Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters
Abstract
1. Introduction
2. Results and Discussion
2.1. Elemental Composition of the Starting Pistachio Nut Shells as well as Activated Biocarbons Prepared via Direct Physical and Chemical Activation
2.2. Acidic–Basic Properties of the Precursor and the Activated Biocarbons Prepared
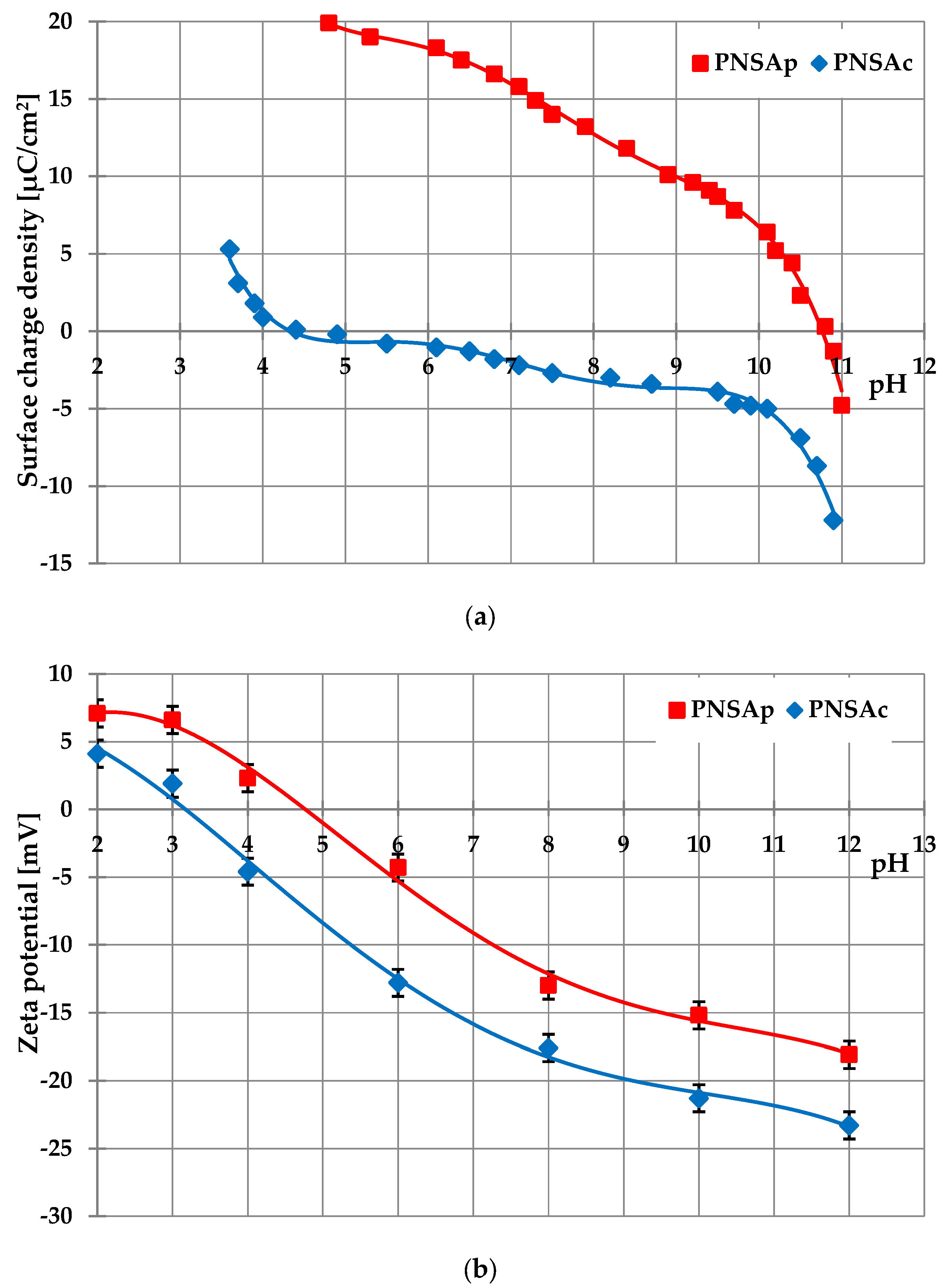
2.3. Surface and Electrokinetic Properties of the Activated Biocarbons Prepared
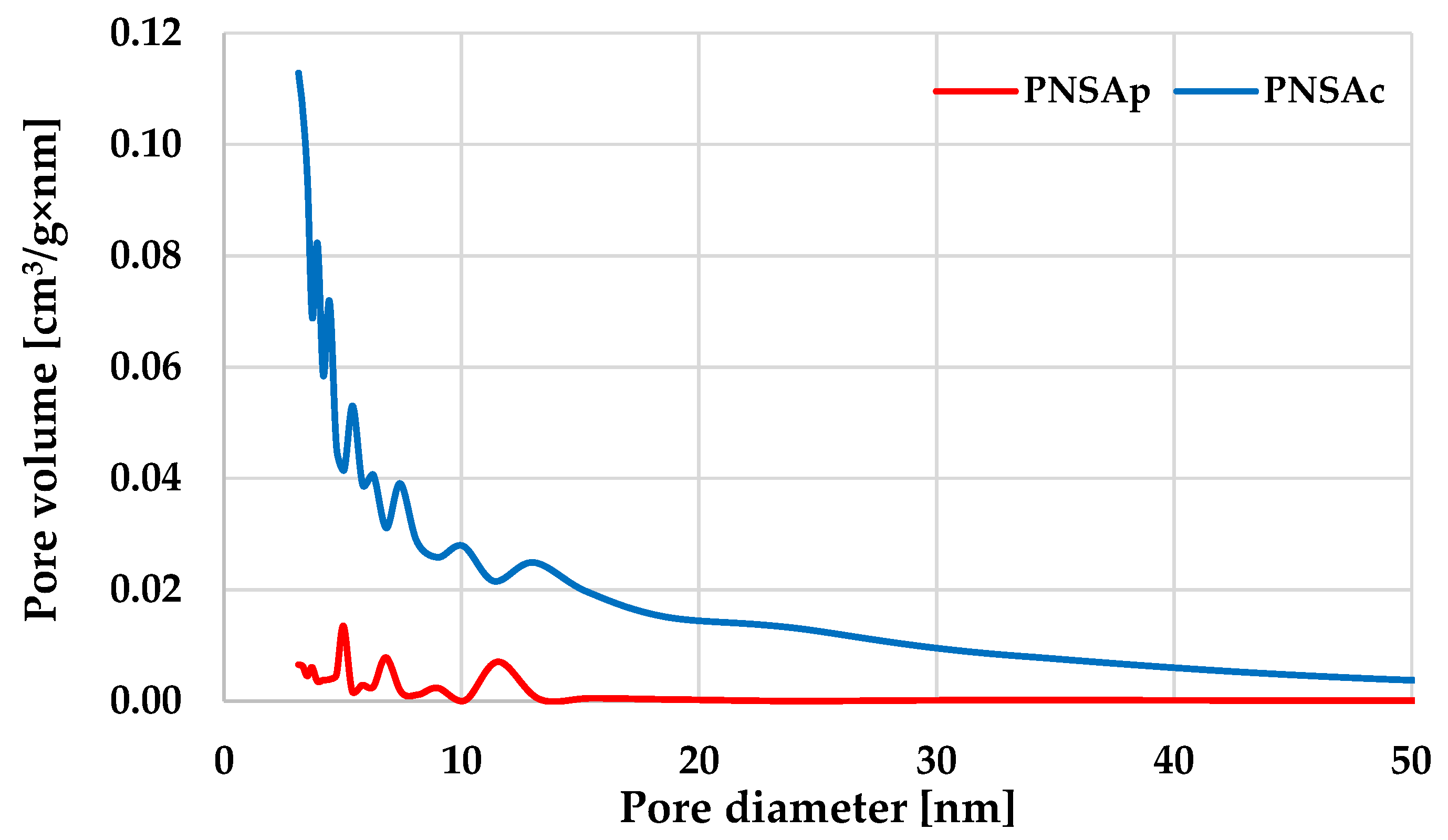
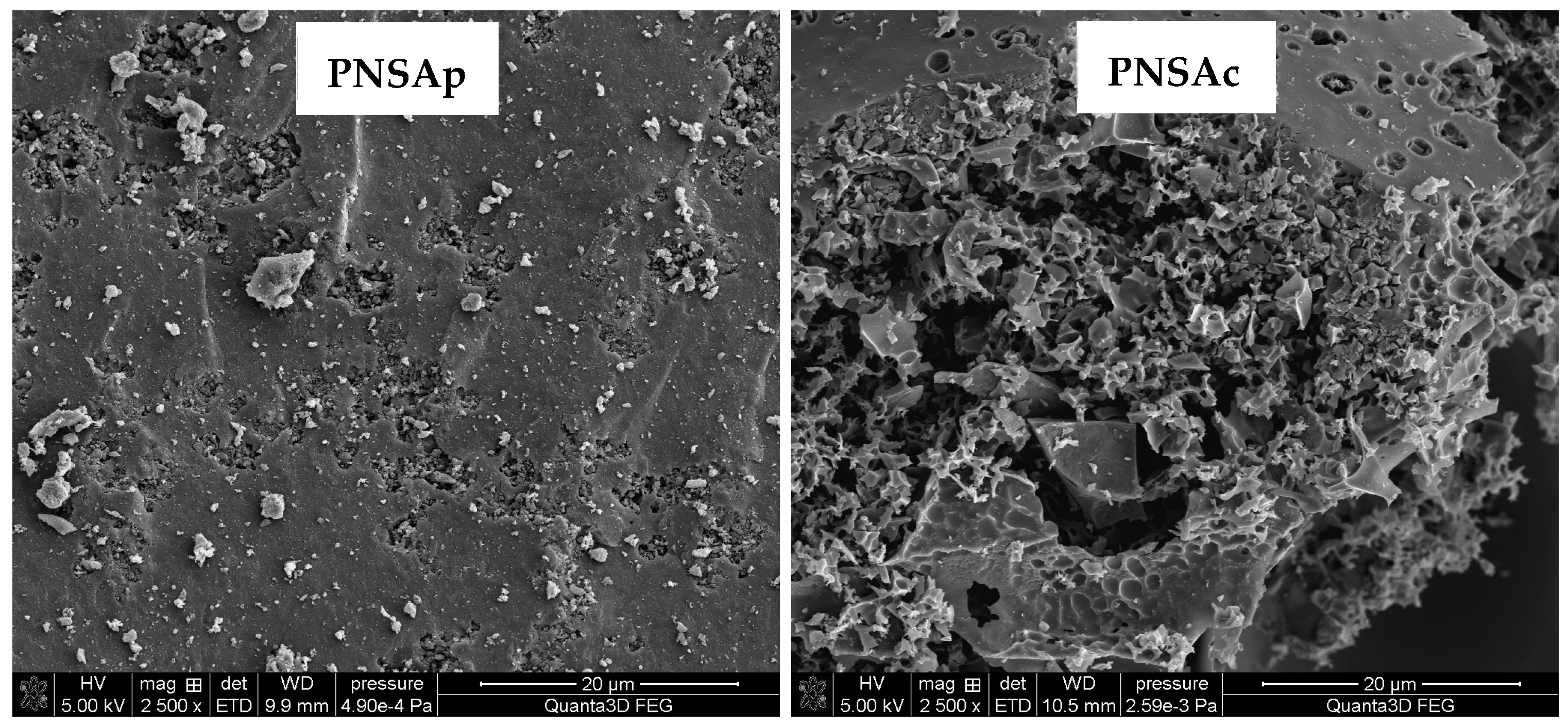
2.4. Textural and Morphological Parameters of the Carbonaceous Materials Obtained from Pistachio Nut Shells
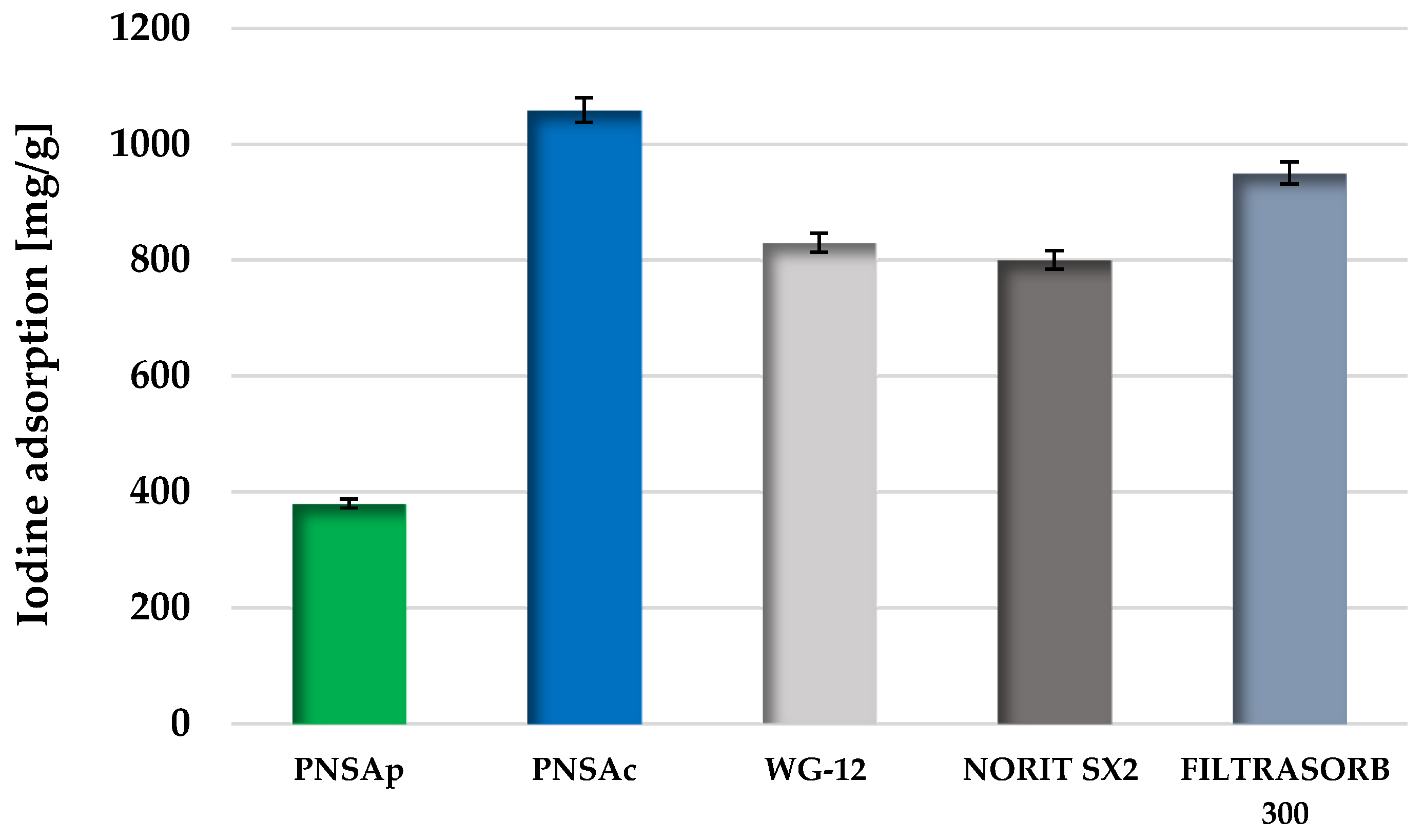
2.5. Sorption Performance of the Activated Biocarbons Prepared from Pistachio Nut Shells in Relation to Iodine and Methylene Blue
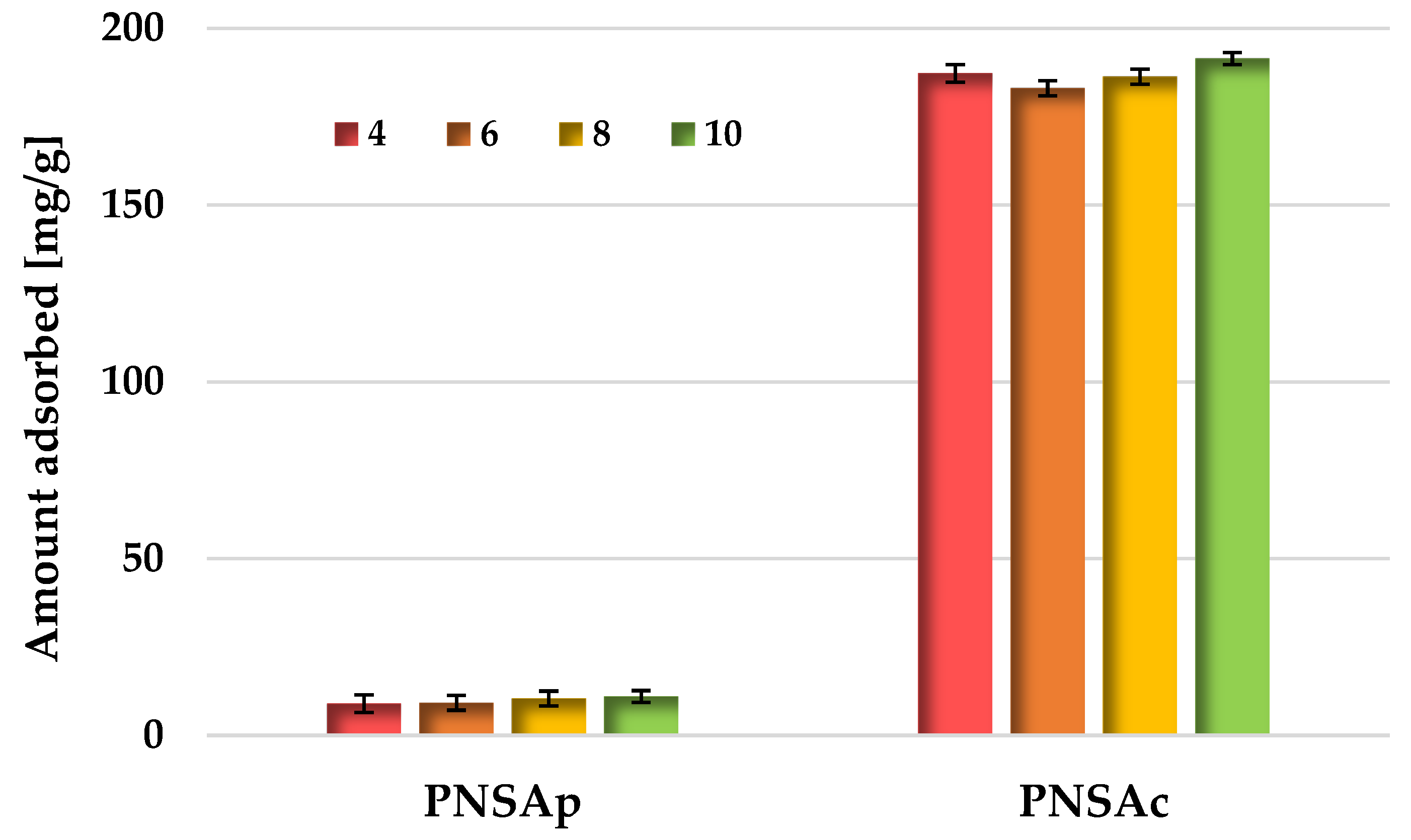
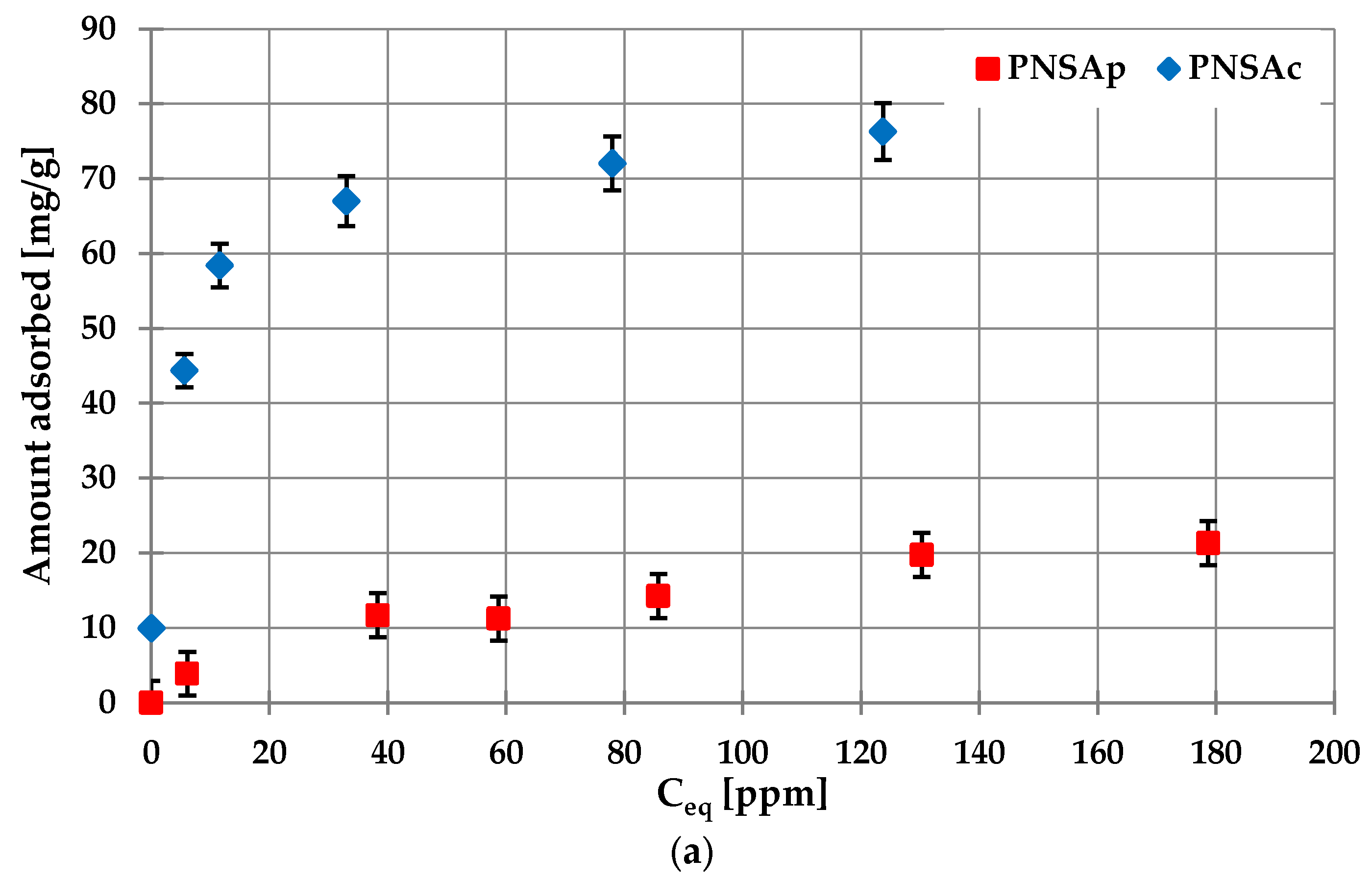
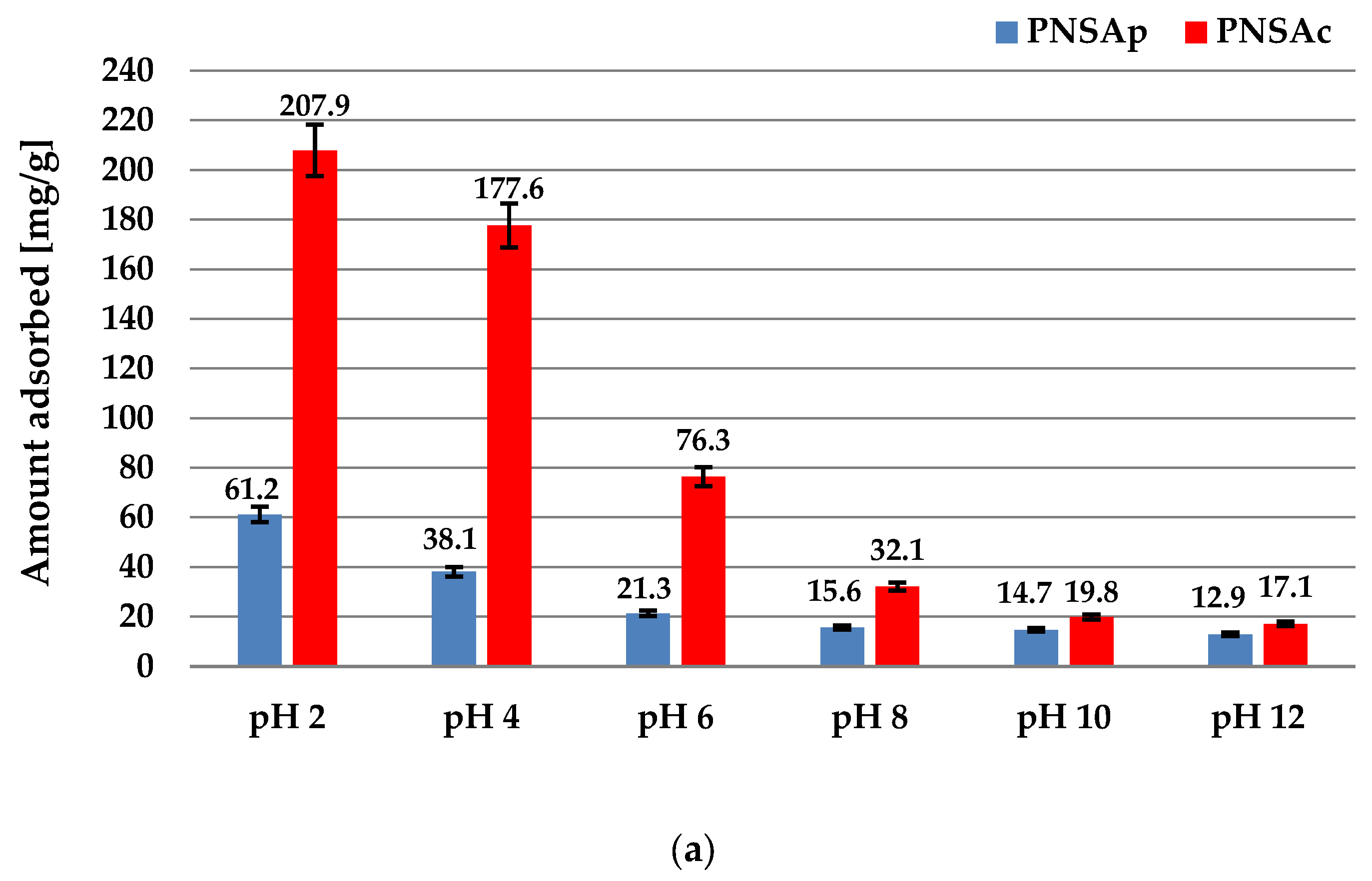
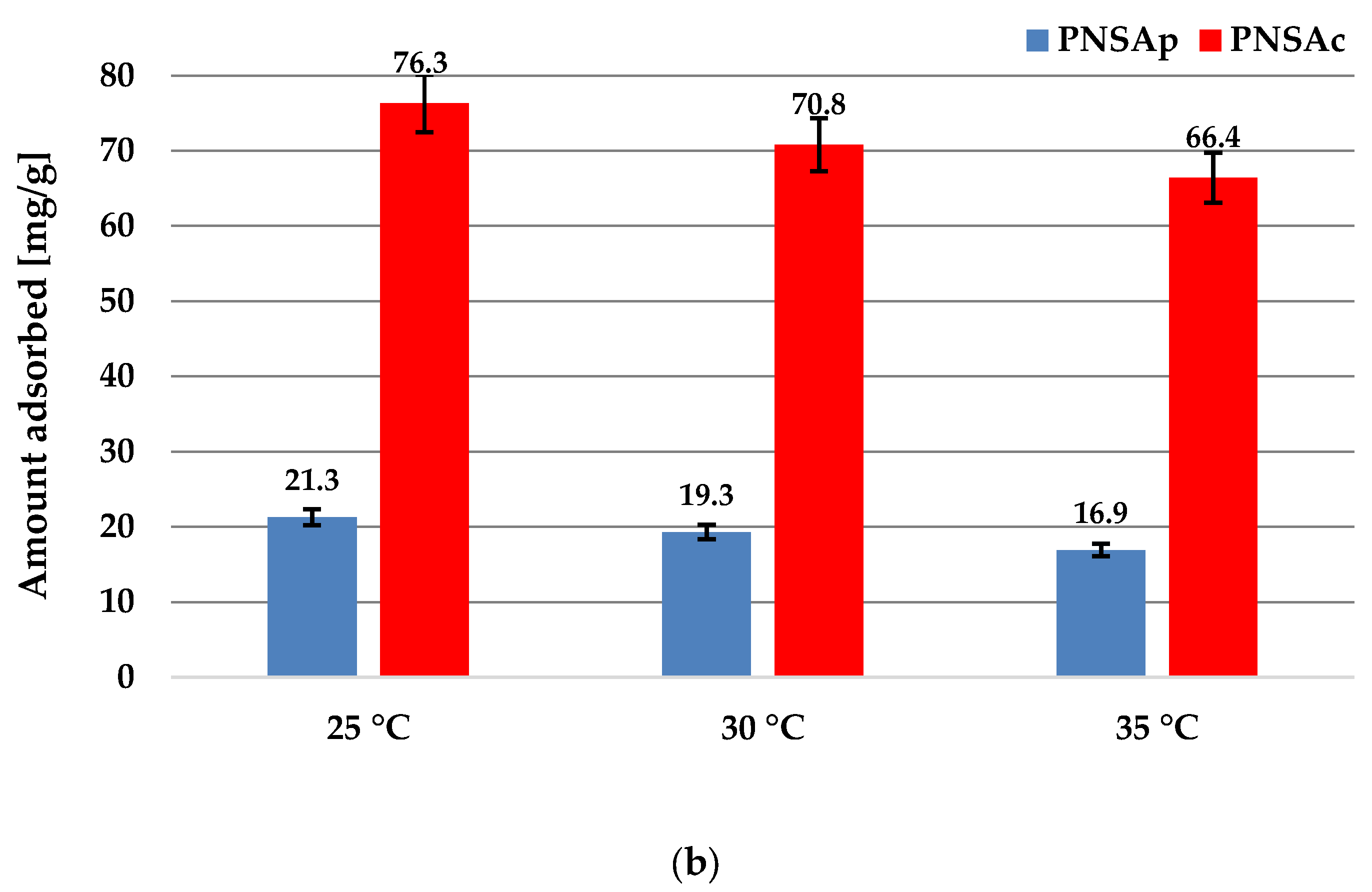
2.6. Sorption Properties of the Activated Biocarbons Prepared towards the Poly(acrylic acid) Polymer
3. Materials and Methods
3.1. Activated Biocarbons Preparation
3.2. Characterization of the Precursor and Activated Biocarbons
3.3. Adsorption of Iodine, Methylene Blue and Poly(acrylic acid)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, D.H. Wastewater Engineering Treatment and Reuse, 4th ed.; Tata McGraw-Hill Publishing Company Limited: New Delhi, India, 2003. [Google Scholar]
- Hung, Y.-T.; Wang, L.K.; Shammas, N.K. Handbook of Environment and Waste Management—Air and Water Pollution Control; World Publishing Co. Pte. Ltd.: Singapore, 2012. [Google Scholar]
- Sun, R.; Luo, X.; Li, Q.X.; Wang, T.; Zheng, X.; Peng, P.; Mai, B. Legacy and emerging organohalogenated contaminants in wild edible aquatic organisms: Implications for bioaccumulation and human exposure. Sci. Total Environ. 2018, 616–617, 38–45. [Google Scholar] [CrossRef]
- Kapelewska, J.; Kotowska, U.; Karpińska, J.; Kowalczuk, D.; Arciszewska, A.; Świrydo, A. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 2018, 137, 292–301. [Google Scholar] [CrossRef]
- Gupta, G.K.; Shukla, P. Insights into the resources generation from pulp and paper industry wastes: Challenges, perspectives and innovations. Bioresour. Technol. 2020, 297, 122496. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Zhao, J.L.; Liu, Y.S.; Liu, W.R.; Zhang, Q.Q.; Yao, L.; Ying, G.-G. Pharmaceuticals and personal care products (PPCPs) and artificial sweeteners (ASs) in surface and ground waters and their application as indication of wastewater contamination. Sci. Total Environ. 2018, 616–617, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Parnaudeau, V.; Nicolardot, B.; Robert, P.; Alavoine, G.; Pages, J.; Duchiron, F. Organic matter characteristics of food processing industry wastewaters affecting their C and N mineralization in soil incubation. Bioresour. Technol. 2006, 97, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Ingaramo, A.; Heluane, H.; Colombo, M.; Cesca, M. Water and wastewater eco-efficiency indicators for the sugar cane industry. J. Clean. Prod. 2009, 17, 487–495. [Google Scholar] [CrossRef]
- Tian, X.; Song, Y.; Shen, Z.; Zhou, Y.; Wang, K.; Jin, X.; Han, Z.; Liu, T. A comprehensive review on toxic petrochemical wastewater pretreatment and advanced treatment. J. Clean. Prod. 2020, 245, 118692. [Google Scholar] [CrossRef]
- Mikhak, Y.; Torabi, M.M.A.; Fouladitajar, A. Chapter 3—Refinery and petrochemical wastewater treatment. In Sustainable Water and Wastewater Processing; Charis, M., Galanakis, E.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–91. [Google Scholar] [CrossRef]
- Dziadel, M.; Ignatowicz, K. Assessment of the Quality of Wastewater Generated During Production at a Tannery Plant. J. Ecol. Eng. 2022, 23, 109–115. [Google Scholar] [CrossRef]
- Kruszelnicka, I.; Ginter-Kramarczyk, D.; Wyrwas, B.; Idkowiak, J. Evaluation of surfactant removal efficiency in selected domestic wastewater treatment plants in Poland. J. Environ. Health Sci. Eng. 2019, 17, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshmi, S.; Raja Lakshmi, P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; van Haute, S.; Zhou, B.; Hapeman, C.J.; Millner, P.D.; Wang, Q.; Luo, Y. Impacts and interactions of organic compounds with chlorine sanitizer in recirculated and reused produce processing water. PLoS ONE 2018, 12, e0208945. [Google Scholar] [CrossRef] [PubMed]
- Buth, J.M.; Ross, M.R.; McNeill, K.; Arnold, W.A. Removal and formation of chlorinated triclosan derivatives in wastewater treatment plants using chlorine and UV disinfection. Chemosphere 2011, 84, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Bhomick, P.C.; Supong, A.; Sinha, D. Organic pollutants in water and its remediation using biowaste activated carbon as greener adsorbent. Int. J. Hydrol. 2017, 1, 91–92. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P.; Szewczuk-Karpisz, K.; Gęca, M.; Jędruchniewicz, K.; Oleszczuk, P. Simultaneous removal of toxic Pb(II) ions, poly(acrylic acid) and Triton X-100 from their mixed solution using engineered biochars obtained from horsetail herb precursor—Impact of post-activation treatment. Sep. Purif. Technol. 2021, 276, 119297. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Biochars and activated carbons as adsorbents of inorganic and organic compounds from multicomponent systems—A review. Adv. Colloid. Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef]
- Kaleta, J.; Kida, M.; Koszelnik, P.; Papciak, D.; Puszkarewicz, A.; Tchórzewska-Cieślak, B. The use of activated carbons for removing organic matter from groundwater. Arch. Environ. Prot. 2017, 43, 32–41. [Google Scholar] [CrossRef]
- Siipola, V.; Tamminen, T.; Källi, A.; Lahti, R.; Romar, H.; Rasa, K.; Keskinen, R.; Hyväluoma, J.; Hannula, M.; Wikberg, H. Effects of biomass type, carbonization process, and activation method on the properties of bio-based activated carbons. BioResources 2018, 13, 5976–6002. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, N.; de Lannoy, H.F. Fast synthesis of high surface area bio-based porous carbons for organic pollutant removal. MethodsX 2021, 8, 101464. [Google Scholar] [CrossRef]
- Gouda, M.S.; Shehab, M.; Soliman, M.M.; Helmy, S.; Salama, S.R. Preparation and characterization of supercapacitor electrodes utilizing catkin plant as an activated carbon source. Delta Univ. Sci. J. 2023, 6, 255–265. [Google Scholar] [CrossRef]
- Saleh, T.S.; Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Design and development of novel composites containing nickel ferrites supported on activated carbon derived from agricultural wastes and its application in water remediation. Materials 2023, 16, 2170. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon, 1st ed.; Elsevier Ltd.: Oxford, UK, 2006; pp. 243–365. [Google Scholar]
- Menéndez-Díaz, J.A.; Martín-Gullón, I. Types of carbon adsorbents and their production. In Activated Carbon Surfaces in Environmental Remediation, 1st ed.; Bandosz, T.J., Ed.; Elsevier Ltd.: New York, NY, USA, 2006; pp. 1–47. [Google Scholar]
- Tsai, W.T.; Jiang, T.J. Mesoporous activated carbon produced from coconut shell using a single-step physical activation process. Biomass Conv. Bioref. 2018, 8, 711–718. [Google Scholar] [CrossRef]
- Olán Ramos, M.; Del Angel, E.M.; Rojo, J.M.; Pacheco-Catalan, D.E.; Pantoja Castro, M.A.; Ortiz, R.S.M. Activated carbons from coconut shell and NiO-based composites for energy storage systems. J. Mater. Sci. Mater. Electron. 2021, 32, 4872–4884. [Google Scholar] [CrossRef]
- Sujiono, E.H.; Zabrian, D.; Zurnansyah; Mulyati; Zharvan, V.; Samnur; Humairah, N.A. Fabrication and characterization of coconut shell activated carbon using variation chemical activation for wastewater treatment application. Results Chem. 2022, 4, 100291. [Google Scholar] [CrossRef]
- Bazan-Woźniak, A.; Nowicki, P.; Pietrzak, R. The influence of activation procedure on the physicochemical and sorption properties of activated carbons prepared from pistachio nutshells for removal of NO2/H2S gases and dyes. J. Clean. Prod. 2017, 152, 211–222. [Google Scholar] [CrossRef]
- Lua, A.C.; Yang, T. Effect of activation temperature on the textural and chemical properties of potassium hydroxide activated carbon prepared from pistachio-nut shell. J. Colloid. Interface Sci. 2004, 274, 594–601. [Google Scholar] [CrossRef]
- Cheng, T.; Li, J.; Ma, X.; Zhou, L.; Wu, H.; Yang, L. The adsorption properties of microporous activated carbon prepared from pistachio nut shell for low concentration VOCs under low-medium temperatures. Environ. Sci. Pollut. Res. 2021, 28, 65216–65228. [Google Scholar] [CrossRef]
- Kayranli, B.; Oğuzhan, G.; Gülden, G.; Mesutoğlu, Ö.Ç. Textile dye removal from aqueous solution by using peanut and pistachio shells. Int. J. Env. Pollut. Env. Model. 2019, 2, 270–276. [Google Scholar]
- Attia, A.A.; Girgis, B.S.; Khedr, S.A. Capacity of activated carbon derived from pistachio shells by H3PO4 in the removal of dyes and phenolics. J. Chem. Technol. Biotechnol. 2003, 78, 611–619. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Sereshti, H.; Ataolahi, S.; Toloutehrani, A.; Ramezani, A.T. Activated carbon derived from pistachio hull biomass for the effective removal of parabens from aqueous solutions: Isotherms, kinetics, and free energy studies. Desalin. Water Treat. 2020, 201, 155–164. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Al-Musawi, T.J.; Kareem, S.L.; Zarrabi, M.; Al-Ma’abreh, A.M. Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab. J. Chem. 2020, 13, 4629–4643. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R. Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: Parametric experiments, kinetics and equilibrium analysis. J. Hazard. Mater. 2010, 183, 724–730. [Google Scholar] [CrossRef]
- Nejadshafiee, V.; Islami, M.R. Intelligent-activated carbon prepared from pistachio shells precursor for effective adsorption of heavy metals from industrial waste of copper mine. Env. Sci. Pollut. Res. 2020, 27, 1625–1639. [Google Scholar] [CrossRef]
- Shirbhate, V.A.; Gulwade, D.P.; Bhandarkar, S.E.; Narsing, S.V. Preparation and spectroscopic characterization of Pistachio nut shell’s activated carbon using ZnCl2 for removal of transition metal ions. Mater. Today Proc. 2020, 29, 1259–1264. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Zhang, Y.; Tan, Y.; Tian, W.; Zhu, L.; Jiang, L. Preparing two-dimensional microporous carbon from Pistachio nutshell with high areal capacitance as supercapacitor materials. Sci. Rep. 2014, 4, 5545. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Khosravi, R. Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour. Technol. 2012, 119, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Skwarek, E.; Janusz, W. The study of the interactions of malonic acid ions with the hydroxyapatite surface in liquid. J. Mol. Liq. 2022, 359, 119370. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P. Simultaneous removal of lead(II) ions and poly(acrylic acid) macromolecules from liquid phase using of biocarbons obtained from corncob and peanut shell precursors. J. Mol. Liq. 2019, 296, 111806. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Urban, T.; Nowicki, P. temperature effect on ionic polymers removal from aqueous solutions using activated carbons obtained from biomass. Materials 2023, 16, 350. [Google Scholar] [CrossRef]
- Wiśniewska, M. Influences of polyacrylic acid adsorption and temperature on the alumina sus-pension stability. Powder Technol. 2010, 198, 258–266. [Google Scholar] [CrossRef]
- Lutfi, M.; Hanafi Susilo, B.; Prasetyo, J.; Sandra Prajogo, U. Characteristics of activated carbon from coconut shell (Cocosnucifera) through chemical activation process. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012134. [Google Scholar] [CrossRef]
- Kra, D.O.; Allou, N.B.; Atheba, P.; Drogui, P.; Trokourey, A. Preparation and characterization of activated carbon based on wood (Acacia auriculeaformis, Côte d’Ivoire). J. Encapsul. Adsorpt. Sci. 2019, 9, 63–82. [Google Scholar] [CrossRef]
- Hanami, Z.A.; Lestari, P. Characterization and application of mangosteen peel activated carbon for ammonia gas removal. Environ. Nat. Resour. J. 2021, 19, 320–329. [Google Scholar] [CrossRef]
- Evwierhoma, E.T.; Madubiko, O.D.; Jaiyeola, A. Preparation and characterization of activated carbon from bean husk. Niger. J. Technol. 2018, 37, 674–678. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Rejer, K.; Pietrzak, R.; Nowicki, P. Biochars and activated biocarbons prepared via conventional pyrolysis and chemical or physical activation of mugwort herb as potential adsorbents and renewable fuels. Molecules 2022, 27, 8597. [Google Scholar] [CrossRef] [PubMed]
- Rosson, E.; Garbo, F.; Marangoni, G.; Bertani, R.; Lavagnolo, M.C.; Moretti, E.; Talon, A.; Mozzon, M.; Sgarbossa, P. Activated Carbon from Spent Coffee Grounds: A Good Competitor of Commercial Carbons for Water Decontamination. Appl. Sci. 2020, 10, 5598. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Statistical modeling of methylene blue dye adsorption by high surface area mesoporous activated carbon from bamboo chip using KOH-assisted thermal activation. Energy Ecol. Environ. 2020, 5, 456–469. [Google Scholar] [CrossRef]
- Thang, N.H.; Khang, D.S.; Hai, T.D.; Nga, D.T.; Tuan, P.D. Methylene blue adsorption mechanism of activated carbon synthesised from cashew nut shells. RSC Adv. 2021, 11, 26563–26570. [Google Scholar] [CrossRef]
- Nedjai, R.; Alkhatib, M.F.R.; Alam, M.Z.; Kabbashi, N.A. Adsorption of methylene blue onto activated carbon developed from baobab fruit shell by chemical activation: Kinetic equilibrium studies. IIUM Eng. J. 2021, 22, 31–49. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of poly (acrylic acid) onto the surface of titanium dioxide and the colloidal stability of aqueous suspension. J. Colloid. Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Urban, T.; Grządka, E.; Zarko, V.I.; Gun’ko, V.M. Comparison of adsorption affinity of polyacrylic acid for surfaces of mixed silica–alumina. Colloid. Polym. Sci. 2014, 292, 699–705. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P.; Urban, T. Influence of surfactants with different ionic character on the structure of poly (acrylic acid) adsorption layer on the activated biocarbons surface–electrokinetic and stability studies. J. Mol. Liq. 2021, 332, 115872. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Simultaneous removal of polymers with different ionic character from their mixed solutions using herb-based biochars and activated carbons. Molecules 2022, 27, 7557. [Google Scholar] [CrossRef]
- Janusz, W. Electrical double layer in the system TiO2 (anathase)/aqueous solution of NaCl. Pol. J. Chem. 1994, 68, 1871–1880. [Google Scholar]
- Ohshima, H.A. Simple Expression for Henry’s Function for the Retardation Effect in Electrophoresis of Spherical Colloidal Particles. J. Colloid. Interface Sci. 1994, 168, 269–271. [Google Scholar] [CrossRef]
- Szymańska, M.; Nowicki, P. Used filter cartridges as potential adsorbents of organic pollutants. Water 2023, 15, 714. [Google Scholar] [CrossRef]
- Crummett, W.B.; Hummel, R.A. The determination of traces of polyacrylamides in water. J. Am. Water Work. Assoc. 1963, 1, 55, 209–219. [Google Scholar] [CrossRef]
- Michaels, A.S.; Morelos, O. Polyelectrolyte adsorption by kaolinite. Ind. Eng. Chem. 1955, 47, 1801–1809. [Google Scholar] [CrossRef]
- Wiśniewska, M. Study of the influence of temperature and the ionic strength of the solution on the adsorption and conformation of poly(acrylic acid) macromolecules on the ZrO2 surface. Adsorpt. Sci. Technol. 2006, 24, 673–686. [Google Scholar] [CrossRef]


| Sample | Ash | Cdaf 1 | Hdaf | Ndaf | Sdaf | Odiff 2 |
|---|---|---|---|---|---|---|
| PNS | 0.2 | 43.9 | 7.0 | 0.2 | 0.1 | 48.8 |
| PNSAp | 1.7 | 91.7 | 0.5 | 0.56 | 0.1 | 7.1 |
| PNSAc | 3.5 | 88.6 | 2.6 | 0.0 | 0.1 | 8.7 |
| Sample | Acidic Groups Content [mmol/g] | Basic Groups Content [mmol/g] | Total Content of Surface Groups [mmol/g] | pH of Aqueous Extracts |
|---|---|---|---|---|
| PNS | 0.65 | 0.24 | 0.89 | 5.29 |
| PNSAp | 0.15 | 0.62 | 0.77 | 9.11 |
| PNSAc | 0.94 | 0.07 | 1.01 | 3.14 |
| Sample | Total 1 | Micropore | Micropore Contribution | Mean Pore Size [nm] | ||
|---|---|---|---|---|---|---|
| Surface Area [m2/g] | Pore Volume [cm3/g] | Area [m2/g] | Volume [cm3/g] | |||
| PNSAp | 31 | 0.059 | - | - | - | 7.256 |
| PNSAc | 1264 | 1.382 | 746 | 0.397 | 0.287 | 4.376 |
| Sample | qexp | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | 1/n | R2 | ||
| PNSAp | 9.04 | 10.41 | 0.157 | 0.916 | 7.179 | 0.126 | 0.376 |
| PNSAc | 183.11 | 182.48 | 0.143 | 0.999 | 153.673 | 0.066 | 0.910 |
| Adsorbent | Maximum Adsorbed Amount [mg/g] | Reference |
|---|---|---|
| Iodine | ||
| Activated carbon from coconut shell | 249 | [47] |
| Activated carbon from acacia wood | 381 | [48] |
| Activated carbon from mangosteen peel | 1153 | [49] |
| Activated carbon from bean husk | 1256 | [50] |
| Activated biocarbon form mugwort | 948 | [51] |
| Activated carbon from pistachio nutshells | 380 and 1089 | This study |
| Methylene blue | ||
| Activated carbon from spent coffee grounds | 179 | [52] |
| Activated carbon from bamboo chips | 305 | [53] |
| Activated carbon from cashew nut shells | 100 | [54] |
| Activated carbon from baobab fruit shell | 114 | [55] |
| Activated carbon from pistachio nutshells | 9 and 183 | This study |
| Poly(acrylic acid) | ||
| Titanium dioxide | 24 | [56] |
| Mixed silica-alumina oxide | 86 | [57] |
| Activated biocarbon from corncobs | 50 | [58] |
| Activated carbon obtained from the nettle herb | 273 | [59] |
| Activated carbon from pistachio nutshells | 61 and 208 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, A.; Wiśniewska, M.; Nowicki, P. Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters. Molecules 2023, 28, 4497. https://doi.org/10.3390/molecules28114497
Wawrzyniak A, Wiśniewska M, Nowicki P. Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters. Molecules. 2023; 28(11):4497. https://doi.org/10.3390/molecules28114497
Chicago/Turabian StyleWawrzyniak, Agata, Małgorzata Wiśniewska, and Piotr Nowicki. 2023. "Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters" Molecules 28, no. 11: 4497. https://doi.org/10.3390/molecules28114497
APA StyleWawrzyniak, A., Wiśniewska, M., & Nowicki, P. (2023). Carbon Adsorbents Obtained from Pistachio Nut Shells Used as Potential Ingredients of Drinking Water Filters. Molecules, 28(11), 4497. https://doi.org/10.3390/molecules28114497








