Effect of Low-Dose Line-Spectrum and Full-Spectrum UV on Major Humoral Components of Human Blood
Abstract
1. Introduction
2. Results and Discussion
- study of the effects of low-dose line-spectrum UV (mercury lamp) on the fluorescence of aromatic amino acids and albumin, oxidative modification of albumin, transport properties of albumin, antioxidant properties of albumin, globulins, uric acid, a mixture of uric acid and albumin, and blood plasma;
- study of the effects of low-dose full-spectrum UV (flash xenon lamp) on the oxidative modification of albumin, its transport and antioxidant properties.
2.1. Mercury Lamp as a Source of UV Radiation
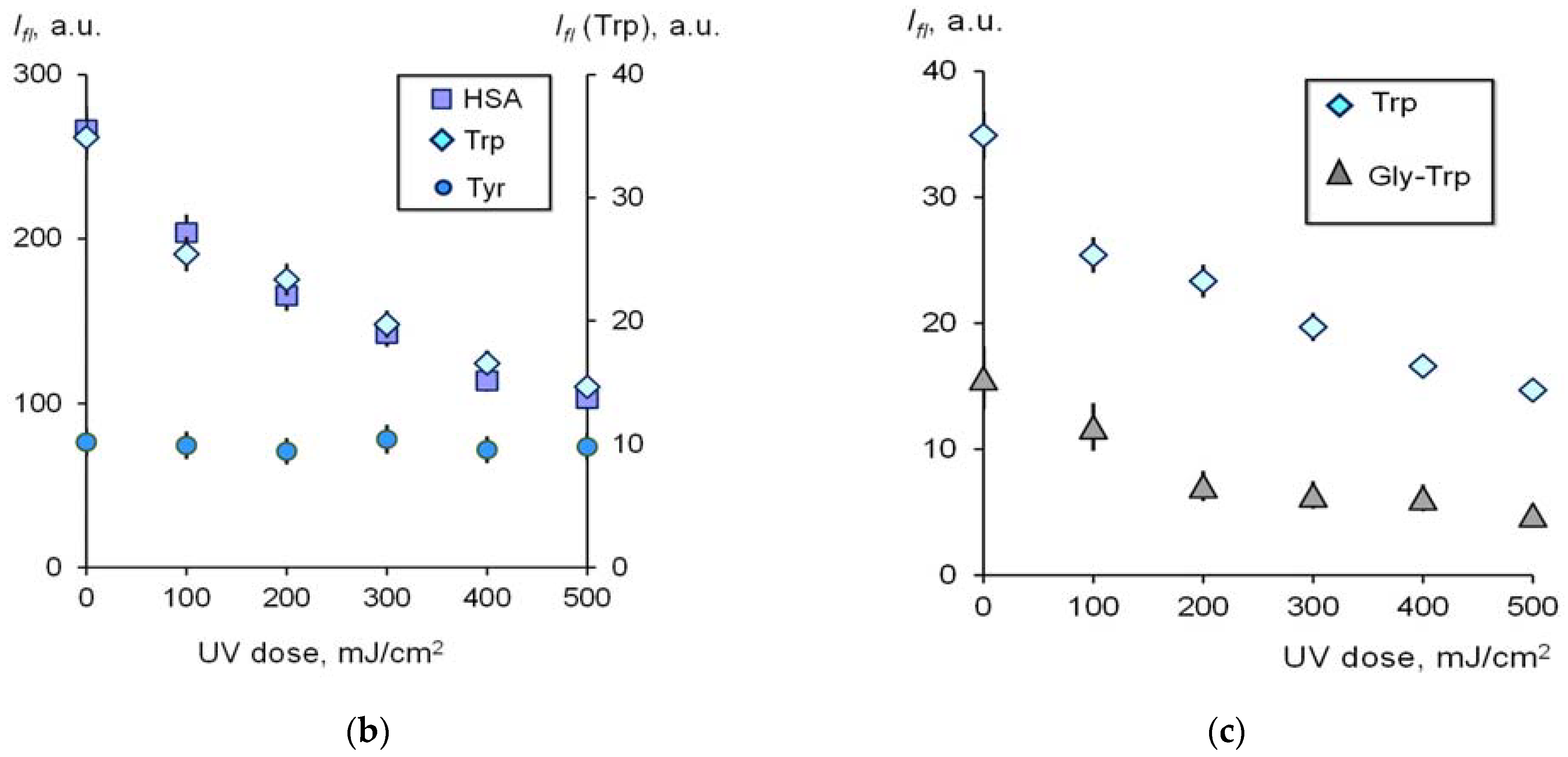
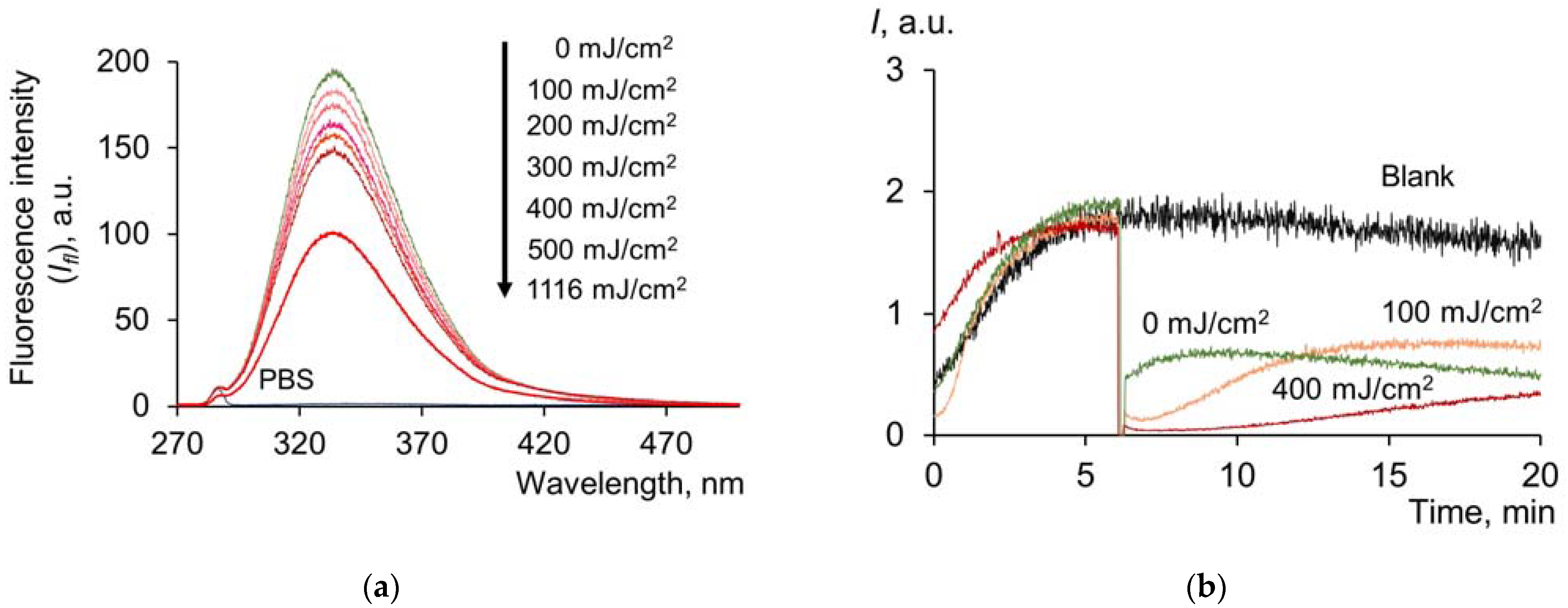
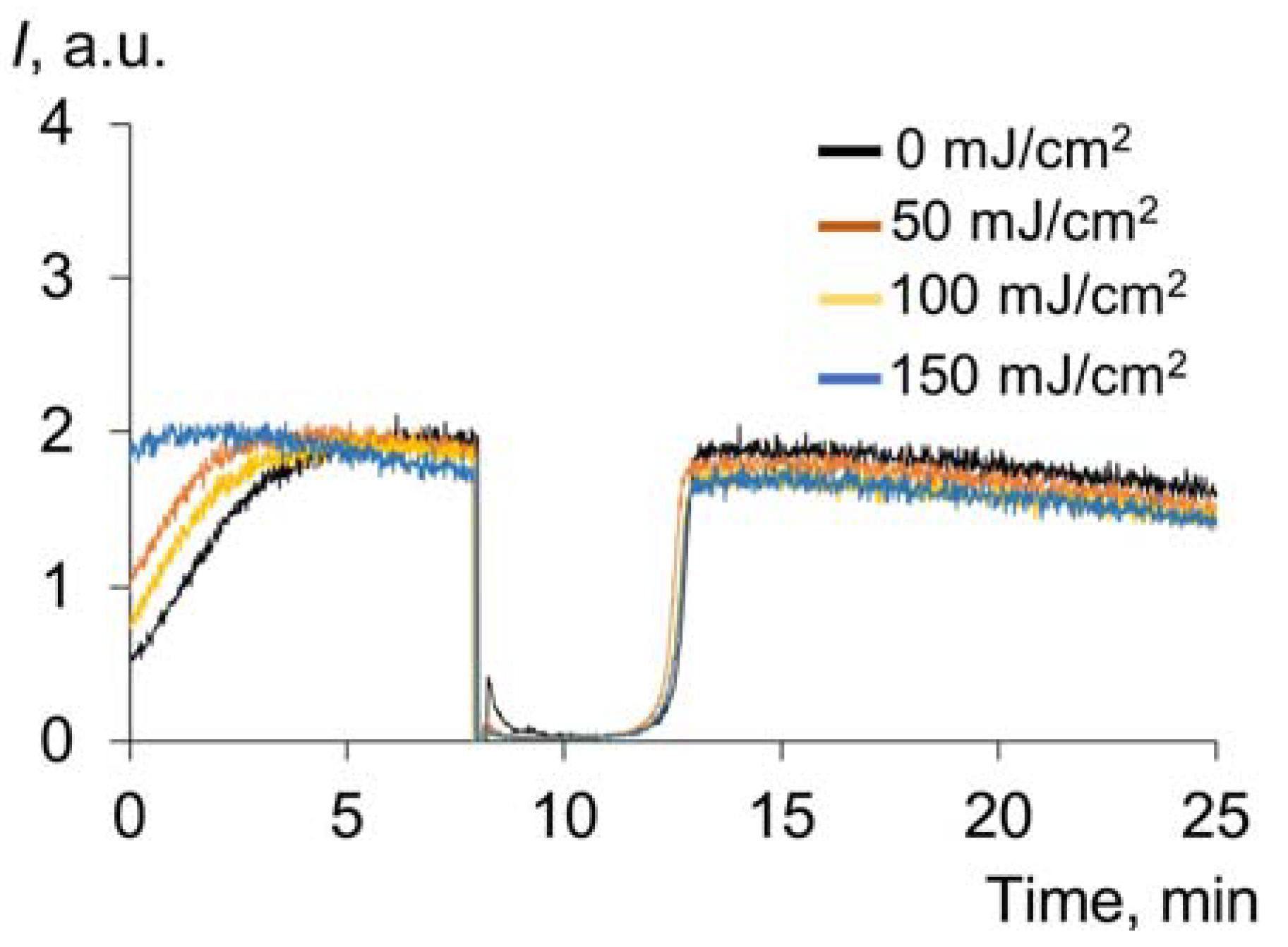
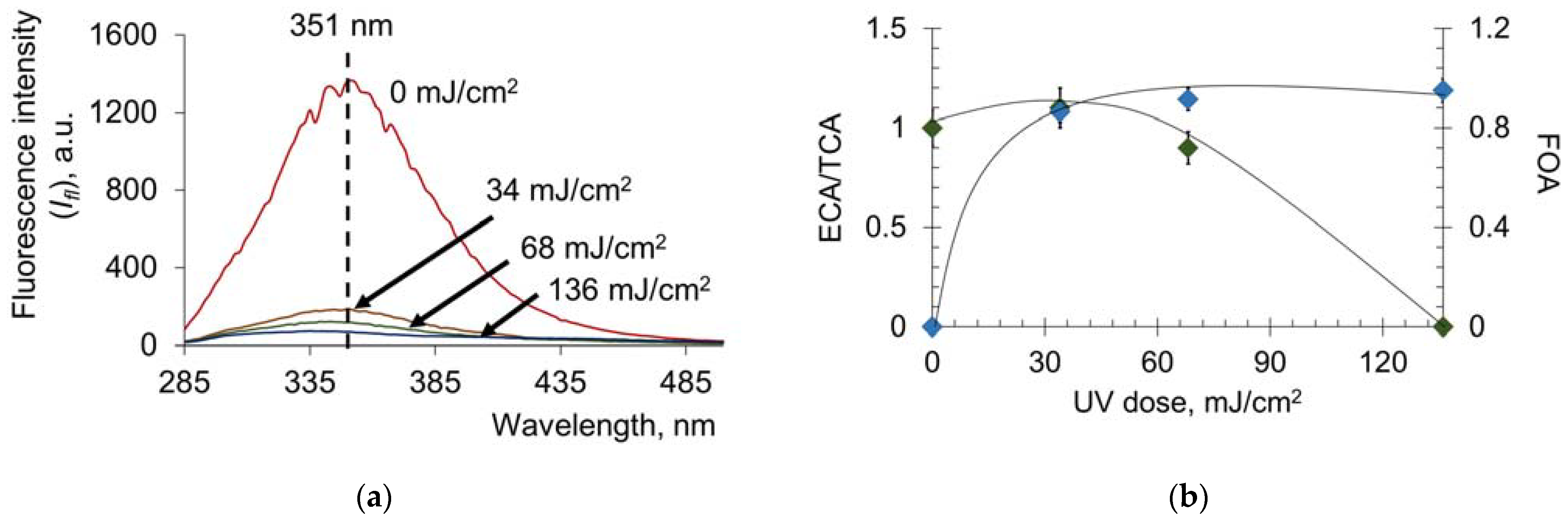
2.1.1. Contribution of Tyrosine and Tryptophan to UV-Induced Oxidative Modification of HSA
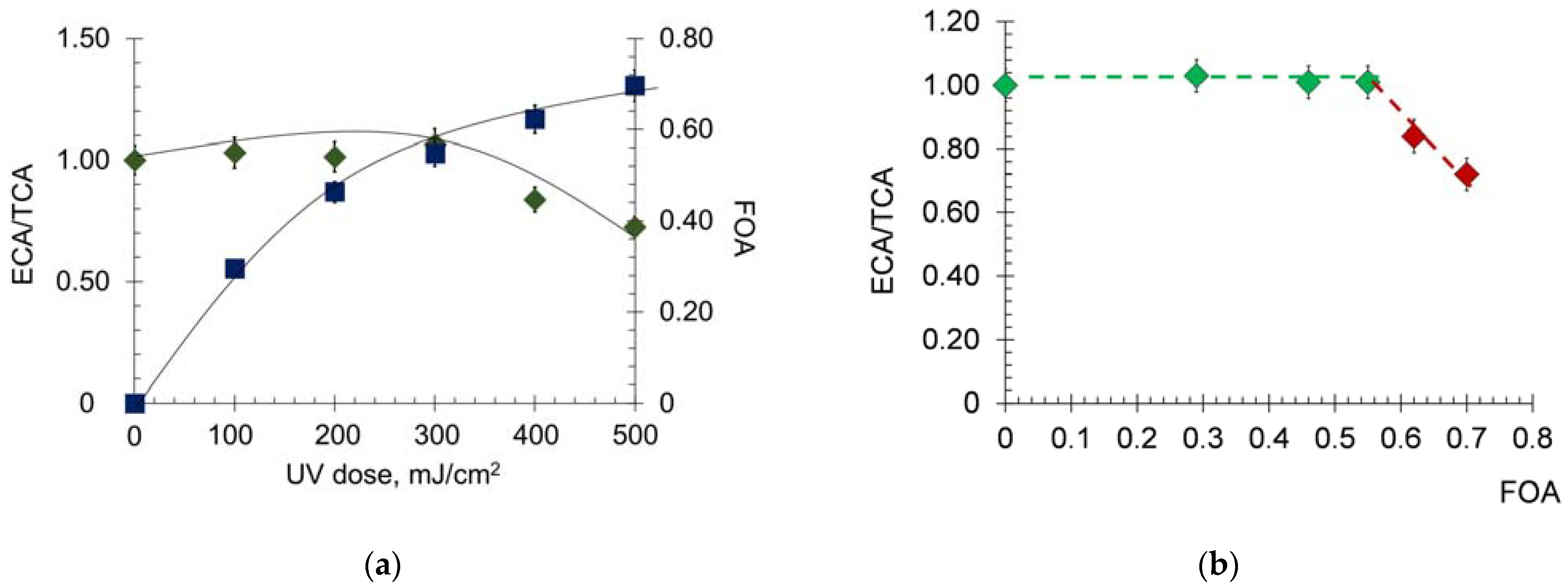
2.1.2. Effect of UV on the Transport Properties of Human Serum Albumin
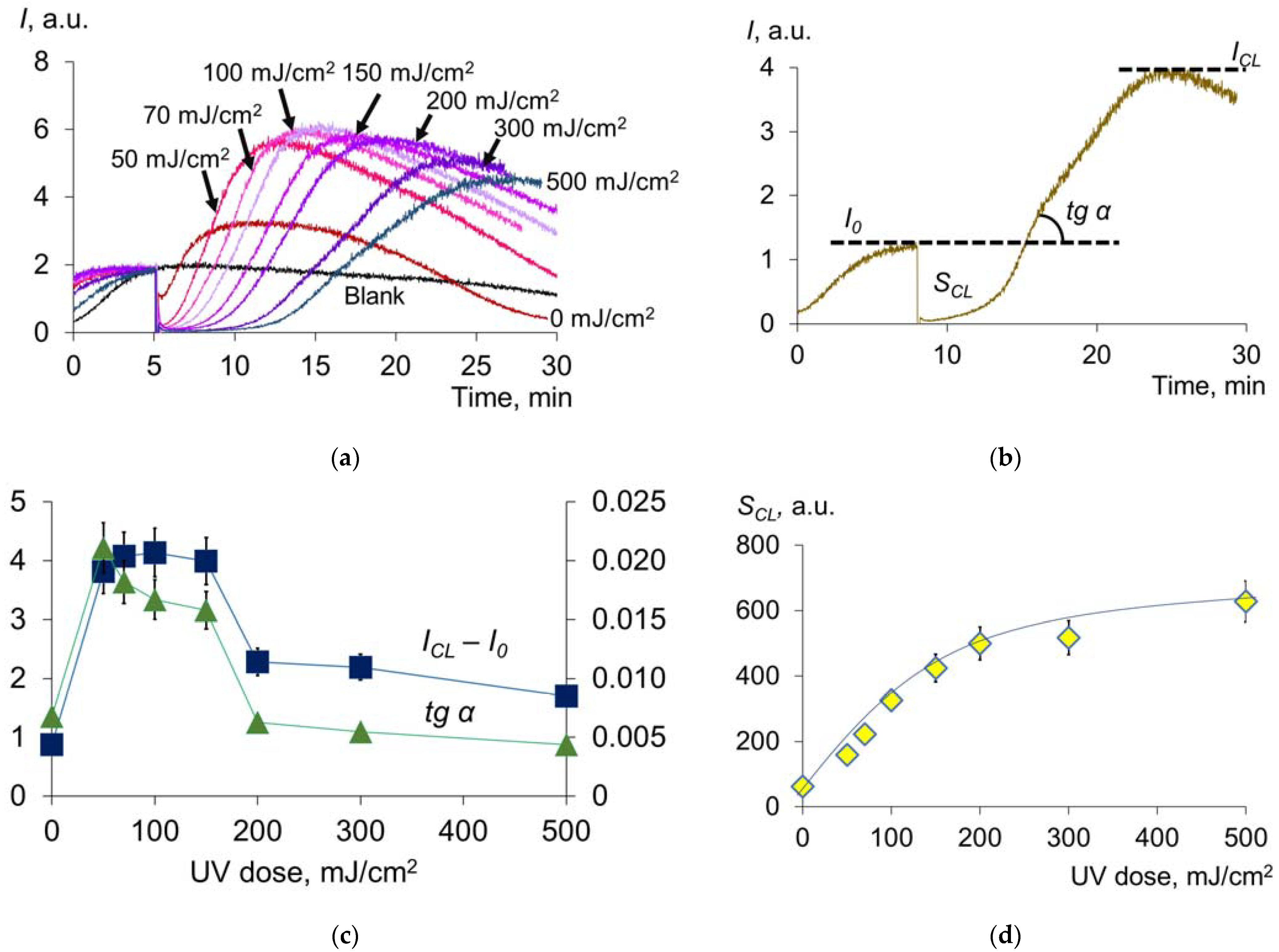
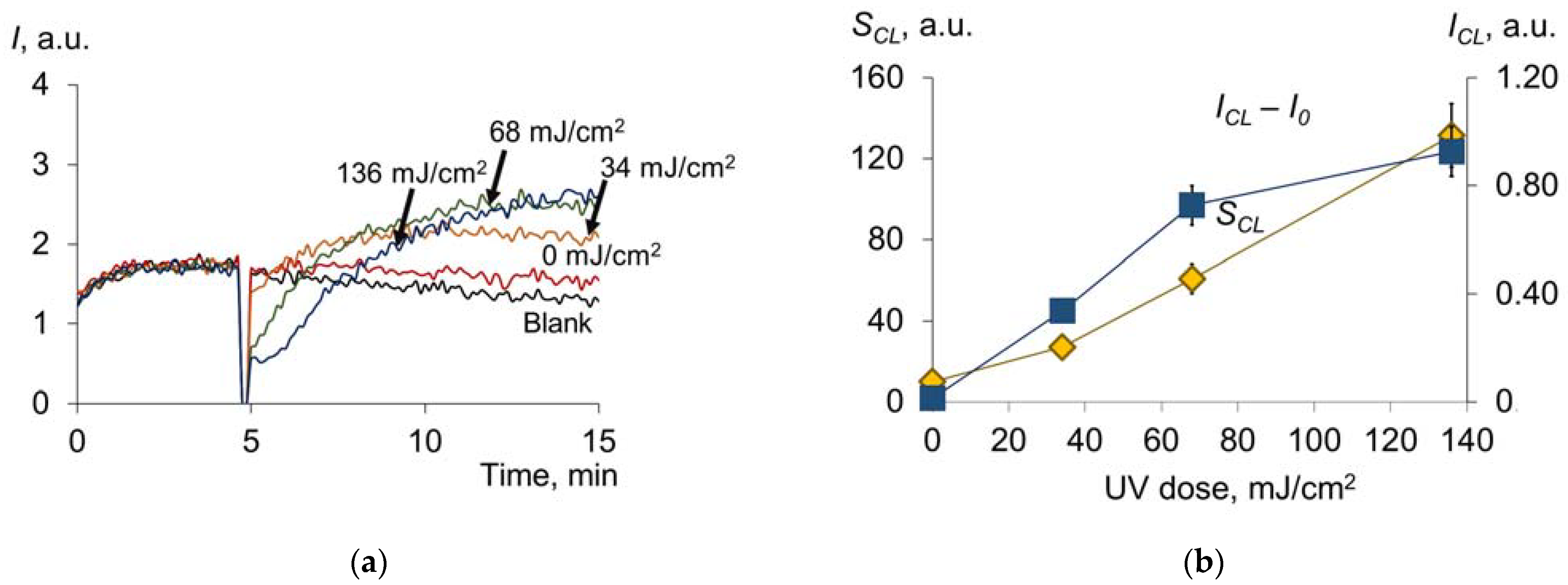
2.1.3. Antioxidant Capacity of Albumin
2.1.4. Changes in Fluorescence and Antioxidant Activity of γ-Globulins under UV Radiation
2.1.5. Effect of UV on the Antioxidant Properties of Uric Acid
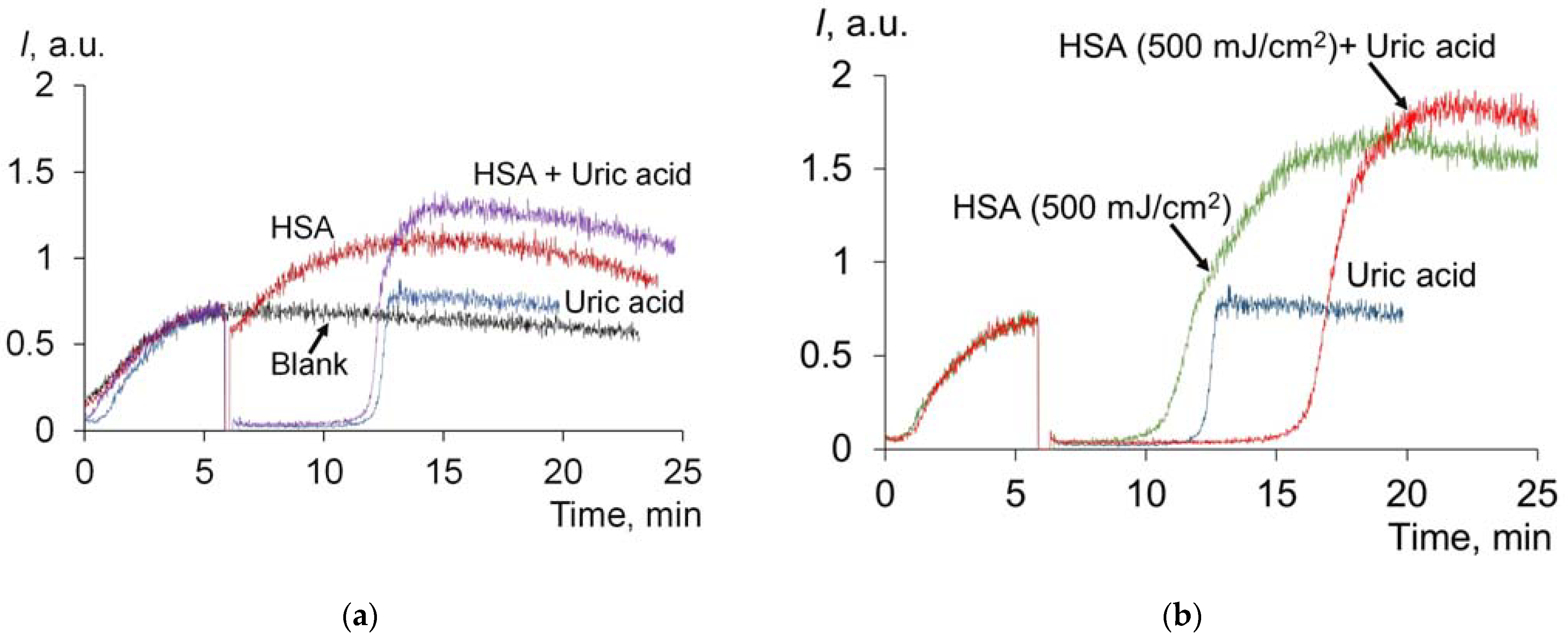
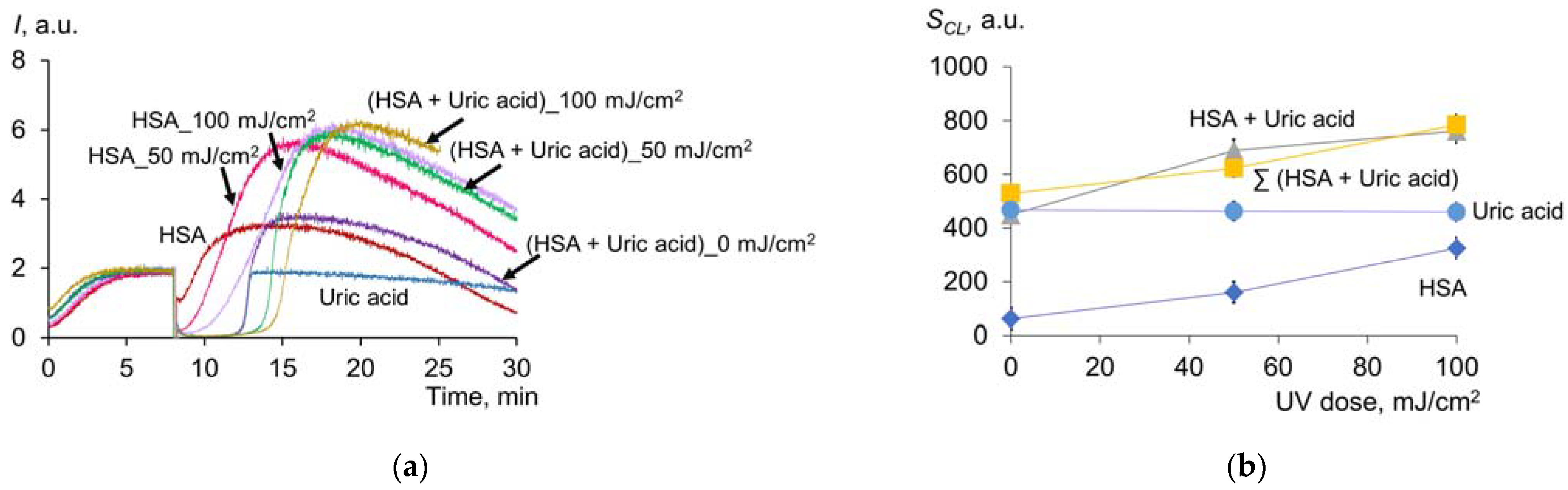
2.1.6. Effect of UV on Antioxidant Properties of Uric Acid and Albumin Mixture
2.1.7. Antioxidant Profile of Blood Plasma after UV Irradiation
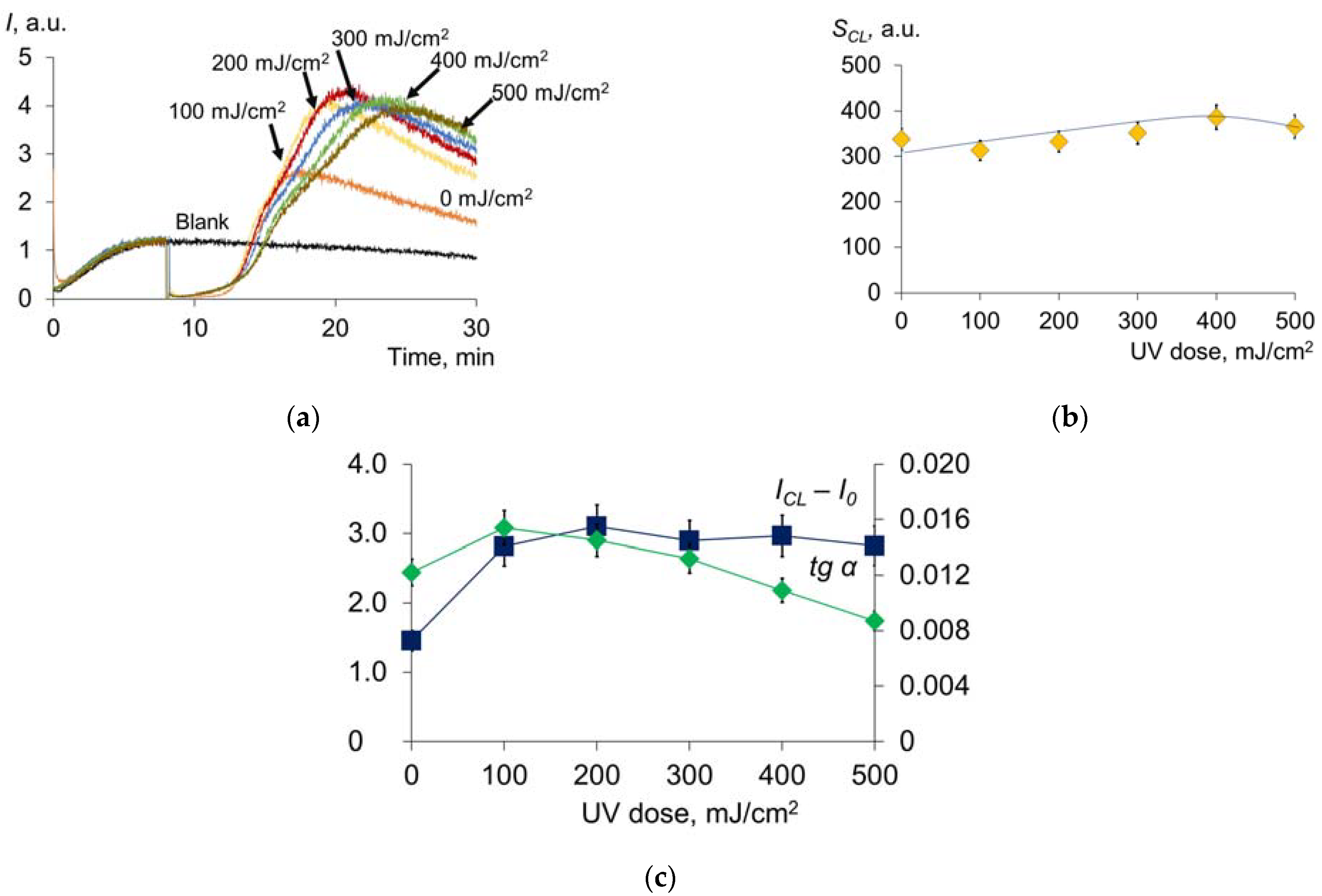
2.2. Pulsed Xenon Lamp (Broadband Spectrum)
3. Materials and Methods
3.1. Reagents and Samples
3.2. Ultraviolet Sources
3.2.1. Mercury Lamp UV
3.2.2. Flash Xenon UV
3.3. Oxidative Modification and Transport Capacity of Albumin
3.3.1. Assessment of Oxidized Albumin Fraction
3.3.2. Albumin Transport Capacity
3.4. Assessment of Thiol Groups
3.5. Antioxidant Capacity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hamblin, M.R. Ultraviolet Irradiation of Blood: “The Cure That Time Forgot”? Adv. Exp. Med. Biol. 2017, 996, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Banik, B.; Castelletto, S. Use of Ultraviolet Blood Irradiation Against Viral Infections. Clin. Rev. Allergy Immunol. 2020, 60, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.; Craciun, L. UV-induced immunosuppressive and anti-inflammatory actions: Mechanisms and clinical applications. Immunotherapy 2009, 1, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Endoh, I.; Girolamo, N.D.; Hampartzoumian, T.; Cameron, B.; Geczy, C.L.; Tedla, N. Ultraviolet B irradiation selectively increases the production of interleukin-8 in human cord blood-derived mast cells. Clin. Exp. Immunol. 2007, 148, 161–167. [Google Scholar] [CrossRef]
- Wenk, J.; Brenneisen, P.; Meewes, C.; Wlaschek, M.; Peters, T.; Blaudschun, M.; Ma, W.; Kuhr, L.; Schneider, L.; Scharffetter-Kochanek, K. UV-induced oxidative stress and photoaging. Curr. Probl. Dermatol. 2001, 29, 83–94. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.L.; Mira, J.P.; Guidet, M. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- Capomaccio, R.; Osório, I.; Ojea-Jiménez, I.; Ceccone, G.; Colpo, P.; Gilliland, D.; Hussain, R.; Siligardi, G.; Rossi, F.; Ricard-Blum, S. Gold nanoparticles increases UV and thermal stability of human serum albumin. Biointerphases 2016, 11, 04B310. [Google Scholar] [CrossRef]
- Michnik, A.; Michalik, K.; Drzazga, Z. Effect of UVC radiation on conformational restructuring of human serum albumin. J. Photochem. Photobiol. B 2008, 90, 170–178. [Google Scholar] [CrossRef]
- Kerwin, B.A.; Remmele, R.L., Jr. Protect from light: Photodegradation and protein biologics. J. Pharm. Sci. 2007, 96, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Meffert, R.; Dose, K.; Rathgeber, G.; Schäfer, H.-J. Ultraviolet crosslinking of DNA-protein complexes via 8-azidoadenine. In Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 323–335. [Google Scholar] [CrossRef]
- Tayyab, S.; Khan, N.J.; Khan, M.A.; Kumar, Y. Behavior of various mammalian albumins towards bilirubin binding and photochemical properties of different bilirubin–albumin complexes. Int. J. Biol. Macromol. 2003, 31, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, H.; Nakanishi, H.; Kashiwagi, Y.; Nakamoto, M.; Tachibana, A.; Hagihara, Y.; Tanabe, T. Changes in Cell Adhesiveness and Physicochemical Properties of Cross-Linked Albumin Films after Ultraviolet Irradiation. Langmuir 2016, 32, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, H. Spectroscopic study on the conformation of serum albumin in film state. J. Biosci. Bioeng. 2019, 127, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Iida, A.; Tanabe, T. Fluorescein isothiocyanate and blue light irradiation alter cell-adhesiveness of cross-linked albumin films for cell patterning. Biosci. Biotechnol. Biochem. 2020, 84, 800–803. [Google Scholar] [CrossRef]
- Samoilova, K.A.; Vasil’eva, Z.F.; Shtil’bans, V.I.; Obolenskaia, K.D.; Shalygina, N.N. Change in the binding capacity of human serum albumin following its exposure to therapeutic doses of UV radiation. Biull. Eksp. Biol. Med. 1987, 104, 676–678. [Google Scholar] [CrossRef]
- Kulms, D.; Schwarz, T. Molecular mechanisms of UV-induced apoptosis. Photodermatol. Photoimmunol. Photomed. 2000, 16, 195–201. [Google Scholar] [CrossRef]
- Monakhov, S.; Korchazhkina, N.; Olisova, O.Y. Narrow-band 311 nm phototherapy of patients with atopic dermatitis. Russ. J. Ski. Vener. Dis. 2012, 15, 25–27. (In Russian) [Google Scholar]
- Grachev, S.V.; Yurinskaya, M.M.; Tikhonenko, S.A.; Vinokuriv, M.G. Mechanisms of apoptosis regulation of human neutrophils at action of endotoxins and apoptosis inducers. Vestn. Novykh Meditsinskikh Tekhnologii 2013, 20, 18–20. (In Russian) [Google Scholar]
- Kuenstner, J.T.; Chamberlin, W.; Naser, S.A.; Collins, M.T.; Dow, C.T.; Aitken, J.M.; Weg, S.; Telega, G.; John, K.; Haas, D.; et al. Resolution of Crohn’s disease and complex regional pain syndrome following treatment of paratuberculosis. World J. Gastroenterol. 2015, 21, 4048–4062. [Google Scholar] [CrossRef]
- Kuenstner, J.T.; Mukherjee, S.; Schafer, Z.; Kuenstner, W.; Petrie, T. A controlled clinical trial of ultraviolet blood irradiation (UVBI) for hepatitis C infection. Cogent Med. 2019, 6, 1614286. [Google Scholar] [CrossRef]
- Kuenstner, J.T.; Mukherjee, S.; Weg, S.; Landry, T.; Petrie, T. The treatment of infectious disease with a medical device: Results of a clinical trial of ultraviolet blood irradiation (UVBI) in patients with hepatitis C infection. Int. J. Infect. Dis. 2015, 37, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, Y.A.; Golubtsov, A.A.; Shashkovsky, S.G. Disinfection of air and open surfaces of premises with pulsed ultraviolet radiation in medical organizations. Poliklinika 2014, 3, 51–54. (In Russian) [Google Scholar]
- Polimova, A.M.; Vladimirova, G.A.; Proskurnina, E.V.; Vladimirov, A.I. Antioxidants as aromatic amino acid oxidation products. Biofizika 2011, 56, 581–586. [Google Scholar] [CrossRef]
- Vorobey, P.; Steindal, A.E.; Off, M.K.; Vorobey, A.; Moan, J. Influence of human serum albumin on photodegradation of folic acid in solution. Photochem. Photobiol. 2006, 82, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, J.; Zhou, P.; Subirade, M. Protective effect of ligand-binding proteins against folic acid loss due to photodecomposition. Food Chem. 2013, 141, 754–761. [Google Scholar] [CrossRef]
- Artyukhov, V.G.; Pantyavin, A.A.; Vashanov, G.A. Vacuum-UV-radiation-induced structural-functional changes in serum albumin molecules. J. Appl. Spectrosc. 2001, 68, 291–298. [Google Scholar] [CrossRef]
- Gryzunov, Y.A.; Dobretsov, G.E. Natural conformation of human serum albumin and its changes in pathology. In Protein Conformation: New Research; Roswell, L.B., Ed.; Nova Publishers: New York, NY, USA, 2008; pp. 125–159. [Google Scholar]
- Vashanov, G.A.; Artyukhov, V.G. Human Serum Albumin in Clinical Medicine, 6th ed.; Geotar: Moscow, Russia, 1998; pp. 56–62. (In Russian) [Google Scholar]
- Zuorro, A.; Lavecchia, R. Protection of Human Albumin against UV-C Irradiation by Natural Antioxidants. Am. J. Biochem. Biotechnol. 2018, 14, 247–254. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation and aging. Free Radic. Res. 2006, 40, 1250–1258. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Batalova, A.A.; Goncharova, N.V. Effect of Bovine Serum Albumin Redox Status on Its Interaction with Paraoxon as Determined by Molecular Modeling. J. Evol. Biochem. Physiol. 2020, 56, 376–379. [Google Scholar] [CrossRef]
- Peters, T. All about Albumin; Biochemistry, Genetics and Medical Applications; Academic Press: San Diego, CA, USA, 1996; pp. 76–132. ISBN 978-0-12-552110-9. [Google Scholar]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.N.; Levin, G. Antioxidative system of the organism and thermo-initiated chemiluminescence method for quantitative evaluation of its state. Biofizika 2013, 58, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Sozarukova, M.M.; Polimova, A.M.; Proskurnina, E.V.; Vladimirov, Y.A. Changes in Kinetics of Chemiluminescence of Plasma as a Measure of Systemic Oxidative Stress in Humans. Biofizika 2016, 61, 337–344. [Google Scholar] [CrossRef]
- Sozarukova, M.M.; Proskurnina, E.V.; Vladimirov, Y.A. Serum albumin as a source of and a target for free radicals in pathology. Bull. Russ. State Med. Univ. 2016, 1, 61–67. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox. Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Belinskaia, D.A.; Razygraev, A.V.; Ukolov, A.I. On the Enzymatic Activity of Albumin. Bioorg. Khim. 2015, 41, 131–144. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Kurano, M.; Yasukawa, K.; Ikeda, H.; Aoki, J.; Yatomi, Y. Redox state of albumin affects its lipid mediator binding characteristics. Free Radic. Res. 2019, 53, 892–900. [Google Scholar] [CrossRef]
- Kawai, K.; Hayashi, T.; Matsuyama, Y.; Minami, T.; Era, S. Difference in redox status of serum and aqueous humor in senile cataract patients as monitored via the albumin thiol-redox state. Jpn. J. Ophthalmol. 2010, 54, 584–588. [Google Scholar] [CrossRef]
- Vasilevski, A.M.; Konoplev, G.A.; Kornilov, N.V. Study of the absorption spectra of albumin and uric acid in the UV region. J. Opt. Technol. 2001, 68, 928–930. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Splenser, A.E.; Valavanidis, A.; Zhang, H.; Uppu, R.M.; Pryor, W.A. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 2000, 376, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Hink, H.U.; Santanam, N.; Dikalov, S.; McCann, L.; Nguyen, A.D.; Parthasarathy, S.; Harrison, D.G.; Fukai, T. Peroxidase properties of extracellular superoxide dismutase: Role of uric acid in modulating in vivo activity. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Patrício, E.S.; Prado, F.M.; da Silva, R.P.; Carvalho, L.A.; Prates, M.V.; Dadamos, T.; Bertotti, M.; Di Mascio, P.; Kettle, A.J.; Meotti, F.C. Chemical characterization of urate hydroperoxide, a pro-oxidant intermediate generated by urate oxidation in inflammatory and photoinduced processes. Chem. Res. Toxicol. 2015, 28, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.J.; Iribarren, C.; Gross, M.D.; Comstock, G.W.; Cutler, R.G. Uric acid and serum antioxidant capacity: A reaction to atherosclerosis? Atherosclerosis 2000, 148, 131–139. [Google Scholar] [CrossRef]
- Johnson, R.J.; Kang, D.H.; Feig, D.; Kivlighn, S.; Kanellis, J.; Watanabe, S.; Tuttle, K.R.; Rodriguez-Iturbe, B.; Herrera-Acosta, J.; Mazzali, M. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension 2003, 41, 1183–1190. [Google Scholar] [CrossRef]
- Fabbrini, E.; Serafini, M.; Colic Baric, I.; Hazen, S.L.; Klein, S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes 2014, 63, 976–981. [Google Scholar] [CrossRef]
- Waring, W. Uric acid: An important antioxidant in acute ischaemic stroke. QJM 2002, 95, 691–693. [Google Scholar] [CrossRef]
- Bowman, G.L.; Shannon, J.; Frei, B.; Kaye, J.A.; Quinn, J.F. Uric acid as a CNS antioxidant. J. Alzheimer’s Dis. 2010, 19, 1331–1336. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C. High plasma uric acid concentration: Causes and consequences. Diabetol. Metab. Syndr. 2012, 4, 12. [Google Scholar] [CrossRef]
- Leighton, S.; Kok, L.F.; Halliday, G.M.; Byrne, S.N. Inhibition of UV-induced uric acid production using allopurinol prevents suppression of the contact hypersensitivity response. Exp. Dermatol. 2013, 22, 189–194. [Google Scholar] [CrossRef]
- Steele, T.H. Uric Acid; William, N.K., Weiner, I.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 51, pp. 257–286. ISBN 978-3-642-66867-8. [Google Scholar]
- Güngör, E.S.; Danişman, N.; Mollamahmutoğlu, L. Relationship between serum uric acid, creatinine, albumin and gestational diabetes mellitus. Clin. Chem. Lab. Med. 2006, 44, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Makarska-Bialokoz, M.; Lipke, A. Study of the binding interactions between uric acid and bovine serum albumin using multiple spectroscopic techniques. J. Mol. Liq. 2019, 276, 595–604. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Q.; Wang, J.; Wang, Q.; Qiao, H. Spectroscopic analyses and studies on respective interaction of cyanuric acid and uric acid with bovine serum albumin and melamine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Cheigh, C.; Park, M.-H.; Chung, M.-S.; Shin, J.-K.; Park, Y.-S. Comparison of intense pulsed light- and ultraviolet (UVC)-induced cell damage in Listeria monocytogenes and Escherichia coli O157:H7. Food Control 2012, 25, 654–659. [Google Scholar] [CrossRef]
- Elmnasser, N.; Dalgalarrondo, M.; Orange, N.; Bakhrouf, A.; Haertle, T.; Federighi, M.; Chobert, J.M. Effect of pulsed-light treatment on milk proteins and lipids. J. Agric. Food Chem. 2008, 56, 1984–1991. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of pulsed light treatment on the aggregation of whey protein isolate. Food Res. Int. 2017, 99, 419–425. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876. [Google Scholar] [CrossRef]
- Pant, M.P.; Mariam, J.; Joshi, A.; Dongre, P. UV radiation sensitivity of bovine serum albumin bound to silver nanoparticles. J. Radiat. Res. Appl. Sci. 2014, 7, 399–405. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Song, K.-B. Effect of γ-irradiation on the molecular properties of Bovine Serum Albumin and β-Lactoglobulin. BMB Rep. 2000, 33, 133–137. [Google Scholar] [CrossRef]
- Gryzunov, Y.A.; Syrejshchikova, T.; Komarova, M.; Misionzhnik, E.Y.; Uzbekov, M.; Molodetskich, A.; Dobretsov, G.; Yakimenko, M. Serum albumin binding sites properties in donors and in schizophrenia patients: The study of fluorescence decay of the probe K-35 using S-60 synchrotron pulse excitation. Nucl. Instrum. Methods Phys. Res. B 2000, 448, 478–482. [Google Scholar] [CrossRef]
- Dobretsov, G.; Syreishchikova, T.; Gryzunov, Y.A.; Smolina, N.; Komar, A. Features of the binding of the fluorescent probe K-35 to albumin. Biophysics 2010, 55, 182–187. [Google Scholar] [CrossRef]
- Azizova, O.; Aseychev, A.; Beckman, E.; Moskvina, S.; Skotnikova, O.; Smolina, N.; Gryzunov, Y.A.; Dobretsov, G. Studies of oxidant-induced changes in albumin transport function with a fluorescent probe K-35. Effect of hypochlorite. Bull. Exp. Biol. Med. 2012, 152, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, G.; Syrejschikova, T.; Smolina, N. On mechanisms of fluorescence quenching by water. Biophysics 2014, 59, 183–188. [Google Scholar] [CrossRef]
- Aseychev, A.; Azizova, O.; Beckman, E.; Skotnikova, O.; Piryazev, A.; Dobretsov, G. Studies of oxidant-induced changes in albumin transport function with a fluorescent probe K-35. Metal-catalyzed oxidation. Bull. Exp. Biol. Med. 2012, 153, 463–467. [Google Scholar] [CrossRef]
- Alekseev, A.V.; Proskurnina, E.V.; Vladimirov, Y.A. Determination of antioxidants by sensitized chemiluminescence using 2, 2′-azo-bis (2-amidinopropane). Mosc. Univ. Chem. Bull. 2012, 67, 127–132. [Google Scholar] [CrossRef]



| UV Dose, mJ/cm2 | Thiols (μM) |
|---|---|
| 100 | 1.63 ± 0.01 |
| 200 | 3.95 ± 0.03 |
| 300 | 5.19 ± 0.04 |
| 400 | 6.48 ± 0.09 |
| 500 | 7.89 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sozarukova, M.M.; Skachko, N.A.; Chilikina, P.A.; Novikov, D.O.; Proskurnina, E.V. Effect of Low-Dose Line-Spectrum and Full-Spectrum UV on Major Humoral Components of Human Blood. Molecules 2023, 28, 4646. https://doi.org/10.3390/molecules28124646
Sozarukova MM, Skachko NA, Chilikina PA, Novikov DO, Proskurnina EV. Effect of Low-Dose Line-Spectrum and Full-Spectrum UV on Major Humoral Components of Human Blood. Molecules. 2023; 28(12):4646. https://doi.org/10.3390/molecules28124646
Chicago/Turabian StyleSozarukova, Madina M., Nadezhda A. Skachko, Polina A. Chilikina, Dmitriy O. Novikov, and Elena V. Proskurnina. 2023. "Effect of Low-Dose Line-Spectrum and Full-Spectrum UV on Major Humoral Components of Human Blood" Molecules 28, no. 12: 4646. https://doi.org/10.3390/molecules28124646
APA StyleSozarukova, M. M., Skachko, N. A., Chilikina, P. A., Novikov, D. O., & Proskurnina, E. V. (2023). Effect of Low-Dose Line-Spectrum and Full-Spectrum UV on Major Humoral Components of Human Blood. Molecules, 28(12), 4646. https://doi.org/10.3390/molecules28124646







