Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al–Air Battery
Abstract
1. Introduction
2. Results and Discussion
2.1. OCP and PDP Analysis
2.2. EIS Measurement
2.3. Hydrogen Evolution Test
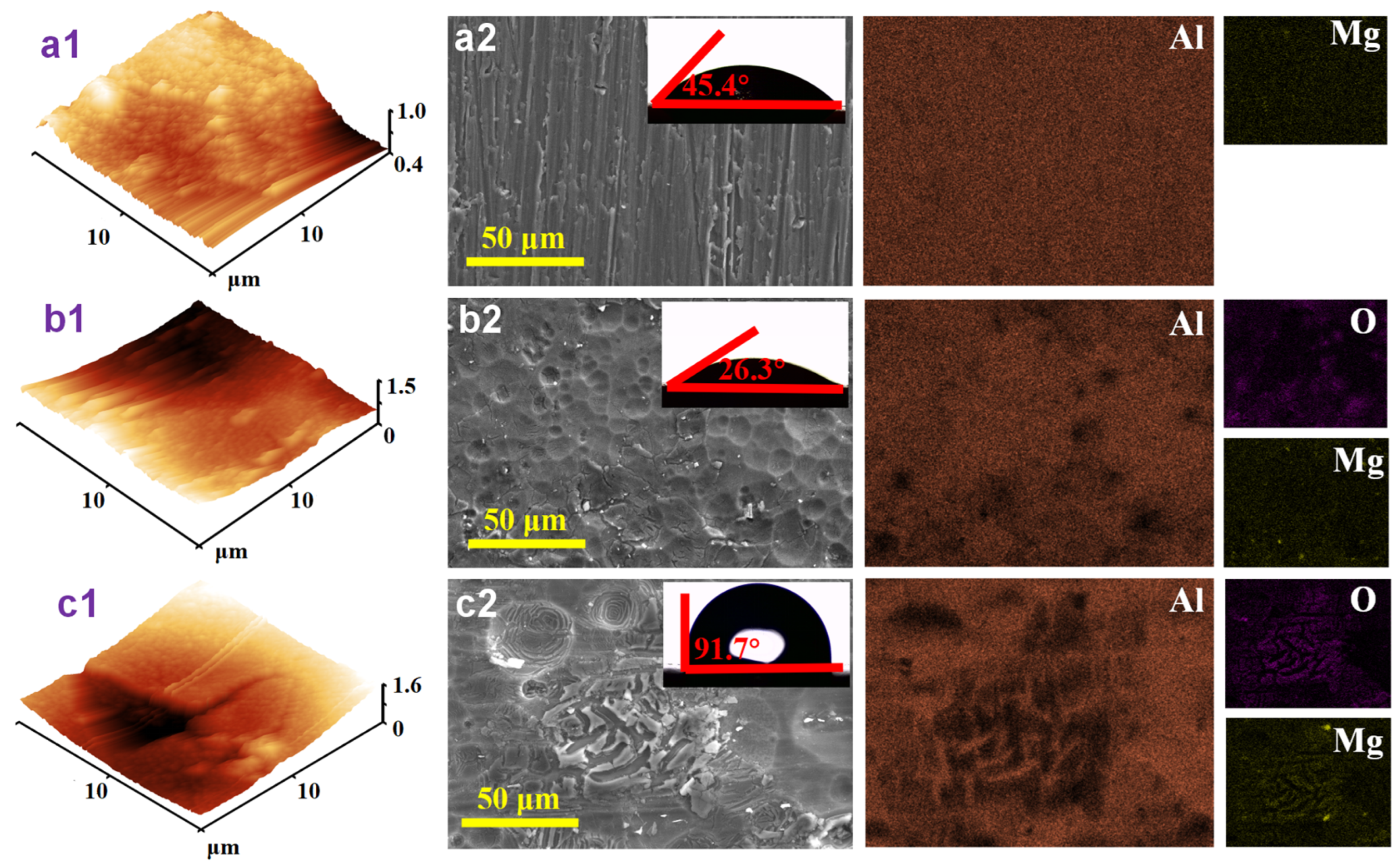
2.4. Surface Analysis
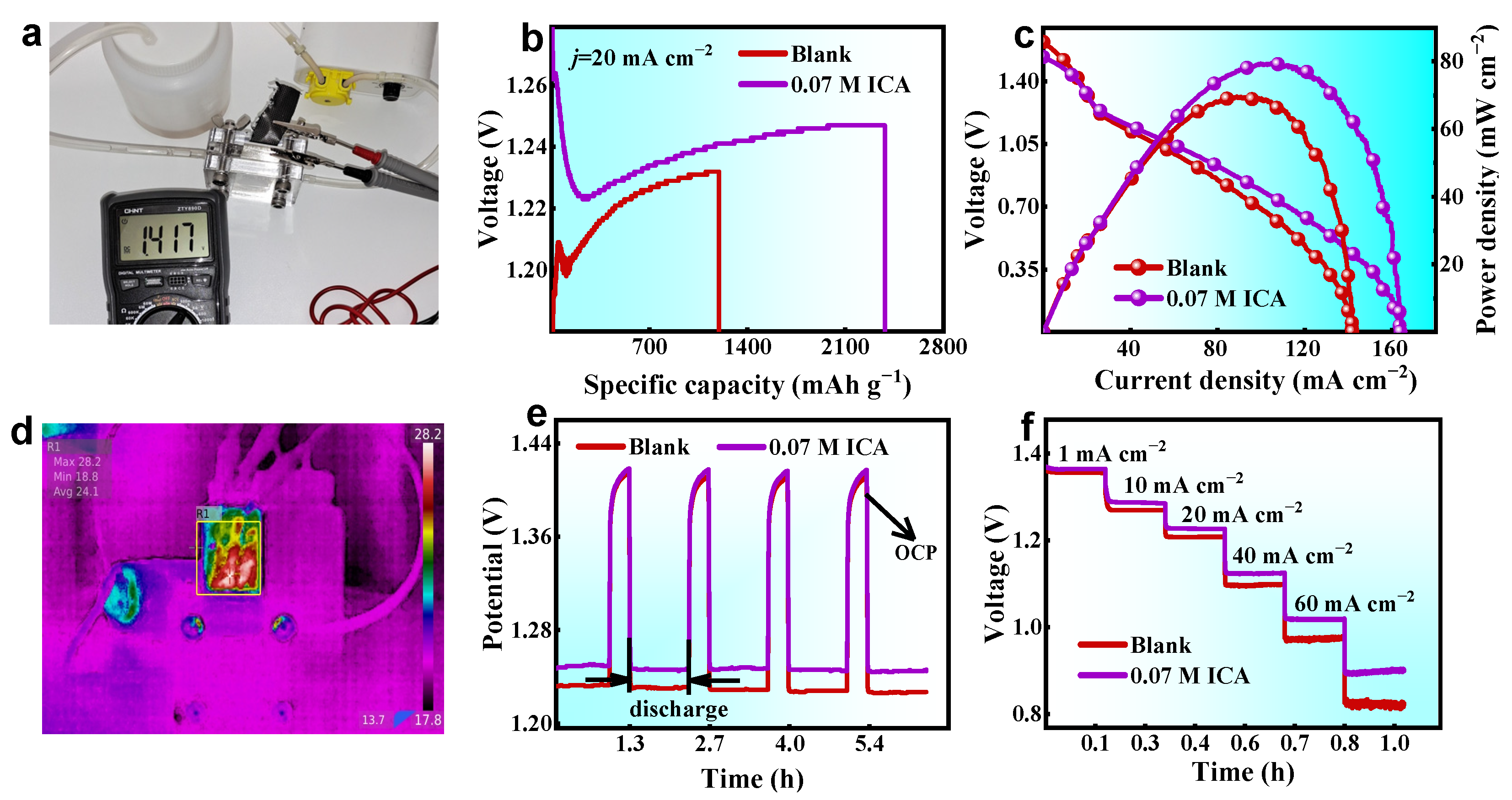
2.5. Battery Performance
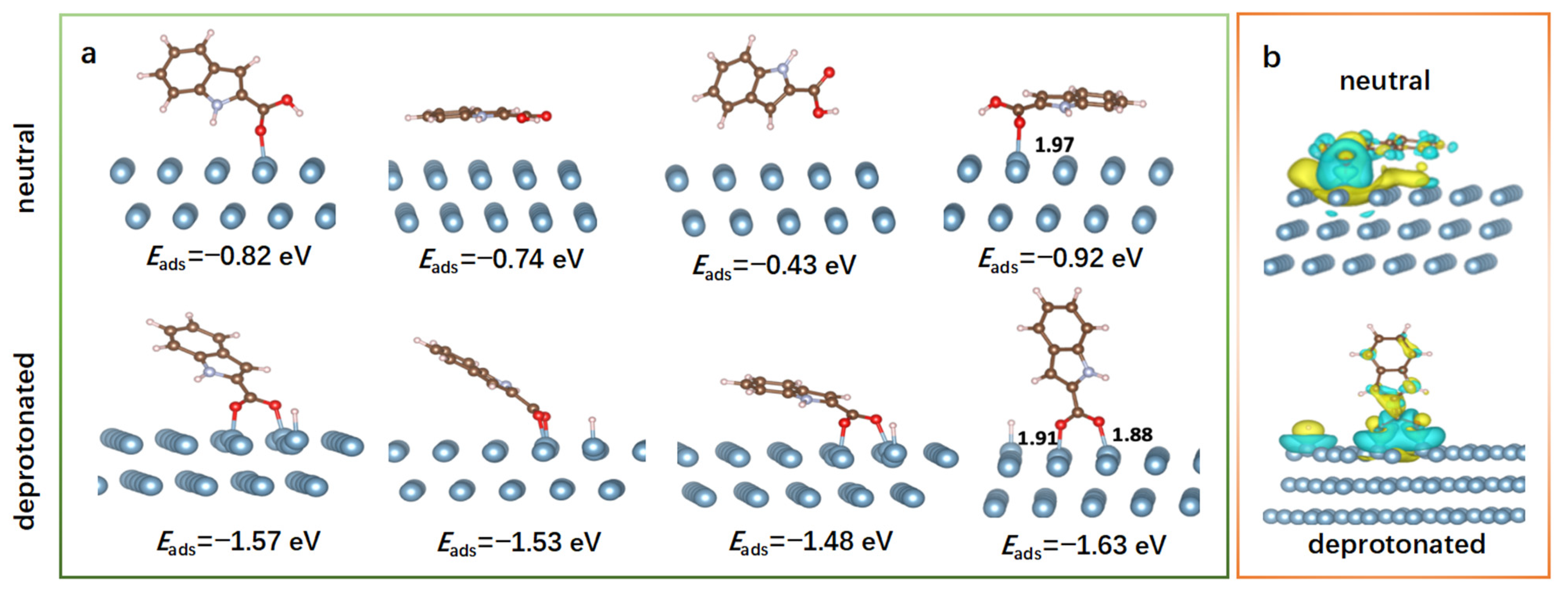
2.6. Theoretical Consideration
3. Experimental
3.1. Materials and Reagents
3.2. Electrochemical Measurements
3.3. Hydrogen Evolution Experiment
3.4. Surface Characteristics of the Aluminum Anode
3.5. Battery Performance Test
3.6. Computational Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-air batteries—A review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Wei, M.; Wang, K.; Zuo, Y.; Wang, H.; Zhang, P.; Zhao, S.; Zhong, D.; Shui, Y.; Pei, P. An advanced alkaline Al-air fuel cell using L-ascorbic acid interface layer upon Al anode via gradient anti-corrosion. ACS Sustain. Chem. Eng. 2023, 11, 3963–3974. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.W.; Zhang, X.B. Recent progress on the development of metal-air batteries. Adv. Sustain. Syst. 2017, 1, 1700036. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.-G.; Xie, Z.; Zhou, Z. Recent progress in rechargeable alkali metal–air batteries. Green Energy Environ. 2016, 1, 4–17. [Google Scholar] [CrossRef]
- Gaele, M.F.; Di Palma, T.M. Polymer electrolytes for Al-air batteries: Current state and future perspectives. Energy Fuels 2022, 36, 12875–12895. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Feng, J.; Zuo, C. Ultrathin Al-air batteries by reducing the thickness of solid electrolyte using aerosol jet printing. Sci. Rep. 2022, 12, 9801. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, Y.; Chen, Y.; Xiao, C.; Ding, S. Development of solid electrolytes in Zn–air and Al–air batteries: From material selection to performance improvement strategies. J. Mater. Chem. A 2021, 9, 4415–4453. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Harchegani, R.K.; Riahi, A.R. Synergistic effect of vanadate and nanoclay hybrid inhibitor on the self-corrosion and discharge activity of Al anode in alkaline aluminum-air batteries. J. Electrochem. Soc. 2023, 170, 030524. [Google Scholar] [CrossRef]
- Kosaba, T.; Muto, I.; Sugawara, Y. Galvanic corrosion of AA5083/Fe in diluted synthetic seawater: Effect of anodizing on local electrochemistry on and around Al6(Fe,Mn) on Al-Matrix. J. Electrochem. Soc. 2022, 169, 020550. [Google Scholar] [CrossRef]
- Pino, M.; Cuadrado, C.; Chacon, J.; Rodriguez, P.; Fatas, E.; Ocon, P. The electrochemical characteristics of commercial aluminium alloy electrodes for Al/air batteries. J. Appl. Electrochem. 2014, 44, 1371–1380. [Google Scholar] [CrossRef]
- Egan, D.R.; Ponce de León, C.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in electrode materials and electrolytes for aluminium–air batteries. J. Power Sources 2013, 236, 293–310. [Google Scholar] [CrossRef]
- Dibetsoe, M.; Olasunkanmi, L.O.; Fayemi, O.E.; Yesudass, S.; Ramaganthan, B.; Bahadur, I.; Adekunle, A.S.; Kabanda, M.M.; Ebenso, E.E. Some phthalocyanine and naphthalocyanine derivatives as corrosion inhibitors for aluminium in acidic medium: Experimental, quantum chemical calculations, QSAR studies and synergistic effect of Iodide ions. Molecules 2015, 20, 15701–15734. [Google Scholar] [CrossRef]
- Moghadam, Z.; Shabani-Nooshabadi, M.; Behpour, M. Electrochemical performance of aluminium alloy in strong alkaline media by urea and thiourea as inhibitor for aluminium-air batteries. J. Mol. Liq. 2017, 242, 971–978. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, S.; Li, W.; Yang, Y.; Yang, L.; Zhang, Z. Inhibition effect and mechanism of inorganic-organic hybrid additives on three-dimension porous aluminum foam in alkaline Al-air battery. J. Power Sources 2020, 448, 227460. [Google Scholar] [CrossRef]
- Luo, L.; Zhu, C.; Yan, L.; Guo, L.; Zhou, Y.; Xiang, B. Synergistic construction of bifunctional interface film on anode via a novel hybrid additive for enhanced alkaline Al-air battery performance. Chem. Eng. J. 2022, 450, 138175. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Mathada, B.S.; Yernale, N.G.; Basha, J.N.; Badiger, J. An insight into the advanced synthetic recipes to access ubiquitous indole heterocycles. Tetrahedron Lett. 2021, 85, 153458. [Google Scholar] [CrossRef]
- Hong, W.-X.; Yao, Z. Synthesis of (2R,3aR,8aR)-6-chloro-3a-hydroxy-l,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indole-2-carboxylic acid methyl ester by reductive cyclization. Chin. J. Chem. 2010, 22, 365–370. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-base molecules as efficient corrosion inhibitors for mild steel surface in 1 M HCl medium: Experimental and theoretical approach. Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef] [PubMed]
- Ganjoo, R.; Sharma, S.; Sharma, P.K.; Dagdag, O.; Berisha, A.; Ebenso, E.E.; Kumar, A.; Verma, C. Coco monoethanolamide surfactant as a sustainable corrosion inhibitor for mild steel: Theoretical and experimental investigations. Molecules 2023, 28, 1581. [Google Scholar] [CrossRef] [PubMed]
- Harchegani, R.K.; Riahi, A.R. Effect of cerium chloride on the self-corrosion and discharge activity of aluminum anode in alkaline aluminum-air batteries. J. Electrochem. Soc. 2022, 169, 030542. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, D.; Lin, T.; Zhang, W.; Li, C.; Gao, L. Effect of quinoline-8-sulfonic acid and CaO as hybrid electrolyte additives on microstructure and property of AA5052 alloy anode for aluminum-air battery. J. Taiwan Inst. Chem. Eng. 2022, 131, 104150. [Google Scholar] [CrossRef]
- Mohamedien, H.A.; Kamal, S.M.; El-Deen, A.G.; Taha, M.; El-Deeb, M.M. Electrochemical and computational estimations of cephalosporin drugs as eco-friendly and efficient corrosion inhibitors for aluminum in alkaline solution. Sci. Rep. 2022, 12, 13333. [Google Scholar] [CrossRef]
- Li, K.; Cheng, R.; Xue, Q.; Zhao, T.; Wang, F.; Fu, C. Construction of a Co/MnO mott-schottky heterostructure to achieve interfacial synergy in the oxygen reduction reaction for aluminum-air batteries. ACS Appl. Mater. Inter. 2023, 17, 9150–9159. [Google Scholar] [CrossRef]
- Mohamedien, H.A.; Kamal, S.M.; Taha, M.; El-Deeb, M.M.; El-Deen, A.G. Experimental and computational evaluations of cefotaxime sodium drug as an efficient and green corrosion inhibitor for aluminum in NaOH solution. Mater. Chem. Phys. 2022, 290, 126546. [Google Scholar] [CrossRef]
- Dong, Y.; Lei, H.; Liu, W. Effect of mixed-shaped silica sol abrasives on surface roughness and material removal rate of zirconia ceramic cover. Ceram. Int. 2020, 46, 23828–23833. [Google Scholar] [CrossRef]
- Shang, J.; Liu, F.; Gu, G.; Meng, L. Effects of Ce(NO3)3 concentration on microstructure and properties of plasma electrolytic oxidation layer on 6061 alloy. Mater. Res. Express 2022, 9, 096513. [Google Scholar] [CrossRef]
- Davo, B.; de Damborenea, J.J. Use of rare earth salts as electrochemical corrosion inhibitors for an Al-Li-Cu (8090) alloy in 3.56% NaCl. Electrochim. Acta 2004, 49, 4957–4965. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Q.; Sun, D.; Luan, J.; Shi, H.; Hu, S.; Tang, Y.; Wang, H. Understanding the synergistic effect of alkyl polyglucoside and potassium stannate as advanced hybrid corrosion inhibitor for alkaline aluminum-air battery. Chem. Eng. J. 2020, 383, 123162. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, C.-W.; Liou, G.-S. Novel aromatic polyamides bearing pendent diphenylamino or carbazolyl groups. J. Polym. Sci. Pol. Chem. 2004, 42, 3302–3313. [Google Scholar] [CrossRef]
- Oteroarean, C. NMR and FTIR spectroscopic studies on the acidity of gallia-silica prepared by a sol-gel route. Micropor. Mesopor. Mat. 2004, 67, 259–264. [Google Scholar] [CrossRef]
- Su, Y.L.; Liu, H.Z.; Wang, J.; Chen, J.Y. Study of salt effects on the micellization of PEO-PPO-PEO block copolymer in aqueous solution by FTIR spectroscopy. Langmuir 2002, 18, 865–871. [Google Scholar] [CrossRef]
- Zlatic, G.; Martinovic, I.; Pilic, Z.; Paut, A.; Mitar, I.; Prkic, A.; Culum, D. Green inhibition of corrosion of aluminium alloy 5083 by artemisia annua L. extract in artificial seawater. Molecules 2023, 28, 2898. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Real, S.; Smedley, S.I.; Urquidi-Macdonald, M. Evaluation of alloy anodes for aluminum-air batteries: IV. electrochemical impedance analysis of pure aluminum in at 25 °C. J. Electrochem. Soc. 1988, 135, 2410. [Google Scholar] [CrossRef]
- Doche, M.L.; Rameau, J.J.; Durand, R.; Novel-Cattin, F. Electrochemical behaviour of aluminium in concentrated NaOH solutions. Corros. Sci. 1999, 41, 805–826. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, S.-R.; Kim, J.-G. Theoretical and experimental study of the crystal orientation effect of the anode on the aluminum-air battery performance. J. Electrochem. Soc. 2023, 169, 120541. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, K.; Si, Y.; Li, Z.; Yu, T.; Liu, X.; Wang, G.; Xie, G.; Jiang, L. High performance of multi-layered alternating Ni–Fe–P and Co–P films for hydrogen evolution. Green Energy Environ. 2022, 7, 75–85. [Google Scholar] [CrossRef]
- Zhang, R.; Ren, X.; Hao, S.; Ge, R.; Liu, Z.; Asiri, A.M.; Chen, L.; Zhang, Q.; Sun, X. Selective phosphidation: An effective strategy toward CoP/CeO2 interface engineering for superior alkaline hydrogen evolution electrocatalysis. J. Mater. Chem. A 2018, 6, 1985–1990. [Google Scholar] [CrossRef]
- Shaikhah, D.; Ritacca, A.G.; Ritacco, I.; Matamorose-Veloza, A.; Taleb, W.; Mohamed-Said, M.; Cowe, B.; Neville, A.; Camellone, M.F.; Barker, R. Engineering of corrosion product-polymer hybrid layers for enhanced CO2 corrosion protection of carbon steel part two: Computational investigation and surface characterisation. Polymer 2022, 250, 124776. [Google Scholar] [CrossRef]
- Damous, M.; Allal, H.; Belhocine, Y.; Maza, S.; Merazig, H. Quantum chemical exploration on the inhibition performance of indole and some of its derivatives against copper corrosion. J. Mol. Liq. 2021, 340, 117136. [Google Scholar] [CrossRef]
- Kunaseth, M.; Poldorn, P.; Junkeaw, A.; Meeprasert, J.; Rungnim, C.; Namuangruk, S.; Kungwan, N.; Inntam, C.; Jungsuttiwong, S. A DFT study of volatile organic compounds adsorption on transition metal deposited graphene. Appl. Surf. Sci. 2017, 396, 1712–1718. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Xu, S. Investigation of indole-3-carboxylic acid as steel inhibitor in 0.1 M H2SO4 solution. J. Ind. Eng. Chem. 2015, 25, 295–303. [Google Scholar] [CrossRef]
- Huong Pham, T.; Lee, W.-H.; Kim, J.-G. Chrysanthemum coronarium leaves extract as an eco-friendly corrosion inhibitor for aluminum anode in aluminum-air battery. J. Mol. Liq. 2022, 347, 118269. [Google Scholar] [CrossRef]
- Pham, T.H.; Lee, W.-H.; Byun, J.-H.; Kim, J.-G. Improving the performance of primary aluminum-air batteries through suppressing water activity by hydrogen bond-rich glycerol solvent additive. Energy Storage Mater. 2023, 55, 406–416. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H. The effect of grain size on aluminum anodes for Al–air batteries in alkaline electrolytes. J. Power Sources 2015, 284, 409–415. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, T.; Li, Z.; Guo, C.; Lai, J.; Tian, Z. Anode interfacial layer construction via hybrid inhibitors for high-performance Al-air batteries. ACS Appl. Mater. Inter. 2021, 13, 51726–51735. [Google Scholar] [CrossRef]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 1994, 6, 8245. [Google Scholar] [CrossRef]
- Wang, D.P.; Gao, L.X.; Zhang, D.Q.; Yang, D.; Wang, H.X.; Lin, T. Experimental and theoretical investigation on corrosion inhibition of AA5052 aluminium alloy by L-cysteine in alkaline solution. Mater. Chem. Phys. 2016, 169, 142–151. [Google Scholar] [CrossRef]
- Poberžnik, M.; Chiter, F.; Milošev, I.; Marcus, P.; Costa, D.; Kokalj, A. DFT study of n-alkyl carboxylic acids on oxidized aluminum surfaces: From standalone molecules to self-assembled-monolayers. Appl. Surf. Sci. 2020, 525, 146156. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for Bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for bader decomposition of charge density. Comp. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
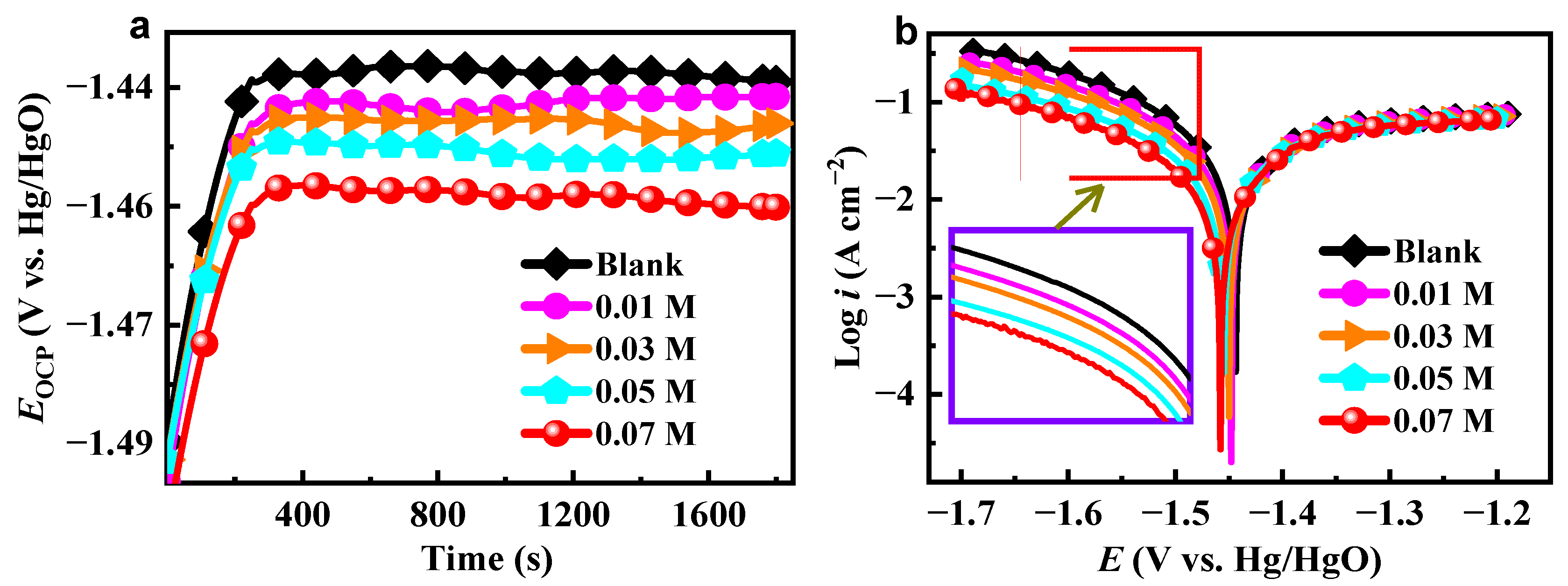
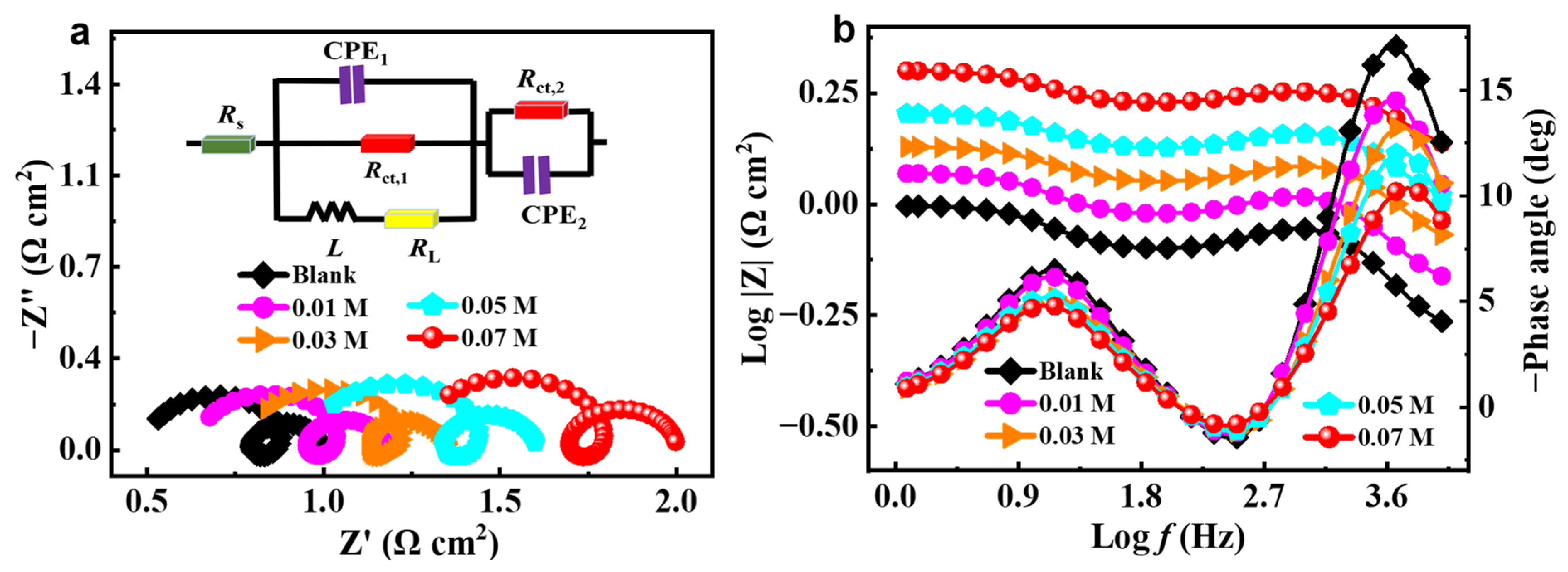
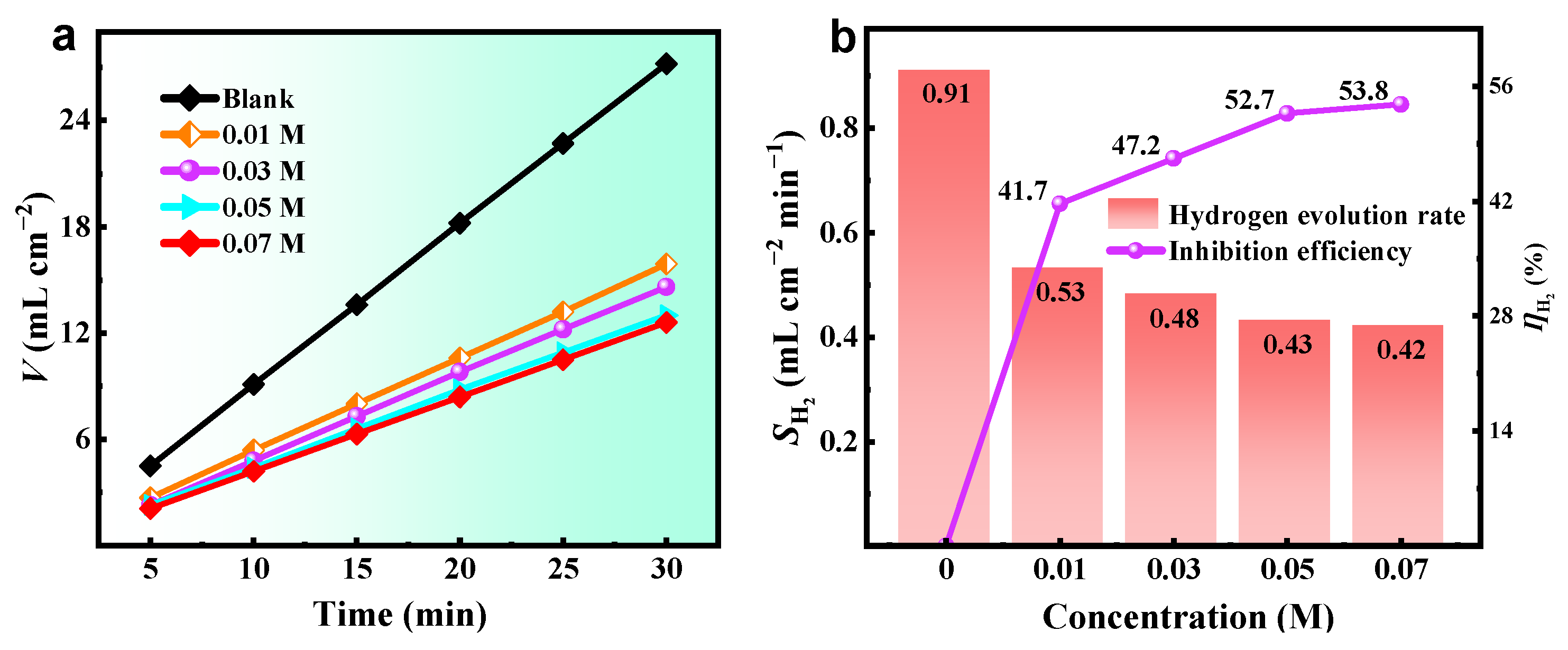





| C (M) | Ecorr (V vs. Hg/HgO) | icorr (mA cm−2) | −βc (mV dec−1) | βa (mV dec−1) | ηPDP (%) |
|---|---|---|---|---|---|
| Blank | −1.4383 | 45.99 | 170.1 | 405.0 | / |
| 0.01 | −1.4413 | 38.24 | 178.6 | 340.1 | 16.9 |
| 0.03 | −1.4450 | 32.90 | 182.2 | 304.9 | 28.5 |
| 0.05 | −1.4491 | 28.80 | 196.2 | 296.0 | 37.4 |
| 0.07 | −1.4553 | 21.14 | 213.3 | 213.6 | 54.0 |
| C (M) | Rs (Ω cm2) | CPE1 | Rct,1 (Ω cm2) | L (10−5 H cm2) | RL (Ω cm2) | CPE2 | Rct,2 (Ω cm2) | Rp (Ω cm2) | χ2 (10−4) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Y0 (10−4 S sn cm−2) | n1 | Y0 (S Sn cm−2) | n2 | ||||||||
| Blank | 0.495 | 1.228 | 1.000 | 0.293 | 4.139 | 0.120 | 0.060 | 1.000 | 0.205 | 0.290 | 1.91 |
| 0.01 | 0.636 | 1.141 | 1.000 | 0.307 | 4.681 | 0.120 | 0.054 | 1.000 | 0.232 | 0.318 | 1.34 |
| 0.03 | 0.779 | 0.883 | 1.000 | 0.341 | 4.380 | 0.123 | 0.054 | 1.000 | 0.230 | 0.320 | 0.99 |
| 0.05 | 0.959 | 0.784 | 1.000 | 0.372 | 5.159 | 0.135 | 0.049 | 1.000 | 0.270 | 0.369 | 0.66 |
| 0.07 | 1.256 | 0.686 | 0.991 | 0.427 | 6.188 | 0.135 | 0.042 | 1.000 | 0.317 | 0.420 | 0.64 |
| C (M) | Weight Loss ∆m (g) | Average Discharge Voltage (V) | Capacity Density (mAh g−1) | Energy Density (Wh kg−1) | Ua (%) |
|---|---|---|---|---|---|
| Blank | 0.0167 | 1.2199 | 1197.6 | 1469.9 | 40.2 |
| 0.07 M ICA | 0.0084 | 1.2398 | 2380.9 | 2951.8 | 79.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Huang, Y.; Ritacca, A.G.; Wang, K.; Ritacco, I.; Tan, Y.; Qiang, Y.; Al-Zaqri, N.; Shi, W.; Zheng, X. Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al–Air Battery. Molecules 2023, 28, 4193. https://doi.org/10.3390/molecules28104193
Guo L, Huang Y, Ritacca AG, Wang K, Ritacco I, Tan Y, Qiang Y, Al-Zaqri N, Shi W, Zheng X. Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al–Air Battery. Molecules. 2023; 28(10):4193. https://doi.org/10.3390/molecules28104193
Chicago/Turabian StyleGuo, Lei, Yue Huang, Alessandra Gilda Ritacca, Kai Wang, Ida Ritacco, Yan Tan, Yujie Qiang, Nabil Al-Zaqri, Wei Shi, and Xingwen Zheng. 2023. "Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al–Air Battery" Molecules 28, no. 10: 4193. https://doi.org/10.3390/molecules28104193
APA StyleGuo, L., Huang, Y., Ritacca, A. G., Wang, K., Ritacco, I., Tan, Y., Qiang, Y., Al-Zaqri, N., Shi, W., & Zheng, X. (2023). Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al–Air Battery. Molecules, 28(10), 4193. https://doi.org/10.3390/molecules28104193









