The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels
Abstract
1. Introduction
1.1. Tissue Engineering and Regenerative Medicine
1.2. Hydrogels as Sophisticated, Biomimetic Materials
1.2.1. Synthetic Hydrogels
1.2.1.1. Polymer-Based Hydrogels
1.2.1.2. Peptide-Based Hydrogels
1.2.2. Natural Hydrogels
1.2.2.1. Protein-Based Hydrogels
1.2.2.2. Polysaccharide-Based Hydrogels
1.2.3. Hydrogels as “Smart” Biomaterials
1.2.3.1. pH-Sensitive Hydrogels
1.2.3.2. Light-Sensitive Hydrogels
1.2.3.3. Enzyme-Responsive Hydrogels
1.2.3.4. Mechanically Sensitive Hydrogels
1.2.3.5. Electrosensitive Hydrogels
1.2.3.6. Temperature-Sensitive Hydrogels
1.3. Enhancing the Complexity of Biomaterials for Tissue Engineering Applications
2. ECM Components That Contribute to Tissue Engineering Strategies
2.1. Collagens
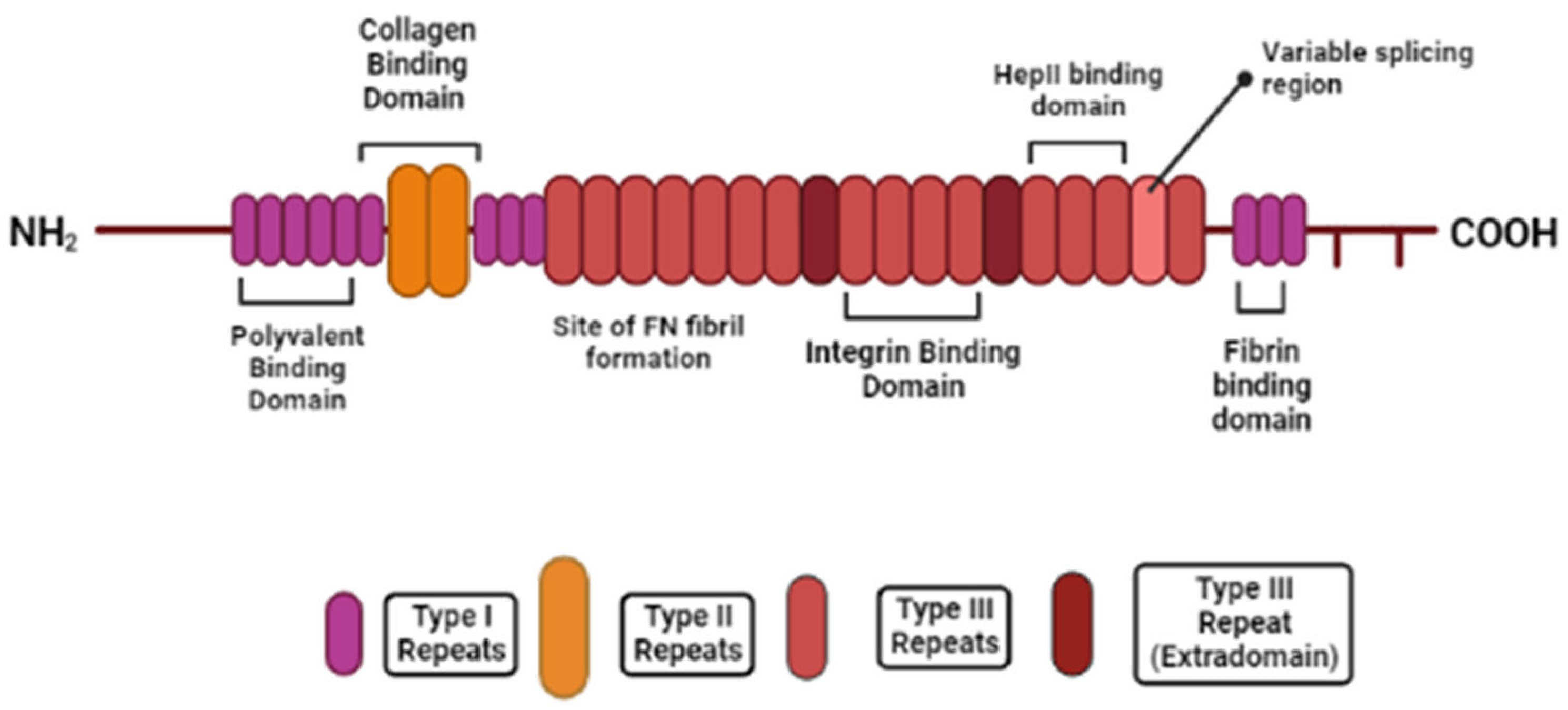
2.2. Fibronectin
2.3. Laminins
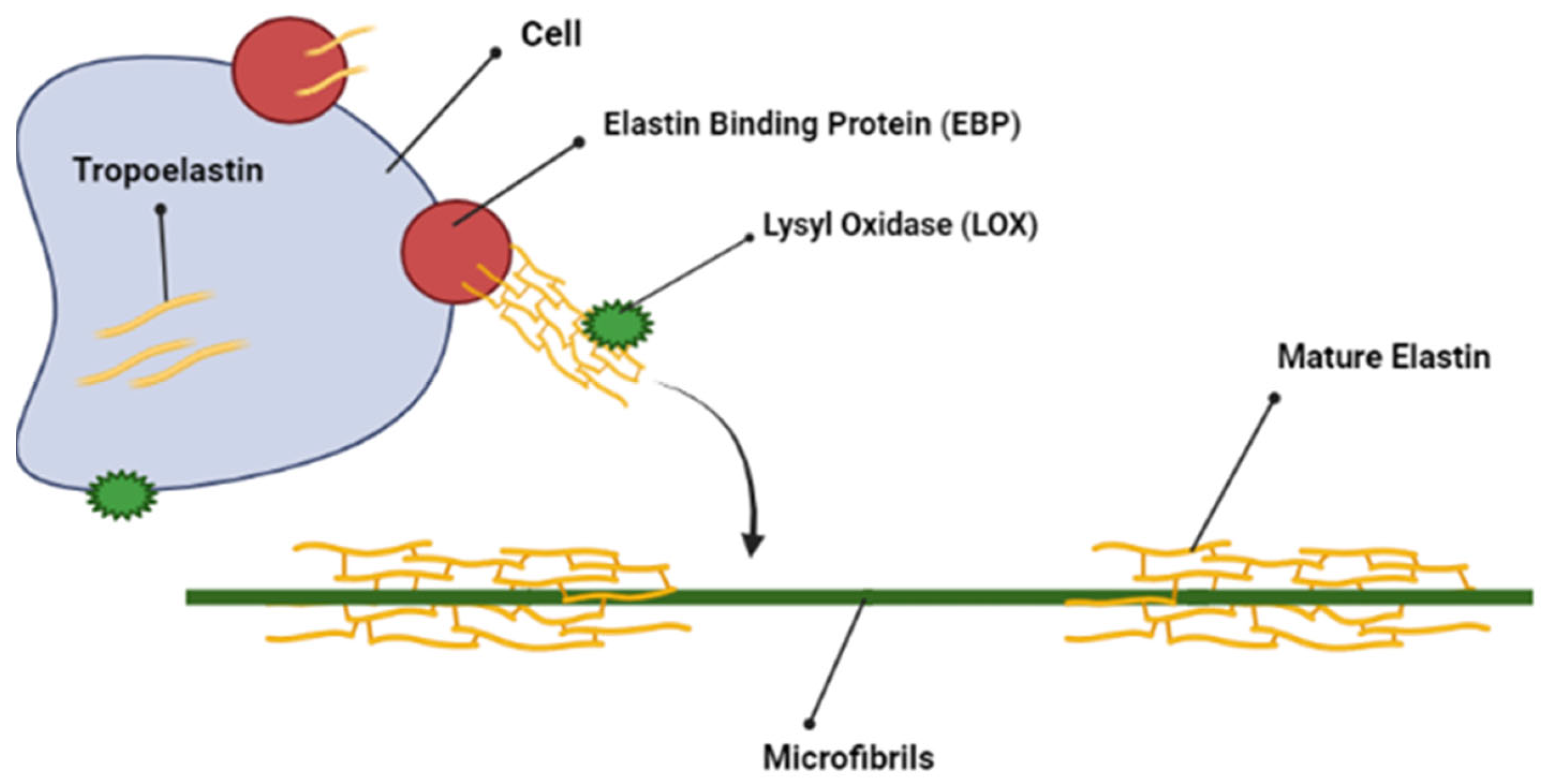
2.4. Elastin
2.5. Proteoglycans and Glycosaminoglycans
3. Composition of ECMs from Various Tissues
3.1. Dental Pulp ECM
3.2. Pulmonary ECM
3.3. Bone ECM
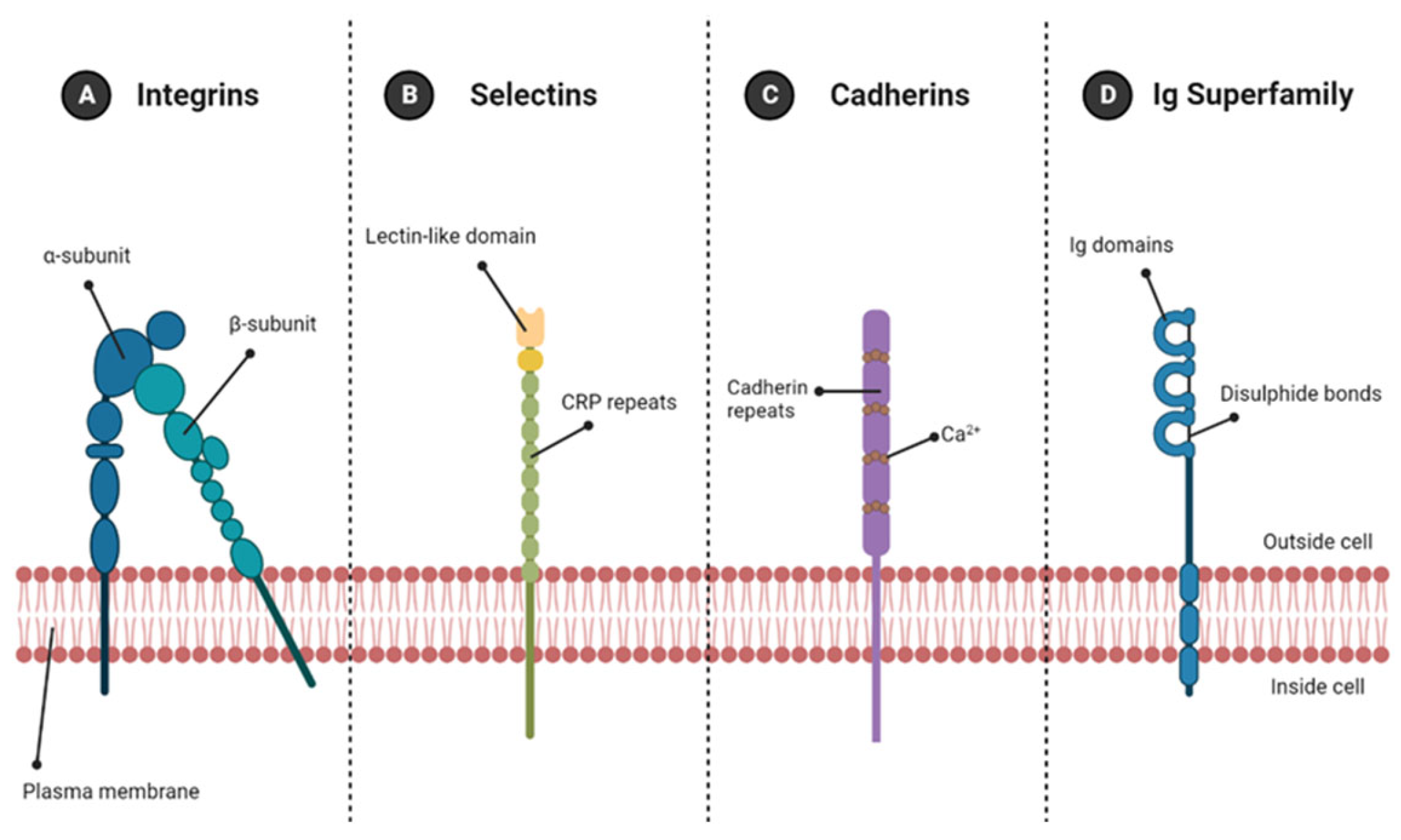
4. Cell Adhesion Molecules
4.1. Cell-ECM Adhesion Molecules
Integrins
4.2. Cell–Cell Adhesion Molecules
4.2.1. Immunoglobulin Superfamily
4.2.2. Cadherins
4.2.3. Selectins
5. ECM-Derived Peptide Sequences and Their Use in Biomimetic Hydrogel Functionalisation
5.1. Fibronectin: RGD Motif
5.2. Collagen: GFOGER Motif
5.3. Collagen: DGEA Motif
5.4. Collagen: P15 Motif
5.5. Fibronectin: LDV Motif
5.6. Laminin: IKVAV Motif
5.7. Laminin: YIGSR Motif
5.8. Elastin: GRKRK Motif
5.9. The Effect of Functionalising Hydrogels with ECM-Derived Peptide Motifs on Gelation
5.10. The Synergy Effect
6. Hydrogels Functionalised with Multiple Adhesion Motifs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Ghayor, C.; Weber, F.E. The Use of Adipose Tissue-Derived Progenitors in Bone Tissue Engineering—A Review. Transfus. Med. Hemother. 2016, 43, 336–343. [Google Scholar] [CrossRef]
- Orciani, M.; Fini, M.; Di Primio, R.; Mattioli-Belmonte, M. Biofabrication and Bone Tissue Regeneration: Cell Source, Approaches, and Challenges. Front. Bioeng. Biotechnol. 2017, 5, 17. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Chapter 10—Hydrogels. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. [Google Scholar]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Seow, W.Y.; Hauser, C.A. Short to ultrashort peptide hydrogels for biomedical uses. Mater. Today 2014, 17, 381–388. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zhu, S.; Liu, H.; Chen, J. Preparation and applications of peptide-based injectable hydrogels. RSC Adv. 2019, 9, 28299–28311. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.S.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Biopolymers 2013, 100, 637–644. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.M.; Yacoub, M.H. Hydrogel scaffolds for tissue engineering: Progress and challenges. Glob. Cardiol. Sci. Pract. 2013, 2013, 316–342. [Google Scholar] [CrossRef]
- Unal, A.Z.; West, J.L. Synthetic ECM: Bioactive Synthetic Hydrogels for 3D Tissue Engineering. Bioconj. Chem. 2020, 31, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. In StemBook; Harvard Stem Cell Institute: Boston, MA, USA, 2008. [Google Scholar]
- Kim, M.; Choi, Y.S.; Yang, S.H.; Hong, H.-N.; Cho, S.-W.; Cha, S.M.; Pak, J.H.; Kim, C.W.; Kwon, S.W.; Park, C.J. Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem. Biophys. Res. Commun. 2006, 348, 386–392. [Google Scholar] [CrossRef]
- Bhang, S.H.; Lim, J.S.; Choi, C.Y.; Kwon, Y.K.; Kim, B.-S. The behavior of neural stem cells on biodegradable synthetic polymers. J. Biomater. Sci. Polym. Ed. 2007, 18, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Zygourakis, K.; Farach-Carson, M.C.; Yaszemski, M.J.; Mikos, A.G. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J. Biomed. Mater. Res. 2004, 69A, 535–543. [Google Scholar] [CrossRef]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and Characterization of Poly(Ethylene Glycol) Photopolymerizable Semi-Interpenetrating Networks for Chondrogenesis of Human Mesenchymal Stem Cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef]
- Afami, M.E.; El Karim, I.; About, I.; Krasnodembskaya, A.D.; Laverty, G.; Lundy, F.T. Multicomponent Peptide Hydrogels as an Innovative Platform for Cell-Based Tissue Engineering in the Dental Pulp. Pharmaceutics 2021, 13, 1575. [Google Scholar] [CrossRef] [PubMed]
- Afami, M.E.; El Karim, I.; About, I.; Coulter, S.M.; Laverty, G.; Lundy, F.T. Ultrashort Peptide Hydrogels Display Antimicrobial Activity and Enhance Angiogenic Growth Factor Release by Dental Pulp Stem/Stromal Cells. Materials 2021, 14, 2237. [Google Scholar] [CrossRef]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.; D’souza, R.; Hartgerink, J.; Schmalz, G. Scaffolds for Dental Pulp Tissue Engineering. Adv. Dent. Res. 2011, 23, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef] [PubMed]
- Davari, N.; Bakhtiary, N.; Khajehmohammadi, M.; Sarkari, S.; Tolabi, H.; Ghorbani, F.; Ghalandari, B. Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers 2022, 14, 986. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; White, L.; Shakesheff, K.; Tatullo, M. Odontogenic Differentiation of Human Dental Pulp Stem Cells on Hydrogel Scaffolds Derived from Decellularized Bone Extracellular Matrix and Collagen Type I. PLoS ONE 2016, 11, e0148225. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef]
- Ryu, J.H.; Kim, I.-K.; Cho, S.-W.; Cho, M.-C.; Hwang, K.-K.; Piao, H.; Piao, S.; Lim, S.H.; Hong, Y.S.; Choi, C.Y. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials 2005, 26, 319–326. [Google Scholar] [CrossRef]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef]
- Joki, T.; Machluf, M.; Atala, A.; Zhu, J.; Seyfried, N.T.; Dunn, I.F.; Abe, T.; Carroll, R.S.; Black, P.M. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat. Biotechnol. 2001, 19, 35–39. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Baker, J.P.; Blanch, H.W.; Prausnitz, J.M. Swelling properties of acrylamide-based ampholytic hydrogels: Comparison of experiment with theory. Polymer 1995, 36, 1061–1069. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Dissemond, J.; Witthoff, M.; Brauns, T.C.; Haberer, D.; Goos, M. pH values in chronic wounds. Evaluation during modern wound therapy. Hautarzt 2003, 54, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, S.J.; Kim, S.I. Properties of smart hydrogels composed of polyacrylic acid/poly(vinyl sulfonic acid) responsive to external stimuli. Smart Mater. Struct. 2004, 13, 317. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I. Controlled drug release via minimization of burst release in pH-response kappa-carrageenan/polyvinyl alcohol hydrogels. Chem. Eng. Res. Des. 2013, 91, 508–519. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Ji, W.; Wu, Q.; Han, X.; Zhang, W.; Wei, W.; Chen, L.; Li, L.; Huang, W. Photosensitive hydrogels: From structure, mechanisms, design to bioapplications. Sci. China Life Sci. 2020, 63, 1813–1828. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Chu, C.-C. Synthesis of photoresponsive hybrid alginate hydrogel with photo-controlled release behavior. Carbohydr. Polym. 2015, 119, 18–25. [Google Scholar] [CrossRef]
- Wang, D.; Wagner, M.; Butt, H.-J.; Wu, S. Supramolecular hydrogels constructed by red-light-responsive host–guest interactions for photo-controlled protein release in deep tissue. Soft Matter 2015, 11, 7656–7662. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Minehan, R.L.; Del Borgo, M.P. Controlled Release of Therapeutics From Enzyme-Responsive Biomaterials. Front. Biomater. Sci. 2022, 1. [Google Scholar] [CrossRef]
- Zhai, Z.; Lei, L.; Song, J.; Song, B.; Pei, X.; Cui, Z. Mechanically-sensitive hydrogels formed from β-cyclodextrin and an anionic surfactant containing a biphenyl group. Soft Matter 2016, 12, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Wade, R.J.; Burdick, J.A. Engineering ECM signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Zheng, C.-X.; Sui, B.-D.; Hu, C.-H.; Qiu, X.-Y.; Zhao, P.; Jin, Y. Reconstruction of structure and function in tissue engineering of solid organs: Toward simulation of natural development based on decellularization. J. Tissue Eng. Regen. Med. 2018, 12, 1432–1447. [Google Scholar] [CrossRef]
- Tam, R.Y.; Owen, S.C.; Shoichet, M.S. ECM-Inspired Chemical Cues: Biomimetic Molecules and Techniques of Immobilization. In Bio-Inspired Materials for Biomedical Engineering; Wiley: Hoboken, NJ, USA, 2014; pp. 5–24. [Google Scholar]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Blackmon, R.L.; Sandhu, R.; Chapman, B.S.; Casbas-Hernandez, P.; Tracy, J.B.; Troester, M.A.; Oldenburg, A.L. Imaging Extracellular Matrix Remodeling In Vitro by Diffusion-Sensitive Optical Coherence Tomography. Biophys. J. 2016, 110, 1858–1868. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ladoux, B.; Mège, R.-M. Mechanobiology of collective cell behaviours. Nat. Rev. Mol. Cell Biol. 2017, 18, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of Fibronectin Extracellular Matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef]
- Qiao, P.; Lu, Z.-R. Fibronectin in the Tumor Microenvironment, in Tumor Microenvironment: Extracellular Matrix Components—Part A; Birbrair, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 85–96. [Google Scholar]
- Durbeej, M. Laminins. Cell Tissue Res. 2009, 339, 259. [Google Scholar] [CrossRef]
- Aumailley, M.; Smyth, N. The role of laminins in basement membrane function. J. Anat. 1998, 193, 1–21. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Patel, D.; Menon, R.; Taite, L.J. Self-Assembly of Elastin-Based Peptides into the ECM: The Importance of Integrins and the Elastin Binding Protein in Elastic Fiber Assembly. Biomacromolecules 2010, 12, 432–440. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.; Esko, J. Essentials of Glycobiology. In Proteoglycans and Glycosaminoglycans—Chapter 11, 4th ed.; National Library of Medicine Cold Spring Harbour Laboratory Press: Woodbury, NY, USA, 1999. [Google Scholar]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; El-Sayed, K.M.F. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A.J. Cells and Extracellular Matrices of Dentin and Pulp: A Biological Basis for Repair and Tissue Engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef]
- Berman, L.H.; Hargreaves, K.M. Cohen’s Pathways of the Pulp, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ito, J.T.; Lourenço, J.D.; Righetti, R.F.; Tibério, I.F.; Prado, C.M.; Lopes, F.D. Extracellular Matrix Component Remodeling in Respiratory Diseases: What Has Been Found in Clinical and Experimental Studies? Cells 2019, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Hackett, T.L.; Osei, E.T. Modeling Extracellular Matrix-Cell Interactions in Lung Repair and Chronic Disease. Cells 2021, 10, 2145. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.-G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Eng. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef]
- Rentsch, C.; Heinemann, S.; Bernhardt, R.; Bischoff, B.; Förster, Y.; Scharnweber, D.; Rammelt, S. ECM Inspired Coating of Embroidered 3D Scaffolds Enhances Calvaria Bone Regeneration. BioMed Res. Int. 2014, 2014, 217078. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell. In Integrins, 4th ed.; National Library of Medicine Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Wong, C.W.; Dye, D.E.; Coombe, D.R. The Role of Immunoglobulin Superfamily Cell Adhesion Molecules in Cancer Metastasis. Int. J. Cell Biol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leshchyns’Ka, I.; Sytnyk, V. Reciprocal Interactions between Cell Adhesion Molecules of the Immunoglobulin Superfamily and the Cytoskeleton in Neurons. Front. Cell Dev. Biol. 2016, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yang, L.; Li, T.; Zhang, Y. Cadherin Signaling in Cancer: Its Functions and Role as a Therapeutic Target. Front. Oncol. 2019, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Maître, J.-L.; Heisenberg, C.-P. Three Functions of Cadherins in Cell Adhesion. Curr. Biol. 2013, 23, R626–R633. [Google Scholar] [CrossRef]
- Tepass, U.; Truong, K.; Godt, D.; Ikura, M.; Peifer, M. Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell Biol. 2000, 1, 91–100. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef]
- Vestweber, D.; Blanks, J.E. Mechanisms That Regulate the Function of the Selectins and Their Ligands. Physiol. Rev. 1999, 79, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A. Structural basis for selectin mechanochemistry. Proc. Natl. Acad. Sci. USA 2009, 106, 91–96. [Google Scholar] [CrossRef]
- Xu, Y.; Kirchner, M. Collagen Mimetic Peptides. Bioengineering 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Schaffner, P.; Dard, M.M. Structure and function of RGD peptides involved in bone biology. Cell. Mol. Life Sci. 2003, 60, 119–132. [Google Scholar] [CrossRef]
- Kudva, A.K.; Luyten, F.P.; Patterson, J. RGD-functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum-derived cells. J. Biomed. Mater. Res. A 2018, 106, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Salinas, C.N.; Anseth, K.S. The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J. Tissue Eng. Regen. Med. 2008, 2, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cui, Z.-K.; Fan, J.; Fartash, A.; Aghaloo, T.L.; Lee, M. Photocrosslinkable chitosan hydrogels functionalized with the RGD peptide and phosphoserine to enhance osteogenesis. J. Mater. Chem. B 2016, 4, 5289–5298. [Google Scholar] [CrossRef] [PubMed]
- Bidarra, S.J.; Barrias, C.C.; Fonseca, K.B.; Barbosa, M.A.; Soares, R.A.; Granja, P.L. Injectable in situ crosslinkable RGD-modified alginate matrix for endothelial cells delivery. Biomaterials 2011, 32, 7897–7904. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Zhang, Z.; He, C.; Chen, X. Bioactive polypeptide hydrogels modified with RGD and N-cadherin mimetic peptide promote chondrogenic differentiation of bone marrow mesenchymal stem cells. Sci. China Chem. 2020, 63, 1100–1111. [Google Scholar] [CrossRef]
- Hong, K.H.; Song, S.-C. 3D hydrogel stem cell niche controlled by host-guest interaction affects stem cell fate and survival rate. Biomaterials 2019, 218, 119338. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Trucco, D.; Saleh, Y.; Gabusi, E.; Dolzani, P.; Lenzi, E.; Vannozzi, L.; Ricotti, L.; Lisignoli, G. RGD-Functionalized Hydrogel Supports the Chondrogenic Commitment of Adipose Mesenchymal Stromal Cells. Gels 2022, 8, 382. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.G.; Morton, L.F.; Peachey, A.R.; Tuckwell, D.S.; Farndale, R.W.; Barnes, M.J. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 2000, 275, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, R.; Öztürk, E.; Vallmajo-Martin, Q.; Millan, C.; Müller, M.; Zenobi-Wong, M. GFOGER-Modified MMP-Sensitive Polyethylene Glycol Hydrogels Induce Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 1165–1174. [Google Scholar] [CrossRef]
- Liu, S.Q.; Tian, Q.; Hedrick, J.L.; Hui, J.H.P.; Ee, P.L.R.; Yang, Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 2010, 31, 7298–7307. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.Y.; Martin, K.E.; García, J.R.; Johnson, C.T.; Theriault, H.S.; Han, W.M.; Zhou, D.W.; Botchwey, E.A.; García, A.J. Integrin-specific hydrogels modulate transplanted human bone marrow-derived mesenchymal stem cell survival, engraftment, and reparative activities. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.Y.; Yang, D.H.; You, S.J.; Kim, H.J.; Chun, H.J. In-situ forming injectable GFOGER-conjugated BMSCs-laden hydrogels for osteochondral regeneration. NPJ Regen. Med. 2023, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Staatz, W.D.; Fok, K.F.; Zutter, M.M.; Adams, S.P.; Rodriguez, B.A.; Santoro, S.A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J. Biol. Chem. 1991, 266, 7363–7367. [Google Scholar] [CrossRef]
- McCann, T.; Mason, W.; Meikle, M.; McDonald, F. A collagen peptide motif activates tyrosine kinase-dependent calcium signalling pathways in human osteoblast-like cells. Matrix Biol. 1997, 16, 273–283. [Google Scholar] [CrossRef]
- Mehta, M.; Madl, C.M.; Lee, S.; Duda, G.N.; Mooney, D.J. The collagen I mimetic peptide DGEA enhances an osteogenic phenotype in mesenchymal stem cells when presented from cell-encapsulating hydrogels. J. Biomed. Mater. Res. Part A 2015, 103, 3516–3525. [Google Scholar] [CrossRef]
- Jha, A.; Moore, E. Collagen-derived peptide, DGEA, inhibits pro-inflammatory macrophages in biofunctional hydrogels. J. Mater. Res. 2021, 37, 77–87. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J. Cell Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Omi, M.; Sun, M.; Cheng, B.; Mao, G.; Liu, T.; Mendonça, G.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Covalent Attachment of P15 Peptide to Ti Alloy Surface Modified with Polymer to Enhance Osseointegration of Implants. ACS Appl. Mater. Interfaces 2019, 11, 38531–38536. [Google Scholar] [CrossRef]
- Zhang, J.; Eisenhauer, P.; Kaya, O.; Vaccaro, A.R.; Diallo, C.; Fertala, A.; Freeman, T.A. P15 peptide stimulates chondrogenic commitment and endochondral ossification. Int. Orthop. 2017, 41, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Qian, J.J.; Bhatnagar, R.S.; Li, S. Enhanced cell attachment and osteoblastic activity by P-15 peptide-coated matrix in hydrogels. Biochem. Biophys. Res. Commun. 2003, 311, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wayner, E.A.; Kovach, N.L. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J. Cell Biol. 1992, 116, 489–497. [Google Scholar] [CrossRef]
- Yamada, K.M. Adhesive recognition sequences. J. Biol. Chem. 1991, 266, 12809–12812. [Google Scholar] [CrossRef]
- Cringoli, M.C.; Romano, C.; Parisi, E.; Waddington, L.J.; Melchionna, M.; Semeraro, S.; De Zorzi, R.; Grönholm, M.; Marchesan, S. Bioadhesive supramolecular hydrogel from unprotected, short d,l-peptides with Phe-Phe and Leu-Asp-Val motifs. Chem. Commun. 2020, 56, 3015–3018. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Josey, B.; Chou, C.J.; Tan, Y.; Zhang, N.; Wen, X. Short Laminin Peptide for Improved Neural Stem Cell Growth. STEM CELLS Transl. Med. 2014, 3, 662–670. [Google Scholar] [CrossRef]
- Tashiro, K.; Sephel, G.C.; Weeks, B.; Sasaki, M.; Martin, G.R.; Kleinman, H.K.; Yamada, Y. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J. Biol. Chem. 1989, 264, 16174–16182. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, W.; Shao, Q.; Li, B.; Yu, D.; Zhou, X.; Parajuli, J.; Xu, H.; Qiu, T.; Yetisen, A.K.; et al. Pentapeptide IKVAV-engineered hydrogels for neural stem cell attachment. Biomater. Sci. 2021, 9, 2887–2892. [Google Scholar] [CrossRef]
- Sun, W.; Incitti, T.; Migliaresi, C.; Quattrone, A.; Casarosa, S.; Motta, A. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 2015, 11, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Yamada, Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J. Biol. Chem. 1987, 262, 17111–17117. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Ishii, E.; Nomizu, M.; Yamada, Y.; Mohri, S.; Kinukawa, N.; Matsuzaki, A.; Oshima, K.; Hara, T.; Miyazaki, S. The laminin-derived peptide YIGSR (Tyr–Ile–Gly–Ser–Arg) inhibits human pre-B leukaemic cell growth and dissemination to organs in SCID mice. Br. J. Cancer 1999, 80, 1898–1904. [Google Scholar] [CrossRef]
- Horák, D.; Matulka, K.; Hlídková, H.; Lapčíková, M.; Beneš, M.J.; Jaroš, J.; Hampl, A.; Dvořák, P. Pentapeptide-modified poly(N,N-diethylacrylamide) hydrogel scaffolds for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98B, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.P.; Seitz, A.K.; Teßmar, J.K.; Blunk, T.; Göpferich, A.M. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials 2010, 31, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Rodgers, U.R.; Bilek, M.M.; Weiss, A.S. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J. Biol. Chem. 2009, 284, 28616–28623. [Google Scholar] [CrossRef]
- Odian, G. Step Polymerization. In Principles of Polymerization; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 39–197. [Google Scholar]
- Moeinzadeh, S.; Jabbari, E. Gelation characteristics, physico-mechanical properties and degradation kinetics of micellar hydrogels. Eur. Polym. J. 2015, 72, 566–576. [Google Scholar] [CrossRef]
- Mauri, E.; Sacchetti, A.; Rossi, F. The Synthesis of RGD-functionalized Hydrogels as a Tool for Therapeutic Applications. J. Vis. Exp. 2016, 116, e54445. [Google Scholar] [CrossRef]
- Mauri, E.; Sacchetti, A.; Vicario, N.; Peruzzotti-Jametti, L.; Rossi, F.; Pluchino, S. Evaluation of RGD functionalization in hybrid hydrogels as 3D neural stem cell culture systems. Biomater. Sci. 2018, 6, 501–510. [Google Scholar] [CrossRef]
- Anderson, J.M.; Andukuri, A.; Lim, D.J.; Jun, H.-W. Modulating the Gelation Properties of Self-Assembling Peptide Amphiphiles. ACS Nano 2009, 3, 3447–3454. [Google Scholar] [CrossRef]
- Tselepis, V.H.; Green, L.J.; Humphries, M.J. An RGD to LDV Motif Conversion within the Disintegrin Kistrin Generates an Integrin Antagonist That Retains Potency but Exhibits Altered Receptor Specificity. J. Biol. Chem. 1997, 272, 21341–21348. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.W.; Turner, S.D.; Mann, B.K. Adhesive and mechanical properties of hydrogels influence neurite extension. J. Biomed. Mater. Res. 2004, 72A, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Roy, S. Controlling Neuronal Cell Growth through Composite Laminin Supramolecular Hydrogels. ACS Biomater. Sci. Eng. 2020, 6, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, K.Y. Dual peptide-presenting hydrogels for controlling the phenotype of PC12 cells. Colloids Surfaces B: Biointerfaces 2017, 152, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.; Benoit, D. Dual peptide-functionalized hydrogels differentially control periodontal cell function and promote tissue regeneration. Biomater. Adv. 2022, 141, 213093. [Google Scholar] [CrossRef] [PubMed]
- Dvořáková, J.; Trousil, J.; Podhorská, B.; Mikšovská, Z.; Janoušková, O.; Proks, V. Enzymatically Cross-linked Hydrogels Based on Synthetic Poly(α-amino acid)s Functionalized with RGD Peptide for 3D Mesenchymal Stem Cell Culture. Biomacromolecules 2021, 22, 1417–1431. [Google Scholar] [CrossRef]


| Class | Type | Genes Encoding Proteins Common in Collagen | Description |
|---|---|---|---|
| Fibril-forming collagens | I II III V XI XXIV XXVII | COL1A1 and COL1A2 COL2A1 COL3A1 COL5A1, COL5A2 and COL5A3 COL11A1, COL11A2, COL11A3 COL24A1 COL27A1 | Quarter-stagger arrangement of fibrils which are packed into stromal tissues such as bone and tendons |
| Network-forming collagens | IV | COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6 | Network structure composed of laminins and basement membrane proteins |
| Fibril-associated collagens | IX XII XIV XVI XIX XX XXI XXII | COL9A1, COL9A2, COL9A3 COL12A1 COL14A1 COL16A1 COL19A1 COL20A1 COL21A1 COL22A1 | Fibril-associated collagens with interrupted triple helices (FACIT); molecular bridges associated with type I and type II collagen fibrils |
| Location | Example | Gene Symbol | Glycosaminoglycan Chain |
|---|---|---|---|
| Intracellular | Serglycin | SRGN | Heparin |
| Cell surface | Betaglycan Syndecan | TGFBR3 SDC | Chondroitin sulfate/Heparan sulfate Heparan sulfate |
| Pericellular | Agrin Collagen XV | AGRN COL15A1 | Heparan sulfate Chondroitin sulfate/Heparan sulfate |
| Extracellular | Biglycan Decorin | BGN DCN | Chondroitin sulfate Dermatan sulfate |
| ECM Peptide | Hydrogel | Cell Application | Cell Type | Reference |
|---|---|---|---|---|
| GFOGER | PEG | Proliferation Differentiation | MSCs | [100] |
| Viability Differentiation | MSCs | [101] | ||
| Viability and Signalling Differentiation | hBMSCs | [102] | ||
| PEG-PCL | Adhesion Spreading Regeneration | BMSCs | [103] | |
| DGEA | Alginate | Adhesion Viability Differentiation | MSCs | [106] |
| P15 | Hyaluronate (with ABM) | Attachment Migration Viability Differentiation | Osteoblast- like HOS cells | [111] |
| RGD | PEG | Viability Proliferation Differentiation | hPDCs | [91] |
| Viability | MSCs | [92] | ||
| Proliferation Differentiation | MSCs | [100] | ||
| Chitosan | Attachment Proliferation Spreading Differentiation | BMSCs | [93] | |
| Vitrogel | Viability Differentiation Adhesion Spreading | hASCs | [97] | |
| Alginate | Viability Spreading Proliferation | HUVECs | [94] | |
| Polypeptide | Viability Spreading Proliferation | BMSCs | [95] | |
| Poly organo- phosphazene | Viability Spreading Proliferation | MSCs | [96] | |
| Hyaluronic acid/Pectin | Differentiation | Chondrocytes | [98] | |
| PHEG | Adhesion Spreading Proliferation Cell–cell and cell–matrix interactions | MSCs | [134] | |
| LDV | Peptide | Viability Spreading | Fibroblasts | [114] |
| IKVAV | PLEOF | Viability Adhesion Differentiation | Neural stem cells | [117] |
| Silk fibroin | Viability Proliferation Differentiation | Neural stem cells | [118] | |
| YIGSR | PEG | Differentiation Attachment Proliferation | Adipocytes | [122] |
| RGDS, IKVAV YIGSR | PEG | Neurite extension | PC12 cells | [130] |
| IKVAV, YIGSR | Peptide | Improved cytotoxicity | C6 glial cells | [131] |
| SHSY5Y neuro- blastoma cells | ||||
| RGD, N-cadherin | PLG | Differentiation | BMSCs | [95] |
| RGD, YIGSR | Alginate | Adhesion Proliferation Differentiation | PC12 cells | [132] |
| RGD, GFOGER | PEG | Viability Mineralisation | Periodontal ligament stem cells | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morwood, A.J.; El-Karim, I.A.; Clarke, S.A.; Lundy, F.T. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules 2023, 28, 4616. https://doi.org/10.3390/molecules28124616
Morwood AJ, El-Karim IA, Clarke SA, Lundy FT. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules. 2023; 28(12):4616. https://doi.org/10.3390/molecules28124616
Chicago/Turabian StyleMorwood, Anna J., Ikhlas A. El-Karim, Susan A. Clarke, and Fionnuala T. Lundy. 2023. "The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels" Molecules 28, no. 12: 4616. https://doi.org/10.3390/molecules28124616
APA StyleMorwood, A. J., El-Karim, I. A., Clarke, S. A., & Lundy, F. T. (2023). The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules, 28(12), 4616. https://doi.org/10.3390/molecules28124616






