Molecular and Electronic Structures of Macrocyclic Compounds Formed at Template Synthesis in the M(II)—Thiocarbohydrazide—Diacetyl Triple Systems: A Quantum-Chemical Analysis by DFT Methods
Abstract
1. Introduction
2. Results
3. Calculation Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mikhailov, O.V.; Kazymova, M.A.; Shumilova, T.A.; Solovieva, S.E. Template synthesis in the M(II)–thiocarbohydrazide–diacetyl triple system (M = Ni, Cu) in a metal(II)hexacyanoferrate(II) gelatin-immobilized matrix. Transit. Metal Chem. 2004, 29, 732–736. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Self-assembly of supramolecular Ni(II) and Cu(II) metalmacrocyclic compounds with tetraazamacrocyclic ligand into a gelatin-immobilized matrix. J. Coord. Chem. 2010, 63, 4309–4318. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Density Functional Theory Calculation of Molecular Structures of (5656) Macrotetracyclic 3d Metal Complexes with 4,12-Dithiooxo-1,8-dioxa-3,6,10,13-tetraazacyclotetradecanedione-5,11. Russ. J. Inorg. Chem. 2012, 57, 981–986. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Structure of (5656) Macrotetracyclic Chelates in the Ternary Systems M(II)–Ethanedithioamide–Acetone (M = Mn, Fe, Co, Ni, Cu, Zn) According to DFT Calculations. Russ. J. Inorg. Chem. 2013, 58, 1073–1078. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Quantum-Chemical Calculation of Molecular Structures of (5656) Macrotetracyclic 3d-Metal Complexes “Self-Assembled” in Quaternary Systems M(II) Ion– Ethanedithioamide–Formaldehyde– Ammonia by the Density Functional Theory Method. Russ. J. Inorg. Chem. 2014, 59, 218–223. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Macrotetracyclic Chelates of Doubly Charged 3d-Element Ions with 1,4,8,11-Tetraazacyclotetradecane-2,3,9,10-tetrathione and Their Molecular Structures according to Density Functional Theory Data. Russ. J. Inorg. Chem. 2014, 59, 1276–1282. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Molecular Structures of (5656) Macrotetracyclic Chelates Formed in the M(II) Ion– Ethanedithioamide– 2-Thiapropanediol-1,3 Systems according to Density Functional Theory Calculations. Russ. J. Inorg. Chem. 2015, 60, 1354–1359. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. DFT Analysis of Molecular Structure of 14-Membered Tetraaza-, Dioxotetraaza- and Hexaazamacroheterocyclic Ligands and Their Metal ComplexesRuss. J. Gen. Chem. 2016, 86, 1102–1107. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. DFT OPBE/TZVP Calculation of M olecular Structures of (5656) Macroheterocyclic Chelates of Double Charged 3d-Element Ions with 1,5,8,11-Tetraazacyclotetradecanetetrathione-2,3,9,10 and Its Dioxa- and Dithia Analogs. Macroheterocycles 2016, 9, 268–276. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Molecular Structures of (5656) Macrotetracyclic Chelates in M(II) Ion–Ethanedithioamide– Methanimine– Hydrogen Cyanide Quaternary Systems by DFT Calculations. Russ. J. Inorg. Chem. 2016, 61, 616–622. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Revs. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Revs. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, M.G.; Bushmarinov, I.S.; Sun, J.; Perdew, J.P.; Lyssenko, K.A. Density functional theory is straying from the path toward the exact functional. Science 2017, 355, 49–52. [Google Scholar] [CrossRef]
- Mikhailov, O.V.; Chachkov, D.V. Molecular Structure of M(N13) Compounds with 12-Membered Nitrogen-Containing Cycle and Axial Nitrogen Atom (M = Mn, Fe): Quantum-Chemical Design by DFT Method. Quantum Rep. 2023, 5, 282–293. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. Heteroligand Iron(V) Complexes Containing Porphyrazine, trans-Di[benzo]porphyrazine or Tetra[benzo]porphyrazine, Oxo and Fluoro Ligands: DFT Quantum-Chemical Study. Int. J. Mol. Sci. 2023, 24, 6442. [Google Scholar] [CrossRef]
- Chachkov, D.V.; Mikhailov, O.V. DFT Method Used for Prediction of Molecular and Electronic Structures of Mn(VI) Macrocyclic Complexes with Porhyrazine/Phthalocyanine and Two Oxo Ligands. Materials 2023, 16, 2394. [Google Scholar] [CrossRef]
- Paulsen, H.; Duelund, L.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Free Energy of Spin-Crossover Complexes Calculated with Density Functional Methods. Inorg. Chem. 2001, 40, 2201–2203. [Google Scholar] [CrossRef]
- Swart, M.; Groenhof, A.R.; Ehlers, A.W.; Lammertsma, K. Validation of Exchange−Correlation Functionals for Spin States of Iron Complexes. J. Phys. Chem. A 2004, 108, 5479–5483. [Google Scholar] [CrossRef]
- Swart, M.; Ehlers, A.W.; Lammertsma, K. Performance of the OPBE exchange-correlation functional. Mol. Phys. 2004, 102, 2467–2474. [Google Scholar] [CrossRef]
- Swart, M. Metal–ligand bonding in metallocenes: Differentiation between spin state, electrostatic and covalent bonding. Inorg. Chim. Acta 2007, 360, 179–189. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an approximation treatment for many-electron systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Head-Gordon, M.; Pople, J.A.; Frisch, M.J. MP2 energy evaluation by direct methods. Chem. Phys. Lett. 1988, 153, 503–506. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Weinhold, F.; Landis, C.R.; Glendening, E.D. What is NBO analysis and how is it useful? Int. Revs. Phys. Chem. 2016, 35, 399–440. [Google Scholar] [CrossRef]
- Ochterski, J.W. Thermochemistry in Gaussian; Gaussian, Inc.: Wallingford, CT, USA, 2000. [Google Scholar]
- Zhabanov, Y.A.; Tverdova, N.V.; Giricheva, N.I.; Girichev, G.V.; Stuzhin, P.A. DFT Study of molecular and electronic structure of magnesium (II) tetra(1,2,5-chalcogenadiazolo) porphyrazines, [TXDPzMg] (X = O, S, Se, Te). J. Porphyr. Phthalocyanines 2017, 21, 439–452. [Google Scholar] [CrossRef]
- Novakova, V.; Donzello, M.P.; Ercolani, C.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part II: Electronic structure, electrochemical, spectral, photophysical and other application related properties. Coord. Chem. Rev. 2018, 361, 1–73. [Google Scholar] [CrossRef]
- Sorokin, A.B. Recent progress on exploring µ-oxo bridged binuclear porphyrinoid complexes in catalysis and material science. Coord. Chem. Rev. 2019, 389, 141–160. [Google Scholar] [CrossRef]
- Pritchard, B.P.; Altarawy, D.; Didier, B.; Gibson, T.D.; Windus, T.L. New Basis Set Exchange: An Open, Up-to-Date Resource for the Molecular Sciences Community. J. Chem. Inf. Model. 2019, 59, 4814–4820. [Google Scholar] [CrossRef]
- Onami, Y.; Koya, R.; Kawasaki, T.; Aizawa, H.; Nakagame, R.; Miyagawa, Y.; Haraguchi, T.; Akitsu, T.; Tsukiyama, K.; Palafox, M.A. Investigation by DFT Methods of the Damage of Human Serum Albumin including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation. Int. J. Mol. Sci. 2019, 20, 2846. [Google Scholar] [CrossRef]
- Otlyotov, A.A.; Ryzhov, I.V.; Kuzmin, I.A.; Zhabanov, Y.A.; Mikhailov, M.S.; Stuzhin, P.A. DFT Study of Molecular and Electronic Structure of Ca(II) and Zn(II) Complexes with Porphyrazine and tetrakis(1,2,5-thiadiazole)porphyrazine. Int. J. Mol. Sci. 2020, 21, 2923. [Google Scholar] [CrossRef]
- Martins, L.S.; Lameira, J.; Kruger, H.G.; Alves, C.N.; Silva, J.R.A. Evaluating the Performance of a Non-Bonded Cu2+ Model Including Jahn-Teller Effect into the Binding of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2020, 21, 4783. [Google Scholar] [CrossRef] [PubMed]
- Latypov, S.K.; Kondrashova, S.A.; Polyancev, F.M.; Sinyashin, O.G. Quantum Chemical Calculations of 31P NMR Chemical Shifts in Nickel Complexes: Scope and Limitations. Organometallics 2020, 39, 1413–1422. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Z.; Marks, T.J.; Darancet, P. Electronic Structure of Metallophthalocyanines, MPc (M = Fe, Co, Ni, Cu, Zn, Mg) and Fluorinated MPc. J. Phys. Chem. A 2021, 125, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Zhabanov, Y.A.; Ryzhov, I.V.; Kuzmin, I.A.; Eroshin, A.V.; Stuzhin, P.A. DFT Study of Molecular and Electronic Structure of Y, La and Lu Complexes with Porphyrazine and Tetrakis(1,2,5-thiadiazole)porphyrazine. Molecules 2021, 26, 113. [Google Scholar] [CrossRef]
- Zhabanov, Y.A.; Eroshin, A.V.; Ryzhov, I.V.; Kuzmin, I.A.; Finogenov, D.N.; Stuzhin, P.A. Molecular Structure, Thermodynamic and Spectral Characteristics of Metal-Free and Nickel Complex of Tetrakis(1,2,5-thiadiazolo) porphyrazine. Molecules 2021, 26, 2945. [Google Scholar] [CrossRef]
- Eroshin, A.V.; Otlyotov, A.A.; Kuzmin, I.A.; Stuzhin, P.A.; Zhabanov, Y.A. DFT Study of the Molecular and Electronic Structure of Metal-Free Tetrabenzoporphyrin and Its Metal Complexes with Zn, Cd, Al, Ga, In. Int. J. Mol. Sci. 2022, 23, 939. [Google Scholar] [CrossRef]
- Ryzhov, I.V.; Eroshin, A.V.; Zhabanov, Y.A.; Finogenov, D.N.; Stuzhin, P.A. DFT Study of Molecular Structure, Electronic and Vibrational Spectra of Tetrapyrazinoporphyrazine, Its Perchlorinated Derivative and Their Al, Ga and In Complexes. Int. J. Mol. Sci. 2022, 23, 5379. [Google Scholar] [CrossRef]
- Eroshin, A.V.; Koptyaev, A.I.; Otlyotov, A.A.; Minenkov, Y.; Zhabanov, Y.A. Iron(II) Complexes with Porphyrin and Tetrabenzoporphyrin: CASSCF/MCQDPT2 Study of the Electronic Structures and UV–Vis Spectra by sTD-DFT. Int. J. Mol. Sci. 2023, 24, 7070. [Google Scholar] [CrossRef]
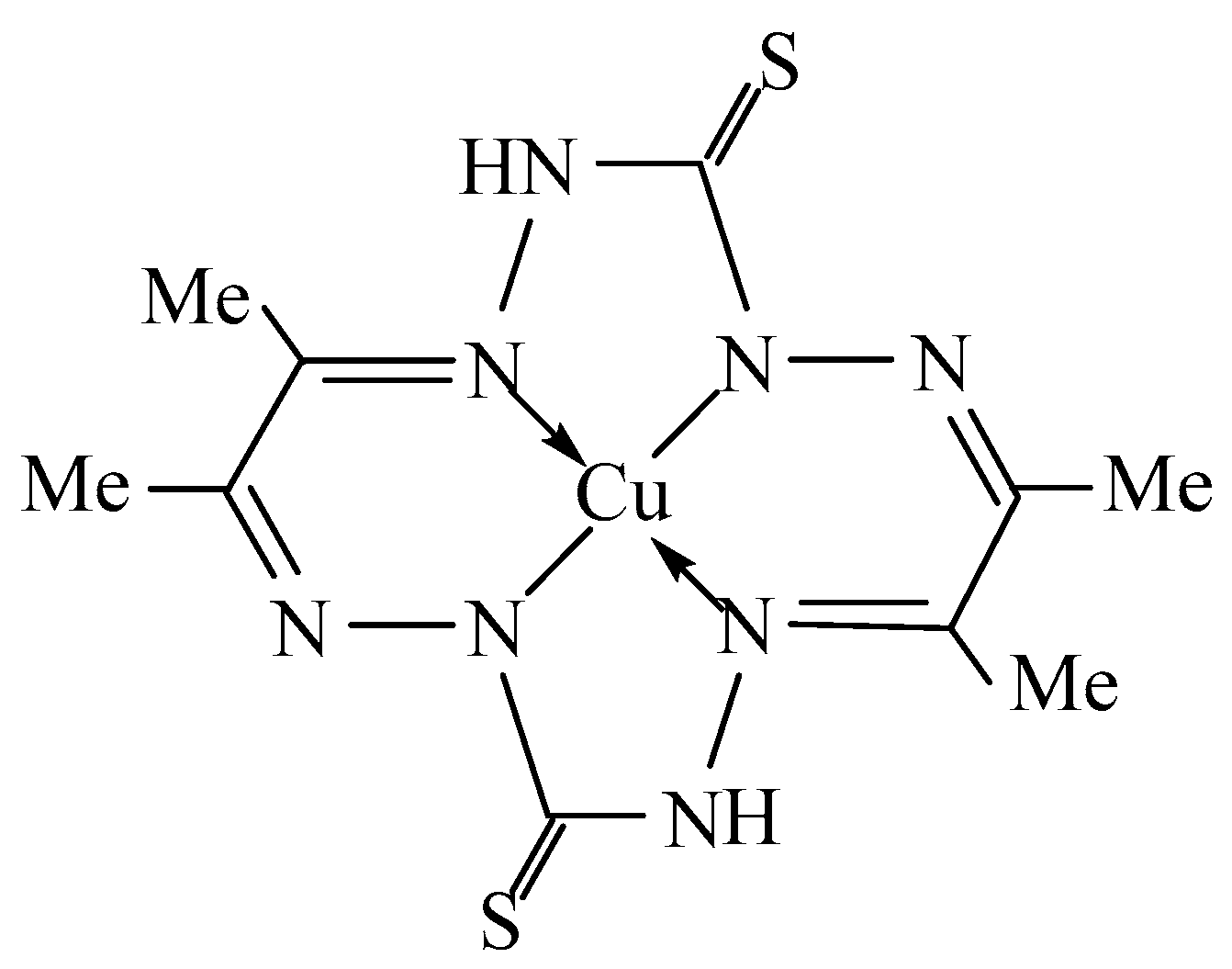
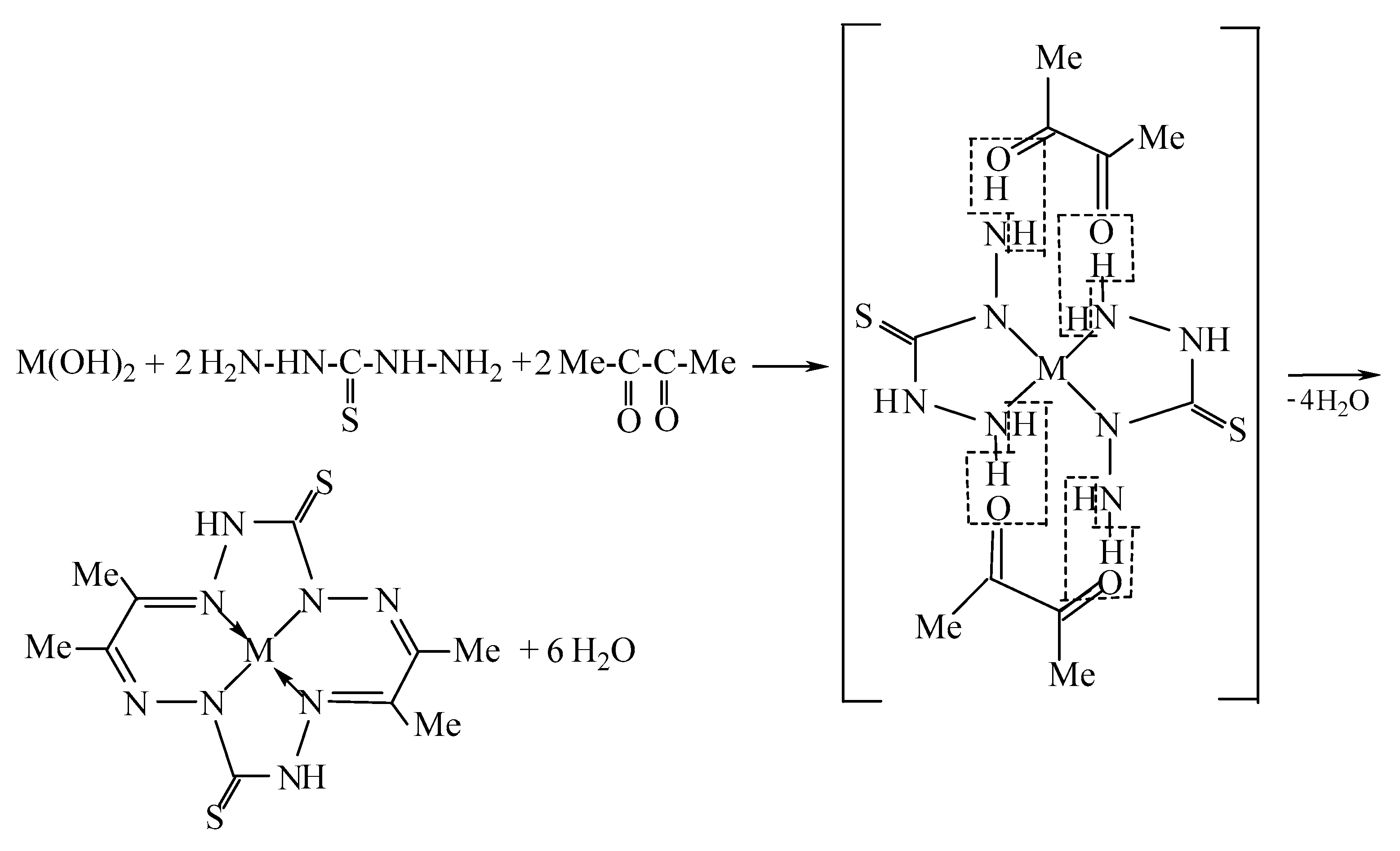

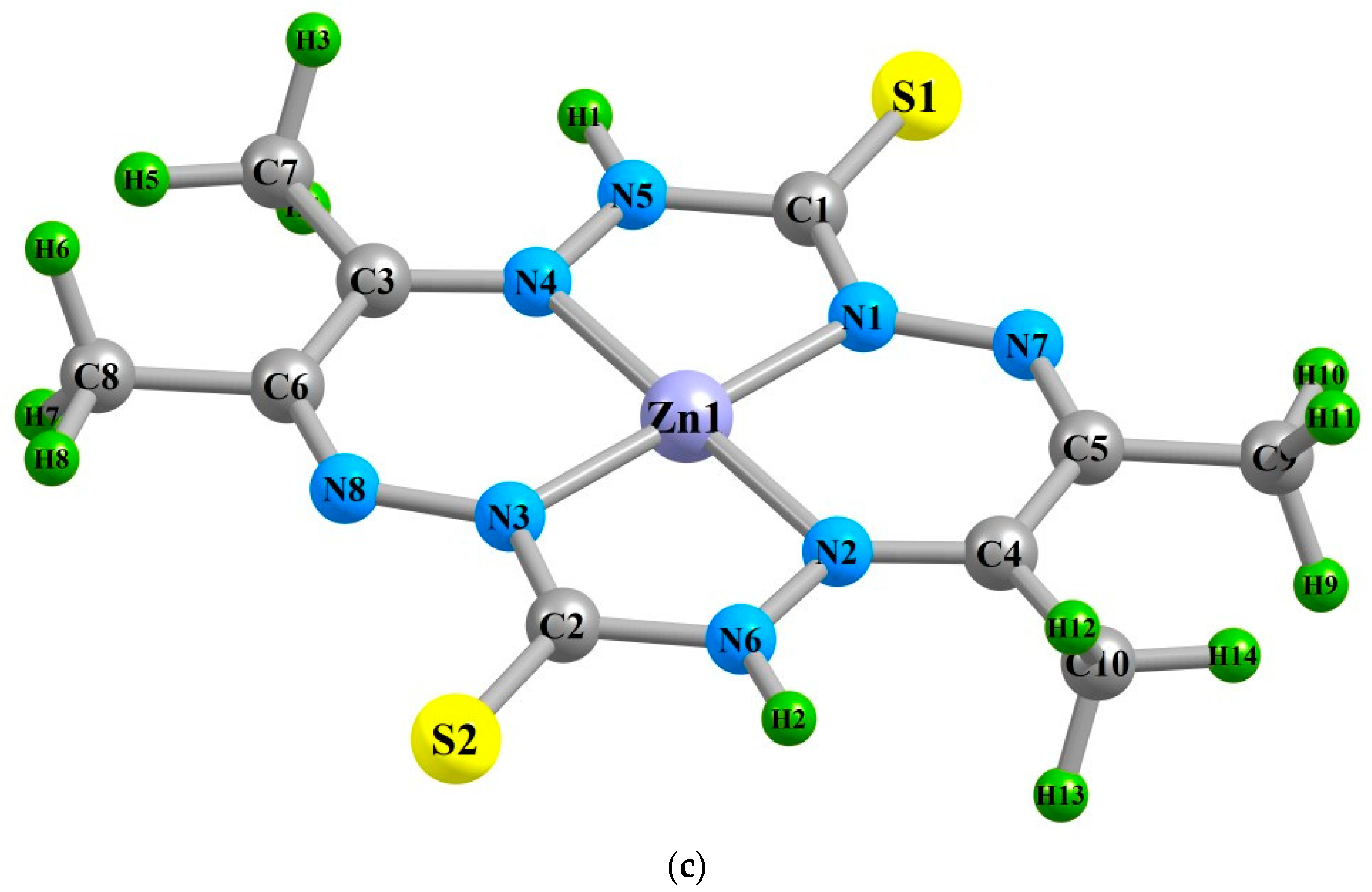
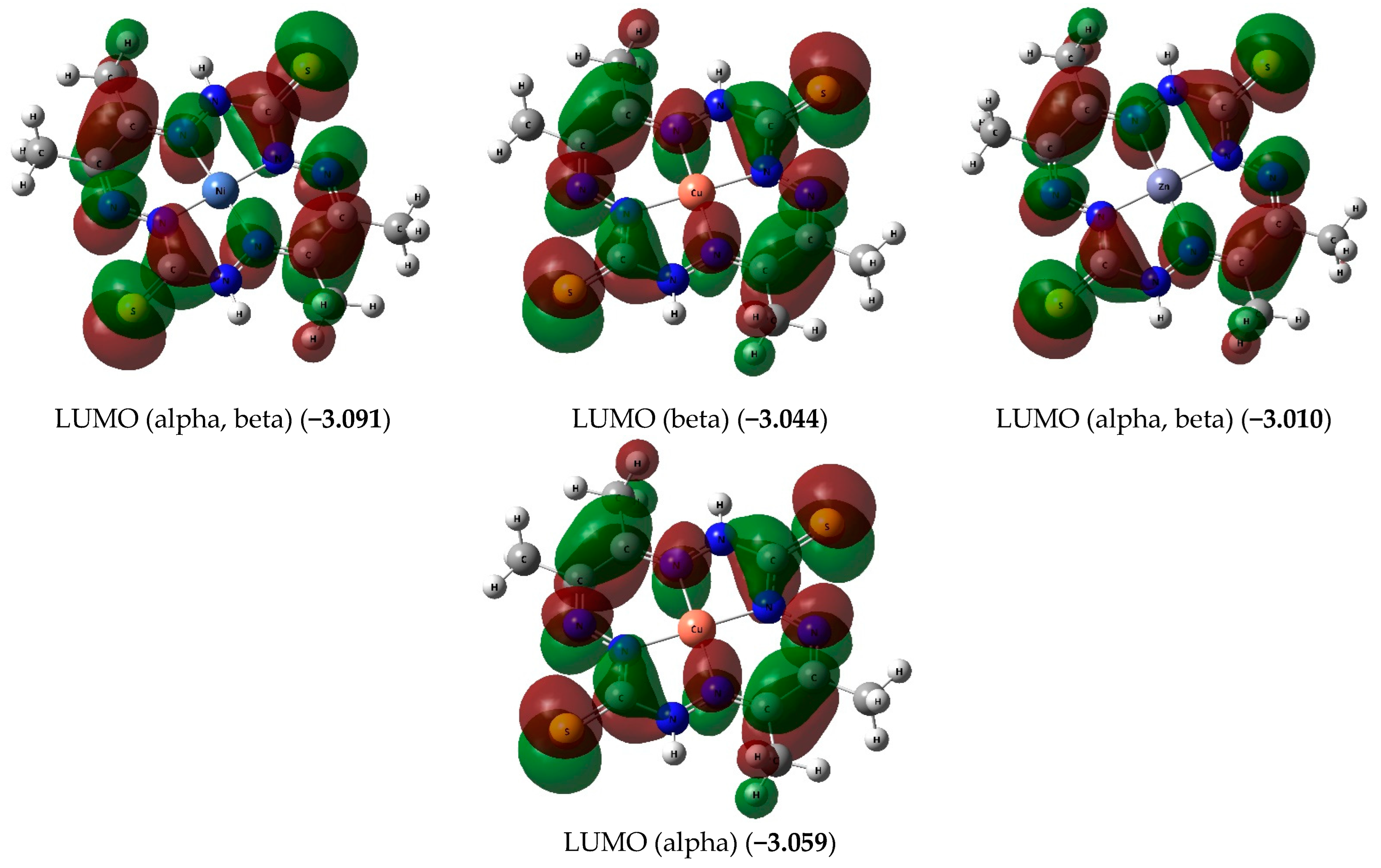

| Complex | NiL | CuL | ZnL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemistry Model | Chemistry Model | Chemistry Model | |||||||
| Structural Parameter | B3PW91/TZVP | M06/TZVP | OPBE/TZVP | B3PW91/TZVP | M06/TZVP | OPBE/TZVP | B3PW91/TZVP | M06/TZVP | OPBE/TZVP |
| Bond lengths in the MN4 chelate node, pm | |||||||||
| (M1N1) | 183.9 | 184.2 | 183.4 | 188.9 | 188.8 | 189.4 | 191.0 | 190.5 | 191.4 |
| (M1N2) | 183.5 | 184.2 | 182.6 | 190.9 | 191.4 | 190.9 | 197.5 | 198.2 | 197.7 |
| (M1N3) | 183.9 | 184.2 | 183.4 | 188.9 | 188.8 | 189.4 | 191.0 | 190.5 | 191.3 |
| (M1N4) | 183.5 | 184.2 | 182.6 | 190.9 | 191.4 | 190.9 | 197.5 | 198.2 | 197.6 |
| Separate bond lengths outside the MN4 chelate node, pm | |||||||||
| (C1S1), (C2S2) | 165.9 | 165.7 | 165.9 | 166.0 | 165.7 | 166.1 | 166.0 | 165.7 | 166.0 |
| (C1N5), (C2N6) | 137.8 | 138.0 | 137.7 | 139.8 | 140.0 | 140.0 | 141.6 | 141.7 | 141.8 |
| (N2N6), (N4N5) | 135.6 | 135.7 | 135.1 | 134.9 | 134.9 | 134.2 | 134.3 | 134.3 | 133.8 |
| (C4C5), (C3C6) | 145.7 | 146.0 | 145.1 | 147.5 | 147.7 | 146.9 | 148.8 | 148.8 | 148.2 |
| (C5N7), (C6N8) | 130.1 | 129.5 | 131.2 | 130.4 | 129.8 | 131.6 | 130.7 | 130.0 | 132.0 |
| (C6C8), (C5C9) | 150.6 | 150.1 | 150.6 | 150.8 | 150.3 | 150.9 | 151.0 | 150.4 | 151.1 |
| (C4C10), (C7C8) | 149.9 | 149.4 | 149.9 | 150.0 | 149.5 | 150.1 | 150.1 | 150.0 | 150.2 |
| Bond angles in the MN4 chelate node, deg | |||||||||
| (N1M1N2) | 94.1 | 94.1 | 94.1 | 94.7 | 94.7 | 94.7 | 95.1 | 95.2 | 95.0 |
| (N2M1N3) | 85.9 | 85.9 | 85.9 | 85.3 | 85.3 | 85.3 | 84.9 | 84.8 | 85.0 |
| (N3M1N4) | 94.1 | 94.1 | 94.1 | 94.7 | 94.7 | 94.7 | 95.1 | 95.2 | 95.0 |
| (N4M1N1) | 85.9 | 85.9 | 85.9 | 85.3 | 85.3 | 85.3 | 84.9 | 84.8 | 85.0 |
| Bond angles sum (BAS) | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 |
| Non-bond angles in the N4 grouping, deg | |||||||||
| (N1N2N3) | 90.1 | 90.0 | 90.2 | 89.4 | 89.2 | 89.5 | 88.1 | 87.7 | 88.2 |
| (N2N3N4) | 89.9 | 90.0 | 89.8 | 90.6 | 90.8 | 90.5 | 91.9 | 92.3 | 91.8 |
| (N3N4N1) | 90.1 | 90.0 | 90.2 | 89.4 | 89.2 | 89.5 | 88.1 | 87.7 | 88.2 |
| (N4N1N2) | 89.9 | 90.0 | 89.8 | 90.6 | 90.8 | 90.5 | 91.9 | 92.3 | 91.8 |
| Non-bond angles sum (NBAS) | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 |
| Bond angles in the 5-numbered (M1N4N5C1N1) chelate ring, deg | |||||||||
| (M1N4N5) | 110.5 | 110.3 | 110.9 | 109.0 | 108.9 | 109.1 | 107.9 | 107.6 | 107.8 |
| (N4N5C1) | 119.1 | 119.2 | 119.3 | 120.5 | 120.5 | 120.9 | 121.1 | 121.0 | 121.6 |
| (N5C1N1) | 109.7 | 109.8 | 109.1 | 111.1 | 111.1 | 110.7 | 111.9 | 112.0 | 111.6 |
| (C1N1M1) | 114.8 | 114.8 | 114.8 | 114.1 | 114.2 | 114.0 | 114.2 | 114.5 | 114.0 |
| (N1M1N4) | 85.9 | 85.9 | 85.9 | 85.3 | 85.3 | 85.3 | 84.9 | 84.8 | 85.0 |
| Bond angles sum (VAS51) | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 |
| Bond angles in the 5-numbered (M1N2N6C2N3) chelate ring, deg | |||||||||
| (M1N2N6) | 110.5 | 110.3 | 110.9 | 109.0 | 108.9 | 109.1 | 107.9 | 107.6 | 107.8 |
| (N2N6C2) | 119.1 | 119.2 | 119.3 | 120.5 | 120.5 | 120.9 | 121.1 | 121.0 | 121.6 |
| (N6C2N3) | 109.7 | 109.8 | 109.1 | 111.1 | 111.1 | 110.7 | 111.9 | 112.0 | 111.6 |
| (C2N3M1) | 114.8 | 114.8 | 114.8 | 114.1 | 114.2 | 114.0 | 114.2 | 114.5 | 114.0 |
| (N3M1N2) | 85.9 | 85.9 | 85.9 | 85.3 | 85.3 | 85.3 | 84.9 | 84.8 | 85.0 |
| Bond angles sum (VAS52) | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 | 540.0 |
| Bond angles in the 6-numbered (M1N1N7C5C4N2) chelate ring, deg | |||||||||
| (M1N1N7) | 128.5 | 128.3 | 128.8 | 127.0 | 126.8 | 127.1 | 126.1 | 125.9 | 126.6 |
| (N1N7C5) | 122.2 | 122.4 | 122.3 | 122.3 | 122.5 | 122.4 | 122.2 | 122.3 | 121.7 |
| (N7C5C4) | 127.0 | 127.2 | 126.8 | 129.5 | 129.5 | 129.6 | 131.6 | 131.6 | 132.1 |
| (C5C4N2) | 120.3 | 120.4 | 119.9 | 120.4 | 120.5 | 120.0 | 120.0 | 120.1 | 119.4 |
| (C4N2M1) | 127.9 | 127.6 | 128.1 | 126.1 | 126.0 | 126.2 | 125.0 | 124.4 | 125.2 |
| (N2M1N1) | 94.1 | 94.1 | 94.1 | 94.7 | 94.7 | 94.7 | 95.1 | 95.2 | 95.0 |
| Bond angles sum (VAS61) | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 719.5 | 720.0 |
| Bond angles in the 6-numbered (M1N3N8C6C3N4) chelate ring, deg | |||||||||
| (M1N3N8) | 128.5 | 128.3 | 128.8 | 127.0 | 126.8 | 127.1 | 126.1 | 125.9 | 126.6 |
| (N3N8C6) | 122.2 | 122.4 | 122.3 | 122.3 | 122.5 | 122.4 | 122.2 | 122.3 | 121.7 |
| (N8C6C3) | 127.0 | 127.2 | 126.8 | 129.5 | 129.5 | 129.6 | 131.6 | 131.6 | 132.1 |
| (C6C3N4) | 120.3 | 120.4 | 119.9 | 120.4 | 120.5 | 120.0 | 120.0 | 120.1 | 119.4 |
| (C3N4M1) | 127.9 | 127.6 | 128.1 | 126.1 | 126.0 | 126.2 | 125.0 | 124.4 | 125.2 |
| (N4M1N3) | 94.1 | 94.1 | 94.1 | 94.7 | 94.7 | 94.7 | 95.1 | 95.2 | 95.0 |
| Bond angles sum (VAS62) | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 720.0 | 719.5 | 720.0 |
| Bond angles outside chelate rings, deg | |||||||||
| (N1C1S1), (N3C2S2) | 130.6 | 130.6 | 130.9 | 130.7 | 130.6 | 131.1 | 130.8 | 130.7 | 131.4 |
| (N5N4C3), (N6N2C4) | 121.6 | 122.1 | 121.0 | 124.8 | 125.2 | 124.6 | 127.2 | 127.6 | 127.1 |
| (N4C3C7), (N2C4C10) | 118.8 | 118.7 | 119.1 | 118.6 | 118.5 | 118.9 | 118.8 | 119.3 | 119.0 |
| (C3C6C8), (C4C5C9) | 118.8 | 118.4 | 119.6 | 117.3 | 117.0 | 117.9 | 116.1 | 115.6 | 116.6 |
| (C8C6N8), (C9C5N7) | 114.1 | 114.4 | 113.6 | 113.2 | 113.5 | 112.4 | 112.2 | 112.8 | 111.3 |
| (C6C3C7), (C5C4C10) | 120.9 | 120.8 | 121.0 | 121.0 | 121.0 | 121.1 | 121.3 | 120.6 | 121.5 |
| Complex | Chemistry Model | The Charges on the Atoms, in Electron Charge Units (ē) | <S**2> | ||||
|---|---|---|---|---|---|---|---|
| M1 | N1 | N2 | N3 | N4 | |||
| NiL | B3PW91/TZVP | +0.379 | −0.316 | −0.187 | −0.316 | −0.187 | 0.0000 |
| M06/TZVP | +0.382 | −0.335 | −0.198 | −0.335 | −0.198 | 0.0000 | |
| OPBE/TZVP | +0.314 | −0.265 | −0.173 | −0.265 | −0.173 | 0.0000 | |
| CuL | B3PW91/TZVP | +0.729 | −0.411 | −0.257 | −0.411 | −0.257 | 0.7500 |
| M06/TZVP | +0.711 | −0.425 | −0.262 | −0.425 | −0.262 | 0.7500 | |
| OPBE/TZVP | +0.672 | −0.364 | −0.245 | −0.364 | −0.245 | 0.7500 | |
| ZnL | B3PW91/TZVP | +1.088 | −0.508 | −0.321 | −0.509 | −0.321 | 0.0000 |
| M06/TZVP | +1.071 | −0.524 | −0.326 | −0.524 | −0.326 | 0.0000 | |
| OPBE/TZVP | +1.085 | −0.475 | −0.318 | −0.475 | −0.318 | 0.0000 | |
| Complex | Chemistry Model | ΔfH0298, kJ/mol | Sf0298, J/mol K | ΔfG0298, kJ/mol |
|---|---|---|---|---|
| NiL | B3PW91/TZVP | 769.1 | 745.5 | 966.6 |
| M06/TZVP | 891.0 | 742.7 | 1089.4 | |
| OPBE/TZVP | 550.1 | 753.4 | 745.3 | |
| CuL | B3PW91/TZVP | 916.4 | 748.5 | 1114.1 |
| M06/TZVP | 1052.2 | 753.7 | 1248.3 | |
| OPBE/TZVP | 753.3 | 760.5 | 947.4 | |
| ZnL | B3PW91/TZVP | 834.8 | 772.5 | 1027.9 |
| M06/TZVP | 983.9 | 766.1 | 1178.9 | |
| OPBE/TZVP | 669.8 | 771.5 | 863.2 |
| Complex | Chemistry Model | ΔrH0 298, kJ | ΔrSr0298, J/K | ΔrG0 298, kJ |
|---|---|---|---|---|
| NiL | B3PW91/TZVP | −411.6 | 46.6 | −425.5 |
| M06/TZVP | −393.1 | 65.6 | −412.7 | |
| CuL | B3PW91/TZVP | −204.7 | 64.1 | −223.8 |
| M06/TZVP | −191.2 | 91.2 | −218.4 | |
| ZnL | B3PW91/TZVP | −77.0 | 80.5 | −101.0 |
| M06/TZVP | −66.7 | 96.0 | −95.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailov, O.V.; Chachkov, D.V. Molecular and Electronic Structures of Macrocyclic Compounds Formed at Template Synthesis in the M(II)—Thiocarbohydrazide—Diacetyl Triple Systems: A Quantum-Chemical Analysis by DFT Methods. Molecules 2023, 28, 4383. https://doi.org/10.3390/molecules28114383
Mikhailov OV, Chachkov DV. Molecular and Electronic Structures of Macrocyclic Compounds Formed at Template Synthesis in the M(II)—Thiocarbohydrazide—Diacetyl Triple Systems: A Quantum-Chemical Analysis by DFT Methods. Molecules. 2023; 28(11):4383. https://doi.org/10.3390/molecules28114383
Chicago/Turabian StyleMikhailov, Oleg V., and Denis V. Chachkov. 2023. "Molecular and Electronic Structures of Macrocyclic Compounds Formed at Template Synthesis in the M(II)—Thiocarbohydrazide—Diacetyl Triple Systems: A Quantum-Chemical Analysis by DFT Methods" Molecules 28, no. 11: 4383. https://doi.org/10.3390/molecules28114383
APA StyleMikhailov, O. V., & Chachkov, D. V. (2023). Molecular and Electronic Structures of Macrocyclic Compounds Formed at Template Synthesis in the M(II)—Thiocarbohydrazide—Diacetyl Triple Systems: A Quantum-Chemical Analysis by DFT Methods. Molecules, 28(11), 4383. https://doi.org/10.3390/molecules28114383







