Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound
Abstract
1. Introduction
2. Results
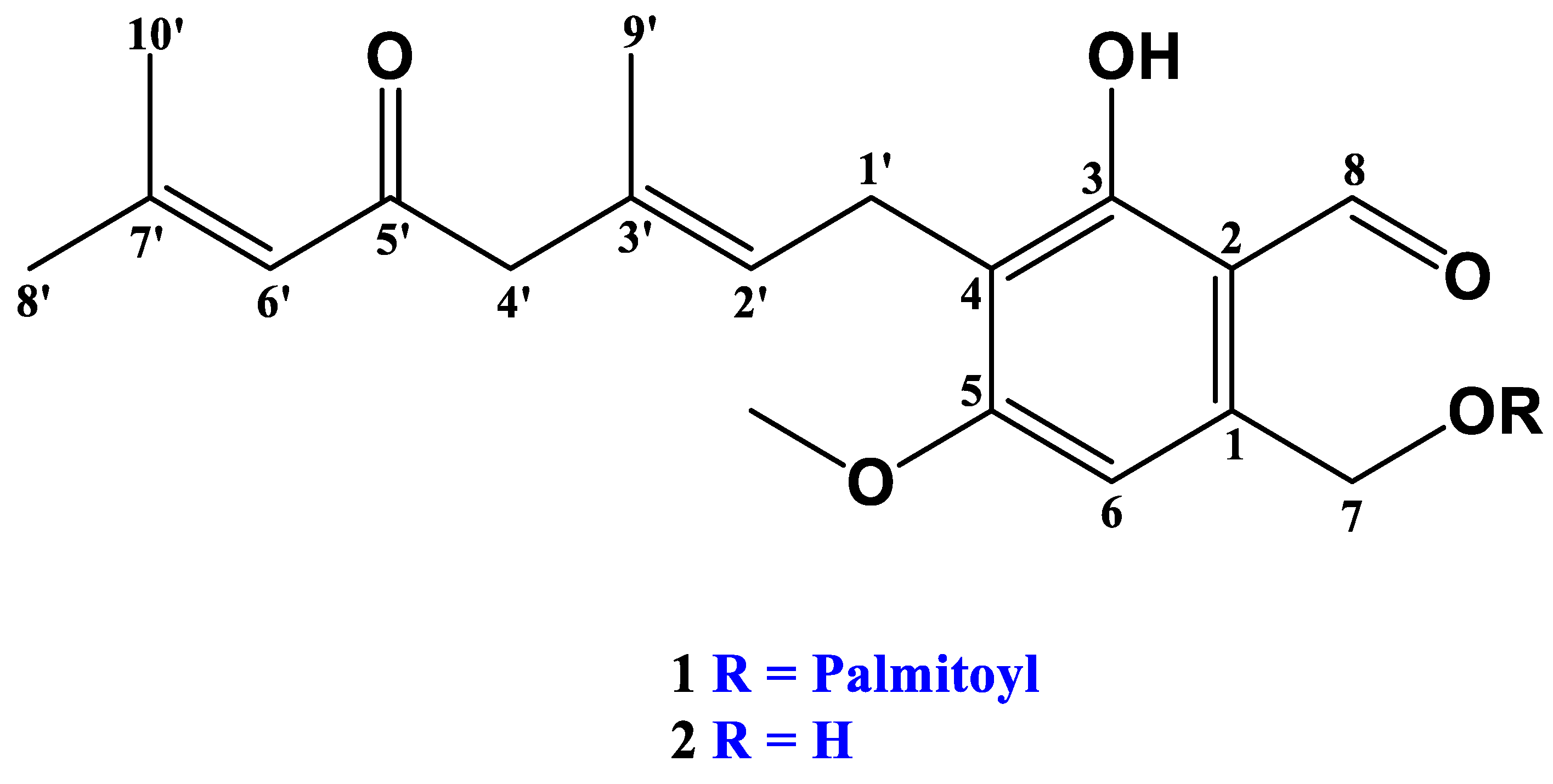
2.1. Lipase Treatment of Hericenone C and n-Hexane Fraction
2.2. Effect on BDNF mRNA Transcription
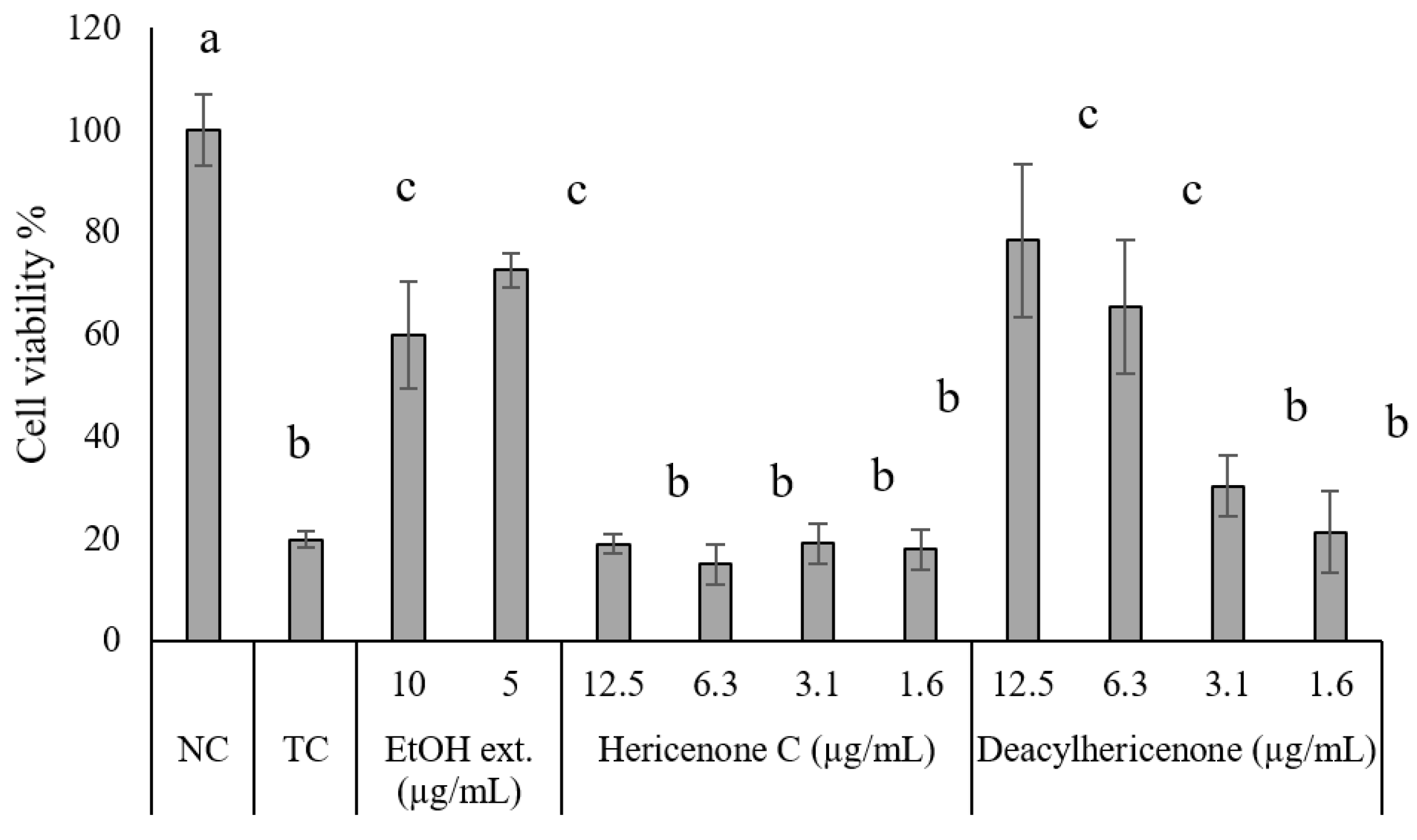
2.3. Hydrogen Peroxide-Induced Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Sample Extraction
4.2. Lipase Treatment of Hericenone C and n-Hexane Fraction
4.3. LC-QTOF-MS Analysis
4.4. NMR Analysis
4.5. Cell Culture
4.6. Effect on BDNF mRNA Transcription
4.7. Hydrogen Peroxide-Induced Oxidative Stress
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kobayashi, S.; Tamura, T.; Koshishiba, M.; Yasumoto, T.; Shimizu, S.; Kintaka, T.; Nagai, K. Total Synthesis, Structure Revision, and Neuroprotective Effect of Hericenones C−H and Their Derivatives. J. Org. Chem. 2021, 86, 2602–2620. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H. Biologically Functional Compounds From Mushroom-Forming Fungi. In Natural Products and Drug Discovery: An Integrated Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–326. [Google Scholar] [CrossRef]
- Ashour, A.; Amen, Y.; Allam, A.E.; Kudo, T.; Nagata, M.; Ohnuki, K.; Shimizu, K. New Isoindolinones from the Fruiting Bodies of the Fungus Hericium erinaceus. Phytochem. Lett. 2019, 32, 10–14. [Google Scholar] [CrossRef]
- Ruan, Y.; Han, C.; Wang, D.; Inoue, Y.; Amen, Y.; Othman, A.; Mittraphab, Y.; Nagata, M.; Shimizu, K. New Benzaldehyde Derivatives from the Fruiting Bodies of Hericium erinaceus with Cytotoxic Activity. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Kobayashi, S. Total Synthesis of Geranyl-Resorcinols Isolated from Mushrooms of Genus Hericium. Synthesis 2023, 55, 417–432. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, Stimulators of Nerve Growth Factor (NGF)-Synthesis, from the Mushroom Hericium Erinaceum. Tetrahedron. Lett. 1991, 32, 4561–4564. [Google Scholar] [CrossRef]
- Bothwell, M. Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, ISBN 978-3-642-45105-8. [Google Scholar]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Chong, P.S.; Poon, C.H.; Roy, J.; Tsui, K.C.; Lew, S.Y.; Phang, M.W.L.; Tan, R.J.Y.; Cheng, P.G.; Fung, M.L.; Wong, K.H.; et al. Neurogenesis-Dependent Antidepressant-like Activity of Hericium erinaceus in an Animal Model of Depression. Chin. Med. 2021, 16, 1–24. [Google Scholar] [CrossRef]
- Chong, P.S.; Fung, M.L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 163. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Phan, C.-W.; Lee, G.-S.; Hong, S.-L.; Wong, Y.-T.; Brkljača, R.; Urban, S.; Nurestri, S.; Malek, A.; Sabaratnam, V. Hericium erinaceus (Bull.: Fr) Pers. Cultivated under Tropical Conditions: Isolation of Hericenones and Demonstration of NGF-Mediated Neurite Outgrowth in PC12 Cells via MEK/ERK and PI3K-Akt Signaling Pathways. Food Funct. 2014, 5, 3160. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve Growth Factor-Inducing Activity of Hericium erinaceus in 1321N1 Human Astrocytoma Cells. Biol. Pharm. Bull. 2008, 31, 1727–1732. [Google Scholar] [CrossRef]
- Ni, J.; Wu, Z.; Meng, J.; Zhu, A.; Zhong, X.; Wu, S.; Nakanishi, H. The Neuroprotective Effects of Brazilian Green Propolis on Neurodegenerative Damage in Human Neuronal SH-SY5Y Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7984327. [Google Scholar] [CrossRef]
- Fox, E.A. A Genetic Approach for Investigating Vagal Sensory Roles in Regulation of Gastrointestinal Function and Food Intake. Auton. Neurosci. 2006, 126–127, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.; Alkahtani, R.; Akbarali, H.I.; Murthy, K.S.; Grider, J.R. Stimulation of Synthesis and Release of Brain-Derived Neurotropic Factor from Intestinal Smooth Muscle Cells by Substance P and Pituitary Adenylate Cyclase-Activating Peptide. Neurogastroenterol. Motil. 2015, 27, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of Cognitive Functions by Oral Intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective Effects of Hericium Erinaceus (Bull.: Fr.) Pers. against High-Dose Corticosterone-Induced Oxidative Stress in PC-12 Cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yin, X.; Cao, C.Y.; Wei, J.; Zhang, Q.; Gao, J.M. Chemical Constituents from Hericium erinaceus and Their Ability to Stimulate NGF-Mediated Neurite Outgrowth on PC12 Cells. Bioorg. Med. Chem. Lett. 2015, 25, 5078–5082. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving Effects of the Mushroom Yamabushitake (Hericium erinaceus) on Mild Cognitive Impairment: A Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium Erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mármol, R.; Chai, Y.; Conroy, J.N.; Khan, Z.; Hong, S.M.; Kim, S.B.; Gormal, R.S.; Lee, D.H.; Lee, J.K.; Lee, M.K.; et al. Hericerin Derivativesactivates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK 1/2 signaling enhancing spatial memory. J. Neurochem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Ismail, M.; Azmi, N.H.; Abu Bakar, M.F.; Basri, H.; Abdullah, M.A. Modulation of Hydrogen Peroxide-Induced Oxidative Stress in Human Neuronal Cells by Thymoquinone-Rich Fraction and Thymoquinone via Transcriptomic Regulation of Antioxidant and Apoptotic Signaling Genes. Oxid. Med. Cell. Longev. 2016, 2016, 2528935. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-Induced Oxidative Damage and LPS-Induced Inflammation in HT22 Hippocampal Neurons and BV2 Microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, S.C.; Chang, J.C.; Lin, W.Y.; Chen, C.C.; Li, C.C.; Hsieh, M.; Chen, H.W.; Chang, T.Y.; Liu, C.S.; et al. The Protective Effect of Erinacine A–Enriched Hericium erinaceus Mycelium Ethanol Extract on Oxidative Stress–Induced Neurotoxicity in Cell and Drosophila Models of Spinocerebellar Ataxia Type 3. Free Radic. Biol. Med. 2023, 195, 1–12. [Google Scholar] [CrossRef]
- Hiraki, E.; Furuta, S.; Kuwahara, R.; Takemoto, N.; Nagata, T.; Akasaka, T.; Shirouchi, B.; Sato, M.; Ohnuki, K.; Shimizu, K. Anti-Obesity Activity of Yamabushitake (Hericium erinaceus) Powder in Ovariectomized Mice, and Its Potentially Active Compounds. J. Nat. Med. 2017, 71, 482–491. [Google Scholar] [CrossRef]
- Nirmaladevi, D.; Venkataramana, M.; Chandranayaka, S.; Ramesha, A.; Jameel, N.M.; Srinivas, C. Neuroprotective Effects of Bikaverin on H2O2-Induced Oxidative Stress Mediated Neuronal Damage in SH-SY5Y Cell Line. Cell. Mol. Neurobiol. 2014, 34, 973–985. [Google Scholar] [CrossRef]
- Lai, P.-L.; Naidu, M.; Sabaratnam, V.; Wong, K.-H.; David, R.P.; Kuppusamy, U.R.; Abdullah, N.; Malek, S.N.A. Neurotrophic Properties of the Lion’s Mane Medicinal Mushroom, Hericium erinaceus (Higher Basidiomycetes) from Malaysia. Int. J. Med. Mushrooms. 2013, 15, 539–554. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamrakar, S.; Wang, D.; Hiraki, E.; Han, C.; Ruan, Y.; Allam, A.E.; Amen, Y.; Katakura, Y.; Shimizu, K. Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound. Molecules 2023, 28, 4549. https://doi.org/10.3390/molecules28114549
Tamrakar S, Wang D, Hiraki E, Han C, Ruan Y, Allam AE, Amen Y, Katakura Y, Shimizu K. Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound. Molecules. 2023; 28(11):4549. https://doi.org/10.3390/molecules28114549
Chicago/Turabian StyleTamrakar, Sonam, Dongmei Wang, Eri Hiraki, Chunguang Han, Yang Ruan, Ahmed E. Allam, Yhiya Amen, Yoshinori Katakura, and Kuniyoshi Shimizu. 2023. "Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound" Molecules 28, no. 11: 4549. https://doi.org/10.3390/molecules28114549
APA StyleTamrakar, S., Wang, D., Hiraki, E., Han, C., Ruan, Y., Allam, A. E., Amen, Y., Katakura, Y., & Shimizu, K. (2023). Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound. Molecules, 28(11), 4549. https://doi.org/10.3390/molecules28114549







