Assisted Reductive Amination for Quantitation of Tryptophan, 5-Hydroxytryptophan, and Serotonin by Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Reductive Amination of Tryptophan and Its Metabolites
2.2. MS/MS Fragmentation and MRM Transition Setting
2.3. Signal Enhancements of Modified Serotonin, TRP, and 5-HTP
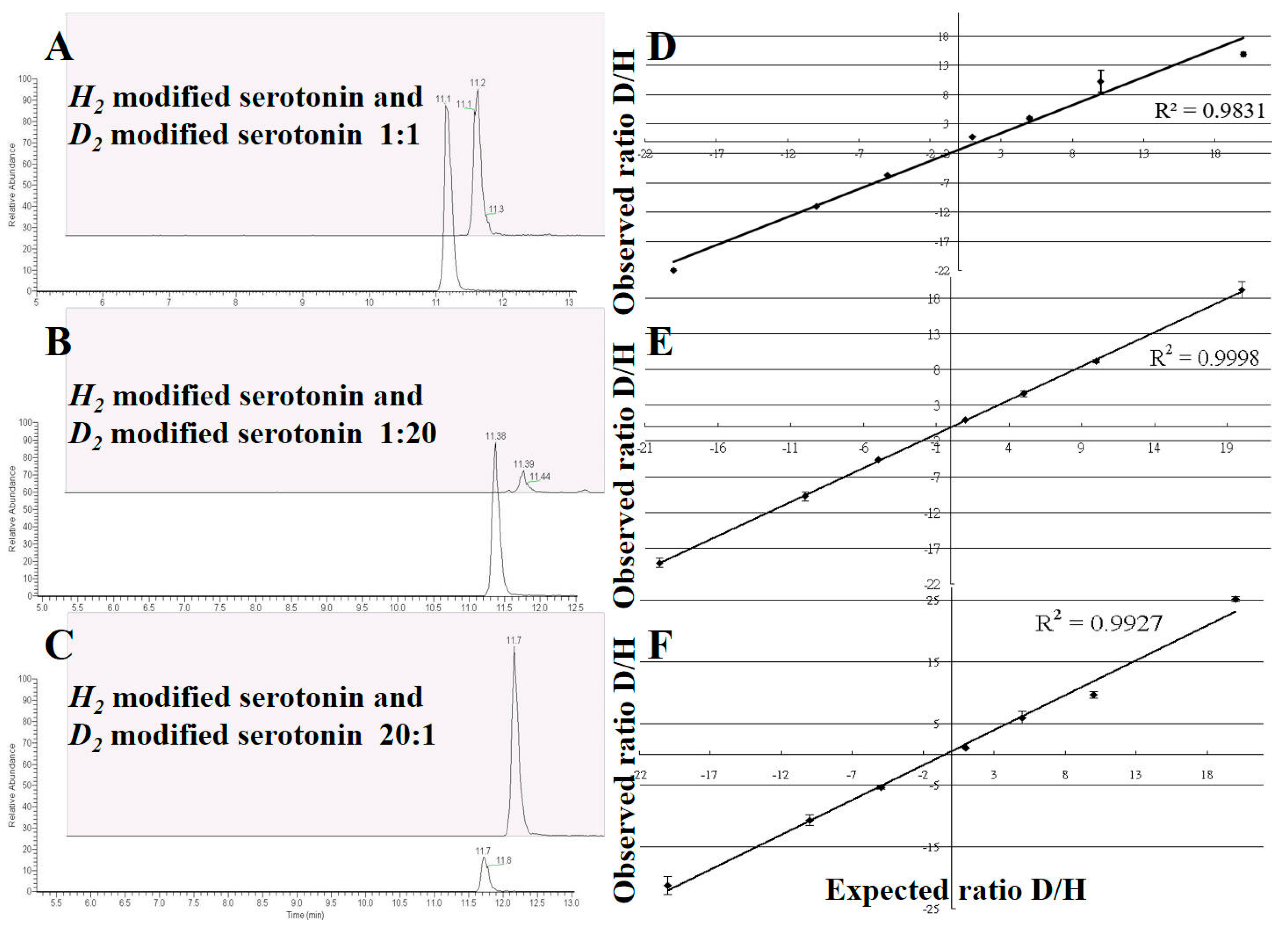
2.4. Method Validation
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Preparation of Derivatized Standards and Internal Standards
3.4. Development of the Detection Method for Serotonin, 5-Hydroxytryptophan, and Tryptophan
3.5. Evulation of the Derivitized Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Stjarne, L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev. Physiol. Biochem. Pharmacol. 1989, 112, 1–137. [Google Scholar] [PubMed]
- Hillyer, J.F.; Estevez-Lao, T.Y.; Mirzai, H.E. The neurotransmitters serotonin and glutamate accelerate the heart rate of the mosquito Anopheles gambiae. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 188, 49–57. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Fakhfouri, G.; Ramezani, V.; Mehr, S.E.; Rahimian, R. The role of serotonin in memory: Interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 2014, 232, 723–738. [Google Scholar] [CrossRef]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Stein, A.; Uncini, T. Urinary neurotransmitter testing: Considerations of spot baseline norepinephrine and epinephrine. Open Access J. Urol. 2011, 3, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Pajski, M.L.; Ross, A.E.; Venton, B.J. Quantitation of dopamine, serotonin and adenosine content in a tissue punch from a brain slice using capillary electrophoresis with fast-scan cyclic voltammetry detection. Anal. Methods 2013, 5, 2704–2711. [Google Scholar] [CrossRef]
- Wang, M.; Lee, F.J.; Liu, F. Dopamine receptor interacting proteins (DRIPs) of dopamine D1-like receptors in the central nervous system. Mol. Cells 2008, 25, 149–157. [Google Scholar]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry Neurosci. 2007, 32, 394–399. [Google Scholar]
- Cote, F.; Thevenot, E.; Fligny, C.; Fromes, Y.; Darmon, M.; Ripoche, M.A.; Bayard, E.; Hanoun, N.; Saurini, F.; Lechat, P.; et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl. Acad. Sci. USA 2003, 100, 13525–13530. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Hoglund, E.; Overli, O.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Azam, B.; Wienecke, J.; Jensen, D.B.; Azam, A.; Zhang, M. Spinal Cord Hemisection Facilitates Aromatic L-Amino Acid Decarboxylase Cells to Produce Serotonin in the Subchronic but Not the Chronic Phase. Neural Plast. 2015, 2015, 549671. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Brotchie, J.M.; Jones, O.T. Locomotor response to L-DOPA in reserpine-treated rats following central inhibition of aromatic L-amino acid decarboxylase: Further evidence for non-dopaminergic actions of L-DOPA and its metabolites. Neurosci. Res. 2010, 68, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Troche, G.; Henry-Lagarrigue, M.; Soppelsa, F.; Legriel, S.; Yehia, A.; Bruneel, F.; Bedos, J.P.; Spreux-Varoquaux, O. Tryptophan pathway catabolites (serotonin, 5-hydroxyindolacetic acid, kynurenine) and enzymes (monoamine oxidase and indole amine 2,3 dioxygenase) in patients with septic shock A prospective observational study versus healthy controls. Medicine 2020, 99, e19906. [Google Scholar] [CrossRef]
- Eser, B.; Ozkan, Y.; Sepici Dincel, A. Determination of Tryptophan and Kynurenine by LC-MS/MS by Using Amlodipine as an Internal Standard. J. Am. Soc. Mass. Spectrom. 2020, 31, 379–385. [Google Scholar] [CrossRef]
- Sadok, I.; Gamian, A.; Staniszewska, M.M. Chromatographic analysis of tryptophan metabolites. J. Sep. Sci. 2017, 40, 3020–3045. [Google Scholar] [CrossRef]
- Ghebregzabher, M.; Rufini, S.; Castellucci, M.G.; Lato, M. Analysis of Some Tryptophan and Phenylalanine Metabolites in Urine by a Straight-Phase High-Performance Liquid-Chromatographic Technique. J. Chromatogr. 1981, 222, 191–201. [Google Scholar] [CrossRef]
- Wonodi, I.; Schwarcz, R. Cortical kynurenine pathway metabolism: A novel target for cognitive enhancement in Schizophrenia. Schizophr. Bull. 2010, 36, 211–218. [Google Scholar] [CrossRef]
- Schwarcz, R.; Pellicciari, R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef]
- Schloss, P.; Williams, D.C. The serotonin transporter: A primary target for antidepressant drugs. J. Psychopharmacol. 1998, 12, 115–121. [Google Scholar] [CrossRef]
- Tan, B.; Chen, J.; Qin, S.; Liao, C.; Zhang, Y.; Wang, D.; Li, S.; Zhang, Z.; Zhang, P.; Xu, F. Tryptophan Pathway-Targeted Metabolomics Study on the Mechanism and Intervention of Cisplatin-Induced Acute Kidney Injury in Rats. Chem. Res. Toxicol. 2021, 34, 1759–1768. [Google Scholar] [CrossRef]
- Lai, Y.; Liu, C.W.; Chi, L.; Ru, H.; Lu, K. High-Resolution Metabolomics of 50 Neurotransmitters and Tryptophan Metabolites in Feces, Serum, and Brain Tissues Using UHPLC-ESI-Q Exactive Mass Spectrometry. ACS Omega 2021, 6, 8094–8103. [Google Scholar] [CrossRef] [PubMed]
- Attwa, M.W.; AlRabiah, H.; Mostafa, G.A.E.; Bakheit, A.H.; Kadi, A.A. Assessment of In Silico and In Vitro Selpercatinib Metabolic Stability in Human Liver Microsomes Using a Validated LC-MS/MS Method. Molecules 2023, 28, 2618. [Google Scholar] [CrossRef] [PubMed]
- Attwa, M.W.; AlRabiah, H.; Mostafa, G.A.E.; Kadi, A.A. Development of an LC-MS/MS Method for Quantification of Sapitinib in Human Liver Microsomes: In Silico and In Vitro Metabolic Stability Evaluation. Molecules 2023, 28, 2322. [Google Scholar] [CrossRef]
- Younis, I.Y.; Khattab, A.R.; Selim, N.M.; Sobeh, M.; Elhawary, S.S.; Bishbishy, M.H.E. Metabolomics-based profiling of 4 avocado varieties using HPLC-MS/MS and GC/MS and evaluation of their antidiabetic activity. Sci. Rep. 2022, 12, 4966. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.L.; Yang, S.; Zhang, W.; Han, F.F.; Xuan, L.L.; Lv, Y.L.; Liu, H.; Liu, L.H. Targeted Metabolomics for Plasma Amino Acids and Carnitines in Patients with Metabolic Syndrome Using HPLC-MS/MS. Dis. Markers 2020, 2020, 8842320. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Shirakawa, H.; Nguyen, T.K.; Aso, H.; Komai, M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016, 13, 56–59. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, F. Evaluation of the Hepatotoxicity of the Zhi-Zi-Hou-Po Decoction by Combining UPLC-Q-Exactive-MS-Based Metabolomics and HPLC-MS/MS-Based Geniposide Tissue Distribution. Molecules 2019, 24, 511. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Jyoti, A.; Mishra, M.K.; Louissaint, N.; Romero, R.; Chugani, D.C.; Kannan, S.; Kannan, R.M. Concurrent quantification of tryptophan and its major metabolites. Anal. Biochem. 2013, 443, 222–231. [Google Scholar] [CrossRef]
- Peterson, Z.D.; Lee, M.L.; Graves, S.W. Determination of serotonin and its precursors in human plasma by capillary electrophoresis-electrospray ionization-time-of-flight mass spectrometry. J. Chromatogr. B 2004, 810, 101–110. [Google Scholar] [CrossRef]
- He, B.; Bi, K.; Jia, Y.; Wang, J.; Lv, C.; Liu, R.; Zhao, L.; Xu, H.; Chen, X.; Li, Q. Rapid analysis of neurotransmitters in rat brain using ultra-fast liquid chromatography and tandem mass spectrometry: Application to a comparative study in normal and insomnic rats. J. Mass. Spectrom. 2013, 48, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Kang, A.; Dai, C.; Liang, Y.; Xie, T.; Xie, L.; Peng, Y.; Wang, G.; Hao, H. Quantitative analysis of neurochemical panel in rat brain and plasma by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2012, 84, 10044–10051. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.T.; Wu, Y.Y.; Chang, Y.T.; Cheng, C.W.; Huang, M.F.; Chen, Z.Y.; Shiue, Y.L.; Liang, S.S. Quantification of the beta(2)-Adrenergic Feed Additives Ractopamine and Salbutamol by Reductive Amination-Assisted Modification. J. Food Prot. 2019, 82, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.T.; Lin, Y.R.; Chen, B.H.; Huang, M.F.; Cheng, C.W.; Shiue, Y.L.; Liang, S.S. A standard addition method to quantify histamine by reductive amination and hydrophilic interaction liquid chromatography coupled with tandem mass spectrometry. Eur. J. Mass. Spectrom. 2019, 25, 412–418. [Google Scholar] [CrossRef]
- Huang, M.F.; Lin, Y.R.; Chang, Y.T.; Shiue, Y.L.; Liang, S.S. Reductive amination assistance for quantification of oseltamivir phosphate and oseltamivir carboxylate by HPLC-MS/MS. J. Chromatogr. B 2018, 1087, 23–28. [Google Scholar] [CrossRef]
- Lin, Y.R.; Wu, C.E.; Huang, M.F.; Wu, Y.Y.; Huang, J.H.; Liu, M.C.; Chen, Z.Y.; Shiue, Y.L.; Liang, S.S. Development of Doxorubicin Quantification by Reductive Amination. Curr. Anal. Chem. 2017, 13, 270–276. [Google Scholar] [CrossRef]
- Lin, Y.R.; Huang, M.F.; Wu, Y.Y.; Liu, M.C.; Huang, J.H.; Chen, Z.Y.; Shiue, Y.L.; Wu, C.E.; Liang, S.S. Reductive amination derivatization for the quantification of garlic components by isotope dilution analysis. Food Chem. 2017, 230, 1–5. [Google Scholar] [CrossRef]
- Tsai, D.C.; Liu, M.C.; Lin, Y.R.; Huang, M.F.; Liang, S.S. A novel reductive amination method with isotopic formaldehydes for the preparation of internal standard and standards for determining organosulfur compounds in garlic. Food Chem. 2016, 197, 692–698. [Google Scholar] [CrossRef]
- Liang, S.S.; Wang, T.N.; Chiu, C.C.; Kuo, P.L.; Huang, M.F.; Liu, M.C.; Tsai, E.M. Reductive amination-assisted quantitation of tamoxifen and its metabolites by liquid phase chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1434, 64–69. [Google Scholar] [CrossRef]
- Liu, M.C.; Lin, Y.R.; Huang, M.F.; Tsai, D.C.; Liang, S.S. Mass spectrometry signal enhancement by reductive amination. Int. J. Mass. Spectrom. 2015, 387, 16–23. [Google Scholar] [CrossRef]
- Cong, M.; Song, J.; Chen, F.; Cao, C.; Wang, S. A surrogate analyte-based LC-MS/MS method for the determination of 5-hydroxytryptamine, kynurenine and tryptophan. Bioanalysis 2020, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]

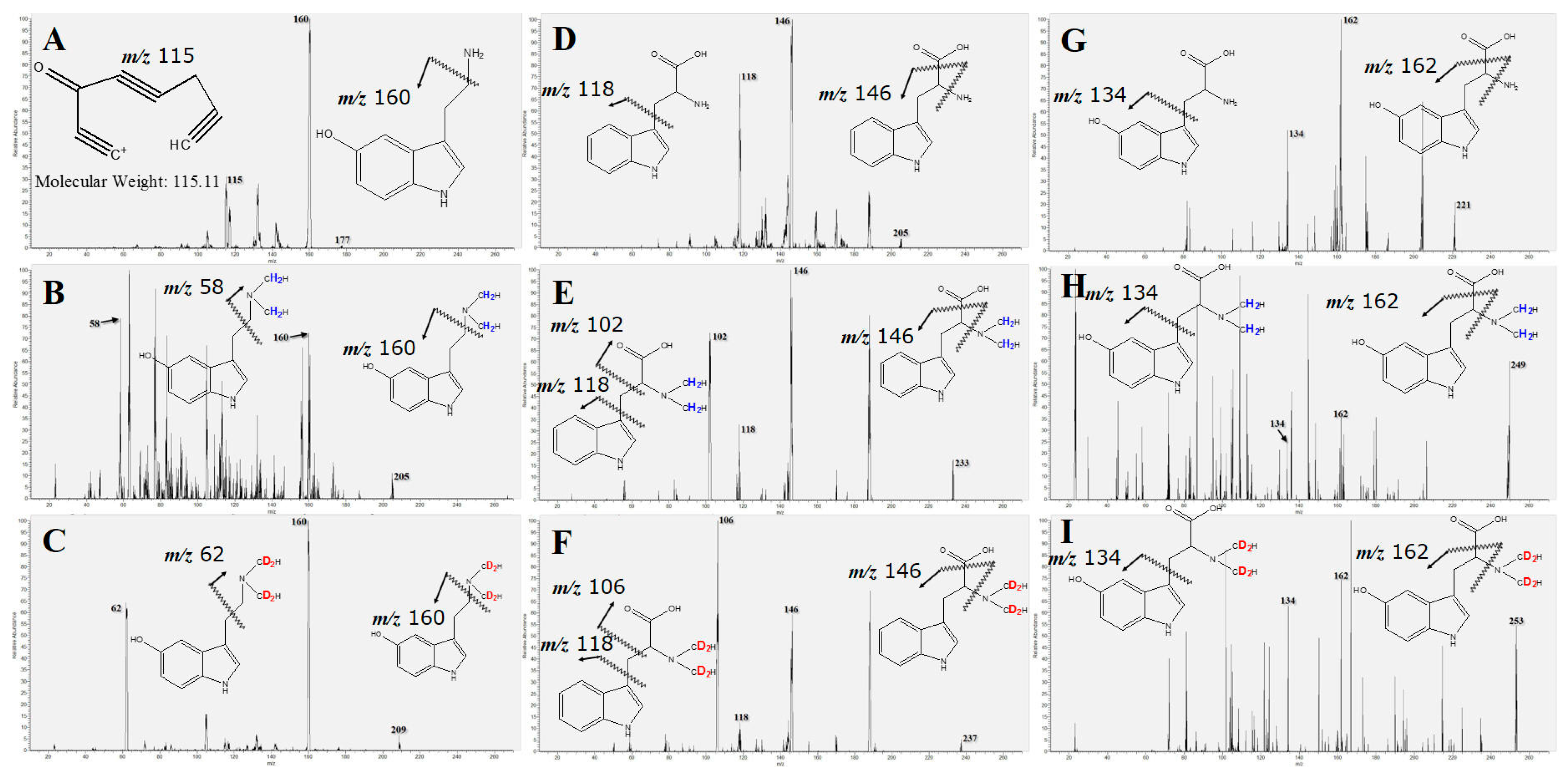

| Name | Molecular Formula | Molecular Weight (Da) | MRM Transitions | LOD # (ng/mL) | LOQ # (ng/mL) | Coefficient of Determination (R2) | |
|---|---|---|---|---|---|---|---|
| Precursor Ion | Product Ions | ||||||
| Serotonin | C10H12N2O | 176.22 | 177 | 115, 160 | --- | --- | --- |
| h2-modified serotonin | C12H16N2O | 204.27 | 205 | 58, 160 | 2.35 | 14.54 | 0.9969 |
| d2-modified serotonin | C12H12D4N2O | 208.29 | 209 | 62, 160 | --- | --- | --- |
| Tryptophan | C11H12N2O2 | 204.23 | 205 | 118, 146 | --- | --- | --- |
| h2-modified tryptophan | C13H16N2O2 | 232.28 | 233 | 102, 118, 146 | 1.96 | 4.36 | 0.9959 |
| d2-modified tryptophan | C13H12D4N2O2 | 236.30 | 237 | 106, 118, 146 | --- | --- | --- |
| 5-HTP | C11H12N2O3 | 220.22 | 221 | 134, 162 | --- | --- | --- |
| h2-modified 5-HTP | C13H16N2O3 | 248.28 | 249 | 134, 162 | 1.39 | 15.36 | 0.9938 |
| d2-modified 5-HTP | C13H12D4N2O3 | 252.30 | 253 | 134, 162 | --- | --- | --- |
| MRM/Average Peak Area (n = 4) | |||||
|---|---|---|---|---|---|
| Unmodified | RSD (%) | d2-Modified | RSD (%) | Enhancement | |
| Serotonin | 55,030,586 | 4.2 | 65,536,481 | 4.5 | 1.19-fold |
| Tryptophan | 6,765,226 | 3.8 | 127,646,745 | 1.2 | 18.87-fold |
| 5-HTP | 2,193,503 | 6.3 | 5,257,500 | 2.4 | 2.40-fold |
| Serotonin | Tryptophan | 5-HTP | ||||
|---|---|---|---|---|---|---|
| Concentration (ng/mL) | Intra-Day | Inter-Day | Intra-Day | Inter-Day | Intra-Day | Inter-Day |
| 500 | 3.5–6.9% | 4.0% | 1.2–3.8% | 6.4% | 0.6–6.1% | 6.7% |
| 100 | 2.2–6.4% | 3.0% | 1.6–8.6% | 7.9% | 1.7–9.7% | 5.7% |
| 50 | 3.4–3.9% | 9.7% | 2.0–10.8% | 3.7% | 4.1–16.3% | 6.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.-S.; Shen, P.-T.; Liang, Y.-Q.; Ke, Y.-W.; Cheng, C.-W.; Lin, Y.-R. Assisted Reductive Amination for Quantitation of Tryptophan, 5-Hydroxytryptophan, and Serotonin by Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules 2023, 28, 4580. https://doi.org/10.3390/molecules28124580
Liang S-S, Shen P-T, Liang Y-Q, Ke Y-W, Cheng C-W, Lin Y-R. Assisted Reductive Amination for Quantitation of Tryptophan, 5-Hydroxytryptophan, and Serotonin by Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules. 2023; 28(12):4580. https://doi.org/10.3390/molecules28124580
Chicago/Turabian StyleLiang, Shih-Shin, Po-Tsun Shen, Yu-Qing Liang, Yi-Wen Ke, Chieh-Wen Cheng, and Yi-Reng Lin. 2023. "Assisted Reductive Amination for Quantitation of Tryptophan, 5-Hydroxytryptophan, and Serotonin by Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry" Molecules 28, no. 12: 4580. https://doi.org/10.3390/molecules28124580
APA StyleLiang, S.-S., Shen, P.-T., Liang, Y.-Q., Ke, Y.-W., Cheng, C.-W., & Lin, Y.-R. (2023). Assisted Reductive Amination for Quantitation of Tryptophan, 5-Hydroxytryptophan, and Serotonin by Ultraperformance Liquid Chromatography Coupled with Tandem Mass Spectrometry. Molecules, 28(12), 4580. https://doi.org/10.3390/molecules28124580






