Nanomaterials with Glucose Oxidase-Mimicking Activity for Biomedical Applications
Abstract
1. Introduction
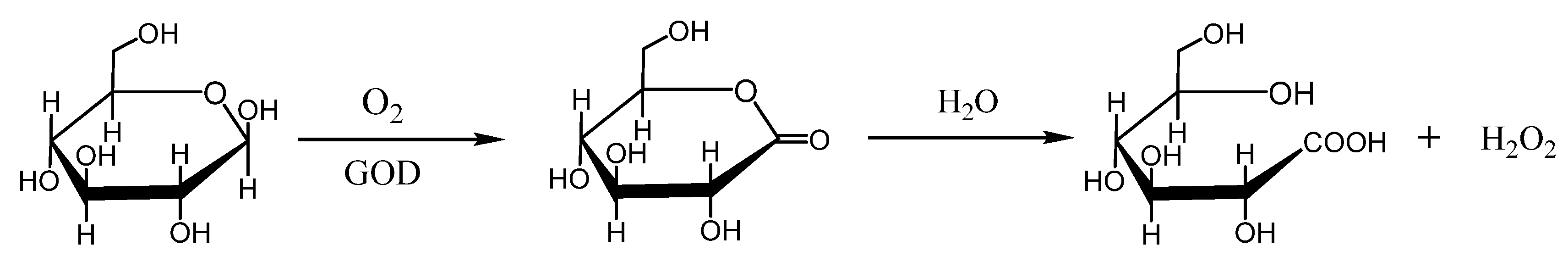
2. Nanomaterials with GOD-like Activities
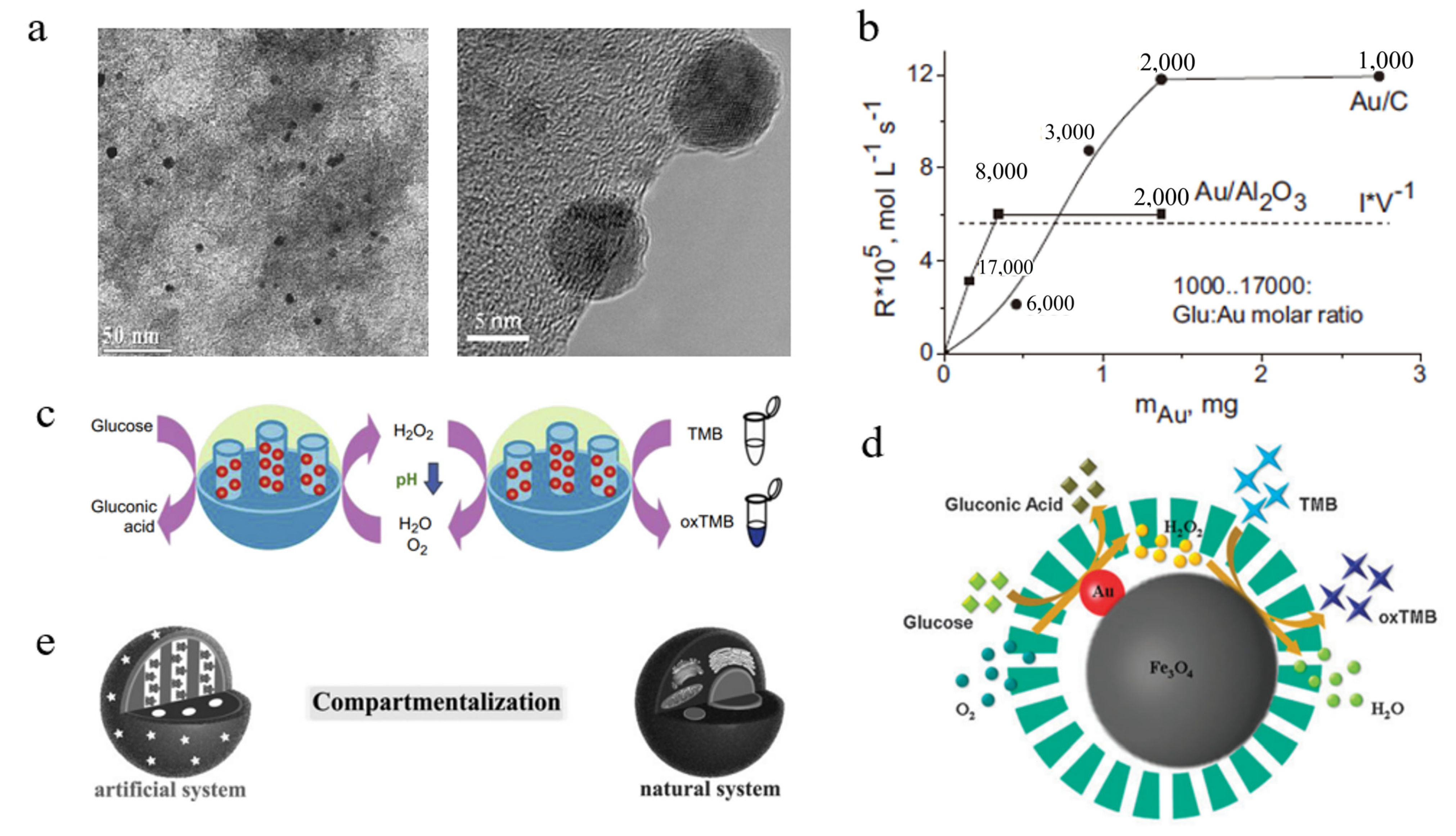
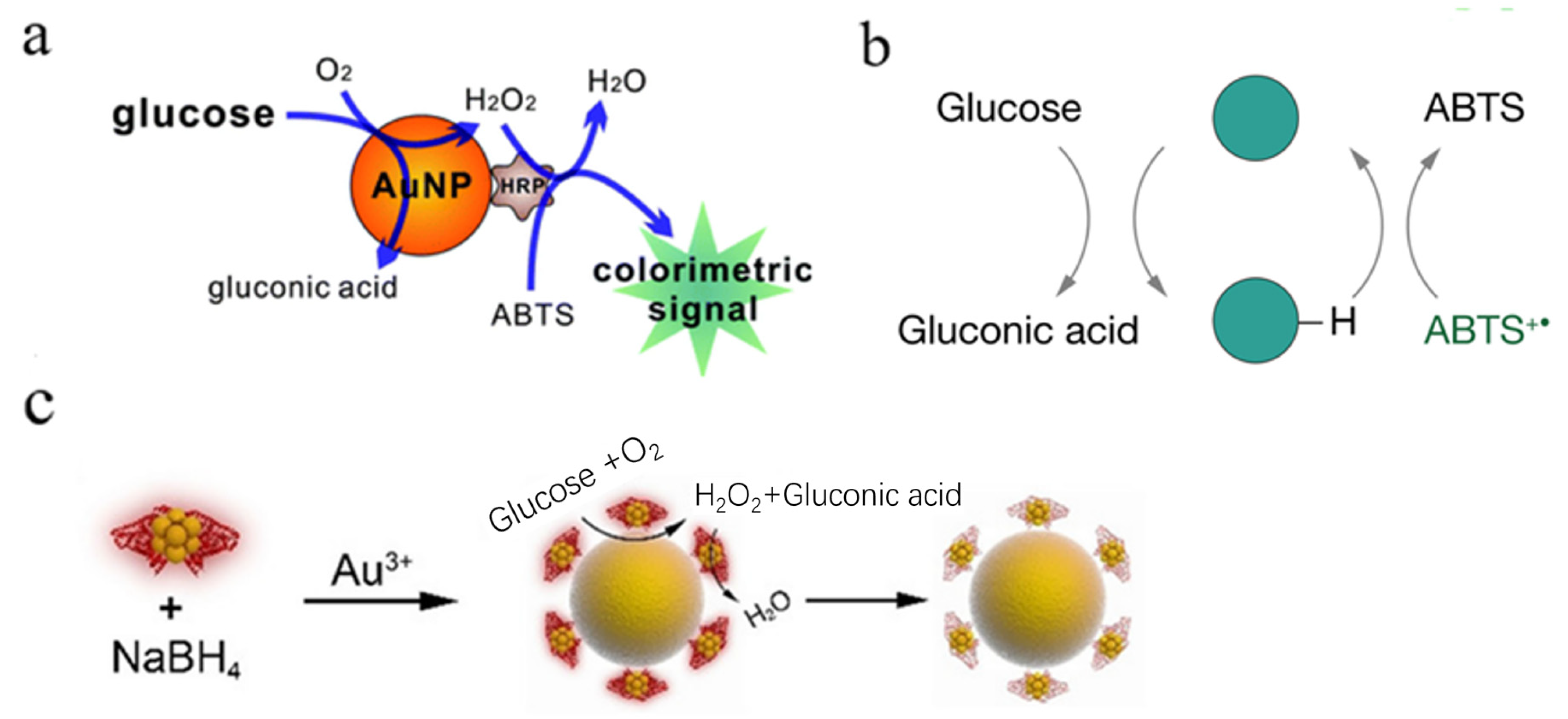
2.1. Au Nanomaterials

2.2. MnO2 Nanomaterials
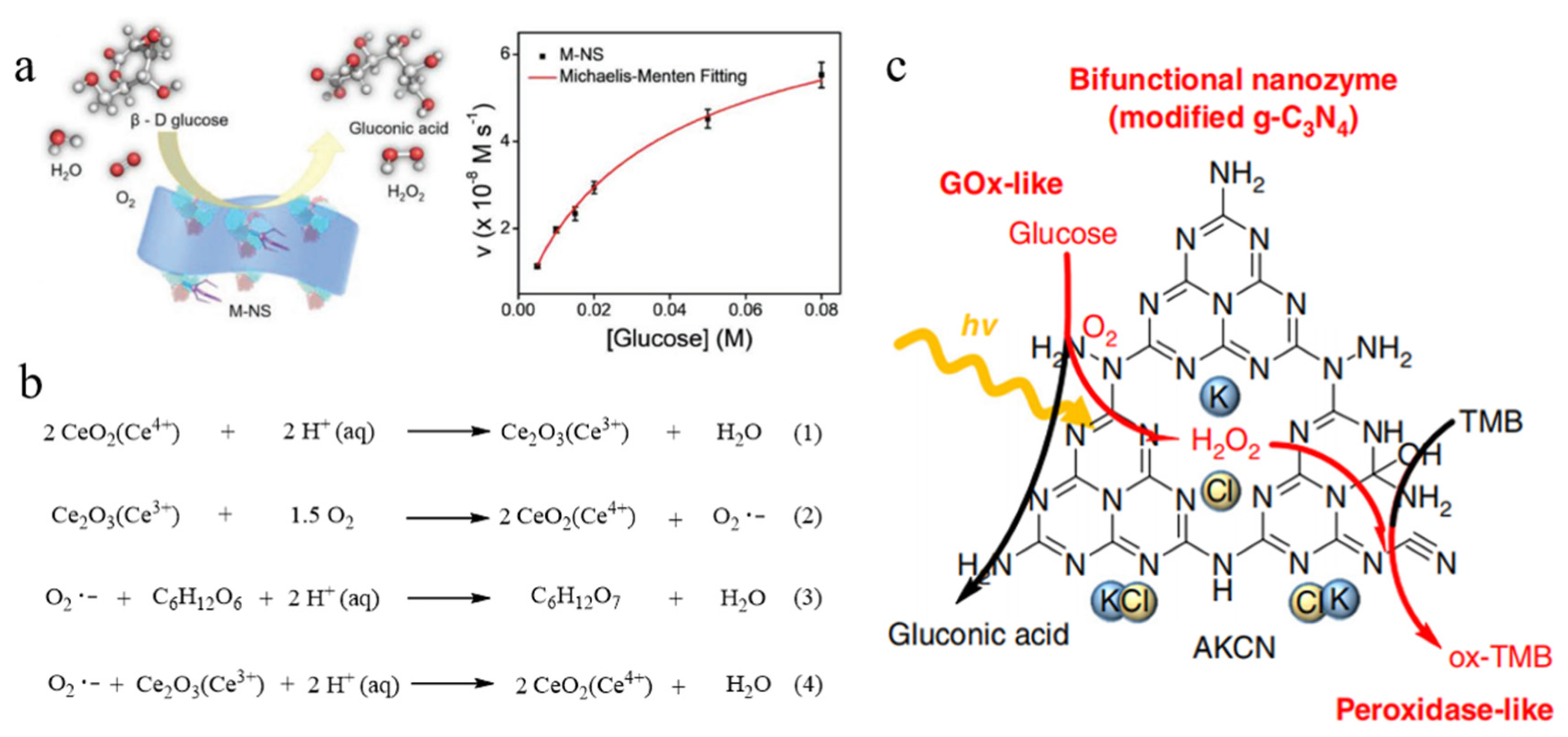
2.3. CeO2 Nanomaterials
2.4. C3N4 Nanomaterials
2.5. Others
3. Activity Regulation of GOD-mimicking Nanomaterials
3.1. Impact of the Physicochemical Parameters in the Catalytic Performance of Nanomaterials
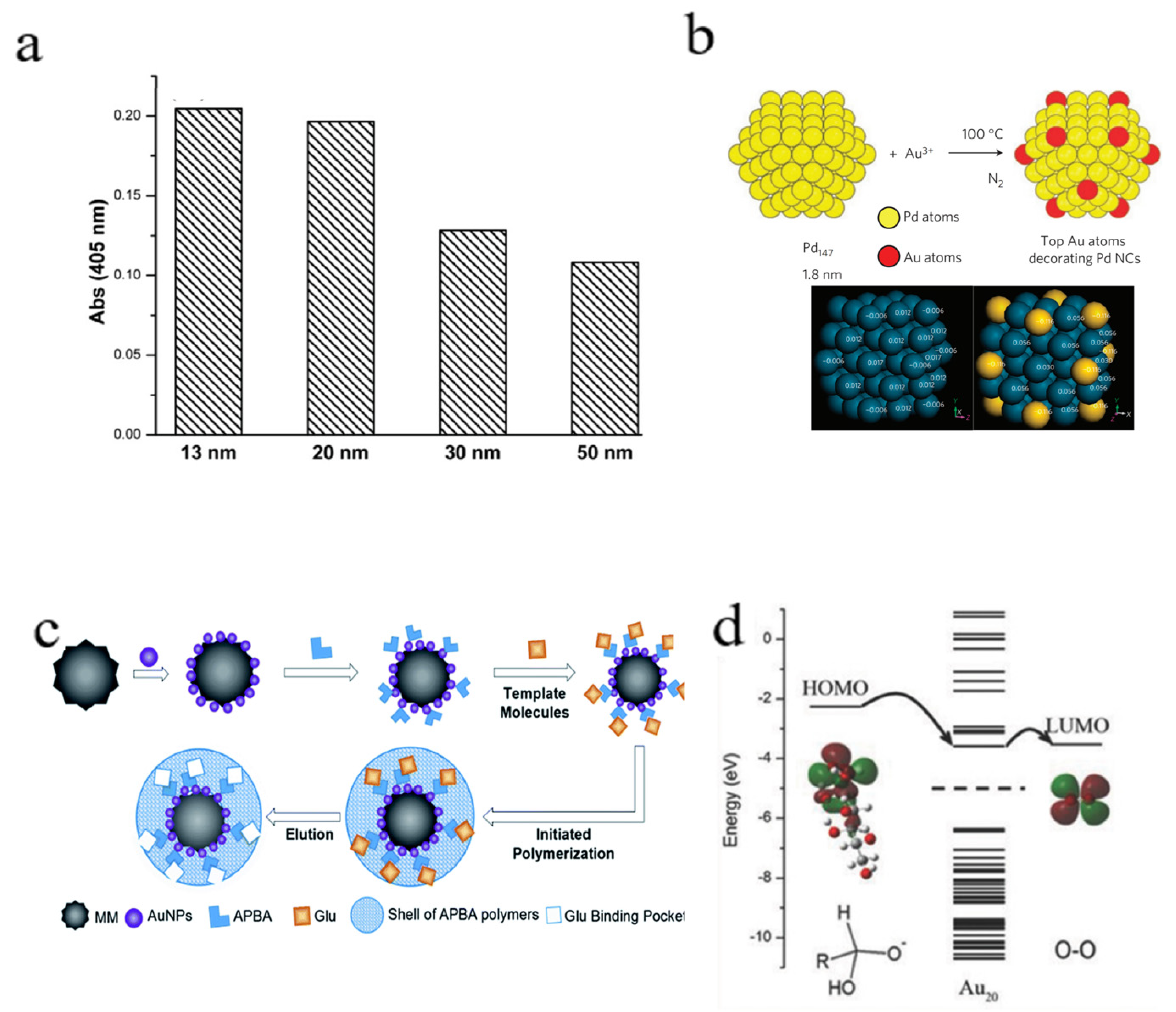
3.1.1. Size and Morphology
3.1.2. Composition
3.1.3. Surface Modification
3.2. Impact of the Environmental Parameters in the Catalytic Performance of Nanomaterials
3.2.1. Environmental pH and Temperature
3.2.2. Light Illumination
4. Biomedical Applications of GOD-mimicking Nanomaterials
4.1. Glucose Detection
4.2. DNA Detection

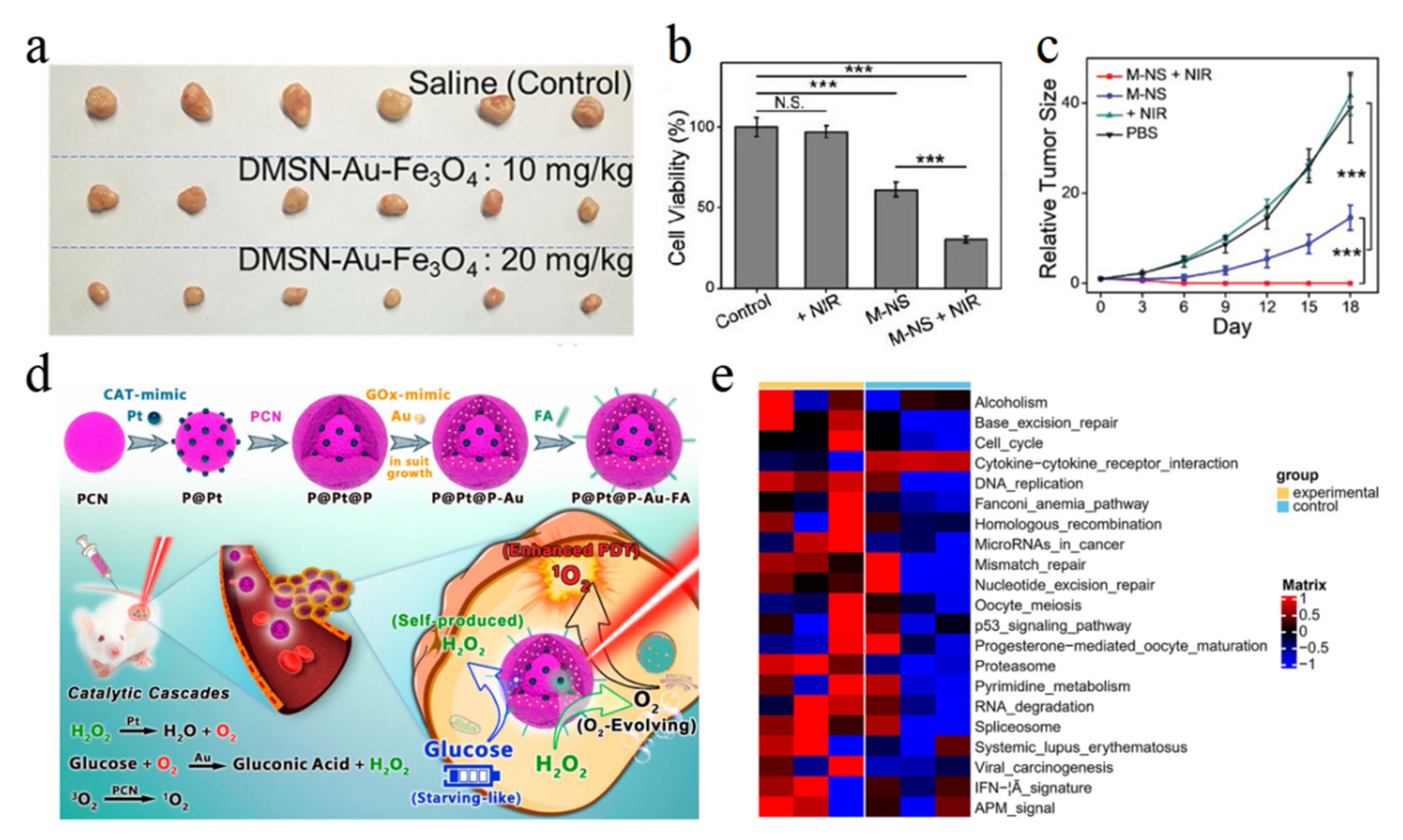
4.3. Tumor Treatment
5. Summary and Perspectives
5.1. Discovery of Novel GOD-mimicking Nanomaterials
5.2. Improvement of the GOD-like Activity
5.3. Regulation of the Catalytic Selectivity
5.4. Expansion of Practical Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, R.; Turner, A. Glucose oxidase: An ideal enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Raba, J.; Mottola, H.A. Glucose oxidase as an analytical reagent. Crit. Rev. Anal. Chem. 1995, 25, 1–42. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Ferri, S.; Kojima, K.; Sode, K. Review of glucose oxidases and glucose dehydrogenases: A bird’s eye view of glucose sensing enzymes. J. Diabetes Sci. Technol. 2011, 5, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Lahmer, F.; Gallezot, P.; Fuertes, P.; Fleche, G. Catalytic Oxidation of Glucose on Bismuth-Promoted Palladium Catalysts. J. Catal. 1995, 152, 116–121. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent advances in nanozyme research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Chong, Y.; Ning, J.; Min, S.; Ye, J.; Ge, C. Emerging nanozymes for potentiating radiotherapy and radiation protection. Chin. Chem. Lett. 2022, 33, 3315–3324. [Google Scholar] [CrossRef]
- Chong, Y.; Liu, Q.; Ge, C. Advances in oxidase-mimicking nanozymes: Classification, activity regulation and biomedical applications. Nano Today 2021, 37, 101076. [Google Scholar] [CrossRef]
- Chong, Y.; Dai, X.; Fang, G.; Wu, R.; Zhao, L.; Ma, X.; Tian, X.; Lee, S.; Zhang, C.; Chen, C.; et al. Palladium concave nanocrystals with high-index facets accelerate ascorbate oxidation in cancer treatment. Nat. Commun. 2018, 9, 4861. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, G.; Feng, W.; Wang, P.; Gao, J.; Liu, J.; Wang, M.; Wang, Q. Detection of glucose based on noble metal nanozymes: Mechanism, activity regulation, and enantioselective recognition. Small 2023, 19, 2205924. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, C.; Su, S.; Li, D.; He, Y.; Huang, Q.; Fan, C. Self-Catalyzed, Self-Limiting Growth of Glucose Oxidase-Mimicking Gold Nanoparticles. ACS Nano 2010, 4, 7451–7458. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Fan, W.; Zhang, W.; Yang, Z.; Li, L.; Wang, Z.; Chiang, Y.-L.; Liu, Y.; Deng, L.; He, L.; et al. Wet/Sono-Chemical Synthesis of Enzymatic Two-Dimensional MnO2 Nanosheets for Synergistic Catalysis-Enhanced Phototheranostics. Adv. Mater. 2019, 31, e1900401. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The Catalytic Activity of “Naked” Gold Particles. Angew. Chem. 2004, 116, 5936–5939. [Google Scholar] [CrossRef]
- Sharifi, M.; Faryabi, K.; Talaei, A.J.; Shekha, M.S.; Ale-Ebrahim, M.; Salihi, A.; Nanakali, N.M.Q.; Aziz, F.M.; Rasti, B.; Hasan, A.; et al. Antioxidant properties of gold nanozyme: A review. J. Mol. Liq. 2020, 297, 112004. [Google Scholar] [CrossRef]
- Xiang, H.; Huang, S.; Zhu, D.; Yu, L.; Liu, R.; Guo, Y.; Xu, L. Au nanozyme driven cascading catalysis in Tollens’ reaction: An insight of glucose oxidase-like mechanism. Chem. Eur. J. 2023, e202300454. [Google Scholar] [CrossRef]
- Chen, J.; Wu, W.; Huang, L.; Ma, Q.; Dong, S. Self-indicative gold nanozyme for H2O2 and glucose sensing. Chem. Eur. J. 2019, 25, 11940–11944. [Google Scholar] [CrossRef]
- Prüße, U.; Herrmann, M.; Baatz, C.; Decker, N. Gold-catalyzed selective glucose oxidation at high glucose concentrations and oxygen partial pressures. Appl. Catal. A Gen. 2011, 406, 89–93. [Google Scholar] [CrossRef]
- Comotti, M.; Della Pina, C.; Falletta, E.; Rossi, M. Aerobic Oxidation of Glucose with Gold Catalyst: Hydrogen Peroxide as Intermediate and Reagent. Adv. Synth. Catal. 2006, 348, 313–316. [Google Scholar] [CrossRef]
- Chen, J.; Ma, Q.; Li, M.; Chao, D.; Huang, L.; Wu, W.; Fang, Y.; Dong, S. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat. Commun. 2021, 12, 3375. [Google Scholar] [CrossRef]
- Li, K.; Wang, K.; Qin, W.; Deng, S.; Li, D.; Shi, J.; Huang, Q.; Fan, C. DNA-directed assembly of gold nanohalo for quantitative plasmonic imaging of single-particle catalysis. J. Am. Chem. Soc. 2015, 137, 4292–4295. [Google Scholar] [CrossRef]
- Beltrame, P.; Comotti, M.; Della Pina, C.; Rossi, M. Aerobic oxidation of glucose: II. Catalysis by colloidal gold. Appl. Catal. A Gen. 2006, 297, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Z.; Chen, Z.; Ren, J.; Qu, X. Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis. Biomaterials 2013, 34, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, X.; Liang, X.; Wang, Z. Preparation of highly efficient Au/C catalysts for glucose oxidation via novel plasma reduction. Catal. Commun. 2012, 25, 92–95. [Google Scholar] [CrossRef]
- He, X.; Tan, L.; Chen, D.; Wu, X.; Ren, X.; Zhang, Y.; Meng, X.; Tang, F. Fe3O4-Au@mesoporous SiO2 microspheres: An ideal artificial enzymatic cascade system. Chem. Commun. 2013, 49, 4643–4645. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Moroz, B.L.; Taran, O.P.; Gromov, N.V.; Pyrjaev, P.A.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Parmon, V.N. Aerobic selective oxidation of glucose to gluconate catalyzed by Au/Al2O3 and Au/C: Impact of the mass-transfer processes on the overall kinetics. Chem. Eng. J. 2013, 223, 921–931. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, L.; Huang, Y.; Ren, J.; Qu, X. Positional assembly of hemin and gold nanoparticles in graphene-mesoporous silica nanohybrids for tandem catalysis. Chem. Sci. 2015, 6, 1272–1276. [Google Scholar] [CrossRef]
- Tao, N.; Li, H.; Deng, L.; Zhao, S.; Ouyang, J.; Wen, M.; Chen, W.; Zeng, K.; Wei, C.; Liu, Y.-N. A cascade nanozyme with amplified sonodynamic therapeutic effects through comodulation of hypoxia and immunosuppression against cancer. ACS Nano 2021, 16, 485–501. [Google Scholar] [CrossRef]
- Liu, C.; Xing, J.; Akakuru, O.U.; Luo, L.; Sun, S.; Zou, R.; Yu, Z.; Fang, Q.; Wu, A. Nanozymes-engineered metal–organic frameworks for catalytic cascades-enhanced synergistic cancer therapy. Nano Lett. 2019, 19, 5674–5682. [Google Scholar] [CrossRef]
- Cai, L.; Hu, C.; Liu, S.; Zhou, Y.; Pang, M.; Lin, J. A covalent organic framework-based multifunctional therapeutic platform for enhanced photodynamic therapy via catalytic cascade reactions. Sci. China Mater. 2020, 64, 488–497. [Google Scholar] [CrossRef]
- Testa, C.; Zammataro, A.; Pappalardo, A.; Sfrazzetto, G.T. Catalysis with carbon nanoparticles. RSC Adv. 2019, 9, 27659–27664. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Xu, X.; Zhang, F.; Xia, J.; Wang, Z. Promoting Nanozyme Cascade Bioplatform by ZIF-Derived N-Doped Porous Carbon Nanosheet-based Protein/Bimetallic Nanoparticles for Tandem Catalysis. ACS Appl. Bio. Mater. 2020, 3, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, Y.; Ran, X.; Ren, J.; Qu, X. Self-Assembly and Compartmentalization of Nanozymes in Mesoporous Silica-based Nanoreactors. Chemistry 2016, 22, 5705–5711. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Qi, W.; Yang, B.; Liu, X.; Zhang, L.; Liu, J.; Su, R.; He, Z. Construction of Supramolecular Nanostructures with High Catalytic Activity by Photoinduced Hierarchical Co-Assembly. Chem. Eur. J. 2019, 25, 7896–7902. [Google Scholar] [CrossRef]
- Gao, S.; Lin, H.; Zhang, H.; Yao, H.; Chen, Y.; Shi, J. Nanocatalytic Tumor Therapy by Biomimetic Dual Inorganic Nanozyme-Catalyzed Cascade Reaction. Adv. Sci. 2019, 6, 1801733. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, D.; Cho, A.; Weon, S.; Lee, S.; Lee, J.; Han, J.W.; Kim, D.P.; Kim, W. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nat. Commun. 2019, 10, 940. [Google Scholar] [CrossRef]
- Han, L.; Zhang, H.; Chen, D.; Li, F. Protein-Directed Metal Oxide Nanoflakes with Tandem Enzyme-Like Characteristics: Colorimetric Glucose Sensing Based on One-Pot Enzyme-Free Cascade Catalysis. Adv. Funct. Mater. 2018, 28, 1800018. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, K.S.; Park, H.G. Glucose oxidase-like activity of cerium oxide nanoparticles: Use for personal glucose meter-based label-free target DNA detection. Theranostics 2020, 10, 4507–4514. [Google Scholar] [CrossRef]
- Rashtbari, S.; Dehghan, G.; Amini, M. An ultrasensitive label-free colorimetric biosensor for the detection of glucose based on glucose oxidase-like activity of nanolayered manganese-calcium oxide. Anal. Chim. Acta 2020, 1110, 98–108. [Google Scholar] [CrossRef]
- Amaniampong, P.N.; Trinh, Q.T.; Li, K.; Mushrif, S.H.; Hao, Y.; Yang, Y. Porous structured CuO-CeO2 nanospheres for the direct oxidation of cellobiose and glucose to gluconic acid. Catal. Today 2018, 306, 172–182. [Google Scholar] [CrossRef]
- Wang, H.; Yang, W.; Wang, X.; Huang, L.; Zhang, Y.; Yao, S. A CeO2@MnO2 core–shell hollow heterojunction as glucose oxidase-like photoenzyme for photoelectrochemical sensing of glucose. Sens. Actuators B Chem. 2020, 304, 127389. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, J.; Long, Y.; Li, J.; Li, W.; Song, S.; Shi, Z.; Zhang, H. CeO2-Encapsulated Hollow Ag–Au Nanocage Hybrid Nanostructures as High-Performance Catalysts for Cascade Reactions. Small 2019, 15, 1903182. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Pramanik, K.; Datta, P.; Sarkar, P. Chemically modified carbon nitride-chitin-acetic acid hybrid as a metal-free bifunctional nanozyme cascade of glucose oxidase-peroxidase for “click off” colorimetric detection of peroxide and glucose. Biosens. Bioelectron. 2020, 154, 112072. [Google Scholar] [CrossRef] [PubMed]
- Witońska, I.; Frajtak, M.; Karski, S. Selective oxidation of glucose to gluconic acid over Pd–Te supported catalysts. Appl. Catal. A Gen. 2011, 401, 73–82. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, G.; Wang, G.; Lu, M.; Liu, J.; Li, K.; Lin, Y. V2O5 Nanobelts Mimick Tandem Enzymes to Achieve Nonenzymatic Online Monitoring of Glucose in Living Rat Brain. Anal. Chem. 2020, 92, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Yu, L.; Kang, Q.; Chen, Y.; Shen, D. A differential photoelectrochemical method for glucose determination based on alkali-soaked zeolite imidazole framework-67 as both glucose oxidase and peroxidase mimics. Mikrochim. Acta 2020, 187, 244. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Yu, Z.; Chen, J.; Dong, Q.; Wu, W.; Fang, Y.; Liu, L.; Dong, S. A revisiting of transition metal phosphide (Cu3P and FeP) nanozymes for two sugar-related reactions. Nano Res. 2023, 16, 189–194. [Google Scholar] [CrossRef]
- Tang, M.; Li, J.; Cai, X.; Sun, T.; Chen, C. Single-Atom Nanozymes for Biomedical Applications: Recent Advances and Challenges. Chem. Asian J. 2022, 17, e202101422. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, X.; Hao, S.; Zhang, Y.; Chen, J.; Zhang, H.; Dong, S. Glucose Oxidase-like Rhodium Single-Atom Nanozymes: A Mimic Platform for Biometabolism and Electrometabolism of Glucose Oxidation at Neutral pH. ACS Energy Lett. 2023, 8, 1697–1704. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Aloui, H.; Kim, B.S. Novel biogenic gold nanoparticles catalyzing multienzyme cascade reaction: Glucose oxidase and peroxidase mimicking activity. Chem. Eng. J. 2021, 421, 127859. [Google Scholar] [CrossRef]
- Hermans, S.; Deffernez, A.; Devillers, M. Au–Pd/C catalysts for glyoxal and glucose selective oxidations. Appl. Catal. A Gen. 2011, 395, 19–27. [Google Scholar] [CrossRef]
- Comotti, M.; Pina, C.D.; Rossi, M. Mono- and bimetallic catalysts for glucose oxidation. J. Mol. Catal. A Chem. 2006, 251, 89–92. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Di, J. Colorimetric detection of glucose based on gold nanoparticles coupled with silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 2011, 11, 49–52. [Google Scholar] [CrossRef]
- Zhang, H.; Kawashima, K.; Okumura, M.; Toshima, N. Colloidal Au single-atom catalysts embedded on Pd nanoclusters. J. Mater. Chem. A 2014, 2, 13498–13508. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Ma, Q.; Chao, D.; Zhu, X.; Liu, L.; Wang, J.; Fang, Y.; Dong, S. Critical evaluation of the glucose oxidase-like activity of gold nanoparticles stabilized by different polymers. Nano Res. 2022, 16, 4758–4766. [Google Scholar] [CrossRef]
- Lang, N.J.; Liu, B.; Liu, J. Characterization of glucose oxidation by gold nanoparticles using nanoceria. J. Colloid Interface Sci. 2014, 428, 78–83. [Google Scholar] [CrossRef]
- Lin, F.; Yushen, T.; Doudou, L.; Haoan, W.; Yan, C.; Ning, G.; Yu, Z. Catalytic gold–platinum alloy nanoparticles and a novel glucose oxidase mimic with enhanced activity and selectivity constructed by molecular imprinting. Anal. Methods 2019, 11, 4586–4592. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Zhang, Y.; Liu, Y. Photo-Controllable Catalysis and Chiral Monosaccharide Recognition Induced by Cyclodextrin Derivatives. Angew. Chem. Int. Ed. Engl. 2021, 60, 7654–7658. [Google Scholar] [CrossRef]
- Zhan, P.; Wang, Z.-G.; Li, N.; Ding, B. Engineering gold nanoparticles with DNA ligands for selective catalytic oxidation of chiral substrates. ACS Catal. 2015, 5, 1489–1498. [Google Scholar] [CrossRef]
- Peng, T.; Miao, J.; Gao, Z.; Zhang, L.; Gao, Y.; Fan, C.; Li, D. Reactivating Catalytic Surface: Insights into the Role of Hot Holes in Plasmonic Catalysis. Small 2018, 14, e1703510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, S.; Wang, H. A surface plasmon-enhanced nanozyme-based fenton process for visible-light-driven aqueous ammonia oxidation. Green Chem. 2018, 20, 4067–4074. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, W.; Li, J.; Liu, H.; Ma, H.; Huang, Q.; Fan, C. Gold nanoparticles-based nanoconjugates for enhanced enzyme cascade and glucose sensing. Analyst 2012, 137, 4435–4439. [Google Scholar] [CrossRef] [PubMed]
- Ruiyi, L.; Xiaobo, W.; Yuanfeng, P.; Pengwu, X.; Haiyan, Z.; Zaijun, L.; Xiulan, S. Synthesis of gold nanocrystals with chiral morphology, chiral ligand and more exposed high-index facets as electrocatalyst for the oxidation of glucose enantiomers with high enantioselectivity and catalytic activity. Catal. Sci. Technol. 2022, 12, 2097–2105. [Google Scholar] [CrossRef]
- Pezhhan, H.; Akhond, M.; Shamsipur, M. Histidine capped-gold nanoclusters mediated fluorescence detection of glucose and hydrogen peroxide based on glucose oxidase-mimicking property of gold nanoparticles via an inner filter effect mechanism. J. Lumin. 2020, 228, 117604. [Google Scholar] [CrossRef]
- Zhang, L.; Vijg, J. Somatic mutagenesis in mammals and its implications for human disease and aging. Annu. Rev. Genet. 2018, 52, 397–419. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Q.; Jing, C.; Li, Y.; Li, D.; Luo, W.; Wen, Y.; He, Y.; Huang, Q.; Long, Y.-T.; et al. Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angew. Chem. Int. Ed. Engl. 2011, 50, 11994–11998. [Google Scholar] [CrossRef]
- Qu, K.; Shi, P.; Ren, J.; Qu, X. Nanocomposite incorporating V2O5 nanowires and gold nanoparticles for mimicking an enzyme cascade reaction and its application in the detection of biomolecules. Chem. Eur. J. 2014, 20, 7501–7506. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Perumal, R.; Barras, A.; Lyskawa, J.; Woisel, P.; Laure, W.; Pradier, C.-M.; Boukherroub, R.; Szunerits, S. Amplified plasmonic detection of DNA hybridization using doxorubicin-capped gold particles. Analyst 2014, 139, 157–164. [Google Scholar] [CrossRef]
- Kim, H.Y.; Song, J.; Park, K.S.; Park, H.G. Simple and label-free strategy for terminal transferase assay using a personal glucose meter. Chem. Commun. 2020, 56, 8912–8915. [Google Scholar] [CrossRef]
- Zhou, H.; Han, T.; Wei, Q.; Zhang, S. Efficient Enhancement of Electrochemiluminescence from Cadmium Sulfide Quantum Dots by Glucose Oxidase Mimicking Gold Nanoparticles for Highly Sensitive Assay of Methyltransferase Activity. Anal. Chem. 2016, 88, 2976–2983. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ni, Q.; Mu, J.; Fan, W.; Liu, L.; Wang, Z.; Li, L.; Tang, W.; Liu, Y.; Cheng, Y.; et al. Solvent-Assisted Self-Assembly of a Metal-Organic Framework Based Biocatalyst for Cascade Reaction Driven Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6822–6832. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; An, P.; Liu, L.; Gao, Z.; Li, Y.; Zhang, Y.; Sun, B.; Zhou, J. A polydopamine-gated biodegradable cascade nanoreactor for pH-triggered and photothermal-enhanced tumor-specific nanocatalytic therapy. Nanoscale 2021, 13, 15677–15688. [Google Scholar] [CrossRef]
| Nanocarrier | Size of Au NPs | Application | Ref |
|---|---|---|---|
| Mesoporous silica | 2.1 nm | Cascade catalysis | [23] |
| Activated carbon | 7–8 nm | Production of gluconate | [24] |
| Fe3O4 | 4.2 nm | Cascade catalysis | [25] |
| Al2O3 | 1.9–16.6 nm | Production of gluconate | [26] |
| Graphene | \ | Cascade catalysis | [27] |
| TiO2 | \ | Tumor therapy | [28] |
| MOF | 2 nm | Cascade catalysis | [29] |
| COF | 3 nm | Cascade catalysis | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, S.; Yu, Q.; Ye, J.; Hao, P.; Ning, J.; Hu, Z.; Chong, Y. Nanomaterials with Glucose Oxidase-Mimicking Activity for Biomedical Applications. Molecules 2023, 28, 4615. https://doi.org/10.3390/molecules28124615
Min S, Yu Q, Ye J, Hao P, Ning J, Hu Z, Chong Y. Nanomaterials with Glucose Oxidase-Mimicking Activity for Biomedical Applications. Molecules. 2023; 28(12):4615. https://doi.org/10.3390/molecules28124615
Chicago/Turabian StyleMin, Shengyi, Qiao Yu, Jiaquan Ye, Pengfei Hao, Jiayu Ning, Zhiqiang Hu, and Yu Chong. 2023. "Nanomaterials with Glucose Oxidase-Mimicking Activity for Biomedical Applications" Molecules 28, no. 12: 4615. https://doi.org/10.3390/molecules28124615
APA StyleMin, S., Yu, Q., Ye, J., Hao, P., Ning, J., Hu, Z., & Chong, Y. (2023). Nanomaterials with Glucose Oxidase-Mimicking Activity for Biomedical Applications. Molecules, 28(12), 4615. https://doi.org/10.3390/molecules28124615








