Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets
Abstract
1. Introduction
2. Results
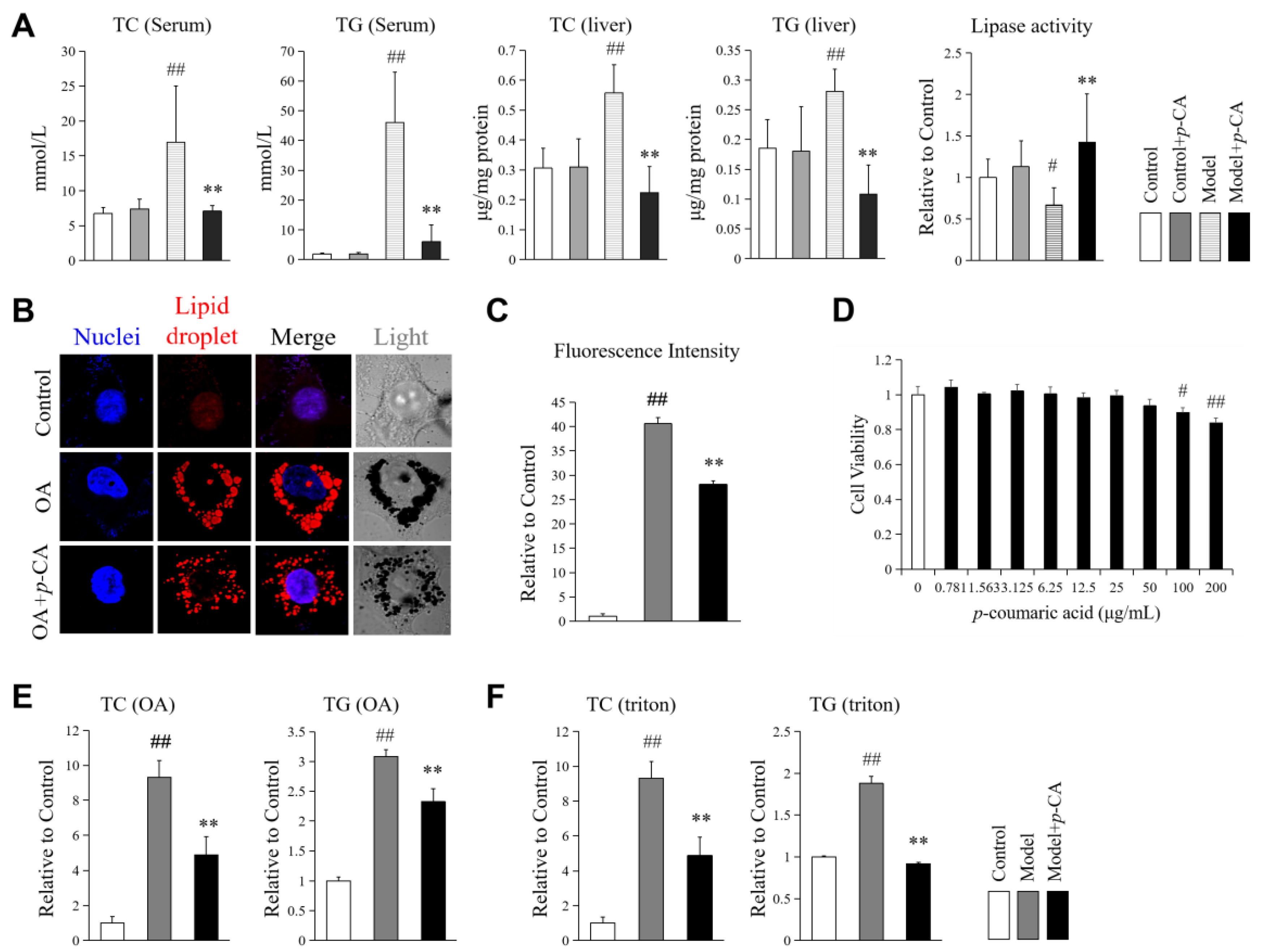
2.1. p-CA Alleviates Lipid Overloading in Hyperlipidemic Mice and HepG2
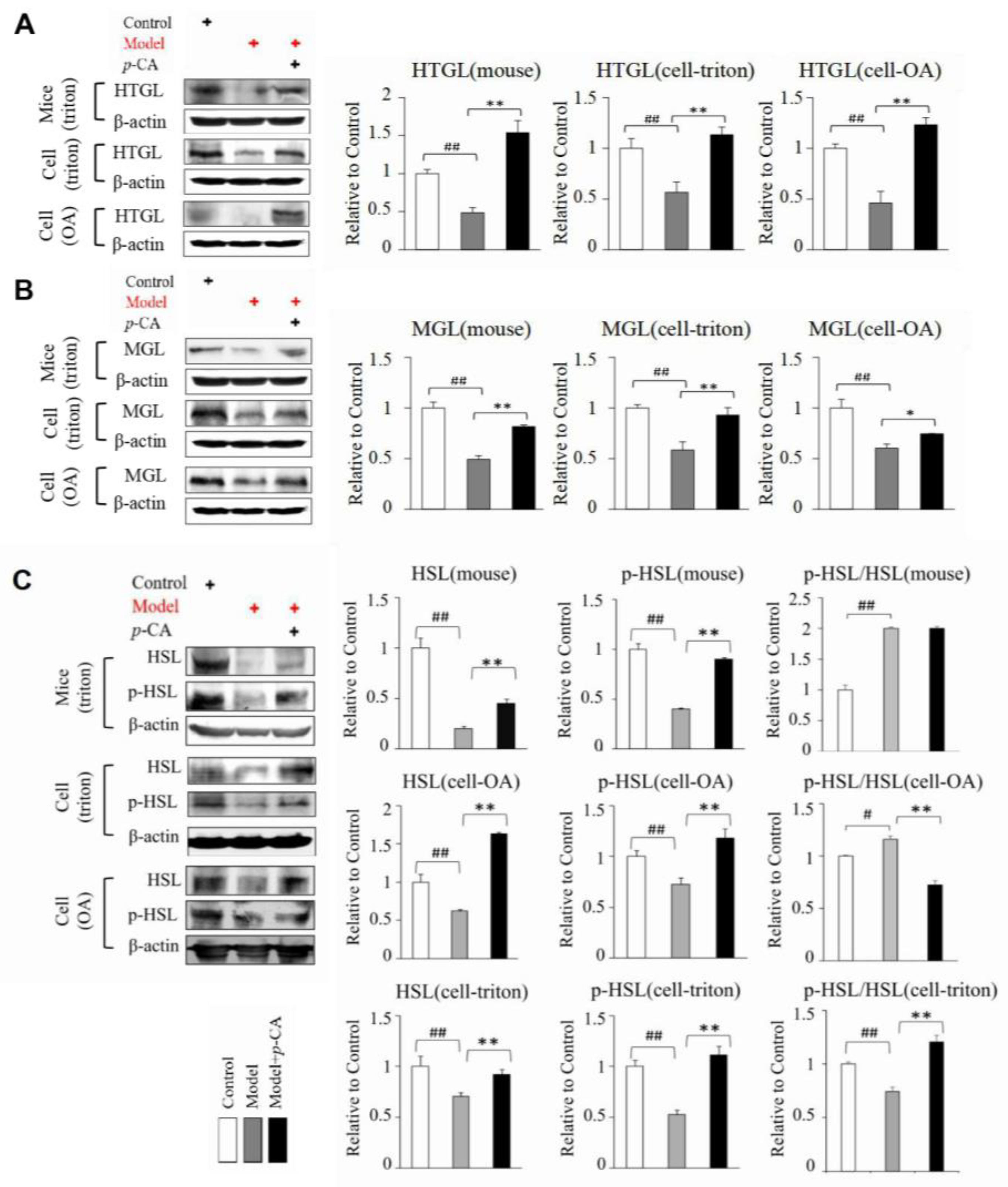
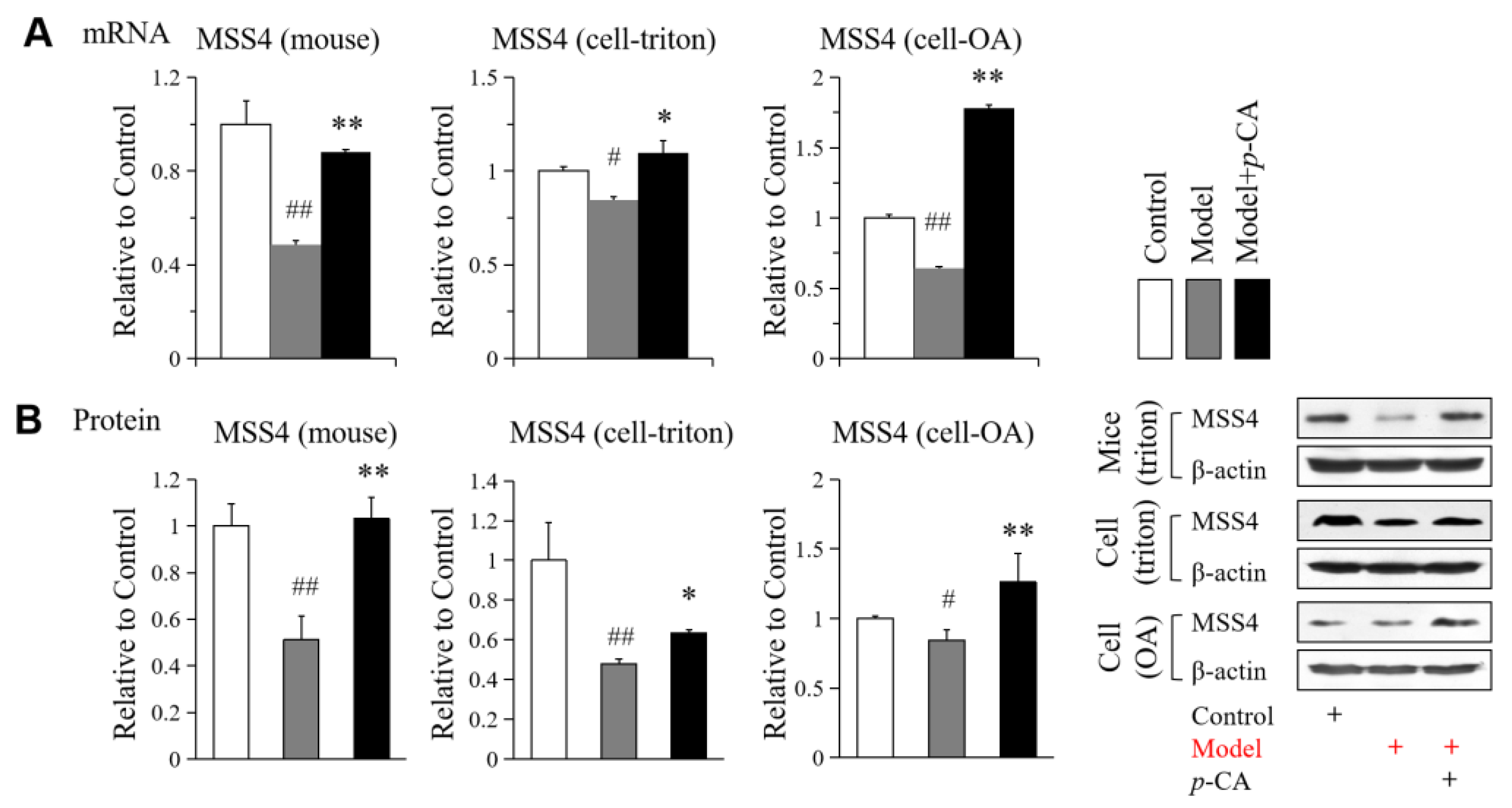
2.2. p-CA Enhances the Expression of MGL HTGL HSL and MSS4 Suppressed by Lipid Overload
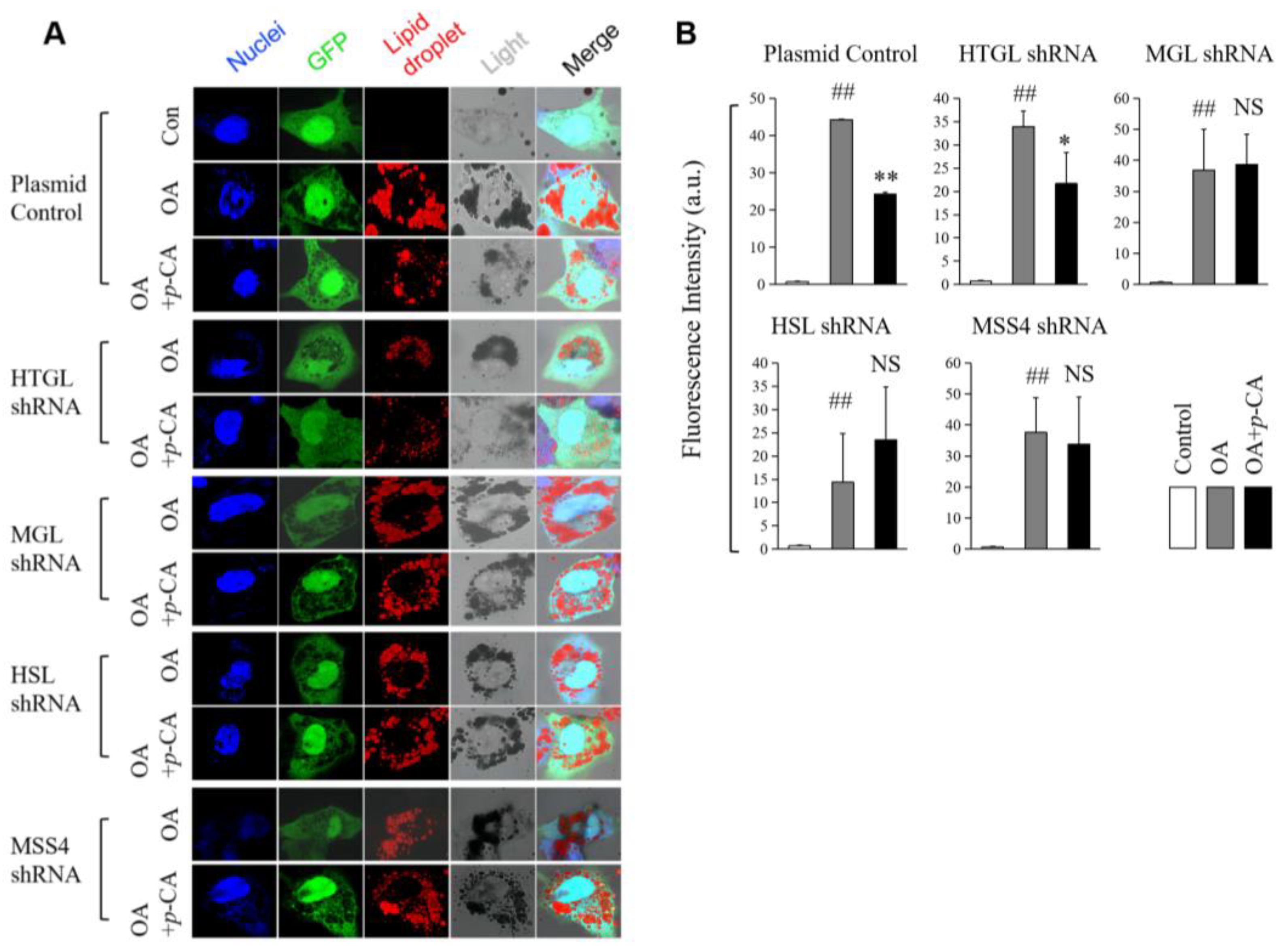
2.3. The Knockdown of MGL HSL and MSS4 Alleviates Lipid-Lowering Effect of p-CA
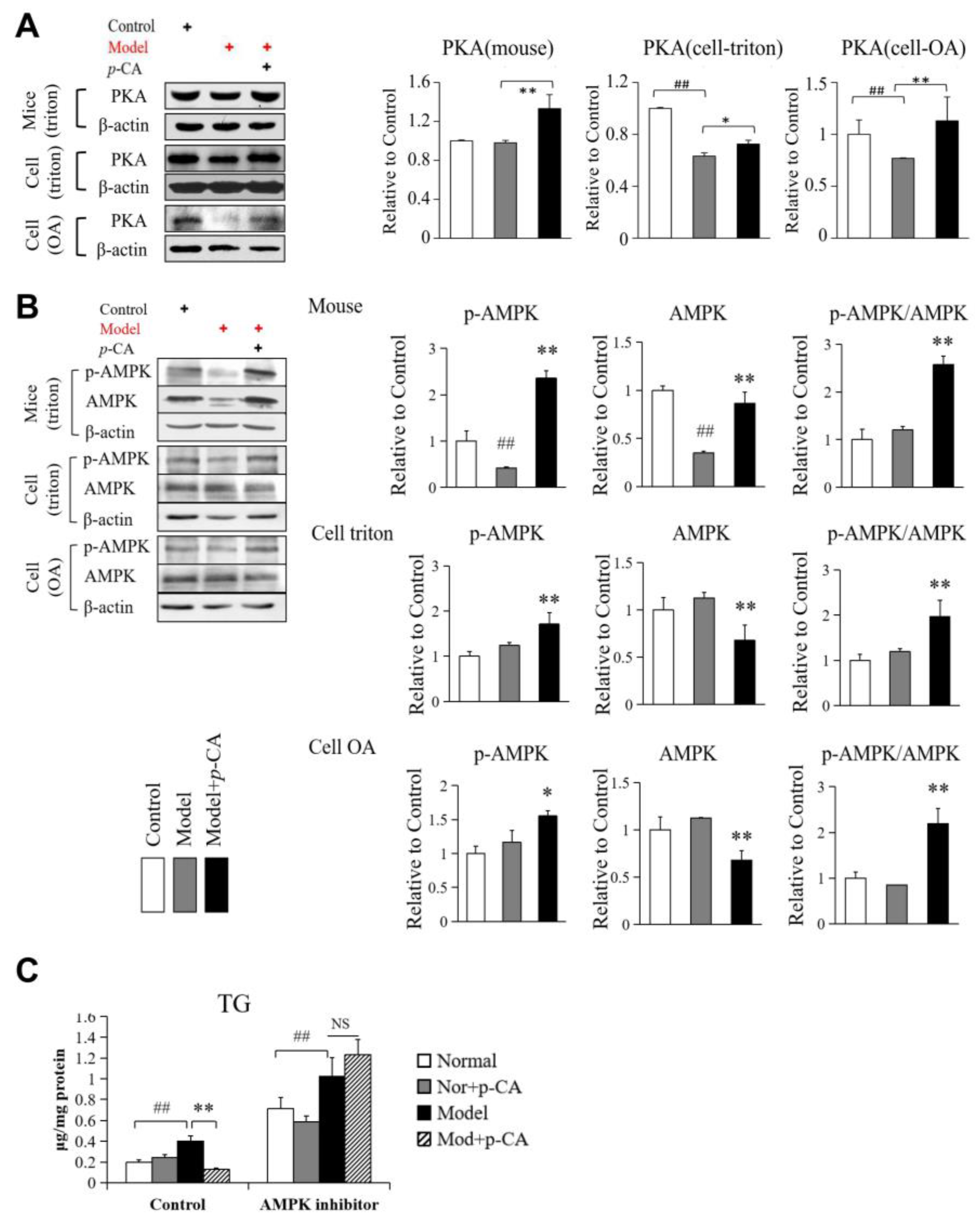
2.4. Activation of AMPK by p-CA May Participate in Alleviating Lipid Accumulation Independent of HSL Phosphorylation
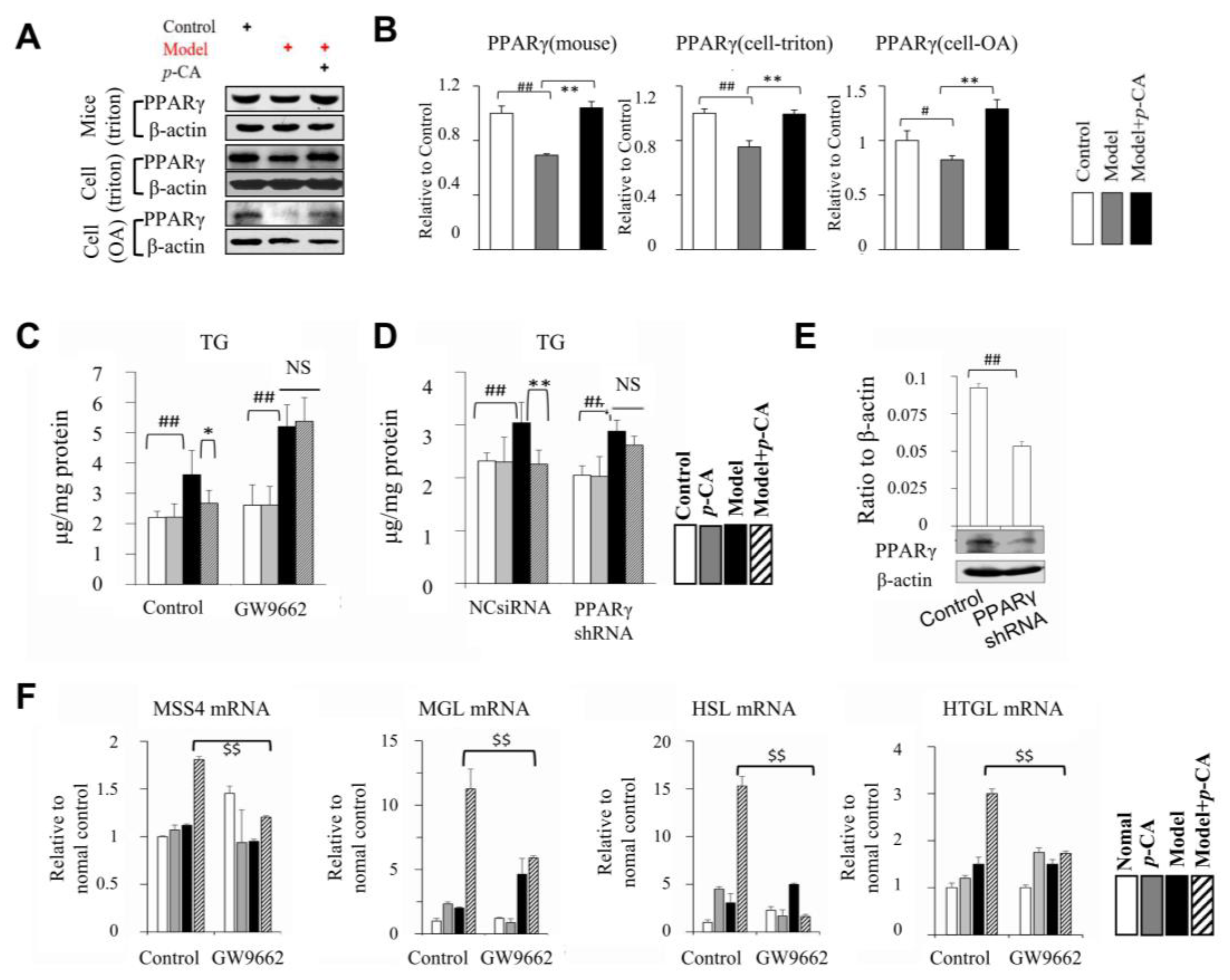
2.5. p-CA Activates PPARα/γ as Transcription Factors Suppressed by Lipid Overload
3. Discussion
4. Materials and Methods
4.1. Experimental Animals, Drugs and Chemicals
4.2. Hyperlipidemic Model in Mice Induced by Triton WR1339
4.3. Cell Culture and Cell Viability Assay
4.4. The Triton WR1339 and Oleic Acid-Induced Hyperlipidemic Model in HepG2 Cells
4.5. The Measurement of TC and TG Content
4.6. The Measurement of Lipase Activity
4.7. Quantitative PCR and Western Blotting
4.8. Lipid Droplet Observed by a Confocal Microscopy
4.9. Construction of MSS4, HSL, MGL, HTGL and PPARγ shRNAs
4.10. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Paternostro, R.; Trauner, M. Current treatment of non-alcoholic fatty liver disease. J. Intern. Med. 2022, 292, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Deng, J.S.; Huang, C.C.; Li, P.Y.; Liang, Y.C.; Chou, C.Y.; Huang, G.J. p-Coumaric-Acid-Containing Adenostemma lavenia Ameliorates Acute Lung Injury by Activating AMPK/Nrf2/HO-1 Signaling and Improving the Anti-oxidant Response. Am. J. Chin. Med. 2019, 47, 1483–1506. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective effects of p-coumaric acid against oxidant and hyperlipidemia-an in vitro and in vivo evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Gholipour, P.; Komaki, A.; Salehi, I.; Rashidi, K.; Esmaeil Khoshnam, S.; Rashno, M. p-Coumaric acid ameliorates cognitive and non-cognitive disturbances in a rat model of Alzheimer’s disease: The role of oxidative stress and inflammation. Int. Immunopharmacol. 2022, 112, 109295. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Reis, M.B.; Coelho, G.D.P.; Gastaldello, G.H.; Peti, A.P.F.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Sorgi, C.A.; Faccioli, L.H.; et al. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021, 278, 114255. [Google Scholar] [CrossRef]
- Goodarzi, G.; Tehrani, S.S.; Panahi, G.; Bahramzadeh, A.; Meshkani, R. Combination Therapy of Metformin and p-Coumaric Acid Mitigates Metabolic Dysfunction Associated with Obesity and Non-Alcoholic Fatty Liver Disease in High-Fat Diet Obese C57BL/6 Mice. J. Nutr. Biochem. 2023, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.A.; Kang, S.I.; Shin, H.S.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. p-Coumaric acid modulates glucose and lipid metabolism via AMP-activated protein kinase in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Nguyen, K.D.A.; Ma, C.T.; Nguyen, Q.T.; Nguyen, H.T.H.; Yang, D.J.; Tran, T.L.; Kim, K.W.; Doan, K.V. p-Coumaric Acid Enhances Hypothalamic Leptin Signaling and Glucose Homeostasis in Mice via Differential Effects on AMPK Activation. Int. J. Mol. Sci. 2021, 22, 1431. [Google Scholar] [CrossRef]
- Dolrahman, N.; Mukkhaphrom, W.; Sutirek, J.; Thong-Asa, W. Benefits of p-coumaric acid in mice with rotenone-induced neurodegeneration. Metab. Brain Dis. 2023, 38, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wu, H.; Fan, J.; Zhang, L.; Li, H.; Guo, H.; Yang, R.; Li, Z. The Mixture of Ferulic Acid and p-Coumaric Acid Suppresses Colorectal Cancer through lncRNA 495810/PKM2 Mediated Aerobic Glycolysis. Int. J. Mol. Sci. 2022, 23, 12106. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Busselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.; Laddha, A.; Nandave, M.; Srinath, S. Dietary Phenolic Acids of Macrotyloma uniflorum (Horse Gram) Protect the Rat Heart Against Isoproterenol-Induced Myocardial Infarction. Phytother. Res. 2016, 30, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Ojha, D.; Patil, K.N. p-Coumaric acid inhibits the Listeria monocytogenes RecA protein functions and SOS response: An antimicrobial target. Biochem. Biophys. Res. Commun. 2019, 517, 655–661. [Google Scholar] [CrossRef]
- Li, J.; Zhao, N.; Xu, R.; Li, G.; Dong, H.; Wang, B.; Li, Z.; Fan, M.; Wei, X. Deciphering the antibacterial activity and mechanism of p-coumaric acid against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2022, 378, 109822. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, L.; La, X.; Wu, H.; Yang, R.; Li, H.; Li, Z. Ferulic Acid and p-Coumaric Acid Synergistically Attenuate Non-Alcoholic Fatty Liver Disease through HDAC1/PPARG-Mediated Free Fatty Acid Uptake. Int. J. Mol. Sci. 2022, 23, 15297. [Google Scholar] [CrossRef]
- Truong, T.M.T.; Seo, S.H.; Chung, S.; Kang, I. Attenuation of hepatic fibrosis by p-Coumaric acid via modulation of NLRP3 inflammasome activation in C57BL/6 mice. J. Nutr. Biochem. 2023, 112, 109204. [Google Scholar] [CrossRef]
- Zhao, X.; Amevor, F.K.; Cui, Z.; Wan, Y.; Xue, X.; Peng, C.; Li, Y. Steatosis in metabolic diseases: A focus on lipolysis and lipophagy. Biomed. Pharmacother. 2023, 160, 114311. [Google Scholar] [CrossRef]
- Fuchs, C.D.; Claudel, T.; Trauner, M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol. Metab. 2014, 25, 576–585. [Google Scholar] [CrossRef]
- Lim, G.B. LIPC variant in combined hypocholesterolaemia. Nat. Rev. Cardiol. 2022, 19, 642. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Yi, L.; Yang, L.; Wang, W.E.; Zeng, C.; Mi, M.; Chen, X. Hepatic PKA inhibition accelerates the lipid accumulation in liver. Nutr. Metab. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, Y.; Bai, M.; Huang, Y.; Yang, H.; Liu, L.; Wang, S.; Yu, C.; Song, Z.; Bao, Y.; et al. Hypericin attenuates nonalcoholic fatty liver disease and abnormal lipid metabolism via the PKA-mediated AMPK signaling pathway in vitro and in vivo. Pharmacol. Res. 2020, 153, 104657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shi, H.; Liu, N.; Tian, J.; Zhao, X.; Steer, C.J.; Han, Q.; Song, G. Activation of microRNA-378a-3p biogenesis promotes hepatic secretion of VLDL and hyperlipidemia by modulating ApoB100-Sortilin1 axis. Theranostics 2020, 10, 3952–3966. [Google Scholar] [CrossRef]
- Khlifi, R.; Lahmar, A.; Dhaouefi, Z.; Kalboussi, Z.; Maatouk, M.; Kilani-Jaziri, S.; Ghedira, K.; Chekir-Ghedira, L. Assessment of hypolipidemic, anti-inflammatory and antioxidant properties of medicinal plant Erica multiflora in triton WR-1339-induced hyperlipidemia and liver function repair in rats: A comparison with fenofibrate. Regul. Toxicol. Pharmacol. 2019, 107, 104404. [Google Scholar] [CrossRef]
- Millar, J.S.; Cromley, D.A.; McCoy, M.G.; Rader, D.J.; Billheimer, J.T. Determining hepatic triglyceride production in mice: Comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 2005, 46, 2023–2028. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, W.; Li, Q.; Chen, Y.; Wu, L.; Dong, Y.; Huang, X.; He, X.; Ou, Z.; Peng, Y. Membrane Protein Amuc_1100 Derived from Akkermansia muciniphila Facilitates Lipolysis and Browning via Activating the AC3/PKA/HSL Pathway. Microbiol. Spectr. 2023, 11, e0432322. [Google Scholar] [CrossRef]
- Przygrodzka, E.; Hou, X.; Zhang, P.; Plewes, M.R.; Franco, R.; Davis, J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology 2021, 162, bqab015. [Google Scholar] [CrossRef]
- Baumann, A.; Burger, K.; Brandt, A.; Staltner, R.; Jung, F.; Rajcic, D.; Lorenzo Pisarello, M.J.; Bergheim, I. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism 2022, 133, 155233. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, J.; Jin, J.; Tian, Y.; Lan, L.; Wang, X.; Zhu, L.; Wang, J. WY-14643 attenuates lipid deposition via activation of the PPARalpha/CPT1A axis by targeting Gly335 to inhibit cell proliferation and migration in ccRCC. Lipids Health Dis. 2022, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Rakhshandehroo, M.; Hooiveld, G.; Muller, M.; Kersten, S. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS ONE 2009, 4, e6796. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, D.; Zhou, L.; Xie, B.; Yu, L.; Yang, H.; Huang, L.; Ye, J.; Deng, H.; Yuan, Y.A.; et al. Rab8a-AS160-MSS4 regulatory circuit controls lipid droplet fusion and growth. Dev. Cell 2014, 30, 378–393. [Google Scholar] [CrossRef]
- Althaher, A.R. An Overview of Hormone-Sensitive Lipase (HSL). Sci. World J. 2022, 2022, 1964684. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yu, H.; Lin, Y.; Sun, K.; Zhang, Y.; Song, Y.; Hui, R.; Chen, J. A functional variant in the exon 5 of PLIN1 reduces risk of central obesity by possible regulation of lipid storage. Biochem. Biophys. Res. Commun. 2015, 456, 896–900. [Google Scholar] [CrossRef]
- Kuo, T.; Chen, T.C.; Lee, R.A.; Nguyen, N.H.T.; Broughton, A.E.; Zhang, D.; Wang, J.C. Pik3r1 Is Required for Glucocorticoid-Induced Perilipin 1 Phosphorylation in Lipid Droplet for Adipocyte Lipolysis. Diabetes 2017, 66, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Pawella, L.M.; Hashani, M.; Eiteneuer, E.; Renner, M.; Bartenschlager, R.; Schirmacher, P.; Straub, B.K. Perilipin discerns chronic from acute hepatocellular steatosis. J. Hepatol. 2014, 60, 633–642. [Google Scholar] [CrossRef]
- Desrochers, G.F.; Filip, R.; Bastianelli, M.; Stern, T.; Pezacki, J.P. microRNA-27b regulates hepatic lipase enzyme LIPC and reduces triglyceride degradation during hepatitis C virus infection. J. Biol. Chem. 2022, 298, 101983. [Google Scholar] [CrossRef]
- Zechner, R. FAT FLUX: Enzymes, regulators, and pathophysiology of intracellular lipolysis. EMBO Mol. Med. 2015, 7, 359–362. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, K.; Staels, B.; Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996, 37, 907. [Google Scholar] [CrossRef]
- Van Dalem, J.; Driessen, J.H.M.; Burden, A.M.; Stehouwer, C.D.A.; Klungel, O.H.; de Vries, F.; Brouwers, M. Thiazolidinediones and Glucagon-like Peptide-1 Receptor Agonists and the Risk of Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology 2021, 74, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, W.T.; Laplante, M.; Berthiaume, M.; Gélinas, Y.; Deshaies, Y. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 2006, 49, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Yajima, H.; Kobayashi, Y.; Kanaya, T.; Horino, Y. Identification of peroxisome-proliferator responsive element in the mouse HSL gene. Biochem. Biophys. Res. Commun. 2007, 352, 526–531. [Google Scholar] [CrossRef]
- Tardelli, M.; Bruschi, F.V.; Claudel, T.; Fuchs, C.D.; Auer, N.; Kunczer, V.; Stojakovic, T.; Scharnagl, H.; Habib, A.; Grabner, G.F.; et al. Lack of monoacylglycerol lipase prevents hepatic steatosis by favoring lipid storage in adipose tissue and intestinal malabsorption. J. Lipid Res. 2019, 60, 1284–1292. [Google Scholar] [CrossRef]
- Gaggini, M.; Morelli, M.; Buzzigoli, E.; DeFronzo, R.A.; Bugianesi, E.; Gastaldelli, A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients 2013, 5, 1544–1560. [Google Scholar] [CrossRef]
- Sobol, M.; Krausová, A.; Yildirim, S.; Kalasová, I.; Fáberová, V.; Vrkoslav, V.; Philimonenko, V.; Marášek, P.; Pastorek, L.; Čapek, M.; et al. Nuclear phosphatidylinositol 4,5-bisphosphate islets contribute to efficient RNA polymerase II-dependent transcription. J. Cell Sci. 2018, 131, jcs211094. [Google Scholar] [CrossRef]
- Martelli, A.M.; Falà, F.; Faenza, I.; Billi, A.M.; Cappellini, A.; Manzoli, L.; Cocco, L. Metabolism and signaling activities of nuclear lipids. Cell Mol. Life Sci. 2004, 61, 1143–1156. [Google Scholar]
- Hoboth, P.; Šebesta, O.; Sztacho, M.; Castano, E.; Hozák, P. Dual-color dSTORM imaging and ThunderSTORM image reconstruction and analysis to study the spatial organization of the nuclear phosphatidylinositol phosphates. MethodsX 2021, 8, 101372. [Google Scholar] [CrossRef] [PubMed]
- Hoboth, P.; Šebesta, O.; Hozák, P. How Single-Molecule Localization Microscopy Expanded Our Mechanistic Understanding of RNA Polymerase II Transcription. Int. J. Mol. Sci. 2021, 22, 6694. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yuan, Z.Y.; Yan, X.J.; Lei, F.; Jiang, J.F.; Yu, X.; Yang, X.W.; Xing, D.M.; Du, L.J. Effects of Angelica dahurica on obesity and fatty liver in mice. Chin. J. Nat. Med. 2016, 14, 641–652. [Google Scholar] [CrossRef] [PubMed]

| Primers(bp) | Sequences | Primers(bp) | Sequences |
|---|---|---|---|
| H-MSS4(154) NM_002871.4 | CTTTTCCTTCCCTCCATGAGAA | M-MSS4(184) NM_145510.1 | CAGCAATCCTGATGGTGATG |
| AGACCAGAAACTTGATGTTGCCCA | CTCCAAGGCCACATAGAAGC | ||
| H-HSL(144) NM_005357.3 | CTCCTCCTATTCCTAATCCTCC | M-HSL(106) NM_010719.5 | CCAGCCTGAGGGCTTACTG |
| CACTTCCTCTTGGGTTTCACTC | CTCCATTGACTGTGACATCTCG | ||
| H-PKA(198) NM_002730.3 | GCGTGAAAGAATTCTTAGCCA | M-PKA(119) NM_008854.5 | AGATCGTCCTGACCTTTGAGT |
| CCACCTTCTGTTTGTCGAGGA | GGCAAAACCGAAGTCTGTCAC | ||
| H-HTGL(276) NM_000236.2 | CTGGTGGTGACATGAACAGC | M-HTGL(207) NM_008280.2 | ATGGGAAATCCCCTCCAAATCT |
| CTTTGCCCAGAGTGATGGGA | GTGCTGAGGTCTGAGACGA | ||
| H-MGL(411) NM_007283.6 | CAATCCTGAATCTGCAACAACTTTC | M-MGL(127) NM_011844.4 | CGGACTTCCAAGTTTTTGTCAGA |
| ATGTTTATTTCATGGAAGACGGAGT | GCAGCCACTAGGATGGAGATG | ||
| H-PPARγ(272) NM_015869.4 | CTGCGAAAGCCTTTTGGTGACTTTATGG | M-PPARγ(272) NM_011146.3 | CTGCGGAAGCCCTTTGGTGACTTTATGG |
| ACAATCTGTCTGAGGTCTGTCATTTTCT | ACGATCTGCCTGAGGTCTGTCATCTTCT | ||
| M-ACSl1(119) NM_007981.4 | TCTTCCCTGTGGTTCCCAG | M-PPARα(76) NM_011144.6 | GACAAGGCCTCAGGGTACCA |
| AAGCTCCGCCTCTTTCCTTT | GCCGAATAGTTCGCCGAAA | ||
| M-AOX1(270) NM_009676.2 | CAAAGATGGAGAACGGCG | M-CPT1A(84) NM_013495.2 | CCTGGGCATGATTGCAAAG |
| GCACTGATGATAGTTGAACCG | ACGCCACTCACGATGTTCTTC | ||
| M-AMPKα1(91) NM_001013367.3 | CTACCTAGCAACCAGCCCAC | M-AMPKα2(75) NM_178143.2 | GAAAGGATGCCGCCTCTCAT |
| TTCGGCAACCAAGAACGGTA | GGGCTTCGTTGTGTTGAGTG |
| Gene Name | Sequences | |
|---|---|---|
| HSL | F | TCCCTCCGATGTCAACTTCTTATTCAAGAGATAAGAAGTTGACATCGGAGGGTTTTTTC |
| R | TCGAGAAAAAACCCTCCGATGTCAACTTCTTATCTCTTGAATAAGAAGTTGACATCGGAGGGA | |
| MGL | F | TCCAATCCTGAATCTGCAACAATTCAAGAGATTGTTGCAGATTCAGGATTGGTTTTTTC |
| R | TCGAGAAAAAACCAATCCTGAATCTGCAACAATCTCTTGAATTGTTGCAGATTCAGGATTGGA | |
| HTGL | F | TGCATGAGATGAAGACCAGATTTTCAAGAGAAATCTGGTCTTCATCTCATGCTTTTTTC |
| R | TCGAGAAAAAAGCATGAGATGAAGACCAGATTTCTCTTGAAAATCTGGTCTTCATCTCATGCA | |
| MSS4 | F | TACGTGGGCAACATCAAGTTTCTTCAAGAGAGAAACTTGATGTTGCCCACGTTTTTTTC |
| R | TCGAGAAAAAAACGTGGGCAACATCAAGTTTCTCTCTTGAAGAAACTTGATGTTGCCCACGTA | |
| PPARγ | F | TGACAACAGACAAATCACCATTTTCAAGAGAAATGGTGATTTGTCTGTTGTCTTTTTTC |
| R | TCGAGAAAAAAGACAACAGACAAATCACCATTTCTCTTGAAAATGGTGATTTGTCTGTTGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Lu, X.; Lei, F.; Sun, H.; Jiang, J.; Xing, D.; Du, L. Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules 2023, 28, 4641. https://doi.org/10.3390/molecules28124641
Yuan Z, Lu X, Lei F, Sun H, Jiang J, Xing D, Du L. Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules. 2023; 28(12):4641. https://doi.org/10.3390/molecules28124641
Chicago/Turabian StyleYuan, Zhiyi, Xi Lu, Fan Lei, Hong Sun, Jingfei Jiang, Dongming Xing, and Lijun Du. 2023. "Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets" Molecules 28, no. 12: 4641. https://doi.org/10.3390/molecules28124641
APA StyleYuan, Z., Lu, X., Lei, F., Sun, H., Jiang, J., Xing, D., & Du, L. (2023). Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules, 28(12), 4641. https://doi.org/10.3390/molecules28124641





