Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications
Abstract
1. Introduction
2. Huitlacoche (Ustilago maydis)
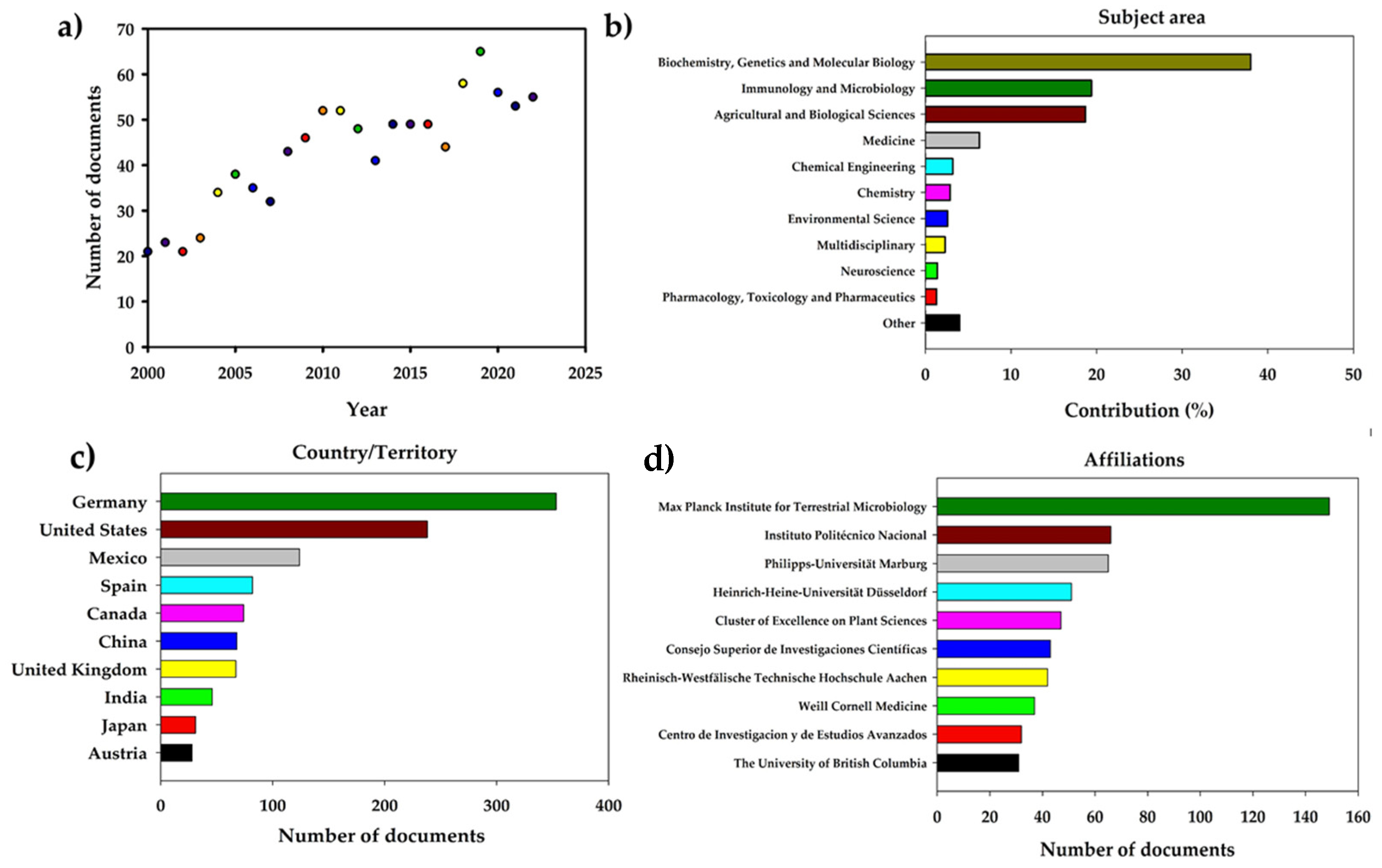
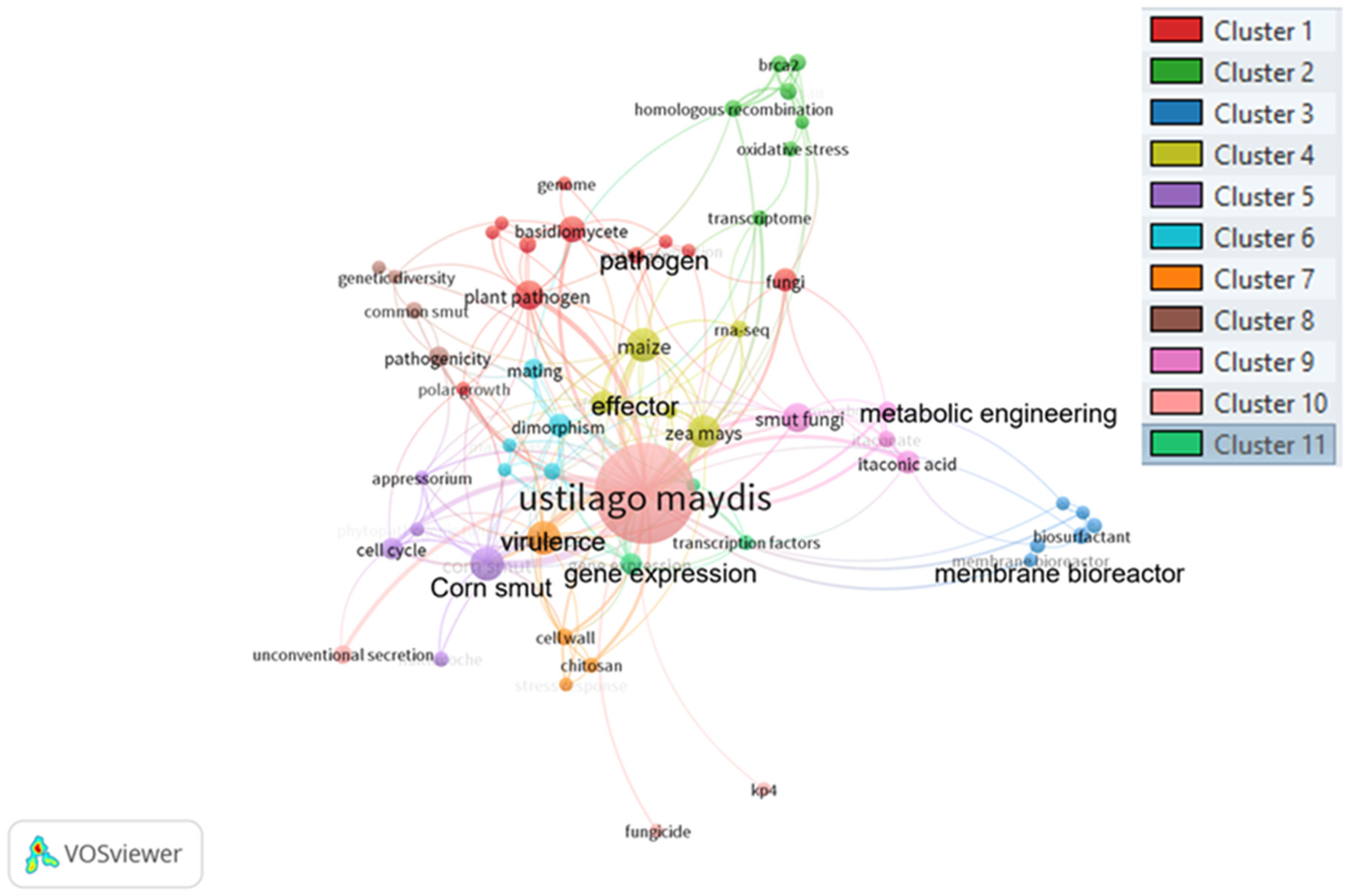
2.1. Literature Search Strategy and Bibliometric Analysis on Ustilago maydis
Huitlacoche (U. maydis) Bibliometric Analysis
3. Biocultural and Gastronomic Importance of Huitlacoche
3.1. Nutrimental Content
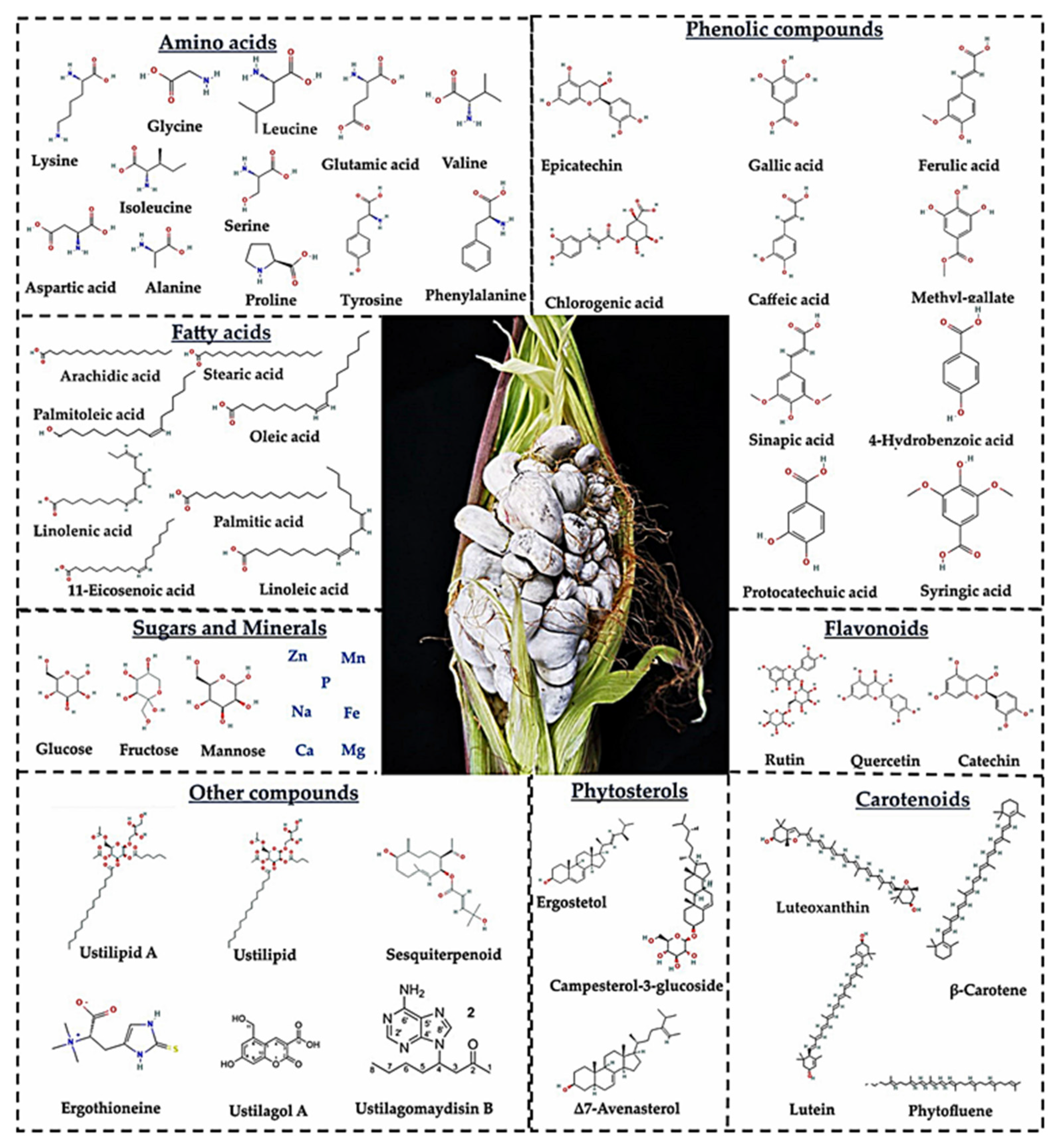
3.2. Mycochemical Compounds of Huitlacoche
3.3. Ustilago maydis as a Biotech Factory
3.4. Potential Technological Applications of Huitlacoche
3.4.1. Antioxidant Capacity
3.4.2. Development of Potential Functional Foods
3.4.3. Biocontrol Agent for Wine Production
3.4.4. Antimicrobial Activity
3.4.5. Miscellaneous Applications
3.4.6. Synthesis of Inorganic Nanoparticles
3.4.7. Bioremediation
3.4.8. Other Investigated Applications
4. Toxicity and Safety Use of Huitlacoche
5. Sustainable Development and Food Security
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pérez-Moreno, J.; Guerin-Laguette, A.; Rinaldi, A.C.; Yu, F.; Verbeken, A.; Hernández-Santiago, F.; Martínez-Reyes, M. Edible mycorrhizal fungi of the world: What is their role in forest sustainability, food security, biocultural conservation and climate change? Plants People Planet 2021, 3, 471–490. [Google Scholar] [CrossRef]
- De Obeso Fernandez Del Valle, A.; Scheckhuber, C.Q. From past to present: Biotechnology in Mexico using algae and fungi. Plants 2021, 10, 2530. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Moreno, J.; Mortimer, P.E.; Xu, J.; Karunarathna, S.C.; Li, H. Global perspectives on the ecological, cultural and socioeconomic relevance of wild edible fungi. Stud. Fungi 2021, 6, 408–424. [Google Scholar] [CrossRef]
- Valverde, M.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef]
- Feldbrügge, M.; Kellner, R.; Schipper, K. The biotechnological use and potential of plant pathogenic smut fungi. Appl. Microbiol. Biotechnol. 2013, 97, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, L.X.; Aguirre-Delgado, A.; Saenz-Hidalgo, H.K.; Buenrostro-Figueroa, J.J.; García, H.S.; Baeza-Jiménez, R. Bioactive ingredients of huitlacoche (Ustilago maydis), a potential food raw material. Food Chem. Mol. Sci. 2022, 4, 100076. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Qi, J.; Han, H.; Wang, P.; Liu, C. Progress in pathogenesis research of Ustilago maydis, and the metabolites involved along with their biosynthesis. Mol. Plant Pathol. 2023, 24, 495–509. [Google Scholar] [CrossRef] [PubMed]
- González-Cervantes, M.E.; Hernández-Uribe, J.P.; Gómez-Aldapa, C.A.; Navarro-Cortez, R.O.; Palma-Rodríguez, H.M.; Vargas-Torres, A. Physicochemical, functional, and quality properties of fettuccine pasta added with huitlacoche mushroom (Ustilago maydis). J. Food Process. Preserv. 2021, 45, e15825. [Google Scholar] [CrossRef]
- Hernández-Carnalla, L.; Olvera-Torres, F.; Teliz-Ramírez, L.E.; Luna-Fernández, V.G.; Palma Salas, M.; Velázquez-Dominguez, A. From pest to nutritious food: Making huitlacoche flour Ustilago maydis. RD-ICUAP 2023, 9, 118–125. [Google Scholar]
- Cruz-Ramírez, L.A.; Valdez-Morales, M.; Chacón-López, M.A.; Rosas-Cárdenas, F.F.; Cruz-Hernández, A. Mexican crops of agroalimentary importance. In Advances in Agricultural and Food Biotechnology; Research Signpost: Trivandrum, India, 2006; pp. 35–53. ISBN 8177362690. [Google Scholar]
- Martinez-Medina, G.A.; Chávez-González, M.L.; Verma, D.K.; Prado-Barragán, L.A.; Martínez-Hernández, J.L.; Flores-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-funcional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. J. Funct. Foods 2021, 77, 104326. [Google Scholar] [CrossRef]
- Guzmán, G. Diversity and use of traditional mexican medicinal fungi. A review. Int. J. Med. Mushrooms 2008, 10, 209–217. [Google Scholar] [CrossRef]
- Wu, H.C.; His, H.Y.; Hsiao, G.; Yen, C.H.; Leu, J.Y.; Wu, C.C.; Chang, S.H.; Huang, S.J.; Lee, T.H. Chemical constituents and bioactive principles from the Mexican truffle and fermented products of the derived fungus Ustilago maydis MZ496986. J. Agric. Food Chem. 2023, 71, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Bautista-González, J.A.; Moreno-Fuentes, A. Los hongos medicinales de México. In La Etnomicología en México; Cromo Edit: Tamaulipas, Mexico, 2015; pp. 14–176. [Google Scholar]
- Aydoğdu, M.; Gölükçü, M. Nutritional value of huitlacoche, maize mushroom caused by Ustilago maydis. Food Sci. Technol. 2017, 37, 531–535. [Google Scholar] [CrossRef]
- Mata, G. Introducción a la etnomicología maya de Yucatán. El conocimiento de los hongos en Pixoy, Valladolid. Rev. Mex. Micol. 1987, 3, 175–187. [Google Scholar]
- Chacón, S. Conocimiento etnoecológico de los hongos en Plan de Palmar, Municipio de Papantla, Veracruz, México. Micol. Neotrop. Appl. 1988, 1, 45–54. [Google Scholar]
- Méndez, R.M.; Ruan-Sotoh, F.; Cano-Contreras, E.J. Conocimiento tradicional de Ustilago maydis en cuatro grupos Mayenses del sureste de México. Etnobiología 2008, 6, 9–23. [Google Scholar]
- Lampman, A.M. General principles of ethnomycological classification among the Tzeltal Maya of Chiapas, Mexico. J. Ethnobiol. 2007, 27, 11–27. [Google Scholar] [CrossRef]
- Shepard, G.H.; Arora, D.; Lampman, A. The grace of the flood: Classification and use of wild mushrooms among the highland Maya of Chiapas. Econ. Bot. 2008, 62, 437–470. [Google Scholar] [CrossRef]
- Ruan-Soto, F.; Cifuentes, J.; Pérez-Ramírez, L.; Ordaz-Velázquez, M.; Caballero, J. Mushrooms of cultural interest in Chiapas Highlands and Lacandon Rainforest, Mexico. Rev. Mex. Biodivers. 2021, 92, e923525. [Google Scholar] [CrossRef]
- Alfaro, H.I.L. Etnomicología y diversidad fúngica tojolabal como aporte a los estudios rurales. In Estudios Rurales en México; CLACSO: Mexico City, Mexico, 2019; pp. 168–196. [Google Scholar]
- Ríos-García, U.; Carrera-Martínez, A.; Martínez-Reyes, M.; Hernández-Santiago, F.; Evangelista, F.R.; Díaz-Aguilar, I.; Olvera-Noriega, J.W.; Pérez-Moreno, J. Traditional knowledge and use of wild mushrooms with biocultural importance in the Mazatec culture in Oaxaca, Mexico, cradle of the ethnomycology. For. Syst. 2023, 32, e007. [Google Scholar] [CrossRef]
- Santiago, F.H.; Moreno, J.P.; Cázares, B.X.; Suárez, J.J.A.; Trejo, E.O.; de Oca, G.M.M.; Aguilar, I.D. Traditional knowledge and use of wild mushrooms by Mixtecs or Ñuu savi, the people of the rain, from Southeastern Mexico. J. Ethnobiol. Ethnomed. 2016, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Aparicio Aparicio, J.C. Taxonomía Mixteca y usos de los hongos en San Miguel el Grande, Oaxaca, México. Etnobiología 2019, 17, 18–30. [Google Scholar]
- López-García, A.; Jiménez-Ruíz, M.; Pérez-Moreno, J. Vocabulary related to the mycological resource in the Chinantec culture from the Northern Mountain Range of the state of Oaxaca, Mexico. Sci. Fungorum 2017, 46, 9–18. [Google Scholar]
- Nellis, N.; Nellis, J.G. Diccionario Zapoteco de Juarez; The Summer Institute of Linguistics, Inc.: Mexico City, Mexico, 1983. [Google Scholar]
- Hunn, E.S. A Zapotec Natural History: Trees, Herbs, and Flowers, Birds, Beasts, and Bugs in the Life of San Juan Gbëë; University of Arizona Press: Tucson, AZ, USA, 2008. [Google Scholar]
- Stubblefield, M.; Stubnlefield, C.M. Diccionario Zapoteco de Mitla; The Summer Institute of Linguistics, Inc.: Mexico City, Mexico, 1991. [Google Scholar]
- Butler, I.M. Diccionario Zapoteco de Yatzachi; The Summer Institute of Linguistics, Inc.: Mexico City, Mexico, 2000; ISBN 9683102891. [Google Scholar]
- Hunn, E.S.; Venegas-Ramírez, Y.; Vásquez-Dávila, M.A. Where do fungi fit? The fungal domain in Mixtepec Zapotec. J. Ethnobiol. 2015, 35, 286–313. [Google Scholar] [CrossRef]
- Estrada-Torres, A.; Aroche, R.M. Acervo etnomicológico entre localidades del municipio de Acambay, Estado de México. Rev. Mex. Micol. 1987, 3, 109–131. [Google Scholar]
- Montoya, A.; Hernandez-Totomoch, O.; Estrada-Torres, A.; Kong, A.; Caballero, J. Traditional knowledge about mushrooms in a Nahua community in the state of Tlaxcala, Mexico. Mycologia 2003, 95, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Valadez Azúa, R. El cuitlacoche, un recurso alimentario mexicano no tan milenario. Antropológicas Boletín 2012, 1, 1–9. [Google Scholar]
- Hernández Vásquez, M.Á. Mythology in the Izalco cosmovisions: A symbolic universe of nahuales and contra nahuales. Rev. Museol. Kóot 2020, 10, 93–115. [Google Scholar] [CrossRef]
- Valadez-Azúa, R.; Moreno-Fuentes, A.; Gómez-Álvarez, G. Cujtlacochi. El Cuitlacoche; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2011; ISBN 9786070221439. [Google Scholar]
- Servín Campuzano, L.S.; Alarcón-Cháires, P.E. Traditional knowledge of wild edible fungi in the p’urhépecha community of Comachuén, Nahuatzen, Michoacán. Acta Univ. 2018, 28, 15–29. [Google Scholar] [CrossRef]
- Martínez-Alfaro, M.A.; Pérez-Silva, E.; Aguirre-Acosta, E. Etnomicología y exploraciones micológicas en la sierra norte de Puebla. Boletín Soc. Botánica México 1983, 18, 51–63. [Google Scholar]
- Villaseñor, I.L.; Cedao-Maldonado, M.; Vargas-Ponce, O. Aprovechamiento y manejo de las plantas, hongos y animales silvestres por los huicholes y nahuas. In La Biodiversidad en Jalisco. Estudio de Estado; CONABIO-SEMADET: Guadalajara, Mexico, 2017; Volumen I, pp. 189–196. [Google Scholar]
- Moreno-Fuentes, Á.; Aguirre-Acosta, E.; Pérez-Ramírez, L. Conocimiento tradicional y científico de los hongos en el estado de Chihuahua, México. Etnobiología 2004, 4, 89–105. [Google Scholar]
- Schumacher, M.; Durán-Díaz, P.; Kurjenoja, A.K.; Gutiérrez-Juárez, E.; González-Rivas, D.A. Evolution and collapse of ejidos in Mexico-To what extent is communal land used for urban development? Land 2019, 8, 146. [Google Scholar] [CrossRef]
- Keleman, A.; Hellin, J.; Flores, D. Diverse varieties and diverse markets: Scale-related maize “Profitability Crossover” in the central mexican highlands. Hum. Ecol. 2013, 41, 683–705. [Google Scholar] [CrossRef]
- Rodríguez-Piña, A.L.; Juárez-Montiel, M.; Hernández-Sánchez, I.E.; Rodríguez-Hernández, A.A.; Bautista, E.; Becerra-Flora, A.; López-Villegas, E.O.; Jiménez-Bremont, J.F. The Ustilago maydis null mutant strains of the RNA-binding protein UmRrm75 accumulate hydrogen peroxide and melanin. Sci. Rep. 2019, 9, 10813. [Google Scholar] [CrossRef] [PubMed]
- Geiser, E.; Wiebach, V.; Wierckx, N.; Blank, L.M. Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol. Biotechnol. 2014, 1, 2. [Google Scholar] [CrossRef]
- Juárez, O.; Guerra, G.; Martínez, F.; Pardo, J.P. The mitochondrial respiratory chain of Ustilago maydis. Biochim. Biophys. Acta-Bioenergy 2004, 1658, 244–251. [Google Scholar] [CrossRef]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- Ho, E.C.H.; Cahill, M.J.; Saville, B.J. Gene discovery and transcript analyses in the corn smut pathogen Ustilago maydis: Expressed sequence tag and genome sequence comparison. BMC Genom. 2007, 8, 334. [Google Scholar] [CrossRef]
- Amador-Rodríguez, K.Y.; Pérez-Cabrera, L.E.; Guevara-Lara, F.; Chávez-Vela, N.A.; Posadas-Del Río, F.A.; Silos-Espino, H.; Martínez-Bustos, F. Physicochemical, thermal, and rheological properties of nixtamalized blue-corn flours and masas added with huitlacoche (Ustilago maydis) paste. Food Chem. 2019, 278, 601–608. [Google Scholar] [CrossRef]
- Merkevičiūte-Venslovė, L.; Venslovas, E.; Mankevičienė, A.; Šlepetienė, A.; Cesevičienė, J. Effect of Ustilago maydis on the nutritive value and aerobic deterioration of maize silage. Agronomy 2022, 13, 111. [Google Scholar] [CrossRef]
- Becker, J.; Tehrani, H.H.; Ernst, P.; Blank, L.M.; Wierckx, N. An optimized Ustilago maydis for itaconic acid production at maximal theoretical yield. J. Fungi 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Ferris, A.C.; Walbot, V. Understanding Ustilago maydis infection of multiple maize organs. J. Fungi 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Parra, J.A.; Pérez España, V.H.; Zavala-González, E.A.; Peralta-Gil, M.; Aparicio Burgos, J.E.; Romero-Cortes, T. Trichoderma Asperellum strains as potential biological control agents against Fusarium verticillioides and Ustilago maydis in maize. Biocontrol Sci. Technol. 2022, 32, 624–647. [Google Scholar] [CrossRef]
- Rehman, F.; Adnan, M.; Kalsoom, M.; Naz, N.; Husnain, M.G.; Ilahi, H.; Ilyas, M.A.; Yousaf, G.; Tahir, R.; Ahmad, U. Seed-borne fungal diseases of maize (Zea mays L.): A review. Agrinula J. Agroteknologi Perkeb. 2021, 4, 43–60. [Google Scholar] [CrossRef]
- Liebal, U.W.; Ullmann, L.; Lieven, C.; Kohl, P.; Wibberg, D.; Zambanini, T.; Blank, L.M. Ustilago maydis metabolic characterization and growth quantification with a genome-scale metabolic model. J. Fungi 2022, 8, 524. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Montiel, M.; Ruiloba de León, S.; Chávez-Camarillo, G.; Hernández-Rodríguez, C.; Villa-Tanaca, L. Huitlacoche (corn smut), caused by the phytopathogenic fungus Ustilago maydis, as a functional food. Rev. Iberoam. Micol. 2011, 28, 69–73. [Google Scholar] [CrossRef]
- Sánchez-Vega, M.; Méndez-López, A.; Salazar-Torres, J.C.; Leal-Robles, A.I.; Martínez-Amador, S.; Pérez-Pérez, J.E. Diversity of insect pests damaging quality of “Huitlacoche” (Corn Smut) at Saltillo, Coahuila, Mexico. Southwest. Entomol. 2023, 44, 627–636. [Google Scholar] [CrossRef]
- Pataky, J.K. Production of cuitlacoche [Ustilago maydis (DS) Corda] on sweet corn. HortScience 2019, 26, 1374–1377. [Google Scholar] [CrossRef]
- Castañeda de León, V.; Martínez-Carrera, D.; Morales, P.; Sobal, M.; Gil-Muñoz, A.; Severiano-Pérez, P.; Leal-Lara, H. Productivity and flavor of diverse genotypes of Ustilago maydis “cuitlacoche” for human consumption. Fungal Biol. 2019, 123, 481–488. [Google Scholar] [CrossRef]
- Aguayo-González, D.J.; Guevara-Lara, F.; Luna-Ruiz, J.D.J.; Pérez-Cabrera, L.E.; García-Munguía, C.A.; García-Munguía, A.M. Pathogenicity of Ustilago maydis strains for production under controlled conditions. Remexca 2021, 12, 513–524. [Google Scholar] [CrossRef]
- Pataky, J.K.; Chandler, M.A. Production of huitlacoche, Ustilago maydis: Timing inoculation and controlling pollination. Mycologia 2003, 95, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Torres-Salcido, G.; Meiners-Mandujano, R.; Morales-Córdova, D.A.; Marina-Carral, V.; Alonso-Torres, G. Family agriculture and localized agrifood system: Local policies for the production of cuitlacoche (Ustilago maydis sp.). Agric. Soc. Desarro. 2015, 12, 199–218. [Google Scholar] [CrossRef]
- Tracy, W.F.; Vargas, C.; Zepeda, L.; Pataky, J.K.; Chandler, M.A. Production and marketing of Huitlacoche. In Issues in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2007; pp. 233–236. [Google Scholar]
- Valdez-Morales, M.; Céspedes-Carlos, L.; Valverde, M.E.; Ramírez-Chávez, E.; Paredes-López, O. Phenolic compounds, antioxidant activity and lipid profile of huitlacoche mushroom (Ustilago maydis) produced in several maize genotypes at different stages of development. Plant Foods Hum. Nutr. 2016, 71, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Salazar-López, J.M.; Martínez-Saldaña, M.C.; Reynoso-Camacho, R.; Chávez-Morales, R.M.; Sandoval-Cardozo, M.L.; Guevara-Lara, F. Antioxidant capacity and phytochemical characterization of ethanolic extracts from raw and cooked huitlacoche (Ustilago maydis-Zea mays). Rev. Mex. Cienc. Farm. 2017, 48, 37–47. [Google Scholar]
- Rosalba Beas, F.; Guadalupe Loarca, P.; Salvador Horacio Guzmán, M.; Rodriguez, M.G.; Nora Lilia Vasco, M.; Fidel Guevara, L. Nutraceutic potential of bioactive components present in huitlacoche from the central zone of Mexico. Rev. Mex. Cienc. Farm. 2011, 42, 36–44. [Google Scholar]
- Pimentel-González, D.J.; Rodríguez-Huezo, M.E.; Campos-Montiel, R.G.; Trapala-Islas, A.; Hernández-Fuentes, A.D. Influence of corn variety on physicochemical characteristics of Huitlacoche (Ustilago maydis). Rev. Mex. Ing. Quim. 2011, 10, 171–178. [Google Scholar]
- Galicia-García, P.R.; Silva-Rojas, H.V.; Mendoza-Onofre, L.E.; Zavaleta-Mancera, H.A.; Córdova-Téllez, L.; Espinosa-Calderón, A. Selection of aggressive pathogenic and solopathogenic strains of Ustilago maydis to improve Huitlacoche production. Acta Bot. Bras. 2016, 30, 683–692. [Google Scholar] [CrossRef]
- González-Cervantes, M.E. Caracterización fisicoquímica y funcional de una pasta elaborada con sémola de trigo y harina de hongo huitlacoche (Ustilago maydis). Investig. Desarro Cienc. Technol. Aliment. 2022, 7, 172–178. [Google Scholar]
- Valdez-Morales, M.; Barry, K.; Fahey, G.C.; Domínguez, J.; de Mejia, E.G.; Valverde, M.E.; Paredes-López, O. Effect of maize genotype, developmental stage, and cooking process on the nutraceutical potential of huitlacoche (Ustilago maydis). Food Chem. 2010, 119, 689–697. [Google Scholar] [CrossRef]
- Amador-Rodríguez, K.Y.; Martínez-Bustos, F.; Pérez-Cabrera, L.E.; Posadas-Del-Río, F.A.; Chávez-Vela, N.A.; Sandoval-Cardoso, M.L.; Guevara-Lara, F. Effect of huitlacoche (Ustilago maydis DC Corda) paste addition on functional, chemical and textural properties of tortilla chips. Food Sci. Technol. 2015, 35, 452–459. [Google Scholar] [CrossRef]
- Haro-Luna, M.X.; Ruan-Soto, F.; Guzmán-Dávalos, L. Traditional knowledge, uses, and perceptions of mushrooms among the Wixaritari and mestizos of Villa Guerrero, Jalisco, Mexico. IMA Fungus 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Molina-Castillo, S.; Espinoza-Ortega, A.; Thomé-Ortiz, H.; Moctezuma-Pérez, S. Gastronomic diversity of wild edible mushrooms in the Mexican cuisine. Int. J. Gastron. Food Sci. 2023, 31, 100652. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H. The relevance of folkloric usage of plant galls as medicines: Finding the scientific rationale. Biomed. Pharmacother. 2018, 97, 240–247. [Google Scholar] [CrossRef]
- Monroy-Gutiérrez, T.; Valle-Guadarrama, S.; Espinosa-Solares, T.; Martínez-Damián, M.T.; Pérez-López, A. Effect of microperforation and temperature on quality of modified atmosphere packaged huitlacoche (Ustilago maydis). CYTA-J. Food 2013, 11, 309–317. [Google Scholar] [CrossRef]
- Munkacsi, A.B.; Stoxen, S.; May, G. Ustilago maydis populations tracked maize through domestication and cultivation in the Americas. Proc. R. Soc. B Biol. Sci. 2008, 275, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Mayett, Y.; Martínez-Carrera, D.; Sánchez, M.; Macías, A.; Mora, S.; Estrada, A. Consumption of edible mushrooms in developing countries: The case of Mexico. In Science and Cultivation of Edible and Medicinal Fungi; International Society for Mushroom Science: Las Vegas, NV, USA, 2004; pp. 687–696. [Google Scholar]
- Reyes-López, R.C.; Montoya, A.; Kong, A.; Cruz-Campuzano, E.A.; Caballero-Nieto, J. Folk classification of wild mushrooms from San Isidro Buensuceso, Tlaxcala, Central Mexico. J. Ethnobiol. Ethnomed. 2020, 16, 53. [Google Scholar] [CrossRef]
- Guzmán, G. Hallucinogenic, medicinal, and edible mushrooms in Mexico and Guatemala: Traditions, myths, and knowledge. Int. J. Med. Mushrooms 2001, 3, 10. [Google Scholar] [CrossRef]
- Haro-Luna, M.X.; Ruan-Soto, F.; Blancas, J.; Guzmán-Dávalos, L. The cultural role played by the ethnomycological knowledge of wild mushrooms for the peoples of highlands and lowlands in Tlaltenango, Zacatecas, Mexico. Mycologia 2022, 114, 645–660. [Google Scholar] [CrossRef]
- Guzmán, G. Fungi in the Maya culture: Past, present and future. In The Lowland Maya Area; Food Products Press: Nueva York, NY, USA, 2003; pp. 315–325. [Google Scholar]
- Salazar-Torres, J.C.; Mendez-López, A.; Álvarez-Hernadez, R.; Sánchez-Vega, M. El Huitlacoche. Alimento Prehispánico Vigente en México. Historia, Aprovechamiento y Técnicas de Producción; Universidad Autónoma Chapingo-Universidad Autónoma Agraria Antonio Narro: Saltillo, Mexico, 2015; Volume 3, ISBN 9786079831691. [Google Scholar]
- León-Ramírez, C.G.; Sánchez-Arreguín, J.A.; Ruiz-Herrera, J. Ustilago maydis, a delicacy of the aztec cuisine and a model for research. Nat. Resour. 2014, 05, 256–267. [Google Scholar] [CrossRef]
- Patel, S. Nutrition, safety, market status quo appraisal of emerging functional food corn smut (huitlacoche). Trends Food Sci. Technol. 2016, 57, 93–102. [Google Scholar] [CrossRef]
- Dahl, K. Corn soot woman’s timeless lesson: Eat your smut. Etnobiología 2009, 7, 94–99. [Google Scholar]
- Estrada, A.F.; Brefort, T.; Mengel, C.; Díaz-Sánchez, V.; Alder, A.; Al-Babili, S.; Avalos, J. Ustilago maydis accumulates β-carotene at levels determined by a retinal-forming carotenoid oxygenase. Fungal Genet. Biol. 2009, 46, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.; Estrada-Torres, A.; Caballero, J. Comparative ethnomycological survey of three localities from La Malinche Volcano, Mexico. J. Ethnobiol. 2002, 22, 103–131. [Google Scholar]
- Sivieri, K.; de Oliveira, S.M.; de Souza Marquez, A.; Pérez-Jiménez, J.; Diniz, S.N. Insights on β-glucan as a prebiotic coadjuvant in the treatment of diabetes mellitus: A review. Food Hydrocoll. Health 2022, 2, 100056. [Google Scholar] [CrossRef]
- Pérez-Lizaur, A.B.; Palacios-González, B.; Castro-Becerra, A.L.; Flores-Galicia, I. Mexican Equivalent Food Systems, 4th ed.; Instituto Nacional de Salud Pública: Mexico City, Mexico, 2014; ISBN 9786070079283. [Google Scholar]
- Lizárraga-Guerra, R.; López, M.G. Content of free amino acids in Huitlacoche (Ustilago maydis). J. Agric. Food Chem. 1996, 44, 2556–2559. [Google Scholar] [CrossRef]
- Pinotti, L.; Manoni, M.; Ferrari, L.; Tretola, M.; Cazzola, R.; Givensa, I. The contribution of dietary magnesium in farm animals and human nutrition. Nutrients 2021, 13, 509. [Google Scholar] [CrossRef]
- Hejazi, J.; Davoodi, A.; Khosravi, M.; Sedaghat, M.; Abedi, V.; Hosseinverdi, S.; Ehrampoush, E.; Homayounfar, R.; Shojaie, L. Nutrition and osteoporosis prevention and treatment. Biomed. Res. Ther. 2020, 7, 3709–3720. [Google Scholar] [CrossRef]
- United Nations. Global Sustainable Development Report 2023. Department of Economic and Social Affairs. Sustainable Development 2023. Available online: https://sdgs.un.org/gsdr/gsdr2023 (accessed on 13 April 2023).
- Wang, S.Q.; Wang, X.N.; Li, Y.Y.; Di, X.X.; Lou, H.X. Identification of purine-derived compounds, ustilagomaydisin A-C, from the plant pathogen Ustilago maydis and their modulating effects on multidrug-resistant (MDR) tumors. Phytochem. Lett. 2014, 10, 193–197. [Google Scholar] [CrossRef]
- Lee, J.; Hilgers, F.; Loeschke, A.; Jaeger, K.E.; Feldbrügge, M. Ustilago maydis Serves as a novel production host for the synthesis of plant and fungal sesquiterpenoids. Front. Microbiol. 2020, 11, 1655. [Google Scholar] [CrossRef]
- Kurz, M.; Eder, C.; Isert, D.; Li, Z.; Paulus, E.F.; Schiell, M.; Toti, L.; Vértesy, L.; Wink, J.; Seibert, G. Ustilipids, acylated β-D-mannopyranosyl D-erythritols from Ustilago maydis and Geotrichum candidum. J. Antibiot. 2003, 56, 91–101. [Google Scholar] [CrossRef]
- Shaffique, S.; Kang, S.M.; Kim, A.Y.; Imran, M.; Khan, M.A.; Lee, I.J. Current knowledge of medicinal mushrooms related to anti-oxidant properties. Sustainability 2021, 13, 7948. [Google Scholar] [CrossRef]
- Guan, R.; Van Le, Q.; Yang, H.; Zhang, D.; Gu, H.; Yang, Y.; Sonne, C.; Lam, S.S.; Zhong, J.; Jianguang, Z.; et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere 2021, 271, 129499. [Google Scholar] [CrossRef] [PubMed]
- Vezza, T.; Canet, F.; de Marañón, A.M.; Bañuls, C.; Rocha, M.; Víctor, V.M. Phytosterols: Nutritional health players in the management of obesity and its related disorders. Antioxidants 2020, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Trautwein, E.A. Consumer purchase behaviour of foods with added phytosterols in six European countries: Data from a post-launch monitoring survey. Food Chem. Toxicol. 2017, 110, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Yang, W.Q.; Zhou, X.Z.; Shao, F.; Shen, T.; Guan, H.Y.; Zheng, J.; Zhang, L.M. Antibacterial and antifungal sesquiterpenoids: Chemistry, resource, and activity. Biomolecules 2022, 12, 1271. [Google Scholar] [CrossRef] [PubMed]
- Bölker, M.; Basse, C.W.; Schirawski, J. Ustilago maydis secondary metabolism-from genomics to biochemistry. Fungal Genet. Biol. 2008, 45, 88–93. [Google Scholar] [CrossRef]
- Wierckx, N.; Miebach, K.; Ihling, N.; Hussnaetter, K.P.; Büchs, J.; Schipper, K. Perspectives for the application of Ustilaginaceae as biotech cell factories. Essays Biochem. 2021, 65, 365. [Google Scholar] [CrossRef]
- Zuther, K.; Mayser, P.; Hettwer, U.; Wu, W.; Spiteller, P.; Kindler, B.L.J.; Karlovsky, P.; Basse, C.W.; Schirawski, J. The tryptophan aminotransferase Tam1 catalyses the single biosynthetic step for tryptophan-dependent pigment synthesis in Ustilago maydis. Mol. Microbiol. 2008, 68, 152–172. [Google Scholar] [CrossRef]
- Liu, Y.; Koh, C.M.J.; Ji, L. Bioconversion of crude glycerol to glycolipids in Ustilago maydis. Bioresour. Technol. 2011, 102, 3927–3933. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; Araiza-Villanueva, M.G.; Pardo, J.P.; Aranda, E.; Guerra-Sánchez, G. New insights of Ustilago maydis as yeast model for genetic and biotechnological research: A review. Curr. Microbiol. 2019, 76, 917–926. [Google Scholar] [CrossRef]
- Cortes-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr. J. Microbiol. Res. 2011, 5, 2512–2523. [Google Scholar] [CrossRef]
- Hewald, S.; Linne, U.; Scherer, M.; Marahiel, M.A.; Kämper, J.; Bölker, M. Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus Ustilago maydis. Appl. Environ. Microbiol. 2006, 72, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Becker, F.; Stehlik, T.; Linne, U.; Bölker, M.; Freitag, J.; Sandrock, B. Engineering Ustilago maydis for production of tailor-made mannosylerythritol lipids. Metab. Eng. Commun. 2021, 12, e00165. [Google Scholar] [CrossRef]
- Yang, X.L.; Awakawa, T.; Wakimoto, T.; Abe, I. Induced production of the novel glycolipid ustilagic acid C in the plant pathogen Ustilago maydis. Tetrahedron Lett. 2013, 54, 3655–3657. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-derived production of itaconic acid as a building block in specialty polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic acid: A promising and sustainable platform chemical. Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar]
- Rafi, M.M.; Hanumanthu, M.G.; Rao, D.M.; Venkateswarlu, K. Production of itaconic acid by Ustilago maydis from agro wastes in solid state fermentation. J. BioSci. Biotechnol. 2014, 3, 163–168. [Google Scholar]
- Becker, J.; Hosseinpour Tehrani, H.; Gauert, M.; Mampel, J.; Blank, L.M.; Wierckx, N. An Ustilago maydis chassis for itaconic acid production without by-products. Microb. Biotechnol. 2020, 13, 350–362. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Engel, M.; Kleineberg, W.; Büttner, L.; Sarikaya, E.; den Hartog, T.; Klankermayer, J.; Leitner, W.; Bölker, M.; et al. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab. Eng. 2016, 38, 427–435. [Google Scholar] [CrossRef]
- Klement, T.; Milker, S.; Jäger, G.; Grande, P.M.; Domínguez de María, P.; Büchs, J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Fact. 2012, 11, 43. [Google Scholar] [CrossRef]
- Schlembach, I.; Hosseinpour Tehrani, H.; Blank, L.M.; Büchs, J.; Wierckx, N.; Regestein, L.; Rosenbaum, M.A. Consolidated bioprocessing of cellulose to itaconic acid by a co-culture of Trichoderma reesei and Ustilago maydis. Biotechnol. Biofuels 2020, 13, 207. [Google Scholar] [CrossRef] [PubMed]
- Winterberg, B.; Uhlmann, S.; Linne, U.; Lessing, F.; Marahiel, M.A.; Eichhorn, H.; Kahmann, R.; Schirawski, J. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol. Microbiol. 2010, 75, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- De Serrano, L.O. Biotechnology of siderophores in high-impact scientific fields. Biomol. Concepts 2017, 8, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Nieter, A.; Haase-Aschoff, P.; Kelle, S.; Linke, D.; Krings, U.; Popper, L.; Berger, R.G. A chlorogenic acid esterase with a unique substrate specificity from Ustilago maydis. Appl. Environ. Microbiol. 2015, 81, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Brundiek, H.; Sa, S.; Evitt, A.; Kourist, R.; Bornscheuer, U.T. The short form of the recombinant CAL-A-type lipase UM03410 from the smut fungus Ustilago maydis exhibits an inherent trans-fatty acid selectivity. Appl. Microbiol. Biotechnol. 2012, 94, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Nieter, A.; Kelle, S.; Takenberg, M.; Linke, D.; Bunzel, M.; Popper, L.; Berger, R.G. Heterologous production and characterization of a chlorogenic acid esterase from Ustilago maydis with a potential use in baking. Food Chem. 2016, 209, 1–9. [Google Scholar] [CrossRef]
- Santos, A.; Navascués, E.; Bravo, E.; Marquina, D. Ustilago maydis killer toxin as a new tool for the biocontrol of the wine spoilage yeast Brettanomyces bruxellensis. Int. J. Food Microbiol. 2011, 145, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.L.; Parsley, N.C.; Bartges, T.E.; Cooke, M.E.; Evans, W.S.; Heil, L.R.; Smith, T.J.; Hicks, L.M. Fungal Secretome Analysis via PepSAVI-MS: Identification of the bioactive peptide KP4 from Ustilago maydis. J. Am. Soc. Mass Spectrom. 2018, 29, 859–865. [Google Scholar] [CrossRef]
- Juárez-Montiel, M.; Romero-Maldonado, A.; Monreal-Escalante, E.; Becerra-Flora, A.; Korban, S.S.; Rosales-Mendoza, S.; Jiménez-Bremont, J.F. The corn smut (‘Huitlacoche’) as a new platform for oral vaccines. PLoS ONE 2015, 10, e0133535. [Google Scholar] [CrossRef]
- Monreal-Escalante, E.; Navarro-Tovar, G.; León-Gallo, A.; Juárez-Montiel, M.; Becerra-Flora, A.; Jiménez-Bremont, J.F.; Rosales-Mendoza, S. The corn smut-made cholera oral vaccine is thermostable and induces long-lasting immunity in mouse. J. Biotechnol. 2016, 234, 1–6. [Google Scholar] [CrossRef]
- Cortés-Camargo, S.; Jiménez-Rosales, A.; Acuña-Avila, P.E. Green synthesis of Ag NPs Using Ustilago maydis as reducing and atabilizing agent. J. Nanotechnol. 2022, 2022, 2494882. [Google Scholar] [CrossRef]
- Bakur, A.; Niu, Y.; Kuang, H.; Chen, Q. Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. AMB Express 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Gómez, J.; Olguín, M.T. Separation of Cr(VI) from aqueous solutions by adsorption on the microfungus Ustilago maydis. Int. J. Environ. Sci. Technol. 2015, 12, 2559–2566. [Google Scholar] [CrossRef]
- Sargin, I.; Arslan, G.; Kaya, M. Microfungal spores (Ustilago maydis and U. digitariae) immobilised chitosan microcapsules for heavy metal removal. Carbohydr. Polym. 2016, 138, 201–209. [Google Scholar] [CrossRef]
- Zepeda, L. The Huitlacoche project: A tale of smut and gold. Renew. Agric. Food Syst. 2006, 21, 224–226. [Google Scholar] [CrossRef]
- Moore, M.; Russell, W.O.; Sachs, E. Chronic leptomeningitis and ependymitis caused by Ustilago, probably U. Zeae (corn smut): Ustilagomycosis, the second reported instance of human infection. Am. J. Pathol. 1946, 22, 761–777. [Google Scholar]
- Mcghie, T.A.; Huber, T.W.; Kassis, C.E.; Jinadatha, C. Ustilago species as a cause of central line-related blood stream infection. Am. J. Med. Sci. 2013, 345, 254–255. [Google Scholar] [CrossRef]
- McNeil, J.C.; Palazzi, D.L. Ustilago as a cause of fungal peritonitis: Case report and review of the literature. J. Pediatr. Infect. Dis. Soc. 2012, 1, 337–339. [Google Scholar] [CrossRef]
- Weber, R.W.; Levetin, E. Allergen of the month—Ustilago maydis. Ann. Allergy Asthma Immunol. 2013, 111, A13. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food allergy from infancy through adulthood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New perspectives in food allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [PubMed]
- Pepeljnjak, S.; Petrik, J.; Klarić, M.Š. Toxic effects of Ustilago maydis and fumonisin B1 in rats. Acta Pharm. 2005, 55, 339–348. [Google Scholar] [PubMed]
- Abbas, H.K.; Shier, W.T.; Plasencia, J.; Weaver, M.A.; Bellaloui, N.; Kotowicz, J.K.; Butler, A.M.; Accinelli, C.; de la Torre-Hernandez, M.E.; Zablotowicz, R.M. Mycotoxin contamination in corn smut (Ustilago maydis) galls in the field and in the commercial food products. Food Control 2017, 71, 57–63. [Google Scholar] [CrossRef]

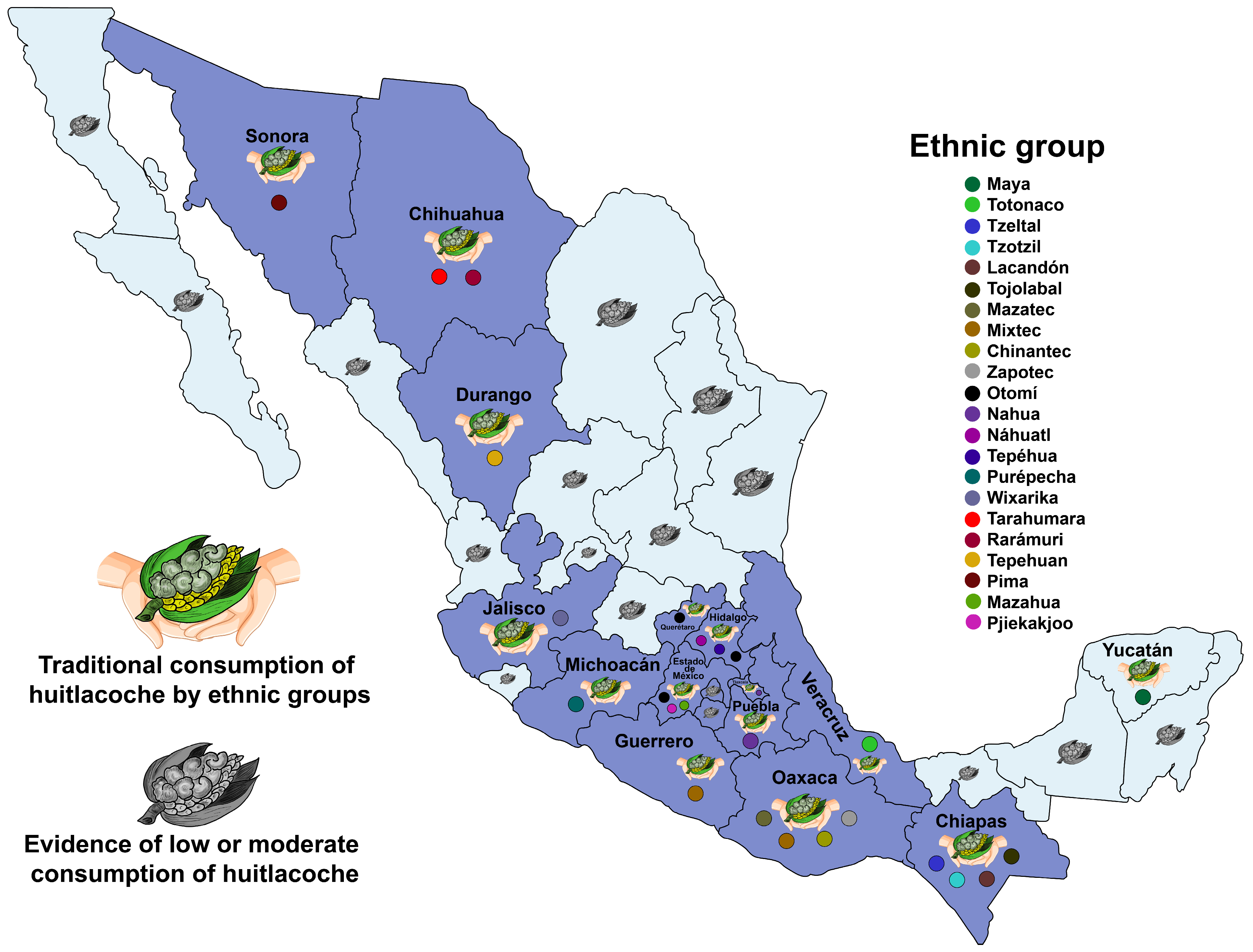




| Ethnic Group | State | Common Name in Traditional Language | English Translation | Ref. |
|---|---|---|---|---|
| Maya | Yucatan | Ta’ chak, ta’ chak ixia | Excrement of the Maya God Chak in the corn | [16] |
| Totonac | Veracruz | Xanat kuxi | Corn flower | [17] |
| Tzeltal | Chiapas | Lu’, sakil ti’bal | Donkey testicles | [18] |
| Tzeltal | Chiapas | Slu ‘il ixim | Corn fungus | [19] |
| Tzotzil | Chiapas | Stok’al ixim, sjo’jal ajan | Corn cloud storm, Corn fungus | [20] |
| Tzotzil | Chiapas | Tok | Cloud | [21] |
| Tzotzil | Chiapas | Xu’ixim, chikin te | Milk from the cornfield, Stick ear | [18] |
| Lacandón | Chiapas | Ta’ urim nar | Corn fungus | [21] |
| Tojolabal | Chiapas | Chikin chu’ | Corn fungus | [22] |
| Mazatec | Oaxaca | Tohíjé | Ball of young corn | [23] |
| Mixtec | Oaxaca | Tɨkámaa | Bad grasshooper | [24] |
| Mixtec | Oaxaca | Tikayá | Round bleached | [25] |
| Mixtec | Guerrero | Xi’i itu’u | Corn fungus | Inedit |
| Chinantec | Oaxaca | Dséc cui | Son or shoot of the cornfield | [26] |
| Zapotec | Oaxaca | Bia’huí’ | Moldy guava | [27] |
| Zapotec | Oaxaca | Mey guiel | Corn fungus | [28] |
| Zapotec | Oaxaca | Xobdam | Owl corn | [29] |
| Zapotec | Oaxaca | Bzodlan | Ear fungus | [30] |
| Zapotec | Oaxaca | Měy-guiêl-do | Corn tassel fungus | [31] |
| Otomí | Estado de México | Kjú tha | Lost ear | [32] |
| Nahua | Tlaxcala | Cuitlacoche | Excrement | [33] |
| Náhuatl | Hidalgo | Kjod kja | Corn fungus | [34] |
| Tepéhua | Hidalgo | Búas | Excrement | [35] |
| Otomí | Hidalgo | Kjo thä, kjo ra mancha | Corn fungus, cornfield fungus, ear fungus | [36] |
| Pjiekakjoo | Estado de México | Nchjo pa | Cornfield fungus | Inedit |
| Purépecha | Michoacan | Terékua poxi | Corn fungus | [37] |
| Nahua | Puebla | Tacatzazamazlat | Excrement fungus | [38] |
| Wixaritari | Jalisco | Ku’u | Corn fungus | [39] |
| Tarahumara | Chihuahua | Weko wiwara | Corn fungus | [36] |
| Rarámuri | Chihuahua | Witáchori | Excrement | [40] |
| Tepehuan | Durango | Jaroi o jurá | Heart | [36] |
| Pima | Sonora | Nanha | Corn smut | [40] |
| Parameter | References | ||||
|---|---|---|---|---|---|
| [6] | [8] | [15] | [69] | [65] | |
| Moisture (g/100 g) | 26.81 | § 8.55 | 90 | 92–96 | 80–86 |
| Ash (g/100 g) | 3.37 | 5.66 | ND | 4–8 | 3.8–5.3 |
| Protein (g/100 g) | 3.27 | 8.08 | 12 | 12–14 | 12.4 |
| Total fat (g/100 g) | 0.73 | 1.14 | 1.8 | 4–6 | 2.9 |
| Carbohydrates (g/100 g) | 57.2 | 64.43 | 45 | 72–86 | 54–65 |
| Total fiber (g/100 g) | * 8.61 | * 12.14 | ND | ¥ 39–60 | ¥ 47–49 |
| Soluble dietary fiber (g/100 g) | ND | ND | ND | 9–29 | ND |
| Insoluble dietary fiber (g/100 g) | ND | ND | ND | 22–51 | ND |
| β-glucans (mg/100 g) | ND | ND | ND | 20–120 | ND |
| Amino Acids | Content (mg/g) | Fatty Acids | Content (%) | Sugars | Content (mg/g) | Minerals | Content (mg/g) |
|---|---|---|---|---|---|---|---|
| Lysine | 3.21 | Oleic acid | 42.49 | Total free sugars | 56–267 | Phosphorous | 0.342 |
| Glycine | 2.44 | Linoleic acid | 26.97 | Glucose | 53–231 | Magnesium | 0.262 |
| Leucine | 2.24 | Palmitic acid | 14.79 | Fructose | 19–138 | Calcium | 0.018 |
| Glutamic acid | 1.90 | 11-Eicosenoic acid | 4.39 | Galactose | 0.2–3.5 | Sodium | 0.012 |
| Aspartic acid | 1.80 | Stearic acid | 3.94 | Arabinose | 0.2–3.3 | Iron | 0.0028 |
| Valine | 1.46 | Arachidic acid | 2.86 | Mannose | 0–1.8 | Zinc | 0.0025 |
| Isoleucine | 1.32 | Palmitoleic acid | 2.10 | Xylose | 0.2 | Manganese | 0.0019 |
| Phenylalanine | 1.16 | Linolenic acid | 0.84 | ||||
| Alanine | 1.05 | Pentadecanoic acid | 0.67 | ||||
| Serine | 1.02 | Margaric acid | 0.51 | ||||
| Tyrosine | 1.00 | Myristic acid | 0.44 | ||||
| Proline | 0.75 | Behenic | * 2.4–5.9 | ||||
| Threonine | 0.62 | Lignoceric | * 1.2–2.7 | ||||
| Methionine | 0.15 | Ergosterol | * 20–97 | ||||
| Ornithine | 0.08 | ||||||
| Tryptophane | 0.05 | ||||||
| References | [89] | [15,63] | [65] | [15] |
| Bioactive Compounds | Content | Ref. |
|---|---|---|
| Phenolic compounds | ||
| Total phenolic compounds (mg GAE/100 g) | 11–1394 | [6,8,15,64,65] |
| Condensed tannins (mg Eq Catechin/100 g) | 32.2–310 | [65] |
| Chlorogenic acid (µg/g) | 15.94 | [64] |
| Methyl-gallate (µg/g) | 4.19 | [64] |
| Epicatechin (µg/g) | 3.16 | [64] |
| Ferulic acid (µg/g) | 358 | [63] |
| Gallic acid (µg/g) | 0.4–1.5 | [63,64] |
| Caffeic acid (µg/g) | 11.2 | [63] |
| Protocatechuic acid (µg/g) | 0.00093 | [64] |
| o-coumaric acid (µg/g) | 5 | [63] |
| p-coumaric acid(µg/g) | 12 | [63] |
| Sinapic acid (µg/g) | 36 | [63] |
| Syringic acid (µg/g) | 0.0158 | [64] |
| 4-Hydroxybenzoic acid (µg/g) | 0.0174 | [64] |
| Flavonoids | ||
| Total flavonoid (mg Catechin/kg) | 28.51 | [15] |
| Anthocyanins (mg/kg cianidin-3-glucoside) | 89.8–226.3 | [65] |
| Rutin (µg/g) | 5 | [63] |
| Catechin (µg/g) | 10–11.42 | [63,64] |
| Quercetin (µg/g) | 33 | [63] |
| Naringenin (µg/g) | 14.1 | [63] |
| Carotenoids (µg/g) | 3.05 | [64] |
| β-Carotene (µg/g) | 15 | [85] |
| β-Cryptoxanthin (µg/g) | 1.13 | [64] |
| Phytofluene (µg/g) | 0.40 | [64] |
| Lutein (µg/g) | 0.31 | [64] |
| Zeaxanthin (µg/g) | 0.31 | [64] |
| Luteoxanthin (µg/g) | 0.63 | [64] |
| Phytosterols | ||
| Ergosterol (µg/g) | 3.24–4.19 | [64] |
| Campesterol-3-β-glucoside (µg/g) | 8.25–12.94 | [64] |
| Δ7-avenasterol (µg/g) | 3.83–5.81 | [64] |
| Δ7-estigmasterol (µg/g) | 4.25–5.92 | [64] |
| Other compounds | ||
| Ustilagol A | Identified | [13] |
| Ustilagol B | Identified | [13] |
| Ustilagol C | Identified | [13] |
| Ustilagol D | Identified | [13] |
| Ustilagol E | Identified | [13] |
| Ustilagol F | Identified | [13] |
| Ustilagomaydisin A | Identified | [93] |
| Ustilagomaydisin B | Identified | [93] |
| Ustilagomaydisin C | Identified | [93] |
| Sesquiterpenoids | Identified | [94] |
| Ustilipid A | NI | [95] |
| Ustilipid B | NI | [95] |
| Ustilipid C | NI | [95] |
| Ergothioneine (µmol/g) | 5.4 | [96] |
| Bioactive Extracts or Compounds | Extraction Method | Method of Antioxidant Capacity | Reference | |||
|---|---|---|---|---|---|---|
| ABTS• | DPPH• | FRAP | ORAC | |||
| Hydroethanolic extract | Maceration | 45.26 a | 13.16 a | ND | ND | [6] |
| Hydroethanolic extract | UAE | 26.45 a | 22.5 a | ND | ND | [6] |
| Methanolic extract | Stirring | ND | 56–74 b | ND | ND | [65] |
| Methanol-Water | Shaking | ND | 186.44 c | ND | ND | [15] |
| Ethanolic extract | Magnetic stirring | 200–312 d | 30–165 d | 117–215 d | ND | [64] |
| Methanol-Water | Shaking | 1652.42 e | 9.50 e | 64.8 e | ND | [8] |
| Methanolic extract | Shaking | ND | ND | ND | 41–76 e | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villagrán, Z.; Martínez-Reyes, M.; Gómez-Rodríguez, H.; Ríos-García, U.; Montalvo-González, E.; Ortiz-Basurto, R.I.; Anaya-Esparza, L.M.; Pérez-Moreno, J. Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications. Molecules 2023, 28, 4415. https://doi.org/10.3390/molecules28114415
Villagrán Z, Martínez-Reyes M, Gómez-Rodríguez H, Ríos-García U, Montalvo-González E, Ortiz-Basurto RI, Anaya-Esparza LM, Pérez-Moreno J. Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications. Molecules. 2023; 28(11):4415. https://doi.org/10.3390/molecules28114415
Chicago/Turabian StyleVillagrán, Zuamí, Magdalena Martínez-Reyes, Horacio Gómez-Rodríguez, Uzziel Ríos-García, Efigenia Montalvo-González, Rosa Isela Ortiz-Basurto, Luis Miguel Anaya-Esparza, and Jesús Pérez-Moreno. 2023. "Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications" Molecules 28, no. 11: 4415. https://doi.org/10.3390/molecules28114415
APA StyleVillagrán, Z., Martínez-Reyes, M., Gómez-Rodríguez, H., Ríos-García, U., Montalvo-González, E., Ortiz-Basurto, R. I., Anaya-Esparza, L. M., & Pérez-Moreno, J. (2023). Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications. Molecules, 28(11), 4415. https://doi.org/10.3390/molecules28114415









