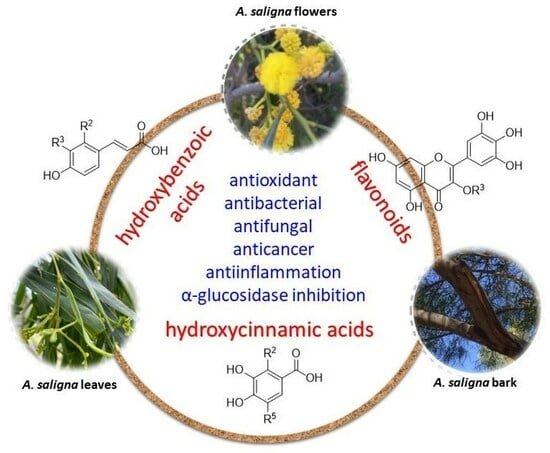
Bioactive Phytochemicals of Acacia saligna
Abstract
1. Introduction
1.1. Acacia Saligna
1.2. Aims
2. Phytochemicals from A. saligna
2.1. Phytochemicals from Flowers
2.2. Phytochemicals from Leaves

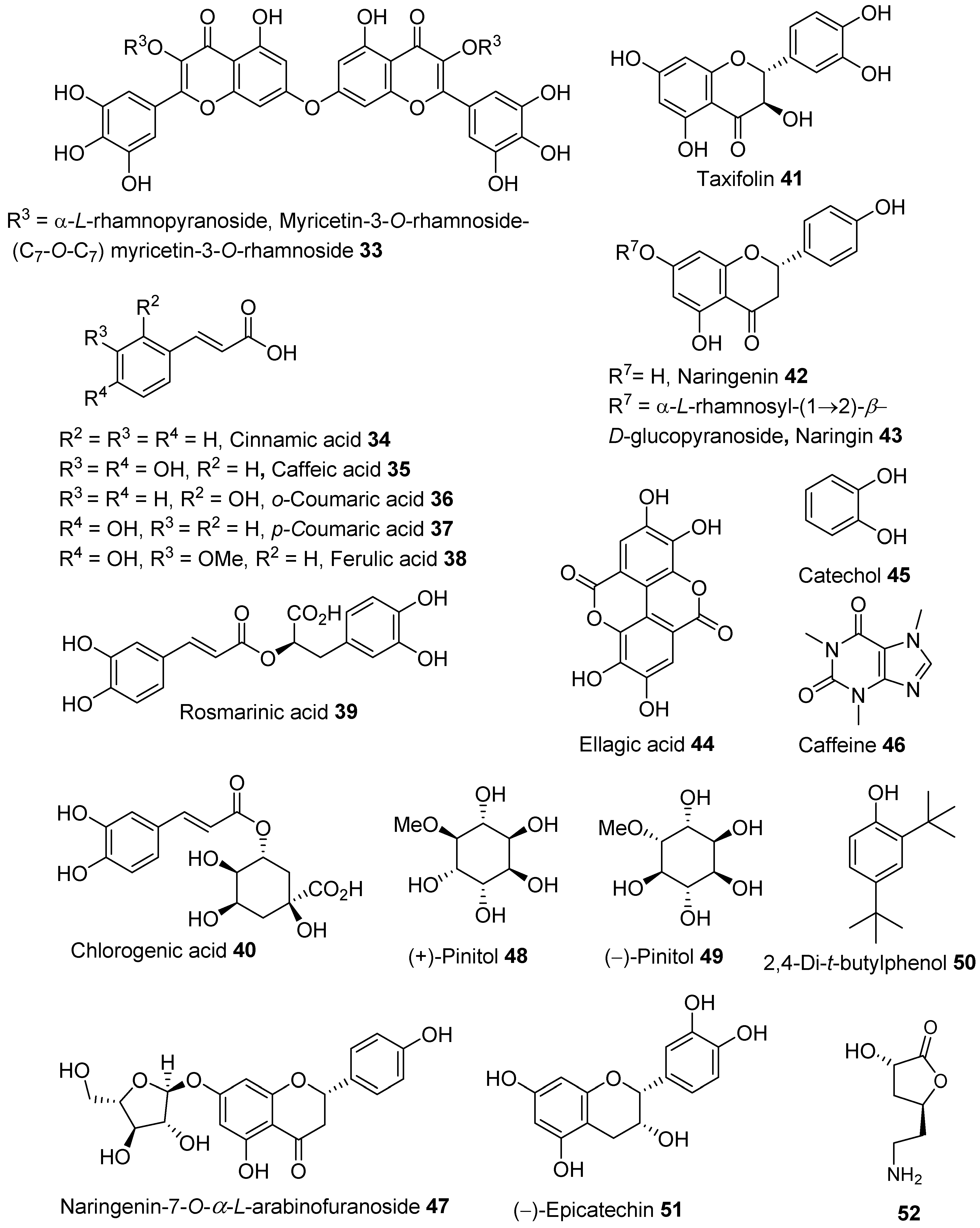
2.3. Phytochemicals from Barks
2.4. Variability in Phytochemical Compositions of A. saligna
- (i)
- flavonoids—isosalipurposide 1, quercetin 3, kaempferol 22, rutin 6, and naringenin 42;
- (ii)
- hydroxycinnamic acids—caffeic acid 35, o-coumaric acid 36, p-coumaric acid 37, and ferulic acid 38; and
- (iii)
- benzoic acid 24 and hydroxybenzoic acids such as gallic acid 25, salicylic acid 32, ellagic acid 44, syringic acid 28, and p-hydroxybenzoic acid 31.
3. Bioactivities of A. saligna Extracts and Identified Phytochemicals
3.1. Antioxidant
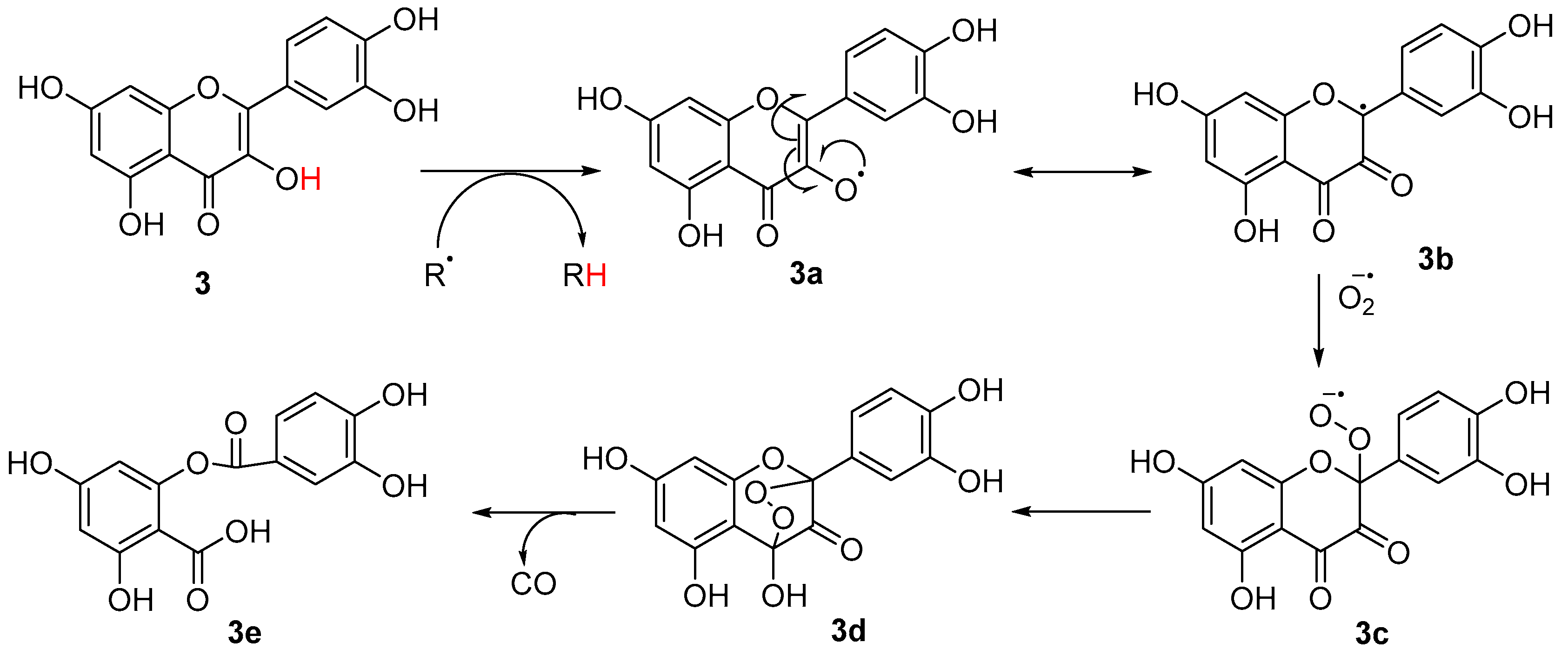
3.1.1. Antioxidant Mechanism of Identified Compounds in A. saligna
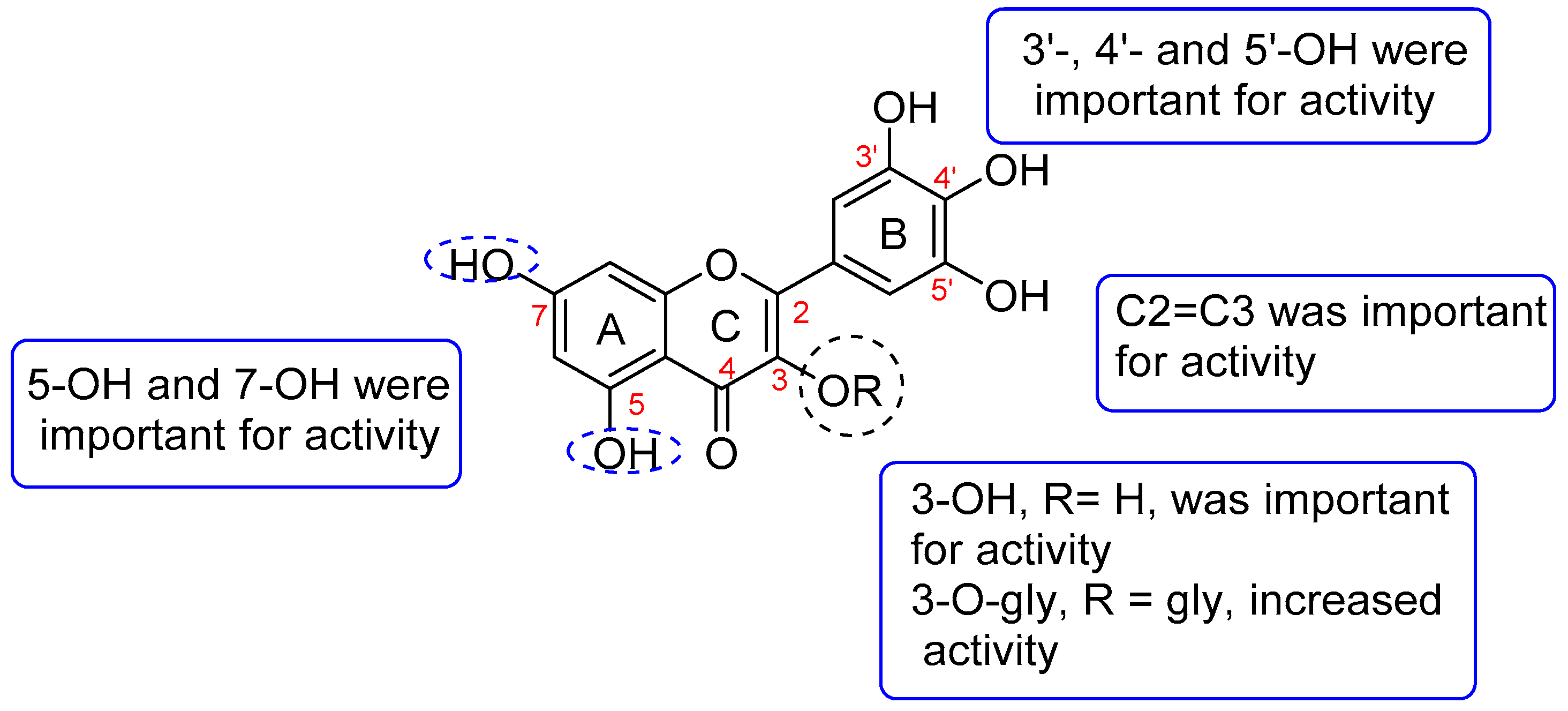
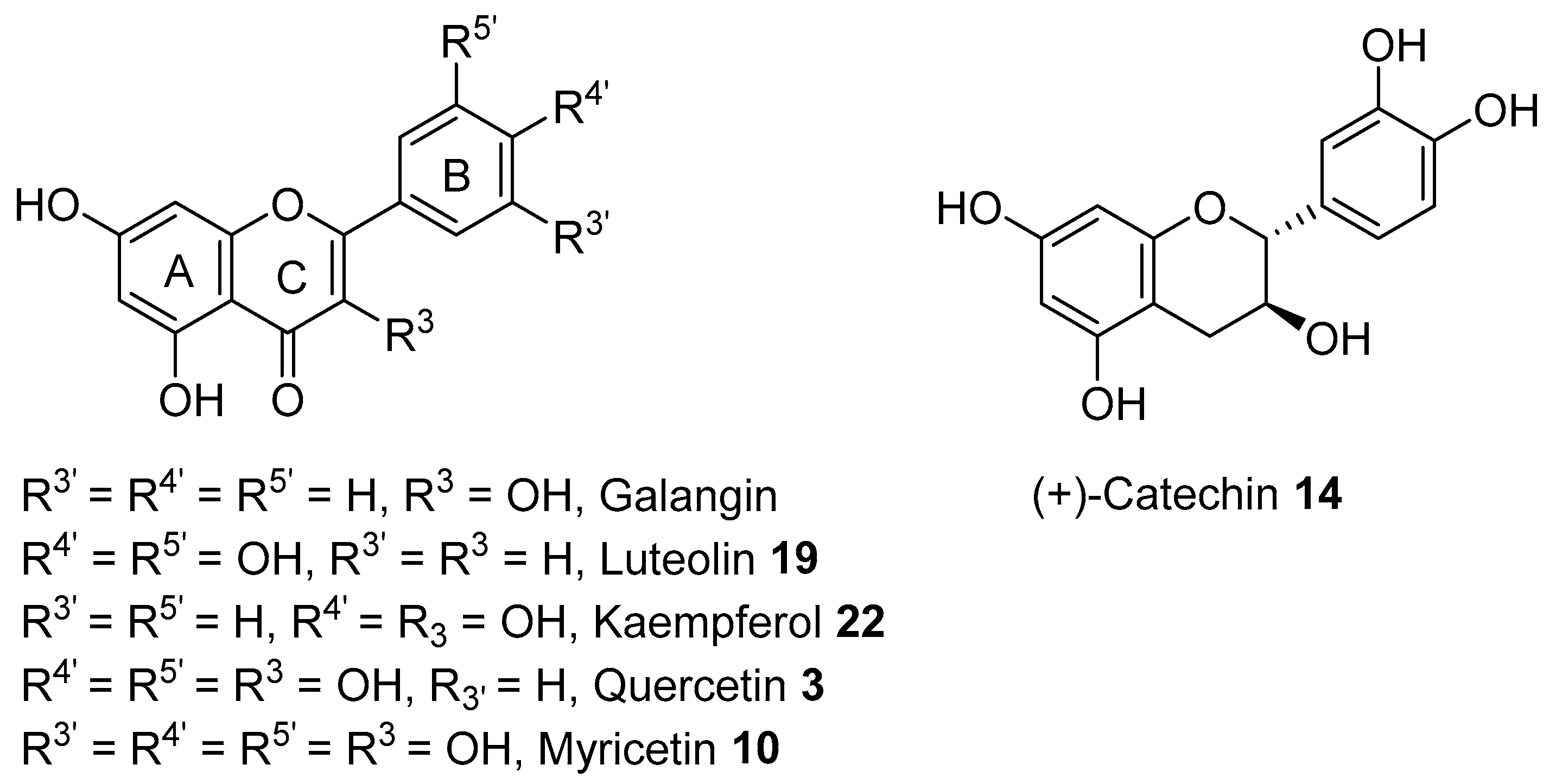
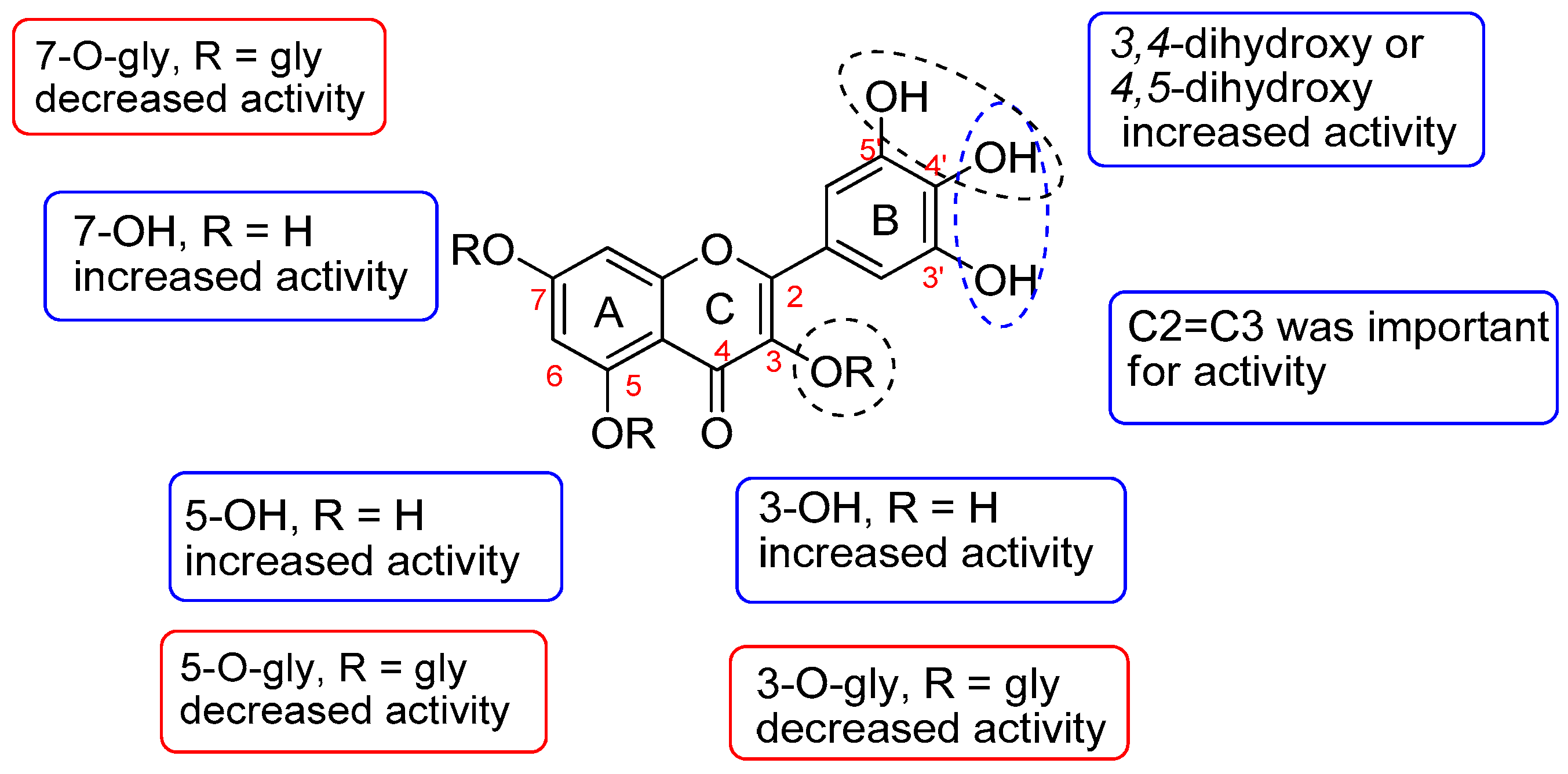
3.1.2. SAR of Antioxidants Compounds Identified in A. saligna
3.2. Antibacterial
3.2.1. Possible Mechanisms of Action (MOA) of Antibacterial Compounds Identified in A. saligna
3.2.2. SAR of Antibacterial Flavonoids Identified in A. saligna
3.3. Antifungal
3.4. Inhibition of α-Glucosidase Enzyme
SAR of Flavonoids Required for the α-Glucosidase Inhibition
3.5. Anti-Inflammation
3.6. Anticancer
4. Toxicity of Bioactive Extracts and Their Safety
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) |
| Ag-NPs | Silver nanoparticles |
| AMPK | Adenosine-monophosphate-activated protein kinase |
| ATCC | American-type culture collection |
| BHT | Butylated hydroxytoluene |
| COX-2 | Cyclooxygenase-2 |
| DMSO | Dimethylsulfoxide |
| DCM | Dichloromethane |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DW | Dried weight |
| EtOAc | Ethyl acetate |
| EtOH | Ethanol |
| GC-FID | Gas chromatography with flame ionisation detection |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| GLUT-4 | Glucose transporter type 4 |
| H. L. Wendl. | Heinrich Ludolph (Ludwig) Wendlan |
| HPLC | High-performance liquid chromatography |
| HPLC-DAD | HPLC–photodiode array detection |
| HPLC-VWD | HPLC-variable wavelength detector |
| IBD | Inflammatory bowel diseases |
| IC50 | Half-maximal inhibitory concentration |
| IL-1b | Interleukin-1 |
| LDL | Low-density lipid |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibition concentration |
| MOA | Mechanism of action |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| NADH | Nicotinamide adenine dinucleotide H |
| NF-kB | Nuclear factor kappa B |
| NMR | Nuclear magnetic resonance |
| PGE2 | Prostaglandin E2 |
| PI3kt | phosphatidylinositol 3-kinase |
| Akt | serine/threonine kinase or serine/threonine kinase (PKB) |
| SAR | Structure-activity relationships |
| T2D | Type 2 diabetes |
| TEAC | Trolox equivalent antioxidant capacity |
| UC | Ulcerative colitis |
| VRE | Vancomycin-resistant enterococci |
References
- Maslin, B.; Miller, J.; Seigler, D. Overview of the generic status of Acacia (Leguminosae: Mimosoideae). Aust. Syst. Bot. 2003, 16, 1–18. [Google Scholar] [CrossRef]
- Wickens, K.; Pennacchio, M. A search for novel biologically active compounds in the phyllodes of Acacia species. Conserv. Sci. West. Aust. 2002, 4, 139–153. [Google Scholar]
- Subhan, N. Phytochemical and Pharmacological Investigations of Australian Acacia: An Ethnomedicine-Guided Bioprospective Approach. Ph.D. Thesis, Charles Sturt University, Bathurst, Australia, 2016. [Google Scholar]
- Cock, I.E. Medicinal and aromatic plants–Australia. In Ethnopharmacology Section, Biological, Physiological and Health Sciences; Encyclopedia of Life Surpport Systems (EOLSS): Queensland, Australia, 2011. [Google Scholar]
- Williams, C. Medicinal Plants in Australia Volume 2: Gums, Resins, Tannin and Essential Oils; Rosenberg Publishing: Kenthurst, Australia, 2011; Volume 2. [Google Scholar]
- Saini, M.L.; Saini, R.; Roy, S.; Kumar, A. Comparative pharmacognostical and antimicrobial studies of Acacia species (Mimosaceae). J. Med. Plants Res. 2008, 2, 378–386. [Google Scholar]
- Batiha, G.E.-S.; Akhtar, N.; Alsayegh, A.A.; Abusudah, W.F.; Almohmadi, N.H.; Shaheen, H.M.; Singh, T.G.; De Waard, M. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Genus Acacia. Molecules 2022, 27, 7340. [Google Scholar] [CrossRef] [PubMed]
- Millar, M.A. Acacia Saligna as a Sustainable Agroforestry Crop for Southern Australia: A Genetic Assessment. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2008. [Google Scholar]
- Orchard, A.E.; Wilson, A.G. Flora of Australia: Mimosaceae, Acacia Part 2; CSIRO Melbourne: Clayton, Australia, 2001; Volume 11B, p. 475. [Google Scholar]
- Maslin, B.R. Studies in the genus Acacia 3: The taxonomy of A. saligna (Labill.) H. Wendl. Nuytsia 1974, 1, 332–340. [Google Scholar] [CrossRef]
- Ghribia, L.; Ghouilaa, H.; Omrib, A.; Besbesb, M.; Janneta, H.B. Antioxidant and anti–acetylcholinesterase activities of extracts and secondary metabolites from Acacia cyanophylla. Asian Pac. J. Trop. Biomed. 2014, 4, S417–S423. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Al-Mana, F.; Al-Yafrsi, M.A. Antioxidant and biological activities of Acacia saligna and Lawsonia inermis natural populations. Plants 2020, 9, 908. [Google Scholar] [CrossRef]
- El Ayeb-Zakhama, A.; Sakka-Rouis, L.; Bergaoui, A.; Flamini, G.; Ben Jannet, H.; Harzallah-Skhiri, F. Chemical composition and allelopathic potential of essential oils obtained from Acacia cyanophylla Lindl. cultivated in Tunisia. Chem. Biodivers. 2015, 12, 615–626. [Google Scholar] [CrossRef]
- Jeddi, K.; Mnif Fakhfakh, L.; Siddique, K.H.; Hessini, K.; Chaieb, M. Effect of Acacia saligna (Labill.) Wendl. extracts on seed germination and seedling performance of three native Mediterranean shrubs. Bot. Lett. 2022, 169, 51–60. [Google Scholar] [CrossRef]
- El-Toumy, S.A.; Salib, J.; Mohamed, W.; Morsy, F. Phytochemical and antimicrobial studies on Acacia saligna leaves. Egypt. J. Chem. 2010, 53, 705–717. [Google Scholar]
- Gumgumjee, N.; Hajar, A. Antimicrobial efficacy of Acacia saligna (Labill.) HL Wendl. and Cordia sinensis Lam. leaves extracts against some pathogenic microorganisms. Int. J. Microbiol. Immunol. Res 2015, 3, 51–57. [Google Scholar]
- Salem, M.Z.; Mohamed, A.A.; Ali, H.M.; Al Farraj, D.A. Characterization of phytoconstituents from alcoholic extracts of four woody species and their potential uses for management of six Fusarium oxysporum isolates identified from some plant hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Buttner, D.H.; Reddy, S.; Koekemoer, T.; van de Venter, M. An in vitro assessment of the potential antidiabetic activity and cytotoxic effects of ethanolic and aqueous extracts from three invasive Australian acacias. S. Afr. J. Bot. 2021, 141, 1–11. [Google Scholar] [CrossRef]
- Shaer, E. Utilization of Acacia saligna as livestock fodder in arid and semi-arid areas in Egypt. Cah. Options Mediterr. 2000, 45, 213–217. [Google Scholar]
- Mousa, M. Effect of feeding acacia as supplements on the nutrient digestion, growth performance, carcass traits and some blood constituents of Awassi lambs under the conditions of North Sinai. Asian J. Anim. Sci. 2011, 5, 102–117. [Google Scholar] [CrossRef]
- Gebru, G.; Tesfay, Y. Utilization of wheat bran and dried Acacia saligna (Labill) HL Wendl leaves by highland rams. Afr. J. Agric. Res. 2017, 12, 1286–1292. [Google Scholar]
- WHO. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Invisible Numbers: The True Extent of Noncommunicable Diseases and What to Do about Them; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Gedara, S.R.; Galala, A.A. New cytotoxic spirostane saponin and biflavonoid glycoside from the leaves of Acacia saligna (Labill.) HL Wendl. Nat. Prod. Res. 2014, 28, 324–329. [Google Scholar] [CrossRef]
- Asmara, A.P.; Prasansuklab, A.; Tencomnao, T.; Ung, A.T. Identification of Phytochemicals in Bioactive Extracts of Acacia saligna Growing in Australia. Molecules 2023, 28, 1028. [Google Scholar] [CrossRef]
- El Sissi, H.; El Sherbeiny, A. The flavanoid components of the leaves of Acacia saligna. Qual. Plant. Mater. Veg. 1967, 14, 257–266. [Google Scholar] [CrossRef]
- Guneidy, R.A.; Amer, M.A.; Hakim, A.E.E.; Abdel-Shafy, S.; Allam, S.A. Effect of polyphenols extracted from Punica granatum and Acacia saligna plants on glutathione S-transferase of the cattle tick Rhipicephalus (Boophilus) annulatus (Acari: Ixodidae). J. Parasit. Dis. 2021, 45, 524–538. [Google Scholar] [CrossRef]
- El-Toumy, S. Flavonoids from Acacia saligna leaves and Evaluation of Antihyperglycaemic Effect of Aqueous Extract. Planta Med. 2006, 72, P_004. [Google Scholar] [CrossRef]
- Verma, N.S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Streeter, J.G.; Lohnes, D.G.; Fioritto, R.J. Patterns of pinitol accumulation in soybean plants and relationships to drought tolerance. Plant Cell Environ. 2001, 24, 429–438. [Google Scholar] [CrossRef]
- Almazroui, M.N.I.; Nazrul Islam, M.; Athar, H.; Jones, P.D.; Rahman, M.A. Recent climate change in the Arabian Peninsula: Annual rainfall and temperature analysis of Saudi Arabia for 1978–2009. Int. J. Climatol. 2012, 32, 953–966. [Google Scholar] [CrossRef]
- Alghamdi, A.G.; Aly, A.A.; Majrashi, M.A.; Ibrahim, H.M. Impact of climate change on hydrochemical properties and quality of groundwater for domestic and irrigation purposes in arid environment: A case study of Al-Baha region, Saudi Arabia. Environ. Earth Sci. 2023, 82, 39–56. [Google Scholar] [CrossRef]
- Bahnasy, M.I.; Khamis, M.H. Distribution pattern of trees diversity in Orman Botanical Garden. Middle East J. Agric. Res 2019, 8, 1–11. [Google Scholar]
- Okba, S.K.; Mazrou, Y.; Mikhael, G.B.; Farag, M.E.; Alam-Eldein, S.M. Magnetized Water and Proline to Boost the Growth, Productivity and Fruit Quality of ‘Taifi’Pomegranate Subjected to Deficit Irrigation in Saline Clay Soils of Semi-Arid Egypt. Horticulturae 2022, 8, 564. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef]
- Zheng, J.H.C.; Yang, B.; Kallio, H.; Liu, P.; Ou, S. Regulation of phytochemicals in fruits and berries by environmental variation-Sugars and organic acids. J. Food Biochem. 2019, 43, e12642–e12660. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Domitrović, R.R.K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem.-Biol. Interact. 2015, 239, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.W.; Chung, S.K. Isolation and identification of myricitrin, an antioxidant flavonoid, from daebong persimmon peel. Prev. Nutr. Food Sci. 2018, 23, 341. [Google Scholar] [CrossRef]
- Chen, W.Z.X.; Lu, Q.; Zhang, L.; Wang, X.; Liu, R. C-ring cleavage metabolites of catechin and epicatechin enhanced antioxidant activities through intestinal microbiota. Food Res. Int. 2020, 135, 109271–109281. [Google Scholar] [CrossRef]
- Miao, J.L.X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Q.; Wang, T.; Liu, J.; Chen, D. Comparison of the antioxidant effects of quercitrin and isoquercitrin: Understanding the role of the 6″-OH group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.-O.L.; Rhee, C.H.; Choung, S.-Y.; Lee, K.-W. Separation of the antioxidant compound quercitrin from Lindera obtusiloba Blume and its antimelanogenic effect on B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2013, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Li, X.; Chen, B.; Xie, Y.; Xie, H.; Chen, D. Antioxidant change in biosynthesis from naringenin chalcone to flavonoid apingenin. ChemistrySelect 2019, 4, 5155–5159. [Google Scholar] [CrossRef]
- Ouyang, X.L.; Li, X.; Lu, W.; Zhao, X.; Chen, D. A null B-ring improves the antioxidant levels of Flavonol: A comparative study between Galangin and 3, 5, 7-Trihydroxychromone. Molecules 2018, 23, 3083. [Google Scholar] [CrossRef]
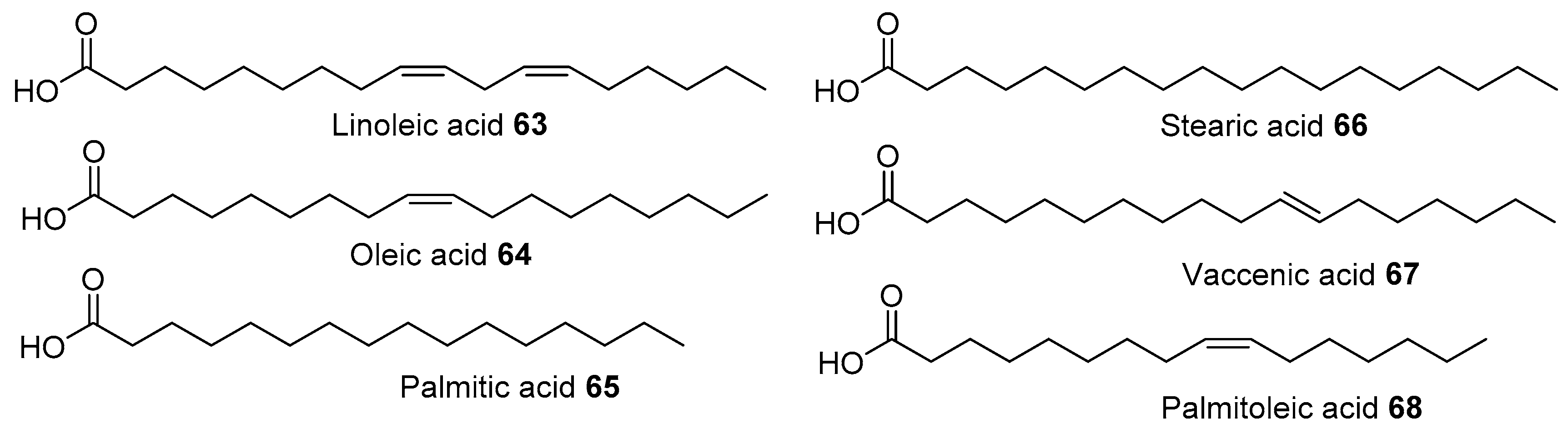
- Youzbachi, N.; Elfalleh, W.; Tlili, N.; Gregoire, S.; Berdeaux, O.; Salles, C.; Triki, S.; Khouja, M.L.; Khaldi, A.; Nasri, N. Unexploited Acacia cyanophylla seeds: Potential food sources of ω6 fatty acids and antioxidants? J. Sci. Food Agric. 2012, 92, 1526–1532. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Nill, K.; Takechi-Haraya, Y.; Playford, M.P.; Nguyen, D.; Yu, Z.-X.; Pryor, M.; Tang, J.; Rojulpote, K.V.; Mehta, N.N. Differential Effect of Dietary Supplementation with a Soybean Oil Enriched in Oleic Acid versus Linoleic Acid on Plasma Lipids and Atherosclerosis in LDLR-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 8385. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure—Antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Nehela, Y.; Taha, N.A.; Elzaawely, A.A.; Xuan, T.D.; Amin, M.; Ahmed, M.E.; El-Nagar, A. Benzoic acid and its hydroxylated derivatives suppress early blight of tomato (Alternaria solani) via the induction of salicylic acid biosynthesis and enzymatic and nonenzymatic antioxidant defense machinery. J. Fungi 2021, 7, 663. [Google Scholar] [CrossRef]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Petrov, A.I.; Kazachenko, A.S.; Kuznetsov, B.N.; Levdansky, V.A. Complexation of rare earth metals by quercetin and quercetin-5′-sulfonic acid in acidic aqueous solution. Main Group Chem. 2018, 17, 17–25. [Google Scholar] [CrossRef]
- Lutoshkin, M.A.; Petrov, A.I.; Kuznetsov, B.N.; Kazachenko, A.S. Aqueous complexation of morin and its sulfonate derivative with lanthanum (III) and trivalent lanthanides. J. Solut. Chem. 2019, 48, 676–688. [Google Scholar] [CrossRef]
- Ferrari, G.V.; Pappano, N.B.; Debattista, N.B.; Montana, M.P. Potentiometric and spectrophotometric study of 3-hydroxyflavone− La (III) complexes. J. Chem. Eng. Data 2008, 53, 1241–1245. [Google Scholar] [CrossRef]
- Meyer, A.S.; Donovan, J.L.; Pearson, D.A.; Waterhouse, A.L.; Frankel, E.N. Fruit hydroxycinnamic acids inhibit human low-density lipoprotein oxidation in vitro. J. Agric. Food Chem. 1998, 46, 1783–1787. [Google Scholar] [CrossRef]
- Natella, F.; Nardini, M.; Felice, M.D.; Scaccini, C. Benzoic and cinnamic acid derivatives as antioxidants: Structure—Activity relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Ashmawy, N.A.; Behiry, S.I.; Al-Huqail, A.A.; Ali, H.M.; Salem, M.Z. Bioactivity of selected phenolic acids and hexane extracts from Bougainvilla spectabilis and Citharexylum spinosum on the growth of pectobacterium carotovorum and Dickeya solani Bacteria: An opportunity to save the environment. Processes 2020, 8, 482. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.C.; Weatherly, M.; Beale, T.; Randriamahefa, A. Atomic force microscopy study of the antimicrobial activity of aqueous garlic versus ampicillin against Escherichia coli and Staphylococcus aureus. J. Sci. Food. Agric. 2009, 89, 958–964. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Tunçel, G.N.C. Antimicrobial effect of some olive phenols in a laboratory medium. Lett. Appl. Microbiol. 1993, 17, 300–302. [Google Scholar] [CrossRef]
- Guzman, J.D.; Mortazavi, P.N.; Munshi, T.; Evangelopoulos, D.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. 2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis. MedChemComm 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ergün, B.Ç.T.; Onurdag, F.; Banoglu, E. Synthesis, antioxidant and antimicrobial evaluation of simple aromatic esters of ferulic acid. Arch. Pharm. Res. 2011, 34, 1251–1261. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Cruz-Martins, N.; Nepovimova, E.; Oleksak, P.; Dhanjal, D.S.; Bhardwaj, S.; Singh, R.; Chopra, C.; Verma, R. Applications of fruit polyphenols and their functionalized nanoparticles against foodborne bacteria: A mini review. Molecules 2021, 26, 3447. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Alvarez, M.D.L.A.; Zarelli, V.E.P.; Pappano, N.B.; Debattista, N.B. Bacteriostatic action of synthetic polyhydroxylated chalcones against Escherichia coli. Biocell 2004, 28, 31–34. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Wu, D.; Kong, Y.; Han, C.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. D-Alanine: D-alanine ligase as a new target for the flavonoids quercetin and apigenin. Int. J. Antimicrob. Agents 2008, 32, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Boesen, T.; Larsen, M.; Schønning, K.; Kromann, H. Antibacterial chalcones-bioisosteric replacement of the 4′-hydroxy group. Bioorganic Med. Chem. 2004, 12, 3047–3054. [Google Scholar] [CrossRef]
- Ávila, H.P.; Smânia, E.d.F.A.; Delle Monache, F.; Júnior, A.S. Structure–activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Khan, M.S.; Khan, R.A.; Khan, J.M. Rutin inhibits mono and multi-species biofilm formation by foodborne drug resistant Escherichia coli and Staphylococcus aureus. Food Control 2017, 79, 325–332. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Gao, L.; Cai, H.; Zhou, Y.; Yang, Y.; Xu, C.; Ding, W.; Chen, J.; Muhammad, I. Rutin inhibits Streptococcus suis biofilm formation by affecting CPS biosynthesis. Front. Pharmacol. 2017, 8, 379. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: A comparative interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef]
- Woźnicka, E.; Kuźniar, A.; Nowak, D.; Nykiel, E.; Kopacz, M.; Gruszecka, J.; Golec, K. Comparative study on the antibacterial activity of some flavonoids and their sulfonic derivatives. Acta Pol. Pharm 2013, 70, 567–571. [Google Scholar]
- Echeverría, J.; Opazo, J.; Mendoza, L.; Urzúa, A.; Wilkens, M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of Chilean flora. Molecules 2017, 22, 608. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Lee, S.F. Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother. Res. 2001, 15, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Tebou, P.; Tamokou, J.-d.-D.; Ngnokam, D.; Voutquenne-Nazabadioko, L.; Kuiate, J.-R.; Bag, P. Flavonoids from Maytenus buchananii as potential cholera chemotherapeutic agents. S. Afr. J. Bot. 2017, 109, 58–65. [Google Scholar] [CrossRef]
- Jeong, M.-H.; Lee, Y.-S.; Cho, J.-Y.; Ahn, Y.-S.; Moon, J.-H.; Hyun, H.-N.; Cha, G.-S.; Kim, K.-Y. Isolation and characterization of metabolites from Bacillus licheniformis MH48 with antifungal activity against plant pathogens. Microb. Pathog. 2017, 110, 645–653. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Jia, Z.; Zhang, T.; Wang, X. Effect of benzoic acid on soil microbial communities associated with soilborne peanut diseases. Appl. Soil Ecol. 2017, 110, 34–42. [Google Scholar] [CrossRef]
- Heflish, A.I.; El Samra, I.; Youssef, N. Occurrence and control of Alternaria alternata, Penicilliun citrinum and Aspergillus flavus mycotoxins in broad bean seeds by benzoic and sorbic acids. Egypt. Acad. J. Biol. Sci. G Microbiol. 2020, 12, 75–87. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Tempesti, T.C.; Alvarez, M.G.; de Araújo, M.F.; Catunda Júnior, F.E.A.; de Carvalho, M.G.; Durantini, E.N. Antifungal activity of a novel quercetin derivative bearing a trifluoromethyl group on Candida albicans. Med. Chem. Res. 2012, 21, 2217–2222. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.d.A.; de Aguiar Cordeiro, R.; Castelo-Branco, D.d.S.C.M.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from Camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Mendoza, L.; Cotoras, M. Alteration of oxidative phosphorylation as a possible mechanism of the antifungal action of p-coumaric acid against Botrytis cinerea. J. Appl. Microbiol. 2017, 123, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.L.; Hirsch, I.B.; Peterson, K.A.; Schneider, D. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2010, 95, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.T.; Chen, J.; Pollock, C.A.; Wong, M.G.; Saad, S. Mouse models of diabetes, obesity and related kidney disease. PLoS ONE 2016, 11, e0162131–e0162146. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Ghani, U. Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015, 103, 133–162. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem.-Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- López-Domènech, S.; Bañuls, C.; de Marañón, A.M.; Abab-Jiménez, Z.; Morillas, C.; Gómez-Abril, S.Á.; Rovira-Llopis, S.; Víctor, V.M.; Hernández-Mijares, A.; Rocha, M. Pinitol alleviates systemic inflammatory cytokines in human obesity by a mechanism involving unfolded protein response and sirtuin 1. Clin. Nutr. 2018, 37, 2036–2044. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type-2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef]
- Dang, N.T.; Mukai, R.; Yoshida, K.-I.; Ashida, H. D-pinitol and myo-inositol stimulate translocation of glucose transporter 4 in skeletal muscle of C57BL/6 mice. Biosci. Biotechnol. Biochem. 2010, 74, 1062–1067. [Google Scholar] [CrossRef]
- Pandi, A.; Kalappan, V.M.; Chandrashekar, N. Effects of d-pinitol on diabetes mellitus: An updated review. Bull. Natl. Res. Cent. 2022, 46, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Wu, T.; Xu, M.; Cai, H.; Zhang, Z. Effects of D-pinitol on insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2015, 63, 6019–6026. [Google Scholar] [CrossRef]
- Silva Junior, J.A.; Silva, A.C.D.; Figueiredo, L.S.; Araujo, T.R.; Freitas, I.N.; Carneiro, E.M.; Ribeiro, E.S.; Ribeiro, R.A. D-Pinitol increases insulin secretion and regulates hepatic lipid metabolism in Msg-obese mice. An. Acad. Bras. Ciências 2020, 92, 1–14. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.; Sousa, J.L.; Tome, S.M.; Ramos, M.J.; Silva, A.M.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, G.; Lin, S.; Gong, D. Inhibitory mechanism of apigenin on α-glucosidase and synergy analysis of flavonoids. J. Agric. Food Chem. 2016, 64, 6939–6949. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, X.; Jiang, Z.; Geng, S.; Ma, H.; Liu, B. Interaction mechanism of flavonoids and α-glucosidase: Experimental and molecular modelling studies. Foods 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.M.; Ammar, N.M.; Abdelhameed, M.F.; Gendy, A.E.-N.G.E.; Ragab, T.I.; Abd-ElGawad, A.M.; Farag, M.A.; Alwahibi, M.S.; Elshamy, A.I. Protective mechanism of Acacia saligna butanol extract and its nano-formulations against ulcerative colitis in rats as revealed via biochemical and metabolomic assays. Biology 2020, 9, 195. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in inflammatory bowel disease: A review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef]
- Comalada, M.; Camuesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef]
- Mak, P.; Leung, Y.-K.; Tang, W.-Y.; Harwood, C.; Ho, S.-M. Apigenin suppresses cancer cell growth through ERβ. Neoplasia 2006, 8, 896–904. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, K.P.; Laroux, F.S.; Fuseler, J.; Wolf, R.E.; Gray, L.; Hoffman, J.; Grisham, M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002, 33, 311–322. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, P.; Zhong, J.; Hu, Y.; Zhuang, S.; Fan, K.; Liu, Z. Quercetin attenuates adhesion molecule expression in intestinal microvascular endothelial cells by modulating multiple pathways. Dig. Dis. Sci. 2018, 63, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Murakami, A.; Tanaka, T.; Ohigashi, H. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: Attenuation of pro-inflammatory gene expression. Biochem. Pharm. 2005, 69, 395–406. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 651593–651607. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, P.; Xu, H. Hyperoside exhibits anticancer activity in non-small cell lung cancer cells with T790M mutations by upregulating FoxO1 via CCAT1. Oncol. Rep. 2020, 43, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Zhang, H.; Zhang, L. Inhibitory effect of hyperoside isolated from Zanthoxylum bungeanum leaves on SW620 human colorectal cancer cells via induction of the p53 signaling pathway and apoptosis. Mol. Med. Rep. 2017, 16, 1125–1132. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D. New colorimetric cytotoxicity assay for anticancer drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Rupérez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. Cell models and their application for studying adipogenic differentiation in relation to obesity: A review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed]

| Domain | Eurayota |
| Kingdom | Plantae |
| Phylum | Spermatophyta |
| Subphylum | Angiospermae |
| Class | Dicotyledonae |
| Order | Fabales |
| Family | Fabaceae |
| Subfamily | Mimosoideae |
| Genus | Acacia |
| Species | Acacia saligna (Labill.) H. L. Wendl. |
| Plant Part/Origin | Type of Extract | IC50 µg/mL | Identified Compounds | Ref |
|---|---|---|---|---|
| Flowers/Tunisia | ethyl acetate | 67.26 | Isosalipurposide 1, quercetin 3, and naringenin 42 | [11] |
| Flowers/Egypt | Aqueous | 461.71 | Quercetin 3, kaempferol 22, naringenin 42, benzoic acid 24, syringic acid 28, p-hydroxybenzoic acid 31, salicylic acid 32, caffeic acid 35, o-coumaric acid 36, p-coumaric acid 37, ferulic acid 38, ellagic acid 44, catechol 45, and caffeine 46 | [12] |
| Leaves/Saudi Arabia | methanolic | 17.0 | Quercetin 3, rutin 6, miquelianin 7, isoquercetin 8, hyperoside 9, gallic acid 25, and p-coumaric acid 37 | [13] |
| Bark/Egypt | Ethanolic | 10.10 | Quercetin 3, rutin 6, kaempferol 22, benzoic acid 24, gallic acid 25, vanillin 29, caffeic acid 35, o-coumaric acid 36, p-coumaric acid 37, ferulic acid 38, rosmarinic acid 39, chlorogenic acid 40, and caffeine 46 | [18] |
| Flowers/ Australia | methanolic | 331.50 | Naringenin 42, isosalipurposide 1, quercitrin 4, D-(+)-pinitol 48, and naringenin-7-O-α-L-arabinofuranoside 47 | [26] |
| Leaves/ Australia | methanolic | 190.10 | (–)-Epicatechin 51, quercitrin 4, myricitrin 11, 2,4-di-t-butylphenol 50, (−)-pinitol 49, and (3S,5S)-3-hydroxy-5-(2-aminoethyl)-dihydrofuran-2(3H)-one 52 | [26] |
| Bark/ Australia | methanolic | 94.24 | (−)-Epicatechin 51, D-(+)-pinitol 48, and sucrose | [26] |
| Plant Parts/Origin | Extracted Solvent | Bacteria | Fungi | Identified Compounds | Ref |
|---|---|---|---|---|---|
| Flowers/Egypt | water | A. tumefaciens, E. cloacae, E. amylovora, P. carotovorum subsp | F. culmorum, R. solani, P. chrysogenum | Quercetin 3, kaempferol 22, naringenin 42, benzoic acid 24, syringic acid 28, p-hydroxybenzoic acid 31, salicylic acid 32, caffeic acid 35, o-coumaric acid 36, p-coumaric acid 37, ferulic acid 38, ellagic acid 44, catechol 45, and caffeine 46 | [12] |
| Leaves/Saudi Arabia | methanol | please refer to Table 4 | please refer to Table 5 | Quercetin 3, rutin 6, miquelianin 7, isoquercetin 8, hyperoside 9, gallic acid 25, and p-coumaric acid 37 | [13] |
| Leaves/Egypt | ethyl acetate | S. aureus, S. pyogens, B.cereus, B. subtilius | NR | Quercetin 3, quercitrin 4, quercetin-3-O-arabinoside 5, myricetin 10, myricitrin 11, myricetin-3-O-arabinoside 12, myricetin-3-O-glucoside 13, (+)-catechin 14, 7-O-Galloyl-cathecin 15, apigenin 16, apigetrin 17, luteolin 19, cynaroside 20, gallic acid 25, and methyl gallate 26 | [16] |
| Leaves/Saudi Arabia | ethanol | E. coli, K. pneumonia, P. aeruginosa, Methicillin-resistant S. aureus (MRSA) | A. niger, A. fumigatus, A. flavus and C. albicans | Gallic acid 25, syringic acid 28, chlorogenic 40, p-hydroxybenzoic 31, vanillic acid 29, p-coumaric acid 37, and salicylic acid 32 | [17] |
| Bark/Egypt | methanol | NR | F. oxysporum | Quercetin 3, rutin 6, kaempferol 22, benzoic acid 24, gallic acid 25, vanillin 29, caffeic acid 35, o-coumaric acid 36, p-coumaric acid 37, ferulic acid 38, rosmarinic acid 39, chlorogenic acid 40, and caffeine 46 | [18] |
| Extract and Compounds | B. cereus | P. aeruginosa | L. monocytogenes | E. coli | M. flavus | S. aureus |
|---|---|---|---|---|---|---|
| MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | |
| A. saliga leaf extract | 0.35, 0.73 | 0.37, 0.79 | 0.47, 0.99 | 0.31, 0.72 | 0.41, 0.85 | 0.30, 0.73 |
| Rutin 6 | 0.11, 0.22 | 0.07, 0.12 | 0.11, 0.21 | 0.12, 0.23 | 0.12, 0.23 | 0.1, 0.251 |
| Hyperoside 9 | 23.3, >500 | 27.2, >500 | 34.3, >500 | 31.2, >500 | 22.42, >500 | 19.54, >500 |
| p-Coumaric acid 37 | 0.12, 0.31 | 0.06, 0.22 | 0.26, 0.58 | 0.12, 0.25 | 0.16, 28.65 | 0.23, 0.47 |
| Quercetin 3 | 30.6, >500 | 31.8, >500 | 43.7, >500 | 38.6, >500 | 28.65, >500 | 21.53, >500 |
| Miquelianin 7 | 35.2, >500 | 25.3, 315.14 | 32.1, >500 | 31.8, >500 | 21.65, 411.17 | 17.1, 427 |
| Streptomycin | 0.07, 0.16 | 0.11, 0.21 | 0.12, 0.21 | 0.11, 0.20 | 0.10, 0.21 | 0.15, 0.31 |
| Extract and Compounds | Aspergillus flavus | Aspergillus ochraceus | L. monocytogenes | Candida albicans | Penicillium funiculosum | Penicillium ochrochloron |
|---|---|---|---|---|---|---|
| MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | MIC, MBC (mg/mL) | |
| A. saliga extract | 0.30, 0.91 | 0.38, 0.95 | 0.48, 1.02 | 0.58, 1.42 | 0.43, 1.01 | 0.44, 1.31 |
| Rutin 6 | 0.21, 0.45 | 0.18, 0.55 | 0.28, 0.62 | 0.25, 0.51 | 0.30, 0.71 | 0.23, 0.43 |
| Hyperoside 9 | 0.10, 0.46 | 0.13, 0.50 | 0.15, 0.52 | 0.21, 1.03 | 0.25, 1.03 | 0.31, 1.19 |
| p-Coumaric acid | 0.22, 0.43 | 0.23, 0.45 | 0.21, 0.41 | 0.32, 0.60 | 0.22, 0.59 | 0.20, 0.40 |
| Quercetin 3 | 0.31, 0.63 | 0.20, 0.75 | 0.21, 0.75 | 0.06, 0.33 | 0.24, 0.70 | 0.29, 0.63 |
| Miquelianin 7 | 0.26, 0.52 | 0.17, 0.61 | 0.18, 0.62 | 0.06, 0.27 | 0.21, 0.60 | 0.26, 0.54 |
| Ketoconazole | 0.20, 0.41 | 0.23, 0.46 | 0.10, 0.21 | 0.22, 0.43 | 2.05, 3.51 | 0.21, 0.40 |
| Compound | IC50 |
|---|---|
| Methanolic flower extract | 34.93 μg/mL |
| Methanolic leaf extract | 38.69 μg/mL |
| Methanolic bark extract | 4.37 μg/mL |
| Isosalipurposide 1 | 116.5 μM |
| Naringenin 42 | 89.71 μM |
| Quercitrin 4 | 177.3 μM |
| Myricitrin 11 | 351.6 μM |
| Naringenin-7-O-α-L-arabinofuranose 47 | 769.1 μM |
| D-(+)-pinitol 48 | 74.69 μM |
| (–)-Pinitol 49 | 164.2 μM |
| (–)-Epicatechin 51 | 63.58 μM |
| 2,4-Di-t-butylphenol 50 | 259 μM |
| (3S,5S)-3-hydroxy-5-(2-aminoethyl) dihydrofuran-2(3H)-one 52 | >1000 a |
| Acarbose | 239.9 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ung, A.T.; Asmara, A.P. Bioactive Phytochemicals of Acacia saligna. Molecules 2023, 28, 4396. https://doi.org/10.3390/molecules28114396
Ung AT, Asmara AP. Bioactive Phytochemicals of Acacia saligna. Molecules. 2023; 28(11):4396. https://doi.org/10.3390/molecules28114396
Chicago/Turabian StyleUng, Alison T., and Anjar P. Asmara. 2023. "Bioactive Phytochemicals of Acacia saligna" Molecules 28, no. 11: 4396. https://doi.org/10.3390/molecules28114396
APA StyleUng, A. T., & Asmara, A. P. (2023). Bioactive Phytochemicals of Acacia saligna. Molecules, 28(11), 4396. https://doi.org/10.3390/molecules28114396