Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium
Abstract
1. Introduction
2. Results and Discussion
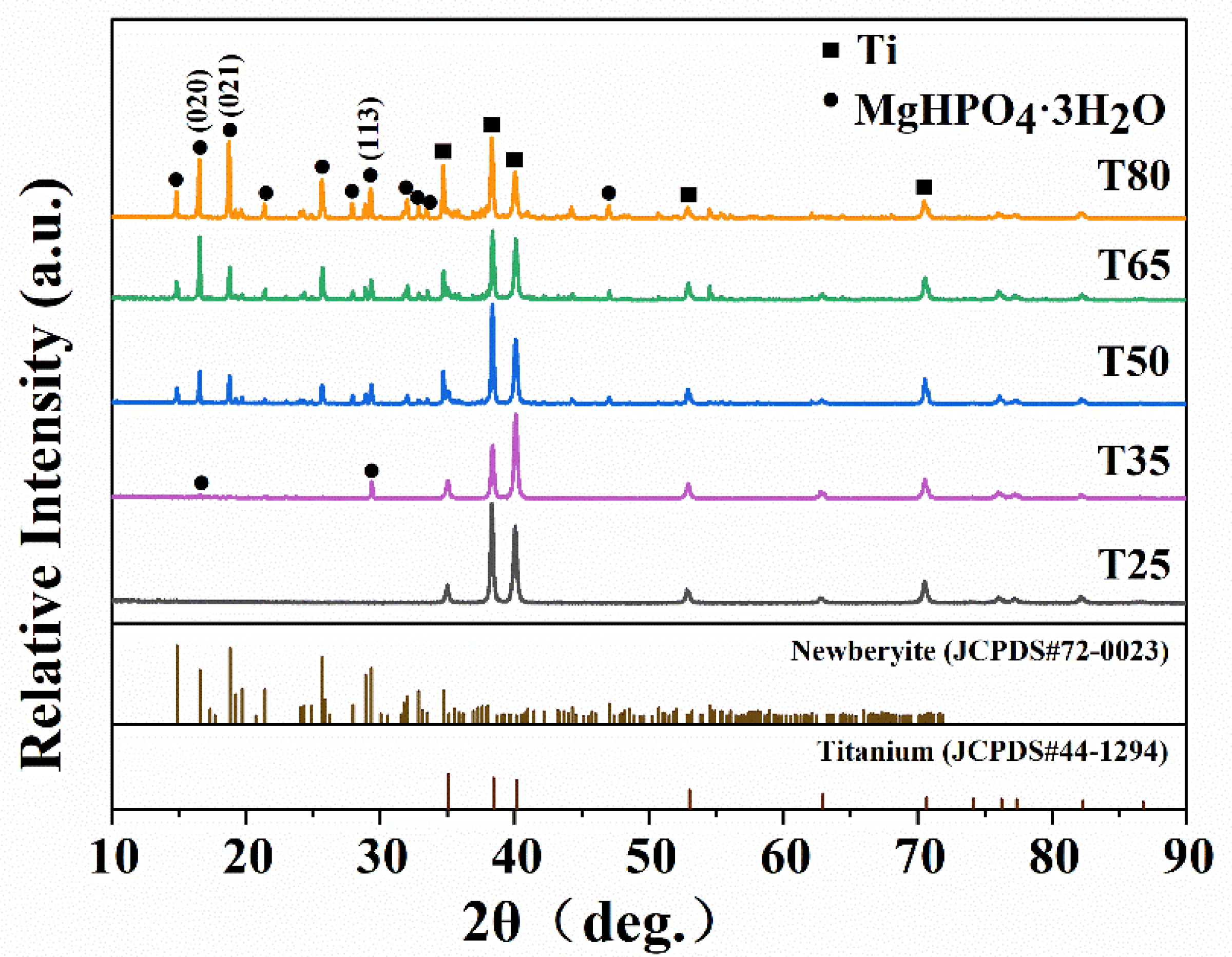
2.1. Phase Composition
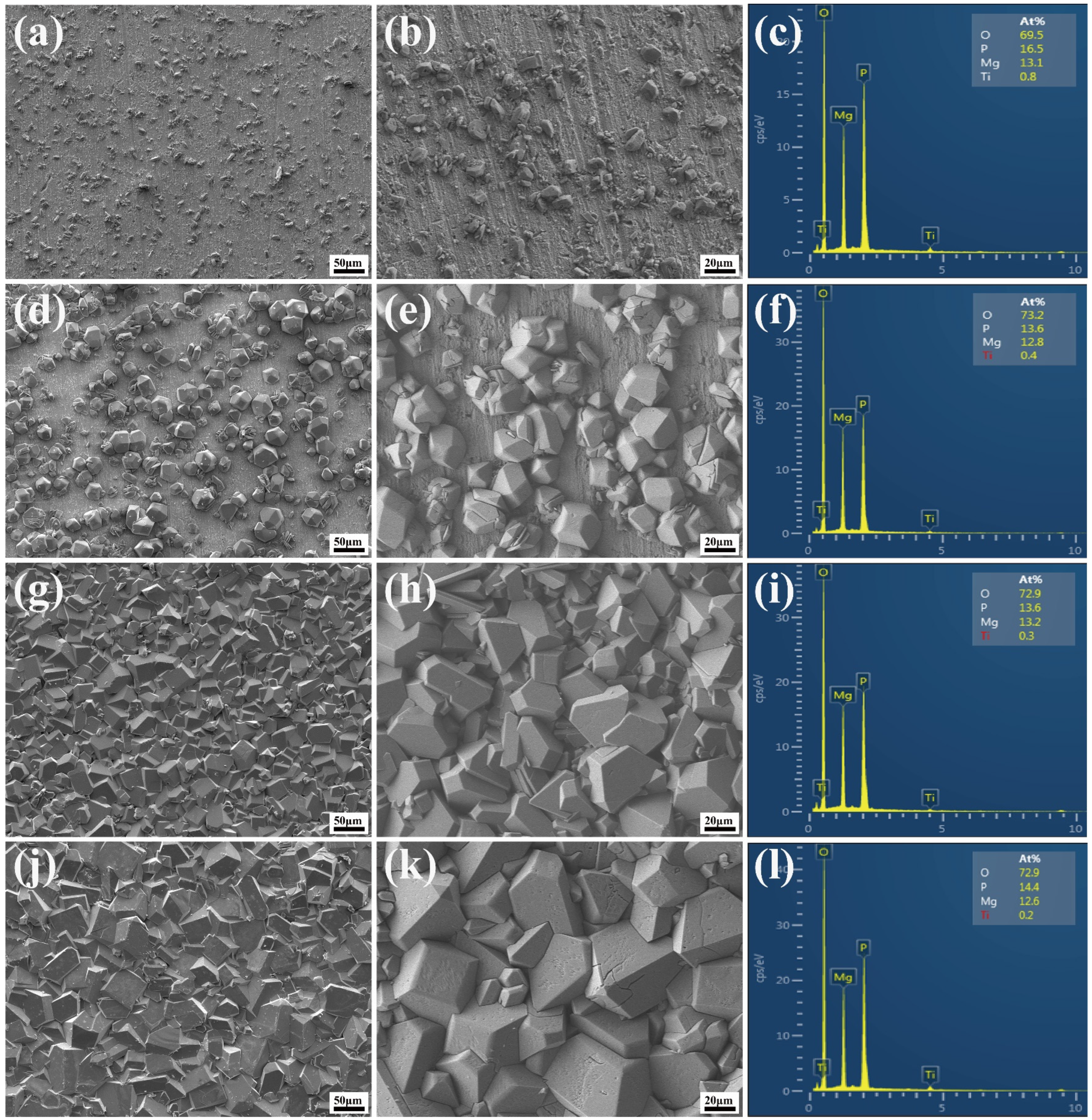
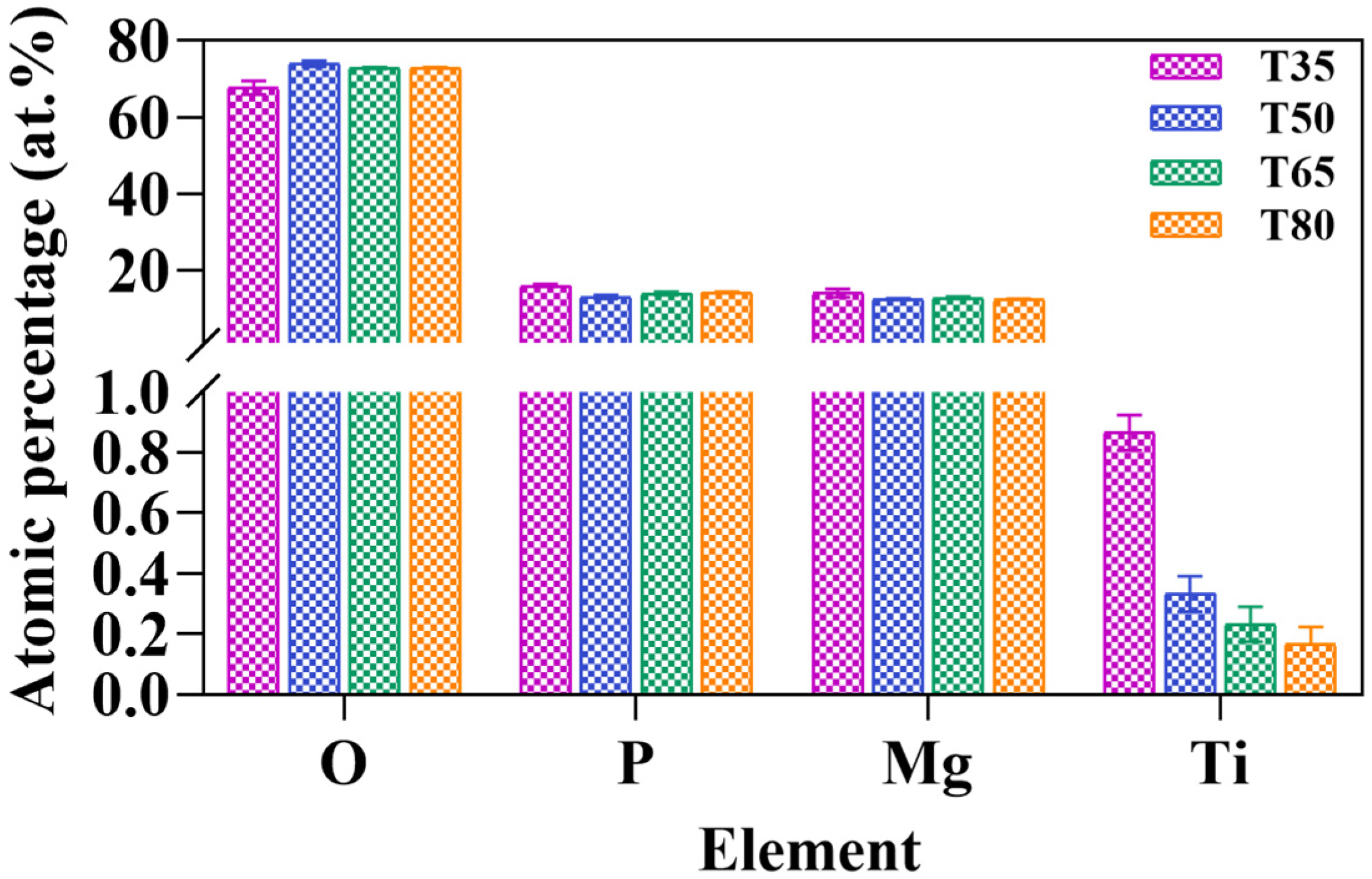
2.2. Microstructure of the Coatings
2.3. Corrosion Characteristics
| Sample | Ecorr (V) | Icorr (×10−8 A/cm2) | βa (V·dec−1) | −βc (V·dec−1) | Rp (×104 Ω·cm2) |
|---|---|---|---|---|---|
| Bare Ti | −0.466 ± 0.009 | 62.50 ± 3.37 | 0.373 ± 0.007 | 0.150 ± 0.004 | 7.430 ± 0.675 |
| T35 | −0.489 ± 0.011 | 468.89 ± 7.42 | 0.639 ± 0.010 | 0.173 ± 0.021 | 1.263 ± 0.022 |
| T50 | −0.473 ± 0.014 | 95.25 ± 3.58 | 0.399 ± 0.006 | 0.153 ± 0.017 | 5.047 ± 0.286 |
| T65 | −0.585 ± 0.017 | 28.22 ± 1.66 | 0.245 ± 0.001 | 0.170 ± 0.002 | 15.43 ± 0.954 |
| T80 | −0.540 ± 0.009 | 21.36 ±1.25 | 0.259 ± 0.003 | 0.163 ± 0.004 | 20.31 ± 0.977 |

| Samples | Bare Ti | T35 | T50 | T65 | T80 |
|---|---|---|---|---|---|
| Rs (Ω⋅cm2) | 106.6 | 98.87 | 102.2 | 104.5 | 124.2 |
| Qdl (×10−5 Ω−1⋅cm−2⋅S−n) | 1.114 | 1.898 | 1.191 | 0.6027 | 1.174 |
| ndl | 0.9005 | 0.841 | 0.8304 | 0.8504 | 0.7715 |
| Rct (×105 Ω⋅cm2) | 3.455 | 0.7902 | 0.9703 | 3.789 | 5.364 |
| Qc (×10−5 Ω−1⋅cm−2 S−n) | 1.677 | 0.6252 | 0.7354 | 1.066 | |
| nc | 0.7685 | 0.5822 | 0.7669 | 0.9544 | |
| Rc (×105 Ω⋅cm2) | 5.292 | 5.377 | 9.324 | 13.11 | |
| χ2 (10−3) | 1.56 | 0.559 | 5.26 | 1.14 | 2.82 |
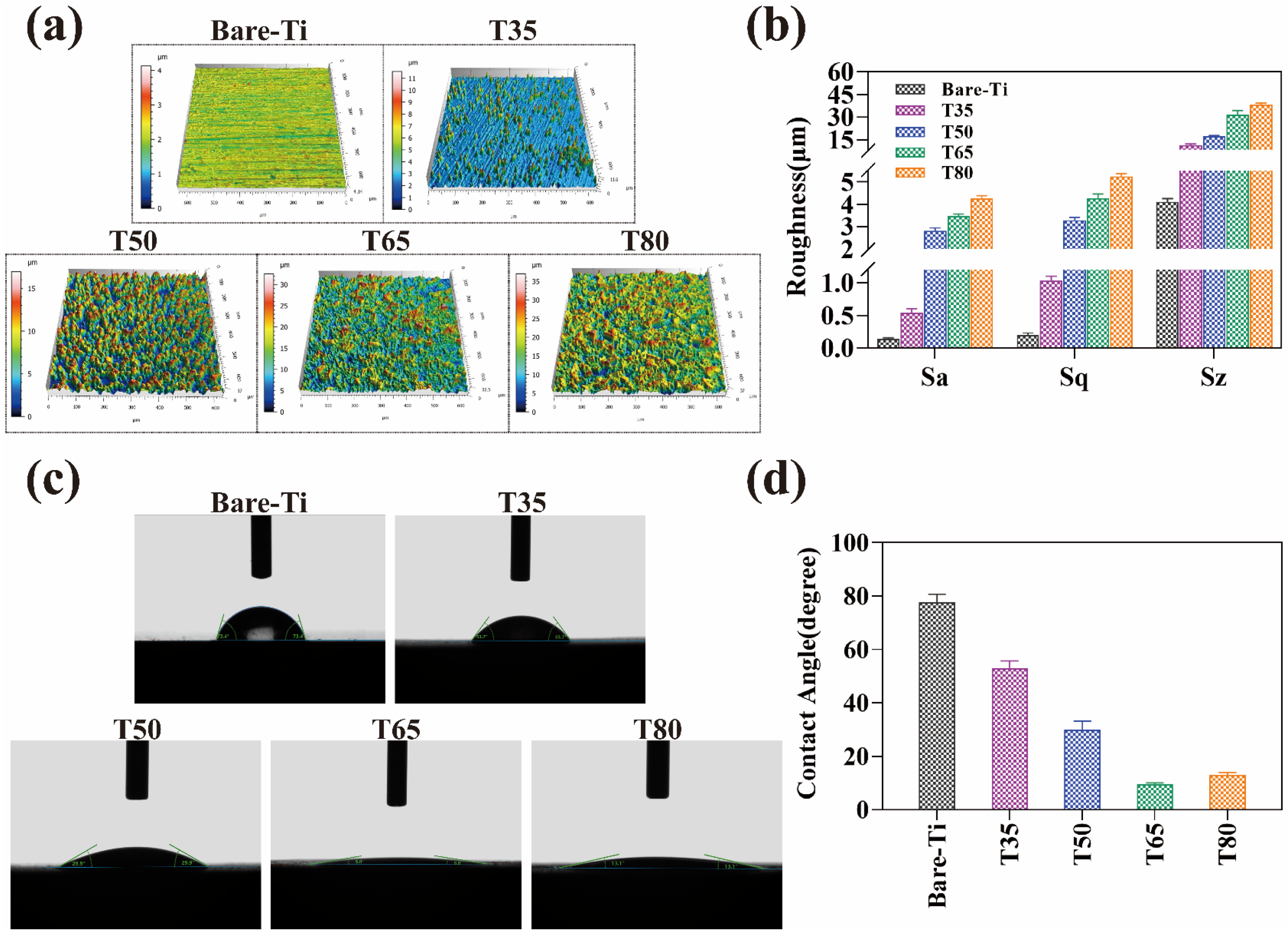
2.4. Surface Roughness and Wettability
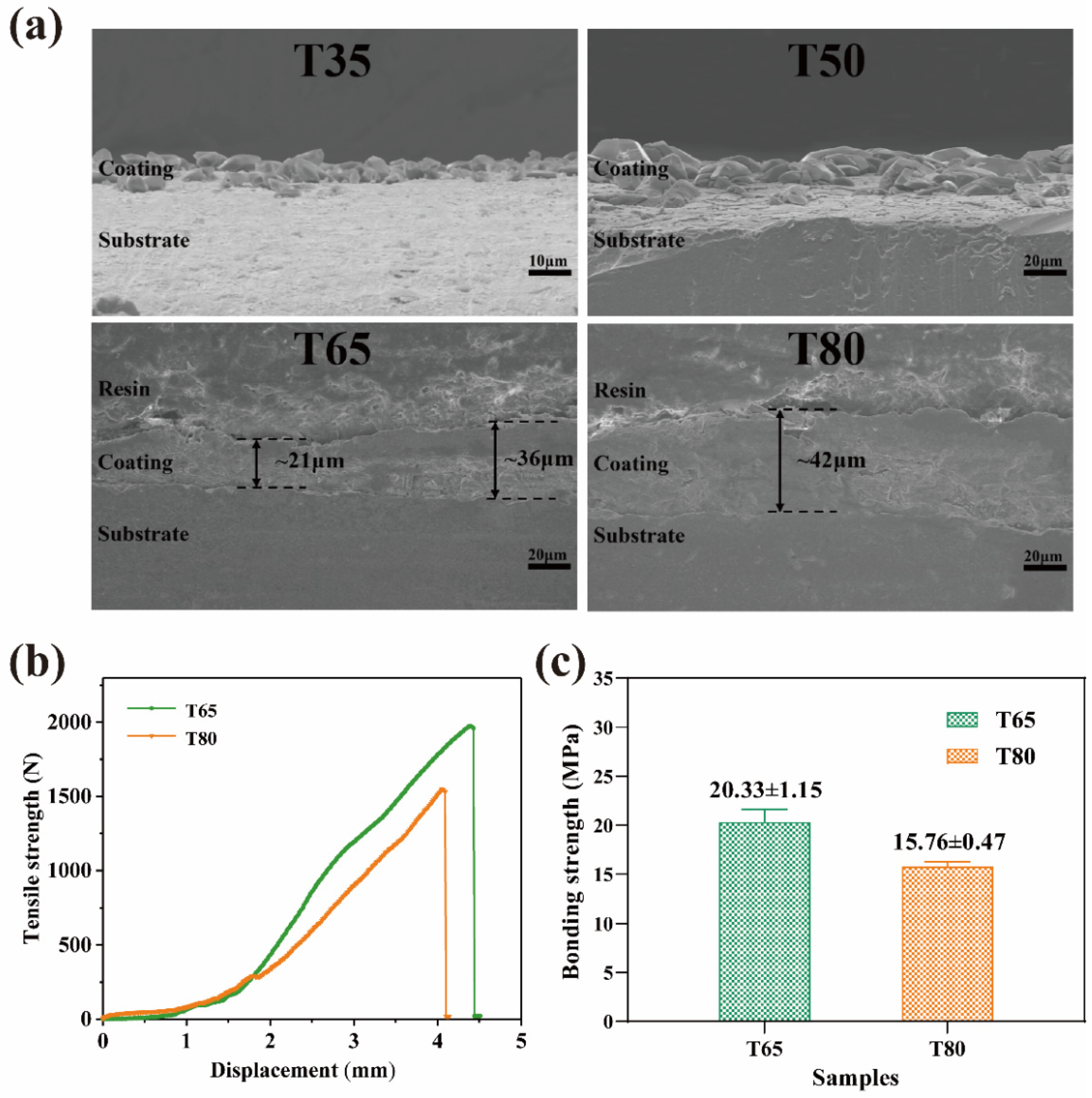
2.5. Coating Thickness and Bonding Strength
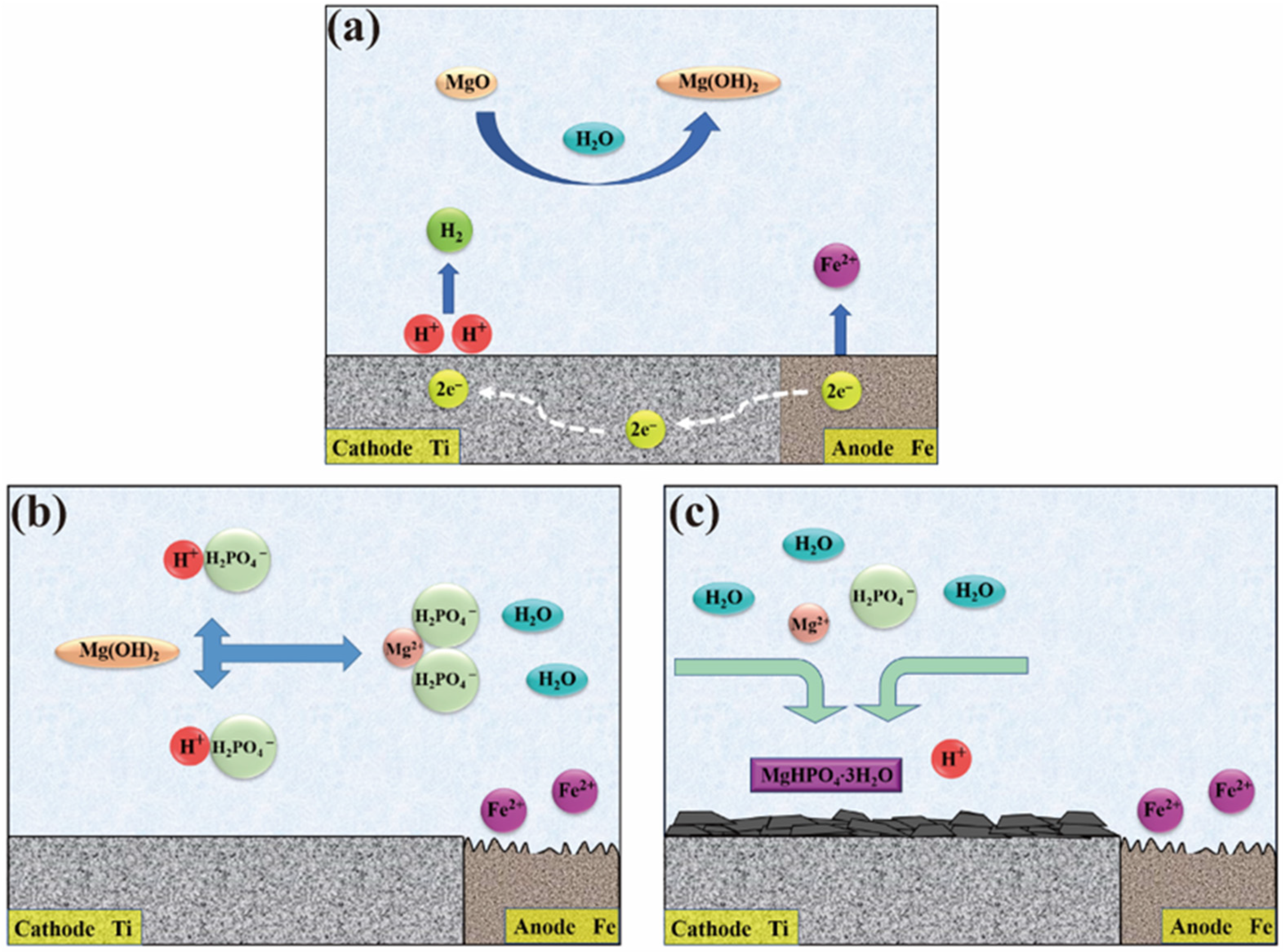
2.6. Formation Mechanism
3. Materials and Methods
3.1. Coating Preparation
| Bath Composition | Concentration | Treatment Conditions |
|---|---|---|
| MgO | 10 g/L | PH = 4.2, t = 25 min, T = 35 °C; 50 °C; 65 °C; 80 °C |
| H3PO4 | 20 mL | |
| HNO3 | 18 mL | |
| NaNO2 | 2 g/L | |
| NaNO3 | 2 g/L | |
| NaOH | 2.5 g/L |
3.2. Coating Characterization
3.3. Tensile Adhesion Tests
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Civantos, A.; Martinez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium Coatings and Surface Modifications: Toward Clinically Useful Bioactive Implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, X.; Tan, L.; Zheng, B.; Muhammed, F.K.; Yang, K.; Liu, Y. In vitro and in vivo characterization of novel calcium phosphate and magnesium (CaP-Mg) bilayer coated titanium for implantation. Surf. Coat. Technol. 2019, 374, 784–796. [Google Scholar] [CrossRef]
- Wen, H.B.; Liu, Q.; De Wijn, J.R.; De Groot, K.; Cui, F.Z. Preparation of bioactive microporous titanium surface by a new two-step chemical treatment. J. Mater. Sci. Mater. Med. 1998, 9, 121–128. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Kyo, Y.; Yadav, A.P.; Nishikata, A.; Tsuru, T. Hydrogen entry behaviour of newly developed Al–Mg–Si coating produced by physical vapour deposition. Corros. Sci. 2011, 53, 3043–3047. [Google Scholar] [CrossRef]
- Kim, H.-W.; Koh, Y.-H.; Li, L.-H.; Lee, S.; Kim, H.-E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar] [CrossRef]
- Vizza, M.; Giurlani, W.; Cerri, L.; Calisi, N.; Leonardi, A.A.; Faro, M.J.; Irrera, A.; Berretti, E.; Perales-Rondón, J.V.; Colina, A.; et al. Electrodeposition of Molybdenum Disulfide (MoS2) Nanoparticles on Monocrystalline Silicon. Molecules 2022, 27, 5416. [Google Scholar] [CrossRef]
- Wen, C.; Guan, S.; Peng, L.; Ren, C.; Wang, X.; Hu, Z. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Appl. Surf. Sci. 2009, 255, 6433–6438. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S. Surface pretreatment by phosphate conversion coatings—A review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Liu, B.; Shi, X.M.; Xiao, G.Y.; Lu, Y.P. In-situ preparation of scholzite conversion coatings on titanium and Ti-6Al-4V for biomedical applications. Colloids Surf. B Biointerfaces 2017, 153, 291–299. [Google Scholar] [CrossRef]
- Cheng, K.; Ren, C.; Weng, W.; Du, P.; Shen, G.; Han, G.; Zhang, S. Bonding strength of fluoridated hydroxyapatite coatings: A comparative study on pull-out and scratch analysis. Thin Solid Films 2009, 517, 5361–5364. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, M.; Ding, C. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials 2000, 21, 841–849. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, P.; Zhang, B.; Tan, L.; Yang, K.; Lin, J. Research on the corrosion resistance and formation of double-layer calcium phosphate coating on AZ31 obtained at varied temperatures. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 264–271. [Google Scholar] [CrossRef]
- Fouladi, M.; Amadeh, A. Effect of phosphating time and temperature on microstructure and corrosion behavior of magnesium phosphate coating. Electrochim. Acta 2013, 106, 1–12. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, S. Fabrication of Magnesium Phosphate Coating by Electrochemical Cathodic Method for Corrosion Protection of Sintered NdFeB Magnets. J. Mater. Eng. Perform. 2021, 30, 1200–1206. [Google Scholar] [CrossRef]
- Zuo, K.-Q.; Xiao, G.-Y.; Du, C.-M.; Liu, B.; Li, Y.-B.; Lu, Y.-P. Controllable phases evolution and properties of zinc-phosphate/strontium-zinc-phosphate composite conversion coatings on Ti: Effect of temperature. Surf. Coat. Technol. 2022, 447, 128885. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Y.; Wu, G.; Sun, M.; Wang, M.; Zhang, Q. Growth characteristics and properties of micro-arc oxidation coating on SLM-produced TC4 alloy for biomedical applications. Appl. Surf. Sci. 2019, 479, 727–737. [Google Scholar] [CrossRef]
- Zaludin, M.A.F.; Zahid Jamal, Z.A.; Derman, M.N.; Kasmuin, M.Z. Fabrication of calcium phosphate coating on pure magnesium substrate via simple chemical conversion coating: Surface properties and corrosion performance evaluations. J. Mater. Res. Technol. 2019, 8, 981–987. [Google Scholar] [CrossRef]
- Zai, W.; Zhang, X.; Su, Y.; Man, H.C.; Li, G.; Lian, J. Comparison of corrosion resistance and biocompatibility of magnesium phosphate (MgP), zinc phosphate (ZnP) and calcium phosphate (CaP) conversion coatings on Mg alloy. Surf. Coat. Technol. 2020, 397, 125919. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef]
- Zuo, K.; Wang, L.; Wang, Z.; Yin, Y.; Du, C.; Liu, B.; Sun, L.; Li, X.; Xiao, G.; Lu, Y. Zinc-Doping Induces Evolution of Biocompatible Strontium-Calcium-Phosphate Conversion Coating on Titanium to Improve Antibacterial Property. ACS Appl. Mater. Interfaces 2022, 14, 7690–7705. [Google Scholar] [CrossRef] [PubMed]
- Zuo, K.; Yin, Y.; Yao, L.; Wang, K.; Yan, Y.; Ma, Z.; Liu, B.; Lu, Y.; Li, X.; Xiao, G. Antibacterial activities and cytocompatibility of zinc-contained strontium phosphate coating on titanium. Mater. Res. Express 2020, 7, 075402. [Google Scholar] [CrossRef]
- Du, C.; Zuo, K.; Ma, Z.; Zhao, M.; Li, Y.; Tian, S.; Lu, Y.; Xiao, G. Effect of Substrates Performance on the Microstructure and Properties of Phosphate Chemical Conversion Coatings on Metal Surfaces. Molecules 2022, 27, 6434. [Google Scholar] [CrossRef]
- Sikder, P.; Bhaduri, S.B. Microwave assisted synthesis and characterization of single-phase tabular hexagonal newberyite, an important bioceramic. J. Am. Ceram. Soc. 2018, 101, 2537–2544. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.N. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable magnesium phosphates in biomedical applications. J. Mater. Chem. B 2022, 10, 2097–2112. [Google Scholar] [CrossRef]
- Massit, A.; El Yacoubi, A.; Kholtei, A.; El Idrissi, B.C. XRD and FTIR Analysis of Magnesium Substituted Tricalcium Calcium Phosphate Using a Wet Precipitation Method. Biointerface Res. Appl. Chem. 2020, 11, 8034–8042. [Google Scholar] [CrossRef]
- Meininger, M.; Wolf-Brandstetter, C.; Zerweck, J.; Wenninger, F.; Gbureck, U.; Groll, J.; Moseke, C. Electrochemically assisted deposition of strontium modified magnesium phosphate on titanium surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 65–71. [Google Scholar] [CrossRef]
- Ibasco, S.; Tamimi, F.; Meszaros, R.; Nihouannen, D.L.; Vengallatore, S.; Harvey, E.; Barralet, J.E. Magnesium-sputtered titanium for the formation of bioactive coatings. Acta Biomater. 2009, 5, 2338–2347. [Google Scholar] [CrossRef] [PubMed]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cai, S.; Ding, Z.; Zhang, M.; Li, Y.; Xu, G. A simple method for the preparation of magnesium phosphate conversion coatings on a AZ31 magnesium alloy with improved corrosion resistance. RSC Adv. 2015, 5, 24586–24590. [Google Scholar] [CrossRef]
- Tamimi, F.; Le Nihouannen, D.; Bassett, D.C.; Ibasco, S.; Gbureck, U.; Knowles, J.; Wright, A.; Flynn, A.; Komarova, S.V.; Barralet, J.E. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater. 2011, 7, 2678–2685. [Google Scholar] [CrossRef]
- Ishizaki, T.; Shigematsu, I.; Saito, N. Anticorrosive magnesium phosphate coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2009, 203, 2288–2291. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Lin, B.; Bhaduri, S.B. Microwave-assisted magnesium phosphate coating on the AZ31 magnesium alloy. Biomed. Mater. 2017, 12, 045026. [Google Scholar] [CrossRef]
- Saffarzade, P.; Amadeh, A.A.; Agahi, N. Study of tribological and friction behavior of magnesium phosphate coating and comparison with traditional zinc phosphate coating under dry and lubricated conditions. Tribol. Int. 2020, 144, 106122. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-Y.; Chen, C.-Z.; Lu, Y.-P.; Geng, X.-W. Hopeite and scholzite coatings formation on titanium via wet-chemical conversion with controlled temperature. Surf. Coat. Technol. 2020, 384, 125330. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-Y.; Lu, Y.-P. Effect of pH on the Phase Composition and Corrosion Characteristics of Calcium Zinc Phosphate Conversion Coatings on Titanium. J. Electrochem. Soc. 2016, 163, C477–C485. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Nadaraia, K.V.; Imshinetskiy, I.M.; Belov, E.A.; Filonina, V.S.; Suchkov, S.N.; Sinebryukhov, S.L.; Gnedenkov, S.V. Composite coatings formed on Ti by PEO and fluoropolymer treatment. Appl. Surf. Sci. 2021, 536, 147976. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, X.; Du, A.; Ma, R.; Zhang, X.; Shi, T.; Fan, Y.; Zhao, X. Investigation on adhesion strength and corrosion resistance of Ti-Zr aminotrimethylene phosphonic acid composite conversion coating on 7A52 aluminum alloy. Appl. Surf. Sci. 2018, 458, 350–359. [Google Scholar] [CrossRef]
- Liu, B.; Yu, W.-L.; Xiao, G.-Y.; Chen, C.-Z.; Lu, Y.-P. Comparative investigation of hydroxyapatite coatings formed on titanium via phosphate chemical conversion. Surf. Coat. Technol. 2021, 413, 127093. [Google Scholar] [CrossRef]
- Ahmadi, S.; Mohammadi, I.; Sadrnezhaad, S.K. Hydroxyapatite based and anodic Titania nanotube biocomposite coatings: Fabrication, characterization and electrochemical behavior. Surf. Coat. Technol. 2016, 287, 67–75. [Google Scholar] [CrossRef]
- Tamar, Y.; Mandler, D. Corrosion inhibition of magnesium by combined zirconia silica sol-gel films. Electrochim. Acta 2008, 53, 5118–5127. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Elkhooly, T.A.; Liu, Y.; Wu, H.; Feng, Q.; Liu, L.; Fang, Y.; Zhu, W.; Hu, T. Effects of titanium surface roughness on the mediation of osteogenesis via modulating the immune response of macrophages. Biomed. Mater. 2018, 13, 045013. [Google Scholar] [CrossRef]
- Zhao, D.-W.; Liu, C.; Zuo, K.-Q.; Su, P.; Li, L.-B.; Xiao, G.-Y.; Cheng, L. Strontium-zinc phosphate chemical conversion coating improves the osseointegration of titanium implants by regulating macrophage polarization. Chem. Eng. J. 2021, 408, 127362. [Google Scholar] [CrossRef]
- Liu, B.; Xiao, G.-Y.; Jiang, C.-C.; Zheng, Y.-Z.; Wang, L.-L.; Lu, Y.-P. Formation initiation and structural changes of phosphate conversion coating on titanium induced by galvanic coupling and Fe2+ ions. RSC Adv. 2016, 6, 75365–75375. [Google Scholar] [CrossRef]
- Ruan, Q.; Zhu, Y.; Zeng, Y.; Qian, H.; Xiao, J.; Xu, F.; Zhang, L.; Zhao, D. Ultrasonic-Irradiation-Assisted Oriented Assembly of Ordered Monetite Nanosheets Stacking. J. Phys. Chem. B 2009, 113, 1100–1106. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Kutbuddin, Y.; Borse, R.N.; Selokar, N.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Ultrasound assisted synthesis of calcium zinc phosphate pigment and its application in nanocontainer for active anticorrosion coatings. Chem. Eng. J. 2013, 231, 345–354. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-B.; Lu, Y.-P.; Du, C.-M.; Zuo, K.-Q.; Wang, Y.-Y.; Tang, K.-L.; Xiao, G.-Y. Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium. Molecules 2023, 28, 4495. https://doi.org/10.3390/molecules28114495
Li Y-B, Lu Y-P, Du C-M, Zuo K-Q, Wang Y-Y, Tang K-L, Xiao G-Y. Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium. Molecules. 2023; 28(11):4495. https://doi.org/10.3390/molecules28114495
Chicago/Turabian StyleLi, Yi-Bo, Yu-Peng Lu, Chun-Miao Du, Kang-Qing Zuo, Yu-Ying Wang, Kang-Le Tang, and Gui-Yong Xiao. 2023. "Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium" Molecules 28, no. 11: 4495. https://doi.org/10.3390/molecules28114495
APA StyleLi, Y.-B., Lu, Y.-P., Du, C.-M., Zuo, K.-Q., Wang, Y.-Y., Tang, K.-L., & Xiao, G.-Y. (2023). Effect of Reaction Temperature on the Microstructure and Properties of Magnesium Phosphate Chemical Conversion Coatings on Titanium. Molecules, 28(11), 4495. https://doi.org/10.3390/molecules28114495






