Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues
Abstract
1. Introduction
2. Results and Discussion
2.1. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.2. 2,2′-Azino-bis(3-ethylbenzothiazoline)-6-sulfonic Acid (ABTS) Assay
2.3. Peroxidase Activity
2.3.1. Horseradish Peroxidase (HRP) Test
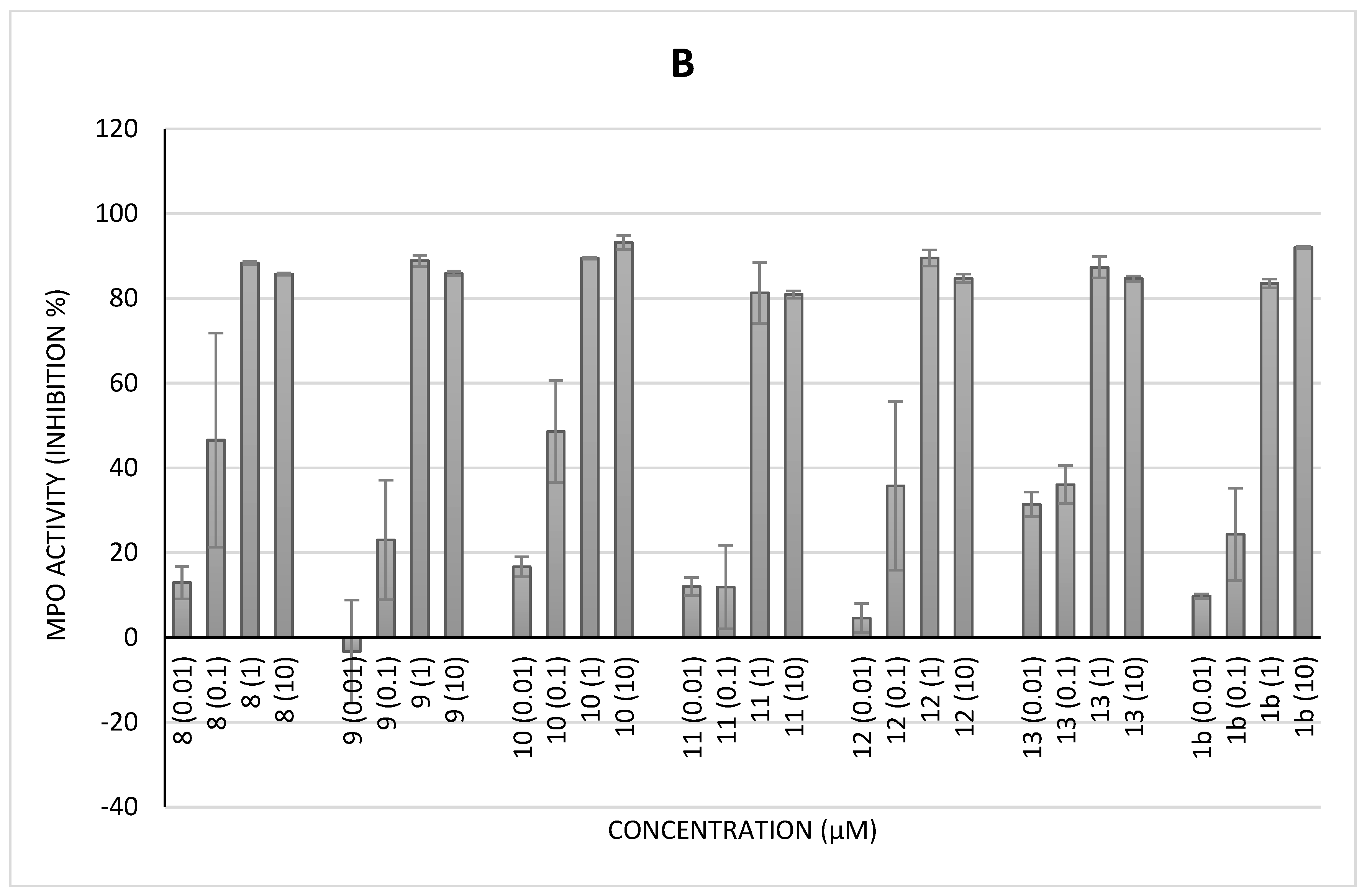
2.3.2. Myeloperoxidase (MPO) Activity (Classical Assay)
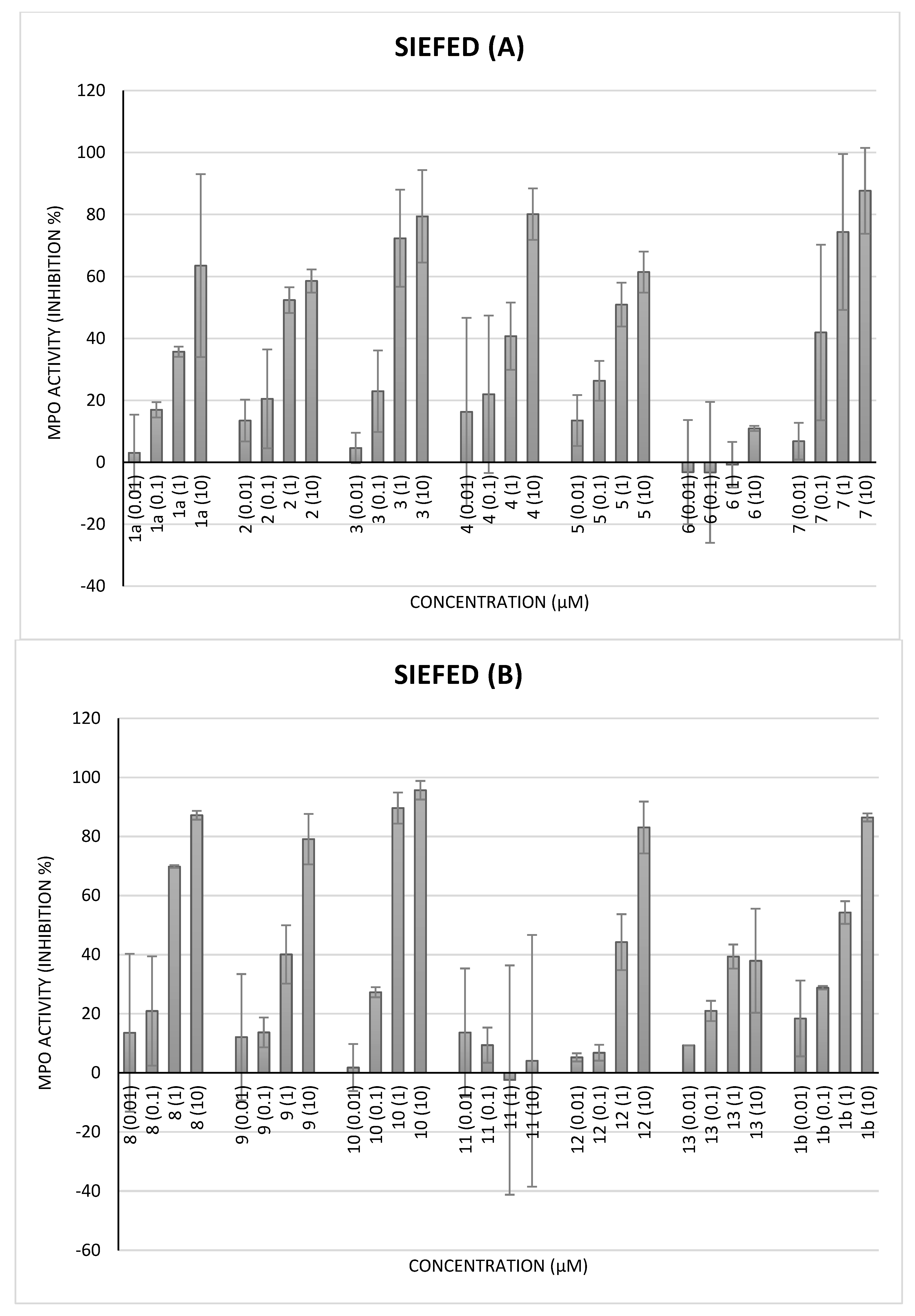
2.3.3. MPO Activity by Specific Immunological Extraction Followed by Enzymatic Detection (Siefed Assay)
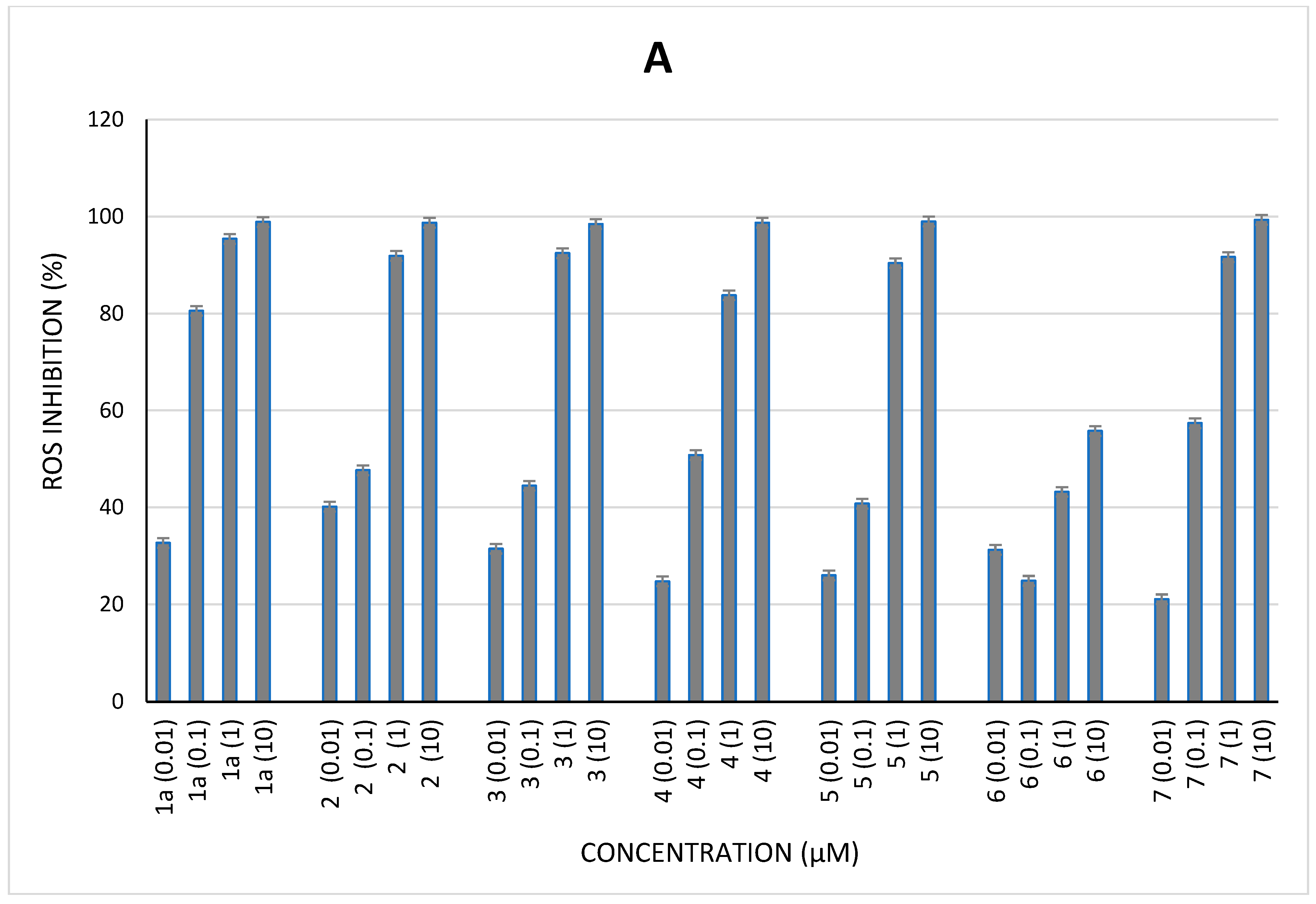
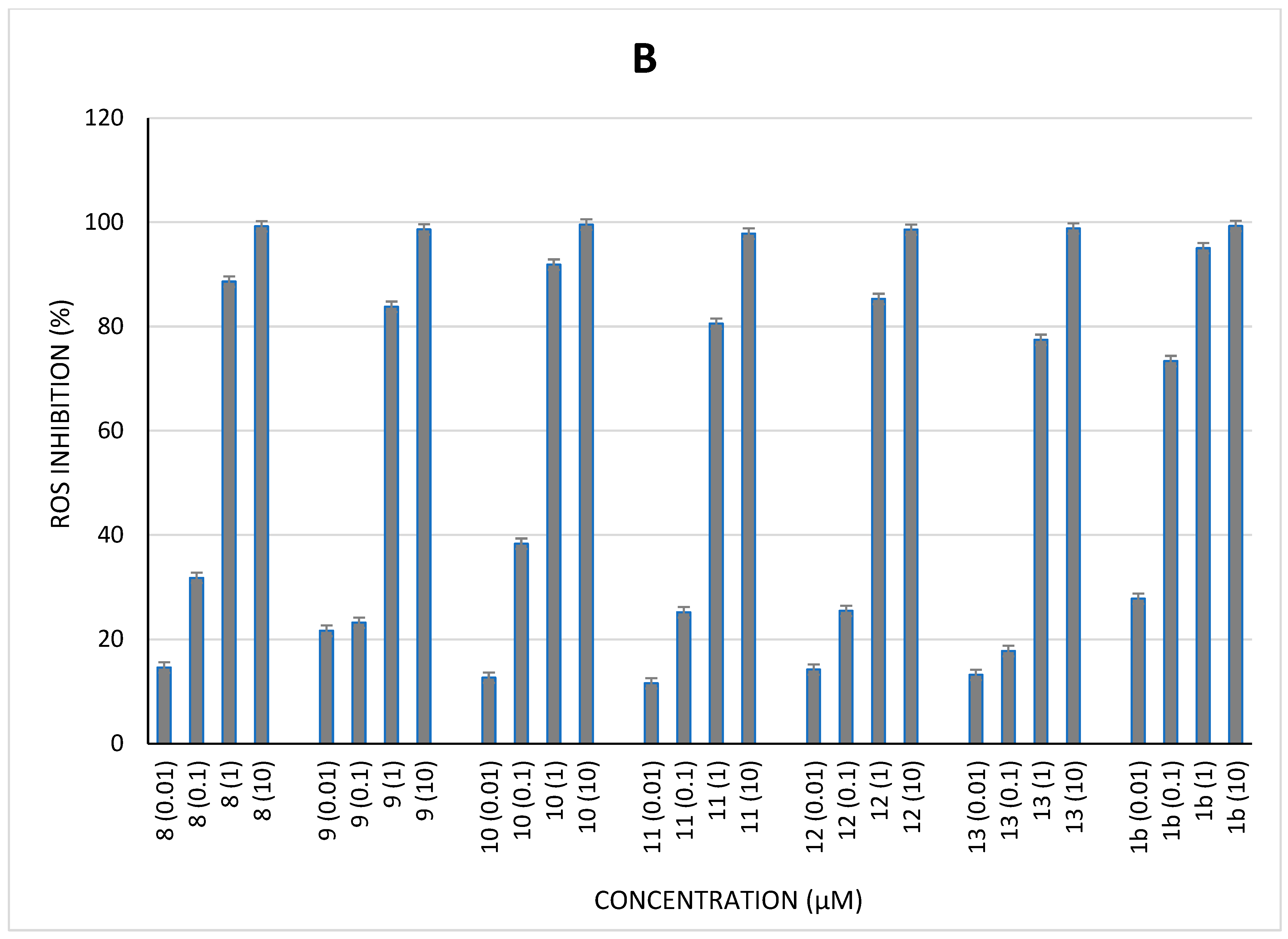
2.4. Cellular Antioxidant Activity
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. In Vitro Radical Assays
4.2.1. ABTS Test
4.2.2. DPPH Test
4.3. Peroxidase Activity
4.3.1. HRP Assay
4.3.2. MPO Assay
- Measurement of myeloperoxidase activity
- Classical assay of MPO activity
- SIEFED assay of MPO activity
4.4. Cellular Assays
Neutrophil ROS Production by Chemiluminescence Investigation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Derosa, G.; Maffioli, P.; Sahebkar, A. Ellagic Acid and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 473–479. [Google Scholar] [CrossRef]
- Muganga, R.; Angenot, L.; Tits, M.; Frédérich, M. In vitro and in vivo antiplasmodial activity of three Rwandan medicinal plants and identification of their active compounds. Planta Med. 2014, 80, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Soh, P.N.; Witkowski, B.; Olagnier, D.; Nicolau, M.L.; Garcia-Alvarez, M.C.; Berry, A.; Benoit-Vical, F. In vitro and in vivo properties of ellagic acid in malaria treatment. Antimicrob. Agents Chemother. 2009, 53, 1100–1106. [Google Scholar] [CrossRef]
- Muganga, R.; Angenot, L.; Tits, M.; Frédérich, M. Antiplasmodial and cytotoxic activities of Rwandan medicinal plants used in the treatment of malaria. J. Ethnopharmacol. 2010, 128, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J. The Potential of Ellagic Acid as a Possible Antimalarial Drug Candidate. Curr. Bioact. Compd. 2010, 6, 161–177. [Google Scholar] [CrossRef]
- Shakeri, A.; Zirak, M.R.; Sahebkar, A. Ellagic Acid: A Logical Lead for Drug Development? Curr. Pharm. Des. 2018, 24, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Gupta, A.C.; Maurya, A.K.; Shanker, K.; Pal, A.; Bawankule, D.U. Ameliorative Effects of Dietary Ellagic Acid Against Severe Malaria Pathogenesis by Reducing Cytokine Storms and Oxidative Stress. Front. Pharmacol. 2021, 12, 777400. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Jereb, M.; Muzlovic, I.; Prabhu, R.M. Clinical review: Severe malaria. Crit. Care 2003, 7, 315–323. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Malaria 2022. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria (accessed on 1 April 2022).
- World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006489-8.
- Imboumy-Limoukou, R.K.; Maghendji-Nzondo, S.; Sir-Ondo-Enguier, P.N.; De Carvalho, J.N.; Tsafack-Tegomo, N.P.; Buekens, J.; Okouga, A.P.; Mouinga-Ondeme, A.; Nolna, S.K.; Lekana-Douki, J.-B. Malaria in children and women of childbearing age: Infection prevalence, knowledge and use of malaria prevention tools in the province of Nyanga, Gabon. Malar. J. 2020, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.S. Current status of malaria and potential for control. Clin. Microbiol. Rev. 2001, 14, 208–226. [Google Scholar] [CrossRef]
- Parhizgar, A.R.; Tahghighi, A. Introducing New Antimalarial Analogues of Chloroquine and Amodiaquine: A Narrative Review. Iran. J. Med. Sci. 2017, 42, 115–128. [Google Scholar] [PubMed]
- Kehr, S.; Sturm, N.; Rahlfs, S.; Przyborski, J.M.; Becker, K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010, 6, e1001242. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383–389. [Google Scholar] [CrossRef]
- Nauseef, W.M. The phagocyte NOX2 NADPH oxidase in microbial killing and cell signaling. Curr. Opin. Immunol. 2019, 60, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Knackstedt, S.L.; Georgiadou, A.; Apel, F.; Abu-Abed, U.; Moxon, C.A.; Cunnington, A.J.; Raupach, B.; Cunningham, D.; Langhorne, J.; Krüger, R.; et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci. Immunol. 2019, 4, eaaw0336. [Google Scholar] [CrossRef] [PubMed]
- Kho, S.; Minigo, G.; Andries, B.; Leonardo, L.; Prayoga, P.; Poespoprodjo, J.R.; Kenangalem, E.; Price, R.N.; Woodberry, T.; Anstey, N.M.; et al. Circulating Neutrophil Extracellular Traps and Neutrophil Activation Are Increased in Proportion to Disease Severity in Human Malaria. J. Infect. Dis. 2019, 219, 1994–2004, Erratum in J. Infect. Dis. 2019, 219, 2026. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.P.; Galloway, L.J.; Riley, E.M. Malaria, anemia, and invasive bacterial disease: A neutrophil problem? J. Leukoc. Biol. 2019, 105, 645–655. [Google Scholar] [CrossRef]
- Sarr, D.; Oliveira, L.J.; Russ, B.N.; Owino, S.O.; Middii, J.D.; Mwalimu, S.; Ambasa, L.; Almutairi, F.; Vulule, J.; Rada, B.; et al. Myeloperoxidase and Other Markers of Neutrophil Activation Associate with Malaria and Malaria/HIV Coinfection in the Human Placenta. Front. Immunol. 2021, 12, 682668. [Google Scholar] [CrossRef]
- Muchtar, N.H.; Nik Mat Zin, N.N.I.; Mohamad, F.S.; Abu-Bakar, N. Ellagic Acid Induces in vitro Alkalinisation of the Digestive Vacuole in Drug-Sensitive Plasmodium falciparum Strain. Malays. J. Med. Sci. 2022, 29, 43–52. [Google Scholar] [CrossRef]
- Degotte, G.; Pendeville, H.; Di Chio, C.; Ettari, R.; Pirotte, B.; Frédérich, M.; Francotte, P. Dimeric Polyphenols to Pave the Way for New Antimalarial Drugs. RSC Med. Chem. 2023, 14, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Tryniszewski, W.; Sarniak, A.; Wlodarczyk, A.; Nowak, P.J.; Nowak, D. Concentration Dependence of Anti- and Pro-Oxidant Activity of Polyphenols as Evaluated with a Light-Emitting Fe2+-Egta-H2O2 System. Molecules 2022, 27, 3453. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.Q.; Cunha, N.; Varela, E.L.P.; Brígido, H.P.C.; Vale, V.V.; Dolabela, M.F.; De Carvalho, E.P.; Percário, S. Oxidative Stress in Malaria: Potential Benefits of Antioxidant Therapy. Int. J. Mol. Sci. 2022, 23, 5949. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Grulke, S.; Deby-Dupont, G.; Deby, C.; Duvivier, H.; Peters, F.; Serteyn, D. Development of an enzyme-linked immunosorbent assay for specific equine neutrophil myeloperoxidase measurement in blood. J. Vet. Diagn. Investig. 2005, 17, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Basu, S.; Parija, L.; Rout, D.; Manna, S.; Dandapat, J.; Debata, P.R. Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells. Biomed Pharmacother. 2016, 81, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, A.D.G.; Gontijo, A.V.L.; Lima, G.M.G.; de Oliveira, M.A.C.; Lepesqueur, L.S.S.; Koga-Ito, C.Y. Ellagic Acid-Cyclodextrin Complexes for the Treatment of Oral Candidiasis. Molecules 2021, 26, 505. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ghosh, S.; Kumar, P.; Basu, B.; Nagpal, K. Ellagic acid-loaded, tween 80-coated, chitosan nanoparticles as a promising therapeutic approach against breast cancer: In-vitro and in-vivo study. Life Sci. 2021, 284, 119927. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Etsè, K.S.; Etsè, K.D.; Nyssen, P.; Mouithys-Mickalad, A. Assessment of anti-inflammatory-like, antioxidant activities and molecular docking of three alkynyl-substituted 3-ylidene-dihydrobenzo[d]isothiazole 1,1-dioxide derivatives. Chem. Biol. Interact. 2021, 344, 109513. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G.; et al. Flavonoid composition, cellular antioxidant activity and (myelo)peroxidase inhibition of a Bryonia alba L. (Cucurbitaceae) leaves extract. J. Pharm. Pharmacol. 2019, 71, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, P.; Mouithys-Mickalad, A.; Minguet, G.; Sauvage, E.; Wouters, J.; Franck, T.; Hoebeke, M. Morphine, a potential inhibitor of myeloperoxidase activity. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2236–2244. [Google Scholar] [CrossRef]
- Benbarek, H.; Deby-Dupont, G.; Deby, C.; Caudron, I.; Mathy-Hartert, M.; Lamy, M.; Serteyn, D. Experimental model for the study by chemiluminescence of the activation of isolated equine leucocytes. Res. Vet. Sci. 1996, 61, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Derochette, S.; Franck, T.; Mouithys-Mickalad, A.; Deby-Dupont, G.; Neven, P.; Serteyn, D. Intra- and extracellular antioxidant capacities of the new water-soluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem. Biol. Interact. 2013, 201, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mouithys-Mickalad, A.; Storms, N.; Franck, T.; Ceusters, J.; de la Rebière de Pouyade, G.; Deby-Dupont, G.; Serteyn, D. Effects of Juglone on Neutrophil Degranulation and Myeloperoxidase Activity Related to Equine Laminitis. Front. Vet. Sci. 2021, 8, 677675. [Google Scholar] [CrossRef] [PubMed]
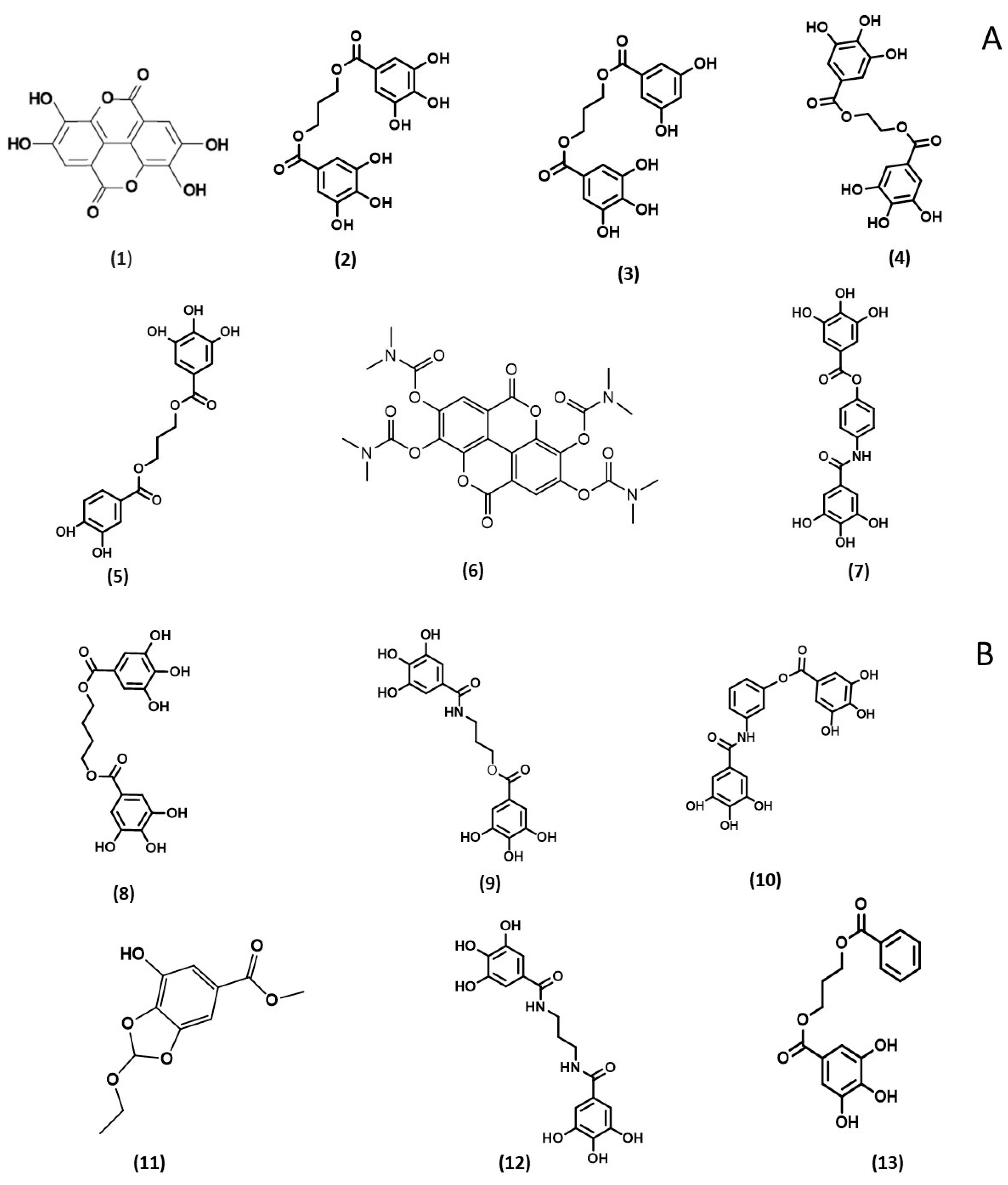

| Compound | DPPH IC50 (µM) | ABTS IC50 (µM) | HRP IC50 (µM) |
|---|---|---|---|
| 1a | 5.64 ± 0.47 | 6.01 ± 0.26 | ND |
| 2 | 2.86 ± 0.13 | 5.50 ±0.16 | 2.28 ± 0.11 |
| 3 | 5.78 ± 0.13 | 5.52 ± 0.34 | 3.19 ± 0.07 |
| 4 | 3.36 ± 0.25 | 5.77 ± 0.01 | 2.84 ± 0.00 |
| 5 | 4.24 ± 0.36 | 5.48 ± 0.23 | 3.73 ± 0.21 |
| 6 | * ND | * ND | * ND |
| 7 | 3.14 ± 0.72 | 4.68 ± 0.13 | 5.81 ± 0.49 |
| 8 | 5.96 ± 0.06 | 6.97 ± 0.04 | 1.48 ± 1.17 |
| 9 | 5.82 ± 0.35 | 6.34 ± 0.39 | 1.81 ± 0.18 |
| 10 | 5.27 ± 0.29 | 6.22 ± 0.16 | 1.02 ± 0.09 |
| 11 | 5.22 ± 0.05 | 6.56 ± 0.37 | 3.55 ± 0.37 |
| 12 | 3.70 ± 0.45 | 5.86 ± 0.18 | 3.06 ± 0.07 |
| 13 | 4.91 ± 0.26 | 6.00 ± 0.31 | 5.46 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degotte, G.; Frederich, M.; Francotte, P.; Franck, T.; Colson, T.; Serteyn, D.; Mouithys-Mickalad, A. Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules 2023, 28, 4516. https://doi.org/10.3390/molecules28114516
Degotte G, Frederich M, Francotte P, Franck T, Colson T, Serteyn D, Mouithys-Mickalad A. Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules. 2023; 28(11):4516. https://doi.org/10.3390/molecules28114516
Chicago/Turabian StyleDegotte, Gilles, Michel Frederich, Pierre Francotte, Thierry Franck, Thomas Colson, Didier Serteyn, and Ange Mouithys-Mickalad. 2023. "Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues" Molecules 28, no. 11: 4516. https://doi.org/10.3390/molecules28114516
APA StyleDegotte, G., Frederich, M., Francotte, P., Franck, T., Colson, T., Serteyn, D., & Mouithys-Mickalad, A. (2023). Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules, 28(11), 4516. https://doi.org/10.3390/molecules28114516







