Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology
Abstract
1. Introduction
2. Botany
3. Traditional Uses
4. Phytochemistry
4.1. Phenylpropanoids
4.2. Sesquiterpenes
4.3. Lignans
4.4. Flavonoids
4.5. Alkaloids
4.6. Amides
4.7. Organic Acids
4.8. Others
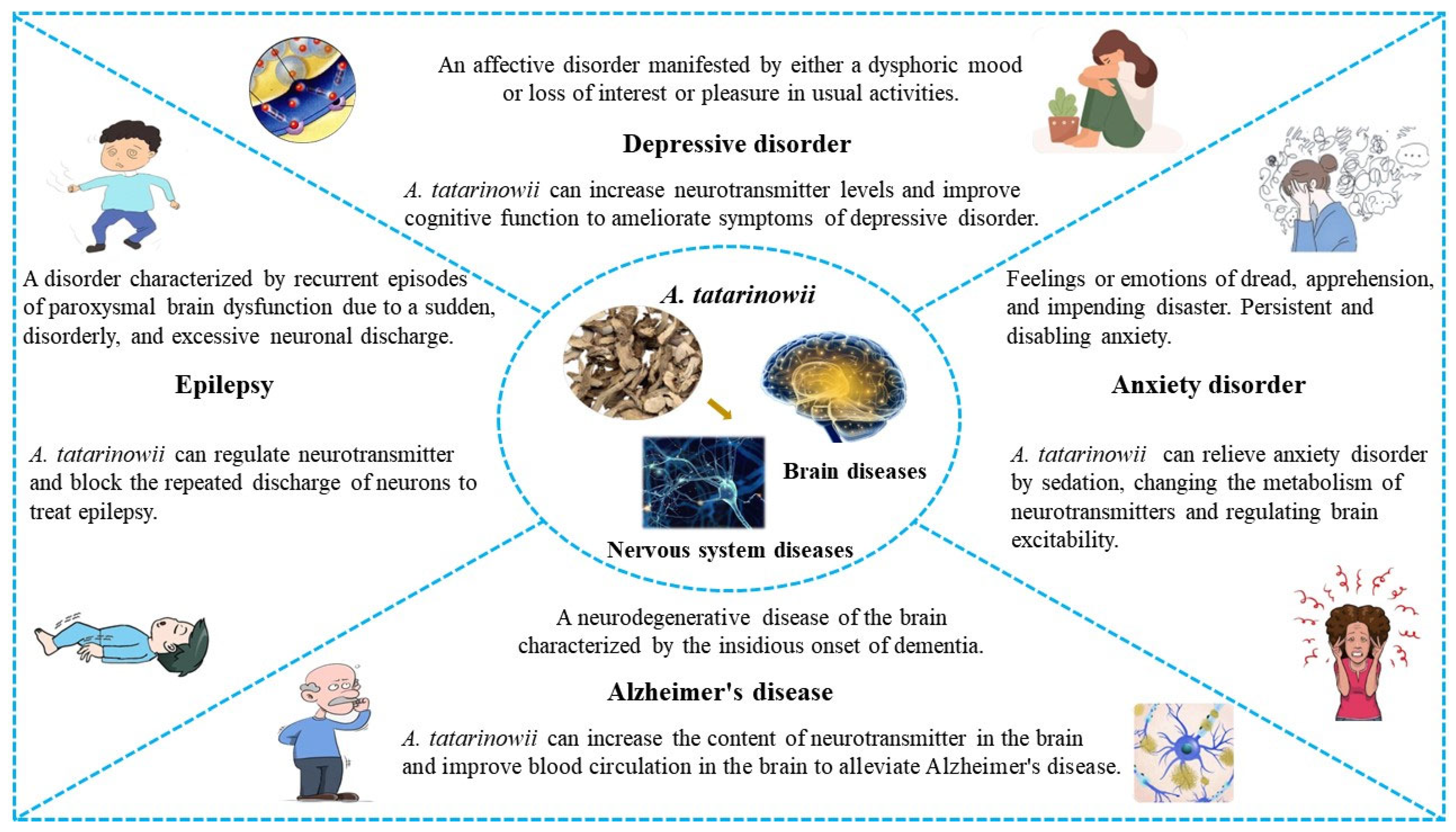
5. Pharmacological Activities
5.1. Antidepressant Properties
5.2. Antiepileptic Properties
5.3. Anticonvulsant Properties
5.4. Antianxiety Properties
5.5. Neuroprotective Properties
5.6. Protective Effects against Alzheimer’s Disease
5.7. Antifatigue Properties
5.8. Antifungal Properties
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.J.; Zhu, Y.Y.; Yi, X.; Zhou, Z.S.; He, Y.J.; Zhou, Y.; Qi, Z.H.; Jin, D.N.; Zhao, L.X.; Luo, X.D. Bioguided isolation, identification and activity evaluation of antifungal compounds from Acorus tatarinowii Schott. J. Ethnopharmacol. 2020, 261, 113119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Shu, H.; Zhang, S.; Luo, B.; Gu, R.; Zhang, R.; Ji, Y.; Li, F.; Long, C. From Folk Taxonomy to Species Confirmation of Acorus (Acoraceae): Evidences Based on Phylogenetic and Metabolomic Analyses. Front. Plant Sci. 2020, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2nd ed.; China Medical Science Press: Beijing, China, 1963. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 11th ed.; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Lee, Y.C.; Kao, S.T.; Cheng, C.Y. Acorus tatarinowii Schott extract reduces cerebral edema caused by ischemia-reperfusion injury in rats: Involvement in regulation of astrocytic NKCC1/AQP4 and JNK/iNOS-mediated signaling. BMC Complement. Med. Ther. 2020, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Zhao, Y.; Sun, Y.; Kong, L.D. Potential effect of herbal antidepressants on cognitive deficit: Pharmacological activity and possible molecular mechanism. J. Ethnopharmacol. 2020, 257, 112830. [Google Scholar] [CrossRef]
- Chellian, R.; Pandy, V.; Mohamed, Z. Pharmacology and toxicology of α- and β-Asarone: A review of preclinical evidence. Phytomedicine 2017, 32, 41–58. [Google Scholar] [CrossRef]
- Lam, K.Y.C.; Wu, Q.Y.; Hu, W.H.; Yao, P.; Wang, H.Y.; Dong, T.T.X.; Tsim, K.W.K. Asarones from Acori Tatarinowii Rhizoma stimulate expression and secretion of neurotrophic factors in cultured astrocytes. Neurosci. Lett. 2019, 707, 134308. [Google Scholar] [CrossRef]
- Zhang, F.H.; Wang, Z.M.; Liu, Y.T.; Huang, J.S.; Liang, S.; Wu, H.H.; Xu, Y.T. Bioactivities of serotonin transporter mediate antidepressant effects of Acorus tatarinowii Schott. J. Ethnopharmacol. 2019, 241, 111967. [Google Scholar] [CrossRef]
- Jaiswal, Y.; Liang, Z.; Ho, A.; Chen, H.; Zhao, Z. Metabolite profiling of tissues of Acorus calamus and Acorus tatarinowii rhizomes by using LMD, UHPLC-QTOF MS, and GC-MS. Planta Med. 2015, 81, 333–341. [Google Scholar] [CrossRef]
- Unger, P.; Melzig, M.F. Comparative study of the cytotoxicity and genotoxicity of alpha- and Beta-asarone. Sci. Pharm. 2012, 80, 663–668. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Xue, W.Z.; Zou, R.X.; Xu, Y.; Du, Y.; Wang, S.; Xu, L.; Chen, Y.Z.; Wang, H.L.; Chen, X.T. β-Asarone Rescues Pb-Induced Impairments of Spatial Memory and Synaptogenesis in Rats. PLoS ONE 2016, 11, e0167401. [Google Scholar] [CrossRef]
- Zhong, R.N.; Wang, X.H.; Wan, L.; Shen, C.Y.; Shen, B.D.; Wang, J.; Han, L.; Yuan, H.L. [Study on preparation of volatile oil from Acorus tatarinowii self-nanoemulsion dropping pills and its protective effect on acute myocardial ischemia injury]. Zhongguo Zhong Yao Za Zhi 2019, 44, 1357–1362. [Google Scholar] [PubMed]
- Lam, K.Y.; Ku, C.F.; Wang, H.Y.; Chan, G.K.; Yao, P.; Lin, H.Q.; Dong, T.T.; Zhang, H.J.; Tsim, K.W. Authentication of Acori Tatarinowii Rhizoma (Shi Chang Pu) and its adulterants by morphological distinction, chemical composition and ITS sequencing. Chin. Med. 2016, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Xu, S.L.; Choi, R.C.; Yan, A.L.; Dong, T.T.; Tsim, K.W. Kai-xin-san, a chinese herbal decoction containing ginseng radix et rhizoma, polygalae radix, acori tatarinowii rhizoma, and poria, stimulates the expression and secretion of neurotrophic factors in cultured astrocytes. Evid. Based Complement. Alternat. Med. 2013, 2013, 731385. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Zhang, X.H.; Wang, Y.Y.; Pan, J.H.; Cui, H.M.; Fang, S.P.; Wu, W.; Zheng, J.; Li, D.J.; Bai, G. Effects of Kaixin Jieyu Decoction () on behavior, monoamine neurotransmitter levels, and serotonin receptor subtype expression in the brain of a rat depression model. Chin. J. Integr. Med. 2014, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Xu, S.L.; Zhu, K.Y.; Lam, K.Y.; Xin, G.; Maiwulanjiang, M.; Li, N.; Dong, T.T.; Lin, H.; Tsim, K.W. Optimizing the compatibility of paired-herb in an ancient Chinese herbal decoction Kai-Xin-San in activating neurofilament expression in cultured PC12 cells. J. Ethnopharmacol. 2015, 162, 155–162. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, L.L.; Zhang, Y.L.; Fan, W.T. [Research on network pharmacology of Acori Tatarinowii Rhizoma combined with Curcumae Radix in treating epilepsy]. Zhongguo Zhong Yao Za Zhi 2019, 44, 2701–2708. [Google Scholar]
- Xiong, W.; Zhao, X.; Xu, Q.; Wei, G.; Zhang, L.; Fan, Y.; Wen, L.; Liu, Y.; Zhang, T.; Zhang, L.; et al. Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 2022, 282, 114598. [Google Scholar] [CrossRef]
- Dang, H.; Sun, L.; Liu, X.; Peng, B.; Wang, Q.; Jia, W.; Chen, Y.; Pan, A.; Xiao, P. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp. Biol. Med. 2009, 234, 785–793. [Google Scholar] [CrossRef]
- Huang, Z.; Mao, Q.Q.; Zhong, X.M.; Feng, C.R.; Pan, A.J.; Li, Z.Y. Herbal formula SYJN protect PC12 cells from neurotoxicity induced by corticosterone. J. Ethnopharmacol. 2009, 125, 456–460. [Google Scholar] [CrossRef]
- Parés-Badell, O.; Barbaglia, G.; Jerinic, P.; Gustavsson, A.; Salvador-Carulla, L.; Alonso, J. Cost of disorders of the brain in Spain. PLoS ONE 2014, 9, e105471. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kwak, T.Y.; Bae, M.H.; Shin, H.K.; Choi, B.T. Therapeutic Potential of Active Components from Acorus gramineus and Acorus tatarinowii in Neurological Disorders and Their Application in Korean Medicine. J. Pharmacopunct. 2022, 25, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qi, P.; Xue, R.; Li, Z.; Zhu, K.; Wan, P.; Huang, C. Qualitative and quantitative analysis of the major constituents in Acorus tatarinowii Schott by HPLC/ESI-QTOF-MS/MS. Biomed. Chromatogr. 2015, 29, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q.; Yang, B.; Li, J.; Yang, C.; Meng, Y.; Kuang, H. GC-MS method for determination and pharmacokinetic study of four phenylpropanoids in rat plasma after oral administration of the essential oil of Acorus tatarinowii Schott rhizomes. J. Ethnopharmacol. 2014, 155, 1134–1140. [Google Scholar] [CrossRef]
- Liang, S.; Ying, S.S.; Wu, H.H.; Liu, Y.T.; Dong, P.Z.; Zhu, Y.; Xu, Y.T. A novel sesquiterpene and three new phenolic compounds from the rhizomes of Acorus tatarinowii Schott. Bioorg. Med. Chem. Lett. 2015, 25, 4214–4218. [Google Scholar] [CrossRef]
- Gao, E.; Zhou, Z.Q.; Zou, J.; Yu, Y.; Feng, X.L.; Chen, G.D.; He, R.R.; Yao, X.S.; Gao, H. Bioactive Asarone-Derived Phenylpropanoids from the Rhizome of Acorus tatarinowii Schott. J. Nat. Prod. 2017, 80, 2923–2929. [Google Scholar] [CrossRef]
- Tong, X.G.; Qiu, B.; Luo, G.F.; Zhang, X.F.; Cheng, Y.X. Alkaloids and sesquiterpenoids from Acorus tatarinowii. J. Asian Nat. Prod. Res. 2010, 12, 438–442. [Google Scholar] [CrossRef]
- Ni, G.; Shi, G.R.; Zhang, D.; Fu, N.J.; Yang, H.Z.; Chen, X.G.; Yu, D.Q. Cytotoxic Lignans and Sesquiterpenoids from the Rhizomes of Acorus tatarinowii. Planta Med. 2016, 82, 632–638. [Google Scholar] [CrossRef]
- Dong, W.; Yang, D.; Lu, R. Chemical constituents from the rhizome of Acorus calamus L. Planta Med. 2010, 76, 454–457. [Google Scholar] [CrossRef]
- Tong, X.G.; Wu, G.S.; Huang, C.G.; Lu, Q.; Wang, Y.H.; Long, C.L.; Luo, H.R.; Zhu, H.J.; Cheng, Y.X. Compounds from Acorus tatarinowii: Determination of absolute configuration by quantum computations and cAMP regulation activity. J. Nat. Prod. 2010, 73, 1160–1163. [Google Scholar] [CrossRef]
- Hao, Z.Y.; Liang, D.; Luo, H.; Liu, Y.F.; Ni, G.; Zhang, Q.J.; Li, L.; Si, Y.K.; Sun, H.; Chen, R.Y.; et al. Bioactive sesquiterpenoids from the rhizomes of Acorus calamus. J. Nat. Prod. 2012, 75, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Wang, W.; Li, L.; Zhang, L.; Zhao, X.F.; Liu, Q.R.; Liu, F.; Yang, M.; Khan, I.A.; et al. New Acorane-Type Sesquiterpene from Acorus calamus L. Molecules 2017, 22, 529. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xue, Y.; Chen, S.; Zhu, H.; Zhang, J.; Li, X.N.; Wang, J.; Liu, J.; Qi, C.; Du, G.; et al. Antioxidant Lignans and Neolignans from Acorus tatarinowii. Sci. Rep. 2016, 6, 22909. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xue, Y.; Liu, J.; Yao, G.; Li, D.; Sun, B.; Zhang, J.; Liu, Y.; Qi, C.; Xiang, M.; et al. (±)-Acortatarinowins A-F, Norlignan, Neolignan, and Lignan Enantiomers from Acorus tatarinowii. J. Nat. Prod. 2015, 78, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, N.; Wang, Y.; Pan, L.C.; Wu, D.; Peng, Q.; Zhang, M.; Wang, H.B.; Sun, W.C. Tatarinan O, a lignin-like compound from the roots of Acorus tatarinowii Schott inhibits osteoclast differentiation through suppressing the expression of c-Fos and NFATc1. Int. Immunopharmacol. 2016, 34, 212–219. [Google Scholar] [CrossRef]
- Ni, G.; Shen, Z.F.; Lu, Y.; Wang, Y.H.; Tang, Y.B.; Chen, R.Y.; Hao, Z.Y.; Yu, D.Q. Glucokinase-activating sesquinlignans from the rhizomes of Acorus tatarinowii Schott. J. Org. Chem. 2011, 76, 2056–2061. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Feng, X.L.; Lu, D.; Gao, H.; Yu, Y.; Yao, X.S. New lignans attenuating cognitive deterioration of Aβ transgenic flies discovered in Acorus tatarinowii. Bioorg. Med. Chem. Lett. 2018, 28, 814–819. [Google Scholar] [CrossRef]
- Luo, X.H.; Zhang, Y.Y.; Chen, X.Y.; Sun, M.L.; Li, S.; Wang, H.B. Lignans from the roots of Acorus tatarinowii Schott ameliorate β amyloid-induced toxicity in transgenic Caenorhabditis elegans. Fitoterapia 2016, 108, 5–8. [Google Scholar] [CrossRef]
- Feng, X.L.; Li, H.B.; Gao, H.; Huang, Y.; Zhou, W.X.; Yu, Y.; Yao, X.S. Bioactive Nitrogenous Compounds from Acorus tatarinowii. Magn. Reson. Chem. 2016, 54, 396–399. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.X.; Zhao, J.P.; Wang, W.; Zhao, X.F.; Xu, B.; Li, L.; Zhang, L.; Ren, J.; Khan, I.A.; et al. A Novel Tropoloisoquinoline Alkaloid, Neotatarine, from Acorus calamus L. Chem. Biodivers. 2017, 14, e1700201. [Google Scholar] [CrossRef]
- Tong, X.G.; Zhou, L.L.; Wang, Y.H.; Xia, C.; Wang, Y.; Liang, M.; Hou, F.F.; Cheng, Y.X. Acortatarins A and B, two novel antioxidative spiroalkaloids with a naturally unusual morpholine motif from Acorus tatarinowii. Org. Lett. 2010, 12, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qiu, J.; Huang, Z.; Yu, Z.; Wang, W.; Hu, H.; You, Y. A comprehensive review of ethnopharmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et Wils. J. Ethnopharmacol. 2022, 284, 114759. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Han, P.; Han, T.; Peng, W.; Wang, X.R. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm. Biol. 2013, 51, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Gao, Z.; Rong, H.; Jin, M.; Zhang, X. β-asarone reverses chronic unpredictable mild stress-induced depression-like bhavior and promotes hippocampal neurogenesis in rats. Molecules 2014, 19, 5634–5649. [Google Scholar] [CrossRef]
- Liao, W.P.; Chen, L.; Yi, Y.H.; Sun, W.W.; Gao, M.M.; Su, T.; Yang, S.Q. Study of antiepileptic effect of extracts from Acorus tatarinowii Schott. Epilepsia 2005, 46, 21–24. [Google Scholar] [CrossRef]
- Liu, H.; Song, Z.; Liao, D.G.; Zhang, T.Y.; Liu, F.; Zhuang, K.; Luo, K.; Yang, L.; He, J.; Lei, J.P. Anticonvulsant and Sedative Effects of Eudesmin isolated from Acorus tatarinowii on mice and rats. Phytother. Res. 2015, 29, 996–1003. [Google Scholar] [CrossRef]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [CrossRef]
- Tian, J.; Tian, Z.; Qin, S.L.; Zhao, P.Y.; Jiang, X.; Tian, Z. Anxiolytic-like effects of α-asarone in a mouse model of chronic pain. Metab. Brain Dis. 2017, 32, 2119–2129. [Google Scholar] [CrossRef]
- Lam, K.Y.C.; Yao, P.; Wang, H.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Asarone from Acori Tatarinowii Rhizome prevents oxidative stress-induced cell injury in cultured astrocytes: A signaling triggered by Akt activation. PLoS ONE 2017, 12, e0179077. [Google Scholar] [CrossRef]
- Wang, N.; Wang, H.; Li, L.; Li, Y.; Zhang, R. β-Asarone Inhibits Amyloid-β by Promoting Autophagy in a Cell Model of Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Saki, G.; Eidi, A.; Mortazavi, P.; Panahi, N.; Vahdati, A. Effect of β-asarone in normal and β-amyloid-induced Alzheimeric rats. Arch. Med. Sci. 2020, 16, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, H.; Tan, N.; Zeng, G.; Zeng, Z.; Chu, H.; Wang, H.; Xia, Z.; Wu, R. The effects of Acorus tatarinowii Schott on 5-HT concentrations, TPH2 and 5-HT1B expression in the dorsal raphe of exercised rats. J. Ethnopharmacol. 2014, 158, 431–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Yu, S.; Li, D.; Yang, M.; Guan, Y.; Zhang, D.; Wan, J.; Liu, S.; Shi, A.; et al. Natural volatile oils derived from herbal medicines: A promising therapy way for treating depressive disorder. Pharmacol. Res. 2021, 164, 105376. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.J.; Baxter, A.J.; Cheng, H.G.; Shidhaye, R.; Whiteford, H.A. The burden of mental, neurological, and substance use disorders in China and India: A systematic analysis of community representative epidemiological studies. Lancet 2016, 388, 376–389. [Google Scholar] [CrossRef]
- Ding, D.; Zhou, D.; Sander, J.W.; Wang, W.; Li, S.; Hong, Z. Epilepsy in China: Major progress in the past two decades. Lancet Neurol. 2021, 20, 316–326. [Google Scholar] [CrossRef]
- Nandakumar, S.; Menon, S.; Shailajan, S. A rapid HPLC-ESI-MS/MS method for determination of β-asarone, a potential anti-epileptic agent, in plasma after oral administration of Acorus calamus extract to rats. Biomed. Chromatogr. 2013, 27, 318–326. [Google Scholar] [CrossRef]
- Huang, C.; Li, W.G.; Zhang, X.B.; Wang, L.; Xu, T.L.; Wu, D.; Li, Y. α-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology 2013, 65, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Liang, L.; Xu, R.; Cheng, P.; Jia, P.; Bai, Y.; Zhang, Y.; Zhao, X.; Zheng, X.; Xiao, C. Revealing the Antiepileptic Effect of α-Asaronol on Pentylenetetrazole-Induced Seizure Rats Using NMR-Based Metabolomics. ACS Omega 2022, 7, 6322–6334. [Google Scholar] [CrossRef]
- Chin, R.F.; Neville, B.G.; Peckham, C.; Bedford, H.; Wade, A.; Scott, R.C.; NLSTEPSS Collaborative Group. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: Prospective population-based study. Lancet 2006, 368, 222–229. [Google Scholar] [CrossRef]
- Sánchez Fernández, I.; Goodkin, H.P.; Scott, R.C. Pathophysiology of convulsive status epilepticus. Seizure 2019, 68, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Miao, J.K.; Li, C.; Li, X.W.; Wu, X.M.; Zhang, X.P. Anticonvulsant activity of acute and chronic treatment with a-asarone from Acorus gramineus in seizure models. Biol. Pharm. Bull. 2013, 36, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Colla, A.R.; Rosa, J.M.; Cunha, M.P.; Rodrigues, A.L. Anxiolytic-like effects of ursolic acid in mice. Eur. J. Pharmacol. 2015, 758, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Descalzi, G.; Chen, T.; Ko, H.G.; Lu, J.; Li, S.; Son, J.; Kim, T.; Kwak, C.; Huganir, R.L.; et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron 2015, 85, 377–389. [Google Scholar] [CrossRef]
- Wang, G.Q.; Cen, C.; Li, C.; Cao, S.; Wang, N.; Zhou, Z.; Liu, X.M.; Xu, Y.; Tian, N.X.; Zhang, Y.; et al. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat. Commun. 2015, 6, 7660. [Google Scholar] [CrossRef]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Zhang, N.; Guo, Y.Y.; Feng, B.; Liu, S.B.; Zhao, M.G. Estrogen receptor GPR30 exerts anxiolytic effects by maintaining the balance between GABAergic and glutamatergic transmission in the basolateral amygdala of ovariectomized mice after stress. Psychoneuroendocrinology 2013, 38, 2218–2233. [Google Scholar] [CrossRef]
- Yan, L.; Mahady, G.; Qian, Y.; Song, P.; Jian, T.; Ding, X.; Guan, F.; Shan, Y.; Wei, M. The Essential Oil from Acori Tatarinowii Rhizome (the Dried Rhizome of Acorus tatarinowii Schott) Prevents Hydrogen Peroxide-Induced Cell Injury in PC12 Cells: A Signaling Triggered by CREB/PGC-1α Activation. Evid. Based Complement. Alternat. Med. 2020, 2020, 4845028. [Google Scholar] [CrossRef]
- Manikandan, S.; Srikumar, R.; Jeya Parthasarathy, N.; Sheela Devi, R. Protective effect of Acorus calamus LINN on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol. Pharm. Bull. 2005, 28, 2327–2330. [Google Scholar] [CrossRef]
- Yang, Y.X.; Chen, Y.T.; Zhou, X.J.; Hong, C.L.; Li, C.Y.; Guo, J.Y. Beta-asarone, a major component of Acorus tatarinowii Schott, attenuates focal cerebral ischemia induced by middle cerebral artery occlusion in rats. BMC Complement. Altern. Med. 2013, 13, 236. [Google Scholar] [CrossRef]
- Lam, K.Y.; Chen, J.; Lam, C.T.; Wu, Q.; Yao, P.; Dong, T.T.; Lin, H.; Tsim, K.W. Asarone from Acori Tatarinowii Rhizoma Potentiates the Nerve Growth Factor-Induced Neuronal Differentiation in Cultured PC12 Cells: A Signaling Mediated by Protein Kinase, A. PLoS ONE 2016, 11, e0163337. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Cho, D.Y.; Kim, I.S.; Seol, S.H.; Choi, D.K. Molecular Mechanisms and Therapeutic Potential of α- and β-Asarone in the Treatment of Neurological Disorders. Antioxidants 2022, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, B.W.; Song, S.Y.; Kim, J.S.; Kim, I.S.; Kwon, Y.S.; Koppula, S.; Choi, D.K. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci. Biotechnol. Biochem. 2012, 76, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, H.; Zhang, K.; Lv, P.; Zheng, L.; Zhao, J. Pro-neurogenic effect of β-asarone on RSC96 Schwann cells in vitro. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, D.; Xu, D.; Wang, T.; Chen, L.; Duan, J. Characterization of polysaccharides with antioxidant and immunological activities from Rhizoma Acori Tatarinowii. Carbohydr. Polym. 2015, 133, 154–162. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Li, X.; Ye, L. Preparation and characterization of solid lipid nanoparticles loaded with alpha-Asarone. PDA J. Pharm. Sci. Technol. 2008, 62, 56–65. [Google Scholar]
- Hu, Y.; Yuan, M.; Liu, P.; Mu, L.; Wang, H. [Effect of Acorus tatarinowii schott on ultrastructure and permeability of blood-brain barrier]. Zhongguo Zhong Yao Za Zhi 2009, 34, 349–351. [Google Scholar]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Mendelsohn, A.R.; Larrick, J.W. Cellular Senescence as the Key Intermediate in Tau-Mediated Neurodegeneration. Rejuvenation Res. 2018, 21, 572–579. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.Y.; Wang, F.; Qu, Y.H.; Zhang, M.; Luo, T.; Wang, H.Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice. Sci. Rep. 2019, 9, 19575. [Google Scholar] [CrossRef]
- Yang, Y.; Xuan, L.; Chen, H.; Dai, S.; Ji, L.; Bao, Y.; Li, C. Neuroprotective Effects and Mechanism of β-Asarone against Aβ1-42-Induced Injury in Astrocytes. Evid. Based Complement. Alternat. Med. 2017, 2017, 8516518. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, G.; Dong, M.; Zhou, L.; Li, J.; Wang, G.; Zou, D.; Wang, R.; Liu, J.; Niu, Y. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 2010, 635, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.T.; Fang, Y.Q.; He, Y.P.; Zhang, S. β-Asarone protects PC12 cells against OGD/R-induced injury via attenuating Beclin-1-dependent autophagy. Acta Pharmacol. Sin. 2012, 33, 737–742. [Google Scholar] [CrossRef] [PubMed]
- An, H.M.; Li, G.W.; Lin, C.; Gu, C.; Jin, M.; Sun, W.X.; Qiu, M.F.; Hu, B. Acorus tatarinowii Schott extract protects PC12 cells from amyloid-beta induced neurotoxicity. Pharmazie 2014, 69, 391–395. [Google Scholar]
- Wang, N.; Wang, H.; Pan, Q.; Kang, J.; Liang, Z.; Zhang, R. The Combination of β-Asarone and Icariin Inhibits Amyloid-β and Reverses Cognitive Deficits by Promoting Mitophagy in Models of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2021, 2021, 7158444. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Xing, G.; Zhou, L.; Dong, M.; Geng, Y.; Li, X.; Li, J.; Wang, G.; Zou, D.; et al. Beta-asarone attenuates neuronal apoptosis induced by Beta amyloid in rat hippocampus. Yakugaku Zasshi 2010, 130, 737–746. [Google Scholar] [CrossRef]
- Zou, D.J.; Wang, G.; Liu, J.C.; Dong, M.X.; Li, X.M.; Zhang, C.; Zhou, L.; Wang, R.; Niu, Y.C. Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Pharmazie 2011, 66, 44–51. [Google Scholar]
- Cotel, F.; Exley, R.; Cragg, S.J.; Perrier, J.F. Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 4774–4779. [Google Scholar] [CrossRef]
- Twomey, R.; Aboodarda, S.J.; Kruger, R.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Zhu, M.J.; Mao, Z.H.; Guo, H.Y.; Zhu, H.Z.; Ding, X.M. [Effects of acorus tatarinowii Schott and alpha asarone on free radicals and nNOS/NO in hippocampus of rats with fatigue movement]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2020, 36, 306–311. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Guarner, J. Emerging and reemerging fungal infections. Semin. Diagn. Pathol. 2019, 36, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Singla, R.K.; Dubey, A.K. Emerging Virulence, Drug Resistance and Future Anti-fungal Drugs for Candida Pathogens. Curr. Top. Med. Chem. 2018, 18, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, Y.; Hou, C.; Wang, D.; Sun, F.; Li, X.; Wang, F.; Yi, H.; Mu, H.; Duan, J. Synthesis of hyaluronan-amikacin conjugate and its bactericidal activity against intracellular bacteria in vitro and in vivo. Carbohydr. Polym. 2018, 181, 132–140. [Google Scholar] [CrossRef]
- Moudgal, V.; Sobel, J. Antifungals to treat Candida albicans. Expert Opin. Pharmacother. 2010, 11, 2037–2048. [Google Scholar] [CrossRef]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- Stegmüller, S.; Schrenk, D.; Cartus, A.T. Formation and fate of DNA adducts of alpha- and beta-asarone in rat hepatocytes. Food Chem. Toxicol. 2018, 116, 138–146. [Google Scholar] [CrossRef]
- Zhong, R.N.; Wang, X.H.; Wang, X.T.; Shen, B.D.; Shen, C.Y.; Wang, J.; Han, L.; Yuan, H.L. [Preparation and quality evaluation of volatile oil from Acori Tatarinowii Rhizoma self-nanoemulsion]. Zhongguo Zhong Yao Za Zhi 2018, 43, 4062–4068. [Google Scholar]
- Zhang, S.; Zhao, L.; Shan, C.; Shi, Y.; Ma, K.; Wu, J. Exploring the biosynthetic pathway of lignin in Acorus tatarinowii Schott using de novo leaf and rhizome transcriptome analysis. Biosci. Rep. 2021, 41, 20210006. [Google Scholar] [CrossRef]


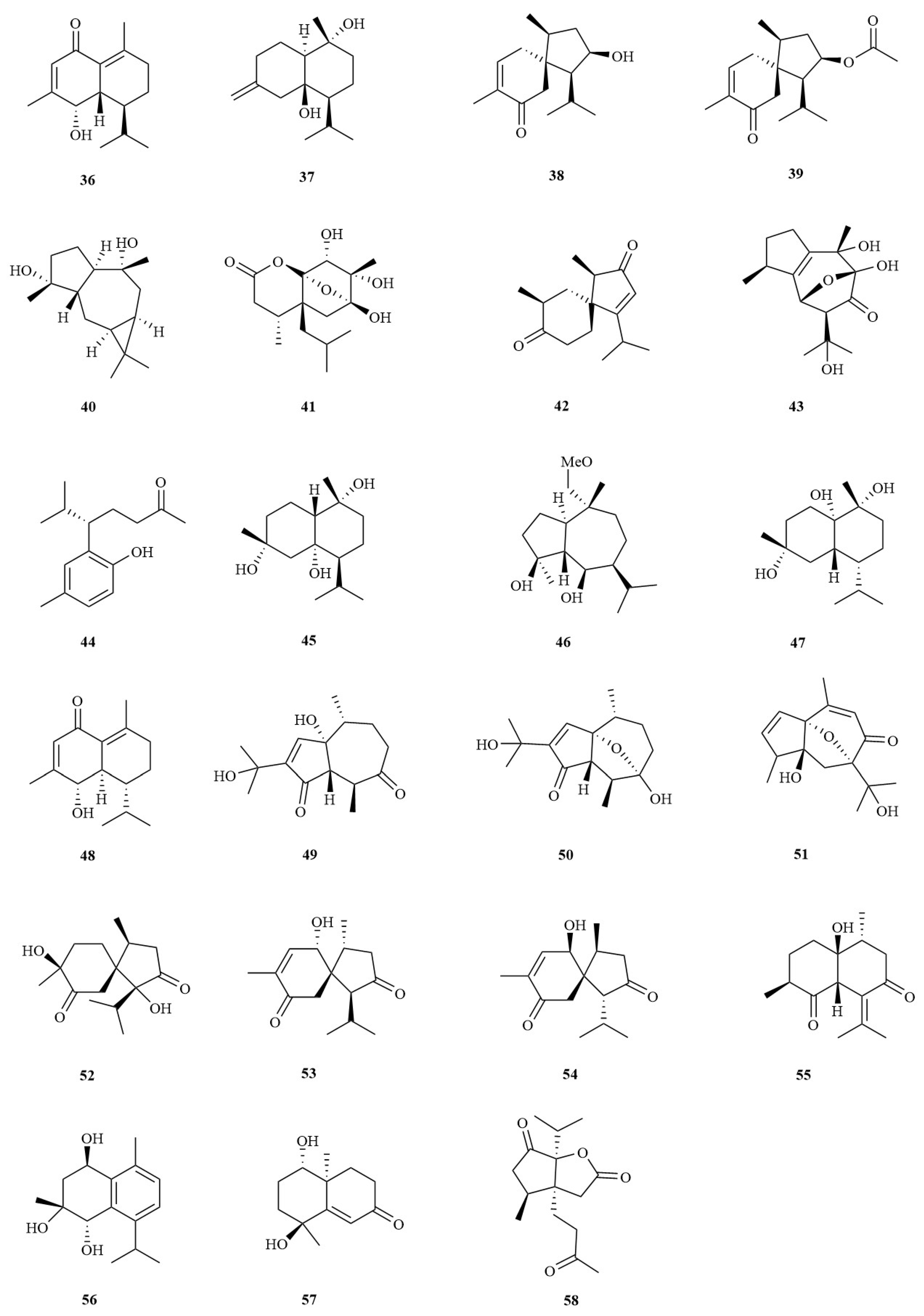

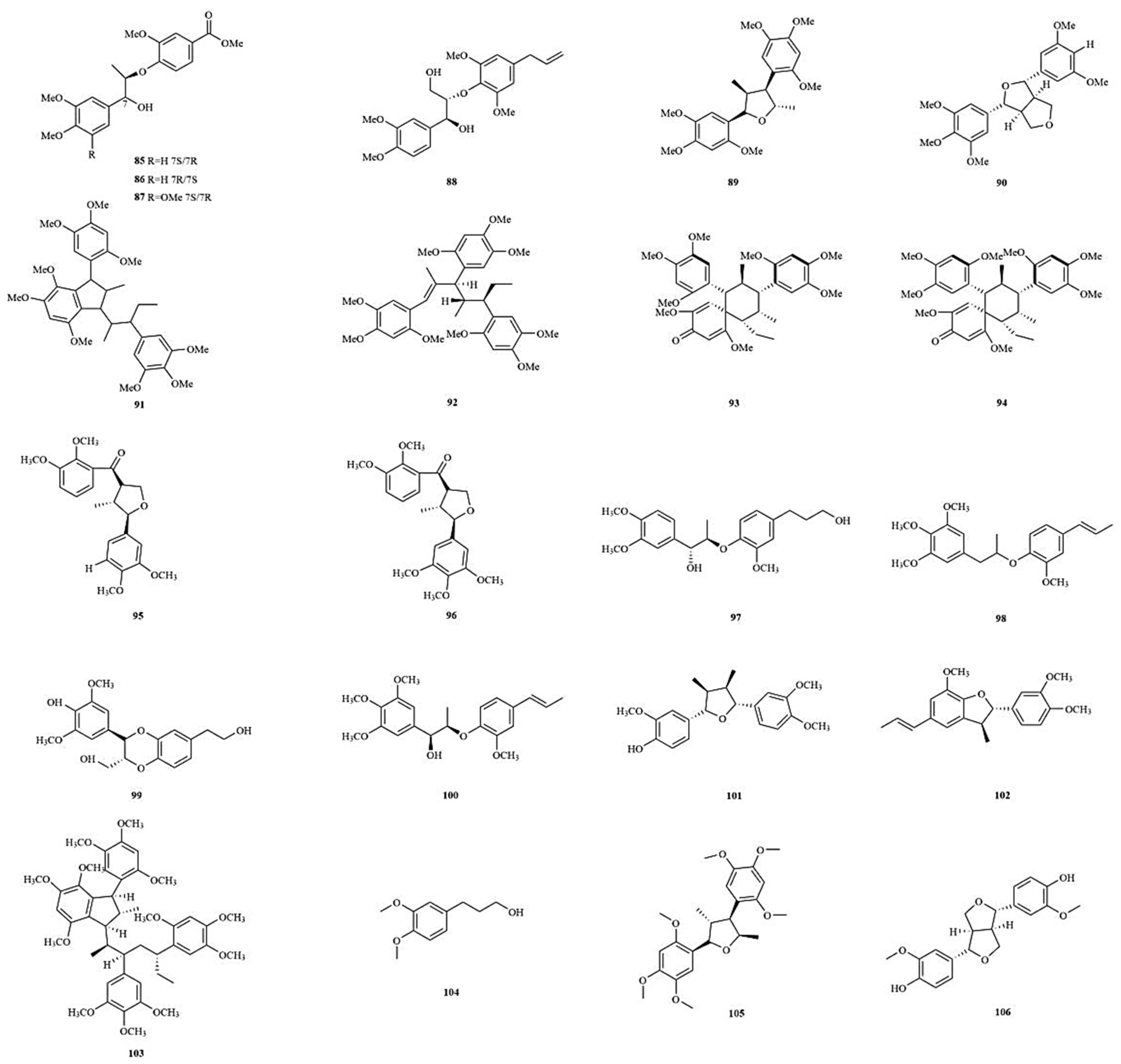
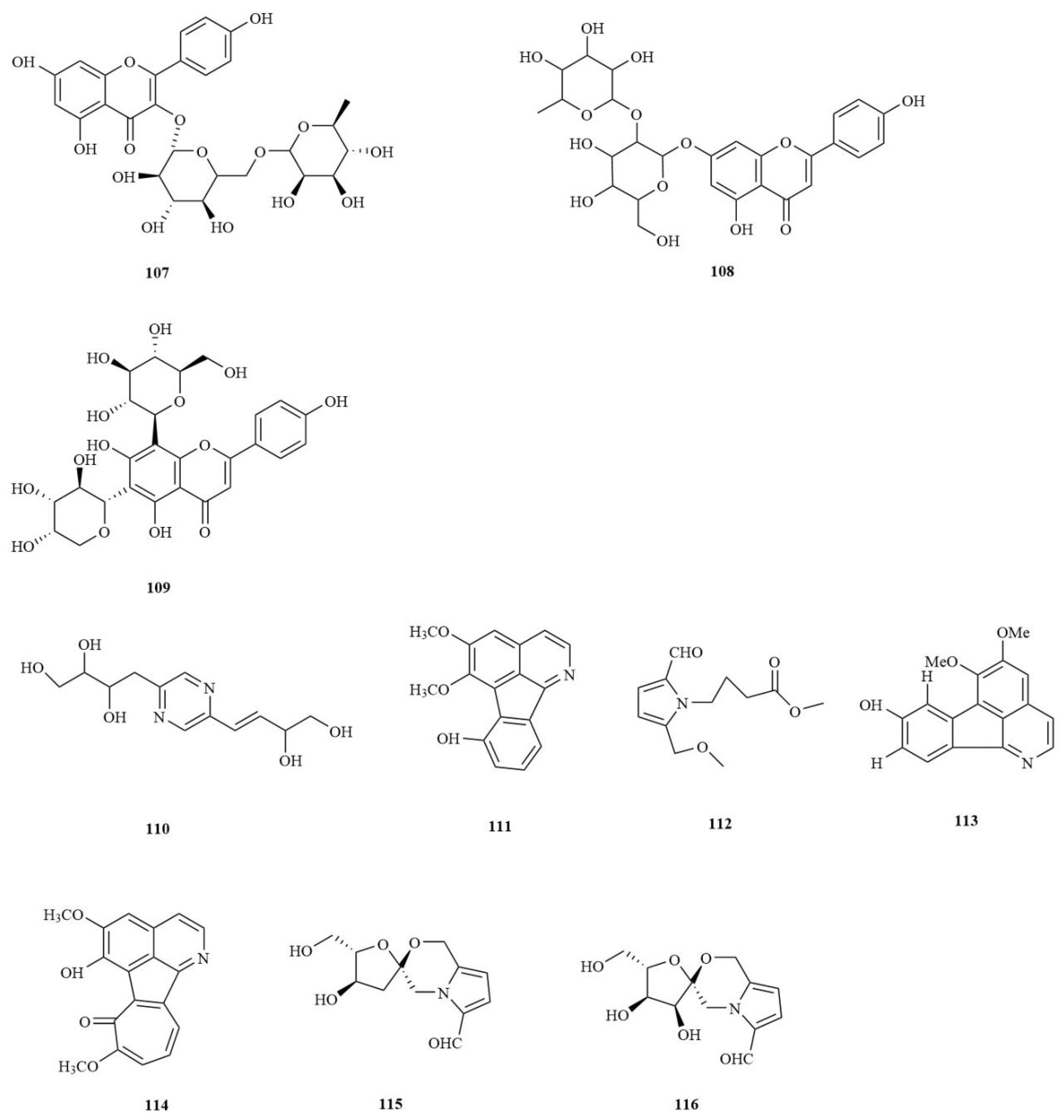
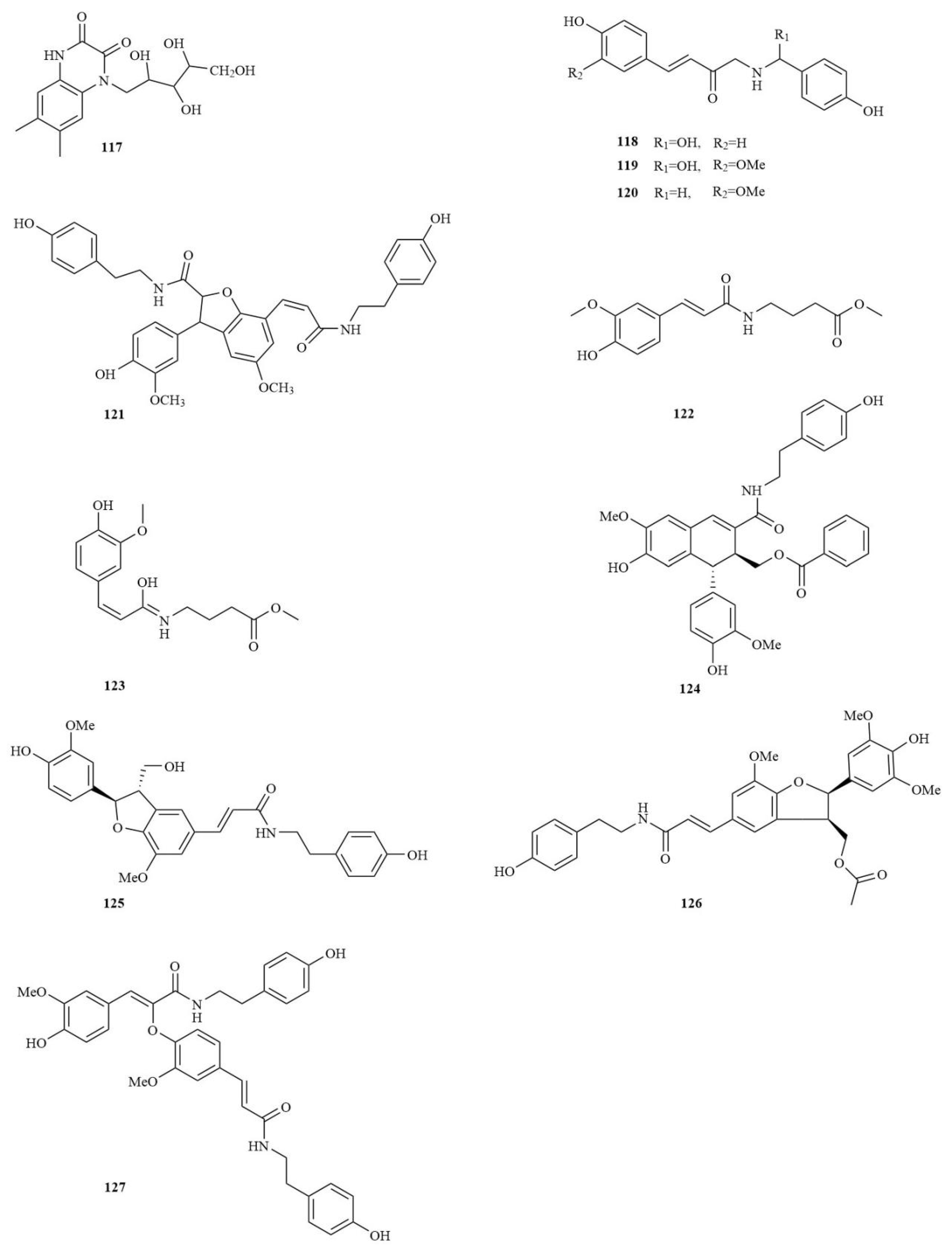
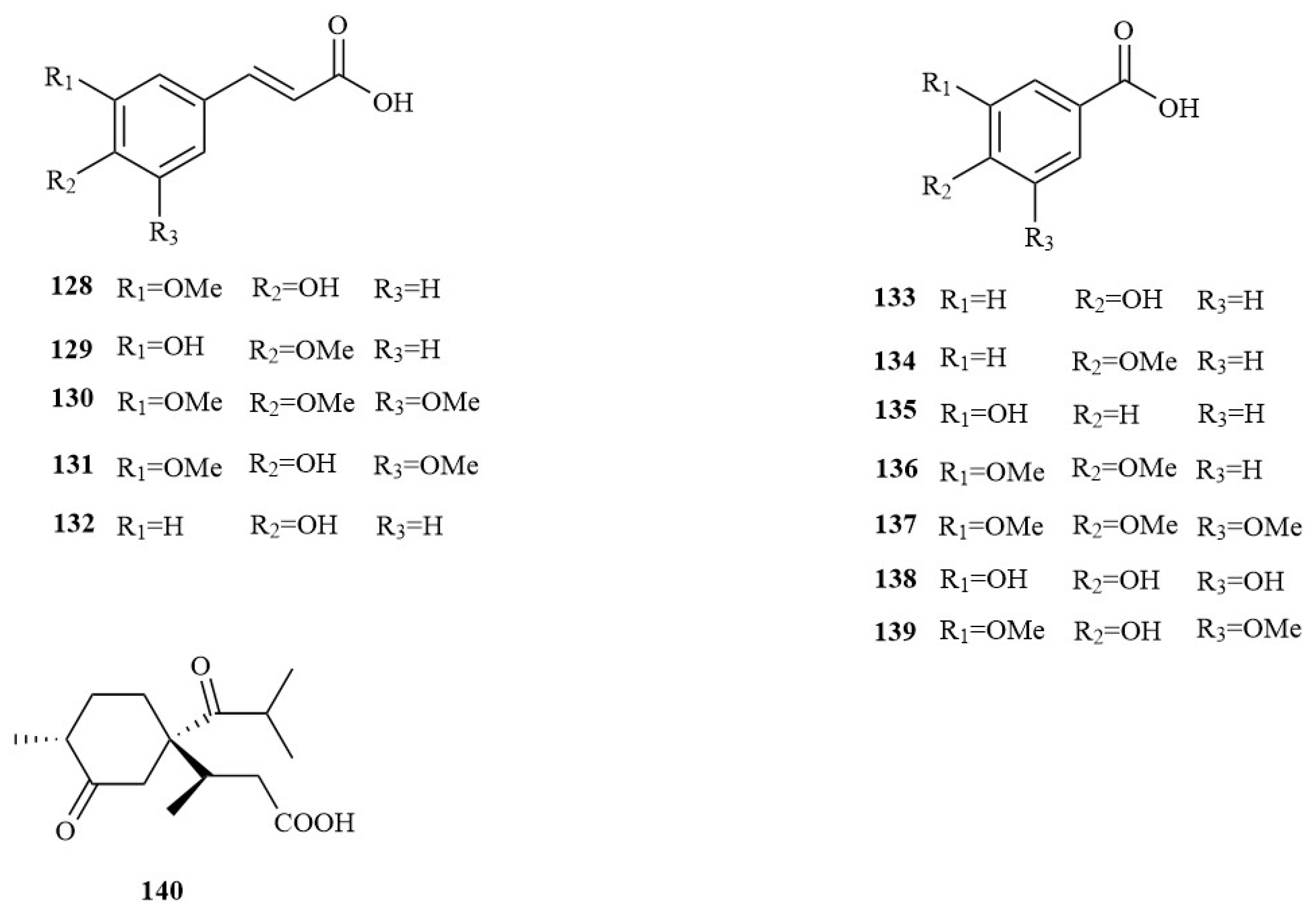

| No. | Chemical Component | Molecular Formula | Extraction Solvent | Plant Parts | Reference |
|---|---|---|---|---|---|
| Phenylpropanoids | |||||
| 1 | Acoramo | C12H16O4 | MeOH | Rhizomes | [25] |
| 2 | (Z)-Coniferyl alcohol | C10H12O3 | MeOH | Rhizomes | [25] |
| 3 | 2,4,5-trimethoxybenzoic acid | C10H12O5 | MeOH | Rhizomes | [25] |
| 4 | Tatarinoids B | C12H16O5 | MeOH | Rhizomes | [25] |
| 5 | Tatarinoids A | C12H16O5 | MeOH | Rhizomes | [25] |
| 6 | 3-(3,4,5-trimethoxyphenyl) propan-1-ol | C12H18O4 | MeOH | Rhizomes | [25] |
| 7 | Acoramone or isoacoramone | C12H16O4 | MeOH | Rhizomes | [25] |
| 8 | Asaronaldehyde | C10H12O4 | MeOH | Rhizomes | [25] |
| 9 | Isoacoramone or acoramone | C12H16O4 | MeOH | Rhizomes | [25] |
| 10 | 1-(2,4,5-trimethoxyphenyl) propan-1,2-dione | C12H14O5 | MeOH | Rhizomes | [25] |
| 11 | (E)-3-(2,4,5- trimethoxyphenyl) acrylaldehyde | C12H14O4 | MeOH | Rhizomes | [25] |
| 12 | 2,4,5-trimethoxyl-2′-butoxy-1,2-phenyl propandiol | C16H26O5 | MeOH | Rhizomes | [25] |
| 13 | α-Asarone | C12H16O3 | MeOH | Rhizomes | [25] |
| 14 | β-Asarone | C12H16O3 | MeOH | Rhizomes | [25] |
| 15 | γ-Asarone | C12H16O3 | MeOH | Rhizomes | [25] |
| 16 | 1-(4-methoxyphenyl) allyl acetate | C12H14O3 | MeOH | Rhizomes | [25] |
| 17 | Cis-methylisoeugenol | C11H14O2 | MeOH | Rhizomes | [26] |
| 18 | Elemicin | C12H16O2 | MeOH | Rhizomes | [26] |
| 19 | Benzoic acid | C8H8O4 | 95% EtOH | Rhizomes | [1] |
| 20 | (R)-4-hydroxy-3-[1-hydroxy-3-(4-hydroxy-3-methoxyphenyl)propan-2-yl]-5-methoxyben-zoic acid | C18H20O7 | Water | Rhizomes | [27] |
| 21 | (R)-1-(1,1-dimethoxypropan-2-yl)-2,4,5-trimethoxybenzene [(−)-R-isoacorphenylpropanoid] | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 22 | (S)-1-(1,1-dimethoxypropan-2-yl)-2,4,5-trimethoxybenzene [(+)-S-isoacorphenylpropanoid] | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 23 | (7R,8R)-7-methoxy-8-hydroxy-dihydroasarone (ent-acoraminol A) | C13H20O5 | 60% EtOH | Rhizomes | [28] |
| 24 | (7S,8R)-7-methoxy-8-hydroxydihydroasarone (ent-acoraminol B) | C13H20O5 | 60% EtOH | Rhizomes | [28] |
| 25 | (7R,8R)-7-ethoxy-8-hydroxydihydroasarone (ent-acoraminol C) | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 26 | (7S,8S)-7-ethoxy-8-hydroxydihydroasarone(acoraminol C) | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 27 | (7S,8R)-7-ethoxy-8-hydroxy-dihydroasarone (ent-acoraminol D) | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 28 | (7R,8S)-7-ethoxy-8-hydroxydihydroasarone (acoraminol D) | C14H22O5 | 60% EtOH | Rhizomes | [28] |
| 29 | Acoraminol A | C13H20O5 | 60% EtOH | Rhizomes | [28] |
| 30 | Acoraminol B | C13H20O5 | 60% EtOH | Rhizomes | [28] |
| 31 | (7R,8R)-7,8-dihydroxydihydroa-sarone | C12H18O5 | 60% EtOH | Rhizomes | [28] |
| 32 | (7S,8R)-7,8-dihydroxydihydroa-sarone | C12H18O5 | 60% EtOH | Rhizomes | [28] |
| 33 | 1-hydroxy-1-(2,4,5-trimethoxyphenyl)propan-2-one | C12H16O5 | 60% EtOH | Rhizomes | [28] |
| 34 | 1-(2,4,5-trimethoxyphenyl)ethanone | C11H14O4 | 60% EtOH | Rhizomes | [28] |
| 35 | Asaraldehyde | C10H12O4 | 60% EtOH | Rhizomes | [28] |
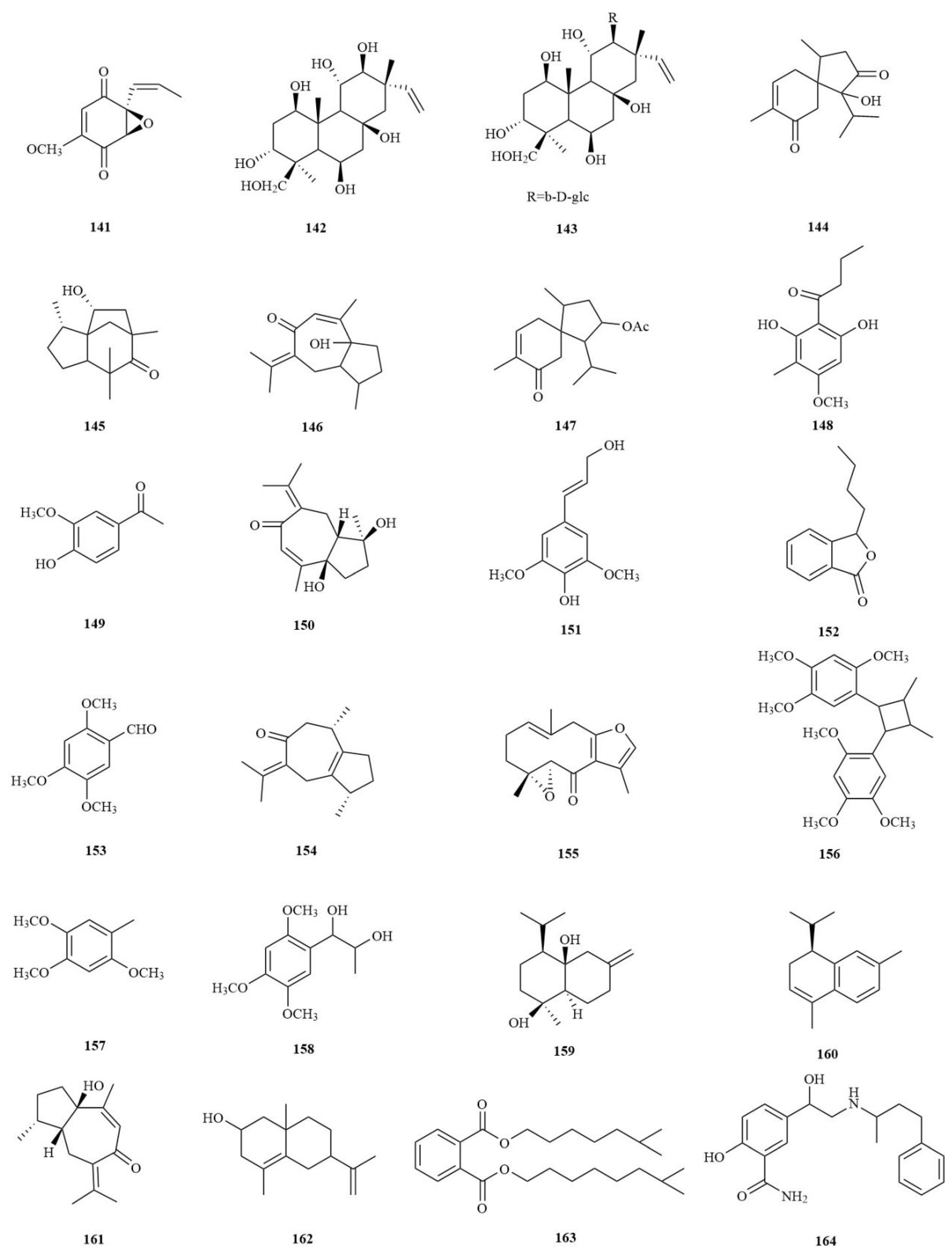
| Sesquiterpenes | |||||
| 36 | 2-oxocadinan-1(10),3-dien-5-ol | C15H22O2 | Water | Rhizomes | [29] |
| 37 | Isocalamediol | C15H26O2 | Water | Rhizomes | [29] |
| 38 | 2-hydroxyacorenone | C15H24O2 | Water | Rhizomes | [29] |
| 39 | 2-acetoxyacorenone | C17H26O3 | Water | Rhizomes | [29] |
| 40 | 4a,10a-aroma-dendranediol | C15H26O2 | Water | Rhizomes | [29] |
| 41 | 6,7,8-trihydroxy-4a-isobutyl-4,7-dimethylhexahydro-6,8a-epoxychromen-2(3H)-one | C15H24O6 | Water | Rhizomes | [27] |
| 42 | Acorusin D | C15H22O2 | 95% EtOH | Rhizomes | [30] |
| 43 | Acorusin E | C15H22O5 | 95% EtOH | Rhizomes | [30] |
| 44 | Litseachromolaevane A | C15H22O2 | 95% EtOH | Rhizomes | [30] |
| 45 | 1β,7α(H)-cadinane- 4α,6α,10α-triol | C15H28O3 | 95% EtOH | Roots | [31] |
| 46 | 1α,5β-guaiane-10α-O-ethyl- 4β,6β-diol | C17H32O3 | 95% EtOH | Rhizomes | [31] |
| 47 | 6β,7β(H)-cadinane-1α,4α, 10α-triol | C15H28O3 | 95% EtOH | Rhizomes | [31] |
| 48 | Tatarinowin A | C15H22O2 | Water | Rhizomes | [32] |
| 49 | Calamusin A | C15H22O4 | 95% EtOH | Rhizomes | [33] |
| 50 | Calamusin B | C15H22O4 | 95% EtOH | Rhizomes | [33] |
| 51 | Calamusin C | C15H20O4 | 95% EtOH | Rhizomes | [33] |
| 52 | Calamusin D | C15H24O4 | 95% EtOH | Rhizomes | [33] |
| 53 | Calamusin E | C15H22O3 | 95% EtOH | Rhizomes | [33] |
| 54 | Calamusin F | C15H22O3 | 95% EtOH | Rhizomes | [33] |
| 55 | Calamusin G | C15H22O3 | 95% EtOH | Rhizomes | [33] |
| 56 | Calamusin H | C15H22O3 | 95% EtOH | Rhizomes | [33] |
| 57 | Calamusin I | C12H18O3 | 95% EtOH | Rhizomes | [33] |
| 58 | Neo-acorane A | C15H22O4 | 95% EtOH | Rhizomes | [34] |
| Lignans | |||||
| 59 | 4,7,9,9′-tetrahydroxy-3,3′-dimethoxy-8-O-4′- neolignan | C20H26O7 | MeOH | Rhizomes | [25] |
| 60 | (7R,8R)-Virolin | C22H28O6 | MeOH | Rhizomes | [25] |
| 61 | Ligraminol D | C21H28O6 | MeOH | Rhizomes | [25] |
| 62 | Tatarinowin | C23H30O8 | MeOH | Rhizomes | [25] |
| 63 | Ligraminol C | C23H28O6 | MeOH | Rhizomes | [25] |
| 64 | Polysphorin | C23H30O6 | MeOH | Rhizomes | [25] |
| 65 | Veraguensin | C22H28O5 | MeOH | Rhizomes | [25] |
| 66 | Magnosalicin | C24H32O7 | MeOH | Rhizomes | [25] |
| 67 | Eudesmin | C22H26O6 | MeOH | Rhizomes | [25] |
| 68 | 2S-(2,6-dimethoxy-4- propenyl-phenoxy)-1 - (3,4,5-trimethoxy-phenyl)- propane-1-one | C23H28O7 | MeOH | Rhizomes | [25] |
| 69 | Diasarone I | C24H32O6 | MeOH | Rhizomes | [25] |
| 70 | 1,3-dimethoxy-2-[1-methyl-2-(3,4,5-trimethoxyphenyl)- ethoxy]-5-(1- propenyl-1-yl)-benzen | C23H30O6 | MeOH | Rhizomes | [25] |
| 71 | (2S,3R)-ceplignan | C18H20O7 | Water | Rhizomes | [27] |
| 72 | (2R,3S)-ceplignan | C18H20O7 | Water | Rhizomes | [27] |
| 73 | Acortatarinowin G | C24H32O8 | 95% EtOH | Rhizomes | [35] |
| 74 | Acortatarinowin H | C22H24O6 | 95% EtOH | Rhizomes | [35] |
| 75 | Acortatarinowin I | C23H30O6 | 95% EtOH | Rhizomes | [35] |
| 76 | Acortatarinowin J | C23H28O7 | 95% EtOH | Rhizomes | [35] |
| 77 | Acortatarinowin K | C22H26O6 | 95% EtOH | Rhizomes | [35] |
| 78 | Acortatarinowin L | C22H28O6 | 95% EtOH | Rhizomes | [35] |
| 79 | Acortatarinowin M | C22H28O9 | 95% EtOH | Rhizomes | [35] |
| 80 | Acortatarinowin N | C21H26O8 | 95% EtOH | Rhizomes | [35] |
| 81 | Tatarinoid C | C21H28O7 | 95% EtOH | Rhizomes | [35] |
| 82 | Saucernetindiol | C20H24O5 | 95% EtOH | Rhizomes | [35] |
| 83 | Machilin-I | C20H24O5 | 95% EtOH | Rhizomes | [35] |
| 84 | Verrucosn | C20H24O5 | 95% EtOH | Rhizomes | [35] |
| 85 | (±)-Acortatarinowin A | C20H24O7 | 95% MeOH | Rhizomes | [36] |
| 86 | (±)-Acortatarinowin B | C20H24O7 | 95% MeOH | Rhizomes | [36] |
| 87 | (±)-Acortatarinowin C | C21H26O8 | 95% MeOH | Rhizomes | [36] |
| 88 | (±)-Acortatarinowin D | C20H24O7 | 95% MeOH | Rhizomes | [36] |
| 89 | (±)-Acortatarinowin E | C22H28O7 | 95% MeOH | Rhizomes | [36] |
| 90 | (±)-Acortatarinowin F | C23H28O7 | 95% MeOH | Rhizomes | [36] |
| 91 | Tatarinan O | C36H48O9 | 95% EtOH | Roots | [37] |
| 92 | Tatanan A | C36H48O9 | 95% EtOH | Rhizomes | [38] |
| 93 | Tatanan B | C35H46O9 | 95% EtOH | Rhizomes | [38] |
| 94 | Tatanan C | C35H46O9 | 95% EtOH | Rhizomes | [38] |
| 95 | Tatarinoid D | C22H26O6 | 60% EtOH | Rhizomes | [39] |
| 96 | Tatarinoid E | C23H28O7 | 60% EtOH | Rhizomes | [39] |
| 97 | Tatarinoid F | C21H28O6 | 60% EtOH | Rhizomes | [39] |
| 98 | Tatarinoid G | C22H28O5 | 60% EtOH | Rhizomes | [39] |
| 99 | Tatarinoid H | C19H22O7 | 60% EtOH | Rhizomes | [39] |
| 100 | [S,R-(E)]-3,4,5-trimethoxy-[1-[2-methoxy-4-(1-propenyl)phenoxy]ethyl]-benzenemethanol | C22H28O6 | 60% EtOH | Rhizomes | [39] |
| 101 | Nectandrin A | C21H26O5 | 60% EtOH | Rhizomes | [39] |
| 102 | (2R,3R)-2-(3,4-dimethoxyphenyl)-2,3-dihydro-7-methoxy-3-methyl-5-(1E)-1-propen-1-yl-benzofuran | C21H24O4 | 60% EtOH | Rhizomes | [39] |
| 103 | Tatarinan T | C48H64O12 | 95% EtOH | Roots | [40] |
| 104 | 3-(3,4-dimethoxyphenyl)propan-1-ol | C11H16O3 | 60% EtOH | Rhizomes | [28] |
| 105 | (±)-Magnosalicin | C24H32O7 | 60% EtOH | Rhizomes | [28] |
| 106 | (±)-Pinoresinol | C20H22O6 | 60% EtOH | Rhizomes | [28] |
| Flavonoids | |||||
| 107 | Kaempferol-3-O- rutinoside | C27H30O15 | MeOH | Rhizomes | [25] |
| 108 | Rhoifolin | C27H30O14 | MeOH | Rhizomes | [25] |
| 109 | Isoschaftoside | C26H28O14 | MeOH | Rhizomes | [25] |
| Alkaloids | |||||
| 110 | 2-(3′,4′-dihydroxy-1′ -butylenyl)-5-(2″,3″,4″-trihydroxybutyl)-pyrazine | C12H18N2O5 | Water | Rhizomes | [29] |
| 111 | Tatarine A | C17H13NO3 | Water | Rhizomes | [29] |
| 112 | 4-(2-formyl-5-methoxymethyl pyrrol- 1-yl)butyric acid methyl ester | C12H17NO4 | Water | Rhizomes | [29] |
| 113 | Tatarine D | C17H13NO3 | 60% EtOH | Rhizomes | [41] |
| 114 | Neotatarine | C18H13NO4 | 95% EtOH | Rhizomes | [42] |
| 115 | Acortatarin A | C12H15NO5 | Water | Rhizomes | [43] |
| 116 | Acortatarin B | C12H15NO6 | Water | Rhizomes | [43] |
| Amides | |||||
| 117 | Tatarine C | C15H20N2O6 | MeOH | Rhizomes | [25] |
| 118 | Tataramide A | C17H17NO4 | MeOH | Rhizomes | [25] |
| 119 | (S)-N-trans-feruloyloctopamine | C18H19NO5 | MeOH | Rhizomes | [25] |
| 120 | N-trans-Feruloyl-tyramine | C18H19NO4 | MeOH | Rhizomes | [25] |
| 121 | Tataramide B | C36H36N2O8 | MeOH | Rhizomes | [25] |
| 122 | (E)-methyl 4-[3-(4-hydroxy-3-methoxyphenyl) acrylamido]butanoate | C15H19NO5 | Water | Rhizomes | [27] |
| 123 | (Z)-methyl-4-[3-(4-hydroxy-3-methoxyphenyl)acrylamido]butanoate enol isomer | C15H21NO5 | Water | Rhizomes | [27] |
| 124 | Acorusin A | C35H33NO8 | 95% EtOH | Rhizomes | [30] |
| 125 | Grossamide K | C28H29NO7 | 95% EtOH | Rhizomes | [30] |
| 126 | Tatarine E | C31H33NO9 | 60% EtOH | Rhizomes | [41] |
| 127 | Cannabisin F | C36H36N2O8 | 60% EtOH | Rhizomes | [41] |
| Organic acids | |||||
| 128 | Ferulic acid | C10H10O4 | Water | Roots and Rhizomes | [9] |
| 129 | Trans-Isoferulic acid | C10H10O4 | Water | Roots and Rhizomes | [9] |
| 130 | 3,4,5-trimethoxycinnamic acid | C12H14O5 | Water | Roots and Rhizomes | [9] |
| 131 | 3,5-dimethoxy-4-hydroxycinnamic acid | C11H12O5 | Water | Roots and Rhizomes | [9] |
| 132 | Trans-4-hydroxycinnamic acid | C9H8O3 | Water | Roots and Rhizomes | [9] |
| 133 | 4-hydroxybenzoic acid | C7H6O3 | Water | Roots and Rhizomes | [9] |
| 134 | Anisic acid | C8H8O3 | Water | Roots and Rhizomes | [9] |
| 135 | 3-hydroxybenzoic acid | C7H6O3 | Water | Roots and Rhizomes | [9] |
| 136 | Veratric acid | C9H10O4 | Water | Roots and Rhizomes | [9] |
| 137 | 3,4,5-trimethoxybenzoic acid | C10H12O5 | Water | Roots and Rhizomes | [9] |
| 138 | Gallic acid | C7H6O5 | Water | Roots and Rhizomes | [9] |
| 139 | Syringic acid | C9H10O5 | Water | Roots and Rhizomes | [9] |
| 140 | Acoric acid | C15H24O4 | 95% EtOH | Rhizomes | [34] |
| Other types | |||||
| 141 | 1-cis-propenyl-1S,6R- epoxy-4-methoxy- 2,5-quinone | C10H10O4 | MeOH | Rhizomes | [25] |
| 142 | Tatarol | C20H34O7 | MeOH | Rhizomes | [25] |
| 143 | Tataroside | C26H44O12 | MeOH | Rhizomes | [25] |
| 144 | Isocalamediol | C15H22O3 | MeOH | Rhizomes | [25] |
| 145 | Calamensesquiterpinenol | C15H24O2 | MeOH | Rhizomes | [25] |
| 146 | 2,3,3a,7,8,8a-hexahydro-3a-hydroxy-1,4-dimethyl- 7-(1-methylethylidene)- 6(1H)-azulenone | C15H22O2 | MeOH | Rhizomes | [25] |
| 147 | 2-acetoxyacorenone | C17H26O3 | MeOH | Rhizomes | [25] |
| 148 | Aspidinol | C12H16O4 | 95% EtOH | Rhizomes | [1] |
| 149 | Apocynin | C9H10O3 | 95% EtOH | Rhizomes | [1] |
| 150 | Aeru-gidiol | C15H22O3 | 95% EtOH | Rhizomes | [1] |
| 151 | Ethanone | C11H14O4 | 95% EtOH | Rhizomes | [1] |
| 152 | 3-butyl-phthalide | C12H14O2 | 95% EtOH | Rhizomes | [1] |
| 153 | Asaraldehyde | C10H12O4 | 95% EtOH | Rhizomes | [1] |
| 154 | Cala-musenone | C15H22O | 95% EtOH | Rhizomes | [1] |
| 155 | Zederone | C15H18O3 | 95% EtOH | Rhizomes | [1] |
| 156 | Bisasaricin | C24H32O6 | 95% EtOH | Rhizomes | [1] |
| 157 | 3,4,5-trimethoxytoluene | C10H14O3 | 95% EtOH | Rhizomes | [1] |
| 158 | 1-(2,4,5-trimethoxyphenyl)-1,2-propanediol | C12H18O5 | 95% EtOH | Rhizomes | [1] |
| 159 | Calamendiol | C15H26O2 | 95% EtOH | Rhizomes | [1] |
| 160 | α-calacorene | C15H20 | 95% EtOH | Rhizomes | [1] |
| 161 | Acotatarone C | C15H22O2 | 95% EtOH | Rhizomes | [1] |
| 162 | Cyperol | C15H24O | 95% EtOH | Rhizomes | [1] |
| 163 | Diisocapryl phthalate | C24H38O4 | 95% EtOH | Rhizomes | [1] |
| 164 | Labetalol | C19H24N2O3 | 95% EtOH | Rhizomes | [1] |
| Pharmacological Activities | Study Design | Models | Results/Mechanisms | Dosages | Reference |
|---|---|---|---|---|---|
| Antidepressant | In vivo | Male C57/BL6 mice | ↑SERT activity ↓SERT activity | 1.56 μg/mL 50–100 μg/mL | [9] |
| In vivo | Male ICR mice | Significantly reduced immobility time | 5, 10, and 20 mg/kg | [46] | |
| In vivo | CUMS rats | Significantly reduced immobility time, reduced the level of sucrose preference, and increased the CREB and BDNF mRNA levels | 25 mg/kg | [47] | |
| Antiepileptic | In vivo | Kunming mice and male SD rats | ↓GABA-IR neuron damage | 100 mg/kg | [48] |
| Anticonvulsant | In vivo | Male ICR mice or SD rats | Up-regulation of GABAA and GAD65 expressions and anti-apoptosis of neurons in the brain | 5, 10, and 20 mg/kg | [49] |
| In vivo | The pain models in mice | Regulate GABA activity | 100 and 200 mg/kg | [50] | |
| Antianxiety | In vivo | Mice (chronic inflammatory mouse model) | Blocked CFA-induced anxiety-like behavior, regulating the balance between GABAergic and glutamatergic transmission in the basolateral (BLA) | 20 mg/kg | [51] |
| Neuroprotective | In vitro | SD rats in cultured astrocytes | The tBHP-induced cell mortality in cultured astrocytes was markedly reduced | 0.5–15 μg/mL | [52] |
| In vivo | Both SD developmental rat pups and adult rats | Pb-induced reduction of spine density in hippocampal CA1 and effectively up-regulated the protein expression of NR2B, Arc, and Wnt7a, as well as the mRNA levels of Arc/Arg3.1 and Wnt7a | (10, 40 mg/kg) and (2.5, 10, 40 mg/kg) | [12] | |
| Protective effects against Alzheimer’s disease | In vitro | PC12 cell | Inhibits Amyloid-β | 12, 24, 36, 72, and 144 μM | [53] |
| In vivo | Male Wistar rats | Significantly increased the levels of antioxidant enzymes, including SOD and GPX | 12.5, 25, and 50 mg/kg | [54] | |
| Antifatigue | In vivo | Adult male SD rats | Suppress the exercise-induced increase in 5-HT synthesis, TPH2 mRNA, and protein expression and prevent the exercise-induced decrease in 5-HT1B mRNA and protein expression in the dorsal raphe | 100 mg/kg | [55] |
| Antifungal | In vivo and in vitro | Six-week-old female Kunming mice and C. albicans strain | Inhibiting the activity of C. albicans and inhibit biofilm formation by regulating the C. albicans protein kinase C pathway. | 8 mg/kg | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Tang, H.-P.; Wang, S.; Hu, W.-J.; Li, J.-Y.; Yu, A.-Q.; Bai, Q.-X.; Yang, B.-Y.; Kuang, H.-X. Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules 2023, 28, 4525. https://doi.org/10.3390/molecules28114525
Wang M, Tang H-P, Wang S, Hu W-J, Li J-Y, Yu A-Q, Bai Q-X, Yang B-Y, Kuang H-X. Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules. 2023; 28(11):4525. https://doi.org/10.3390/molecules28114525
Chicago/Turabian StyleWang, Meng, Hai-Peng Tang, Shuang Wang, Wen-Jing Hu, Jia-Yan Li, Ai-Qi Yu, Qian-Xiang Bai, Bing-You Yang, and Hai-Xue Kuang. 2023. "Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology" Molecules 28, no. 11: 4525. https://doi.org/10.3390/molecules28114525
APA StyleWang, M., Tang, H.-P., Wang, S., Hu, W.-J., Li, J.-Y., Yu, A.-Q., Bai, Q.-X., Yang, B.-Y., & Kuang, H.-X. (2023). Acorus tatarinowii Schott: A Review of Its Botany, Traditional Uses, Phytochemistry, and Pharmacology. Molecules, 28(11), 4525. https://doi.org/10.3390/molecules28114525





