Abstract
Substituent-regulated cyclization of conjugated alkynes with acid catalysis was developed in this paper, and it provides a straightforward synthesis of cyclic-(E)-[3]dendralenes. Depending on the electronic effect of the aromatic ring pairing, a variety of phosphinyl quintuplet/hexa cyclo-[3]dendralenes with diverse substitution patterns are accessible, with good efficiency and high stereoselectivity. This self-cyclization process achieves the first precise construction of a phosphinylcyclo-(E)-[3]dendralene from conjugated alkynes to aromatization.
1. Introduction
Dendralenes, commonly known as cross-conjugated oligoalkenes, are an important class of oligo-olefinic hydrocarbon structures, exclusively comprising sp2-hybridized carbons [1]. Until the turn of the century, dendralenes had received little attention, most likely due to the erroneous assumption that they were too unstable to be handled in the laboratory using standard equipment and methods [2,3]. These formations were difficult to synthesize for a long time and were believed to be unstable entities from their discovery in 1955, which limited further research. A noticeable breakthrough in the practical synthesis of dendranlenes was reported by Sherburn and co-workers in 2009, finding that dendralenes are actually stable and also have surprising reactivities [4]. Subsequently, dendralenes attracted increased interest because of their unusual structure and electronic phenomenon, with widespread applications of dendralenes in polymer chemistry [5,6], theoretical chemistry [7,8,9,10], materials chemistry [11,12], electrochemistry [13], and synthetic chemistry [14,15,16,17,18,19,20,21,22,23,24,25,26], among which, cyclo-[3]dendralene, as the primary member of the family, has attracted the greatest attention owing to its wide existence in naturally occurring products and engagement in synthetic compounds [27]. For linear dendralenes, the physical and chemical properties of the annulenes are dominated by this parity-dependent behavior. Alternation of melting points in families of compounds with even- and odd-length chains has also been reported, and this is related to the property of the packing of molecules in crystal lattices. In these systems, an alternation in behavior does not extend to any other physical or chemical property [28]. Notably, when all double bonds in a dendralene molecule are in the same plane, the endo-type dendralenes will produce significant site resistance. Single bonds will then be rotated to reduce the site resistance, making the molecule resemble a “propeller” shape. However, cyclic-dendralenes limit the rotation of the single bond, which will affect the arrangement of the double bond and the chemical properties of the molecule [28]. Although cyclic-(E)-[3]dendralenes are also important skeletons in natural products, as shown in Scheme 1 [29,30], only a handful of cases have been devoted to synthesizing these stereo-structures, sometimes with unsatisfied stereoselectivity.
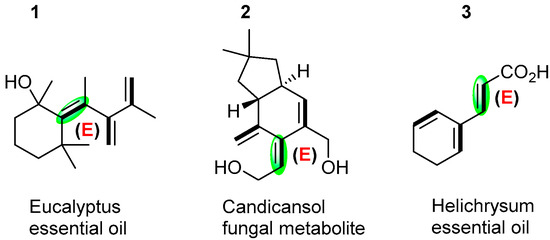
Scheme 1.
Naturally occurring cyclo-(E)-[3]dendralenes.
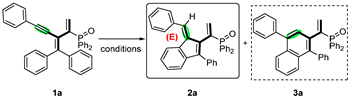
The accurate construction of cyclo-[3]dendralenes is challenging. The palladium-catalyzed coupling of allenynes is an efficient way to obtain these cross-conjugated polyenes. In particular, only two papers documented the construction of cyclic-(E)-[3]dendralenes. In 2017, our group disclosed a palladium-catalyzed coupling of allenylphosphine oxide and N-tosyl-hydrazone affording diverse phosphinyl cyclic-(E)-[3]dendralenes [29]. However, limited examples were successful in acceptable yields and stereoselectivity (Scheme 2a). Later on, Backvall’s group revealed the synthesis of cyclic-(E)-[3]dendralenes with palladium-catalyzed oxidative arylating carbocyclization of allenynes, though just one case was reported (Scheme 2b) [30].
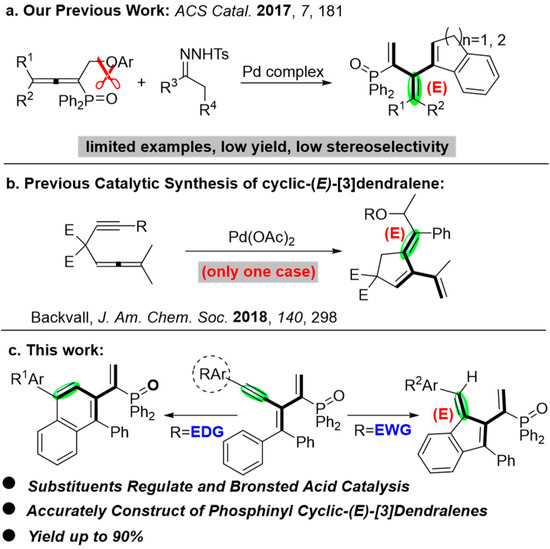
Scheme 2.
(a) Our previous work [29]; (b) previous catalytic synthesis of (E)-[3]dendralenes [30]; (c) this work.
2. Results and Discussion
Inspired by the aforementioned studies and our continuous work on allenyl-phosphine oxides [31,32,33,34,35,36,37], mechanistically, an ideal approach to access phosphinyl cyclic-(E)-[3]dendralenes can be an acid-catalyzed cyclization and substituent-regulated conjugation of alkynes (Scheme 2c).
Initially, phosphinyl-conjugated enynes were prepared according to our previous study, and substrate 1a was selected as a model. As shown in Table 1, solvents and acids played crucial roles in producing the target phosphinyl cyclic-(E)-[3]dendralenes. Successful research indicated that using AgSbF6 directly in 1,4-dioxane, the self-cyclization reaction produced phosphinyl cyclic-(E)-[3]dendralenes (2a) in a 78% yield (entry 3). Interestingly, DCM was proven to be the best choice and enhanced the isolated yield to 90% (entry 1). However, it is accompanied by a small amount of 3a production. As a comparison, other solvents, including DMF, acetonitrile, and toluene led to negative results. Afterward, systematic screenings of the conditions were further performed using varieties of Lewis acid catalysts (entries 6–11). Although no acid was better than that of trifluoroacetic acid, it is quite clear that weak lewis acids, such as AgCl and AgOAc, rendered the decomposition of starting materials, leading to only trace amounts of the products. The target product 2a was then achieved in yields of 40% (entry 8) and 61% (entry 9) by changing the optimum conditions and the type of Lewis acid. It is worth mentioning that a small amount of six-membered ring products (3a) appeared when the type of catalyst was changed (entry 1, entry 13), indicating that regioselective cyclization reactions might be feasible. The target product can hardly be obtained after reducing the ratios of acid catalysts from 30 to 10 mol% (entry 12). Thus, by using TFA as a promoter in DCM with no additives, an efficient self-cyclization reaction of conjugated enynes was achieved to produce phosphinyl cyclic-(E)-[3]dendralenes. Moreover, the X-ray structure of 2a was obtained, and this confirmed the relative stereochemistry of products and revealed multiple double bonds in [3]dendralenes with a “fan-like” dimensional orientation.

Table 1.
Optimization of the reaction conditions a.
Encouraged by the preliminary results, we continued to investigate the substrate scope of various phosphinyl-conjugated enynes, and the results are depicted in Scheme 3 and Scheme 4. The stereoselectivity and dimensional orientation of all functional moieties were elucidated by the X-ray structure of 2a (CCDC 2214047). It is worth mentioning that the electronic effect of the variation in the R1 and R2 groups would render further stereodiversity to produce cyclic-[3]dendralenes. In general, good to excellent yields of the corresponding cyclic-(E)-[3]dendralene products were obtained from para-substituted aryl alkynes containing electron-withdrawing groups, such as fluorine, chlorine, and nitro (2b–e). In addition, para-substituted aryl alkynes with cyano and ester groups were also tested and, to our delight, were found to be applicable to the system, with good yields ranging from 79 to 85% (2d and 2f). We investigated the substrate scope of enynes, and the results are depicted in Scheme 4. Quite interestingly, phosphinyl-conjugated enynes bearing electron-donating groups, including methyl, methoxy, t-butyl, and phenyl at the para-positions; methyl at the meta-position was well-tolerated in the system, affording the six-membered ring products (3g–3l) in medium to excellent yields, among which, para-methoxy- and meta-methyl-derivated conjugated enynes were good candidates as well, and the corresponding products 3g and 3i were obtained in 80% and 84% yields. The stereoselectivity was elucidated through the X-ray structure of 3i (CCDC 2068909). In addition, pyridine-yl and thiophene-yl enynes were also tested, although the conditions needed to be altered and, to our delight, were found to be applicable to the system, with moderate yields (3m and 3n) ranging from 60% to 65% (see Supporting Information (SI) for details).
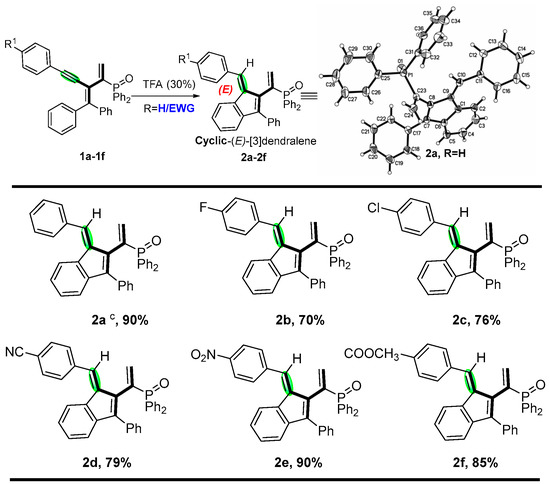
Scheme 3.
Substrate scope on alkynes a,b. a Phosphinyl-conjugated enynes (1a, 0.1 mmol), catalyst (30 mol%), in 3 mL refluxing solvent for 3 h. b Isolated yields. c The stereochemistry was determined using X-ray.
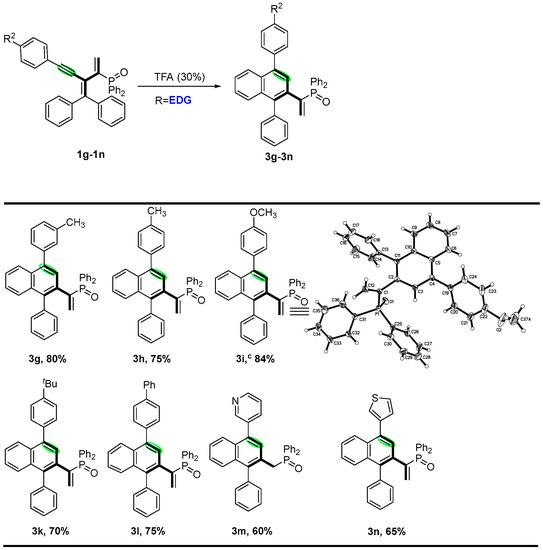
Scheme 4.
Substrate scope on allenylphosphine oxides and the formation of (Z,Z)-[3]Dendralenes a,b. a Phosphinyl-conjugated enynes (1a, 0.1 mmol), catalyst (30 mol%), in 3 mL refluxing solvent for 3 h. b Isolated yields. c The stereochemistry of 3i was determined using X-ray.
Finally, in order to study the effect of olefins next to the diphenylphosphine oxide group in this reaction, as shown in Scheme 5, compound 1p was synthesized and reacted under template conditions. It is noteworthy that these double bonds are crucial for the modulation reaction. If the functional group attached to the phosphorus oxygen is not an olefin, the terminal aryl substituent of the alkyne is not affected by the regulation of this reaction. For example, a substrate of 1g′ does not give the six-membered ring product 2p according to the previous pattern but only the five-membered ring product 2p′. This approach offers a novel complementary strategy to synthesize stereodivergent indene skeletal compounds.
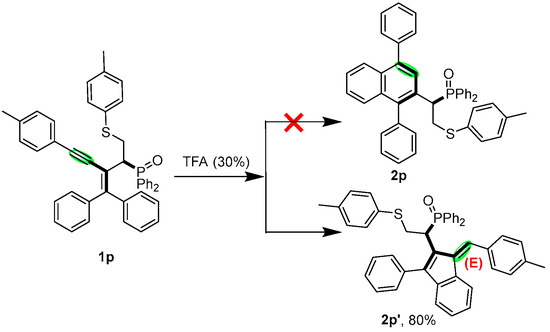
Scheme 5.
Control experiments.
Based on the acid-catalyzed conjugated alkyne chemistry, self-cyclization process, and previous reports [38], a plausible mechanism is proposed in Scheme 6. Initially, the protonation of alkyne leads to the formation of an alkenyl carbocation species 1 and 1′. Further dearomatization of electrophilic addition intermediates 2 and 2′ gives the target products 2a′ and 3a′, respectively.
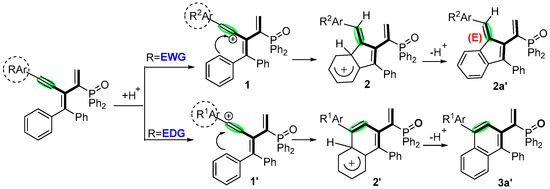
Scheme 6.
Proposed mechanism.
3. Experimental Section
General Procedures for Substrate I Preparation [35].
Diphenylphosphine chloride (40 mmol) in CH2Cl2 (50 mL) was added dropwise to a stirred and cooled (0 °C) solution of acetylenic alcohol (40 mmol) in anhydrous ether (50 mL) and pyridine (3.88 mL, 48 mmol) under nitrogen. The stirring was maintained 1 h at 0 °C and at room temperature overnight. The solution was then quenched with cold water and extracted with CH2Cl2, and the organic layer was dried with Na2SO4. Concentration in vacuo gave the crude product that was subjected to flash chromatography on silica gel eluting with ethyl acetate and petroleum ether.
Note: The acetylenic alcohols were synthesized from terminal alkynes with corresponding ketones mediated by n-BuLi [36].
General Procedures for Substrate II Preparation [36].
To a 25 mL vial, allenylphosphine oxides (1 mmol), alkynes (2 mmol), potassium carbonate (K2CO3) (2 mmol), DMSO (10 mL), and Pd-NPS (1 mol%, 16 mg) were added. The reaction was then allowed to react at 100 °C for a certain time until the complete consumption of starting materials monitored via TLC. The reaction mixture was extracted with EtOAc (10 mL × 3). The combined organic extract was washed with brine and dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure, and the residue was purified using column chromatography on silica gel using petroleum ether/ethylacetate (1.5/1) as the eluent to afford the enynes. After this was finished, the compound was determined through NMR. Further, 1a–1n are known compounds; please refer to [37].
General Procedures for Substituents Regulate the Cyclization of Conjugated Alkynes Constructing Cyclo-(Z)-[3]Dendralenes.
A 10 mL flask equipped with a magnetic stirrer was charged with conjugated alkynes (1a, 50 mg, 0.1 mmol), TFA (3.4 mg, 30 mol%), and 3 mL DCM. The reaction mixture was then heated to 50 °C for 3 h until the complete consumption of 1a monitored via TLC. Subsequently, all of the volatiles were removed under vacuum, and the crude product was purified on flash chromatography (eluent: 1:1 (v/v) of ethyl acetate/petroleum ether) to afford product 2a (45 mg, 90%) as a yellow solid.
Products of 2p′. (E)-(1-(1-(4-methylbenzylidene)-3-phenyl-1H-inden-2-yl)-2-(p-tolylthio)ethyl)diphenylphosphine oxide (2p′). A yellow solid (92 mg, 80% yield), m.p.: 154.4–155.9 °C. 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 8.5 Hz, 2H), 7.66–7.46 (m, 7H), 7.45–7.23 (m, 10H), 7.22–7.05 (m, 5H), 6.95 (d, J = 8.1 Hz, 2H), 6.88 (d, J = 8.1 Hz, 2H), 5.76 (d, J = 7.5 Hz, 1H), 3.97 (m, 1H), 3.89–3.71 (m, 1H), 3.44 (m, 1H), 2.53 (s, 3H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 140.2 (d, J = 7.5 Hz), 137.8, 137.5, 137.1, 135.9, 133.2, 133.1, 132.6, 132.4, 132.1, 131.8, 131.7, 131.6, 131.4, 131.2, 130.9, 130.34 (d, J = 15.8 Hz), 130.3, 129.56 (d, J = 5.1 Hz), 129.6, 129.4, 129.0, 128.8, 128.7, 128.2, 128.0, 127.8, 127.7, 127.3, 127.1, 126.8, 126.1, 125.7, 125.6, 43.6, 43.0, 35.4, 21.4, 21.0. 31P NMR (162 MHz, CDCl3) δ 33.47 (s). HRMS (ESI): ([M + H]+) calcd for C44H38OPS+: 645.2381, found: 645.2379. IR (film) ν 3581, 2973, 2921, 1677, 1539, 1479, 1241, 1109, 1027, 939, 741, and 689 cm−1.
Products of 2a. (E)-(1-(1-benzylidene-3-phenyl-1H-inden-2-yl)vinyl)diphenylphosphine oxide (2a). A yellow solid (84 mg, 83% yield), m.p.: 137.4–139.1 °C. 1H NMR (400 MHz, CDCl3) δ 7.50 (m, 4H), 7.45–7.32 (m, 9H), 7.29–7.22 (m, 7H), 7.18–7.09 (m, 2H), 7.07 (s, 1H), 6.97 (t, J = 7.3 Hz, 1H), 6.72 (m, 1H), 6.46 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 143.7, 141.9, 141.1, 139.7, 137.4, 136.5, 135.4, 134.4, 133.9, 131.9 (d, J = 9.6 Hz), 131.5 (d, J = 2.7 Hz), 130.5, 129.4 (d, J = 13.1 Hz), 128.3, 128.2, 128.1, 128.0, 127.8, 127.7, 125.5, 123.1, 120.2. 31P NMR (162 MHz, CDCl3) δ 26.04 (s). HRMS (ESI): ([M + H]+) calcd for C36H29OP+: 507.1878, found: 507.1876. IR (film) ν 3677, 2985, 2907, 2221, 1592, 1574, 1496, 1438, 1172, 1077, 916, 739, and 695 cm−1.
Products of 2b. (E)-(1-(1-(4-fluorobenzylidene)-3-phenyl-1H-inden-2-yl)vinyl)diphenylphosphine oxide (2b). A yellow solid (89 mg, 85% yield), m.p.: 107.4–109.1 °C. 1H NMR (400 MHz, CDCl3) δ 7.53–7.43 (m, 8H), 7.43–7.32 (m, 12H), 7.28–7.20 (m, 16H), 7.19–7.04 (m, 8H), 7.02–6.95 (m, 4H), 6.72 (d, J = 1.6 Hz, 1H), 6.67 (d, J = 1.6 Hz, 1H), 6.49 (d, J = 1.6 Hz, 1H), 6.40 (d, J = 1.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 161.4), 143.7, 142.0, 141.2, 140.6 139.7, 137.4, 135.3, 134.25 (d, J = 11.1 Hz), 133.7, 132.19 (d, J = 48.1 Hz), 132.0, 131.9, 131.6, 131.5, 131.2, 131.1, 130.4, 129.4, 128.3, 128.1, 128.0, 127.9, 127.8, 125.5, 122.9, 120.3, 115.5, 115.2, 100.0. 31P NMR (162 MHz, CDCl3) δ 26.15 (s). HRMS (ESI): ([M + H]+) calcd for C36H26FOP: 525.1784, found: 525.1783. IR (film) ν 3677, 2985, 2900, 2213, 1502, 1396, 1245, 1061, 924, 723, and 694 cm−1.
Products of 2c. (E)-(1-(1-(4-chlorobenzylidene)-3-phenyl-1H-inden-2-yl)vinyl)diphenylphosphine oxide (2c). A yellow solid (82 mg, 77% yield), m.p.: 111.6–113.7 °C. 1H NMR (400 MHz, CDCl3) δ 7.54–7.31 (m, 26H), 7.22 (m, 16H), 7.13 (m, 4H), 6.98 (m, 4H), 6.73 (s, 1H), 6.69 (s, 1H), 6.49 (s, 1H), 6.40 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 143.7, 141.6, 134.9, 134.1, 133.7 (d, J = 17.0 Hz), 131.9 (d, J = 9.6 Hz), 131.6, 130.7, 129.4, 128.5, 128.3, 128.2, 128.1, 128.0, 127.9, 125.6, 122.9, 120.4. 31P NMR (162 MHz, CDCl3) δ 26.47 (s). HRMS (ESI): ([M + H]+) calcd for C36H27ClOP+: 541.1488, found: 537.2322. IR (film), ν 3059, 2947, 2837, 2385, 1544, 1423, 1422, 1343, 1023, 881, 735, and 675 cm−1.
Products of 2d. (E)-4-((2-(1-(diphenylphosphoryl)vinyl)-3-phenyl-1H-inden-1-ylidene)methyl)benzonitrile (2d). A yellow solid (98 mg, 87% yield), m.p.: 125.7–127.6 °C. 1H NMR (400 MHz, CDCl3) δ 7.68 (d, J = 8.2 Hz, 4H), 7.55–7.32 (m, 24H), 7.31–7.20 (m, 15H), 7.20–7.06 (m, 4H), 7.02–6.94 (m, 4H), 6.67 (d, J = 1.3 Hz, 1H), 6.62 (d, J = 1.3 Hz, 1H), 6.49 (d, J = 1.4 Hz, 1H), 6.39 (d, J = 1.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 143.9, 143.0, 141.5, 137.8, 134.0, 133.3, 132.3, 132.1, 131.9, 131.8, 131.7, 131.6, 131.3, 131.3, 130.3, 130.0, 129.3, 128.45 (d, J = 20.2 Hz), 128.13 (d, J = 12.0 Hz), 125.9, 122.9, 120.6, 118.8, 111.6, 100.0. 31P NMR (162 MHz, CDCl3) δ 26.27 (s). HRMS (ESI): ([M + H]+) calcd for C37H27NOP+: 532.1830, found: 532.1830. IR (film) ν 3675, 2970, 2906, 2231, 1537, 1338, 1244, 1044, 891, and 694 cm−1.
Products of 2e. (E)-(1-(1-(4-nitrobenzylidene)-3-phenyl-1H-inden-2-yl)vinyl)diphenylphosphine oxide (2e). A yellow solid (65 mg, 56% yield), m.p.: 186.3–188.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 7.5 Hz, 2H), 7.63–7.47 (m, 12H), 7.45–7.26 (m, 10H), 7.17 (s, 5H), 6.14 (d, J = 12.1 Hz, 1H), 6.05 (d, J = 12.1 Hz, 1H), 5.89 (d, J = 42.0 Hz, 1H), 5.46 (d, J = 19.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 145.2 (d, J = 7.5 Hz), 143.4 (d, J = 94.9 Hz), 142.4, 142.1, 140.9, 139.7, 136.0, 135.5 (d, J = 10.1 Hz), 133.7 (d, J = 7.2 Hz), 133.0, 132.7, 131.9, 131.8, 131.5 (d, J = 2.6 Hz), 130.9, 130.0, 129.8, 128.9, 128.3, 128.2, 127.9, 127.6, 127.3 (d, J = 8.1 Hz), 127.0, 126.9, 126.3. 31P NMR (162 MHz, CDCl3) δ 28.67 (s). HRMS (ESI): ([M + H]+) calcd for C42H34OP+: 585.2342, found: 585.2341. IR (film) ν 3062, 3015, 1599, 1487, 1435, 1195, 1111, 966, 860, and 690 cm−1.
Products of 2f. Methyl-(E)-4-((2-(1-(diphenylphosphoryl)vinyl)-3-phenyl-1H-inden-1-ylidene)methyl)benzoate (2f). A yellow solid (98 mg, 91% yield), m.p.: 185.5–187.4 °C. 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 8.2 Hz, 4H), 7.46 (M, 13H), 7.35 (M, 9H), 7.23 (t, J = 7.9 Hz, 9H), 7.13 (M, 5H), 7.07–6.92 (m, 5H), 6.76 (s, 1H), 6.71 (s, 1H), 6.54 (d, J = 4.6 Hz, 1H), 6.45 (s, 1H), 3.97 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 152.2, 152.1, 132.3, 132.2, 131.9, 131.8, 131.7, 130.3 (d, J = 7.0 Hz), 130.2, 128.4, 128.3, 128.2, 128.0, 127.9, 127.8, 127.6, 89.5, 21.2. 31P NMR (162 MHz, CDCl3) δ 27.80 (s). HRMS (ESI): ([M + H]+) calcd for C37H32O2P+:C38H30O3P:565.1933, found: 565.1931. IR (film) ν 3676, 2985, 2901, 1605, 1524, 1455, 1255, 1108, 1021, 945, 765, and 696 cm−1.
Products of 3g. Diphenyl(1-(1-phenyl-4-(m-tolyl)naphthalen-2-yl)vinyl)phosphine oxide (3g). A yellow solid (70 mg, 67% yield), m.p.: 179.5–181.6 °C. 1H NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.1 Hz, 1H), 7.62–7.52 (m, 7H), 7.40 (m, 16H), 7.25–6.99 (m, 14H), 6.00 (m, 1H), 5.93 (d, J = 8.2 Hz, 1H), 2.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 143.8, 139.0, 138.5, 137.2, 137.0, 134.8 133.6, 133.2, 133.1, 132.4, 132.3 (d, J = 2.7 Hz), 131.9, 131.8, 131.4, 131.1, 130.8, 130.0, 128.9, 128.5, 128.3, 127.8, 127.5, 127.3, 127.0, 125.9, (d, J = 5.1 Hz), 21.3. 31P NMR (162 MHz, CDCl3) δ 28.58 (s). HRMS (ESI): ([M + H]+) calcd for C37H30OP+: 521.2034, found: 521.2033. IR (film) ν 3645, 3408, 3235, 2985, 2917, 1634, 1594, 1435, 1245, 1093, 834, 758, and 695 cm−1.
Products of 3h. Diphenyl(1-(1-phenyl-4-(p-tolyl)naphthalen-2-yl)vinyl)phosphine oxide (3h). A yellow solid (65 mg, 61% yield), m.p.: 167.3–168.8 °C. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.1 Hz, 1H), 7.67–7.47 (m, 7H), 7.46–7.32 (m, 9H), 7.30–7.21 (m, 4H), 7.17 (dd, J = 6.4, 3.0 Hz, 2H), 7.11 (s, 1H), 6.09–5.96 (m, 1H), 5.93 (s, 1H), 2.47 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 144.7, 143.8, 139.0 (s), 138.7 (d, J = 47.4 Hz), 137.2, 137.0, 134.8, 133.6, 133.2 (d, J = 8.5 Hz), 132.3 (d, J = 9.6 Hz), 131.9 (d, J = 2.7 Hz), 131.4, 131.1, 130.8, 130.0, 128.9 128.4 (d, J = 12.0 Hz), 127.8, 127.4 (d, J = 16.2 Hz), 127.0, 125.9 (d, J = 5.6 Hz), 21.3. 31P NMR (162 MHz, CDCl3) δ 28.17 (s), HRMS (ESI): ([M + H]+) calcd for C37H30OP+: 521.2034, found: 521.2039. IR (film) ν 3645, 3051, 2968, 2900, 2228, 1603, 1503, 1432, 1177, 950, 865, and 694 cm−1.
Products of 3i. (1-(4-(4-methoxyphenyl)-1-phenylnaphthalen-2-yl)vinyl)Diphenylphosphine oxide (3i). A yellow solid (91 mg, 80% yield), m.p.: 181.5–183.4 °C. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.3 Hz, 1H), 7.68–7.46 (m, 7H), 7.46–7.31 (m, 9H), 7.30–7.22 (m, 2H), 7.15 (dd, J = 6.4, 3.0 Hz, 2H), 7.09 (s, 1H), 6.99 (d, J = 8.6 Hz, 2H), 6.01 (d, J = 22.2 Hz, 1H), 5.93 (s, 1H), 3.90 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.0, 144.4, 138.7, 138.5, 135.1, 133.6, 133.1, 132.5, 132.3, 132.2, 132.0 (d, J = 2.7 Hz), 131.5 (d, J = 10.3 Hz), 131.1, 130.5, 128.4 (d, J = 12.1 Hz), 127.8, 127.4 (d, J = 18.5 Hz), 127.0, 125.9, 113.7, 55.4. 31P NMR (162 MHz, CDCl3) δ 28.86 (s). HRMS (ESI): ([M + H]+) calcd for C37H30O2P: 537.1983, found: 537.1986. IR (film) ν 3365, 2765, 2656, 2344, 1581, 1492, 1488, 1385, 1055, 883, 745, and 635 cm−1.
Products of 3j. (1-(4-(4-(tert-butyl)phenyl)-1-phenylnaphthalen-2-yl)vinyl)diphenylphosphine oxide (3j). A yellow solid (62 mg, 56% yield), m.p.: 195.2–197.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.3 Hz, 1H), 7.55 (m, 6H), 7.52–7.32 (m, 13H), 7.33–7.24 (m, 4H), 7.13 (m, 4H), 5.95 (dd, J = 29.9, 16.8 Hz, 2H), 1.43 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 150.2, 144.8, 138.9, 138.5, 137.1, 134.8, 133.6, 132.27 (d, J = 9.7 Hz), 131.8, 131.4, 131.0, 129.8, 128.4 (d, J = 12.0 Hz), 127.7, 127.4, 127.0, 126.0 (d, J = 18.2 Hz), 125.1, 124.7, 34.6, 31.6,31.5. 31P NMR (162 MHz, CDCl3) δ 28.82 (s). HRMS (ESI): ([M + H]+) calcd for C40H36OP+: 563.2504, found: 563.2501. IR (film) ν 3012, 2945, 2832, 2375, 1545, 1423, 1425, 1315, 1054, 945, 738, and 645 cm−1.
Products of dendralenes. (1-(4-([1,1’-biphenyl]-4-yl)-1-phenylnaphthalen-2-yl)vinyl)diphenylphosphine oxide (3k). A yellow solid (87 mg, 83% yield), m.p.: 112.1–113.3 °C. 1H NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.3 Hz, 1H), 7.70 (m, 4H), 7.57 (m, 9H), 7.48–7.34 (m, 12H), 7.22–7.11 (m, 3H), 6.00 (d, J = 25.8 Hz, 1H), 5.92 (d, J = 4.3 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 140.8, 140.1, 139.2, 138.9,138.8, 138.5 (d, J = 11.0 Hz), 134.9 (d, J = 9.2 Hz), 133.7, 133.3, 133.2, 132.4,132.3, 131.9,131.8, 131.4, 131.0, 130.53 (s), 130.8, 128.9, 128.5, 128.4, 127.8, 127.6,127.5, 127.2, 127.1, 126.9, 126.1, 126.0, 125.8. 31P NMR (162 MHz, CDCl3) δ 28.27 (s). HRMS (ESI): ([M + H]+) calcd for C42H32OP+: 583.2191, found: 583.2195. IR (film) ν 3668, 2983, 2902, 2214, 1605, 1401, 1254, 1065, 894, and 693 cm−1.
Products of 3i. Diphenyl(1-(1-phenyl-4-(pyridin-3-yl)naphthalen-2-yl)vinyl)phosphine oxide (3i). A yellow solid (74 mg, 70% yield), m.p.: 146.2–148.5 °C. 1H NMR (400 MHz, CDCl3) δ 8.40 (s, 1H), 8.00–7.60 (m, 5H), 7.59–6.82 (m, 18H), 5.97 (dd, J = 54.8, 28.6 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 150.8, 141.2, 140.2, 138.7, 132.1 (d, J = 10.0 Hz), 131.0, 130.2 (d, J = 18.0 Hz), 128.5, 128.4, 128.0, 127.8, 127.7, 126.7, 123.0, 100.0, 96.4. 31P NMR (162 MHz, CDCl3) δ 28.62 (s). HRMS (ESI): ([M + H]+) calcd for C35H27NOP+: 508.1830, found: 508.1835. IR (film) ν 3653, 3382, 2933, 2223, 1548, 1435, 1173, 957, 795, and 695 cm−1.
Products of 3m. Diphenyl(1-(1-phenyl-4-(thiophen-3-yl)naphthalen-2-yl)vinyl)phosphine oxide (3m). A yellow solid (76 mg, 75% yield), m.p.: 173.2–174.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.08 (d, J = 8.4 Hz, 1H), 7.60–7.49 (m, 5H), 7.48–7.33 (m, 9H), 7.20 (M, 1H), 7.14 (M, 3H), 5.97 (dd, J = 30.5, 20.9 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 140.5, 139.0, 138.2, 135.6, 133.7 (d, J = 29.3 Hz), 132.4, 132.3, 132.1, 131.3, 131.2, 129.6, 128.6, 128.5, 128.4, 127.8, 127.7, 127.6, 127.4, 127.1, 127.0, 126.2, 126.1, 126.0, 125.7, 125.5, 125.3, 123.7. 31P NMR (162 MHz, CDCl3) δ 30.37 (s). HRMS (ESI): ([M + H]+) calcd for C34H26OPS+: 513.1442, found: 513.1447. IR (film) ν 3675, 3050, 2223, 1678, 1585, 1437, 1314, 126.6, 117.03, 112.7, 903, and 853 cm−1.
4. Conclusions
In summary, we revealed that an unprecedented substituent electron effect regulates the cyclization reactions of conjugated alkynes via Lewis acid catalysis, providing a novel and efficient method for the construction of cyclic-(E)-[3]dendralenes from alkynes with remarkable functional group tolerance and good yields. This strategy features easily accessible substrates, operational simplicity, and expands the pool of cyclic-[3]dendralenes in organic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28114382/s1. Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org. Reference [39] is cited in the Supplementary Materials.
Author Contributions
Methodology, Y.-T.X., W.L., M.Y. and T.G.; Validation, Y.-T.X., Y.-X.L., T.-T.B. and X.W.; Writing—original draft, Y.-T.X. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the Foundation Research Project of Jiangsu Province (the Natural Science Foundation, No. BK20191305), Ph.D. Foundation of Henan University of Technology (No. 2021BS026), Colleges and Universities Key Research Program Foundation of Henan Province (No. 22A150037), the Innovative Funds Plan of Henan University of Technology (No. 2020ZKCJ29), Natural Science Foundation of Henan Province (No. 232300420381, 232300421375), Natural Science Project of Zhengzhou Science and Technology Bureau (No. 22ZZRDZX08), and Science and Technology Foundation of Henan Province (No. 222102230069).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study can be found in the article or in the associated Supplementary Materials.
Acknowledgments
Authors wish to thank all colleagues and lab technicians that helped in developing this study.
Conflicts of Interest
The authors declare no competing financial interests.
Sample Availability
Samples of the compounds 2b–2f and 3g–n are available from the authors.
References
- Sherburn, M.S. Preparation and Synthetic Value of π-Bond-Rich Branched Hydrocarbons. Acc. Chem. Res. 2015, 48, 1961. [Google Scholar] [CrossRef]
- Mackay, E.G.; Sherburn, M.S. Demystifying the dendralenes. Pure Appl. Chem. 2013, 85, 1227. [Google Scholar] [CrossRef]
- Hopf, H. Forgotten hydrocarbons prepared. Nature 2009, 460, 183. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.D.; Bojase, G.; Paddon-Row, M.N.; Sherburn, M.S. Practical Synthesis of the Dendralene Family Reveals Alternation in Behavior. Angew. Chem. Int. Ed. 2009, 48, 4836. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Amamoto, S.; Kishi, H.; Takeshita, H.; Miya, M.; Shiomi, T. Anionic Polymerization of 2-Phenyl[3]dendralene and 2-(4-Methoxyphenyl)[3]dendralene. Macromolecules 2013, 46, 7282. [Google Scholar] [CrossRef]
- Takamura, Y.; Takenaka, K.; Toda, T.; Takeshita, H.; Miya, M.; Shiomi, T. Anionic Polymerization of 2-Hexyl [3]dendralene. Macromol. Chem. Phys. 2018, 219, 1700046. [Google Scholar] [CrossRef]
- Ravinder, P.; Subramanian, V. Theor. Density functional theory studies on the Diels–Alder reaction of [3]dendralene with C60: An attractive approach for functionalization of fullerene. Chem. Acc. 2012, 131, 1128. [Google Scholar] [CrossRef]
- Paddon-Row, M.N.; Sherburn, M.S. On the origin of the alternating Diels–Alder reactivity in [n]dendralenes. Chem. Commun. 2012, 48, 832. [Google Scholar] [CrossRef]
- Saglam, M.F.; Fallon, T.; Paddon-Row, M.N.; Sherburn, M.S. Discovery and Computational Rationalization of Diminishing Alternation in [n]Dendralenes. J. Am. Chem. Soc. 2016, 138, 1022. [Google Scholar] [CrossRef]
- Hosoya, H. Mathematical Features of the Genealogy of Acyclic Conjugated Polyenes. Bull. Chem. Soc. Jpn. 2019, 92, 205. [Google Scholar] [CrossRef]
- Breiten, B.; Wu, Y.-L.; Jarowski, P.D.; Gisselbrecht, J.-P.; Boudon, C.; Griesser, M.; Onitsch, C.; Gescheidt, G.; Schweizer, W.B.; Langer, N.; et al. Donor-substituted octacyano [4]dendralenes: A new class of cyano-rich non-planar organic acceptors. Chem. Sci. 2011, 2, 88. [Google Scholar]
- Shoji, T.; Miura, K.; Araki, T.; Maruyama, A.; Ohta, A.; Sekiguchi, R.; Ito, S.; Okujima, T. Synthesis of 2-Methyl-1-azulenyl Tetracyanobutadienes and Dicyanoquinodimethanes: Substituent Effect of 2-Methyl Moiety on the Azulene Ring toward the Optical and Electrochemical Properties. J. Org. Chem. 2018, 83, 6690. [Google Scholar] [CrossRef]
- Michinobu, T.; Diederich, F. The [2 + 2] Cycloaddition-Retroelectrocyclization (CA-RE) Click Reaction: Facile Access to Molecular and Polymeric Push-Pull Chromophores. Angew. Chem. Int. Ed. 2018, 57, 3552. [Google Scholar] [CrossRef]
- Pronin, S.V.; Shenvi, R.A. Synthesis of a Potent Antimalarial Amphilectene. J. Am. Chem. Soc. 2012, 134, 19604. [Google Scholar] [PubMed]
- Volla, C.M.R.; Bäckvall, J.-E. Palladium-Catalyzed Aerobic Domino Oxidative Carbocyclization-Alkynylation of Allenynes. Angew. Chem. Int. Ed. 2013, 52, 14209. [Google Scholar]
- Fallon, T.; Willis, A.C.; Paddon-Row, M.N.; Sherburn, M.S. Furanodendralenes. J. Org. Chem. 2014, 79, 3185. [Google Scholar] [PubMed]
- Bartholomeyzik, T.; Mazuela, J.; Pendrill, R.; Deng, Y.; Bäckvall, J.-E. Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes: Control of Selectivity and Role of H2O. Angew. Chem. Int. Ed. 2014, 53, 8696. [Google Scholar] [CrossRef]
- Thies, N.; Haak, E. Ruthenium-Catalyzed Synthesis of 2,3-Cyclo[3]dendralenes and Complex Polycycles from Propargyl Alcohols. Angew. Chem. Int. Ed. 2015, 54, 4097. [Google Scholar] [CrossRef]
- Tan, S.M.; Willis, A.C.; Paddon-Row, M.N.; Sherburn, M.S. Multicomponent Diene-Transmissive Diels–Alder Sequences Featuring Aminodendralenes. Angew. Chem. Int. Ed. 2016, 55, 3081. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, K.; Shibata, Y.; Tanaka, K. Rhodium-Catalyzed Cross-Cyclotrimerization and Dimerization of Allenes with Alkynes. Angew. Chem. Int. Ed. 2016, 55, 6753. [Google Scholar] [CrossRef]
- Lippincott, D.J.; Linstadt, R.T.H.; Maser, M.R.; Lipshutz, B.H. Synthesis of Functionalized [3], [4], [5] and [6]Dendralenes through Palladium-Catalyzed Cross-Couplings of Substituted Allenoates. Angew. Chem. Int. Ed. 2017, 56, 847. [Google Scholar] [CrossRef]
- Qiu, Y.; Posevins, D.; Bäckvall, J.-E. Selective Palladium-Catalyzed Allenic C−H Bond Oxidation for the Synthesis of [3]Dendralenes. Angew. Chem. Int. Ed. 2017, 56, 13112. [Google Scholar] [CrossRef]
- Rivera-Chao, E.; Fañanás-Mastral, M. Synthesis of Stereodefined Borylated Dendralenes through Copper-Catalyzed Allylboration of Alkynes. Angew. Chem. Int. Ed. 2018, 57, 9945. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, P.; Deng, Y.; Shao, Z. Regioselectivity Switch in Palladium-Catalyzed Allenylic Cycloadditions of Allenic Esters: [4 + 1] or [4 + 3] Cycloaddition/Cross-Coupling. Angew. Chem. Int. Ed. 2019, 131, 4758. [Google Scholar] [CrossRef]
- Li, H.; Gontla, R.; Flegel, J.; Merten, C.; Ziegler, S.; Antonchick, A.P.; Waldmann, H. Enantioselective Formal C(sp3)−H Bond Activation in the Synthesis of Bioactive Spiropyrazolone Derivatives. Angew. Chem. Int. Ed. 2019, 58, 307. [Google Scholar] [CrossRef]
- Li, S.C.; Hou, B.; Wang, J.B. Palladium-Catalyzed Oxidative Coupling of the Allenic C–H Bond with α-Diazo Esters: Synthesis of [3]Dendralenes. J. Org. Chem. 2021, 86, 5371–5379. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.; Sherburn, M.S. Dendralenes Branch Out: Cross-Conjugated Oligoenes Allow the Rapid Generation of Molecular Complexity. Angew. Chem. Int. Ed. 2012, 51, 2298. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.C.; Zhang, C.Y.; Wu, L. Recent Progress in the Synthesis of Dendralenes: A Decade Update. Chin. J. Org. Chem. 2021, 41, 1081. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, L.; Chen, Y.-Z.; Zhu, J.; Wu, L. Palladium-Catalyzed Coupling of Allenylphosphine Oxides with N-Tosylhydrazones toward Phosphinyl [3]Dendralenes. ACS Catal. 2017, 7, 181. [Google Scholar] [CrossRef]
- Bartholomeyzik, T.; Pendrill, R.; Lihammar, R. Kinetics and Mechanism of the Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes. J. Am. Chem. Soc. 2018, 140, 298. [Google Scholar] [CrossRef]
- Wei, K.; Luo, K.; Liu, F.; Wu, L.; Wu, L.-Z. Visible-Light-Driven Selective Alkenyl C–P Bond Cleavage of Allenylphosphine Oxides. Org. Lett. 2019, 21, 1994. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mao, M.; Ji, H.-J.; Xu, J.-Y.; Wu, L. Palladium-Catalyzed Cleavage of α-Allenylic Aryl Ether toward Pyrazolemethylene-Substituted Phosphinyl Allenes and Their Transformations via Alkenyl C–P(O) Cleavage. Org. Lett. 2017, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, J.; Ma, J.; Wu, L.; Zhang, W.-H. Visible-Light-Driven α-Allenylic C–O Bond Cleavage and Alkenyl C–S Formation: Metal-Free and Oxidant-Free Thiolation of Allenyl Phosphine Oxides. Org. Lett. 2017, 19, 6308. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.T.; Wu, J.J.; Zhang, C.Y.; Mao, M.; Ji, Y.G.; Wu, L.; Zhang, W.H. Cascade Alkynylation and Highly Selective Hydrogenation Catalyzed by Binaphthyl-Palladium Nanoparticles Accessing Phosphinyl (Z)-[3]Dendralenes. Org. Lett. 2019, 21, 6383. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, L.; Lu, A.-M.; Yang, F.; Wu, L. α-Allenyl Ethers as Starting Materials for Palladium Catalyzed Suzuki–Miyaura Couplings of Allenylphosphine Oxides with Arylboronic Acids. J. Org. Chem. 2015, 80, 673. [Google Scholar] [CrossRef]
- Liu, P.; Deng, C.-L.; Lei, X.; Lin, G. Tandem Amination/Cycloisomerization of Aryl Propargylic Alcohols with 2-Aminopyridines as an Expedient Route to Imidazo [1,2-a]pyridines. Eur. J. Org. Chem. 2011, 2011, 7308–7316. [Google Scholar] [CrossRef]
- Xia, Y.T.; Xie, X.Y.; Cui, S.H.; Ji, Y.G.; Wu, L. Secondary phosphine oxides stabilized Au/Pd nanoalloys: Metal components-controlled regioselective hydrogenation toward phosphinyl (Z)-[3]dendralenes. Chem. Commun. 2019, 55, 11699. [Google Scholar] [CrossRef]
- Franco, M.; Azzena, U.; Melloni, G. A novel synthesis of methylene-1H-indenes (benzofulvenes) by cyclisation of phenyl-substituted but-1-en-3-ynes. J. Chem. Soc. Perkin Trans. 1993, 1, 2957. [Google Scholar]
- Luo, X.-L.; Chen, X.-W.; Chen, L.; Zhang, K.; Chen, Y.-B. Xanthate-mediated synthesis of (E)-alkenes by semi-hydrogenation of alkynes using water as the hydrogen donor. Chem. Commun. 2019, 55, 2170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).