Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Fresh Olive Oils
2.2. Indexes of Lipid Nutritional Quality
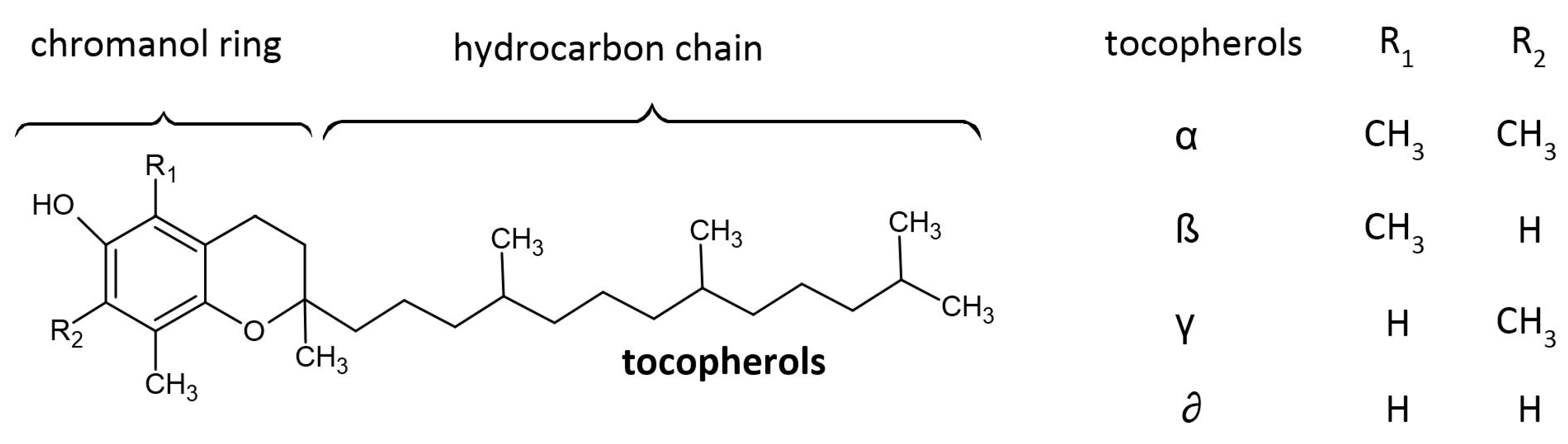
2.3. Tocopherols Content
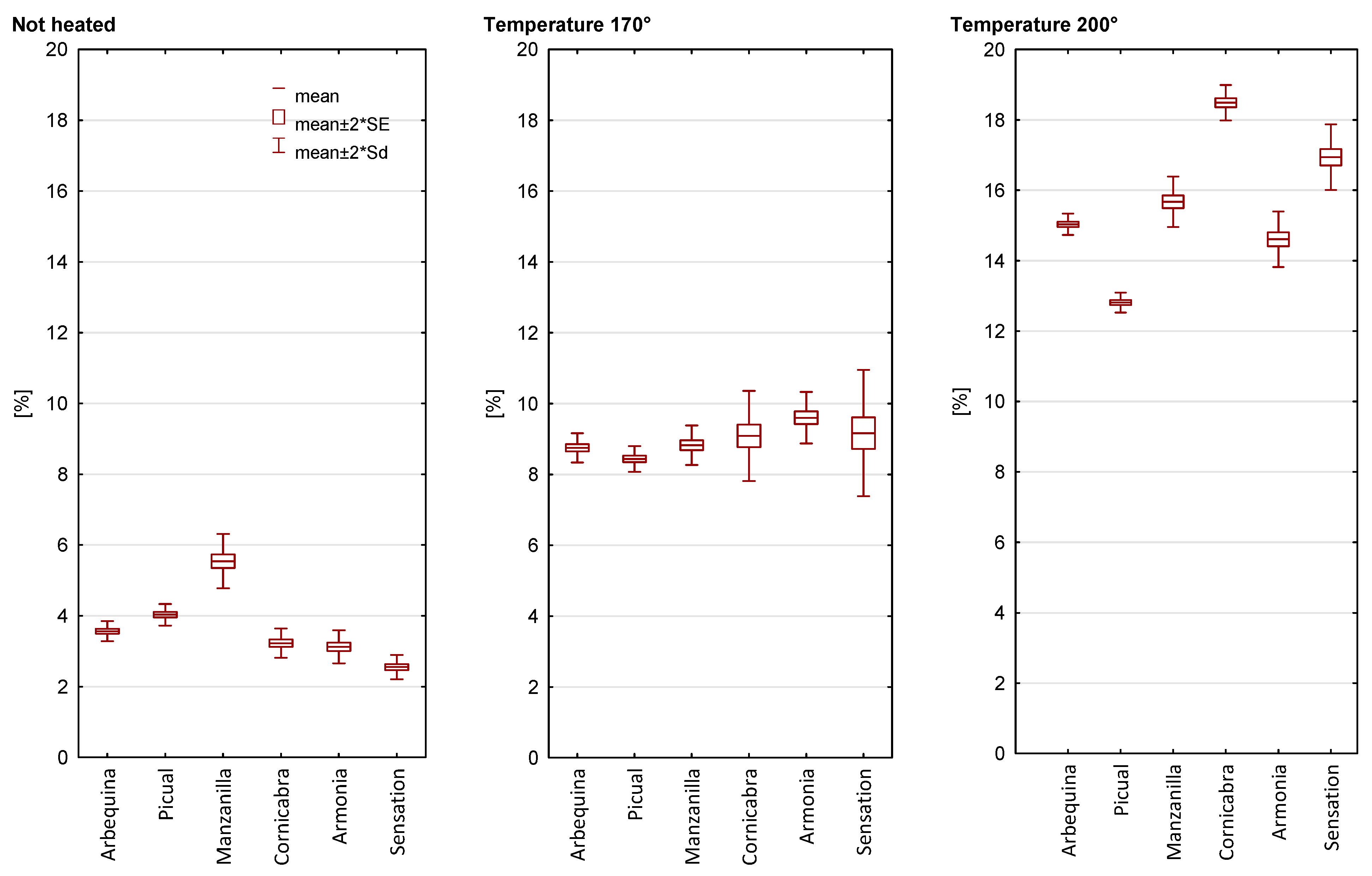
2.4. Total Polar Compounds Content
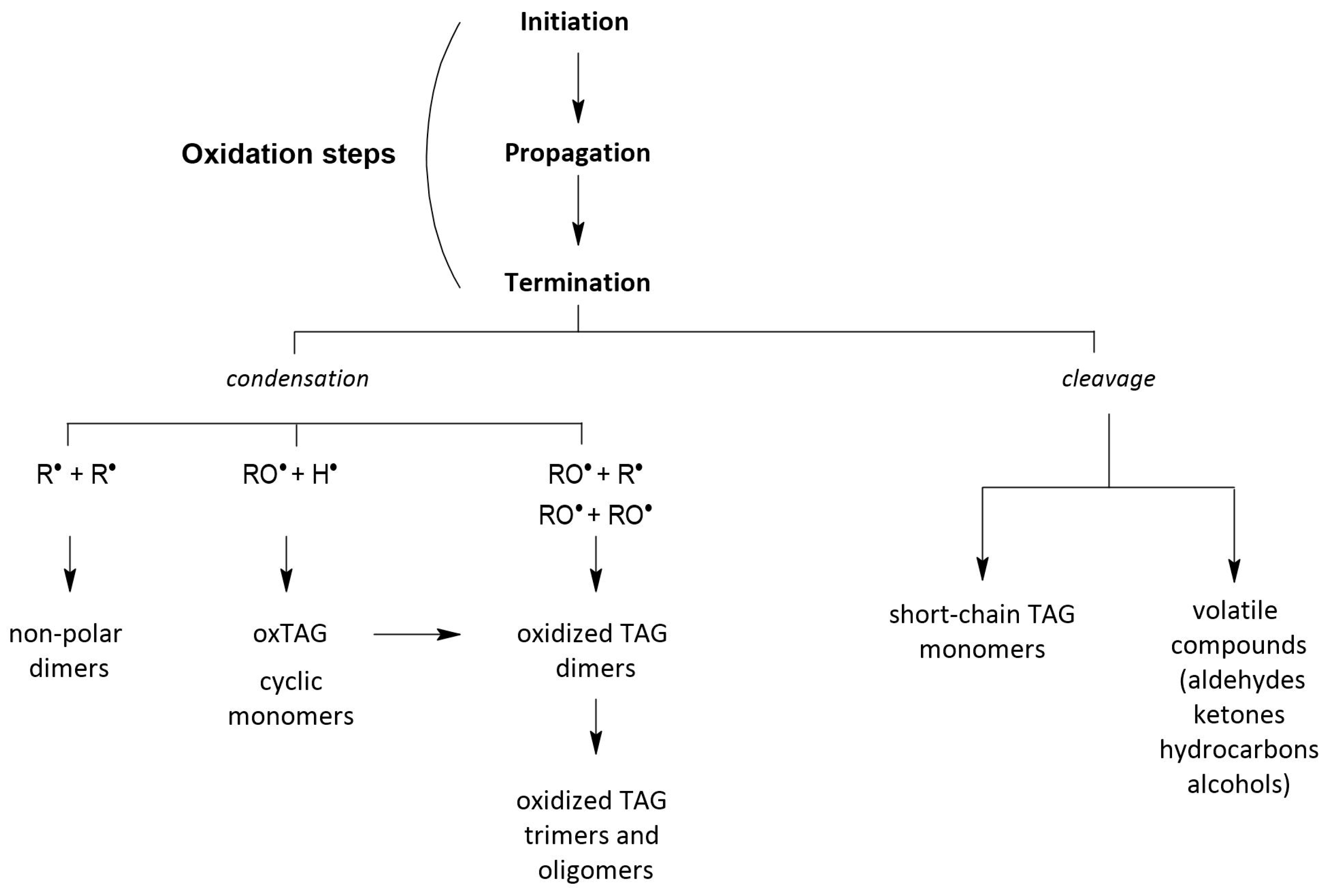
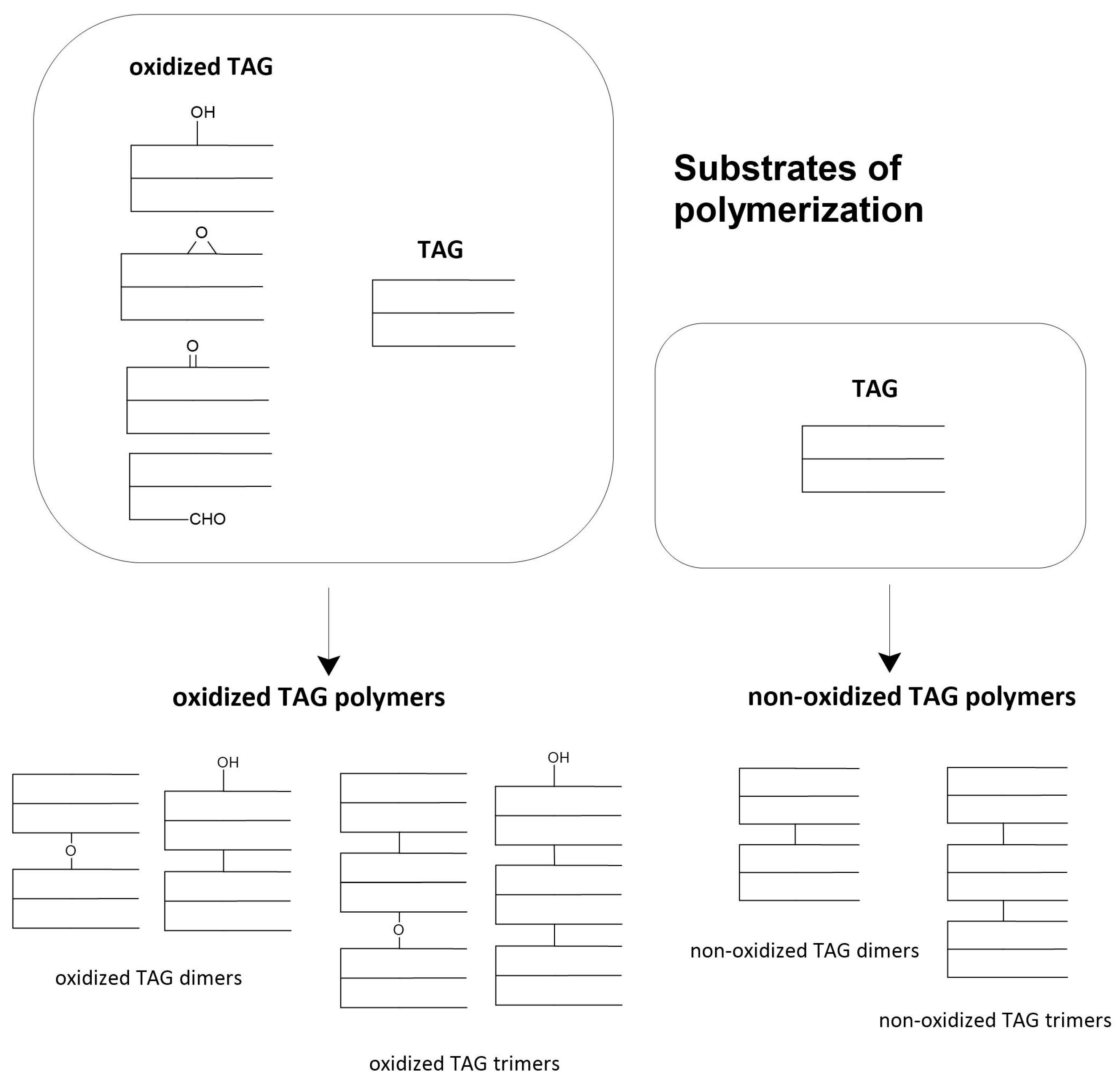
2.5. Polimeryztion of Triacyloglycerols
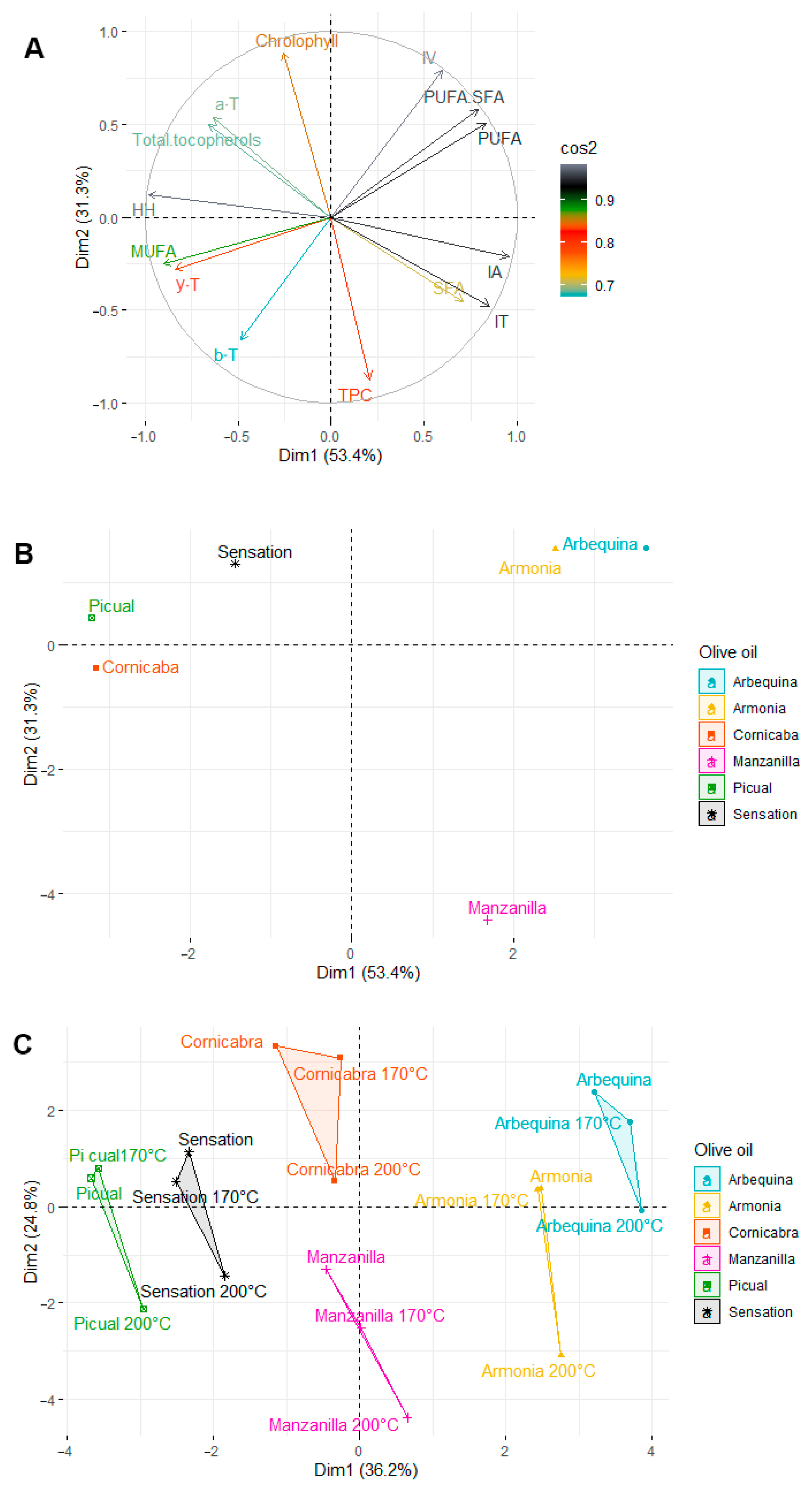
2.6. Principal Component Analysis
3. Materials and Methods
3.1. Materials
3.2. Heating Procedure
3.3. Fatty Acid Composition Analysis
3.4. Calculated Iodine Value (CIV)
3.5. Indexes of Lipid Nutritional Quality
3.5.1. Polyunsaturated Fatty Acid/Saturated Fatty Acid (PUFA/SFA)
3.5.2. Index of Atherogenicity (IA)
3.5.3. Index of Thrombogenicity (IT)
3.5.4. Hypocholesterolemic/Hypercholesterolemic (HH) Ratio
3.6. Chlorophyll Content Analysis
3.7. Tocochromanols Analysis
3.8. Total Polar Compounds (TPC) Analysis
3.9. Polymerized Triacylglycerols (PTAG) Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Biotechnol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Rossi, R. The EU Olive and Olive Oil Sector Main Features, Challenges and Prospects; European Parliament: Brussels, Belgium, 2017. [Google Scholar]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef]
- Nomikos, N.N.; Nomikos, G.N.; Kores, D.S. The use of deep friction massage with olive oil as a means of prevention and treatment of sports injuries in ancient times. Arch. Med. Sci. 2010, 6, 642–645. [Google Scholar] [CrossRef]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Frankel, E.N. Nutritional and biological properties of extra virgin olive oil. J. Agric. Food Chem. 2011, 59, 785–792. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Phenolic compounds and squalene in olive oils: The concentration and antioxidant potential of total phenols, simple phenols, secoiridoids, lignansand squalene. Food Chem. Toxicol. 2000, 38, 647–659. [Google Scholar] [CrossRef]
- Wani, T.A.; Masoodi, F.A.; Gani, A.; Baba, W.N.; Rahmanian, N.; Akhter, R.; Wani, I.A.; Ahmad, M. Olive oil and its principal bioactive compound: Hydroxytyrosol—A review of the recent literature. Trends Food Sci. Technol. 2018, 77, 77–90. [Google Scholar] [CrossRef]
- Shahidi, F.; De Camargo, A.C. Tocopherols and tocotrienols in common and emerging dietary sources: Occurrence, applications, and health benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Martakos, I.; Kostakis, M.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Tocopherols, and Squalene in Greek Olive Oils: A Study of the Influence of Cultivation and Oil-Production Parameters. Foods 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D.; Mène-Saffrané, L. Vitamin E; Elsevier: Amsterdam, The Netherlands, 2011; Volume 59, ISBN 9780123858535. [Google Scholar]
- Boskou, D. Olive oil. World Rev. Nutr. Diet 2007, 97, 180–210. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gándara, J. Characterisation of extra virgin olive oils from Galician autochthonous varieties and their co-crushings with Arbequina and Picual cv. Food Chem. 2015, 176, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, C.; Yang, X.; Lei, J.; Chen, H.; Zhang, C.; Wang, C. Parameters Related to Virgin Olive Oil from Wudu (China). Foods 2023, 12, 7. [Google Scholar] [CrossRef]
- Thomsen, C.; Storm, H.; Holst, J.J.; Hermansen, K. Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am. J. Clin. Nutr. 2003, 77, 605–611. [Google Scholar] [CrossRef]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef]
- Nieto, G.; Lorenzo, J.M. Use of olive oil as fat replacer in meat emulsions. Curr. Opin. Food Sci. 2021, 40, 179–186. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef]
- Centrone, M.; Ranieri, M.; Di Mise, A.; D’Agostino, M.; Venneri, M.; Valenti, G.; Tamma, G. Health benefits of olive oil and by-products and possible innovative applications for industrial processes. Funct. Foods Health Dis. 2021, 11, 295–309. [Google Scholar] [CrossRef]
- De Alzaa, F.; Guillaume, C.; Ravetti, L. Evaluation of Chemical and Nutritional Changes in Chips, Chicken Nuggets, and Broccoli after Deep-Frying with Extra Virgin Olive Oil, Canola, and Grapeseed Oils. J. Food Qual. 2021, 2021, 7319013. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; Rinaldi de Alvarenga, J.F.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Cooking with extra-virgin olive oil: A mixture of food components to prevent oxidation and degradation. Trends Food Sci. Technol. 2022, 123, 28–36. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N. Virgin Olive Oil as Frying Oil. Compr. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Velasco, J.; Dobarganes, C. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci. Technol. 2002, 104, 661–676. [Google Scholar] [CrossRef]
- Velasco, J.; Marmesat, S.; Dobarganes, M.C. Chemistry of Frying. In Advances in Deep-Fat Frying of Foods; Sumnu, S.G., Sahin, S., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 33–57. [Google Scholar]
- Alvis, A.; Vélez, C.; Rada-Mendoza, M.; Villamiel, M.; Villada, H.S. Heat transfer coefficient during deep-fat frying. Food Control 2009, 20, 321–325. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kulczyński, B. Degradation of tocopherol molecules and its impact on the polymerization of triacylglycerols during heat treatment of oil. Molecules 2019, 24, 4555. [Google Scholar] [CrossRef]
- Kmiecik, D.; Fedko, M.; Rudzińska, M.; Siger, A.; Gramza-Michałowska, A.; Kobus-Cisowska, J. Thermo-oxidation of phytosterol molecules in rapeseed oil during heating: The impact of unsaturation level of the oil. Foods 2021, 10, 50. [Google Scholar] [CrossRef]
- Shaker, M.A.; Marwa, A.A.; Aml, R.T.; Ahmed, A.E. Identification of cyclic fatty acids in frying oil by NMR. Food Res. 2022, 6, 128–137. [Google Scholar] [CrossRef]
- Gabrielle Alves de Carvalho, A.; Olmo-García, L.; Rachel Antunes Gaspar, B.; Carrasco-Pancorbo, A.; Naciuk Castelo-Branco, V.; Guedes Torres, A. Evolution of the metabolic profile of virgin olive oil during deep-frying: Assessing the transfer of bioactive compounds to the fried food. Food Chem. 2022, 380, 132205. [Google Scholar] [CrossRef]
- Feleke, D.G.; Gebeyehu, G.M.; Admasu, T.D. Effect of deep-fried oil consumption on lipid profile in rats. Sci. Afr. 2022, 17, e01294. [Google Scholar] [CrossRef]
- Vonk, R.J.; Kalivianakis, M.; Minich, D.M.; Bijleveld, C.M.A.; Verkade, H.J. The metabolic importance of unabsorbed dietary lipids in the colon. Scand. J. Gastroenterol. 1997, 32, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Kmiecik, D.; Gramza-Michałowska, A.; Korczak, J. Anti-polymerization activity of tea and fruits extracts during rapeseed oil heating. Food Chem. 2018, 239, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Schreiner, M. Stabilities of tocopherols and phenolic compounds in virgin olive oil during thermal oxidation. J. Food Sci. Technol. 2018, 55, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; De la Rosa, R.; Sánchez-Ortiz, A.; Cano, J.; Pérez, A.G.; Sanz, C.; Arias-Calderón, R.; Velasco, L.; León, L. Chemical components influencing oxidative stability and sensorial properties of extra virgin olive oil and effect of genotype and location on their expression. LWT 2021, 136, 110257. [Google Scholar] [CrossRef]
- Fernández-Cuesta, A.; León, L.; Velasco, L.; De la Rosa, R. Changes in squalene and sterols associated with olive maturation. Food Res. Int. 2013, 54, 1885–1889. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; Sánchez, J.; De Miguel, C.; Martín-Vertedor, D. Antioxidant capacity of the phenolic fraction and its effect on the oxidative stability of olive oil varieties grown in the southwest of Spain. Grasas Aceites 2014, 65, 115–125. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An increase in the Omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Dell’agli, M.; Bosisio, E. Minor polar compounds of olive oil: Composition, factors of variability and bioactivity. Stud. Nat. Prod. Chem. 2002, 27, 697–734. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Marosvölgyi, T.; Szabo, E.; Koczka, V.; Verzar, Z.; Figler, M.; Decsi, T. Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods 2022, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Bruscatto, M.H.; Zambiazi, R.C.; Sganzerla, M.; Pestana, V.R.; Otero, D.; Lima, R.; Paiva, F. Degradation of tocopherols in rice bran oil submitted to heating at different temperatures. J. Chromatogr. Sci. 2009, 47, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kowalczewski, P.Ł. Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids. Foods 2022, 11, 1081. [Google Scholar] [CrossRef]
- Gertz, C. Chemical and physical parameters as quality indicators of used frying fats. Eur. J. Lipid Sci. Technol. 2000, 102, 566–572. [Google Scholar] [CrossRef]
- Karakaya, S.; Şimşek, Ş. Changes in Total Polar Compounds, Peroxide Value, Total Phenols and Antioxidant Activity of Various Oils Used in Deep Fat Frying. J. Am. Oil Chem. Soc. 2011, 88, 1361–1366. [Google Scholar] [CrossRef]
- Allouche, Y.; Jiménez, A.; Gaforio, J.J.; Uceda, M.; Beltrán, G. How heating affects extra virgin olive oil quality indexes and chemical composition. J. Agric. Food Chem. 2007, 55, 9646–9654. [Google Scholar] [CrossRef]
- Ambra, R.; Lucchetti, S.; Pastore, G. A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds. Molecules 2022, 27, 661. [Google Scholar] [CrossRef]
- Falade, A.O.; Oboh, G.; Okoh, A.I. Potential Health Implications of the Consumption of Thermally-Oxidized Cooking Oils—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 95–105. [Google Scholar] [CrossRef]
- Debnath, S.; Rastogi, N.K.; Gopala Krishna, A.G.; Lokesh, B.R. Effect of frying cycles on physical, chemical and heat transfer quality of rice bran oil during deep-fat frying of poori: An Indian traditional fried food. Food Bioprod. Process. 2012, 90, 249–256. [Google Scholar] [CrossRef]
- Da Silva, T.O.; Pereira, P.A.d.P. Influence of time, surface-to-volume ratio, and heating process (continuous or intermittent) on the emission rates of selected carbonyl compounds during thermal oxidation of palm and soybean oils. J. Agric. Food Chem. 2008, 56, 3129–3135. [Google Scholar] [CrossRef] [PubMed]
- Rudzińska, M.; Hassanein, M.M.M.; Abdel-Razek, A.G.; Kmiecik, D.; Siger, A.; Ratusz, K. Influence of composition on degradation during repeated deep-fat frying of binary and ternary blends of palm, sunflower and soybean oils with health-optimised saturated-to-unsaturated fatty acid ratios. Int. J. Food Sci. Technol. 2018, 53, 1021–1029. [Google Scholar] [CrossRef]
- Hammouda, B.I.; Márquez-Ruiz, G.; Holgado, F.; Freitas, F.; Da Silva, G.; Bouaziz, M. Comparative study of polymers and total polar compounds as indicators of refined oil degradation during frying. Eur. Food Res. Technol. 2019, 245, 967–976. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Ce 1h-05. Determination of Cis-, Trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-Ruminant Animal Oils and Fats by Capillary GLC, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- AOCS. AOCS Official Method Cd 1c-85. Calculated Iodine Value, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cc 13i-96 Determination of chlorophyll Pigments in Crude Vegetable Oils. In Official Methods and Recommended Practices; American Oil Chemists’ Society: Urbana, IL, USA, 1993. [Google Scholar]
- Siger, A.; Michalak, M.; Rudzińska, M. Canolol, tocopherols, plastochromanol-8, and phytosterols content in residual oil extracted from rapeseed expeller cake obtained from roasted seed. Eur. J. Lipid Sci. Technol. 2016, 118, 1358–1367. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method 982.27. Polar Components in Frying Fats, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- AOCS. AOCS Official Method 993.25. Polymerized Triglycerides in Oils and Fats, 6th ed.; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]


| Arbequina | Picual | Manzanilla | Cornicabra | Armonia | Sensation | |
|---|---|---|---|---|---|---|
| Fatty acid composition [%] | ||||||
| C 16:0 | 15.61 ± 0.16 c | 13.00 ± 0.07 a | 14.58 ± 0.28 b | 13.19 ± 0.14 a | 14.76 ± 0.08 b | 13.60 ± 0.13 a |
| C 16:1 | 1.28 ± 0.06 b | 0.91 ± 0.01 a | 1.26 ± 0.06 b | 1.02 ± 0.04 a | 1.18 ± 0.01 bc | 1.04 ± 0.03 ac |
| C 18:0 | 2.10 ± 0.04 a | 2.17 ± 0.03 a | 3.22 ± 0.04 b | 3.58 ± 0.10 c | 2.07 ± 0.03 a | 2.11 ± 0.06 a |
| C 18:1 | 69.49 ± 0.45 c | 79.02 ± 0.07 d | 74.38 ± 0.30 a | 75.73 ± 0.17 b | 71.65 ± 0.13 d | 75.53 ± 0.47 ab |
| C 18:2 | 9.89 ± 0.07 e | 3.12 ± 0.06 b | 4.75 ± 0.08 a | 4.49 ± 0.06 a | 8.90 ± 0.07 d | 5.99 ± 0.07 c |
| C 18:3 | 0.64 ± 0.04 ab | 0.83 ± 0.01 bc | 0.73 ± 0.04 abc | 0.85 ± 0.08 c | 0.63 ± 0.03 a | 0.74 ± 0.06 abc |
| C 22:0 | 0.41 ± 0.03 a | 0.37 ± 0.04 a | 0.51 ± 0.03 ab | 0.56 ± 0.04 b | 0.41 ± 0.04 a | 0.42 ± 0.03 ab |
| C 20:1 | 0.32 ± 0.03 a | 0.27 ± 0.03 a | 0.28 ± 0.01 a | 0.27 ± 0.01 a | 0.13 ± 0.00 b | 0.29 ± 0.04 a |
| C 22:0 | 0.12 ± 0.00 a | 0.12 ± 0.03 a | 0.14 ± 0.03 ab | 0.15 ± 0.01 b | 0.10 ± 0.01 a | 0.12 ± 0.01 ab |
| C 22:1 | 0.08 ± 0.01 a | 0.12 ± 0.03 a | 0.09 ± 0.03 a | 0.09 ± 0.03 a | 0.10 ± 0.00 a | 0.10 ± 0.03 a |
| C 24:0 | 0.06 ± 0.01 a | 0.07 ± 0.01 a | 0.06 ± 0.00 a | 0.07 ± 0.00 a | 0.07 ± 0.01 a | 0.06 ± 0.01 a |
| SFA | 18.30 ± 0.24 cd | 15.73 ± 0.04 a | 18.51 ± 0.24 d | 17.55 ± 0.28 bc | 17.41 ± 0.04 b | 16.31 ± 0.24 a |
| MUFA | 71.17 ± 0.35 c | 80.32 ± 0.00 e | 76.01 ± 0.28 a | 77.11 ± 0.25 b | 73.06 ± 0.14 d | 76.96 ± 0.37 ab |
| PUFA | 10.53 ± 0.11 e | 3.95 ± 0.04 b | 5.48 ± 0.04 a | 5.34 ± 0.03 a | 9.53 ± 0.10 d | 6.73 ± 0.13 c |
| ω6/ω3 ratio | 15.48 ± 0.92 e | 3.76 ± 0.13 a | 6.52 ± 0.5 c | 5.31 ± 0.6 b | 14.14 ± 0.52 e | 8.11 ± 0.52 d |
| CIV | 80.09 ± 0.07 b | 76.56 ± 0.07 a | 75.59 ± 0.20 c | 76.37 ± 0.34 a | 79.98 ± 0.07 b | 78.55 ± 0.05 d |
| Tocopherols [mg/1 g of oils] | ||||||
| α-T | 27.73 ± 0.60 a | 30.85 ± 0.64 c | 21.43 ± 0.30 b | 34.85 ± 0.32 d | 28.27 ± 0.58 a | 27.68 ± 0.69 a |
| β-T | 0.16 ± 0.05 a | 0.27 ± 0.07 ab | 0.48 ± 0.10 c | 0.50 ± 0.07 c | 0.25 ± 0.05 ab | 0.40 ± 0.07 bc |
| γ-T | 0.47 ± 0.09 a | 0.64 ± 0.11 ab | 0.59 ± 0.03 a | 0.82 ± 0.11 b | 0.49 ± 0.09 a | 0.60 ± 0.06 a |
| Total T | 28.36 ± 0.51 a | 31.75 ± 0.50 c | 22.49 ± 0.38 b | 36.16 ± 0.31 d | 29.00 ± 0.64 a | 28.68 ± 0.74 a |
| Chlorophyll [1 mg phenophytin/1 kg of oil] | ||||||
| 17.30 ± 0.25 a | 20.27 ± 0.36 b | 9.51 ± 0.28 c | 14.30 ± 0.41 d | 17.79 ± 0.59 a | 19.89 ± 0.45 b | |
| Total Polar Compounds [%] | ||||||
| 3.57 ± 0.14 ab | 4.03 ± 0.15 b | 5.54 ± 0.38 d | 3.23 ± 0.21 a | 3.13 ± 0.23 a | 2.55 ± 0.17 c | |
| Samples | Arbequina | Picual | Manzanilla | Cornicabra | Armonia | Sensation |
|---|---|---|---|---|---|---|
| Not heated | - | - | - | - | - | - |
| 170 °C | - | - | - | - | 0.79 ± 0.03 | - |
| 200 °C | 2.06 ± 0.14 b | 1.14 ± 0.1 a | 3.24 ± 0.25 d | 2.14 ± 0.12 b | 5.17 ± 0.23 e | 2.28 ± 0.10 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmiecik, D.; Fedko, M.; Małecka, J.; Siger, A.; Kowalczewski, P.Ł. Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process. Molecules 2023, 28, 4247. https://doi.org/10.3390/molecules28104247
Kmiecik D, Fedko M, Małecka J, Siger A, Kowalczewski PŁ. Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process. Molecules. 2023; 28(10):4247. https://doi.org/10.3390/molecules28104247
Chicago/Turabian StyleKmiecik, Dominik, Monika Fedko, Justyna Małecka, Aleksander Siger, and Przemysław Łukasz Kowalczewski. 2023. "Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process" Molecules 28, no. 10: 4247. https://doi.org/10.3390/molecules28104247
APA StyleKmiecik, D., Fedko, M., Małecka, J., Siger, A., & Kowalczewski, P. Ł. (2023). Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process. Molecules, 28(10), 4247. https://doi.org/10.3390/molecules28104247










