Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives
Abstract
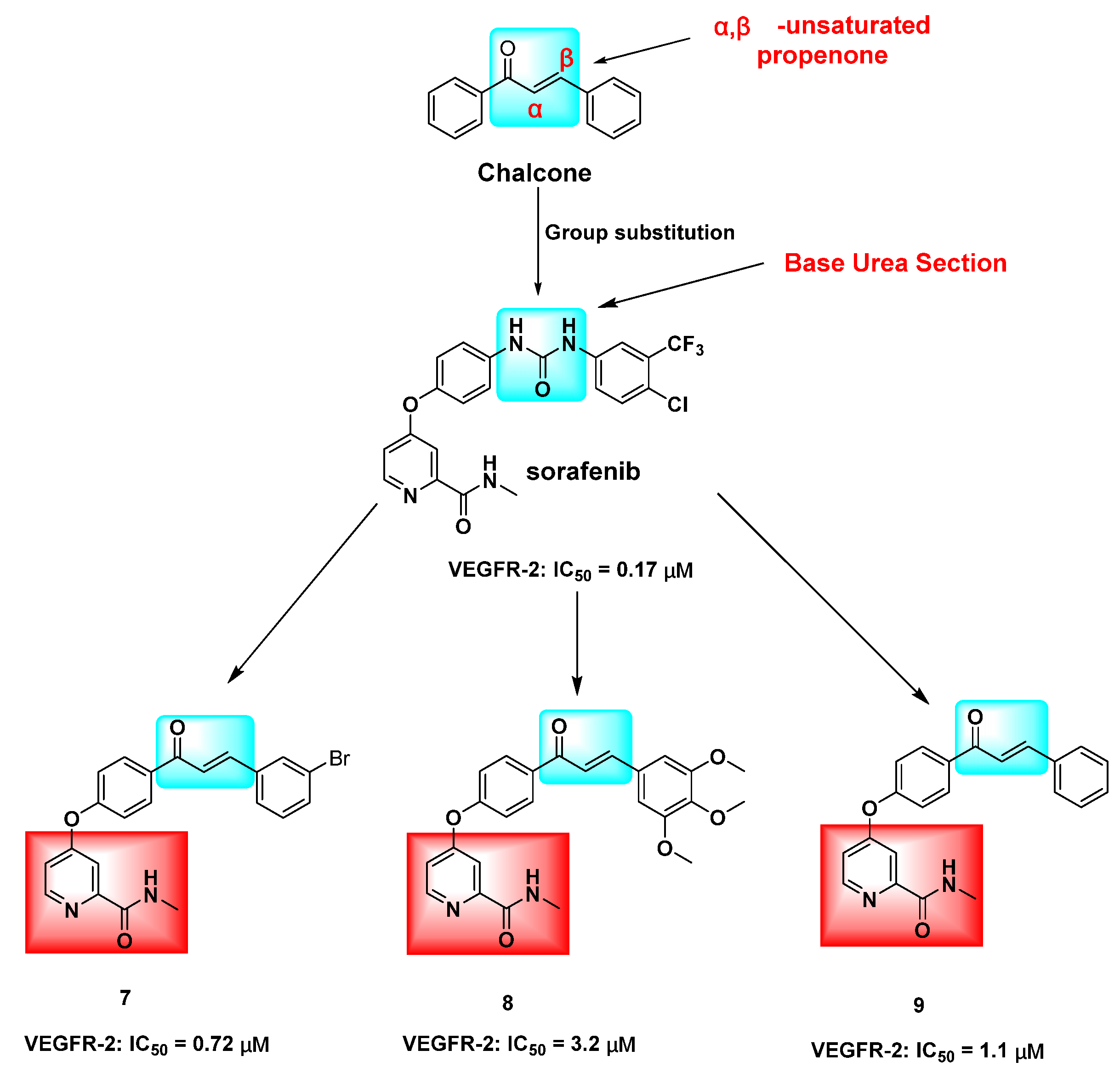
1. Introduction
2. Results and Discussion
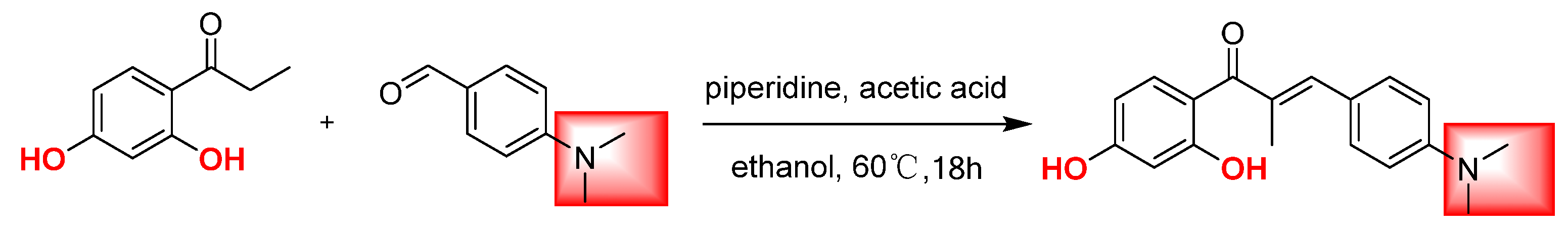
2.1. Chemistry
2.2. In Vitro Activity Assays
2.2.1. Anti-Cervical Cancer Activity Assay
2.2.2. Structure–Effect Relationship Analysis
2.2.3. In Vitro Anti-HUVEC Cell Activities
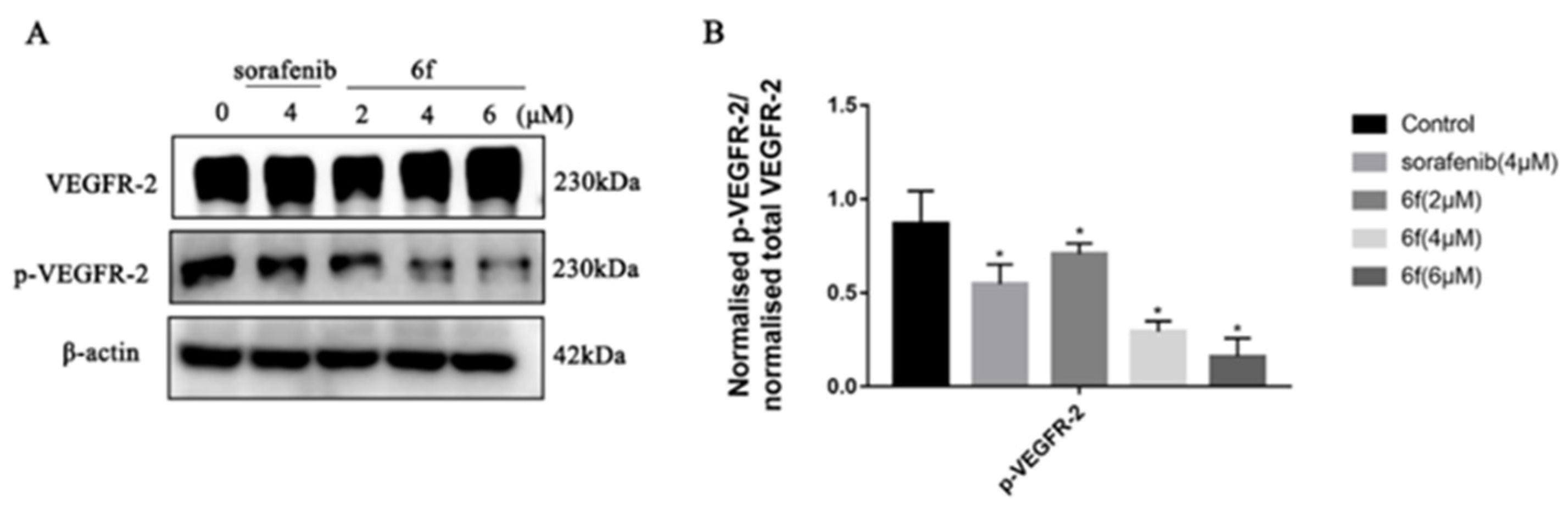
2.2.4. In Vitro VEGFR-2 Inhibitory Assay
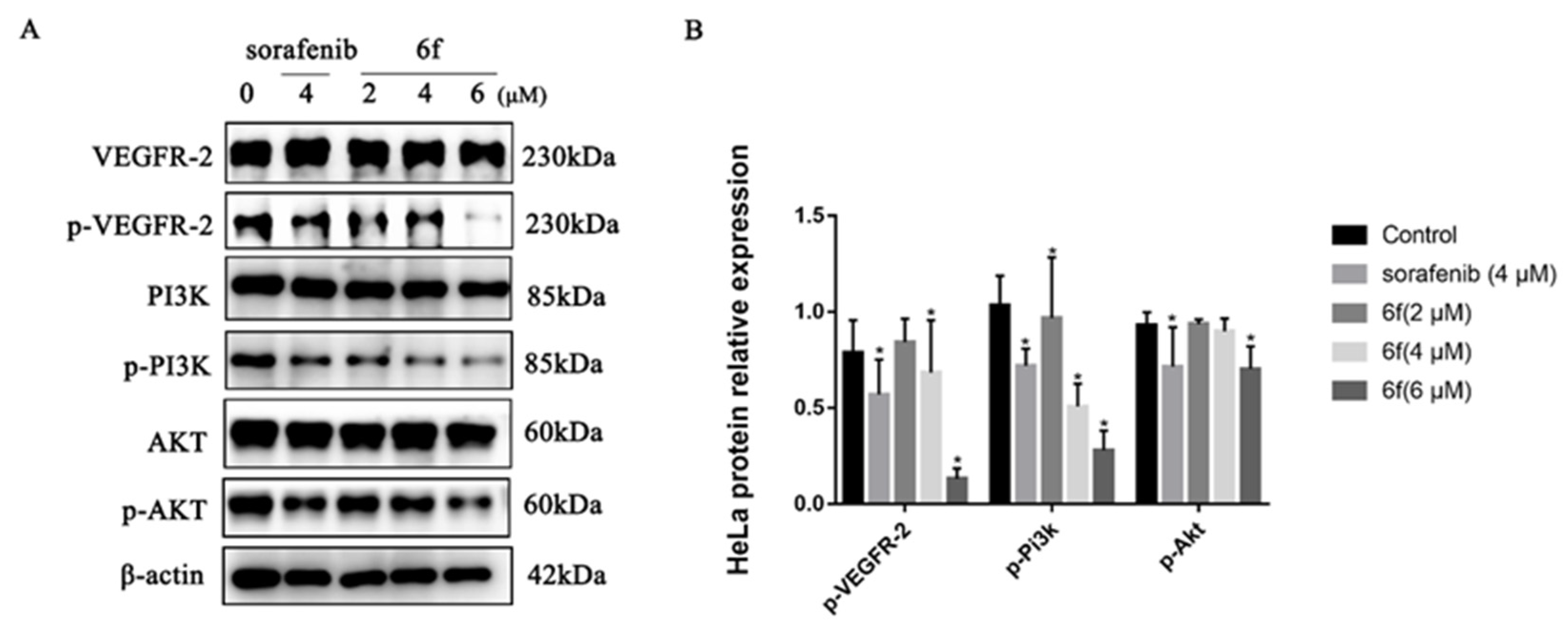
2.2.5. Compound 6f Blocked the PI3K/AKT Pathway of HeLa Cells
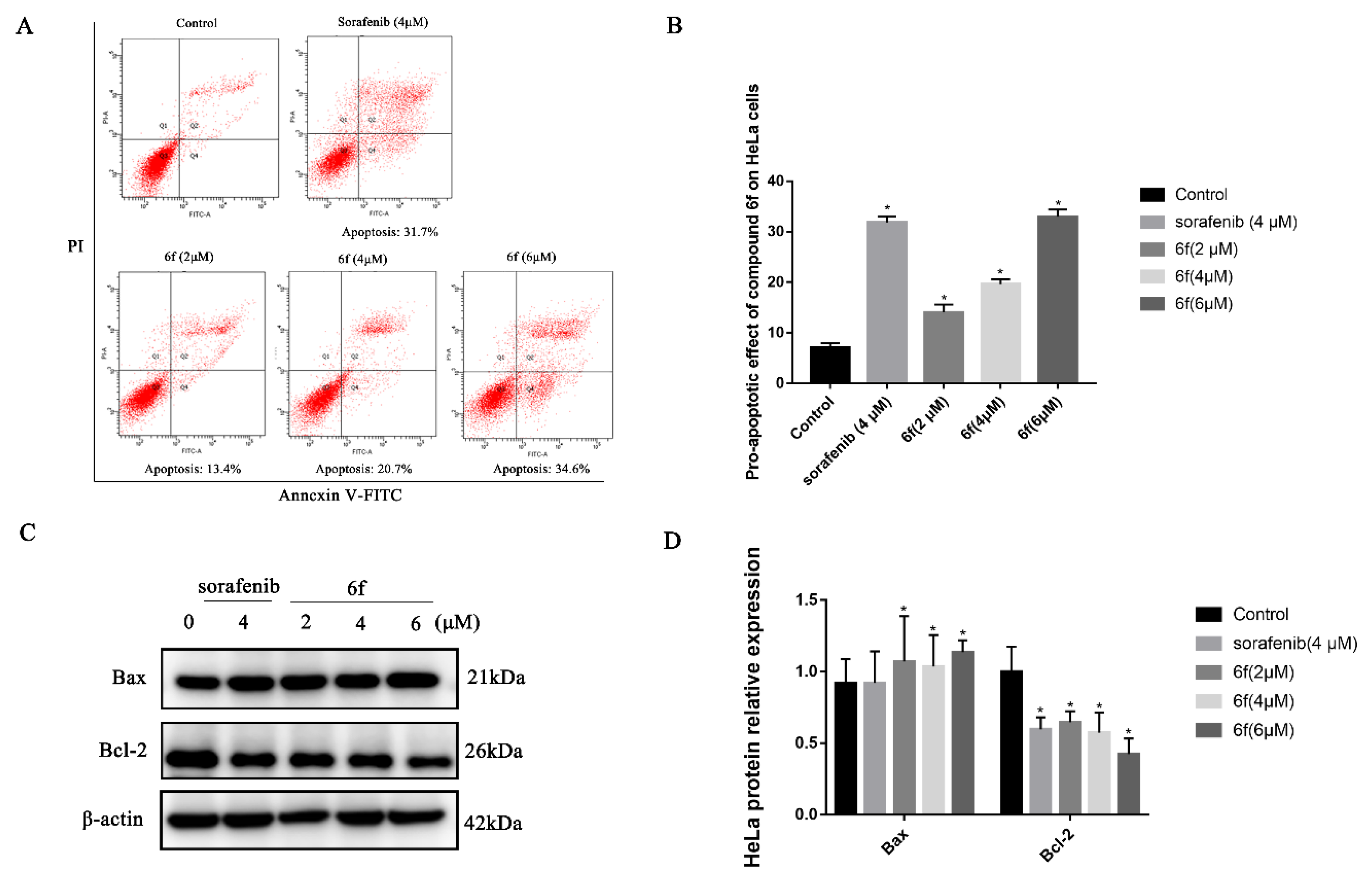
2.2.6. Compound 6f Induced the Apoptosis of Hela Cells
2.2.7. Compound 6f Inhibited the Migration and Invasion of HeLa Cells
2.2.8. In Vitro Anti-HeLa/DDP Cell Activities
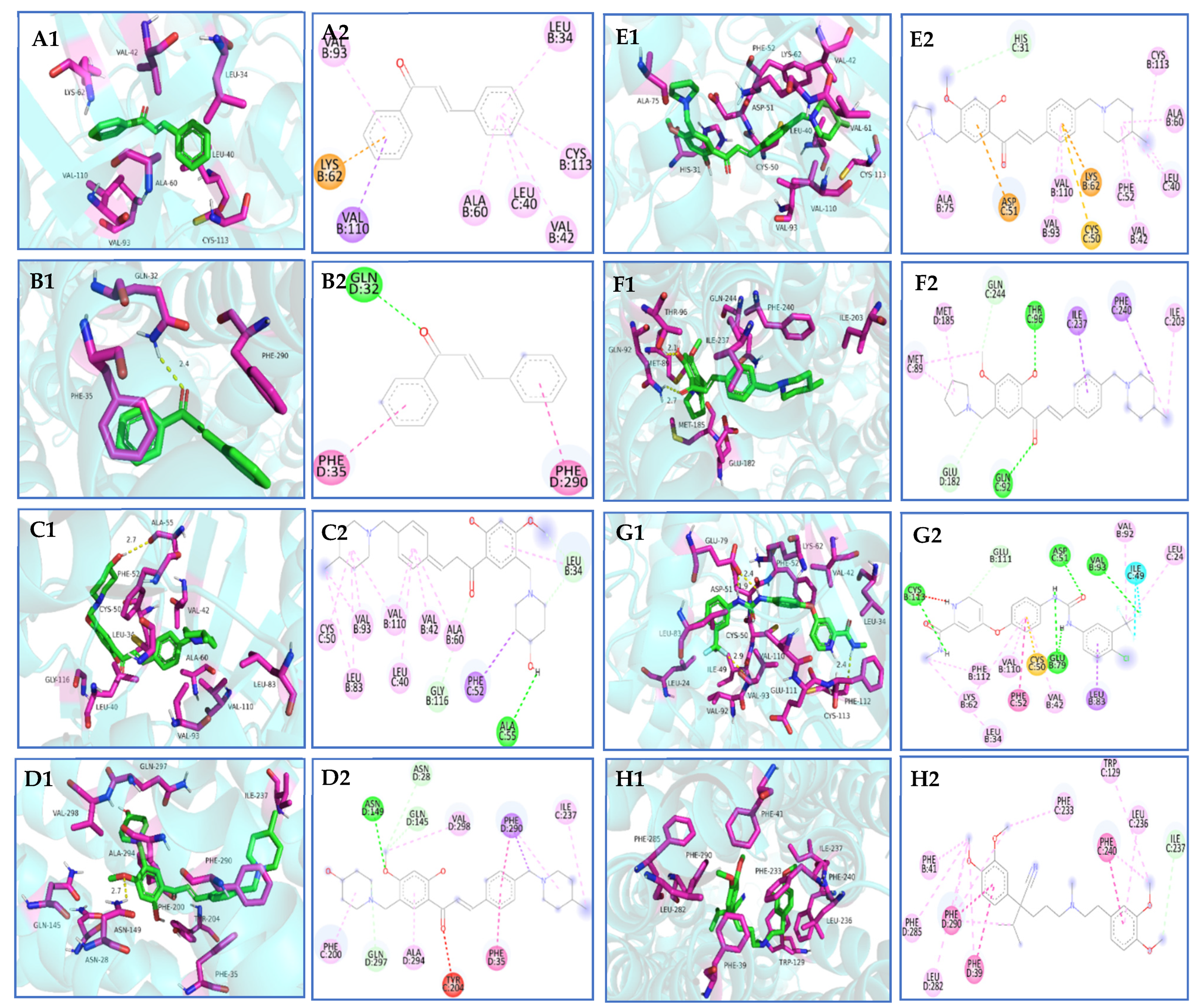
2.2.9. Molecular Docking
3. Material and Methods
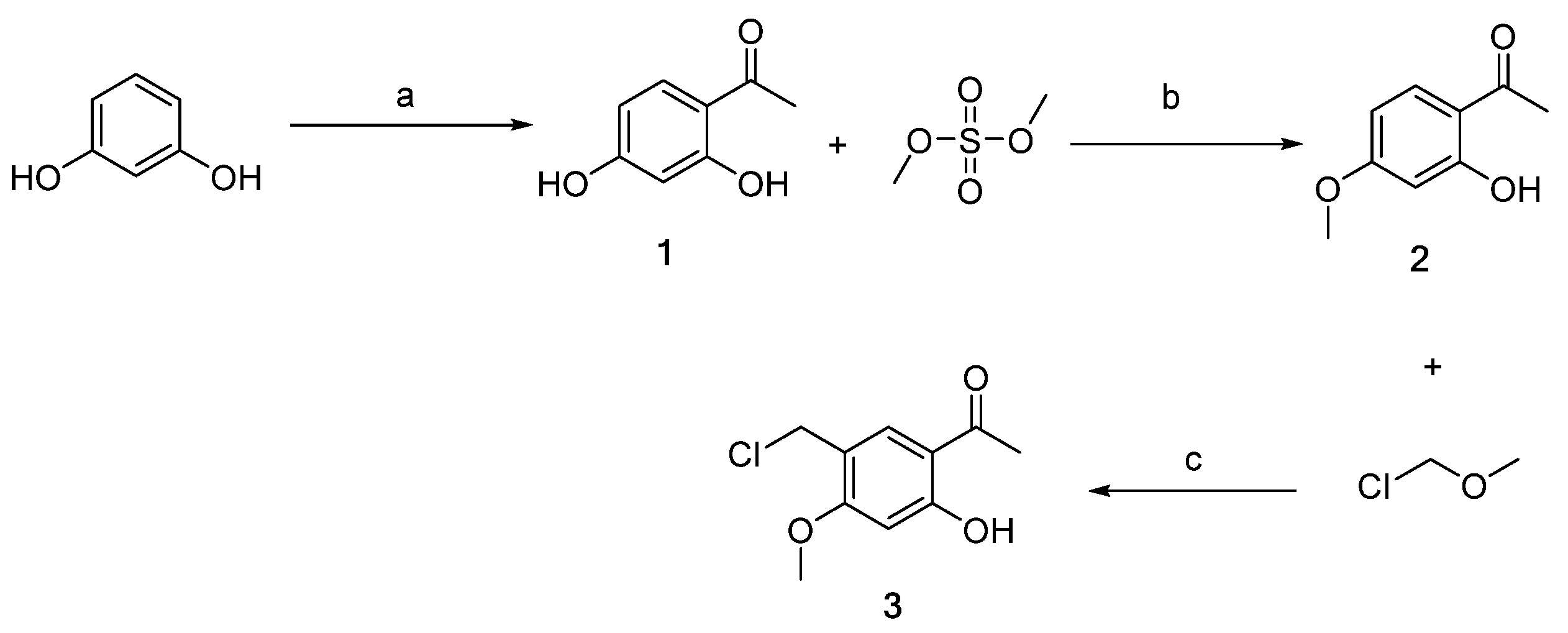
3.1. Chemistry
3.2. Synthesis and Structural Characterization
3.2.1. Synthesis of Intermediate 1 [39]
3.2.2. Synthesis of Intermediate 2 [40]
3.2.3. Synthesis of Intermediate 3
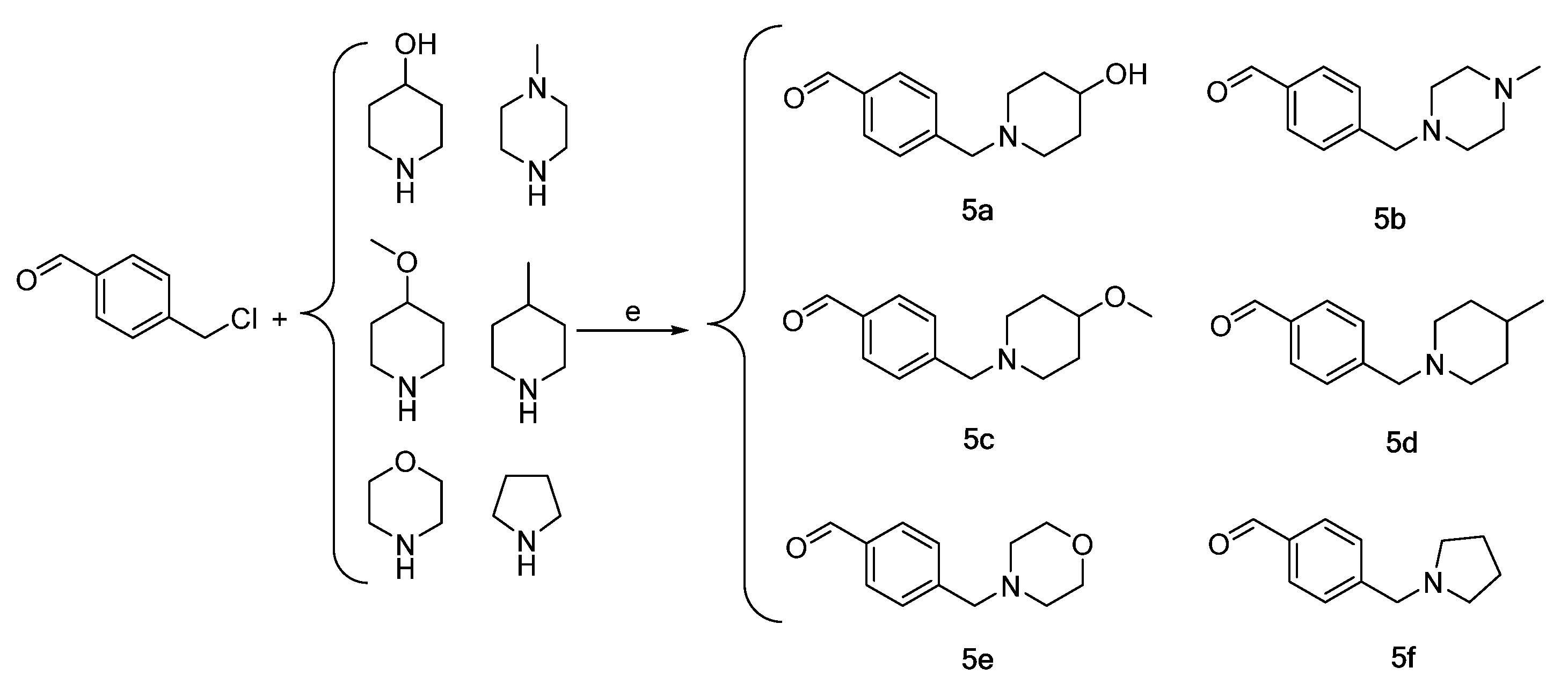
3.2.4. Synthesis of Intermediates 4a–4f
3.2.5. Preparation of Intermediates 5a–5f
3.2.6. Synthesis of Target Compounds
3.3. Biological Assays
3.3.1. Cell Lines and Cell Culture
3.3.2. In Vitro Cytotoxicity Evaluation
3.3.3. In Vitro Anti-HUVEC Cell Activities and Western Blot Analysis
3.3.4. VEGFR-2 Inhibition Test
3.3.5. Effect of Compound 6f on PI3K/AKT Signaling Pathway
3.3.6. Apoptosis Analysis
3.3.7. Transwell Migration and Invasion Assay
3.3.8. Anti-Cisplatin-Resistant Cervical Cancer Activity
3.3.9. Molecular Docking Experiment
3.3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.K.; Balbhadra, S.S.; Choudhary, J.; Kohli, D.V. Exploring pharmacological significance of chalcone scaffold: A review. Cur. Med. Chem. 2012, 19, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Y.; Li, M.; Zhang, Z.; Wang, J.; Ri, M.; Jin, C.; Xu, G.; Piao, L.; Jin, H.; et al. Licochalcone a inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the nf-κb and ras/raf/mek pathways. J. Ethnopharmacol. 2021, 273, 113989. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, C.; Dong, P.; Guo, Y.; Mu, N. Molecular regulation of cervical cancer growth and invasion by vegfa. Tumour. Biol. 2014, 35, 11587–11593. [Google Scholar] [CrossRef]
- Hammes, L.S.; Tekmal, R.R.; Naud, P.; Edelweiss, M.I.; Kirma, N.; Valente, P.T.; Syrjänen, K.J.; Cunha-Filho, J.S. Up-regulation of vegf, c-fms and cox-2 expression correlates with severity of cervical cancer precursor (cin) lesions and invasive disease. Gynecol. Oncol. 2008, 110, 445–451. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Pallares, J.; Montero-Conde, C.; Inglada-Pérez, L.; Castelblanco, E.; Landa, I.; Leskelä, S.; Leandro-García, L.J.; López-Jiménez, E.; Letón, R.; et al. Overexpression and activation of egfr and vegfr2 in medullary thyroid carcinomas is related to metastasis. Endocr. Relat. Cancer 2010, 17, 7–16. [Google Scholar] [CrossRef]
- Qiu, H.; Li, J.; Liu, Q.; Tang, M.; Wang, Y. Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with paclitaxel. Cell Cycle 2018, 17, 1235–1244. [Google Scholar] [CrossRef]
- Long, W.; Zhang, L.; Wang, Y.; Xie, H.; Wang, L.; Yu, H. Research progress and prospects of autophagy in the mechanism of multidrug resistance in tumors. J. Oncol. 2022, 2022, 7032614. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Kania, K.D.; Blauz, A.; Ciszewski, W.M. The lactate receptor (hcar1/gpr81) contributes to doxorubicin chemoresistance via abcb1 transporter up-regulation in human cervical cancer hela cells. J. Physiol. Pharmacol. 2017, 68, 555–564. [Google Scholar]
- Lee, Y.S.; Lim, S.S.; Shin, K.H.; Kim, Y.S.; Ohuchi, K.; Jung, S.H. Anti-angiogenic and anti-tumor activities of 2′-hydroxy-4′-methoxychalcone. Biol. Pharm. Bull 2006, 29, 1028–1031. [Google Scholar] [CrossRef]
- Varinska, L.; van Wijhe, M.; Belleri, M.; Mitola, S.; Perjesi, P.; Presta, M.; Koolwijk, P.; Ivanova, L.; Mojzis, J. Anti-angiogenic activity of the flavonoid precursor 4-hydroxychalcone. Eur. J. Pharmacol. 2012, 691, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via vegf/vegfr-2 signaling pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Feng, L.; Huang, C.; Ding, H.; Zhang, X.; Wang, Z.; Li, Y. Prenylflavone derivatives from broussonetia papyrifera, inhibit the growth of breast cancer cells in vitro and in vivo—Sciencedirect. Phytochem. Lett. 2013, 6, 331–336. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Santali, E.Y.; El-Haggar, R. Novel piperazine-chalcone hybrids and related pyrazoline analogues targeting vegfr-2 kinase; design, synthesis, molecular docking studies, and anticancer evaluation. J. Enzym. Inhib. Med. Chem. 2021, 36, 307–318. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of abc transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- Yin, H.; Dong, J.; Cai, Y.; Shi, X.; Wang, H.; Liu, G.; Tang, Y.; Liu, J.; Ma, L. Design, synthesis and biological evaluation of chalcones as reversers of p-glycoprotein-mediated multidrug resistance. Eur. J. Med. Chem. 2019, 180, 350–366. [Google Scholar] [CrossRef]
- Wang, M.; Xu, S.; Wu, C.; Liu, X.; Tao, H.; Huang, Y.; Liu, Y.; Zheng, P.; Zhu, W. Design, synthesis and activity of novel sorafenib analogues bearing chalcone unit. Bioorg. Med. Chem. Let. 2016, 26, 5450–5454. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef] [PubMed]
- Vergelli, C.; Ciciani, G.; Cilibrizzi, A.; Crocetti, L.; Mannelli, L.D.C.; Ghelardini, C.; Guerrini, G.; Iacovone, A.; Giovannoni, M.P. Synthesis of five and six-membered heterocycles bearing an arylpiperazinylalkyl side chain as orally active antinociceptive agents. Bioorg. Med. Chem. 2015, 23, 6237–6245. [Google Scholar] [CrossRef] [PubMed]
- Safrygin, A.V.; Irgashev, R.A.; Slepukhin, P.A.; Röschenthaler, G.-V.; Sosnovskikh, V.Y. Synthesis of 5-aryl-2-hydroxy-2-(trifluoromethyl)furan-3(2h)-ones and their reactions with aromatic 1,2-diamines, hydrazine and hydroxylamine. Tetrahedron 2015, 71, 8535–8543. [Google Scholar] [CrossRef]
- Edlund, C.; Oh, H.; Nord, C.E. In vitro activity of linezolid and eperezolid against anaerobic bacteria. Clin. Microbiol. Infect. 1999, 5, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Fasciolo, G.; Nicolini, A.; Vacca, N.; Viglierchio, P. Efficacy and safety of moguisteine in comparison with levodropropizine in patients with cough associated with chronic obstructive pulmonary disease, lung cancer, or pulmonary fibrosis. Curr. Ther. Res. 1994, 55, 251–261. [Google Scholar] [CrossRef]
- Fagan, V.; Johansson, C.; Gileadi, C.; Monteiro, O.; Dunford, J.E.; Nibhani, R.; Philpott, M.; Malzahn, J.; Wells, G.; Farham, R.; et al. A chemical probe for tudor domain protein spindlin1 to investigate chromatin function. J. Med. Chem. 2019, 62, 9008–9025. [Google Scholar] [CrossRef]
- Nakagawa, T.; Matsushima, T.; Kawano, S.; Nakazawa, Y.; Kato, Y.; Adachi, Y.; Abe, T.; Semba, T.; Yokoi, A.; Matsui, J.; et al. Lenvatinib in combination with golvatinib overcomes hepatocyte growth factor pathway-induced resistance to vascular endothelial growth factor receptor inhibitor. Cancer Sci. 2014, 105, 723–730. [Google Scholar] [CrossRef]
- Sisko, J.T.; Tucker, T.J.; Bilodeau, M.T.; Buser, C.A.; Ciecko, P.A.; Coll, K.E.; Fernandes, C.; Gibbs, J.B.; Koester, T.J.; Kohl, N.; et al. Potent 2-[(pyrimidin-4-yl)amine}-1,3-thiazole-5-carbonitrile-based inhibitors of vegfr-2 (kdr) kinase. Bioorg. Med. Chem. Let. 2006, 16, 1146–1150. [Google Scholar] [CrossRef]
- de Souza, P.S.; Bibá, G.C.C.; Melo, E.D.D.N.; Muzitano, M.F. Chalcones against the hallmarks of cancer: A mini-review. Nat. Prod. Res. 2021, 36, 4809–4826. [Google Scholar] [CrossRef]
- Ren, B.-Z.; Ablise, M.; Yang, X.-C.; Liao, B.-E.; Yang, Z. Synthesis and biological evaluation of α-methyl-chalcone for anti-cervical cancer activity. Med. Chem. Res. 2017, 26, 1871–1883. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Molecular and biological aspects. Curr. Top Microbiol. Immunol. 1999, 237, 25–57. [Google Scholar]
- Rizvi, S.U.F.; Siddiqui, H.L.; Nisar, M.; Khan, N.; Khan, I. Discovery and molecular docking of quinolyl-thienyl chalcones as anti-angiogenic agents targeting vegfr-2 tyrosine kinase. Bioorg. Med. Chem. Let. 2012, 22, 942–944. [Google Scholar] [CrossRef]
- Tapia, O.; Riquelme, I.; Leal, P.; Sandoval, A.; Aedo, S.; Weber, H.; Letelier, P.; Bellolio, E.; Villaseca, M.; Garcia, P.; et al. The pi3k/akt/mtor pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014, 465, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; Wolf, K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat. Rev. Cancer 2003, 3, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Huang, M.; Lin, Q.; Fang, Q.; Liu, J.; Chen, X.; Liu, L.; Zhan, X.; Shan, H.; et al. The multi-molecular mechanisms of tumor-targeted drug resistance in precision medicine. Biomed. Pharm. 2022, 150, 113064. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. Autodock vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Higashi, F.; Nishiyama, S.; Koshio, M. Preparation of polyarylates from dihydroxyaceto-phenones by the solution polycondensation with tscl/dmf/py as a condensing agent. Macromol. Chem. Phys. 2001, 202, 1812–1816. [Google Scholar] [CrossRef]
- Reddy, M.V.B.; Tsai, W.-J.; Qian, K.; Lee, K.-H.; Wu, T.-S. Structure-activity relationships of chalcone analogs as potential inhibitors of adp- and collagen-induced platelet aggregation. Bioorg. Med. Chem. 2011, 19, 7711–7719. [Google Scholar] [CrossRef]





| Compounds | 48h IC50 (μM) ± SD | ||
|---|---|---|---|
| HeLa | SiHa | H8 | |
| 6a | 72.90 ± 0.12 | >100 | >100 |
| 6b | 40.13 ± 0.11 | 57.11 ± 1.30 | 82.55 ± 5.21 |
| 6c | 32.17 ± 0.14 | 44.49 ± 0.38 | 34.02 ± 4.14 |
| 6d | >100 | >100 | >100 |
| 6e | 56.74 ± 0.59 | >100 | 75.69 ± 0.49 |
| 6f | 6.52 ± 0.42 *#● | 7.88 ± 0.52 *#● | 30.06 ± 2.51 #● |
| 6g | 47.05 ± 0.11 | 85.04 ± 0.47 | 72.86 ± 1.08 |
| 6h | >100 | >100 | >100 |
| 6i | 46.15 ± 0.35 | 77.02 ± 0.22 | 71.64 ± 0.48 |
| 6j | 37.74 ± 0.22 | 47.65 ± 0.19 | 58.46 ± 0.19 |
| 6k | 15.22 ± 0.08 * | 24.83 ± 0.17 * | 30.08 ± 0.44 #● |
| 6l | >100 | >100 | >100 |
| 6m | 52.43 ± 0.42 | >100 | >100 |
| 6n | >100 | >100 | >100 |
| 6o | 65.77 ± 1.36 | 63.68 ± 2.18 | 80.02 ± 8.92 |
| 6p | 79.93 ± 2.40 | 66.04 ± 0.49 | 85.44 ± 3.92 |
| 6q | 88.17 ± 0.72 | >100 | >100 |
| 6r | 25.53 ± 1.21 | 36.60 ± 1.94 | 42.18 ± 0.80 |
| 6s | 30.93 ± 0.39 | 36.15 ± 1.71 | 35.43 ± 0.61 |
| 6t | 30.90 ± 0.18 | 35.95 ± 6.30 | 42.11 ± 0.31 |
| 6u | 28.01 ± 0.14 | 28.22 ± 0.50 | 42.52 ± 0.85 |
| Chalcone | 74.01 ± 4.48 | 66.45 ± 2.88 | 77.71 ± 6.85 |
| Cisplatin | 13.60 ± 1.63 | 21.60 ± 2.90 | 24.75 ± 1.37 |
| Sorafenib | 10.78 ± 0.15 | 14.99 ± 1.20 | 18.41 ± 1.04 |
| Compounds | 48 h IC50 (μM) ± SD |
|---|---|
| Chalcone | 77.51 ± 4.20 |
| 6f | 7.14 ± 0.91 * |
| 6k | 20.15 ± 1.19 * |
| Sorafenib | 9.20 ± 1.22 * |
| Compounds | 48h IC50 (μM) ± SD |
|---|---|
| Chalcone | >20 |
| 6f | 0.75 ± 0.05 * |
| 6k | 1.67 ± 0.18 * |
| Sorafenib | 0.56 ± 0.04 * |
| Compounds | 48h IC50 (μM) ± SD | ||
|---|---|---|---|
| HeLa | HeLa/DDP | RI | |
| Cisplatin | 13.60 ± 1.63 | 100.03 ± 7.94 | 7.36 |
| Paclitaxel | 20.10 ± 1.05 | 124.87 ± 5.30 | 6.21 |
| Doxorubicin | 10.60 ± 0.50 | 41.63 ± 2.05 | 3.93 |
| Sorafenib | 10.78 ± 0.15 | 12.40 ± 0.54 | 1.15 |
| Chalcone | 74.01 ± 4.48 | 91.00 ± 6.22 | 1.23 |
| 6f | 6.52 ± 0.42 | 7.74 ± 0.36 *# | 1.19 |
| 6k | 15.22 ± 0.08 | 17.26 ± 2.07 *# | 1.13 |
| Cisplatin + verapamil (6 μM) | 13.97 ± 0.73 | 35.12 ± 4.14 | 2.51 |
| Cisplatin + 6f (0.25 μM) | 14.47 ± 1.19 | 79.32 ± 4.53 # | 5.48 |
| Cisplatin + 6f (0.5 μM) | 13.20 ± 1.01 | 40.17 ± 2.75 # | 3.04 |
| Cisplatin + 6f (1.0 μM) | 13.69 ± 0.82 | 19.69 ± 1.04 # | 1.44 |
| Ligand | Name of Protein | PDB ID | The Lowest Binding Energy (kcal/mol) | |
|---|---|---|---|---|
| Compd | Hydrogen Bonds | |||
| Chalcone | VEGFR-2 | 4ASD | −9.179 | - |
| P-gp | 7O9W | −8.507 | 1 | |
| Compound 6f | VEGFR-2 | 4ASD | −9.074 | 1 |
| P-gp | 7O9W | −9.823 | 1 | |
| Compound 6k | VEGFR-2 | 4ASD | −8.646 | - |
| P-gp | 7O9W | −8.826 | 2 | |
| Sorafenib | VEGFR-2 | 4ASD | −11.403 | 5 |
| Verapamil | P-gp | 7O9W | −7.507 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Liu, Z.-Y.; Ablise, M.; Maimaiti, A.; Mutalipu, Z.; Alimujiang, Y.; Aihaiti, A. Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives. Molecules 2023, 28, 4537. https://doi.org/10.3390/molecules28114537
Yang Z, Liu Z-Y, Ablise M, Maimaiti A, Mutalipu Z, Alimujiang Y, Aihaiti A. Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives. Molecules. 2023; 28(11):4537. https://doi.org/10.3390/molecules28114537
Chicago/Turabian StyleYang, Zheng, Zheng-Ye Liu, Mourboul Ablise, Aikebaier Maimaiti, Zuohelaguli Mutalipu, Yusupuwajimu Alimujiang, and Aizitiaili Aihaiti. 2023. "Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives" Molecules 28, no. 11: 4537. https://doi.org/10.3390/molecules28114537
APA StyleYang, Z., Liu, Z.-Y., Ablise, M., Maimaiti, A., Mutalipu, Z., Alimujiang, Y., & Aihaiti, A. (2023). Design, Synthesis, and Anti-Cervical Cancer and Reversal of Tumor Multidrug Resistance Activity of Novel Nitrogen-Containing Heterocyclic Chalcone Derivatives. Molecules, 28(11), 4537. https://doi.org/10.3390/molecules28114537






