCA was studied by analyzing and measuring the pH values of aqueous solutions of eight HCAs—glycolic acid, D-(−)-lactic acid, (R)-(−)-mandelic acid, D-gluconic acid, L-(−)-malic acid, L-(+) -tartaric acid, D-(−)-tartaric acid, and citric acid—after adding boric acid. The functions for the pH variations of the HCAs with the amount of boric acid at specified concentrations were obtained by polynomial fitting. By differentiating the fitted pH functions, the functions of the pH rate of change with the change in the boric acid dosage at specified concentrations were obtained. The complexation reactions of boric acid and HCAs were analyzed, and the formulas for calculating the ionization equilibrium constants of monoligand complexes and diligand complexes were deduced. The effects of these two complexes on the ionization equilibrium were compared.
- (2)
- Boric acid can react with the hydroxyl groups of HCA to form complexes, which promotes the ionization equilibrium of HCA to move in the positive direction. HCA molecules contain strong electron donor groups, and the boric acid complex became more stable with a greater proton donating ability. The acidities of the combination of boric acid and HCAs were in the following order: citric acid > tartaric acid > mandelic acid > malic acid > grape acid > lactic acid > glycolic acid.
- (3)
- The compound catalyst composed of tartaric acid and boric acid was used to catalyze the esterification of palmitic acid and methanol, and the yield of methyl ester was up to 98%. The short-chain alcohol was favorable to promote the dissolution of the complex catalyst. The catalyst was mainly present in the alcohol after the esterification reaction was finished, and the product ester was neutral. This facilitated the recovery of catalyst and excess alcohol, which was beneficial to environmentally friendly production.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Blevins, D.G.; Lukaszewski, K.M. Boron in plant structure and function. Annu. Rev. Plant Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; El-Hack, M.E.A.; Swelum, A.A.; Perillo, A.; Losacco, C. The vital roles of boron in animal health and production: A comprehensive review. J. Trace Elem. Med. Biol. 2018, 50, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Rebstöcková, M.; Bartušek, M. Reactions of boric acid with oxalic, glycolic and tartaric acids. Collect. Czechoslov. Chem. Commun. 1977, 42, 627–636. [Google Scholar] [CrossRef]
- Larsson, R.; Nunziata, G.; Pedersen, S.B.; Holme, D.; Srensen, N.A. An Infrared Spectroscopic Investigation on the Complexes formed between Boric Acid and Lactic Acid in Aqueous Solution. Acta Chem. Scand. 1970, 24, 2156–2168. [Google Scholar] [CrossRef]
- Pizer, R.; Selzer, R. The boric acid/lactic acid system. Equilibria and reaction mechanism. Inorg. Chem. 1984, 23, 3023–3026. [Google Scholar] [CrossRef]
- Maseda, M.; Miyazaki, Y.; Takamuku, T. Thermodynamics for complex formation of boric acid and borate with hydroxy acids and diols. J. Mol. Liq. 2021, 341, 117343. [Google Scholar] [CrossRef]
- Lopez, J.R.; Martin, L.; Wallis, J.D.; Akutsu, H.; Nakazawa, Y.; Yamada, J.-I.; Kadoya, T.; Coles, S.J.; Wilson, C. Enantiopure and racemic radical-cation salts of B(malate)2− anions with BEDT-TTF. Dalton Trans. 2016, 45, 9285–9293. [Google Scholar] [CrossRef]
- Fujita, T.; Yamamoto, T.; Morita, Y.; Chen, H.; Shimizu, Y.; Kanai, M. Chemo-and Enantioselective Pd/B Hybrid Catalysis for the Construction of Acyclic Quaternary Carbons: Migratory Allylation of O-Allyl Esters to α-C-Allyl Carboxylic Acids. J. Am. Chem. Soc. 2018, 140, 5899–5903. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, M.X.; Xu, Y.; Wang, L.J.; Qiao, X.Q. Determination of the enantiomers of salmeterol xinafoate in salmeterol fluticasone powder inhalant by chiral nonaqueous capillary electrophoresis. Chin. J. Chromatogr. 2021, 39, 1355–1361. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Jackson, P. Physicochemical studies of complex formation involving weak acids. Part IX. Complex formation between boric and tartaric acids. J. Chem. Soc. 1934, 1002–1010. [Google Scholar] [CrossRef]
- Tsuzuki, Y. The Nature of the Complex Formation between Boric Acid and Organic Polyoxy Compounds. Bull. Chem. Soc. Jpn. 1941, 16, 23–31. [Google Scholar] [CrossRef]
- Meng, Z.L.; Qin, R.X.; Wen, R.S.; Li, G.Q.; Liang, Z.Y.; Xie, J.K.; Yang, Z.Q.; Zhou, Y.H. Study on the Hydration of α-Pinene Catalyzed by α-Hydroxycarboxylic Acid-Boric Acid Composite Catalysts. Molecules 2023, 28, 3202. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.L.; Qin, R.X.; Wen, R.S.; Li, G.Q.; Liang, Z.Y.; Xie, J.K.; Zhou, Y.H.; Yang, Z.Q. Study on Synthesizing Isobornyl Acetate/Isoborneol from Camphene Using α-Hydroxyl Carboxylic Acid Composite Catalyst. Molecules 2023, 28, 1875. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Z. Synthesis of Fatty Acid Methyl Ester from Pistacia Chinensis Seed Oil Catalyzed by Magnetic Cellulose Microspheres; Northeast Forestry University: Harbin, China, 2019. [Google Scholar]
- Wang, T.R.; Cui, G.M.; Ma, Y. Preparation of biodiesel from oil with high acid value by one step method. J. Sol. Energy 2019, 3, 838–842. [Google Scholar]
- Su, Y.; Huang, G.L.; Lv, T.T. Preparation of biodiesel from castor oil catalyzed by binuclear acidic ionic liquid [DABCO-PS][HSO4]. China Oils Fats 2016, 10, 72–76. [Google Scholar]
- Xu, Y.J.; Yan, S.T.; Li, J.M.; Shou, F.Y.; Chen, Y.Q.; Yang, S.F.; Yao, L.Y.; Han, X.X. Preparation of biodiesel from palmitic acid catalyzed by silica-supported sulfonic acid functionalized ionic liquid. J. Chin. Cereals Oils 2019, 10, 42–48. [Google Scholar]
- Zhou, Y.S.; Mei, X.Y.; Cai, Z.; Wang, Q.; Duanmu, J.H.; Li, F.H.; Han, X.X. Preparation of biodiesel from palmitic acid catalyzed by metal oxides and its kinetics. J. Chin. Cereals Oils 2021, 2, 60–66. [Google Scholar]
- Talebian-Kiakalaieh, A.; Tarighi, S. Hierarchical faujasite zeolite-supported heteropoly acid catalyst for acetalization of crude-glycerol to fuel additives. J. Ind. Eng. Chem. 2019, 79, 452–464. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhang, S.; Feng, D.X.; Jian, F.F. Esterification of fatty acid in water with PCPs-PTA as catalyst. China Oils Fats 2017, 42, 78–81. [Google Scholar]
- Bao, C.J.; Jiang, S.C.; Deng, Y.L.; Liao, J.Y.; Li, R.; Tan, Y.Z.; Yu, J.X.; Han, X.X. Optimization of Preparation of Biodiesel from Palmitic Acid by Response Surface Methodology and Its Kinetics Study. J. Chin. Cereals Oils 2022, 37, 114–120. [Google Scholar]
- Saka, S.; Kusdiana, D. Biodisesl Fuel from Rapeseed Oil as Prepared in Supercritical Methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Santin, C.M.T.; Michelin, S.; Scherer, R.P.; Valério, A.; Oliveira, J.V. Comparison of Macauba and Soybean Oils as Substrates for the Enzymatic Biodiesel Production in Ultrasound-assisted System. Ultrason. Sonochem. 2016, 35, 525–528. [Google Scholar] [CrossRef] [PubMed]
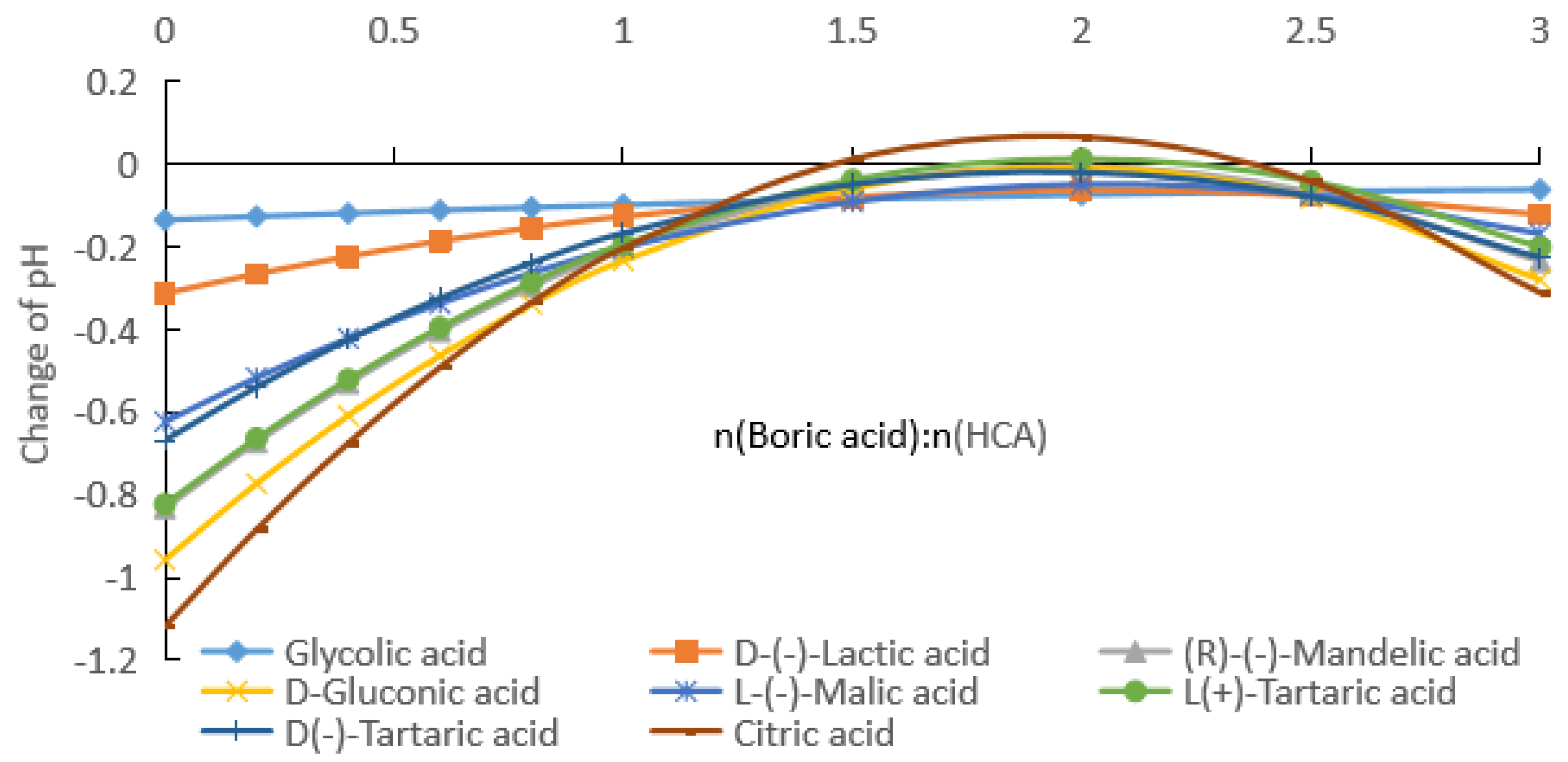
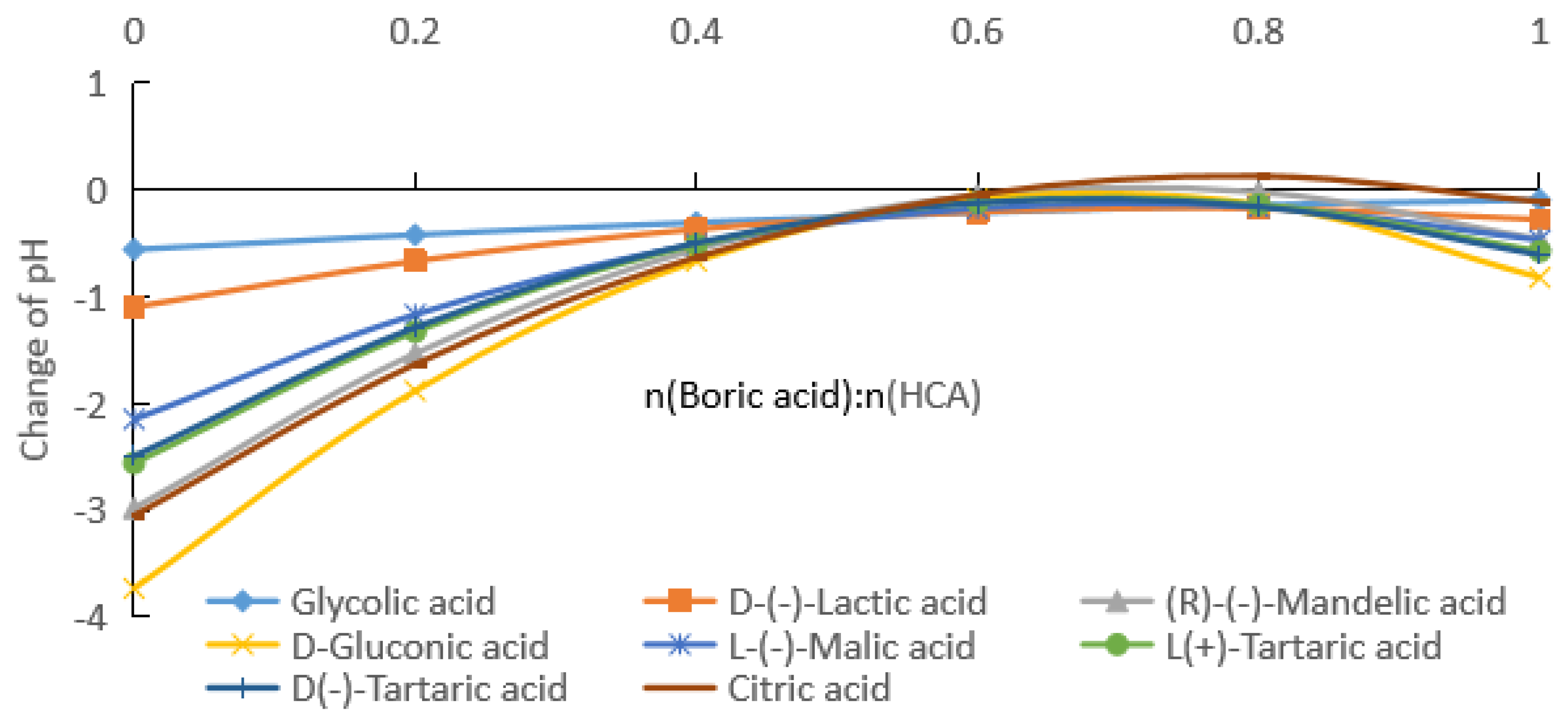

| Potency (mol/kg) | pKa | GA | D-(−)LA | R-(−)MA | D-GlcA | L-(−)H2Mi | L-(+)TA | D-(−)TA | CA |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | pKa (1:1) | 2.93 | 2.46 | 1.9 | 2.33 | 2.08 | 1.5 | 1.39 | 1.29 |
| pKa (1:2) | 1.86 | 1.6 | 0.67 | 1.21 | 0.87 | 0.11 | −0.05 | −0.21 | |
| 0.2 | pKa (1:1) | 3.53 | 2.47 | 1.72 | 2.09 | 1.89 | 1.28 | 1.84 | 1.09 |
| pKa (1:2) | 2.3 | 1.66 | 0.74 | 1.22 | 0.96 | 0.08 | 0.89 | −0.26 | |
| 0.5 | pKa (1:1) | 3.05 | 2.22 | 1.36 | 1.61 | 1.47 | 0.93 | 0.87 | 0.72 |
| pKa (1:2) | 2.7 | 1.83 | 0.83 | 1.14 | 0.97 | 0.26 | 0.17 | −0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Chen, H.; Wen, R.; Li, G.; Meng, Z. Effect of Boric Acid on the Ionization Equilibrium of α-Hydroxy Carboxylic Acids and the Study of Its Applications. Molecules 2023, 28, 4723. https://doi.org/10.3390/molecules28124723
Qin R, Chen H, Wen R, Li G, Meng Z. Effect of Boric Acid on the Ionization Equilibrium of α-Hydroxy Carboxylic Acids and the Study of Its Applications. Molecules. 2023; 28(12):4723. https://doi.org/10.3390/molecules28124723
Chicago/Turabian StyleQin, Rongxiu, Haiyan Chen, Rusi Wen, Guiqing Li, and Zhonglei Meng. 2023. "Effect of Boric Acid on the Ionization Equilibrium of α-Hydroxy Carboxylic Acids and the Study of Its Applications" Molecules 28, no. 12: 4723. https://doi.org/10.3390/molecules28124723
APA StyleQin, R., Chen, H., Wen, R., Li, G., & Meng, Z. (2023). Effect of Boric Acid on the Ionization Equilibrium of α-Hydroxy Carboxylic Acids and the Study of Its Applications. Molecules, 28(12), 4723. https://doi.org/10.3390/molecules28124723






