Materials Based on Co, Cu, and Cr as Activators of PMS for Degrading a Representative Antibiotic—The Strategy for Utilization in Water Treatment and Warnings on Metal Leaching
Abstract
1. Introduction
2. Results and Discussion
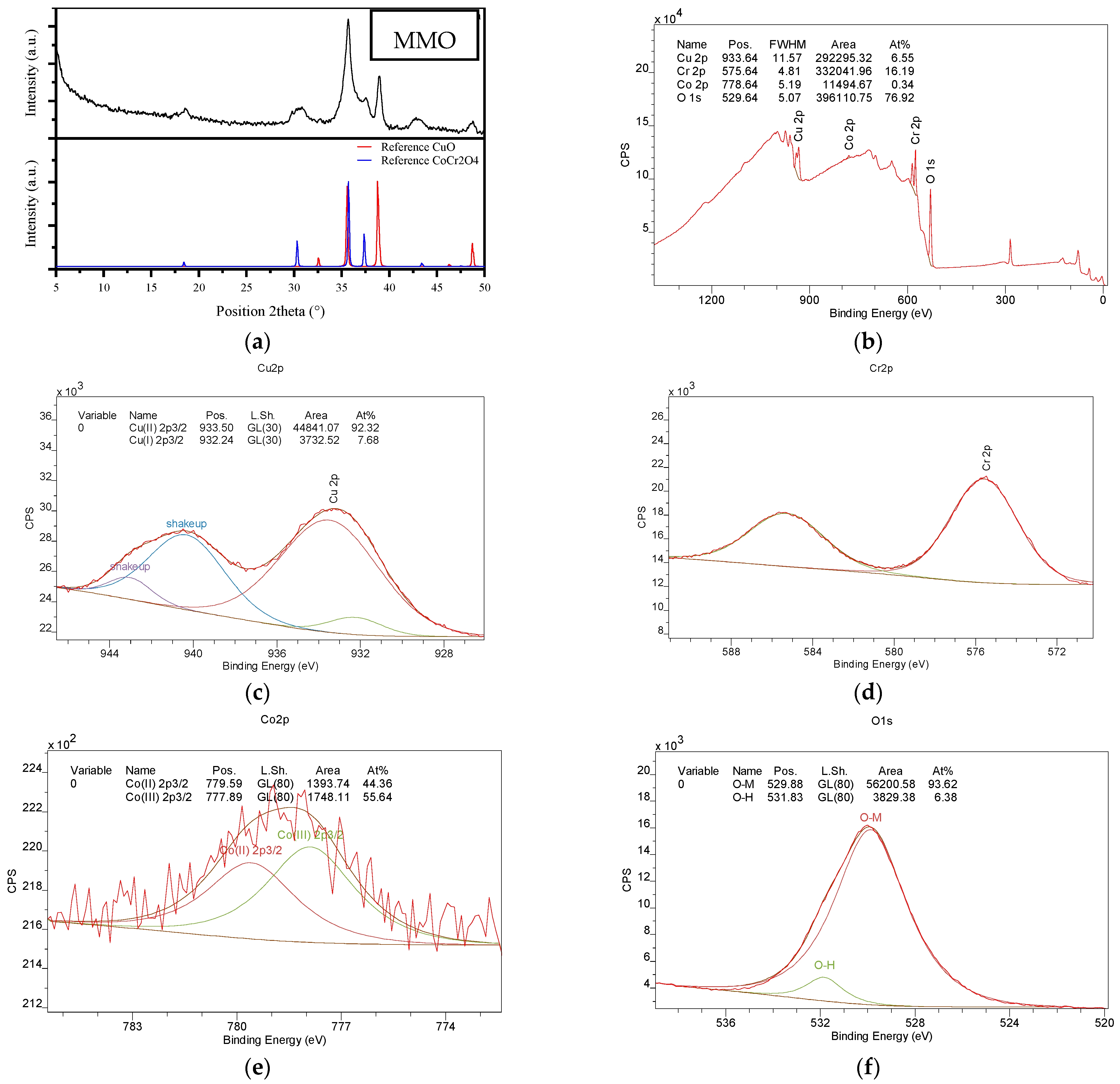
2.1. Characterization of the Φy
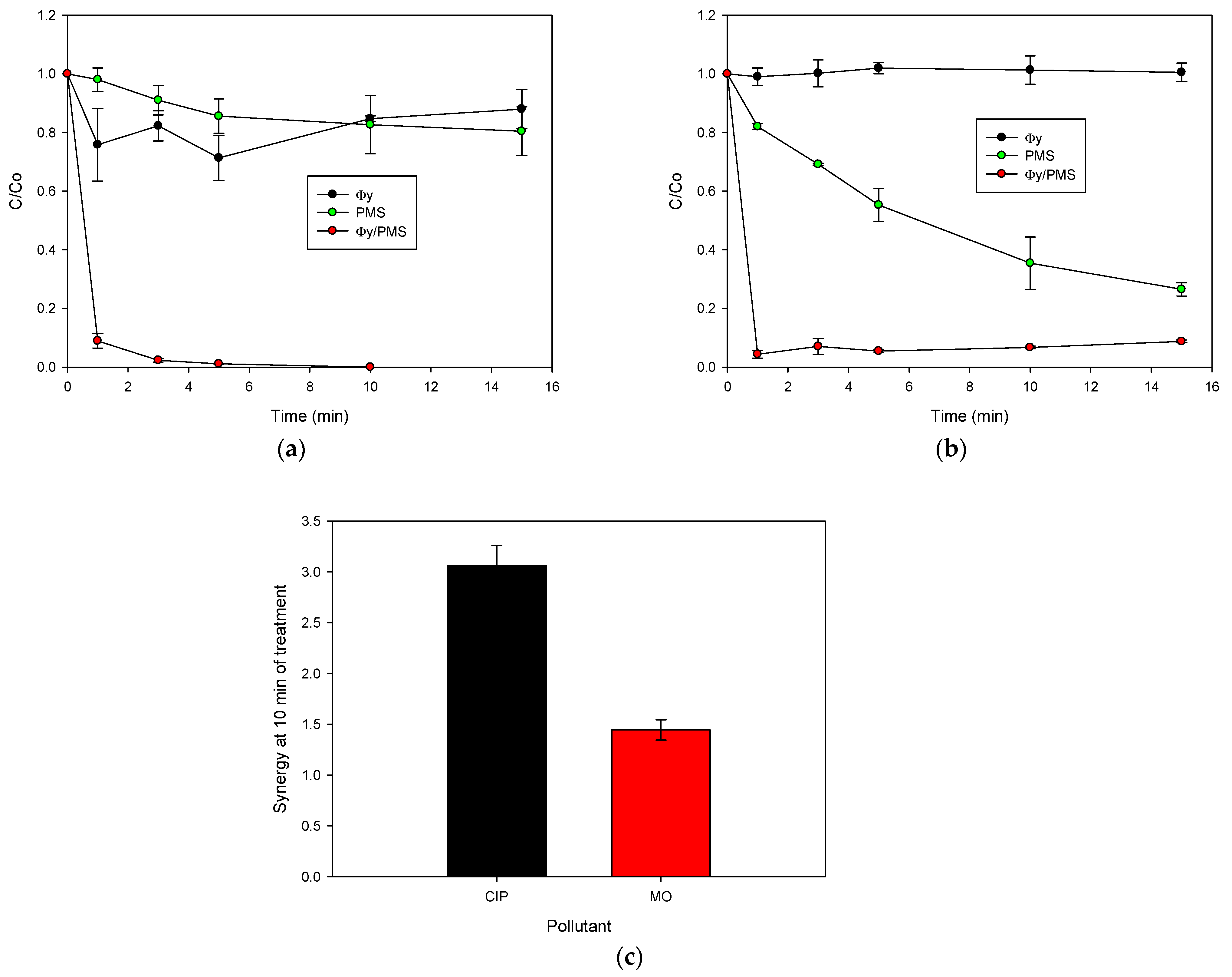
2.2. Activation of PMS for the CIP Degradation
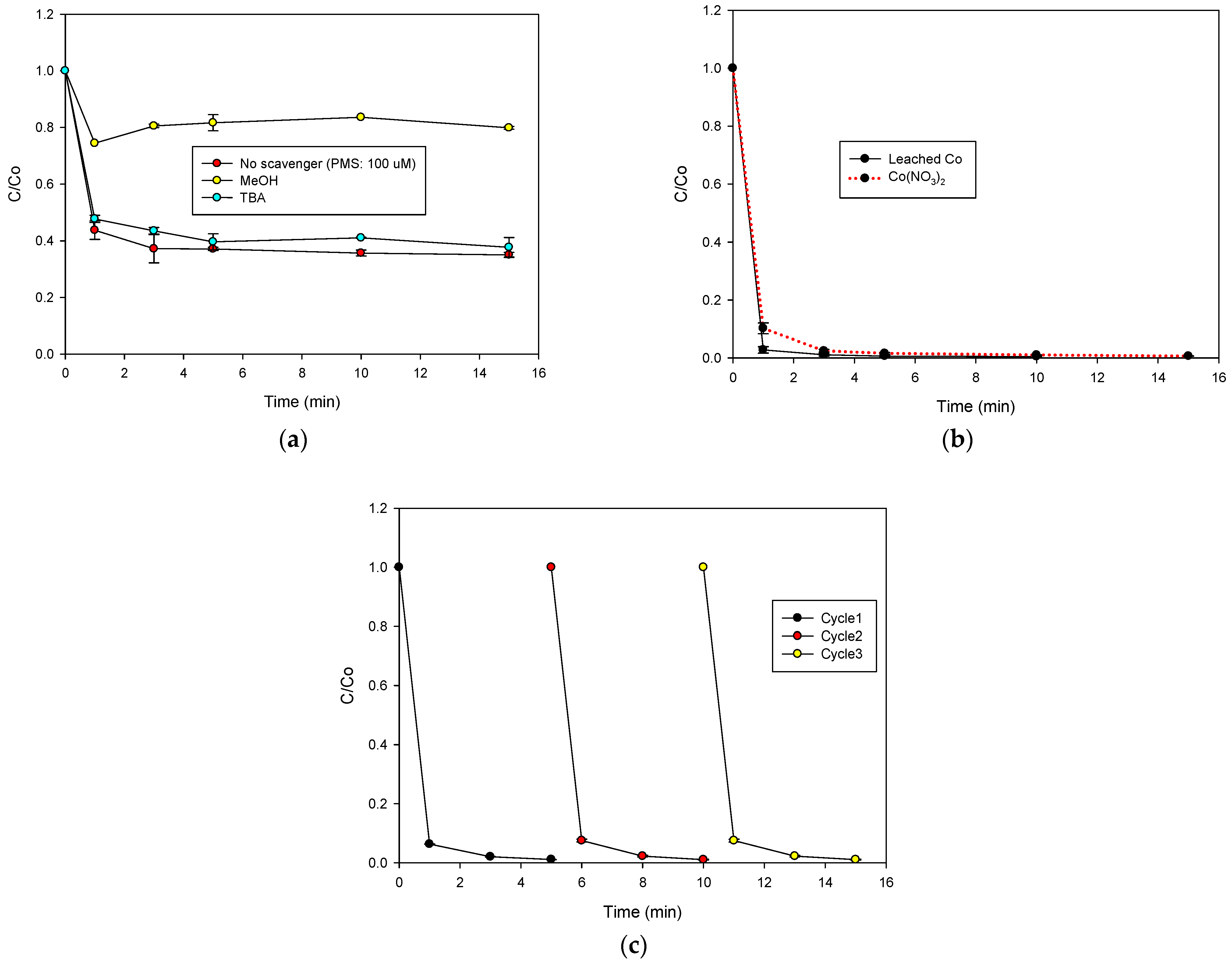
2.3. Action Routes of the Φy/PMS System
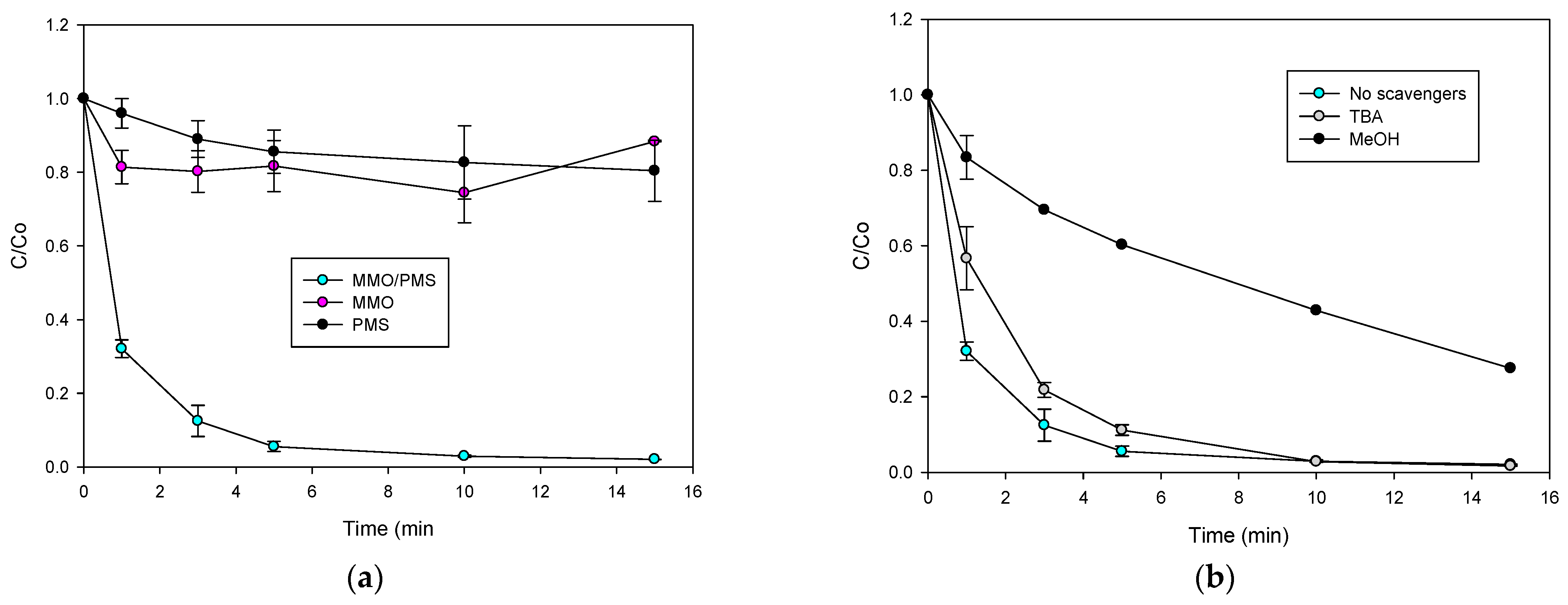
2.4. PMS Activation Using MMO
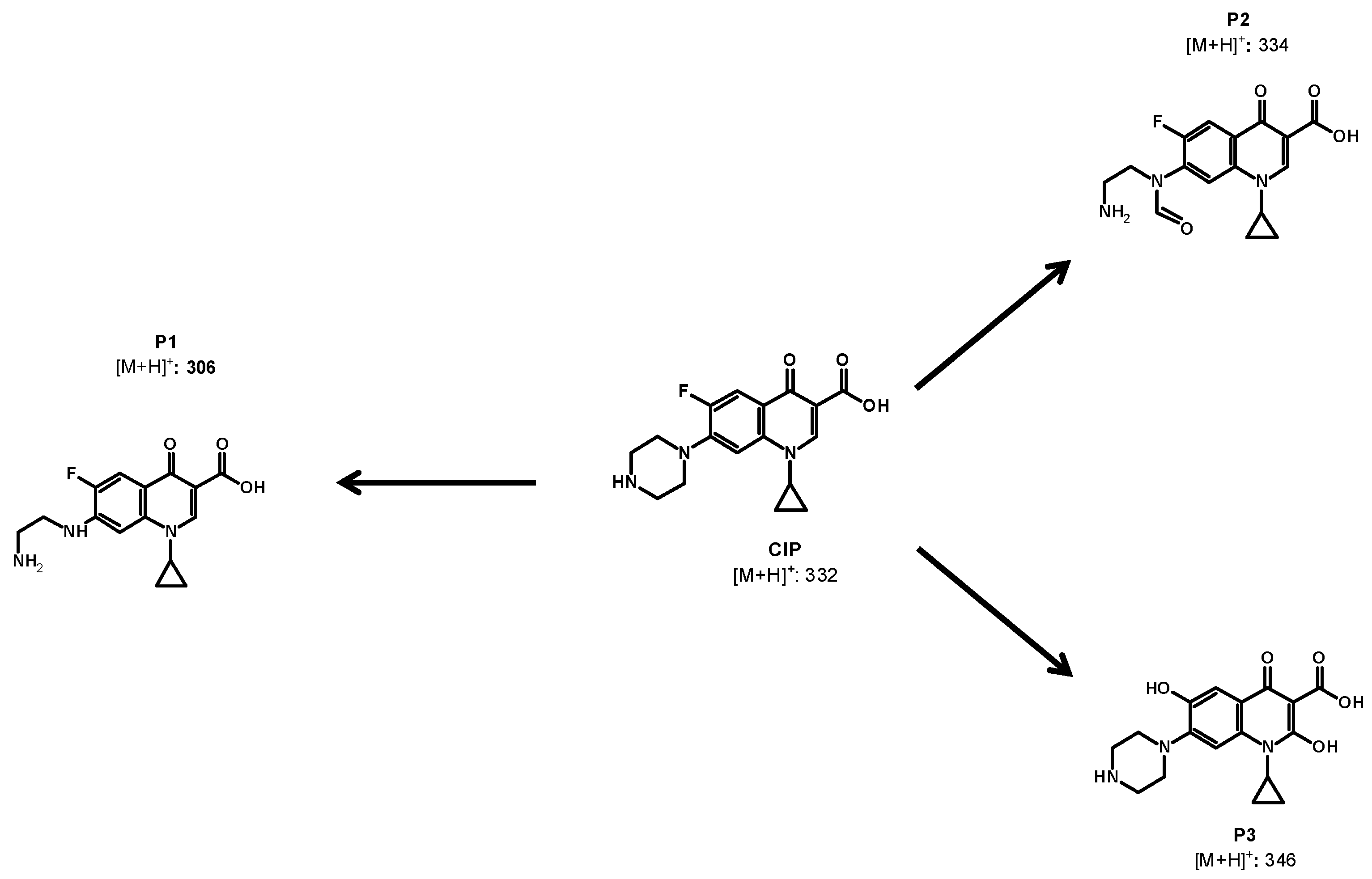
2.5. Primary Transformations of CIP Using the MMO/PMS System
| Biological Activity + | Pa * for CIP | Pa for P1 | Pa for P2 | Pa for P3 |
|---|---|---|---|---|
| Anti-infective | 0.823 | 0.448 | 0.275 | 0.360 |
| DNA synthesis inhibitor | 0.786 | 0.529 | 0.493 | 0.500 |
| Topoisomerase II inhibitor | 0.751 | 0.581 | 0.505 | 0.313 |
| Antibiotic Quinolone-like | 0.567 | 0.172 | 0.138 | 0.053 |
| DNA gyrase inhibitor | 0.468 | 0.166 | 0.067 | 0.122 |
| DNA topoisomerase IV inhibitor | 0.222 | 0.070 | 0.052 | 0.031 |
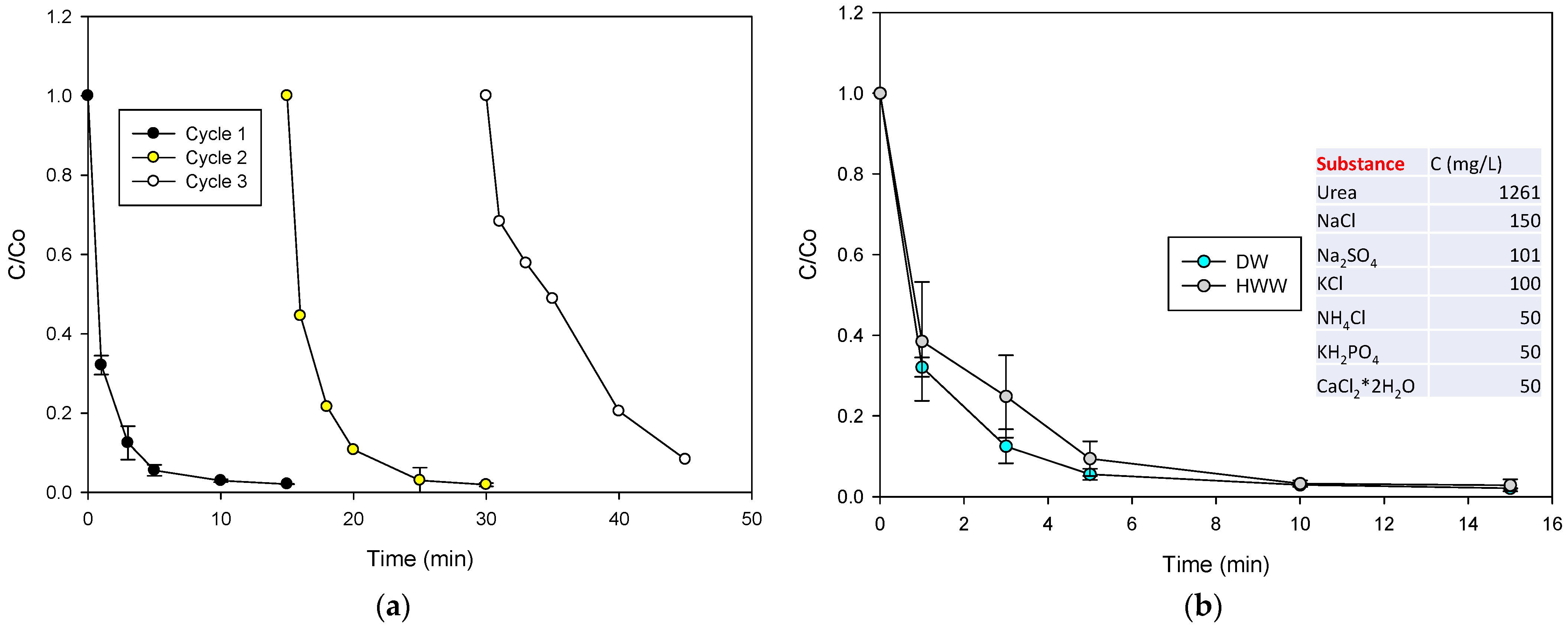
2.6. Reuse of the Catalyst and Treatment in a Complex Matrix CIP Degradation and Using the MMO/PMS System
3. Materials and Methods
3.1. Reagents
3.2. Reaction Systems for Pollutant Removal
3.3. Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brausch, J.M.; Connors, K.; Brooks, B.W.; Rand, G.M. Human pharmaceuticlas in the aquatic environment: A rewiew of recent toxicological studies and considerations for toxicity testing. Rev. Environ. Contam. Toxicol. 2012, 218, 1–99. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, J.R.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.A.; Moncayo-Lasso, A.; Hernández, F. An investigation into the occurrence and removal of pharmaceuticals in colombian wastewater. Sci. Total Environ. 2018, 62, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Ibáñez, M.; Botero-Coy, A.-M.; Bade, R.; Bustos-López, M.C.; Rincón, J.; Moncayo, A.; Bijlsma, L. LC-QTOF MS screening of more than 1,000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal. Bioanal. Chem. 2015, 407, 6405–6416. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Grübel, K.; Padil, V.V.T.; Černík, M.; Dionysiou, D.D. Chemistry of persulfates in water and wastewater treatment: A review. Chem. Eng. J. 2017, 330, 44–62. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.F.; Zhang, L.; Xu, H.Y. Enhancement strategies for efficient activation of persulfate by heterogeneous cobalt-containing catalysts: A review. Chemosphere 2022, 291, 132954. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Chromate-induced activation of hydrogen peroxide for oxidative degradation of aqueous organic pollutants. Environ. Sci. Technol. 2010, 44, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Li, W.; Lan, Y.; Li, Y. CrPO4 as a recycled material supported Co3O4 as an efficient peroxymonosulfate catalyst for phenacetin elimination from aqueous solution. Chem. Eng. J. 2022, 433, 133558. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.-F.; Yan, S.-C.; Wei, W.-W.; Zhang, Q.; Liu, G.; Cai, Z.; Yu, L. Photocatalytic transformation of climbazole and 4-chlorophenol formation using a floral array of chromium-substituted magnetite nanoparticles activated with peroxymonosulfate. Environ. Sci. Nano 2019, 6, 2986–2999. [Google Scholar] [CrossRef]
- Arboleda, J.; Echavarria, A.; Palacio, L.A. Synthesis and characterization of (NH4)1.5Cu2Cr2O8(OH)1.5⋅H2O. Powder Diffr. 2009, 24, 244–246. [Google Scholar] [CrossRef]
- Kiejza, D.; Kotowska, U.; Poli, W.; Karpi, J. Peracids-New oxidants in advanced oxidation processes: The use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern—A review. Sci. Total Environ. 2021, 790, 148195. [Google Scholar] [CrossRef]
- National Library of Medicine Methyl Orange Properties. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/23673835 (accessed on 23 February 2023).
- Rao, V.P.R.; Satyanarayana, D.; Suryanarayana, A. Methyl orange as a redox indicator in titrations with ceric ammonium nitrate in perchloric acid. Fresenius’ Z. Anal. Chem. 1962, 191, 202–204. [Google Scholar] [CrossRef]
- He, X.; Mezyk, S.P.; Michael, I.; Fatta-Kassinos, D.; Dionysiou, D.D. Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J. Hazard. Mater. 2014, 279, 375–383. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt. Environ. Sci. Technol. 2003, 37, 4790–4797. [Google Scholar] [CrossRef]
- Yu, D.; He, J.; Xie, T.; Xu, Q.; Li, G.; Du, L.; Huang, J.; Yang, J.; Li, W.; Wang, J. Peroxymonosulfate activation using a composite of copper and nickel oxide coated on SBA-15 for the removal of sulfonamide antibiotics. Environ. Res. 2022, 206, 112301. [Google Scholar] [CrossRef]
- Ball, D.L.; Edwards, J.O. The catalysis of the decomposition of Caro’s acid. J. Phys. Chem. 1958, 62, 343–345. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, G.; Chen, Y.; Han, B.; Xia, K.; Zhou, C. Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: A critical review. Environ. Sci. Water Res. Technol. 2021, 7, 1197–1211. [Google Scholar] [CrossRef]
- Xie, R.C.; Batchelor-McAuley, C.; Rauwel, E.; Rauwel, P.; Compton, R.G. Electrochemical Characterisation of Co@Co(OH)2 Core-Shell Nanoparticles and their Aggregation in Solution. ChemElectroChem 2020, 7, 4259–4268. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Awala, H.; Gilson, J.-P.; Retoux, R.; Boullay, P.; Goupil, J.-M.; Valtchev, V.; Mintova, S. Template-free nanosized faujasite-type zeolites. Nat. Mater. 2015, 14, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Ferraro, F.; Silva-Agredo, J.; Torres-Palma, R.A. Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res. 2017, 122, 128–138. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Clennan, E.L.; Pace, A. Advances in singlet oxygen chemistry. Tetrahedron 2005, 61, 6665–6691. [Google Scholar] [CrossRef]
- Salma, A.; Thoröe-Boveleth, S.; Schmidt, T.C.; Tuerk, J. Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater. 2016, 313, 49–59. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Nguyen, T.H.; Zheng, N. The chemistry of amine radical cations produced by visible light photoredox catalysis. Beilstein J. Org. Chem. 2013, 9, 1977–2001. [Google Scholar] [CrossRef]
- Dennis, W.; Hull, L.; Rosenblatt, D. Oxidations of amines. IV. Oxidative fragmentation. J. Org. Chem. 1967, 32, 3783–3787. [Google Scholar] [CrossRef]
- Nicolaescu, A.R.; Wiest, O.; Kamat, P.V. Radical-induced oxidative transformation of quinoline. J. Phys. Chem. A 2003, 107, 427–433. [Google Scholar] [CrossRef]
- DrugBank Ciprofloxacin. Available online: https://go.drugbank.com/drugs/DB00537 (accessed on 19 January 2023).
- Andersson, M.I.; MacGowan, A.P. Development of the quinolones. J. Antimicrob. Chemother. 2003, 51 (Suppl. S1), 1–11. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Alovero, F.L.; Pan, X.; Morris, J.E.; Manzo, R.H.; Fisher, L.M. Engineering the Specificity of Antibacterial Fluoroquinolones: Benzenesulfonamide Modifications at C-7 of Ciprofloxacin Change Its Primary Target in Streptococcus pneumoniae from Topoisomerase IV to Gyrase. Antimicrob. Agents Chemother. 2000, 44, 320–325. [Google Scholar] [CrossRef] [PubMed]
- W2D Team-PharmaExpert PASS Online. Available online: http://www.pharmaexpert.ru/passonline/index.php (accessed on 2 May 2021).
- Serna-Galvis, E.A.; Martínez-Mena, Y.L.; Arboleda-Echavarría, J.; Hoyos-Ayala, D.A.; Echavarría-Isaza, A.; Torres-Palma, R.A. Zeolite 4A activates peroxymonosulfate toward the production of singlet oxygen for the selective degradation of organic pollutants. Chem. Eng. Res. Des. 2023, 193, 121–131. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, X.; Liu, Y.; Xia, S.; Zhao, J. Activation of peroxymonosulfate by a floating oxygen vacancies-CuFe2O4 photocatalyst under visible light for efficient degradation of sulfamethazine. Sci. Total Environ. 2022, 824, 153630. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Pang, Y.; Lan, Y.; Zhou, L. Activation of peroxymonosulfate with CuCo2O4@kaolin for the efficient degradation of phenacetin. Chem. Eng. J. 2020, 401, 126014. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, G.; Yuan, S.; Zhan, X.; Wang, W.; Hu, Z.H. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways. J. Photochem. Photobiol. A Chem. 2019, 371, 151–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Pang, S.Y.; Ma, J.; Duan, J.; Guo, Q.; Li, J.; Yang, Y. Oxidation of steroid estrogens by peroxymonosulfate (PMS) and effect of bromide and chloride ions: Kinetics, products, and modeling. Water Res. 2018, 138, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.Y.; Guo, Y.G.; Xiao, D.X.; Wang, Z.H.; Lu, S.Y.; Liu, J.S. Rapid dye degradation with reactive oxidants generated by chloride-induced peroxymonosulfate activation. Environ. Sci. Pollut. Res. 2013, 20, 6317–6323. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, D.; Yin, K.; Yang, C.; Luo, S.; Crittenden, J.C. Degradation of thiacloprid via unactivated peroxymonosulfate: The overlooked singlet oxygen oxidation. Chem. Eng. J. 2020, 388, 124264. [Google Scholar] [CrossRef]
- Dodd, M.C.; Shah, A.D.; Von Gunten, U.; Huang, C.H. Interactions of fluoroquinolone antibacterial agents with aqueous chlorine: Reaction kinetics, mechanisms, and transformation pathways. Environ. Sci. Technol. 2005, 39, 7065–7076. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Jojoa-Sierra, S.D.; Berrio-Perlaza, K.E.; Ferraro, F.; Torres-Palma, R.A. Structure-reactivity relationship in the degradation of three representative fluoroquinolone antibiotics in water by electrogenerated active chlorine. Chem. Eng. J. 2017, 315, 552–561. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Sidhu, H.; Bae, H.; Ogram, A.; Connor, G.O. Azithromycin and Ciprofloxacin Can Promote Antibiotic. Appl. Environ. Microbiol. 2021, 87, e00373-21. [Google Scholar] [CrossRef]
- European Union, Decision 2020/1161/EU Commision Implementing Decision (EU) 2020/1161-4 August 2020-establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Off. J. Eur. Union 2020, 257, 32–35.
- Dai, Y.; Peng, Q.; Liu, K.; Tang, X.; Zhou, M.; Jiang, K.; Zhu, B. Activation of peroxymonosulfate by chrysotile to degrade dyes in water: Performance enhancement and activation mechanism. Minerals 2021, 11, 400. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Dong, X.; Sun, Z.; Duan, X.; Ren, B.; Zheng, S.; Dionysiou, D.D. Highly efficient activation of peroxymonosulfate by natural negatively-charged kaolinite with abundant hydroxyl groups for the degradation of atrazine. Appl. Catal. B Environ. 2019, 247, 10–23. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, J.; Zhao, H.; Zhang, S.; Nie, T.; Zhang, Y.; Lu, J. Enhanced peroxymonosulfate activation by coupling zeolite-supported nano-zero-valent iron with weak magnetic field. Sep. Purif. Technol. 2020, 230, 115886. [Google Scholar] [CrossRef]
- Sun, X.; Xu, D.; Dai, P.; Liu, X.; Tan, F.; Guo, Q. Efficient degradation of methyl orange in water via both radical and non-radical pathways using Fe-Co bimetal-doped MCM-41 as peroxymonosulfate activator. Chem. Eng. J. 2020, 402, 125881. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Jiang, Y.; Lu, J.; Li, H.; Du, X.; Wang, Z. Efficient peroxymonosulfate activation and less metallic leaching through kaolin@MnCo2O4 for bisphenol A degradation in environmental remediation. Appl. Surf. Sci. 2022, 585, 152705. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Luo, C.; Ma, J.; Zhou, Y.; Yang, Y. Oxidation Kinetics of Bromophenols by Nonradical Activation of Peroxydisulfate in the Presence of Carbon Nanotube and Formation of Brominated Polymeric Products. Environ. Sci. Technol. 2017, 51, 10718–10728. [Google Scholar] [CrossRef] [PubMed]
- Serna-Galvis, E.A.; Cáceres-Peña, A.C.; Torres-Palma, R.A. Elimination of representative fluoroquinolones, penicillins, and cephalosporins by solar photo-Fenton: Degradation routes, primary transformations, degradation improvement by citric acid addition, and antimicrobial activity evolution. Environ. Sci. Pollut. Res. 2020, 27, 41381–41393. [Google Scholar] [CrossRef] [PubMed]

| Sample | Metals * (%w/w) | |||
|---|---|---|---|---|
| Cu | Cr | Co | ||
| Φy | 28.30 | 5.87 | 9.39 | |
| Property | Vmicro (cm3 g−1) | Vtotal (cm3 g−1) | SBET (m2 g−1) | Sext (m2 g−1) |
| Value | 0.01 | 0.203 | 38.2 | 18.0 |
| Sample | Metals * (%w/w) | |||
|---|---|---|---|---|
| CuO | CoxCu1−xCr2O4 | |||
| MMO | 28.5 | 71.5 | ||
| Property | Vmicro. (cm3 g−1) | Vtotal (cm3 g−1) | SBET (m2 g−1) | Sext. (m2 g−1) |
| Value | 0.00 | 0.230 | 36.8 | 36.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Galvis, E.A.; Mendoza-Merlano, C.; Torres-Palma, R.A.; Echavarría-Isaza, A.; Hoyos-Ayala, D.A. Materials Based on Co, Cu, and Cr as Activators of PMS for Degrading a Representative Antibiotic—The Strategy for Utilization in Water Treatment and Warnings on Metal Leaching. Molecules 2023, 28, 4536. https://doi.org/10.3390/molecules28114536
Serna-Galvis EA, Mendoza-Merlano C, Torres-Palma RA, Echavarría-Isaza A, Hoyos-Ayala DA. Materials Based on Co, Cu, and Cr as Activators of PMS for Degrading a Representative Antibiotic—The Strategy for Utilization in Water Treatment and Warnings on Metal Leaching. Molecules. 2023; 28(11):4536. https://doi.org/10.3390/molecules28114536
Chicago/Turabian StyleSerna-Galvis, Efraím A., Carlos Mendoza-Merlano, Ricardo A. Torres-Palma, Adriana Echavarría-Isaza, and Dora A. Hoyos-Ayala. 2023. "Materials Based on Co, Cu, and Cr as Activators of PMS for Degrading a Representative Antibiotic—The Strategy for Utilization in Water Treatment and Warnings on Metal Leaching" Molecules 28, no. 11: 4536. https://doi.org/10.3390/molecules28114536
APA StyleSerna-Galvis, E. A., Mendoza-Merlano, C., Torres-Palma, R. A., Echavarría-Isaza, A., & Hoyos-Ayala, D. A. (2023). Materials Based on Co, Cu, and Cr as Activators of PMS for Degrading a Representative Antibiotic—The Strategy for Utilization in Water Treatment and Warnings on Metal Leaching. Molecules, 28(11), 4536. https://doi.org/10.3390/molecules28114536








