Increasing Higher Alcohols and Acetates in Low-Alcohol Beer by Proteases
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Experiments and Mashing with Proteases
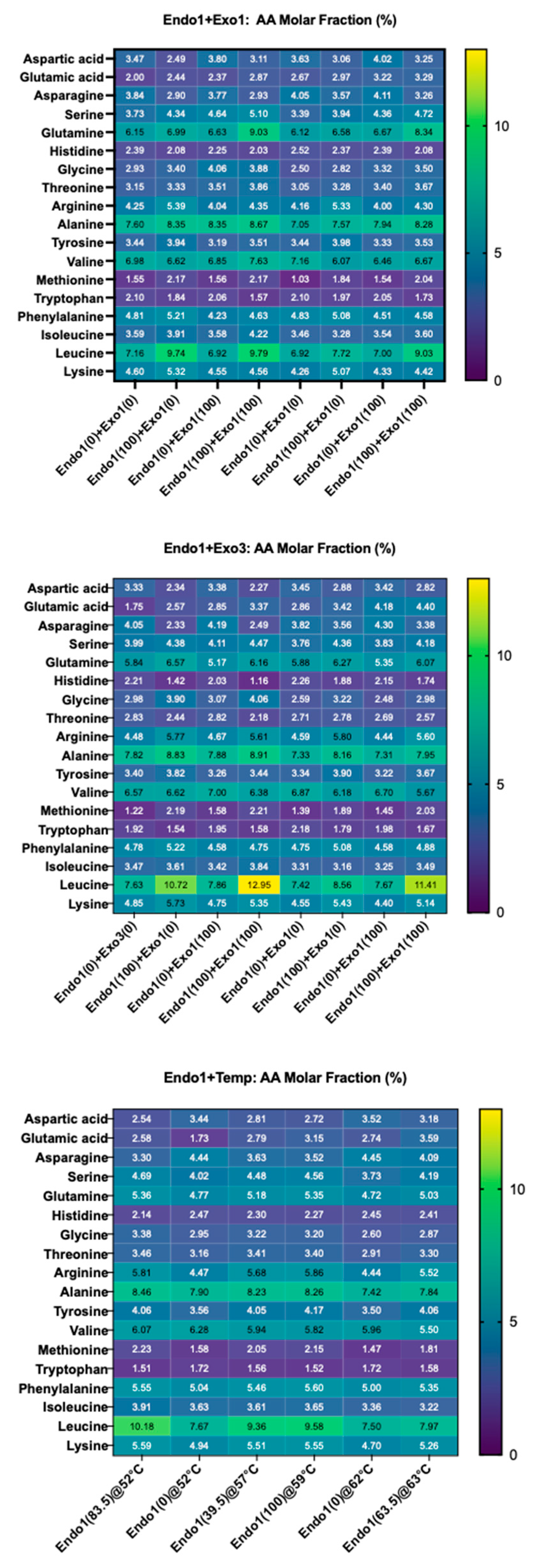
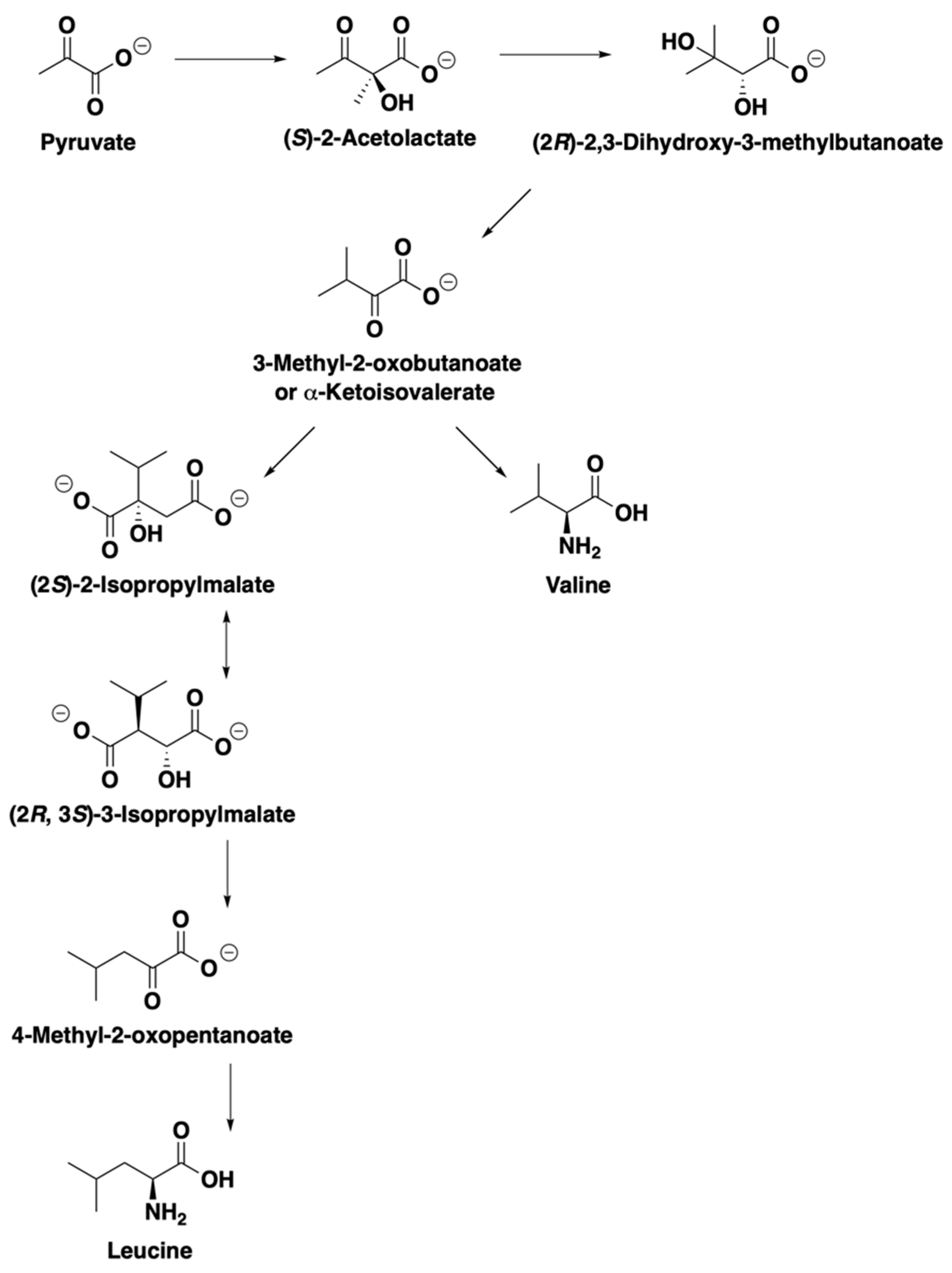
2.1.1. Design of Experiments to Maximize the Leucine Molar Fraction in Wort
Effect Screening of Endoprotease, Exoprotease, and Temperature
Response Surface Model of Endoprotease Endo1 and Temperature
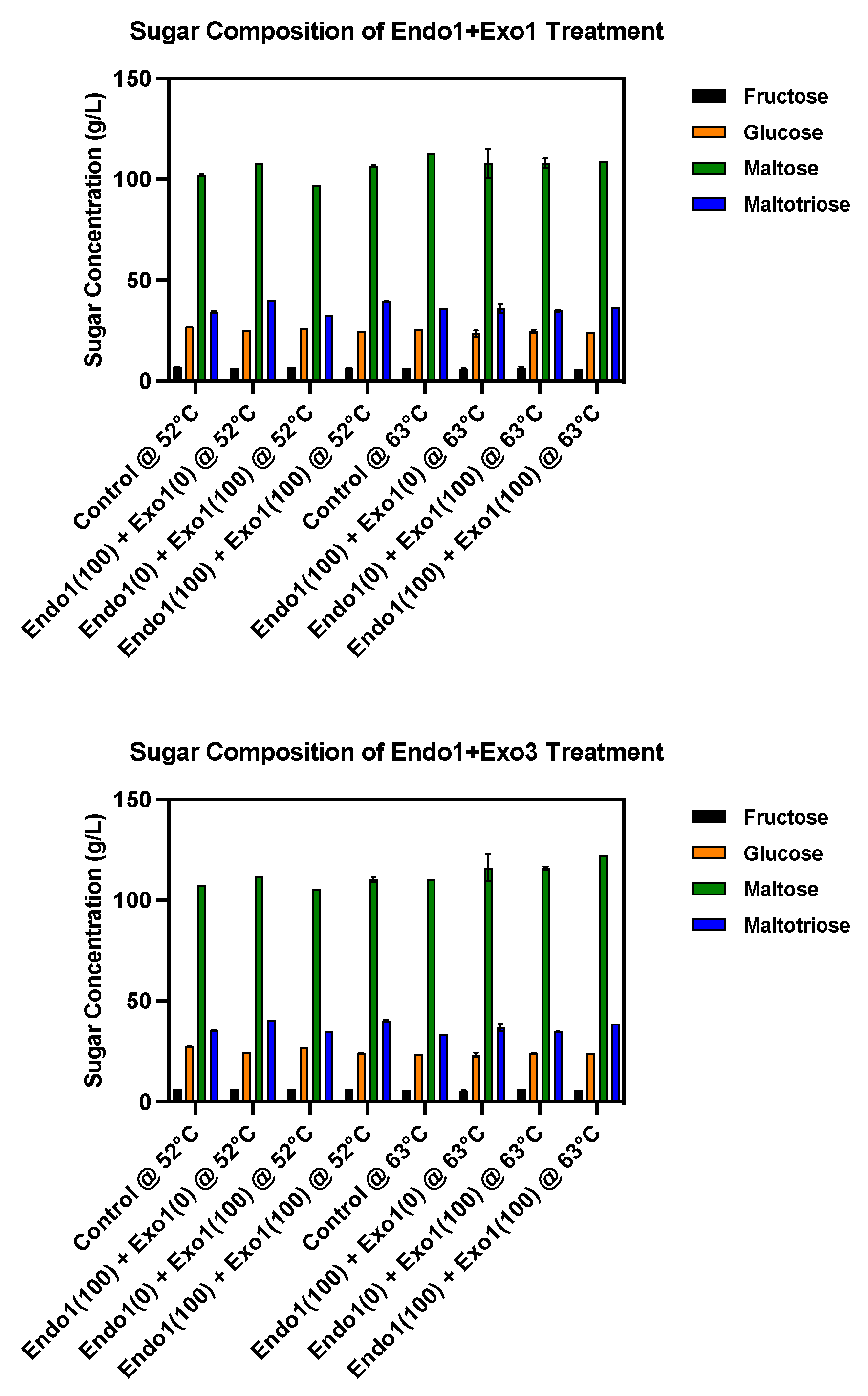
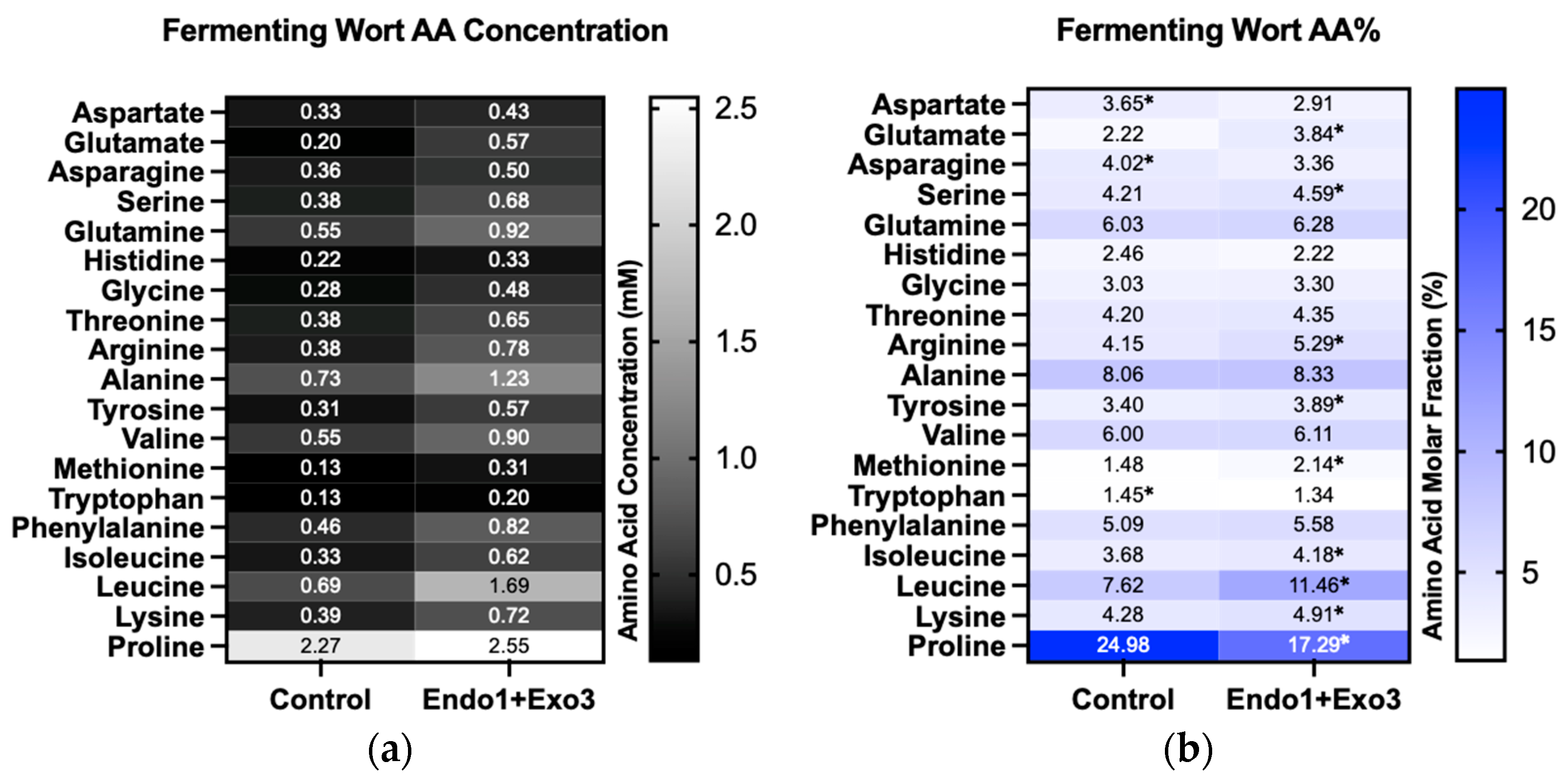
2.1.2. Fermenting Wort Production and Amino Acid Molar Fraction
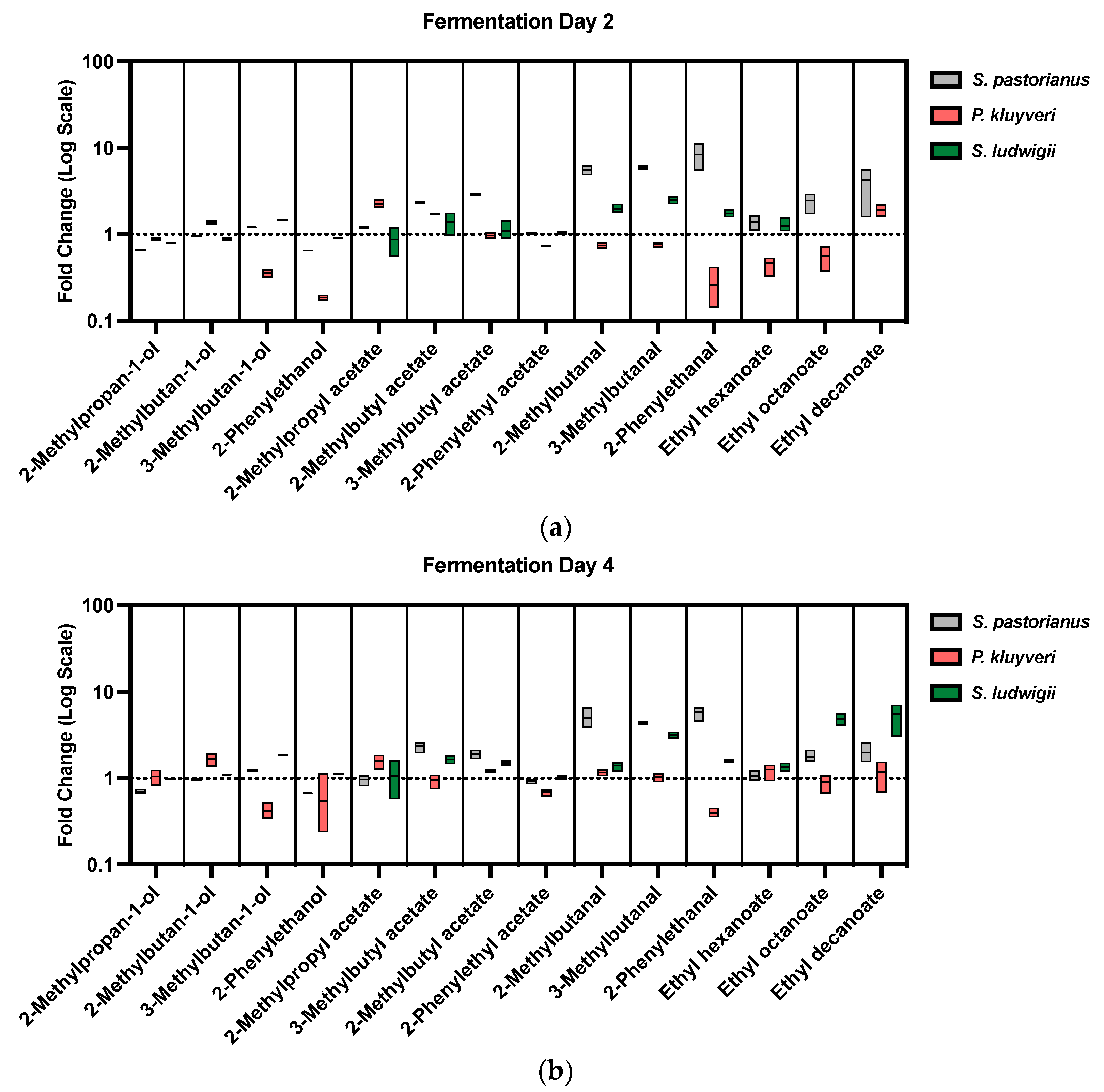
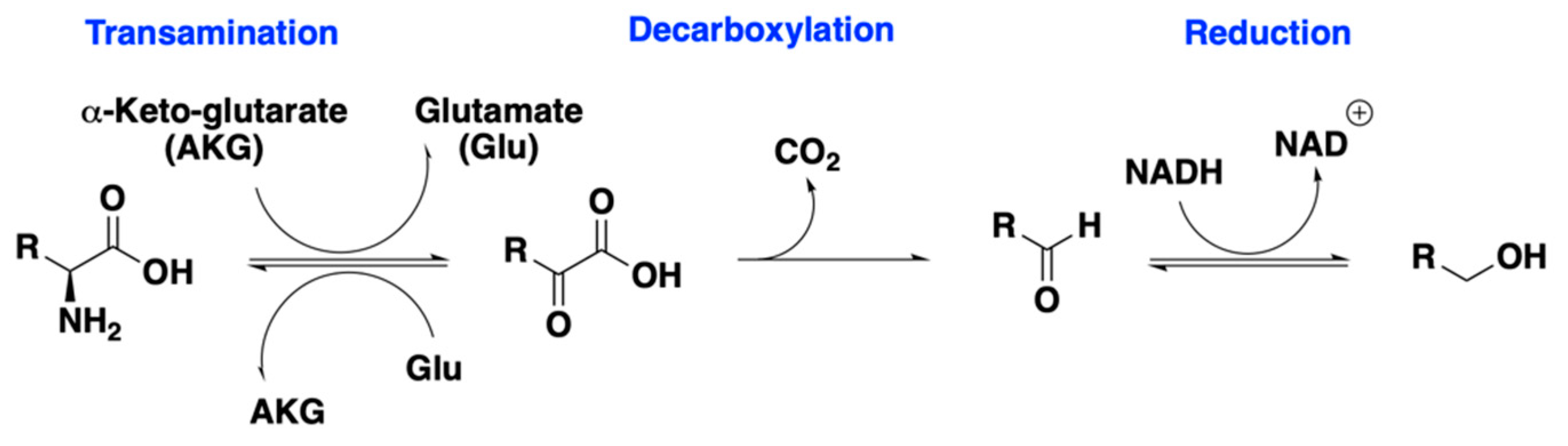
2.2. Fermentation and Aroma Output
3. Materials and Methods
3.1. Mashing and Wort Production
3.2. Fermentation and Volatile Measurement
3.3. Design of Experiments and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

| Yeast | Control | Endo1 + Exo3 | ||
|---|---|---|---|---|
| Attenuation 1 | ABV% 2 | Attenuation | ABV% | |
| S. pastorianus | 60.9% | 2.59 ± 0.05 | 61.2% | 2.57 ± 0.02 |
| P. kluyveri | 8.2% | 0.426 ± 0.002 | 7.6% | 0.386 ± 0.002 |
| S. ludwigii | 8.3% | 0.395 ± 0.002 | 7.7% | 0.360 ± 0.004 |
| Compounds | Saccharomyces pastorianus | Pichia kluyveri | Saccharomycodes ludwigii | ||||
|---|---|---|---|---|---|---|---|
| Ctrl | Protease | Ctrl | Protease | Ctrl | Protease | ||
| Higher alcohol * | 3-methylbutan-1-ol | 27.18 ± 2.40 b | 33.48 ± 1.02 a | 10.71 ± 0.70 c | 4.47 ± 1.03 d | 14.18 ± 0.89 c | 26.47 ± 0.69 b |
| 2-methylbutan-1-ol | 7.58 ± 0.79 a | 7.39 ± 0.31 a | 2.56 ± 0.12 c | 4.27 ± 0.76 b | 1.39 ± 0.11 c | 1.52 ± 0.03 c | |
| 2-methylpropan-1-ol | 2.33 ± 0.14 a | 1.61 ± 0.12 b | 1.44 ± 0.08 b | 1.51 ± 0.32 b | 0.39 ± 0.03 c | 0.39 ± 0.01 c | |
| 2-phenylethanol | 36.61 ± 4.52 b | 24.62 ± 0.37 c | 13.61 ± 1.50 d | 4.02 ± 1.13 e | 40.94 ± 2.22 ab | 46.14 ± 0.96 a | |
| Higher acetate * | 3-methylbutyl acetate | 5.92 ± 0.65 b | 13.84 ± 1.96 b | 131.37 ± 16.81 a | 125.11 ± 23.32 a | 0.33 ± 0.05 b | 0.55 ± 0.06 b |
| 2-methylbutyl acetate | 3.34 ± 0.31 d | 6.40 ± 0.80 c | 28.07 ± 1.06 b | 34.93 ± 1.90 a | 0.21 ± 0.02 e | 0.31 ± 0.02 e | |
| 2-methylpropyl acetate | 0.51 ± 0.04 c | 0.49 ± 0.07 c | 0.88 ± 0.13 b | 1.39 ± 0.27 a | 0.02 ±0.01 d | 0.02 ± 0.01 d | |
| 2-phenylethyl acetate | 18.07 ± 2.48 c | 16.80 ± 1.12 c | 206.19 ± 18.52 a | 143.53 ± 14.92 b | 21.04 ± 1.40 c | 21.90 ± 1.37 c | |
| Medium-chain fatty acid ethyl esters | ethyl hexanoate | 6.90 ± 0.60 a | 7.30 ± 1.11 a | 0.34 ± 0.15 b | 0.43 ± 0.10 b | 0.12 ± 0.02 b | 0.16 ± 0.02 b |
| ethyl octanoate | 44.37 ± 10.82 b | 78.21 ± 14.92 a | 0.38 ± 0.18 c | 0.34 ± 0.08 c | 0.02 ± 0.04 c | 0.08 ± 0.07 c | |
| ethyl decanoate | 18.51 ± 1.97 b | 36.84 ± 9.80 a | 0.44 ± 0.06 c | 0.52 ± 0.20 c | 0.02 ± 0.04 c | 0.13 ± 0.05 c | |
| Other esters | ethyl acetate | 5.66 ± 0.40 b | 7.93 ± 1.08 ab | 14.07 ± 5.09 a | 13.61 ± 4.13 a | 1.05 ± 0.04 b | 0.98 ± 0.04 b |
| 3-methylpropanoate | 0.028 ± 0.02 c | 0.03 ± 0.01 c | 2.2 ± 0.5 a | 1.0 ± 0.2 b | 0.0172 ± 0.0006 c | 0.019 ± 0.001 c | |
| Aldehyde off-flavor | 3-methylbutanal | 0.19 ± 0.06 b | 0.95 ± 0.27 a | 0.023 ± 0.004 b | 0.027 ± 0.002 b | 0.028 ± 0.002 b | 0.04 ± 0.01 b |
| 2-methylbutanal | 0.27 ± 0.09 b | 1.18 ± 0.05 a | 0.03 ± 0.01 c | 0.031 ± 0.04 c | 0.027 ± 0.002 c | 0.09 ± 0.01 c | |
| 2-phenylethanal | 0.04 ± 0.01 b | 0.24 ± 0.04 a | 0.04 ± 0.02 b | 0.018 ± 0.003 b | 0.04 ± 0.01 b | 0.065 ± 0.003 b | |
| Alcohol by volume (ABV%) | - | 2.59 ± 0.05 | 2.57 ± 0.02 | 0.426 ± 0.02 | 0.386 ± 0.002 | 0.395 ± 0.002 | 0.360 ± 0.004 |
References
- Bellut, K.; Arendt, E.K. Chance and Challenge: Non-Saccharomyces Yeasts in Nonalcoholic and Low Alcohol Beer Brewing-A Review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Branyik, T.; Silva, D.P.; Baszczynski, M.; Lehnert, R.; Silva, J. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Muller, M.; Bellut, K.; Tippmann, J.; Becker, T. Physical Methods for Dealcoholization of Beverage Matrices and their Impact on Quality Attributes. Chembioeng Rev. 2017, 4, 310–326. [Google Scholar] [CrossRef]
- Muller, C.; Neves, L.E.; Gomes, L.; Guimaraes, M.; Ghesti, G. Processes for alcohol-free beer production: A review. Food Sci. Technol. 2020, 40, 273–281. [Google Scholar] [CrossRef]
- Blanco, C.A.; Andres-Iglesias, C.; Montero, O. Low-alcohol Beers: Flavor Compounds, Defects, and Improvement Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Alves, K.M.P.; da Silva, B.J.G.; Scheer, A.D. Beer aroma recovery and dealcoholisation by a two-step pervaporation process. J. Inst. Brew. 2020, 126, 67–76. [Google Scholar] [CrossRef]
- Andres-Iglesias, C.; Blanco, C.A.; Garcia-Serna, J.; Pando, V.; Montero, O. Volatile Compound Profiling in Commercial Lager Regular Beers and Derived Alcohol-Free Beers after Dealcoholization by Vacuum Distillation. Food Anal. Methods 2016, 9, 3230–3241. [Google Scholar] [CrossRef]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; De Schutter, D.P.; Daenen, L.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Application of Non-Saccharomyces Yeasts Isolated from Kombucha in the Production of Alcohol-Free Beer. Fermentation 2018, 4, 19. [Google Scholar] [CrossRef]
- Bellut, K.; Krogerus, K.; Arendt, E.K. Lachancea fermentati Strains Isolated from Kombucha: Fundamental Insights, and Practical Application in Low Alcohol Beer Brewing. Front. Microbiol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Bruner, J.; Fox, G. Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations. Fermentation 2020, 6, 16. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Comitini, F.; Ciani, M. Torulaspora delbrueckii in the brewing process: A new approach to enhance bioflavour and to reduce ethanol content. Food Microbiol. 2016, 56, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 11. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dornberg, T.; Jacob, F.; Methner, F.J.; Wagner, R.S.; Hutzler, M. Review: Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Capece, A.; De Fusco, D.; Pietrafesa, R.; Siesto, G.; Romano, P. Performance of Wild Non-Conventional Yeasts in Fermentation of Wort Based on Different Malt Extracts to Select Novel Starters for Low-Alcohol Beers. Appl. Sci. 2021, 11, 801. [Google Scholar] [CrossRef]
- Andres-Iglesias, C.; Blanco, C.A.; Blanco, J.; Montero, O. Mass spectrometry-based metabolomics approach to determine differential metabolites between regular and non-alcohol beers. Food Chem. 2014, 157, 205–212. [Google Scholar] [CrossRef]
- Bauwens, J.; Van Opstaele, F.; Eggermont, L.; Weiland, F.; Jaskula-Goiris, B.; De Rouck, G.; De Brabanter, J.; Aerts, G.; De Cooman, L. Comprehensive analytical and sensory profiling of non-alcoholic beers and their pale lager beer counterparts. J. Inst. Brew. 2021, 127, 385–405. [Google Scholar] [CrossRef]
- Piddocke, M.P.; Fazio, A.; Vongsangnak, W.; Wong, M.L.; Heldt-Hansen, H.P.; Workman, C.; Nielsen, J.; Olsson, L. Revealing the beneficial effect of protease supplementation to high gravity beer fermentations using “-omics” techniques. Microb. Cell. Fact. 2011, 10, 14. [Google Scholar] [CrossRef]
- Lei, H.J.; Zheng, L.Y.; Wang, C.X.; Zhao, H.F.; Zhao, M.M. Effects of worts treated with proteases on the assimilation of free amino acids and fermentation performance of lager yeast. Int. J. Food Microbiol. 2013, 161, 76–83. [Google Scholar] [CrossRef]
- Procopio, S.; Sprung, P.; Becker, T. Effect of amino acid supply on the transcription of flavour-related genes and aroma compound production during lager yeast fermentation. Lwt-Food Sci. Technol. 2015, 63, 289–297. [Google Scholar] [CrossRef]
- Lin, C.L.; Petersen, M.A.; Mauch, A.; Gottlieb, A. Towards lager beer aroma improvement via selective amino acid release by proteases during mashing. J. Inst. Brew. 2022, 128, 15–21. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.A.; Anderson, N.G. Design of Experiments (DoE) and Process Optimization. A Review of Recent Publications. Org. Process Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Leca, J.M.; Pereira, A.C.; Vieira, A.C.; Reis, M.S.; Marques, J.C. Optimal design of experiments applied to headspace solid phase microextraction for the quantification of vicinal diketones in beer through gas chromatography-mass spectrometric detection. Anal. Chim. Acta 2015, 887, 101–110. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.F.; Granato, D.; Barana, A.C. Development and optimization of a mixed beverage made of whey and water-soluble soybean extract flavored with chocolate using a simplex-centroid design. Food Sci. Technol. 2018, 38, 413–420. [Google Scholar] [CrossRef]
- Pineau, N.; Moser, M.; Rawyler, F.; Lepage, M.; Antille, N.; Rytz, A. Design of experiment with sensory data: A pragmatic data analysis approach. J. Sens. Stud. 2019, 34, 9. [Google Scholar] [CrossRef]
- Plesch, G.; Puzio, P.; Blau, A.; Looser, R.; Wendel, B.; Kamlage, B.; Schmitz, O. Process for the Production of Fine Chemicals. WO2006069610 2006. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2006069610 (accessed on 11 May 2023).
- Boulton, C.; Quain, D. The Biochemistry of Fermentation. In Brewing Yeast and Fermentation; Boulton, C., Quain, D., Eds.; Blackwell Science Ltd.: Hoboken, NJ, USA, 2006; pp. 69–142. [Google Scholar]
- Magasanik, B.; Kaiser, C.A. Nitrogen regulation in Saccharomyces cerevisiae. Gene 2002, 290, 1–18. [Google Scholar] [CrossRef]
- Beltran, G.; Novo, M.; Rozes, N.; Mas, A.; Guillamon, J.M. Nitrogen catabolite repression in Saccharomyces cerevisiae during wine fermentations. FEMS Yeast Res. 2004, 4, 625–632. [Google Scholar] [CrossRef]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The Impact of Single Amino Acids on Growth and Volatile Aroma Production by Saccharomyces cerevisiae Strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef]
- Vicente, J.; Calderon, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Grauslund, M.; Didion, T.; Kiellandbrandt, M.C.; Andersen, H.A. BAP2, a gene encoding a permease for branched-chain amino acids in Saccharomyces cerevisiae. Biochim. Et Biophys. Acta-Mol. Cell Res. 1995, 1269, 275–280. [Google Scholar] [CrossRef]
- Kodama, Y.; Omura, F.; Ashikari, T. Isolation and characterization of a gene specific to lager brewing yeast that encodes a branched-chain amino acid permease. Appl. Environ. Microbiol. 2001, 67, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Omura, F.; Miyajima, K.; Ashikari, T. Control of higher alcohol production by manipulation of the BAP2 gene in brewing yeast. J. Am. Soc. Brew. Chem. 2001, 59, 157–162. [Google Scholar]
- Cherry, J.M.; Hong, E.L.; Amundsen, C.; Balakrishnan, R.; Binkley, G.; Chan, E.T.; Christie, K.R.; Costanzo, M.C.; Dwight, S.S.; Engel, S.R.; et al. Saccharomyces Genome Database: The genomics resource of budding yeast. Nucl. Acids Res. 2012, 40, D700–D705. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaw, G.B. Leucine biosynthesis in fungi: Entering metabolism through the back door. Microbiol. Mol. Biol. Rev. 2003, 67, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kispal, G.; Steiner, H.; Court, D.A.; Rolinski, B.; Lill, R. Mitochondrial and cytosolic branched-chain amino acid transaminases from yeast, homologs of the myc oncogene-regulated Eca39 protein. J. Biol. Chem. 1996, 271, 24458–24464. [Google Scholar] [CrossRef]
- Ljungdahl, P.O.; Daignan-Fornier, B. Regulation of Amino Acid, Nucleotide, and Phosphate Metabolism in Saccharomyces cerevisiae. Genetics 2012, 190, 885–929. [Google Scholar] [CrossRef]
- Jastrzebowska, K.; Gabriel, I. Inhibitors of amino acids biosynthesis as antifungal agents. Amino Acids 2015, 47, 227–249. [Google Scholar] [CrossRef]
- Godard, P.; Urrestarazu, A.; Vissers, S.; Kontos, K.; Bontempi, G.; van Helden, J.; Andre, B. Effect of 21 different nitrogen sources on global gene expression in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 2007, 27, 3065–3086. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma-a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography-mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar] [CrossRef]
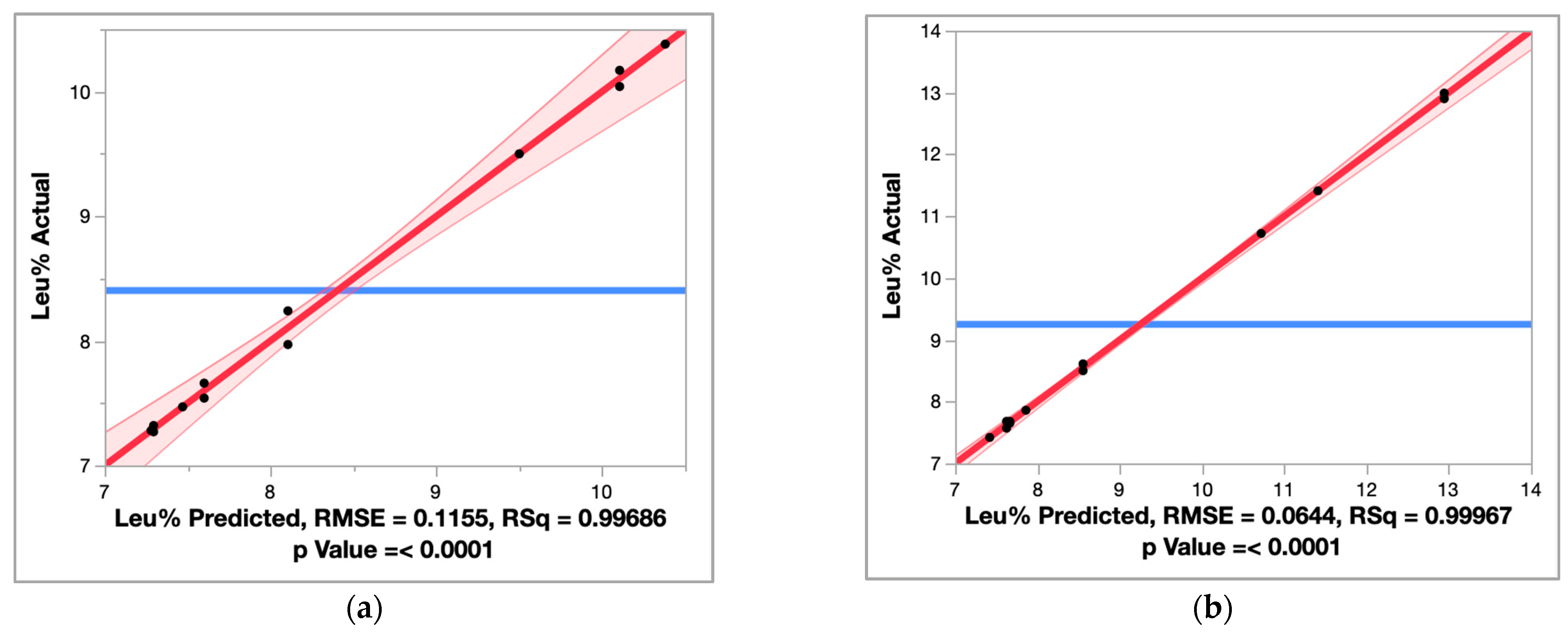


| Run | Endoprotease (mg EP/kg-Grist) | Exoprotease (mg EP/kg-Grist) | Temperature (°C) | Endo1 + Exo1 Leu% | Endo1 + Exo3 Leu% |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 52 | 7.54 | 7.68 |
| 2 | 0 | 0 | 52 | 7.66 | 7.57 |
| 3 | 100 | 0 | 52 | 10.38 | 10.72 |
| 4 | 0 | 100 | 52 | 7.47 | 7.86 |
| 5 | 100 | 100 | 52 | 10.17 | 12.90 |
| 6 | 100 | 100 | 52 | 10.04 | 12.99 |
| 7 | 0 | 0 | 63 | 7.28 | 7.42 |
| 8 | 100 | 0 | 63 | 7.97 | 8.50 |
| 9 | 100 | 0 | 63 | 8.24 | 8.61 |
| 10 | 0 | 100 | 63 | 7.27 | 7.65 |
| 11 | 0 | 100 | 63 | 7.32 | 7.68 |
| 12 | 100 | 100 | 63 | 9.50 | 11.41 |
| Term | Parameter Estimate | p Value | Term | Parameter Estimate | p Value |
|---|---|---|---|---|---|
| Endo1(0, 100) | 1.055625 | 0.00001 | Endo1(0, 100) | 1.6325 | 0.00000 |
| Exo1(0, 100) | 0.125625 | 0.02374 | Exo3(0, 100) | 0.695 | 0.00000 |
| Temp(52, 63) | −0.421875 | 0.00028 | Temp(52, 63) | −0.5125 | 0.00001 |
| Endo1 × Exo1 | 0.154375 | 0.01201 | Endo1 × Exo3 | 0.575 | 0.00001 |
| Endo1 × Temp | −0.298125 | 0.00108 | Endo1 × Temp | −0.4125 | 0.00003 |
| Exo1 × Temp | 0.226875 | 0.00303 | Exo3 × Temp | 0.08 | 0.01540 |
| Endo1 × Exo1 × Temp | 0.190625 | 0.00573 | Endo1 × Exo3 × Temp | 0.0775 | 0.01712 |
| Run | Endo1 (mg EP/kg-Grist) | Temperature (°C) | Leu% | Predicted Leu% |
|---|---|---|---|---|
| 1 | 83.5 | 52 | 10.18 | 10.18 |
| 2 | 0 | 52 | 7.67 | 7.67 |
| 3 | 39.5 | 57 | 9.32 | 9.36 |
| 4 | 39.5 | 57 | 9.39 | 9.36 |
| 5 | 100 | 59 | 9.58 | 9.58 |
| 6 | 100 | 59 | 9.59 | 9.58 |
| 7 | 0 | 62 | 7.50 | 7.50 |
| 8 | 63.5 | 63 | 7.97 | 7.97 |
| Term | Parameter Estimate | p Value |
|---|---|---|
| Endo1(0, 100) | 0.8660797 | 0.0004 |
| Temp(52, 63) | −0.762476 | 0.0006 |
| Temp × Temp | −0.842294 | 0.0012 |
| Endo1 × Temp | −0.515997 | 0.0021 |
| Endo1 × Endo1 | −0.375853 | 0.0056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.L.; Petersen, M.A.; Gottlieb, A. Increasing Higher Alcohols and Acetates in Low-Alcohol Beer by Proteases. Molecules 2023, 28, 4419. https://doi.org/10.3390/molecules28114419
Lin CL, Petersen MA, Gottlieb A. Increasing Higher Alcohols and Acetates in Low-Alcohol Beer by Proteases. Molecules. 2023; 28(11):4419. https://doi.org/10.3390/molecules28114419
Chicago/Turabian StyleLin, Claire Lin, Mikael Agerlin Petersen, and Andrea Gottlieb. 2023. "Increasing Higher Alcohols and Acetates in Low-Alcohol Beer by Proteases" Molecules 28, no. 11: 4419. https://doi.org/10.3390/molecules28114419
APA StyleLin, C. L., Petersen, M. A., & Gottlieb, A. (2023). Increasing Higher Alcohols and Acetates in Low-Alcohol Beer by Proteases. Molecules, 28(11), 4419. https://doi.org/10.3390/molecules28114419







