Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa
Abstract
1. Introduction
2. Results
2.1. SL Accumulation in Flower Heads in Arnica Taxa
2.2. SLs in Leaves
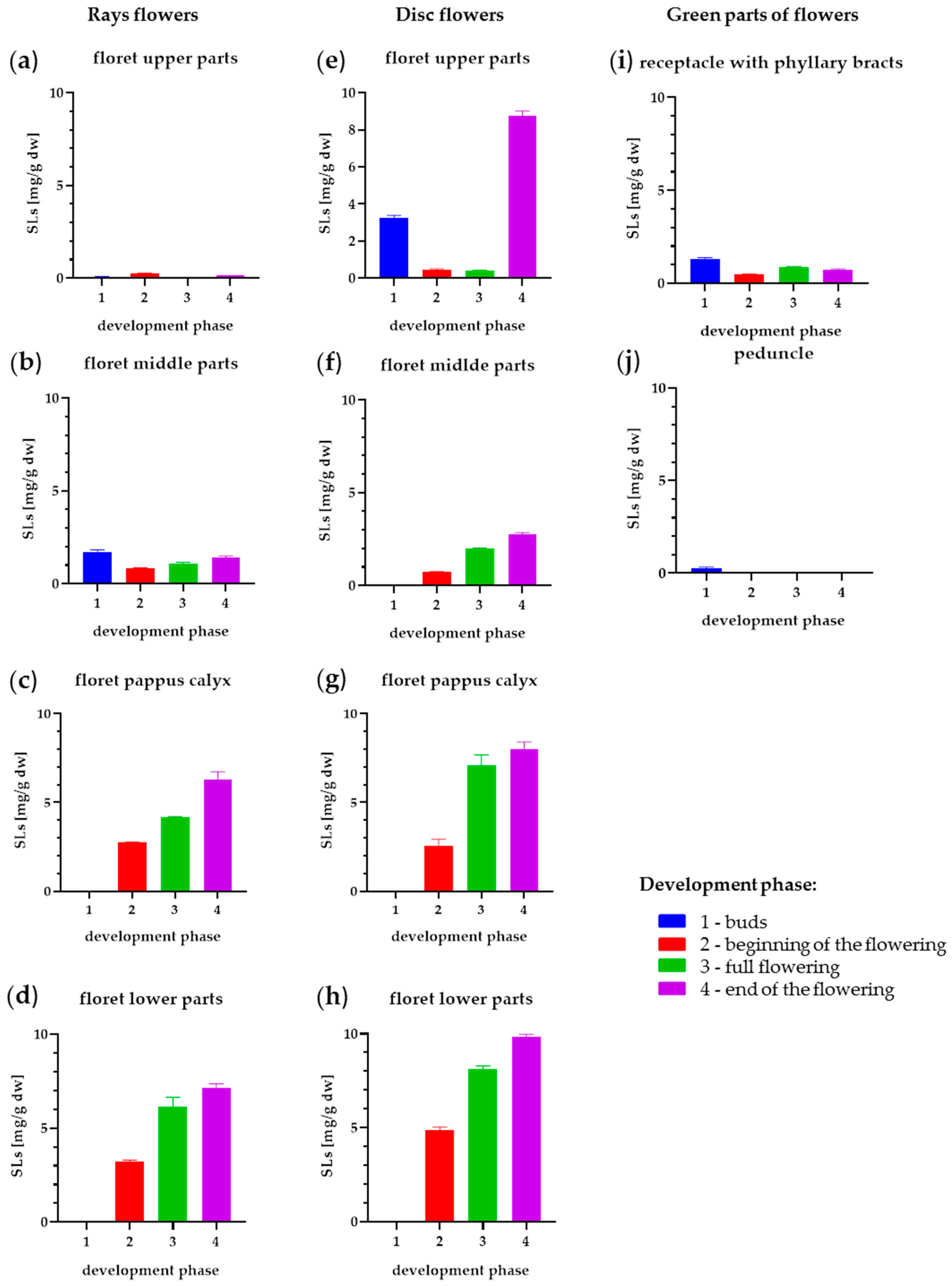
2.3. Influence of Flower Development Stages of SLs
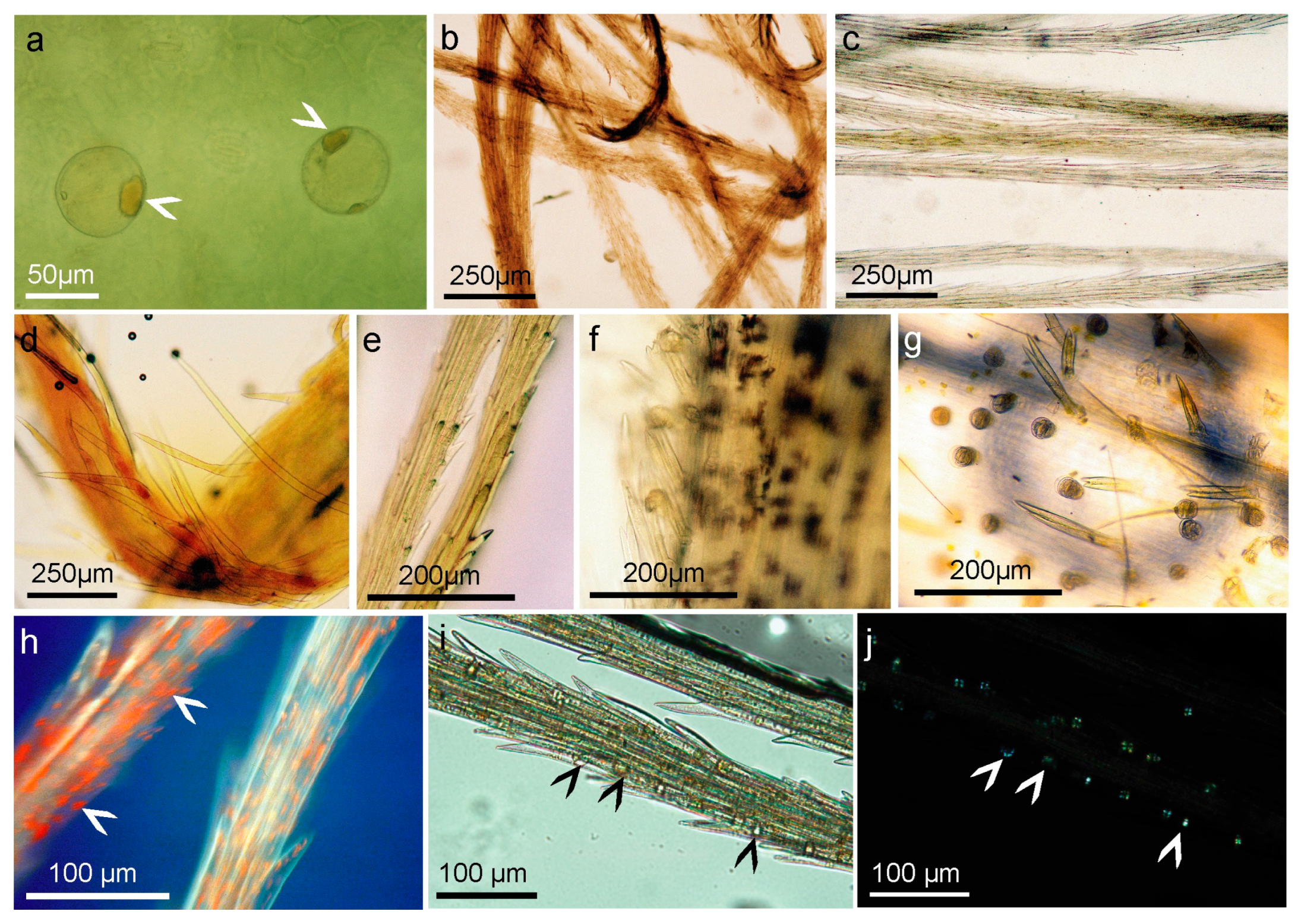
2.4. Structure and Morphology of Flower Trichomes
2.5. Histochemical Analysis
3. Discussion
3.1. SL Accumulation in Flower Heads in Arnica Taxa
3.2. Histochemistry Studies
4. Materials and Methods
4.1. Plant Material
4.2. Reagents
4.3. LC-QTOF-MS Quantitative Analysis and Method Validation
Method Validation
- Linearity
- Accuracy and precision
- Recovery
- Limit of detection and limit of quantification
4.4. Extraction of SLs
4.5. Sample Preparation for Histochemistry Analysis
4.6. Immunolocalization of Farnesyl Policlonal Antibody
4.7. Histochemistry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviation
| ABA | abscisic acid |
| C/EBPβ | CCAAT/enhancer binding protein |
| CV | coefficient of variation |
| DAMPs | damage-associated molecular patterns |
| DH | 11α, 13-dihydrohelenalin |
| DHA | acetyldihydrohelenalin |
| DHIB | isobutyryldihydrohelenalin |
| DHIV | isovaleryldihydrohelenalin |
| DHM | methacryloyldihydrohelenalin |
| DHMB | 2-methylbutyryldihydrohelenalin |
| DHT | tigloyldihydrohelenalin |
| DMAPP | dimethylallyl pyrophosphate |
| FDS | farnesyl diphosphate synthase |
| FPP | farnesyl pyrophosphate |
| GM-CSF | regulatory link between granulocyte macrophage-colony stimulating factor |
| H | helenalin |
| HA | helenalin acetate |
| HIB | isobutyrylhelenalin |
| HIV | isovalerylhelenalin |
| HM | methacryloylhelenalin |
| HMB | 2-methylbutyrylhelenalin |
| HPLC | high-performance liquid chromatography |
| HT | tigloylhelenalin |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MALDI-MS | matrix-assisted laser desorption/ionization-mass spectrometry imaging |
| MAMPs | microbe-associated molecular patterns |
| MEP | 2-C-methyl-D-erythritol-4-phosphate |
| MVA | mevalonate |
| NF-κB | nuclear factor kappa-light-chain-enhancer |
| PAP | primary cause of lung disease |
| PPI | isopentenyl diphosphate |
| RSD | relative standard deviation |
| SD | standard deviation |
| SLs | sesquiterpene lactones |
| SNARE | soluble N-ethylmaleimide-sensitive-factor attachment protein receptor |
| STPS | sesquiterpene synthases |
| UHPLC-MS | ultra-high performance liquid chromatography-mass spectrometry |
References
- Rodríguez-Chávez, J.L.; Egas, V.; Linares, E.; Bye, R.; Hernández, T.; Espinosa-García, F.J.; Delgado, G. Mexican Arnica (Heterotheca Inuloides Cass. Asteraceae: Astereae): Ethnomedical Uses, Chemical Constituents and Biological Properties. J. Ethnopharmacol. 2017, 195, 39–63. [Google Scholar] [CrossRef] [PubMed]
- Kriplani, P.; Guarve, K.; Baghael, U.S. Arnica montana L.—A Plant of Healing: Review. J. Pharm. Pharmacol. 2017, 69, 925–945. [Google Scholar] [CrossRef] [PubMed]
- Duthen, S.; Gadéa, A.; Trempat, P.; Boujedaini, N.; Fabre, N. Comparison of the Phytochemical Variation of Non-Volatile Metabolites within Mother Tinctures of Arnica montana Prepared from Fresh and Dried Whole Plant Using UHPLC-HRMS Fingerprinting and Chemometric Analysis. Molecules 2022, 27, 2737. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Keeling, C.I.; Ghisalberti, E.L.; Barbour, E.L.; Plummer, J.A.; Bohlmann, J. Isolation of CDNAs and Functional Characterisation of Two Multi-Product Terpene Synthase Enzymes from Sandalwood, Santalum album L. Arch. Biochem. Biophys. 2008, 477, 121–130. [Google Scholar] [CrossRef]
- Diaz-Chavez, M.L.; Moniodis, J.; Madilao, L.L.; Jancsik, S.; Keeling, C.I.; Barbour, E.L.; Ghisalberti, E.L.; Plummer, J.A.; Jones, C.G.; Bohlmann, J. Biosynthesis of Sandalwood Oil: Santalum Album CYP76F Cytochromes P450 Produce Santalols and Bergamotol. PLoS ONE 2013, 8, e75053. [Google Scholar] [CrossRef]
- Weitzel, C.; Simonsen, H.T. Cytochrome P450-Enzymes Involved in the Biosynthesis of Mono- and Sesquiterpenes. Phytochem. Rev. 2015, 14, 7–24. [Google Scholar] [CrossRef]
- Herz, W. Biogenetic Aspects of Sesquiterpene Lactone Chemistry. Isr. J. Chem. 1977, 16, 32–44. [Google Scholar] [CrossRef]
- Seaman, F.C. Sesquiterpene Lactones as Taxonomic Characters in the Asteraceae. Bot. Rev. 1982, 48, 121–595. [Google Scholar] [CrossRef]
- Barbero, M.; Prandi, C. Pseudoguaianolides: Recent Advances in Synthesis and Applications. Nat. Prod. Commun. 2018, 13, 241–248. [Google Scholar] [CrossRef]
- Roberts, M.; Schlessinger, R. Total Synthesis of Dl-Helenalin. J. Am. Chem. Soc. 1979, 101, 7626–7627. [Google Scholar] [CrossRef]
- Grieco, P.; Ohfune, Y.; Majetich, G.; Wang, C. Pseudoguaianolides. 2. Stereocontrolled Total Synthesis of the Helenanolide Dl-Helenalin. J. Am. Chem. Soc. 1982, 104, 4233–4240. [Google Scholar] [CrossRef]
- Money, T.; Wong, M.K.C. A Formal, Enantiospecific Synthesis of Pseudoguaianolides. Tetrahedron 1996, 52, 6307–6324. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant Volatile Terpenoid Metabolism: Biosynthetic Genes, Transcriptional Regulation and Subcellular Compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- Roberts, S. Production and Engineering of Terpenoids in Plant Cell Culture. Nat. Chem. Biol. 2007, 3, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Fray, R.G.; Wallace, A.; Fraser, P.D.; Valero, D.; Hedden, P.; Bremley, P.M.; Grierson, D. Constitutive Expression of a Fruit Phytoene Synthase Gene in Transgenic Tomatoes Causes Dwarfism by Redirecting Metabolites from the Gibberellin Pathway. Plant J. 1995, 8, 693–701. [Google Scholar] [CrossRef]
- Pasoreck, E.K.; Su, J.; Silverman, I.M.; Gosai, S.J.; Gregory, B.D.; Yuan, J.S.; Daniell, H. Terpene Metabolic Engineering via Nuclear or Chloroplast Genomes Profoundly and Globally Impacts Off-Target Pathways through Metabolite Signalling. Plant Biotechnol. J. 2016, 14, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Lange, B.M.; Ahkami, A. Metabolic Engineering of Plant Monoterpenes, Sesquiterpenes and Diterpenes—Current Status and Future Opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Subramaniyan, M.; Rawat, K.; Qureshi, M.I.; Malhotra, P.; Mohmmed, A.; Cornish, K.; Daniell, H.; Kumar, S. Compartmentalized Metabolic Engineering for Artemisinin Biosynthesis and Effective Malaria Treatment by Oral Delivery of Plant Cells. Mol. Plant 2016, 9, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an Anti-Inflammatory Sesquiterpene Lactone from Arnica, Selectively Inhibits Transcription Factor NF-ΚB. Biol. Chem. 1997, 378, 951–961. [Google Scholar] [CrossRef]
- Hall, I.H.; Lee, K.H.; Starnes, C.O.; Sumida, Y.; Wu, R.Y.; Waddell, T.G.; Cochran, J.W.; Gerhart, K.G. Anti-Inflammatory Activity of Sesquiterpene Lactones and Related Compounds. Pharm. Sci. 1979, 68, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, A.; Steinmann, S.; Henrich, S.M.; Schmidt, T.J.; Klempnauer, K.H. Helenalin Acetate, a Natural Sesquiterpene Lactone with Anti-Inflammatory and Anti-Cancer Activity, Disrupts the Cooperation of CCAAT-Box/Enhancer-Binding Protein Beta (C/EBPβ) and Co-Activator P300. J. Biol. Chem. 2016, 291, 26098–26108. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Lyss, G.; Pahl, H.L.; Merfort, I. Helenanolide Type Sesquiterpene Lactones. Part 5: The Role of Glutathione Addition Under Physiological Conditions. Bioorganic Med. Chem. 1999, 7, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pineres, A.J.; Castro, V.; Mora, G.; Schmidt, T.J.; Pahl, H.L.; Merfort, I.; Garcı, A.J.; Mora, G.; Schmidt, T.J.; Strunck, E.; et al. Mechanism of Signal Transduction: Cysteine 38 in P65/NF-ΚB Plays a Crucial Role in DNA Binding Inhibition by Sesquiterpene Lactones. J. Biol. Chem. 2001, 276, 39713–39720. [Google Scholar] [CrossRef] [PubMed]
- Dörr, D.; Obermayer, B.; Weiner, J.M.; Zimmermann, K.; Anania, C.; Wagner, L.K.; Lyras, E.M.; Sapozhnikova, V.; Lara-Astiaso, D.; Prósper, F.; et al. C/EBPβ Regulates Lipid Metabolism and Pparg Isoform 2 Expression in Alveolar Macrophages. Sci. Immunol. 2022, 7, eabj0140. [Google Scholar] [CrossRef] [PubMed]
- Arnicae flos; European Pharmacopoeia (EP 10.8); European Directorate for the Quality of Medicines and HealthCare: Strasbourg, France, 2022; pp. 6849–6851.
- Douglas, J.A.; Smallfield, B.M.; Burgess, E.J.; Perry, N.B.; Anderson, R.E.; Douglas, M.H.; Glennie, V.L.A. Sesquiterpene Lactones in Arnica montana: A Rapid Analytical Method and the Effects of Flower Maturity and Simulated Mechanical Harvesting on Quality and Yield. Planta Med. 2004, 70, 166–170. [Google Scholar]
- Perry, N.B.; Burgess, E.J.; Rodríguez Guitián, M.A.; Franco, R.R.; Mosquera, E.L.; Smallfield, B.M.; Joyce, N.I.; Littlejohn, R.P. Sesquiterpene Lactones in Arnica Montana: Helenalin and Dihydrohelenalin Chemotypes in Spain. Planta Med. 2009, 75, 660–666. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Rančić, D.; Ristić, M.; Vujisić, L.; Radanović, D.; Dajić-Stevanović, Z. Rhizome and Root Yield of the Cultivated Arnica Montana L., Chemical Composition and Histochemical Localization of Essential Oil. Ind. Crops Prod. 2012, 39, 177–189. [Google Scholar] [CrossRef]
- Sugier, D. Plon i Skład Chemiczny Surowca Arniki Górskiej (Arnica montana L.) w Zależności Od Sposobu Zakładania Plantacji i Terminu Zbioru Koszyczków Kwiatowych. Agron. Sci. 2013, 68, 51–62. [Google Scholar] [CrossRef]
- Seemann, A.; Wallner, T.; Poschlod, P.; Heilmann, J. Variation of Sesquiterpene Lactone Contents in Different Arnica montana Populations: Influence of Ecological Parameters. Planta Med. 2010, 76, 837–842. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Gawlik-Dziki, U. Propagation and Introduction of Arnica montana L. into Cultivation: A Step to Reduce the Pressure on Endangered and High-Valued Medicinal Plant Species. Sci. World J. 2013, 2013, 414363. [Google Scholar] [CrossRef] [PubMed]
- Willuhn, G.; Rottger, P.-M.; Uwe, M.; Matthiesen, U. Helenalin-and 11,1 3-Dihydrohelenalinester from Flowers of Arnica montana. Planta Med. 1983, 49, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Spitaler, R.; Schlorhaufer, P.D.; Ellmerer, E.P.; Merfort, I.; Bortenschlager, S.; Stuppner, H.; Zidorn, C. Altitudinal Variation of Secondary Metabolite Profiles in Flowering Heads of Arnica montana Cv. ARBO. Phytochemistry 2006, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.; Trendafilova, A.; Vitkova, A.; Petrova, M.; Zayova, E.; Antonova, D. Developmental and Environmental Effects on Sesquiterpene Lactones in Cultivated Arnica montana L. Chem. Biodivers. 2016, 13, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Olofsson, L.M.; Lindahl, A.L.; Lundgren, A.; Brodelius, M.; Brodelius, P.E. Localization of Enzymes of Artemisinin Biosynthesis to the Apical Cells of Glandular Secretory Trichomes of Artemisia annua L. Phytochemistry 2009, 70, 1123–1128. [Google Scholar] [CrossRef]
- Livingston, S.J.; Bae, E.J.; Unda, F.; Hahn, M.G.; Mansfield, S.D.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichome Cell Walls Undergo Remodeling to Store Specialized Metabolites. Plant Cell Physiol. 2021, 62, 1944–1962. [Google Scholar] [CrossRef]
- Amrehn, E.; Aschenbrenner, A.K.; Heller, A.; Spring, O. Localization of Sesquiterpene Lactone Biosynthesis in Cells of Capitate Glandular Trichomes of Helianthus annuus (Asteraceae). Protoplasma 2016, 253, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.A.; Pina, E.S.; Silva, D.B.; Pereira, A.M.S.; da Silva, M.F.D.G.F.; da Costa, F.B.; Lopes, N.P.; Pupo, M.T. Biosynthetic Pathway of Sesquiterpene Lactones in Smallanthus sonchifolius and Their Localization in Leaf Tissues by MALDI Imaging. Chem. Commun. J. 2013, 3, 10715–10722. [Google Scholar] [CrossRef]
- Silva, D.B.; Aschenbrenner, A.K.; Lopes, N.P.; Spring, O. Direct Analyses of Secondary Metabolites by Mass Spectrometry Imaging (MSI) from Sunflower (Helianthus annuus L.) Trichomes. Molecules 2017, 22, 774. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Dudai, N.; Tadmor, Y.; Katzir, I.; Ravid, U.; Putievsky, E.; Joel, D.M. Histochemical Localization of Citral Accumulation in Lemongrass Leaves (Cymbopogon citratus (DC.) Stapf., Poaceae). Ann. Bot. 1998, 81, 35–39. [Google Scholar] [CrossRef]
- Merfort, I. Review of the Analytical Techniques for Sesquiterpenes and Sesquiterpene Lactones. J. Chromatogr. A 2002, 967, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Picman, A.K.; Ranieri, R.I.; Towers, G.H.N.; Lam, J. Visualization Reagents for Sesquiterpene Lactones and Polyacetylenes on Thin-Layer Chromatograms. J. Chromatogr. 1980, 189, 187–198. [Google Scholar] [CrossRef]
- Schmiderer, C.; Andrea, P.T.; Duwe, V.K.; Novak, J. Arnica montana Subsp. Atlantica: Really a Subspecies? Genet. Resour. Crop Evol. 2018, 65, 1829–1838. [Google Scholar]
- Vera, M.; Romero, R.G.; Rodrguez-Guitián, M.A.; Barros, R.M.; Real, C.; Bouza, C. Phylogeography and Genetic Variability of the Arnica montana Chemotypes in NW Iberian Peninsula. Silvae Genet. 2015, 63, 293–300. [Google Scholar] [CrossRef]
- Smallfield, B.M.; Douglas, M.H. Arnica montana a Grower’s Guide for Commercial Production in New Zealand; New Zealand Institute for Crop and Food Research Limited: Christchurch, New Zealand, 2008. [Google Scholar]
- Wagner, S.; Suter, A.; Merfort, I. Skin Penetration Studies of Arnica Preparations and of Their Sesquiterpene Lactones. Planta Med. 2004, 70, 897–903. [Google Scholar] [CrossRef]
- Lyss, G.; Knorre, A.; Schmidt, T.J.; Pahl, H.L.; Merfort, I. The Anti-Inflammatory Sesquiterpene Lactone Helenalin Inhibits the Transcription Factor NF-ΚB by Directly Targeting P65. J. Biol. Chem. 1998, 273, 33508–33516. [Google Scholar] [CrossRef] [PubMed]
- Siedle, B.; García-Piñeres, A.J.; Murillo, R.; Schulte-Mönting, J.; Castro, V.; Rüngeler, P.; Klaas, C.A.; Da Costa, F.B.; Kisiel, W.; Merfort, I. Quantitative Structure-Activity Relationship of Sesquiterpene Lactones as Inhibitors of the Transcription Factor NF-ΚB. J. Med. Chem. 2004, 47, 6042–6054. [Google Scholar] [CrossRef] [PubMed]
- Lass, C.; Vocanson, M.; Wagner, S.; Schempp, C.M.; Nicolas, J.F.; Merfort, I.; Martin, S.F. Anti-Inflammatory and Immune-Regulatory Mechanisms Prevent Contact Hypersensitivity to Arnica montana L. Exp. Dermatol. 2008, 17, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Sugier, P.; Rysiak, A.; Sugier, D.; Winiarczyk, K.; Wołkowycki, D.; Kołos, A. Differentiation and Propagation Potential of Arnica montana L. Achenes as a Consequence of the Morphological Diversity of Flowers and the Position of Flower Heads on the Plant. Plants 2022, 11, 3424. [Google Scholar] [CrossRef]
- Barkhordari, A.; Jafari-Gharabaghlou, D.; Turk, Z. Potential Anti-Cancer Effect of Helenalin as a Natural Bioactive Compound on the Growth and Telomerase Gene Expression in Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2023, 24, 133–140. [Google Scholar] [CrossRef]
- Drogosz, J.; Janecka, A. Helenalin—A Sesquiterpene Lactone with Multidirectional Activity. Curr. Drug Targets 2018, 20, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Widen, J.C.; Kempema, A.M.; Baur, J.W.; Skopec, H.M.; Edwards, J.T.; Brown, T.J.; Brown, D.A.; Meece, F.A.; Harki, D.A. Helenalin Analogues Targeting NF-KB P65: Thiol Reactivity and Cellular Potency Studies of Varied Electrophiles. ChemMedChem 2018, 13, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Falniowski, A.; Bazos, I.; Hodálová, I.; Lansdown, R.; Petrova, A. Arnica montana. The IUCN Red List of Threatened Species 2013: e.T162327A5574104. 2013. Available online: https://www.gbif.org/species/176793983 (accessed on 24 May 2023).
- Luijten, S.H.; Dierick, A.; Gerard, J.; Oostermeijer, B.; Raijmann, L.E.L.; Den Nijs, H.C.M. Population Size, Genetic Variation, and Reproductive Success in a Rapid Declining, Self-Incompatible Perennial (Arnica montana) in The Netherlands. Conserv. Biol. 2000, 14, 1776–1787. [Google Scholar] [PubMed]
- Forycka, A.; Buchwald, W. Badania Zasobów Naturalnych Roślin Leczniczych Objętych w Polsce Ochroną Prawną Badania Zasobów Naturalnych Roślin Leczniczych Objętych w Polsce Ochroną Prawną. Herba Pol. 2008, 54, 82–112. [Google Scholar]
- Buza, L.; Young, A.; Thrall, P. Genetic Erosion, Inbreeding and Reduced Fitness in Fragmented Populations of the Endangered Tetraploid Pea swainsona Recta. Biol. Conserv. 2000, 93, 177–186. [Google Scholar] [CrossRef]
- Ritsema, T.; Smeekens, S. Fructans: Beneficial for Plants and Humans. Curr. Opin. Plant Biol. 2003, 6, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pontes, A.G.O.; Silva, K.L.; Fonseca, S.G.D.C.; Soares, A.A.; Feitosa, J.P.D.A.; Braz-Filho, R.; Romero, N.R.; Bandeira, M.A.M. Identification and Determination of the Inulin Content in the Roots of the Northeast Brazilian Species Pombalia calceolaria L. Carbohydr. Polym. 2016, 149, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, A.V.; Simpson, J. The Sweet Taste of Adapting to the Desert: Fructan Metabolism in Agave Species. Front. Plant Sci. 2020, 11, 324. [Google Scholar] [CrossRef]
- Pérez-López, A.V.; Simpson, J.; Clench, M.R.; Gomez-Vargas, A.D.; Ordaz-Ortiz, J.J. Localization and Composition of Fructans in Stem and Rhizome of Agave tequilana Weber Var. Azul. Front. Plant Sci. 2021, 11, 2309. [Google Scholar] [CrossRef]
- Vijn, I.; Smeekens, S. Fructan: More than a Reserve Carbohydrate? Plant Physiol. 1999, 120, 351–359. [Google Scholar] [CrossRef]
- Versluys, M.; Kirtel, O.; Toksoy Öner, E.; Van den Ende, W. The Fructan Syndrome: Evolutionary Aspects and Common Themes among Plants and Microbes. Plant Cell Environ. 2018, 41, 16–38. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, W. Multifunctional Fructans and Raffinose Family Oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [PubMed]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In Vitro and in Vivo Antioxidant Activities of Inulin. PLoS ONE 2018, 13, e0192273. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.J. Tansley Review No. 5 Fructans and the Metabolism of Sucrose in Vascular Plants. New Phytol. 1986, 104, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Vilhalva Divina, A.A.; Cortelazzo Angelo, L.; Carvalho, A.L.; Maria Angela, M.; Figueiredo-Ribeiro Rita de Cássia, L. Histochemistry and Ultrastructure of Campuloclinium chlorolepis (Asteraceae) Tuberous Roots Accumulating Fructan: Evidences of Functions Other than Reserve Carbohydrate. Aust. J. Bot. 2011, 59, 46–52. [Google Scholar] [CrossRef]
- Bengoechea, C.; López-Castejón, M.L.; Márquez, S.; Salinas, V.; Puppo, C.; Guerrero, A. Gelation Properties of Calcium-Inulin Gels. Food Hydrocoll. 2019, 97, 105239. [Google Scholar] [CrossRef]
- Sulborska, A. Structure and Distribution of Glandular and Non-Glandular Trichomes on above-Ground Organs in Inula helenium L. (Asteraceae). Acta Agrobot. 2013, 66, 25–34. [Google Scholar] [CrossRef]
- Muravnik, L.E.; Kostina, O.V.; Zaporozhets, N.L. Structure and Functions of the Glandular Trichomes in Three Arnica Species (Asteraceae), Depending on Their Location on Leaves and Flowers. Flora Morphol. Distrib. Funct. Ecol. Plants 2022, 290, 152047. [Google Scholar] [CrossRef]
- Konarska, A.; Weryszko-Chmielewska, E.; Matysik-Woźniak, A.; Sulborska, A.; Polak, B.; Dmitruk, M.; Piotrowska-Weryszko, K.; Stefańczyk, B.; Rejdak, R. Histochemical and Phytochemical Analysis of Lamium album Subsp. Album l. Corolla: Essential Oil, Triterpenes, and Iridoids. Molecules 2021, 26, 4166. [Google Scholar]
- dos Santos Tozin, L.R.; de Melo Silva, S.C.; Rodrigues, T.M. Non-Glandular Trichomes in Lamiaceae and Verbenaceae Species: Morphological and Histochemical Features Indicate More than Physical Protection. N. Zeal. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, S.; Chen, F.; Chen, F.; Jiang, Y. Exploring the Relationship between Trichome and Terpene Chemistry in Chrysanthemum. Plants 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Vergauwen, R.; Moglia, A.; Hideg, É.; Van Den Ende, W. Towards Understanding Vacuolar Antioxidant Mechanisms: A Role for Fructans? J. Exp. Bot. 2013, 64, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Vereyken, I.J.; Chupin, V.; Demel, R.A.; Smeekens, S.C.M.; De Kruijff, B. Fructans Insert between the Headgroups of Phospholipids. Biochim. Biophys. Acta-Biomembr. 2001, 1510, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.L.; Hayashi, M.; Hara-Nishimura, I. Membrane Dynamics and Multiple Functions of Oil Bodies in Seeds and Leaves. Plant Physiol. 2018, 176, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hála, M.; Žárský, V. Protein Prenylation in Plant Stress Responses. Molecules 2019, 24, 3906. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Cheng, S.S.; Cheung, M.Y.; Law, C.H.; Lam, H.M. The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants. Membranes 2022, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Woollard, A.A.; Moore, I. The Functions of Rab GTPases in Plant Membrane Traffic. Curr. Opin. Plant Biol. 2008, 11, 610–619. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Turnbull, D.; Hemsley, P.A. Fats and Function: Protein Lipid Modifications in Plant Cell Signalling. Curr. Opin. Plant Biol. 2017, 40, 63–70. [Google Scholar] [CrossRef]
- Bock, L.V.; Hutchings, B.; Grubmüller, H.; Woodbury, D.J. Chemomechanical Regulation of SNARE Proteins Studied with Molecular Dynamics Simulations. Biophys. J. 2010, 99, 1221–1230. [Google Scholar] [CrossRef]
- Greaves, J.; Prescott, G.R.; Gorleku, O.A.; Chamberlain, L.H. Regulation of SNAP-25 Trafficking and Function by Palmitoylation. Biochem. Soc. Trans. 2010, 38, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Kromer, K.; Kreitschitz, A.; Kleinteich, T.; Gorb, S.N.; Szumny, A. Oil Secretory System in Vegetative Organs of Three Arnica Taxa: Essential Oil Synthesis, Distribution and Accumulation. Plant Cell Physiol. 2016, 57, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Johansen Donald, A. Plant Microtechnique; McGraw-Hill Book Company, Inc.: London, UK, 1940. [Google Scholar]
- Kreitschitz, A.; Gorb, S.N. How Does the Cell Wall ‘Stick’ in the Mucilage? A Detailed Microstructural Analysis of the Seed Coat Mucilaginous Cell Wall. Flora Morphol. Distrib. Funct. Ecol. Plants 2017, 229, 9–22. [Google Scholar] [CrossRef]
- Adams, R.; Weerner, H. Helenalin I. Isolation and Properties. J. Am. Chem. Soc. 1949, 71, 2546–2551. [Google Scholar] [CrossRef]
- O’Brien, P.; Siraki, A.; Shangari, N. Aldehyde Sources, Metabolism, Molecular Toxicity Mechanisms, and Possible Effects on Human Health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef]
- David, R.; Carde, J.P. Coloration Differentielle des Inclusions Lipidique et Terpeniques des Pseudophylles du Pin Maritime Au Moyen du Reactif Nadi. Comptes Rendus L’académie Sci. 1964, 258, 1338–1340. [Google Scholar]
- Nachlas, M.M.; Davidson, M.B.; Goldberg, J.D.; Seligman, A.M. Colorimetric Method for the Measurement of Isocitric Dehydrogenase Activity. J. Lab. Clin. Med. 1963, 62, 148–158. [Google Scholar] [PubMed]
- Stahl, E. Mikro-Azulennachweismethode für Schafgarbe (Achillea mllefollium L. und Andere Arten der Gattung). Dtsch. Apoth. Ztg. 1953, 93, 197–200. [Google Scholar]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene Lactones: More Than Protective Plant Compounds with High Toxicity. CRC. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Willuhn, G. Arnica montana L.-Portrat Einer Arzneipflanze. Pharm. Ztg. 1991, 136, 2453. [Google Scholar]
- Bomme, U. Anbau und Züchtung von Arnica montana L. Z Arznei-Gewürzpfla. 1999, 4, 202–203. [Google Scholar]
- Aiello, N.; Bontempo, R.; Vender, C.; Ferretti, V.; Innocenti, G.; Dall’Acqua, S. Morpho-Quantitative and Qualitative Traits of Arnica montana L. Wild Accessions of Trentino, Italy. Ind. Crops Prod. 2012, 40, 199–203. [Google Scholar] [CrossRef]
- Clauser, M.; Aiello, N.; Scartezzini, F.; Innocenti, G.; Dall’acqua, S. Differences in the Chemical Composition of Arnica montana Flowers from Wild Populations of North Italy. Nat. Prod. Commun. 2014, 9, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Radanovic, D.; Markovic, T.; Antic-Mladenovic, S.; Pljevljakušic, D.; Ristic, M.; Krivokuca-Dokic, D. Yield and Quality of Arnica (Arnica montana and Arnica chamissonis Var. Foliosa) Cultivated in Serbia. In Proceedings of the 1st International Scientific Conference on Medicinal, Aromatic and Spice Plants, Nitra, Slovak Republic, 5–6 December 2007; pp. 157–161. [Google Scholar]




| A. montana L. | A. montana cv. Arbo | A. chamissonis Less. | ||||
|---|---|---|---|---|---|---|
| Buds | Full Flowering | Buds | Full Flowering | Buds | Full Flowering | |
| DH | 0.04 ± 0.02 | 0.09 ± 0.06 | 0.02 ± 0.01 | 0.04 ± 0.01 | - | - |
| H | 0.09 ± 0.04 | 0.27 ± 0.05 | 0.14 ± 0.03 | 0.35 ± 0.09 | 0.24 ± 0.03 | 0.13 ± 0.05 |
| DHA | - | 0.03 ± 0.02 | 0.23 ± 0.01 | 0.52 ± 0.11 | - | - |
| HA | - | - | 0.18 ± 0.03 | 0.54 ± 0.12 | - | - |
| DHM | 0.53 ± 0.17 | 0.94 ± 0.46 | 0.54 ± 0.07 | 0.73 ± 0.12 | - | - |
| HM | 0.19 ± 0.08 | 0.72 ± 0.21 | 0.56 ± 0.06 | 1.44 ± 0.23 | - | - |
| DHIB | 0.38 ± 0.04 | 1.48 ± 0.76 | 0.46 ± 0.40 | 1.43 ± 0.28 | - | - |
| HIB | 0.15 ± 0.06 | 0.97 ± 0.26 | 1.07 ± 0.23 | 4.06 ± 0.87 | - | - |
| DHT | 1.70 ± 0.20 | 2.28 ± 0.87 | 0.75 ± 0.17 | 1.13 ± 0.19 | - | - |
| HT | 0.82 ± 0.06 | 2.49 ± 0.75 | 1.09 ± 0.09 | 2.75 ± 0.38 | 0.02 ± 0.01 | 0.01 ± 0.01 |
| DHMB/DHIV | 0.59 ± 0.11 | 1.22 ± 0.52 | 1.23 ± 0.11 | 2.47 ± 0.45 | - | - |
| HMB/HIV | 0.33 ± 0.06 | 1.15 ± 0.32 | 3.41 ± 0.37 | 9.42 ± 1.45 | - | - |
| Total H | 1.58 ± 0.31 | 5.59 ± 1.55 | 6.45 ± 0.75 | 18.57 ± 3.13 | 0.26 ± 0.03 | 0.14 ± 0.06 |
| Total DH | 3.25 ± 0.53 | 6.03 ± 2.69 | 3.24 ± 0.12 | 6.32 ± 1.17 | - | - |
| Total SL | 4.84 ± 0.67 | 11.63 ± 1.69 | 9.69 ± 0.68 | 24.88 ± 4.28 | 0.26 ± 0.03 | 0.14 ± 0.06 |
| Arnica montana | Arnica montana cv. Arbo | ||||
|---|---|---|---|---|---|
| Non-grandural Trichomes | Grandural Trichomes | Diameter of Grandular Trichomes [µm] | Non-grandural Trichomes | Grandural Trichomes | Diameter of Grandular Trichomes [µm] |
| 16.95 ± 1.54 | 15.55 ± 3.33 | 32.33 ± 3.06 | 22.96 ± 2.63 | 18.09 ± 3.57 | 34.48 ± 3.83 |
| Histochemical Tests | Color of Reaction Products | References |
|---|---|---|
| Legal’s reaction for methylene and methyl ketone. | Red to dark brown; concentration dependent after adding CH3COOH red | Positive for helenalin [88] |
| Zimmermann reaction for methyl ketone and aldehyde. | Reddish purple | Positive for helenalin [88] |
| Schiff’s reagent for aldehydes. | Red or magenta | Negative for helenalin [41,88,89] |
| NADI for cytochrome oxidase | Stains lipids blue; fat inclusions, red-purple; and terpenes and resins, indigo to violet, according the proportion of oil and resin acids | [90] |
| Succinate dehydrogenase with Neo-T | Light blue | [91] |
| Vanillin for lactones | Stains helenalin yellow; after heating, turns reddish orange | [43] |
| p-dimethylamino-benzaldehyde for lactones | Greyish yellow | [43] |
| EP reaction for azulene and proazulenes | Green, blue or dark blue | [92] |
| 1% EDTA in water lactones | Greyish black | [42] |
| 2,4-Dinitrophenylhydrazine test for aldehydes and ketones, Brady’s reagent | Stains aliphatic carbonyls yellow; red to orange for aromatic carbonyls Precipitation on slides coated with glycerol, visible after several days | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parafiniuk, A.; Kromer, K.; Fleszar, M.G.; Kreitschitz, A.; Wiśniewski, J.; Gamian, A. Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa. Molecules 2023, 28, 4379. https://doi.org/10.3390/molecules28114379
Parafiniuk A, Kromer K, Fleszar MG, Kreitschitz A, Wiśniewski J, Gamian A. Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa. Molecules. 2023; 28(11):4379. https://doi.org/10.3390/molecules28114379
Chicago/Turabian StyleParafiniuk, Agata, Krystyna Kromer, Mariusz G. Fleszar, Agnieszka Kreitschitz, Jerzy Wiśniewski, and Andrzej Gamian. 2023. "Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa" Molecules 28, no. 11: 4379. https://doi.org/10.3390/molecules28114379
APA StyleParafiniuk, A., Kromer, K., Fleszar, M. G., Kreitschitz, A., Wiśniewski, J., & Gamian, A. (2023). Localization of Sesquiterpene Lactones Biosynthesis in Flowers of Arnica Taxa. Molecules, 28(11), 4379. https://doi.org/10.3390/molecules28114379








