Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases
Abstract
1. Introduction
1.1. Achillea millefolium L. Aster Family—Asteraceae
1.2. Acorus calamus L. Aroid Family—Araceae
1.3. Agropyron repens L. Lacquer Family—Gramineae
1.4. Artemisia absinthium L. Aster Family—Asteraceae
1.5. Bidens tripartita L. Aster Family—Asteraceae
1.6. Capsella bursa-pastoris L. Cabbage Family—Brassicaceae
1.7. Chelidonium majus L. Poppy Family—Papaveraceae
1.8. Cichorium intybus L. Aster Family—Asteraceae
1.9. Equisetum arvense L. Horsetail Family—Equisetaceae
1.10. Eryngium planum L. Seler Family—Apiaceae
1.11. Glycyrrhiza glabra L. Legume Family—Fabaceae
1.12. Gnaphalium uliginosum L. Aster Family—Asteraceae
1.13. Humulus lupulus L. Hemp Family—Cannabaceae
1.14. Juglans regia L. Walnut Family—Juglandaceae
1.15. Matricaria recutita L. Aster Family—Asteraceae
1.16. Ononis spinosa L. Legume Family—Fabaceae
1.17. Onopordum acanthium L. Aster Family—Asteraceae
1.18. Orchis maculata L. Orchid Family—Orchidaceae
1.19. Pastinaca sativa L. Seler Family—Apiaceae
1.20. Plantago major L. Family—Plantaginaceae
1.21. Ribes nigrum L. Saxifrage Family—Saxifragaceae
1.22. Rosa canina L. Rose Family—Rosaceae
1.23. Solanum dulcamara L. Solanaceae Family—Solanaceae
1.24. Sorbus aucuparia L. Rose Family—Rosaceae
1.25. Symphytum officinale L. Borage Family—Boraginaceae
1.26. Tanacetum vulgare L. Aster Family—Asteraceae
1.27. Taraxacum officinale Web. Family—Compositae
1.28. Thymus serpyllum L. Lamiaceae Family—Lamiaceae
1.29. Vaccinium myrtillus L. Cowberry Family—Ericaceae
1.30. Viscum album L. Family Beltflower—Lorantliaceae
2. Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kelly, K. History of Medicine. Early Civilizations Prehistoric Times to 500 C.E; (Facts on File); Infobase Publishing: New York, NY, USA, 2009; pp. 29–50. Available online: https://kupdf.net/download/the-history-of-medicine-2009_5af98606e2b6f51b389016b7_pdf (accessed on 14 May 2023).
- Jumagaliyeva, K.V.; Sarmurzina, N.; Kairgalieva, G.K. History of traditional medicine of the Kazakh people. J. Samara Sci. Cent. RAS Hist. Sci. 2020, 1, 117–126. (In Russian) [Google Scholar] [CrossRef]
- World Health Organization. WHO Monographs on Medicinal Plants Widespread in CGM (NHH); World Health Organization: Geneva, Switzerland, 2010; p. 455. ISBN 978 92 4 459772 9. (In Russian) [Google Scholar]
- Grudzinskaya, L.M.; Gemejiyeva, N.G.; Karzhaubekova, Z.Z. The Kazakhstan medicinal flora survey in a leading families volume. Bull. Karaganda Univ. Ser. Biol. Med. Geogr. 2020, 4, 39–51. [Google Scholar] [CrossRef]
- Gubanov, I.A.; Kiseleva, K.V.; Novikov, V.S.; Tikhomirov, V.N. Illustrated determinant of plants of Central Russia. Mosc. Assoc. Sci. Publ. CMC Inst. Technol. Res. 2004, 3, 11. (In Russian) [Google Scholar]
- Dekker, J. The Evolutionary Ecology of Weeds and Invasive Plants. Evolut. Ecol. 2010, 197. Available online: https://e.eruditor.one/file/2696081/ (accessed on 14 May 2023).
- Kurbanov, S.A. Agriculture: A Textbook for Universities; Yurayt Publishing House: Moscow, Russia, 2023; p. 252. [Google Scholar]
- Ivanović, M.; Grujić, D.; Cerar, J.; Islamčević Razboršek, M.; Topalić-Trivunović, L.; Savić, A.; Kolar, M. Extraction of Bioactive Metabolites from Achillea millefolium L. with Choline Chloride Based Natural Deep Eutectic Solvents: A Study of the Antioxidant and Antimicrobial Activity. Antioxidants 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, S.G.; Ogar, N.P.; Inelova, Z.A.; Karamanidi, E.E. The family spectrum of the flora of the Toraigyr mountains. Bull. Treas. Biol. Ser. 2012, 54, 7–10. (In Russian) [Google Scholar]
- Karami, P.; Zandi, M.; Ganjloo, A. Evaluation of physicochemical, mechanical, and antimicrobial properties of gelatin-sodium alginate-yarrow (Achillea millefolium L.) essential oil film. J. Food Process. Preserv. 2022, 46, 16632. [Google Scholar] [CrossRef]
- Ayoobi, F.; Shamsizadeh, A.; Fatemi, I.; Vakilian, A.; Allahtavakoli, M.; Hassanshahi, G.; Moghadam-Ahmadi, A. Bio-effectiveness of the main flavonoids of Achillea millefolium in the pathophysiology of neurodegenerative disorders—A review. Iran. J. Basic Med. Sci. 2017, 20, 604. [Google Scholar] [CrossRef]
- Kiseleva, T.L. Kinetic synergism in phytotherapy: Traditional drugs from the point of view of modern scientific concepts. Tradit. Med. 2011, 2, 50–57. (In Russian) [Google Scholar]
- Musaev, F.A.; Zakharova, O.A.; Musaeva, R.F. Medicinal Plants (Textbook). Int. J. Exp. Educ. 2014, 77–78. Available online: https://expeducation.ru/ru/article/view?id=6220 (accessed on 14 May 2023). (In Russian).
- Anishchenko, L.V. Encyclopedia of Medicinal Plants; AST: Moscow, Russia, 2017; p. 208. ISBN 978-5-17-100053-0. (In Russian) [Google Scholar]
- Aslanova, D.; Karomatov, I.D. Yarrow is common in folk and scientific herbal medicine. Biol. Integr. Med. 2018, 1, 167–186. (In Russian) [Google Scholar]
- Vazirinejad, R.; Ayoobi, F.; Arababadi, M.K.; Eftekharian, M.M.; Darekordi, A.; Goudarzvand, M.; Shamsizadeh, A. Effect of aqueous extract of Achillea millefolium on the development of experimental autoimmune encephalomyelitis in C57BL/6 mice. Indian J. Pharmacol. 2014, 46, 303. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Masłowski, M.; Aleksieiev, A.; Miedzianowska, J.; Strzelec, K. Potential application of peppermint (Mentha piperita L.), german chamomile (Matricaria chamomilla L.) and yarrow (Achillea millefolium L.) as active fillers in natural rubber biocomposites. Int. J. Mol. Sci. 2021, 22, 7530. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Maver, T.; Maver, U.; Stana Kleinschek, K.; Smrke, D.M.; Kreft, S. A Review of Herbal Medicines in Wound Healing. Int. J. Dermatol. 2015, 54, 740–751. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Pakhomova, E.E.; Pakhomova, A.E.; Pakhomova, Y.V.; Karabintseva, N.O.; Ovsyanko, E.V. Evaluation of wound healing, antimicrobial, anti-inflammatory effects of essential oils. J. Sib. Med. Sci. 2015, 6, 70. [Google Scholar]
- Ghobadian, Z.; Ahmadi, M.R.H.; Rezazadeh, L.; Hosseini, E.; Kokhazadeh, T.; Ghavam, S. In vitro evaluation of Achillea millefolium on the production and stimulation of human skin fibroblast cells (HFS-PI-16). Med. Arch. 2015, 69, 212. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.T.; Hwang, E.; Kang, H.; Park, B.; Seo, S.A.; Yi, T.H. Anti-inflammatory effects of Achillea millefolium on atopic dermatitis-like skin lesions in NC/Nga mice. Am. J. Chin. Med. 2020, 48, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Abdul-hafiz, I.Y.; Egorov, M.A.; Suchenko, L.T. Antibacterial activity of essential oil and alcohol extracts of air marsh (Acorus calamus) and camel thorn (Alhagi pseudalhagi), collected in the Astrakhan region. Vestn. Altai State Agrar. Univ. 2011, 3, 50–53. (In Russian) [Google Scholar]
- Guryev, A.M.; Pozhan, I.S. Research of the Chemical Composition of Rhizomes Acorus calamus L.; Collection of Articles on the Materials of the Fourth Congress of Young Scientists and Specialists; Sciences about Man: Tomsk, Russia, 2003; p. 197. [Google Scholar]
- Kim, H.; Han, T.H.; Lee, S.G. Anti-inflammatory activity of a water extract of Acorus calamus L. leaves on keratinocyte HaCaT cells. J. Ethnopharmacol. 2009, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Khwairakpam, A.D.; Damayenti, Y.D.; Deka, A.; Monisha, J.; Roy, N.K.; Padmavathi, G.; Kunnumakkara, A.B. Acorus calamus: A bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Medicinal properties of Acorus calamus. J. Drug Deliv. Therapeutics. 2013, 3, 143–144. [Google Scholar] [CrossRef]
- Yende, S.; Harle, U.; Rajgure, D.; Tuse, T.; Vyawahare, N. Pharmacological profile of Acorus calamus: An overview. Pharmacogn. Rev. 2008, 2, 23. [Google Scholar]
- Singh, R.; Sharma, P.K.; Malviya, R. Pharmacological Properties and Ayurvedic Value of Indian Buch Plant (Acorus calamus): A Short Review. Adv. Biol. Res. 2011, 5, 145–154. Available online: http://www.idosi.org/abr/5/3.pdf (accessed on 14 May 2023).
- Marongiu, B.; Piras, A.; Porcedda, S.; Scorciapino, A. Chemical composition of the essential oil and supercritical CO2 extract of Commiphora myrrha (Nees) Engl. and of Acorus calamus L. J. Agric. Food Chem. 2005, 53, 7939–7943. [Google Scholar] [CrossRef]
- Balakumbahan, R.; Rajamani, K.; Kumanan, K. Acorus calamus: An overview. J. Med. Plants Res. 2010, 4, 2740–2745. [Google Scholar]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn. (Sweet flag) and other Acorus species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [PubMed]
- Chamorro, M.M.A.; Collado, S.A.V.; Márquez, D. Effectiveness of Using Renalof in the Elimination of Kidney Stones under 10 mm Located in the Renal-Ureteral Tract. Open J. Nephrol. 2021, 11, 78. [Google Scholar] [CrossRef]
- Atabayeva, S.; Sarsenbayev, B.; Prasad, M.N.V.; da Silva, J.A.T.; Kenzhebayeva, S.; Usenbekov, B.; Kotuhov, Y. Accumulation of Trace Metals in Grasses of Kazakhstan: Relevance to Phytostabilization of Mine Waste and Metal-Smelting Areas. AAJPSB Spec. Issue Kazakhstan Plant Sci. Biotechnol. 2010, 1, 91–97. [Google Scholar]
- Neagu, E.; Păun, G.; Moroeanu, V.; Ungureanu, O.; Radu, G.L. Antioxidant and Antidiabetic Properties of Polyphenolic-Rich Extracts of Apium graveolens and Agropyrum repens. Rev. Roum. Chim. 2019, 64, 909–913. [Google Scholar] [CrossRef]
- Bortolami, M.; Di Matteo, P.; Rocco, D.; Feroci, M.; Petrucci, R. Metabolic Profile of Agropyron repens (L.) P. Beauv. Rhizome Herbal Tea by HPLC-PDA-ESI-MS/MS Analysis. Molecules 2022, 27, 4962. [Google Scholar] [CrossRef]
- Tsubanova, N.A.; Barska, A.V.; Cherniavski, E.S. Clinical efficiency of preparations based on medical plant raw materials in the treatment of urolithiasis. Fam. Med. 2019, 81, 80–87. [Google Scholar] [CrossRef][Green Version]
- Al-Snafi, A.E. Chemical constituents and pharmacological importance of Agropyron repens—A review. Res. J. Pharmacol. Toxicol. 2015, 1, 37–41. [Google Scholar]
- Beydokthi, S.S.; Sendker, J.; Brandt, S.; Hensel, A. Traditionally used medicinal plants against uncomplicated urinary tract infections: Hexadecyl coumaric acid ester from the rhizomes of Agropyron repens (L.) P. Beauv. with Antiadhesive Activity against Uropathogenic E. coli. Fitoterapia 2017, 117, 22–27. [Google Scholar] [CrossRef]
- Anghel, N.; Melinte, V. Polysaccharide-Based Matrix Doped with Plant Extract for Medical and Cosmetic Applications. Cellul. Chem. Technol. 2022, 56, 283–291. [Google Scholar] [CrossRef]
- Petrova, A.P.; Krasnov, E.A.; Saprykina, E.V.; Subbotina, Y.A.; Ermilova, E.V. The Chemical Composition of Wheat Grass and the Study of Its Antioxidant Activity in Allergic Contact Dermatitis. Chem. Pharm. J. 2009, 43, 30–32. (In Russian) [Google Scholar] [CrossRef]
- Yousefi, M.; Zahedi, S.; Reverter, M.; Adineh, H.; Hoseini, S.M.; Van Doan, H.; Hoseinifar, S.H. Enhanced growth performance, oxidative capacity and immune responses of common carp, cyprinus carpio fed with Artemisia absinthium extract-supplemented Diet. Aquaculture 2021, 545, 737167. [Google Scholar] [CrossRef]
- Kabdulkarimova, K.K.; Dinzhumanova, R.; Olzhayeva, R.; Karimova, A.A.; Uzbekova, S.I.; Orazalina, A.; Lauenova, S.A. Determination of the chemical composition and antioxidant activity of Artemisia vulgaris and Artemisia absinthium growing in the conditions of the Semey Region. Open Access Maced. J. Med. Sci. 2022, 10, 1512–1519. [Google Scholar] [CrossRef]
- Dyusebaeva, M.A.; Kurmanbaeva, A.K.; Nurlybekova, A.K.; Aisa, H.A.; Jenis, J. Amino-acid and fatty-acid compositions of two Artemisia species. Chem. Nat. Compd. 2018, 54, 1208–1210. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Ekiert, H. Artemisia absinthium L.—Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Rivero-Perez, N. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Amidon, C.; Barnett, R.; Cathers, J.; Chambers, B.; Hamilton, L.; Kellett, A.; Kennel, E.; Montowski, J.; Thomas, M.A.; Watson, B. Artemisia—An Essential Guide from the Herb Society of America; Caroline, A., Thomas, M., Kennel, E., Eds.; The Herb Society of America: Kirtland, OH, USA, 2014. [Google Scholar]
- Ahamad, J. A Pharmacognostic Review on Artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Bordean, M.E.; Muste, S.; Marțiș, G.S.; Mureșan, V.; Buican, B.C. Health effects of wormwood (Artemisia absinthium L.): From Antioxidant to Nutraceutical. J. Agroalim. Proc. Technol. 2021, 27, 211–218. [Google Scholar]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Kouda, A.; Tahiri, M.; Zaid, A. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Hbika, A.; Bouyanzer, A.; Saadi, M.; El Ammari, L.; Benali, M.; Majidi, L.; Zarrouk, A. Structural Study and Thermal Stability of Artemetin Extracted from Artemisia absinthium L. Chem. Data Collect. 2022, 40, 100880. [Google Scholar] [CrossRef]
- Benkhaled, A.; Boudjelal, A.; Napoli, E.; Baali, F.; Ruberto, G. Phytochemical Profile, Antioxidant Activity and Wound Healing Properties of Artemisia absinthium Essential Oil. Asian Pac. J. Trop. Biomed. 2020, 10, 496. [Google Scholar] [CrossRef]
- Tran, T.A.; Ho, M.T.; Song, Y.W.; Cho, M.; Cho, S.K. Camphor Induces Proliferative and Anti-senescence Activities in Human Primary Dermal Fibroblasts and Inhibits UV-Induced Wrinkle Formation in Mouse Skin. Phytother. Res. 2015, 12, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Karolina, Ś.; Elżbieta, S.; Jan, O.; Joanna, K.A. Micelle mediated extraction as a new method of obtaining the infusion of Bidens tripartite. Acta Biochim. Pol. 2016, 63, 543–548. [Google Scholar]
- Uysal, S.; Ugurlu, A.; Zengin, G.; Baloglu, M.C.; Altunoglu, Y.C.; Mollica, A.; Mahomoodally, M.F. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem. Toxicol. 2018, 111, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Calitz, C.; Plessis, L.; Gouws, C.; Steyn, D.; Steenekamp, J.; Muller, C.; Hamman, S. Herbal hepatotoxicity: Current status, examples, and challenges. Expert Opin Drug Metab. Toxicol. 2015, 11, 1551–1565. [Google Scholar] [CrossRef]
- Boyko, N.N.; Bondarev, A.V.; Zhilyakova, E.T.; Pisarev, D.I.; Novikov, O.O. Phytodrugs, analysis of Russian Federation pharmaceutical market. Research Result. Med. Pharm. 2017, 3, 30–38. (In Russian) [Google Scholar]
- Oproshanskaya, T.V. Fatty acids from Bidens tripartita HERB. Chem. Nat. Comp. 2015, 51, 944–945. [Google Scholar] [CrossRef]
- Rodin, M.N.; Bokov, D.O.; Kovaleva, T.Y.; Bobkova, N.V.; Sergunova, E.V.; Strelyaeva, A.V.; Bobkova, N.V.; Sergunova, E.V.; Strelyaeva, A.V.; Khasanova, S.R. Composition of biologically active compounds, biological and pharmacological activity of the three-part beggarticks (Bidens tripartita L.). Nveo—Nat. Volatiles Essent. Oils J. 2021, 8, 11039–11053. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharm. 2014, 4, 177. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Wróblewska, M.; Winnicka, K.; Wieczorek, P.; Majewski, P.; Celi’nska-Janowicz, K.; Sawczuk, R.; Miltyk, W.; Tryniszewska, E.; Tomczyk, M. Novel gel formulations as topical carriers for the essential oil of Bidens tripartita for the treatment of candidiasis. Molecules 2018, 23, 2517. [Google Scholar] [CrossRef]
- Karazhan, N.V.; Buzuk, G.N. Comparative study of morphological and anatomical-diagnostic signs of species of Bur-marigold herb. Pharm. Bull. 2013, 1, 12–19. [Google Scholar]
- Tomczykowa, M.; Leszczyńska, K.; Tomczyk, M.; Tryniszewska, E.; Kalemba, D. Composition of the Essential Oil of Bidens tripartita L. Roots and Its Antibacterial and Antifungal Activities. J. Med. Food 2011, 4, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C. Encyclopedia of Herbal Medicine; Dorling Kindersley: London, UK, 2016; p. 336. [Google Scholar]
- Arkhipov, O.A.; Zhuravleva, V.V.; Alexandrova, M.V.; Alexandrov, T.V. Safety of Herbal Medicines: Clinical and Pharmacological Aspects Demidova. Sci. Cent. Expert Eval. Med. Prod. 2020, 8, 165–177. [Google Scholar] [CrossRef]
- Mironov, A.N.; Sakaeva, I.V.; Sakanyan, E.I.; Korsun, L.V.; Mochikina, O.A. Current approaches to standartization of herbal substasnce. Vedomosti Nauchnogo tsentra ekspertizy sredstv meditsinskogo primeneniya. Bull. Sci. Cent. Expert Eval. Med. Prod. 2013, 2, 52–56. (In Russian) [Google Scholar]
- Sambukova, T.V.; Ovchinnikov, B.V.; Ganapolski, V.P.; Yatmanov, A.N.; Shabanov, P.D. Prospects for phytopreparations use in modem pharmacology. Obzory po klinicheskoy farmakologii i lekarstvennoy terapii. Rev. Clin. Pharmacol. Drug Ther. 2017, 15, 56–63. (In Russian) [Google Scholar] [CrossRef]
- Orhan, N.; İçöz, Ü.G.; Altun, L.; Aslan, M. Anti-hyperglycaemic and antioxidant effects of Bidens tripartita and quantitative analysis on its active principles. Iran. J. Basic Med. Sci. 2016, 19, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Olisova, O.Y.; Snarskaya, E.S.; Gladko, V.V.; Burova, E.P. Russian traditional medicine in dermatology. Clin Dermatol. 2018, 36, 325–337. [Google Scholar] [CrossRef]
- Kaskoniene, V.; Kaškonas, P.; Maruška, A.; Ragažinskienė, O. Essential oils of Bidens tripartita L. collected during period of 3 years composition variation analysis. Acta Physiol. Plant 2012, 7, 1056–1064. [Google Scholar]
- Khatamov, H.M.; Suyarov, A.A.; Kireev, V.V.; Ziyadullaev, S.H.; Muhtorov, S.M.; Alimzhanova, L.I. Efficiency of a dense extract of the sum of fl avonoids in the form of ointment at treatment contact allergic dermatitis in experiment. Immunology 2020, 41, 269–273. (In Russian) [Google Scholar] [CrossRef]
- Dar, M.A.; Ahad, P.; Masoodi, M.H.; Mir, S.R.; Akbar, S. Lady’s Purse (Capsella bursa-pastoris L.): Current Perspective on Its Ethnopharmacological, Therapeutic Potential, and Phytochemistry. In Edible Plants Health Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer: Berlin/Heidelberg, Germany, 2022; pp. 425–455. [Google Scholar]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Capsella bursa-pastoris—A review. Int. J. Pharmacol. Toxicol. 2015, 5, 76–81. [Google Scholar]
- Riaz, I.; Bibi, Y.; Ahmed, N. Evaluation of nutritional, phytochemical, antioxidant and cytotoxic potential of Capsella bursa-pastoris, a wild vegetable from potohar region of Pakistan. Kuwait J. Sci. 2021, 48, 1–11. [Google Scholar] [CrossRef]
- Grosso, C.; Vinholes, J.; Silva, L.R.; Pinho, P.G.d.; Gonçalves, R.F.; Valentão, P.; Jäger, A.K.; Andrade, P.B. Chemical composition and biological screening of Capsella bursa-pastoris. Rev. Bras. Farmacogn. 2011, 21, 635–643. [Google Scholar] [CrossRef]
- Cha, J.M.; Kim, D.H.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phytochemical Constituents of Capsella bursa-pastoris and Their Anti-inflammatory Activity. Nat. Prod. Sci. 2018, 24, 132–138. [Google Scholar] [CrossRef]
- Sushchuk, N.A.; Kolesnik, Y.S.; Kislichenko, V.S.; Kuznecova, V.Y. Investigation of the component composition of volatile fractions of shepherd’s purse grass and black currant buds. Bull. Tajik Natl. Univ. Nat. Sci. Ser. 2013, 1/3, 84–88. (In Russian) [Google Scholar]
- Song, N.; Xu, W.; Guan, H.; Liu, X.; Wang, Y.; Nie, X. Several flavonoids from Capsella bursa-pastoris (L.) Medic. Asian J. Tradit. Med. 2007, 2, 218–222. [Google Scholar]
- Xie, L.K.; Xu, X.J.; Wu, X.; Wang, M.-J.; Gao, C.-F.; Wang, D.-M.; Ren, S.-M.; Pan, Y.-N.; Liu, X.-Q. Capsella bursa-pastoris (L.) Medic. extract alleviate cataract development by regulating the mitochondrial apoptotic pathway of the lens epithelial cells. J. Ethnopharmacol. 2022, 284, 114783. [Google Scholar] [CrossRef]
- Hasan, R.N.; Ali, M.R.; Shakier, S.M.; Khudhair, A.M.; Hussin, M.S.; Kadum, Y.A.; Mohammed, A.I.; Abbas, A.A. Antibacterial activity of aqueous and alcoholic extracts of Capsella Bursa against selected pathogenic bacteria. Am. J. BioScience 2013, 1, 6–10. [Google Scholar] [CrossRef]
- Cha, J.M.; Suh, W.S.; Lee, T.H.; Subedi, L.; Kim, S.Y.; Lee, K.R. Phenolic Glycosides from Capsella bursa-pastoris (L.) Medik and Their Anti-inflammatory Activity. Molecules 2017, 22, 1023. [Google Scholar] [CrossRef]
- Wani, M.A.; Jan, N.; Qazi, H.A.; Andrabi, K.I.; John, R. Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol. Plant. 2018, 40, 1–14. [Google Scholar] [CrossRef]
- Lee, K.E.; Shin, J.; Hong, I.S.; Cho, N.P.; Cho, S.D. Effect of methanol extracts of Cnidium officinale Makino and Capsella bursa-pastoris on the apoptosis of HSC-2 human oral cancer cells. Exp. Ther. Med. 2013, 5, 789–792. [Google Scholar] [CrossRef]
- Kubínová, R.; Spačková, V.; Svajdlenka, E.; Lučivjanská, K. Antioxidant activity of extracts and HPLC analysis of flavonoids from Capsella bursa-pastoris (L.) Medik. Ceska A Slov. Farm. Cas. Ceske Farm. Spol. A Slov. Farm. Spol. 2013, 62, 174–176. [Google Scholar]
- Ma, Q.; Guo, Y.; Wei, R.; Sang, Z.; Liu, W.; Gao, L.; Liu, T. Flavonoids from Capsella bursa-pastoris and their hepatoprotective activities in vitro. Rev. Bras. Farmacogn. 2016, 26, 710–713. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Bussmann, R.W. Traditional and ethnomedicinal dermatology practices in Pakistan. Clin. Dermatol. 2018, 36, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H. Utilisation of plant genetic resources for valuable raw materials in foods, cosmetics, and pharmaceutical products. Schr. Zu Genet. Ressour. 2003, 182–191. [Google Scholar]
- Ghoreschi, K.; Brück, J.; Kellerer, C.; Deng, C.; Deng, C.; Peng, H.; Rothfuss, O.; Hussain, R.Z.; Gocke, A.R.; Respa, A.; et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J. Exp. Med. 2011, 24, 2291–2303. [Google Scholar] [CrossRef]
- Shivraj, H.N.; Hui, W.; Arti, N.; Xianmin, L.; Huilin, D.; Baskar, V.; Elwira, S.; Gansukh, E.; Guoyin, K. Comparative analysis of metabolic variations, antioxidant potential and cytotoxic effects in different parts of Chelidonium majus L. Food Chem. Toxicol. 2021, 156, 112483. [Google Scholar] [CrossRef]
- Maji, A.K.; Banerji, P. Chelidonium majus L. (Greater Celandine)—A Review on Its Phytochemical and Therapeutic Perspectives. Int. J. Herb. Med. 2015, 3, 10–27. Available online: https://www.florajournal.com/vol3issue1/may2015/2-6-10.1.pdf (accessed on 14 May 2023). [CrossRef]
- Heba, F.; Gomaa, N.N.; Fadl, W.M.A.; Elmashad, D.M.A.; Fathia, A.M.; Khaled, G.A. Protective efficiency of Chelidonium majus extract against hepatoimmune and DNA changes induced by aflatoxin B1. J. Appl. Pharm. Sci. 2022, 12, 140–149. [Google Scholar] [CrossRef]
- Maciej, S.; Sławomir, D.; Beata, P.; Kamil, S.; Ireneusz, S.; Daniel, Z.; Rob, V.; Sylwia, Z.; Paweł, K.; Magdalena, W. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium majus Isoquinoline Alkaloids. Molecules 2022, 27, 2815. [Google Scholar] [CrossRef]
- Nawrot, J.; Wilk, J.M.; Nawrot, S.; Nawrot, K.; Wilk, B.; Dawid, P.R.; Urbanska, M.; Micek, I.; Nowak, G.; Gornowicz, P.J. Milky sap of greater celandine (Chelidonium majus L.) and anti-viral properties. Int. Ional J. Environ. Res. Public Health 2020, 17, 1540. [Google Scholar] [CrossRef]
- Jyoti, B.S. Chelidonium majus L.—A review on pharmacological activities and clinical effects. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 238. [Google Scholar]
- Madjeed, H.K.; Dawood, S.H.; Hameed, N.M.; Mahdi, R.A.; Alkhafaje, W.K.; Mahdi, R.A.; Alkhafaje, W.K.; Salaam, A.E.; Hussein, H.A.; Hmod, F.K.; et al. Investigation of in vitro Cytotoxicity of Chelidonium majus against Leishmania major. Arch. Razi Inst. 2022, 77, 1211–1214. [Google Scholar] [CrossRef]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties—A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, P.; Kumar, A.; Mishra, A.K.; Dixit, R.K.; Kumar, A.; Kumar, R.; Gupta, A.K. Kasni (Cichorium intybus L.) A propitious traditional medicinal herb. Int. J. Pharmacogn. 2015, 8, 368–380. [Google Scholar]
- Laurenov, G.V.; Lavrenov, V.K. Encyclopedia of Medicinal Plants; Publishing House “Donechchina”: Donetsk, Ukraine, 2016; Volume 2, p. 1440. [Google Scholar]
- Khaled, N.R.; Monica, B. Antimicrobial and antioxidant effects of Cichorium intybus aerial parts and Chemical profile. Egyp. J Chem. Artic. 2021, 64, 4689–4696. [Google Scholar] [CrossRef]
- Cicillin, A. Medicinal Plants in and around the Country; Complete Encyclopedia; Litres: Moscow, Russia, 2014; p. 4966. Available online: https://www.tursar.ru/page-joy.php?j=1650 (accessed on 14 May 2023).
- Harsahay, M.; Basant, B.; Swati, A.; Madhu, B. Evaluation of phytochemicals, antioxidant property and effects of Cichorium intybus cultivated at foothill area of Uttarakhand on hyperglycemic rats. IP Int. J. Comp. Adv. Pharm. 2022, 7, 54–64. [Google Scholar]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; Garcia-Viguera, C.; Bodroza-Solarov, M.; Ilic, N. (Cichorium intybus L.) as a food ingredient-Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Khayrullina, Z.A.; Canarian, A.V. Phytochemical composition of chicory products (Cichoriumintybus L.). J. Bull. Int. Cold Acad. 2016, 21–25. [Google Scholar]
- Süntar, I.; Akkol, E.K.; Keles, H.; Yesilada, E.; Sarker, S.D.; Baykal, T. Comparative evaluation of traditional prescriptions from Cichorium intybus L. for wound healing: Stepwise isolation of an active component by in vivo bioassay and its mode of activity. J. Ethnopharmacol. 2012, 143, 299–309. [Google Scholar] [CrossRef]
- Popova, E.A.; Shatalova, T.A.; Michnik, L.A.; Michnik, O.V.; Hayrapetova, A.Y. Study of sales of medicinal plants by retail pharmacies and level of their consumption in sanatoriums on kmv. Mod. Prob. Sci. Edu. 2015, 3, 263. [Google Scholar]
- Lebeda, A.F.; Giurenko, N.I.; Isaikina, A.P.; Sobko, V.G. Med. Plants; The Most Complete Encyclopedia; ACT-Press: Moscow, Russia, 2010; p. 494. [Google Scholar]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Carmo, M.A.V.; Azevedo, L.; Marques, M.B.; Granato, D. Neiva Red Chicory (Cichorium Intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Mercurio, D.G.; Melo, M.O.; Closs-Gonthier, B. Cichorium intybus root extract: A “vitamin D-like” active ingredient to improve skin barrier function. J. Dermatol. Treat. 2017, 28, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Makia, R.; Al Halbosiy, M.M.; Al Mashhadani, M.H. Pharmacology of the species Equisetum (Equisetum arvense). GSC Biol. Pharm. Sci. 2022, 18, 290–294. [Google Scholar] [CrossRef]
- Galina, S. Wild medical plants in the phytocenoses of the Northern Kazakhstan. Med. Health Sci. J. 2012, 13, 128. [Google Scholar]
- Amber, N.P.; Iris, L.; Dunja, Š.; Bernd, M.L. Differential Accumulation of Metabolites and Transcripts Related to Flavonoid, Styrylpyrone, and Galactolipid Biosynthesis in Equisetum Species and Tissue Types. Metabolites 2022, 12, 403. [Google Scholar] [CrossRef]
- Botirov, E.H.; Bonacheva, V.M.; Kolomiets, N.E. Chemical Composition and Biological Activity of Metabolites of Plants of the Genus Equisetum L. Chemistry of Plant Raw Materials; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; pp. 5–26. [Google Scholar] [CrossRef]
- Raghda, M.; Khulood, W.A.; Mohammad, M.F.; Mohammed, H.A. Phytochemistry of the Genus Equisetum (Equisetum arvense). GSC Biol. Pharm. Sci. 2022, 18, 283–289. [Google Scholar] [CrossRef]
- Nagai, T.; Myoda, T.; Nagashima, T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005, 91, 389–394. [Google Scholar] [CrossRef]
- Niko, R.; Gordana, S.; Radosav, P. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 2006, 20, 85–88. [Google Scholar]
- Dragana, D.Č.; Jasna, M.Č.; Gordana, M.B.; Sonja, M.D.; Gordana, S.Ć.; Vesna, T.T.; Bratislav, T.S. Antioxidative and Antiproliferative Activities of Different Horsetail (Equisetum arvense L.) Extracts. J. Med. Food 2010, 13, 452–459. [Google Scholar]
- Bhragual, D.D.; Kumar, N.; Garg, V.K.; Sharma, P.K. Review on plants havin g hepatoprotective activity. J. Pharm. Res. 2010, 3, 2077–2082. [Google Scholar]
- Aldaas, S. Cytotoxic and Antibacterial Activity of an Extract from a Saudi Traditional Medicinal Plant Equisetum arvense. Ph.D. Thesis, King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, 2011. Available online: https://core.ac.uk/download/pdf/132719332.pdf (accessed on 14 May 2023).
- Zia-Ur-Rehman; Gurgul, A.; Youn, I.; Maldonado, A.; Wahid, F.; Che, C.T.; Khan, T. UHPLC-MS/MS-GNPS based phytochemical investigation of Equisetum arvense L. and evaluation of cytotoxicity against human melanoma and ovarian cancer cells. Saudi J. Biol. Sci. 2022, 29, 103271. [Google Scholar] [CrossRef]
- Navdeep, S.S.; Sarabjit, K.; Divneet, C. Equietum arvense: Pharmacology and phytochemistry—A review. Asian J. Pharm. Clin. Res. 2010, 3, 146–150. [Google Scholar]
- Hayat, A.; Temamogullari, F.; Yilmaz, R.; Karabulut, O. Effect of Equisetun arvense on wound contraction of Full-Thicnes Skin Wounds in Rabbits. J. Anim. Vet. Adv. 2011, 10, 81–83. [Google Scholar]
- Wang, L.; Zhang, L.; Zheng, G.; Luo, H.; El-Kott, A.F.; El-Kenawy, A.E. Equisetum arvense L. aqueous extract: A novel chemotherapeutic supplement for treatment of human colon carcinoma. Arch. Med. Sci. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.M.; Jardim, T.V.; Araújo, Y.C.L.; Arantes, A.C.; de Sousa, A.C.; Barroso, W.K.S.; Sousa, A.L.L.; da Cunha, L.C.; Cirilo, H.N.C.; Bara, M.T.F.; et al. Equisetum arvense: New evidences supports medical use in daily clinic. Pharmacogn. Rev. 2019, 13, 50–58. [Google Scholar] [CrossRef]
- Oh, H.; Kim, D.H.; Cho, J.H.; Kim, Y.C. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J. Ethnopharmacol. 2004, 95, 421–424. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Lee, S.I.; Chang, I.S.; Kang, H.H.; Lee, O.S. 2005 Cosmetic composition containing as available ingredient the extracts of Equisetum arvense L. KR Patent WO2007004771A1, 30 June 2005. [Google Scholar]
- Dos Santos, D.S.; Barreto, R.D.S.S.; Serafini, M.R.; Gouveia, D.N.; Marques, R.S.; de Carvalho Nascimento, L.; de Carvalho Nascimento, J.; Guimaraes, A.G. Phytomedicines Containing Matricaria Species for the Treatment of Skin Diseases: A Biotechnological Approach. Fitoterapia 2019, 138, 104267. [Google Scholar] [CrossRef]
- Wörz, A.; Diekmann, H. Classification and evolution of the genus Eryngium, L. (Apiaceae-Saniculoideae): Results of fruit anatomical and petal morphological studies. Plant Divers. Evol. 2010, 128, 387–408. [Google Scholar] [CrossRef]
- Wörz, A. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae). Bot. Jahrbücher Syst. Pflanzengesch. Pflanzengeogr. 2005, 253–259. [Google Scholar] [CrossRef]
- Amantayeva, M.E.; Kozhanova, K.K. The study of plants of the genus Eryngium as promising sources for obtaining phytosubstances. Bull. KazNMU 2019, 1, 449–451. (In Russian) [Google Scholar]
- Kartal, M.; Mitaine-Offer, A.C.; Abu-Asaker, M.; Miyamoto, T.; Calis, I.; Wagner, H.; Lacaille-Dubois, M.A. Two new triterpene saponins from Eryngium campestre. Chem. Pharm. Bull. 2005, 53, 1318–1320. [Google Scholar] [CrossRef]
- Dalar, A.; Türker, M.; Zabaras, D.; Konczak, I. Phenolic composition, antioxidant and enzyme inhibitory activities of Eryngium bornmuelleri leaf. Plant Foods Hum. Nutr. 2014, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in disguise: A review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Colloca, C.B.; Espinar, L.A.; Sosa, V.E. Triterpenoid saponins from Eryngium agavifolium. NPAIJ 2014, 10, 61–68. [Google Scholar]
- Conea, S.; Vlase, L.; Chirila, I. Comparative study on the polyphenols and pectin of three Eryngium species and their antimicrobial activity. Cellul. Chem. Technol. 2016, 27, 363. [Google Scholar] [CrossRef]
- Kikowska, M.; Budzianowski, J.; Krawczyk, A.; Thiem, B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012, 34, 2425–2433. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Masullo, M.; Thiem, B.; Piacente, S.; Stochmal, A.; Oleszek, W. Three new triterpene saponins from roots of Eryngium planum. Nat. Prod. Res. 2014, 28, 653–660. [Google Scholar] [CrossRef]
- Rodrigues, T.L.; Silva, M.E.; Gurgel, E.S.; Oliveira, M.S.; Lucas, F.C. Eryngium foetidum L. (Apiaceae): A literature review of traditional uses, chemical composition, and pharmacological activities. Evid.-Based Complement. Altern. Med. 2022, 2022, 15. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Savin, S.; Lucian Radu, G. Chemical and bioactivity evaluation of Eryngium planum and Cnicus benedictus polyphenolic-rich extracts. BioMed Res. Int. 2019, 2019, 10. [Google Scholar] [CrossRef]
- Chockalingam, N.; Muruhan, S. Anti-inflammatory properties of rosmarinic acid—A review. Int. J. Res. Pharm. Sci. 2017, 8, 656–662. [Google Scholar]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Kuatbay, E.; Ustenova, G.; Arykbaeva, A. Prospects of the flat-leaved bluebird (Eryngium planum L.) In the prevention and treatment of dermatological diseases. Bull. Bashkir State Med. Univ. 2019, 4, 173–178. (In Russian) [Google Scholar]
- Kikowska, M.; Dlugaszewska, J.; Kubicka, M.M.; Kedziora, I.; Budzianowski, J.; Thiem, B. In vitro antimicrobial activity of extracts and their fractions from three Eryngium L. species. Herba Pol. 2016, 62, 67–77. [Google Scholar] [CrossRef]
- Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical constituents and pharmacological activities of Eryngium L. (Apiaceae). Pharm. Crops 2012, 3, 99–120. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef]
- Ishmuratova, M.Y.; Imanbayeva, A.A.; Tuyakova, A.T.; Kopbaeva, G.B. Study of common licorice (Glycyrrhiza glabra) reserves in Atyrau and Western-Kazakhstan regions. Biosci. Biotechnol. Res. Asia 2016, 13, 1429. [Google Scholar] [CrossRef]
- Alexyuk, P.G.; Bogoyavlenskiy, A.P.; Alexyuk, M.S.; Turmagambetova, A.S.; Zaitseva, I.A.; Omirtaeva, E.S.; Berezin, V. Adjuvant activity of multimolecular complexes based on Glycyrrhiza glabra saponins, lipids, and influenza virus glycoproteins. Arch. Virol. 2019, 164, 1793–1803. [Google Scholar] [CrossRef]
- Khan Ahmadi, M.M.; Naghdi Badi, H.; Akhondzadeh, S.; Khalighi-Sigaroodi, F.; Mehrafarin, A.; Shahriari, S.; Hajiaghaee, R. A Review on Medicinal Plant of Glycyrrhiza glabra L. J. Med. Plants 2013, 12, 1–12. [Google Scholar]
- Wang, K.L.; Yu, Y.C.; Chen, H.Y.; Chiang, Y.F.; Ali, M.; Shieh, T.M.; Hsia, S.M. Recent Advances in Glycyrrhiza glabra (Licorice)-Containing Herbs Alleviating Radiotherapy-and Chemotherapy-Induced Adverse Reactions in Cancer Treatment. Metabolites 2022, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Parvaiz, M.; Hussain, K.; Khalid, S.; Hussnain, N.; Iram, N.; Hussain, Z.; Ali, M.A. A review: Medicinal importance of Glycyrrhiza glabra L. (Fabaceae family). Global J. Pharmacol. 2014, 8, 8–13. [Google Scholar] [CrossRef]
- Anagha, K.; Manasi, D.; Priya, L.; Meera, M. Antimicrobial activity of yashtimadhu (Glycyrrhiza glabra L.)—A review. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 329–336. [Google Scholar]
- Sukirti, U.; Ashoke, G.; Singh, V. Research Article Hair Growth Promotant Activity of Petroleum Ether Root Extract of Glycyrrhiza glabra L. (Fabaceae) in Female Rats Tropical. J. Pharm. Res. 2012, 11, 753–758. [Google Scholar] [CrossRef][Green Version]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive effect of a combination of Artocarpus lakoocha and Glycyrrhiza glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals 2020, 13, 310. [Google Scholar] [CrossRef]
- Nukebay, A.K. Application in medicine of extracts isolated from liqorice root Glycyrrhiza glabra L. In Proceedings of the Conference Pharmaceutical Science and Practice: Problems, Achievements, Development Prospects, Kharkov, Ukraine, 15–16 April 2021; National University of Pharmacy, Kharkov: Kharkov, Ukraine, 2021; pp. 108–109. (In Russian). [Google Scholar]
- Fatoki, T.H.; Ajiboye, B.O.; Aremu, A.O. In Silico Evaluation of the Antioxidant, Anti-Inflammatory, and Dermatocosmetic Activities of Phytoconstituents in Licorice (Glycyrrhiza glabra L.). Cosmetics 2023, 10, 69. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and their constituents as active cosmeceutical ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Baumann, L.S. Less-known botanical cosmeceuticals. Dermatol. Ther. 2007, 20, 330–342. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, W.; Piao, H.; Xu, W.; Shi, H.; Zhao, C. The genus Gnaphalium L. (Compositae): Phytochemical and pharmacological characteristics. Molecules 2013, 18, 8298–8318. [Google Scholar] [CrossRef]
- Pozdnyakova, Y.; Omarova, G.; Murzatayeva, A. Wild Plants of Central Kazakhstan with Antibiotic Properties and Effect. Int. J. Agric. Biol. 2022, 27, 259–269. [Google Scholar] [CrossRef]
- Wang, L.J.; Su, S.; Wu, J.; Du, H.; Li, S.S.; Huo, J.W.; Wang, L.S. Variation of anthocyanins and flavonols in Vaccinium uliginosum berry in Lesser Khingan Mountains and its antioxidant activity. Food Chem. 2014, 160, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Chirikova, N.K.; Kashchenko, N.I. Spinacetin, a new caffeoylglycoside, and other phenolic compounds from Gnaphalium uliginosum. Chem. Nat. Compd. 2015, 51, 1085–1090. [Google Scholar] [CrossRef]
- Sharonova, N.L.; Terenzhev, D.A.; Bushmeleva, K.N.; Gumerova, S.K.; Lyubina, A.P.; Fitsev, I.M.; Belov, T.G. Phytochemical Contents, Antimicrobial and Antioxidant Properties of Gnaphalium uliginosum L. Ethanolic Extract and Essential Oil for Agricultural Uses. Asian J. Chem. 2019, 11, 2672–2678. [Google Scholar]
- Lubsandorzhieva, P.B.; Rendyuk, T.D.; Dargaeva, T.D.; Ferubko, E.V. Pharmacognostic Study of Collection and Study of its Hepatoprotective Activity. Pharmacogn. J. 2021, 13, 713–721. [Google Scholar] [CrossRef]
- Shikov, A.N.; Kundracikova, M.; Palama, T.L.; Pozharitskaya, O.N.; Kosman, V.M.; Makarov, V.G.; Verpoorte, R. Phenolic constituents of Gnaphalium uliginosum L. Phytochem. Lett. 2010, 3, 45–47. [Google Scholar] [CrossRef]
- Goun, E.A.; Petrichenko, V.M.; Solodnikov, S.U.; Suhinina, T.V.; Kline, M.A.; Cunningham, G.; Miles, H. Anticancer and antithrombin activity of Russian plants. J. Ethnopharmacol. 2002, 81, 337–342. [Google Scholar] [CrossRef]
- Sõukand, R.; Kalle, R.; Pieroni, A. Homogenisation of biocultural diversity: Plant ethnomedicine and its diachronic change in Setomaa and Võromaa, Estonia, in the last century. Biology 2022, 11, 192. [Google Scholar] [CrossRef]
- Deev, M.V.; Schmidt, S.V. 2004 “Antipsoriaz” Cream. RU Patent 2 246 935 C1, April 2004. (In Russian). [Google Scholar]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and present use, and future potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Bizaj, K.; Škerget, M.; Košir, I.J.; Knez, Ž. (Humulus lupulus L.) Essential Oils and Xanthohumol Derived from Extraction Process Using Solvents of Different Polarity. Horticulturae 2022, 8, 368. [Google Scholar] [CrossRef]
- Balciunaitiene, A.; Viskelis, P.; Viskelis, J.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Streimikyte, P.; Liaudanskas, M.; Bartkiene, E.; Lele, V. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Zita, H.; Marie-Luise, F.; Fabian, G.; Martin, H.; Birgit, H.; Anja, C.; Kay, S.; Christoph, M.S.; Ute, W. The Anti-Inflammatory Effect of Humulus lupulus Extract in vivo Depends on the Galenic System of the Topical Formulation. Pharmaceuticals 2022, 15, 350. [Google Scholar] [CrossRef]
- Natarajan, P.; Katta, S.; Andrei, I.; Ambati, V.B.R.; Leonida, M.; Haas, G.J. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine 2008, 15, 194–201. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Guiomar, L.S.L. Evaluation of Humulus lupulus L. Therapeutic Properties for the Treatment of Skin Diseases. Ph.D. Thesis, Universidade Beira Interior, Covilhã, Portugal, 2020. [Google Scholar]
- Taha, N.A.; Al-Wadaan, M.A. Significance and use of walnut, Juglans regia Linn: A review. Adv. J. Microbiol. Res. 2021, 15, 1–10. [Google Scholar]
- Dzhangaliev, A.D.; Salova, T.N.; Turekhanova, R.M. Wild Fruit Plants of Kazakhstan; KazgosINTI: Almaty, Kazakhstan, 2001; p. 135. [Google Scholar]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.; PerezCamino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food. Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Makarenkova, O.G.; Shevyakova, L.V.; Bessonov, V.V. Natural trace elements of nuts are an integral part of a healthy diet. Nutr. Issues 2016, 85, 202. (In Russian) [Google Scholar]
- Bennacer, A.; Sahir-Halouane, F.; Aitslimane-Aitkaki, S.; Oukali, Z.; Oliveira, I.V.; Rahmouni, N.; Aissaoui, M. Structural characterization of phytochemical content, antibacterial, and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal. Agric. Biotechnol. 2022, 40, 102304. [Google Scholar] [CrossRef]
- Vasipov, V.V.; Vytovtov, A.A. Walnut (Juglans regia L.)—A promising source of biologically active substances. Food Ecol. Qual. 2016, 1, 223–228. (In Russian) [Google Scholar]
- Ivanova, R.A.; Elisovetskaya, D.S. Antioxidant Activity of Extracts from Various Types of Unripe Nuts Juglans Spp. In Medicinal Plants: Biodiversity, Technology, Application; GSAU: Grodno, Russia, 2014; pp. 129–131. Available online: https://www.ggau.by/downloads/prints/lekarstwennyje_trawy.pdf#page=130 (accessed on 14 May 2023). (In Russian)
- Gupta, A.; Behl, T.; Panichayupakaranan, P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019, 16, 100142. [Google Scholar] [CrossRef]
- Paudel, P.; Satyal, P.; Dosoky, N.S.; Maharjan, S.; Setzer, W.N. Juglans regia and J. nigra, two trees important in traditional medicine: A comparison of leaf essential oil compositions and biological activities. Nat. Prod. Commun. 2013, 8, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Dar, M.Y.; Wani, B.A.; Shah, W.A.; Bhat, B.A.; Ganai, B.A.; Bhat, K.A.; Anand, R.; Qurishi, M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine 2012, 19, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Bittner Fialová, S.; Rendeková, K.; Mučaji, P.; Nagy, M.; Slobodníková, L. Antibacterial activity of medicinal plants and their constituents in the context of skin and wound infections, considering European legislation and folk medicine—A review. Int.J. Mol. Sci. 2021, 22, 10746. [Google Scholar] [CrossRef] [PubMed]
- Schwindl, S.; Kraus, B.; Heilmann, J. Phytochemical study of Juglans regia L. leaves. Phytochemistry 2017, 144, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Boulfia, M.; Lamchouri, F.; Toufik, H. Mineral analysis, in vitro evaluation of alpha-amylase, alpha-glucosidase, and beta-galactosidase inhibition, and antibacterial activities of Juglans regia L. bark extracts. BioMed Res. Int. 2021, 14. [Google Scholar] [CrossRef]
- Khattak, P.; Khalil, T.F.; Bibi, S.; Jabeen, H.; Muhammad, N.; Khan, M.A.; Liaqat, S. Juglans regia (Walnut Tree) Bark in Dentistry: Walnut Tree Bark in Dentistry. Pak. BioMed. J. 2022, 5, 152–156. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical Constituents, Nutritional, Pharmacological and Therapeutic Importance of Juglans regia—A Review. IOSR J. Pharm. 2018, 8, 1–21. Available online: http://med.utq.edu.iq/wp-content/uploads/sites/7/2021/07/chemical-constituents-nutritional-pharmcological-and-therapeutic-importance-of-juglans-regia-a-review.pdf (accessed on 14 May 2023).
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2021, 35, 2076–2081. [Google Scholar] [CrossRef]
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.G.; Muntean, D.M.; Popa, D.S. Antitussive, antioxidant, and anti-inflammatory effects of a walnut (Juglans regia L.) septum extract rich in bioactive compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef]
- Amel, B.; Saida, C.H. Contribution to the Ethnobotanical, Phytochemical, Antimicrobial and Antioxidant Study of the Leaves Aqueous Extract of the Common Walnut” Juglans regia L. Int. J. Pharmacol. Phytochem. Ethnomedicine 2016, 7, 41–52. [Google Scholar]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Walnut (Juglans regia)-Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas: Nutritional & Health Benefits; Springer: Berlin/Heidelberg, Germany, 2021; pp. 269–281. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crops Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.; Estevinho, L. Bioactive properties and chemical bookcomposition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol 2008, 46, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Al-Snafi, A.; Esteves da Silva, J.C.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef]
- Makubaeva, A.I.; Adekenova, A.S.; Rakhataeva, A.; Mamyrkhan, H. Therapeutic and Cosmetic Agents Based on Biologically Active Substances of Matricaria chamomilla L. and Hypericum perforatum L. Chem. J. Kazakhstan 2020, 4, 105–112. [Google Scholar]
- Höferl, M.; Wanner, J.; Tabanca, N.; Ali, A.; Gochev, V.; Schmidt, E.; Kaul, V.K.; Singh, V.; Jirovetz, L. Biological activity of Matricaria chamomilla essential oils of various chemotypes. Planta Med. Int. Open 2020, 7, 114–121. [Google Scholar] [CrossRef]
- Obead, A.R. Novelty effect of extract of alcohol for Matricaria chamomilla on bacterial growth. Plant Arch. 2019, 19, 1850–1852. [Google Scholar]
- Almosawi, M.B.H. A study of chemical composition and effective materials in chamomile flowers (Matricaria chamomilla). Plant Arch. 2020, 20, 311–312. [Google Scholar]
- Asgharzade, S.; Rabiei, Z.; Rafieian-Kopaei, M. Effects of Matricaria chamomilla Extract on Motor Coordination Impairment Induced by Scopolamine in Rats. Asian Pac. J. Trop. Biomed. 2015, 5, 829–833. [Google Scholar] [CrossRef]
- Golkhani, S.; Vahdati, A.; Modaresi, M.; Edalatmanesh, M.A. The Effects of Matricaria chamomilla Extract during Neonatal Period of Rats on Pituitary-Gonadal Hormone Axis and Changes in Testicular Tissue of Male Progenies. Middle East J. Fam. Med. 2017, 15, 126–132. [Google Scholar] [CrossRef][Green Version]
- Rafraf, M.; Zemestani, M.; Asghari-Jafarabadi, M. Effectiveness of Chamomile Tea on Glycemic Control and Serum Lipid Profile in Patients with Type 2 Diabetes. J. Endocrinol. Investig. 2015, 38, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Bayliak, M.M.; Dmytriv, T.R.; Melnychuk, A.V.; Strilets, N.V.; Storey, K.B.; Lushchak, V.I. Chamomile as a Potential Remedy for Obesity and Metabolic Syndrome. EXCLI J. 2021, 20, 1261. [Google Scholar] [CrossRef] [PubMed]
- Awaad, A.A.; El-Meligy, R.M.; Zain, G.M.; Safhi, A.A.; Al Qurain, N.A.; Almoqren, S.S.; Zain, Y.M.; Sesh Adri, V.D.; Al-Saikhan, F.I. Experimental and Clinical Antihypertensive Activity of Matricaria chamomilla Extracts and Their Angiotensin-Converting Enzyme Inhibitory Activity. Phytother. Res. 2018, 32, 1564–1573. [Google Scholar] [CrossRef]
- Silveira, E.S.; Bezerra, S.B.; Ávila, K.S.; Rocha, T.M.; Pinheiro, R.G.; de Queiroz, M.G.R.; Leal, L.K.A. Gastrointestinal effects of standardized brazilian phytomedicine (arthur de carvalho drops®) containing Matricaria recutita, Gentiana lutea and Foeniculum vulgare. Pathophysiology 2019, 26, 349–359. [Google Scholar] [CrossRef]
- Saidi, R.; Heidari, H.; Sedehi, M.; Safdarian, B. Evaluating the Effect of Matricaria chamomilla and Melissa officinalis on Pain Intensity and Satisfaction with Pain Management in Patients after Orthopedic Surgery. J. Herbmed Pharmacol. 2020, 9, 339–345. [Google Scholar] [CrossRef]
- Niknam, S.; Tofighi, Z.; Faramarzi, M.A.; Abdollahifar, M.A.; Sajadi, E.; Dinarvand, R.; Toliyat, T. Polyherbal Combination for Wound Healing: Matricaria Chamomilla L. and Punica Granatum L. DARU J. Pharm. Sci. 2021, 29, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D. Amerolative Influence of Chamomile (Matricaria recutita L.) on Synthetic Food Additive Induced Probable Toxicity in Male Albino Rats. J. Food Dairy Sci. 2021, 12, 161–170. [Google Scholar] [CrossRef]
- Gomes-Carneiro, M.R.; Dias, D.M.; De-Oliveira, A.C.A.X.; Paumgartten, F.J. Evaluation of Mutagenic and Antimutagenic Activities of α-Bisabolol in the Salmonella/Microsome Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 585, 105–112. [Google Scholar] [CrossRef]
- Tai, Y.; Wang, H.; Yao, P.; Sun, J.; Guo, C.; Jin, Y.; Yang, L.; Chen, Y.; Shi, F.; Yu, L.; et al. Biosynthesis of α-Bisabolol by Farnesyl Diphosphate Synthase and α-Bisabolol Synthase and Their Related Transcription Factors in Matricaria recutita L. Int. J. Mol. Sci. 2023, 24, 1730. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, M.Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The traditional uses, constituents and pharmacological effects of Ononis spinosa. IOSR J. Pharm. 2020, 10, 53–59. [Google Scholar]
- Gampe, N.; Darcsi, A.; Kursinszki, L.; Béni, S. Separation and characterization of homopipecolic acid isoflavonoid ester derivatives isolated from Ononis spinosa L. root. J. Chromatogr. B 2018, 1091, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gampe, N.; Darcsi, A.; Lohner, S.; Béni, S.; Kursinszki, L. Characterization and identification of isoflavonoid glycosides in the root of Spiny restharrow (Ononis spinosa L.) by HPLC-QTOF-MS, HPLC–MS/MS and NMR. J. Pharm. Biomed. Anal. 2016, 123, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Altuner, E.M.; Çeter, T.; Lşlek, C. Investigation of antifungal activity of Ononis spinosa L. ASH used for the therapy of skin infections as folk remedies. Mikrobiyoloji Bul. 2010, 44, 633–639. [Google Scholar]
- Thuwaini, M.M. Natural sources as promising future anticancer therapies—A review. GSC Biol. Pharm. Sci. 2022, 19, 84–113. [Google Scholar] [CrossRef]
- Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; CFR Ferreira, I.; Soković, M.D. Methanolic extract of the herb Ononis spinosa L. is an antifungal agent with no cytotoxicity to primary human cells. Pharmaceuticals 2020, 13, 78. [Google Scholar] [CrossRef]
- Dimitrova-DyulgerovA, I.; Stoyanova, A. Lipid Composition of Carduus Thoermeri Weinm. Onopordum acanthium L. and Silybum marianum L., Growing in Bulgaria. Bulg. J. Agricult. Sci. 2014, 20, 622–627. Available online: https://www.agrojournal.org/20/03-18.pdf (accessed on 14 May 2023).
- Al-Snafi, A.E. Constituents and pharmacology of Onopordum acanthium. IOSR J. Pharm. 2020, 10, 7–14. [Google Scholar]
- Bruno, M.; Maggio, A.; Rosselli, S.; Safder, M.; Bancheva, S. The metabolites of the genus Onopordum (Asteraceae): Chemistry and biological properties. Curr. Org. Chem. 2011, 15, 888–927. [Google Scholar] [CrossRef]
- Tonguc, M.U.H.A.M.M.E.T.; ERBAŞ, S. Evaluation of fatty acid compositions and some seed characters of common wild plant species of Turkey. Turk. J. Agric. For. 2012, 36, 673–679. [Google Scholar] [CrossRef]
- Garsiya, E.R.; Konovalov, D.A.; Shamilov, A.A.; Glushko, M.P.; Orynbasarova, K.K. Traditional medicine plant, Onopordum acanthium L. (Asteraceae): Chemical composition and pharmacological research. Plants 2019, 8, 40. [Google Scholar] [CrossRef]
- Mobli, M.; Qaraaty, M.; Amin, G.; Haririan, I.; Hajimahmoodi, M.; Rahimi, R. Scientific evaluation of medicinal plants used for the treatment of abnormal uterine bleeding by Avicenna. Arch. Gynecol. Obstet. 2015, 292, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mamedov, N.; Mehdiyeva, N.P.; Craker, L.E. Medicinal plants used in traditional medicine of the Caucasus and North America. J. Med. Act. Plants 2015, 4, 42–66. [Google Scholar] [CrossRef]
- Ryzhov, V.M.; Belchenko, A.S. Issues of Diagnostics of Prickly Tartar Fruit (Onopordum acanthium L.) as a Promising Medicinal Plant Raw Material. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2014, 16, 1025–1029. Available online: https://cyberleninka.ru/article/n/issledovanie-perspektivy-kompleksnoy-pererabotki-nadzemnoy-chasti-tatarnika-kolyuchego-onopordum-acanthium-l (accessed on 3 April 2023). (In Russian).
- Sharifi, N.; Souri, E.; Ziai, S.A.; Amin, G.; Amini, M.; Amanlou, M. Isolation, identification and molecular docking studies of a new isolated compound, from Onopordon acanthium: A novel angiotensin converting enzyme (ACE) inhibitor. J. Ethnopharmacol. 2013, 148, 934–939. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Réthy, B.; Zupkó, I.; Máthé, I.; Rédei, T.; Hohmann, J. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part II. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1109–1115. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Zupkó, I.; Molnár, J.; Forgo, P.; Hohmann, J. Bioactivity-guided isolation of antiproliferative compounds from the roots of Onopordum acanthium. Nat. Prod. Commun. 2014, 9, 337–340. [Google Scholar] [CrossRef]
- Polyakova, V.S.; Nikolaeva, T.V.; Setko, N.P.; Voronina, L.G. The Role of Apoptosis Induced by Heavy Metals in the Development of Autoimmune Diseases. Mod. Probl. Sci. Educ. 2016, 6. Available online: https://science-education.ru/ru/article/view?id=26018 (accessed on 3 April 2023). (In Russian).
- Bowen, A.R.; Hanks, A.N.; Murphy, K.J.; Florell, S.R.; Grossman, D. Proliferation, apoptosis, and survivin expression in keratinocytic neoplasms and hyperplasia. Am. J. Dermatopathol. 2004, 26, 177–181. [Google Scholar] [CrossRef]
- Naumov, S.Y.; Vatanskaya, I.Y. Medicinal Plants in the Flora of the Volga-Akhtuba Floodplain. Sci. Notes Cape Martian Nat. Reserve 2017, 8, 112–117. Available online: https://cyberleninka.ru/article/n/lekarstvennye-rasteniya-vo-flore-volgo-ahtubinskoy-poymy (accessed on 3 April 2023). (In Russian).
- Vakhrameeva, M.G.; Denisova, L.V.; Nikitina, S.V.; Samsonov, S.K. Orchidei of Our Country; Science: Moscow, Russia, 1991; p. 224. [Google Scholar]
- Teoh, E.S. Sources of medicinal orchids and conservation. In Medicinal Orchids of Asia; Springer: Berlin/Heidelberg, Germany, 2016; pp. 691–727. [Google Scholar] [CrossRef]
- Khadartsev, A.A.; Sukhiy, G.T.; Volochaeva, M.V.; Platonov, V.V.; Dunaeva, I.V. Chromato-mass spectrometry of ethanol extract of spotted (orcmaculate, orcmacular family). Her. New Med. Technol. 2019, 4, 1–20. [Google Scholar] [CrossRef]
- Arora, M.; Mahajan, A.; Sembi, J.K. A Review on phytochemical and pharmacological potential of family Orchidaceae. Int. J. Pharm. Pharm. Res. 2017, 8, 9–24. [Google Scholar] [CrossRef]
- Brinkmann, J.A. Quick Scan of Orchidaceae Species in European Commerce as Components of Cosmetic. Food Med. Prod. 2014, 1, 22. [Google Scholar]
- Yapo, B.M. Pectic substances: From simple pectic polysaccharides to complex pectins—A new hypothetical model. Carbohydr. Polym. 2011, 86, 373–385. [Google Scholar] [CrossRef]
- Loseva, A.I.; Pozdnyakova, A.V.; Prosekov, A.Y.; Ostapova, E.V.; Al’tshuler, O.G. Callus Orchis maculata L. as a source of bioactive substances: Biotechnology of cultivation. Bull. SUSU. Ser. Food Biotechnol. 2021, 9, 13–22. [Google Scholar]
- Al-Snafi, A.E. Pharmacological potential of Orchis mascula—A review. IOSR J. Pharm. 2020, 10, 1–6. [Google Scholar]
- Rajamalar, P.; Kavisri, M.; Elangovan, M.; Vairamani, S.; Shanmugam, A.; Elumalai, P.; Seedevi, P. Chemical characterization of Orchis mascula and its antibacterial efficiency against clinical isolated human pathogenic bacteria. Biomass convers. Biorefinery 2022, 1, 9. [Google Scholar] [CrossRef]
- Filippava, S.N.; Ditchenko, T.I.; Lohvina, H.O.; Yurin, V.M. Development of an effective method for deposition of callus cultures of valuable medicinal plants. Proc. BSU 2015, 10, 205–220. (In Russian) [Google Scholar]
- Gantait, S.; Das, A.; Mitra, M.; Chen, J.T. Secondary metabolites in orchids: Biosynthesis, medicinal uses, and biotechnology. S. Afr. J. Bot. 2021, 139, 338–351. [Google Scholar] [CrossRef]
- Kenari, H.M.; Kordafshari, G.; Moghimi, M.; Eghbalian, F.; TaherKhani, D. Review of pharmacological properties and chemical constituents of Pastinaca sativa. J. Pharmacopunct. 2021, 24, 14. [Google Scholar] [CrossRef]
- Kupriyanov, A.N.; Klyuykov, E.V.; Ukrainskaya, U.A.; Kupriyanov, O.A. Review of umbrella species (Apiaceae Lindl.) of the Kazakh upland. Bot. Stud. Sib. Kazakhstan 2017, 23, 3–29. [Google Scholar]
- Augustin, I.F.; Butnariu, M. A review about Pastinaca sativa L. ssp. sylvestris [Mill.] secondary metabolite diversity and inducibility. J. Appl. Biotechnol. Bioeng. 2022, 9, 5–6. [Google Scholar] [CrossRef]
- Averill, K.M.; DiTommaso, A. Wild parsnip (Pastinaca sativa): A troublesome species of increasing concern. Weed Technol. 2007, 21, 279–287. [Google Scholar] [CrossRef]
- Winter, J.C.; Thieme, K.; Eule, J.C.; Saliu, E.M.; Kershaw, O.; Gehlen, H. Photodermatitis and Ocular Changes in Nine Horses after Ingestion of Wild Parsnip (Pastinaca sativa). BMC Vet. Res. 2022, 18, 80. Available online: https://link.springer.com/article/10.1186/s12917-022-03162-2 (accessed on 14 May 2023). [CrossRef]
- Symonenko, N.; Shpychak, O.; Mishchenko, O.; Kyslychenko, V.; Shpychak, T.; Grashchenkova, S. Antioxidant and anti-cytolytic activity of parsnip (Pastinaca sativa L.) herb thick extract in conditions of catecholamine myocardiodystrophy in rats. Sci. Rise Pharm. Sci. 2022, 1, 70–76. [Google Scholar] [CrossRef]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.; Ranđelović, V.N.; Krivošej, Z.Đ.; Marin, P.D. Antimicrobial potential of essential oil from Pastinaca sativa L. Biol. Nyssana 2014, 5, 31–35. [Google Scholar]
- Jianu, C.; Goleț, I.; Stoin, D.; Cocan, I.; Lukinich-Gruia, A.T. Antioxidant activity of Pastinaca sativa L. ssp. sylvestris [Mill.] Rouy and Camus essential oil. Molecules 2020, 25, 869. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Petruczynik, A.; Dragan, A.; Wianowska, D.; Dawidowicz, A.L.; Sowa, I. Influence of the extraction mode on the yield of some furanocoumarins from Pastinaca sativa fruits. J. Chromatogr. B 2004, 800, 181–187. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Guo, X. Physicochemical Properties, Structures, Bioactivities and Future Prospective for Polysaccharides from Plantago L. (Plantaginaceae): A Review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef]
- Baitenov, M.S. Flora of Kazakhstan; Gylym: Almaty, Kazakhstan, 2001; Volume 2, pp. 190–191. (In Russian) [Google Scholar]
- Haddadian, K.K.; Haddadian, K.K.; Zahmatkash, M. A review of Plantago plant. Indian J. Tradit Know 2014, 13, 5. [Google Scholar]
- Kassaw, E.; Yohannes, T.; Bizualem, E. In vitro antibacterial activity of Plantago lanceolata against some selected standard pathogenic bacterial. Int. J. Biotechnol. 2018, 7, 44–50. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Mikaili, P.; Moloudizargari, M.; Aghajanshakeri, S.; Javaherypour, S. Therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. J. Basic Appl. Sci. Res. 2013, 3, 212–221. [Google Scholar]
- Abate, L.; Bachheti, R.K.; Tadesse, M.G.; Bachheti, A. Ethnobotanical Uses, Chemical Constituents, and Application of Plantago lanceolata L. J. Chem. 2022, 2022, 1532031. [Google Scholar] [CrossRef]
- Arslan, E.; Aygan, A.; Kocabaş, Y.Z. Antimicrobial Activity of Plantago major Grown in Kahramanmaraş Against Bacteria Causing Hospital Infections. Ecology 2018. (In Turkish) [Google Scholar]
- Kartini, K.; Wati, N.; Gustav, R.; Wahyuni, R.; Anggada, Y.F.; Hidayani, R.; Raharjo, A.; Islamie, R.; Putra, S.E.D. Wound Healing Effects of Plantago major Extract and Its Chemical Compounds in Hyperglycemic Rats. Food Biosci. 2021, 41, 100937. [Google Scholar] [CrossRef]
- Iskandarova, S.F.; Murotov, S.B. Determination of biologically active substances of a dry extract obtained on the basis of plantain leaves. Sci. Time 2018, 2, 48–51. (In Russian) [Google Scholar]
- Núñez Guillén, M.E.; da Silva Emim, J.A.; Souccar, C.; Lapa, A.J. Analgesic and Anti-Inflammatory Activities of the Aqueous Extract of Plantago major L. Int. J. Pharmacogn. 1997, 35, 99–104. [Google Scholar] [CrossRef]
- Najafian, Y.; Hamedi, S.S.; Farshchi, M.K.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and Modern Phytotherapy: A Narrative Review. Electron. Physician 2018, 10, 6390. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A Review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, M.B.A.; Sulaiman, M.W.A.W.; Sengupta, P.; Susanti, D. Chemical Constituents and Medical Benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef]
- Nemereshina, O.N.; Gusev, N.F.; Malkova, T.L. Biologically active substances of the large plantain (Plantago major L.) of the steppe zone. News Orenbg. State Agrar. Univ. 2018, 3, 113–117. (In Russian) [Google Scholar]
- Yazdian, M.A.; Gheisari, M.; Khodadoost, M.; Barikbin, B.; Yazdian, M.; Askarfarashah, M.; Kamalinejad, M. Evaluation of Plantago major aqueous extract in treatment of acute urticarial. Int. J. Biosci. 2014, 5, 182–188. [Google Scholar]
- Yazdian, M.A.; Khodadoost, M.; Gheisari, M.; Kamalinejad, M.; Ehsani, A.H.; Barikbin, B. A Hypothesis on the Possible Potential of Plantago major in the Treatment of Urticaria. Hong Kong Med. J. 2014, 3, 123–126. [Google Scholar] [CrossRef]
- Pasalar, M.; Tabatabaei, F.; Bradley, R.; Tajadini, H.; Kamali, M.; Hasheminasab, F.S.; Parvizi, M.M. Mechanistic support of traditional Persian medicine for the treatment of acne vulgaris: A scoping review. J. Cosmet. Dermatol. 2022, 6, 2338–2348. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Magazhanov, Z.M.; Bektursunova, M.Z. Research on biologically active substances of some fruit crops growing in the southeast of Kazakhstan. Food Process. Tech. Technol. 2016, 43, 30–35. [Google Scholar]
- Pieszka, M.; Migdał, W.; Gąsior, R.; Rudzińska, M.; Bederska-Łojewska, D.; Pieszka, M.; Szczurek, P. Native oils from apple, blackcurrant, raspberry, and strawberry seeds as a source of polyenoic fatty acids, tocochromanols, and phytosterols: A health implication. J. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Ma, E.Z.; Khachemoune, A. Flavonoids and their therapeutic applications in skin diseases. Arch. Dermatol. Res. 2023, 315, 321–331. [Google Scholar] [CrossRef]
- Rani, L.; Sharma, N.; Singh, S.; Grewal, A.S. Therapeutic potential of vitamin c: An overview of various biological activities. Int. J. Pharm. Qual. Assur. 2019, 10, 605–612. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdylo, A. Comparison of Phenolic Compounds and Antioxidant Potential between Selected Edible Fruits and Their Leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Popova, T.S.; Popov, D.M.; Tereshina, N.S. The Study of Flavonoids of Buds and Leaves of Black Currant by HPLC. Pharmaciya 2015, 1, 13–15. Available online: https://pharmaciyajournal.ru/sites/default/files/fulltext-pdf/25419218-2015-01-04.pdf (accessed on 14 May 2023). (In Russian).
- Mikhailova, I.V.; Filippova, Y.V.; Kuzmicheva, N.A.; Vinokurova, N.V.; Ivanova, E.V.; Voronkova, I.P. Black currant as a promising source of polyphenolic antioxidants. Int. Res. J. 2021, 109, 28–32. (In Russian) [Google Scholar]
- Cao, L.; Park, Y.; Lee, S.; Kim, D.O. Extraction, identification, and health benefits of anthocyanins in blackcurrants (Ribes nigrum L.). Appl. Sci. 2021, 11, 1863. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic composition, mineral content, and beneficial bioactivities of leaf extracts from black currant (Ribes nigrum L.), raspberry (Rubus idaeus), and aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Ashigai, H.; Komano, Y.; Wang, G.; Kawachi, Y.; Sunaga, K.; Yamamoto, R.; Takata, R.; Miyake, M.; Yanai, T. Effect of administrating polysaccharide from black currant (Ribes nigrum L.) on atopic dermatitis in NC/Nga mice. Biosci. Microbiota Food. Health 2018, 37, 19–24. [Google Scholar] [CrossRef]
- Aidarkhanova, G.S. Biodiversity and ecological safety of rose hips (Rosa L.) in East Kazakhstan. In Proceedings of the International Scientific Conference “Perspectives of Medicinal Plant Science”, Mosocw, Russian, 1–2 November 2018; VILAR: Moscow, Russia, 2018; pp. 101–105. [Google Scholar]
- Ikhsanov, Y.S.; Tasmagambetova, G.E.; Litvinenko, Y.A.; Burasheva, G.S.; Seitimova, G.A. Phytochemical composition of lipophilic fraction of plants of the plant Rosa canina L. genus Rosa. Proc. Natl. Acad. Sci. Repub. Kazakhstan Chem. Technol. Ser. 2020, 2, 69–74. [Google Scholar] [CrossRef]
- Kizatova, M.; Serik, B. Chemical composition and application of dog rose hips in various industries. Med. Pharm. 2023, 140, 533–536. [Google Scholar]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef]
- Ahmad, N.; Anwar, F. Rose hip (Rosa canina L.) oils. In Essential oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 667–675. [Google Scholar] [CrossRef]
- Kiralan, M.; Yildirim, G. Rosehip (Rosa canina L.) Oil. In Fruit Oils: Chemistry & Functionality; Springer Nature: Basel, Switzerland, 2019; pp. 803–814. [Google Scholar] [CrossRef]
- Patel, P.; Prasad, A.; Srivastava, K.; Singh, S.S.; Chakrabarty, D.; Misra, P. Updates on steroidal alkaloids and glycoalkaloids in Solanum spp.: Biosynthesis, in vitro production and pharmacological values. Stud. Nat. Prod. Chem. 2021, 69, 99–127. [Google Scholar] [CrossRef]
- Isabelle, P.; Monica, B. Highlighting the compounds with pharmacological activity from some medicinal plants from the area of Romania. Med. Aromat. Plants 2021, 10, 370. [Google Scholar]
- Marzouk, A.M.; Deans, S.G.; Nash, R.J.; Gray, A.I. Transformed root cultures of Solanum dulcamara L.: A model for studying production of secondary metabolites. In Genetic Transformation; INTECH Open Access Publisher: Rijeka, Croatia, 2011; pp. 271–290. [Google Scholar]
- Zha, X.; Sun, H.; Hao, J.; Zhang, Y. Efficient Synthesis of Solasodine, O-Acetylsolasodine, and Soladulcidine as Anticancer Steroidal Alkaloids. Chem. Biodivers. 2007, 4, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.T.; Stoyanova, M.A.; Ivanova, T.A.; Stoyanova, A.S.; Dimitrova-Dyulgerova, I.Z. Phytochemical composition of leaves and stems of Solanum nigrum L. and Solanum dulcamara L. (Solanaceae) from Bulgaria. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1031, 1. [Google Scholar] [CrossRef]
- Morais, M.G.; Saldanha, A.A.; Azevedo, L.S.; Mendes, I.C.; Rodrigues, J.P.C.; Amado, P.A.; dos Santos Lima, L.A.R. Antioxidant and anti-inflammatory effects of fractions from ripe fruits of Solanum lycocarpum St. Hil. (Solanaceae) and putative identification of bioactive compounds by GC–MS and LC-DAD-MS. Food Res. Int. 2022, 156, 111145. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Merecz-Sadowska, A.; Rijo, P.; Mori, M.; Hatziantoniou, S.; Górski, K.; Sitarek, P. Hidden in plants—A review of the anticancer potential of the Solanaceae family in in vitro and in vivo studies. Cancers 2022, 14, 1455. [Google Scholar] [CrossRef]
- Sabudak, T.; Kaya, O.; Cukurova, E. A new biflavonoid from Solanum dulcamara L. and investigation of anti-hyperglycaemic activity of its fruit extract. Nat. Prod. Res. 2015, 29, 308–314. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, B.; Bakshi, N. Biological activity of alkaloids from Solanum dulcamara L. Nat. Prod. Res. 2009, 23, 719–723. [Google Scholar] [CrossRef]
- Fallahzadeh, A.R.; Mohammadi, S. Assessment of the antinociceptive, anti-inflammatory, and acute toxicity effects of Solanum dulcamara essential oil in male mice. J. Babol Univ. Med. Sci. 2020, 22, 162–168. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant alkaloids: Structures and bioactive properties. Plant-Deriv. Bioact. Chem. Mode Action 2020, 85–117. [Google Scholar] [CrossRef]
- Mutlu, E.C.; Turker, A.U. Efficient plant regeneration of bittersweet [Solanum dulcamara L.], a medicinal plant. Acta Soc. Bot. Pol. 2008, 77, 275–280. Available online: file:///C:/Users/user/Downloads/Efficient_plant_regeneration_of_bit%20.pdf (accessed on 14 May 2023).
- Neha, T.; Verma, S.K. Aspects of Phenolic Compounds in Pharmacological Activities of Solanum Family. Mol. Biol. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Bujak, T. Saponins as natural raw materials for increasing the safety of bodywash cosmetic use. J. Surfactants Deterg. 2018, 21, 767–776. [Google Scholar] [CrossRef]
- Khalighi, S.F.; Ahvazi, M.; Yazdani, D.; Kashefi, M. Cytotoxicity and antioxidant activity of five plant species of Solanaceae family from Iran. J. Med. Plants. 2012, 11, 43–53. [Google Scholar]
- Milutinović, M.; Nakarada, Đ.; Božunović, J.; Todorović, M.; Gašić, U.; Živković, S.; Mišić, D. Solanum dulcamara L. Berries: A Convenient Model System to Study Redox Processes in Relation to Fruit Ripening. Antioxidants 2023, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Minkhaidarov, V.Y. Medicinal & Food Plants of the Far East; PGSHA: Ussuriysk, Russia, 2015; 329p. (In Russian) [Google Scholar]
- Shchulipenko, I.M.; Shchulipenko, L.I. Green pharmacy of nature: Past and present. Phytotherapy 2010, 4, 5–9. (In Ukrainian) [Google Scholar]
- McAllister, H. The Genus Sorbus: Mountain Ash and Other Rowans; Royal Botanic Gardens, Kiew: Richmond, VA, USA; Surrey, UK, 2005. [Google Scholar]
- Lykholat, Y.L.; Didur, O.O.; Khromykh, N.O.; Davydov, V.R.; Borodai, Y.S.; Kravchuk, K.V.; Lykholat, T.Y. Comparative analysis of the antioxidant capacity and secondary metabolites accumulation in the fruits of rowan (Sorbus aucuparia L.) and some closely related species. Ecol. Noospherology 2021, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chikov, P.S. Medicinal Plants; Medicine: Moscow, Russia, 2002; 496p. (In Russian) [Google Scholar]
- Isaikina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.I.; Ulrich, A.V.; Fedorova, E.P.; Shilova, A.V. Sorbus aucuparia L. fruit is a source of the drug for increasing the efficiency of tumor chemotherapy. Rus. J. Bioorg. Chem. 2018, 44, 899–905. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Zdunić, G.M.; Krstić-Milošević, D.B.; Šircelj, H.J.; Stešević, D.D.; Pljevljakušić, D.S. Sorbus aucuparia and Sorbus aria as a source of antioxidant phenolics, tocopherols, and pigments. Chem. Biodivers. 2017, 14, 1700329. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, G.P.; Pancake, K.F. Medicinal Plant Material, Pharmacognosy; SpecLit.: St. Petersburg, Russia, 2004; 765p. (In Russian) [Google Scholar]
- Bussmann, R.W.; Paniagua, Z.; Narel, Y.; Sikharulidze, S.; Kikvidze, Z.; Kikodze, D.; Tchelidze, D.; Batsatsashvili, K.; Robbie, E. Plants in the spa–the medicinal plant market of Borjomi, Sakartvelo (Republic of Georgia), Caucasus. Indian J. Tradit. Knowl. 2017, 16, 25–34. [Google Scholar]
- Popoviciu, D.R.; Negreanu-Pîrjol, T. Carotenoid, Flavonoid and Total Phenolic Content of Sorbus torminalis Fruits. Rom. Arab. Int. J. Geobiodivers. 2019, 3, 20–25. [Google Scholar]
- Sirotina, K.; Kazimova, K.; Shcherbakova, Y.; Akhmadullina, F.; Nikitin, E. Study of the antioxidant activity of rowan extracts (Sorbus aucuparia) by biotesting method. IOP Conf. Ser. Earth Environ. Sci. 2022, 949, 012032. [Google Scholar] [CrossRef]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.I.; Tchaikovsky, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’Kov, G.N. Antitumor effects of Sorbus aucuparia L. extract highly saturated with anthocyans and their mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef] [PubMed]
- KC, B.; Gyawali, S.; Luintel, S.; Sharma, H.P.; Kunwar, R.M.; Bussmann, R.W.; Paniagua-Zambrana, N.Y. Sorbus cuspidata (Spach) Hedl Rosaceae. In Ethnobotany of the Himalayas; Springer: Cham, Switzerland, 2021; pp. 1917–1926. [Google Scholar] [CrossRef]
- Koromatov, I.D.; Rasulova, H.N. Healing properties of mountain ash. Biol. Integr. Med. 2017, 7, 133–139. (In Russian) [Google Scholar]
- Salehi, B.; Sharopov, F.; Boyunegmez, T.T.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Martins, N. Symphytum species: A comprehensive review on chemical composition, food applications and phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef]
- Prozorova, T.A.; Chernykh, I.B. Forage Plants of Kazakhstan; Pavlodar: Almaty, Kazakhstan, 2004; p. 170. (In Russian) [Google Scholar]
- Mahmoudzadeh, E.; Nazemiyeh, H.; Valizadeh, H.; Khaleseh, F.; Mohammadi, S.; Hamedeyazdan, S. Nanoencapsulation of n-butanol extract of Symphytum kurdicum and Symphytum asperrimum: Focus on phytochemical analysis, anti-oxidant and antibacterial activity. Iran. J. Basic Med. Sci. 2022, 25, 364. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carretero, A. Comparative assessment of phytochemical profiles of comfrey (Symphytum officinale L.) root extracts obtained by different extraction techniques. Molecules 2020, 25, 837. [Google Scholar] [CrossRef]
- Cao, Z.; Guo, Y.; Liu, Z.; Zhang, H.; Zhou, H.; Shang, H. Ultrasonic enzyme-assisted extraction of comfrey (Symphytum officinale L.) polysaccharides and their digestion and fermentation behaviors in vitro. Process Biochem. 2022, 112, 98–111. [Google Scholar] [CrossRef]
- Vanitha, A.; Kavinprashantha, R.; Mugendhira-na, S.; Shashikanth, J. Conservation Of Symphytum officinale L. At Cmprh Garden, Emerald. J. Univ. Shanghai Sci. Technol. 2022, 24, 261–272. [Google Scholar] [CrossRef]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Wójciak-Kosior, M. Proliferative and antioxidant activity of Symphytum officinale root extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Skalicka-Woźniak, K.; Minceva, M.; Luca, S.V. Symphytum ibericum Steven: LC–HRMS/MS-based phytochemical profile, in vitro antioxidant and enzyme inhibitory potential. Chem. Biol. Technol. Agric. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Le, V.; Dolganyuk, V.; Sukhikh, A.; Babich, O.; Ivanova, S.; Prosekov, A.; Dyshlyuk, L. Phytochemical analysis of Symphytum officinale root culture extract. Appl. Sci. 2021, 11, 4478. [Google Scholar] [CrossRef]
- Vaezi, S.; Haghighi, H.M.; Farzad, S.A.; Arabzadeh, S.; Kalalinia, F. Bone Regeneration by Homeopathic Symphytum officinale. Regen. Eng. Transl. Med. 2020, 7, 548–555. [Google Scholar] [CrossRef]
- Seigner, J.; Junker-Samek, M.; Plaza, A.; D ‘Urso, G.; Masullo, M.; Piacente, S.; de Martin, R. A Symphytum officinale root extract exerts anti-inflammatory properties by affecting two distinct steps of NF-κB signaling. Front. Pharmacol. 2019, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Potarniche, A.V.; Rus, V.; Diaconeasa, Z.; Mocan, A.; Mihaiu, M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019, 145, 104369. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Marineci, C.D.; Păun, G.; Ungureanu, O.; Neagu, E.; Chiriță, C.; Negreș, S. Chitosan supports containing Impatiens noli-tangere and Symphytum officinale hydroalcoholic extracts in burns treatment: Antimicrobial and healing effects. Farmacia 2021, 69, 948–953. [Google Scholar] [CrossRef]
- Grollier, J.-F.; Allec, J.; Fourcadier, C.; Rosenbaum, G.; Darmenton, P. Cosmetic Compositions for the Treatment of the Hair and Skin Contain in the form of a Powder Particles Resulting from the Pulverization of at Least One Plant Substance and a Cohesion Agent. U.S. Patent 4,569,839, 11 February 1986. [Google Scholar]
- Habtemariam, S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2016. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Mahmoudzadeh, E.; Nazemiyeh, H.; Hamedeyazdan, S. Anti-inflammatory Properties of the Genus Symphytum L.: A Review. Iran. J. Pharm. Res. 2022, 21, e123949. [Google Scholar] [CrossRef]
- Uehara, A.; Akiyama, S.; Iwashina, T. Foliar flavonoids from Tanacetum vulgare var. boreale and their geographical variation. Nat. Prod. Commun. 2015, 10, 403–405. [Google Scholar] [CrossRef]
- Aidarbayeva, D.K.; Sholpankulova, G.; Jarylkapova, S.; Shokanova, A. Natural resources of some medicinal plants of Kazakhstan. Int. Multidiscip. Sci. GeoConference SGEM 2018, 18, 385–391. [Google Scholar] [CrossRef]
- Räisänen, R.; Primetta, A.; Nikunen, S.; Honkalampi, U.; Nygren, H.; Pihlava, J.M.; von Wright, A. Examining safety of biocolourants from fungal and plant sources-examples from Cortinarius and Tapinella, Salix and Tanacetum spp. and Dyed Woollen Fabrics. Antibiotics 2020, 9, 266. [Google Scholar] [CrossRef]
- Vilhelmova, N.; Simeonova, L.; Nikolova, N.; Pavlova, E.; Gospodinova, Z.; Antov, G.; Nikolova, I. Antiviral, cytotoxic and antioxidant effects of Tanacetum vulgare L. Crude Extract In Vitro. Folia Med. 2020, 62, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Puvača, N. Tanacetum vulgare L.—A Systematic Review. J. Agron. Technol. Eng. Manag. 2020, 3, 416–422. [Google Scholar]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Apetrei, C.; Mihai, C.T.; Gheldiu, A.-M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulovic, S.; Sokovic, M.; Krstić-Milošević, D.; Ristić, M.; Galic, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Senkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Z.; et al. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch. Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Boisnic, S.; Branchet, M.C.; Soto, P. Inulin and dermatology. J. Cosmet. Dermatol. 2018, 17, 968–971. [Google Scholar] [CrossRef]
- Choi, J.H.; Shin, K.M.; Kim, N.Y.; Hong, J.P.; Lee, Y.S.; Kim, H.J.; Park, H.J.; Lee, K.T. Taraxinic acid, a hydrolysate of sesquiterpene lactone glycoside from the Taraxacum coreanum NAKAI, induces the differentiation of human acute promyelocytic leukemia HL-60 cells. Biol. Pharm. Bull. 2002, 25, 1446–1450. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Nguyen, T.T.; Le, H.T.N.; Nguyen, T.T.T.; Bach, L.G.; Nguyen, T.D.; Vo, D.V.N.; Tra, T.V. The sunflower plant family for bioenergy, environmental remediation, nanotechnology, medicine, food and agriculture: A review, Environ. Chem. Lett. 2021, 19, 3701–3726. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002, 366, 653–661. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Łysoń, E.; Telesiński, A. The chemical composition and antioxidant properties of common dandelion leaves compared to Sea buckthorn. Can. J. Plant Sci. 2017, 97, 1165–1174. [Google Scholar] [CrossRef]
- Modaresi, M.; Resalatpour, N. The effect of Taraxacum officinale hydroalcoholic extract on blood cells in mice. Adv. Hematol. 2012, 2012, 653412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gemejiyeva, N.G.; Grudzinskaya, L.M. Current State and Prospects for Studies on the Diversity of Medicinal Flora in Kazakhstan. In Vegetation of Central Asia and Environs; Springer: Berlin/Heidelberg, Germany, 2018; pp. 239–262. Available online: https://link.springer.com/chapter/10.1007/978-3-319-99728-5_9 (accessed on 14 May 2023).
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and Their Isomers: Color Pigments in Fruits and Vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Quantitative UPLC-MS/MS analysis of chlorogenic acid derivatives in antioxidant fractionates from dandelion (Taraxacum officinale) root. Int. J. Food Sci. Technol. 2015, 50, 766–773. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Concepts in functional foods: The case of inulin and oligofructose. J. Nutr. 1999, 129, 1398S–1401S. [Google Scholar] [CrossRef]
- Kisiel, W.; Barszcz, B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia 2000, 71, 269–273. [Google Scholar] [CrossRef]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and haemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.F.; Li, W.; Xu, G.Y.; Wang, K.R.; Li, L.; Wu, J.S. Updates and advances on pharmacological properties of Taraxacum mongolicum Hand.-Mazz and its potential applications. Food Chem. 2022, 373, 131380. [Google Scholar] [CrossRef]
- Ata, S.; Farooq, F.; Javed, S. Elemental profile of 24 common medicinal plants of Pakistan and its direct link with traditional uses. J. Med. Plants Res. 2011, 5, 6164–6168. [Google Scholar] [CrossRef]
- Sweeney, B.; Vora, M.; Ulbricht, C.; Basch, E. Evidence-based systematic review of dandelion (Taraxacum officinale) by natural standard research collaboration. J. Herb. Pharmacother. 2005, 5, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Modaresi, M. A comparative analysis of the effects of garlic, elderberry and black seed extract on the immune system in mice. J. Anim. Vet. Adv. 2012, 11, 458–461. [Google Scholar] [CrossRef]
- Blumental, M.; Cladbery, A.; Brinkman, J. Herbal Medicine: Expanded Commission E Monographs; Integrative Medicine Communications: Newton, MA, USA, 2000. [Google Scholar]
- Mahesh, A.; Jeyachandran, R.; Cindrella, L.; Thangadurai, D.; Veerapur, V.; Muralidhara Rao, D. Hepatocurative potential of sesquiterpene lactones of Taraxacum officinale on carbon tetrachloride induced liver toxicity in mice. Acta Biol. Hung. 2010, 61, 175–190. [Google Scholar] [CrossRef]
- Liu, W.; Yu, Q.; Wang, F.; Li, Y.; Zhang, G.; Tao, S. Taraxasterol attenuates melanoma progression via inactivation of reactive oxygen species-mediated PI3K/Akt signaling pathway. Hum. Exp. Toxicol. 2022, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Poljački, M.; Mimica-Dukić, N.; Boža, P.; Vujanović, L.J.; Ðuran, V.; Stojanović, S. Sesquiterpene lactone mix patch testing supplemented with dandelion extract in patients with allergic contact dermatitis, atopic dermatitis and non-allergic chronic inflammatory skin diseases. Contact Dermat. 2004, 51, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Im, D.Y.; Lee, K.I. Nitric oxide production inhibitory and scavenging activity and tyrosinase inhibitory activity of extracts from Taraxacum officinale and Taraxacum coreanum. Korean J. Med. Crop. Sci. 2011, 19, 362–367. [Google Scholar] [CrossRef]
- Kadeeja Sinoobiya, T.T.; Shijikumar, P.S.; Sirajudheen, M.K.; Baboo, R. A Review on Pharmacological Activity of Dandelion Plant. Int. J. Pharm. Pharm. Res. 2020, 18, 18–30. Available online: https://ijppr.humanjournals.com/wp-content/uploads/2020/07/2.Kadeeja-Sinoobiya.T.T-Shijikumar-P-S-Sirajudheen-M-K-RV-Celestin-Baboo.pdf (accessed on 14 May 2023).
- Singh, A.; Malhotra, S.; Subban, R. Dandelion (Taraxacum officinale)-Hepatoprotective Herb with Therapeutic Potential. Pharmacogn. Rev. 2008, 2, 163. Available online: https://www.phcogrev.com/sites/default/files/PhcogRev-2-3-163.pdf (accessed on 14 May 2023).
- Jeon, H.J.; Kang, H.J.; Jung, H.J.; Kang, Y.S.; Lim, C.J.; Kim, Y.M.; Park, E.H. Anti-inflammatory activity of Taraxacum officinale. J. Ethnopharmacol. 2008, 115, 82–88. [Google Scholar] [CrossRef]
- Al-Eisawi, Z.; Abderrahman, S.M.; Al-Khalaf, I.F.; Al-Abbassi, R.; Bustanji, Y.K. Taraxacum officinale Extracts Exhibit Safe and Selective Anticancer Activity. Nat. Prod. J. 2022, 12, 69–77. [Google Scholar] [CrossRef]
- Epure, A.; Parvu, A.; Vlase, L.; Benedec, D.; Hanganu, D.; Vlase, A.; Oniga, I. Polyphenolic compounds, antioxidant activity and nephroprotective properties of Romanian Taraxacum officinale. Farmacia 2022, 70, 47–53. [Google Scholar] [CrossRef]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Pârvu, A.E. Protective effects of Taraxacum officinale L. (Dandelion) root extract in experimental acute on chronic liver failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Ðordević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Godevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Orazbayeva, P.Z.; Akhmetalimova, A.M.; Ivasenko, S.A.; Loseva, I.V.; Ishmuratova, M.Y. Distribution of some plants of the Thyme genus on the territory of Central Kazakhstan. Mod. Asp. Use Plant Raw Mater. Raw Mater. Nat. Orig. Med. 2017, 2017, 170–172. (In Russian) [Google Scholar]
- Jovanović, A.A.; Balanč, B.; Petrović, P.; Pravilović, R.; Djordjević, V. Pharmacological potential of Thymus serpyllum L. (wild thyme) extracts and essential oil: A review. J. Eng. Process. Manag. 2021, 13, 32–41. [Google Scholar] [CrossRef]
- Khudonogova, E.G.; Kiseleva, T.V. The content of essential oils in the aboveground part of creeping thyme. Sib. Bull. Agric. Sci. 2010, 7, 110–113. (In Russian) [Google Scholar]
- Konovalov, D.A.; Orobinskaya, V.N.; Pisarenko, O.N. Antioxidants of fruits and vegetables. Mod. Sci. Innov. 2013, 4, 76–83. (In Russian) [Google Scholar]
- Goncharova, T.A. Encyclopedia of Medicinal Plants; M.: Publishing house of MSP: Moscow, Russia, 2001; Volume 1, pp. 528–560. (In Russian) [Google Scholar]
- Chaadaeva, H.H.; Boitsova, O.A. Anatomical features of the structure of Thymus serpyllum L., growing on the territory of the Orel region. Sci. Notes Orel State Univ. Ser. Nat. Tech. Med. Sci. 2010, 2, 134–141. (In Russian) [Google Scholar]
- Bazuk, A.G.; Yurchenko, R.A.; Vinarsky, V.A.; Buzuk, G.N. Comparative pharmacognostic analysis of Thyme herb. Bull. Pharm. 2011, 3, 19–24. (In Russian) [Google Scholar]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef] [PubMed]
- Tadele, A.; Urga, K.; Gemeda, N.; Lemma, H.; Melaku, D.; Mudie, K. Antimicrobial activity of topical formulations containing Thymus vulgaris essential oil on major pathogens causing skin diseases. Ethiop. Pharm. J. 2009, 26, 103–110. [Google Scholar] [CrossRef]
- Udintsev, S.N.; Zhilyakova, T.P.; Melnikov, D.P. Vegetable feed additives prospects for the use of Grass and Thyme meal. Pig Breed. 2010, 5, 18–21. (In Russian) [Google Scholar]
- Michalczyk, A.; Ostrowska, P. Essential oils and their components in combating fungal pathogens of animal and human skin. J. Med. Mycol. 2021, 31, 101118. [Google Scholar] [CrossRef]
- Tadić, V.M.; Nešić, I.; Martinović, M.; Rój, E.; Brašanac, V.S.; Maksimović, S.; Žugić, A. Old Plant, New Possibilities: Wild Bilberry (Vaccinium myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 2021, 10, 465. [Google Scholar] [CrossRef]
- Podwyszynska, M.; Mynett, K.; Markiewicz, M.; Pluta, S.; Marasek, C.A. Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.). Agronomy 2021, 11, 2584. [Google Scholar] [CrossRef]
- Kubentayev, S.A.; Suleimenov, A.N.; Kotukhov, J.A.; Danilova, A.N.; Sumbembayev, A.A. Phytocenotic characteristics and stocks of the main medicinal plants of the South-Western Altai (East Kazakhstan). Eur. J. BioSci. 2018, 12, 355–368. [Google Scholar]
- Tung, Y.T.; Wu, M.F.; Lee, M.C.; Wu, J.H.; Huang, C.C.; Huang, W.C. Antifatigue Activity and Exercise Performance of Phenolic-Rich Extracts from Calendula officinalis, Ribes nigrum, and Vaccinium myrtillus. Nutrients 2019, 11, 1715. [Google Scholar] [CrossRef]
- Musilova, J.; Frankova, H.; Lidikova, J.; Vollmannová, A.; Bojňanská, T.; Jurítková, J. The content of bioactive substances and their antioxidant effects in the European blueberry (Vaccinium myrtillus L.) influenced by different ways of their processing. J. Food Process. Preserv. 2022, 46, 16549. [Google Scholar] [CrossRef]
- Chehri, A.; Yarani, R.; Yousefi, Z.; Shakouri, S.K.; Ostadrahimi, A.; Mobasseri, M.; Araj-Khodaei, M. Phytochemical and pharmacological anti-diabetic properties of bilberries (Vaccinium myrtillus), recommendations for future studies. Prim. Care Diabetes 2022, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pires, T.C.; Caleja, C.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C. Vaccinium myrtillus L. fruits as a novel source of phenolic compounds with health benefits and industrial applications—A review. Curr. Pharm. Des. 2020, 26, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Govindaraghavan, S. Pharmacopeial HPLC identification methods are not sufficient to detect adulterations in commercial bilberry (Vaccinium myrtillus) extracts. Anthocyanin profile provides additional clues. Fitoterapia 2014, 99, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Güder, A.; Gür, M.; Engin, M.S. Antidiabetic and antioxidant properties of bilberry (Vaccinium myrtillus Linn.) fruit and their chemical composition. J. Agric. Sci. Technol. 2015, 17, 387–400. [Google Scholar]
- Drozd, J.; Anuszewska, E. Bilberry plant—Prospects of new applications in prevention and supportive treatment of civilisation diseases. Prz. Med. Uniw. Rzesz. Inst. Leków. 2013, 2, 226–235. [Google Scholar]
- Kitagawa, S.; Yoshii, K.; Morita, S.Y.; Teraoka, R. Efficient topical delivery of chlorogenic acid by an oil-in-water microemulsion to protect skin against UV-induced damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef]
- Menshikova, E.B.; Lankan, V.Z.; Kandalinzeva, N.V. Phenolic Antioxidants in Biology and Medicine: Structure, Properties, Mechanisms of Action; Lap Lambert Academic Publishing: Saarbrücken, Germany, 2012; p. 492. [Google Scholar]
- Kurkin, V.A.; Ryazanova, T.K. New Approaches in the Field of Standardization of Raw Materials and Preparations of Chernika Common; Izvestia of the Samara Science Center of the Russian Academy of Sciences: Samara, Russia, 2011; Volume 1, p. 13. [Google Scholar]
- Yakimenko, O.V.; Grigorievskaya, A.Y.; Ternovets, M.A. Mistletoe Viscum album L. (Loranthaceae) and “Witch’s Broom” (Proliferation). Series: Geography. Geoecology. Bull. VSU 2019, 2, 82–85. (In Russian) [Google Scholar]
- Kleszken, E.; Timar, A.V.; Memete, A.R.; Miere, F.; Vicas, S.I. On overview of bioactive compounds, biological and pharmacological effects of mistletoe (Viscum album L.). Pharmacophore 2022, 13, 10–26. [Google Scholar] [CrossRef]
- Peñaloza, E.; Holandino, C.; Scherr, C.; Araujo, P.I.P.d.; Borges, R.M.; Urech, K.; Baumgartner, S.; Garrett, R. Comprehensive Metabolome Analysis of Fermented Aqueous Extracts of Viscum album L. by Liquid Chromatography–High Resolution Tandem Mass Spectrometry. Molecules 2020, 25, 4006. [Google Scholar] [CrossRef]
- Vergara-Barberán, M.; Lerma-García, M.J.; Nicoletti, M.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Fasoli, E.; Righetti, P.G. Proteomic fingerprinting of mistletoe (Viscum album L.) via combinatorial peptide ligand libraries and mass spectrometry analysis. J. Proteom. 2017, 164, 52–58. [Google Scholar] [CrossRef]
- Orhan, D.D.; Küpeli, E.; Yesilada, E.; Ergun, F. Anti-inflammatory and antinociceptive activity of flavonoids isolated from Viscum album ssp. album. Z. Für Nat. C 2006, 61, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Blinova, K.F. Botanical-Pharmacognostic Dictionary: A Reference Guide; Higher School: Moscow, Russia, 2013; p. 117. (In Russian) [Google Scholar]
- Kyosev, P. Medicinal Plants: The Most Complete Reference Book; Eksmo: Moscow, Russia, 2011; p. 888. (In Russian) [Google Scholar]
- Majeed, M.; Rehman, R.U. Phytochemistry, Pharmacology, and Toxicity of an Epiphytic Medicinal Shrub Viscum album L. (White Berry Mistletoe). Med. Aromat. Plants Healthc. Ind. Appl. 2021, 287–301. [Google Scholar] [CrossRef]
- Jäger, T.; Holandino, C.; Melo, M.N.D.O.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Baumgartner, S. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants 2021, 10, 1726. [Google Scholar] [CrossRef]
- Golovkin, B.N.; Rudenskaya, R.N.; Trofimova, I.A.; Schroeter, A.I.; Semikhov, V.F. Biologically Active Substances of Plant Origin; Science: Moscow, Russia, 2002; p. 202. (In Russian) [Google Scholar]
- Kwon, Y.S.; Chun, S.Y.; Kim, M.K.; Nan, H.Y.; Lee, C.; Kim, S. Mistletoe extract targets the STAT3-FOXM1 pathway to induce apoptosis and inhibits metastasis in breast cancer cells. Am. J. Chin. Med. 2021, 49, 487–504. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.; Schwermer, M.; Eisenbraun, J.; Schramm, A.; Zuzak, T.J. Anticancer effects of Viscum album Fraxini extract on medulloblastoma cells in vitro. Complement. Med. Res. 2021, 28, 15–22. [Google Scholar] [CrossRef]
- Szurpnicka, A.; Zjawiony, J.K.; Szterk, A. Therapeutic potential of mistletoe in CNS-related neurological disorders and the chemical composition of Viscum species. J. Ethnopharmacol. 2019, 231, 241–252. [Google Scholar] [CrossRef]
- Turova, A.D. Medicinal Plants and Their Use; Medicine: Moscow, Russia, 2013; p. 203. (In Rissian) [Google Scholar]
- Sayakova, G.M.; Khamitova, A.E.; Olataeva, Z.N. Creation of New Dosage Forms from Domestic Plant Materials of Thick-Fruited Sophora (Sophora pachycarpa) and White Mistletoe (Viscum album) as Promising Sources of Biologically Active Substances. Bull. KazNMU 2018, 4, 217–220. Available online: https://cyberleninka.ru/article/n/sozdanie-novyh-lekarstvennyh-form-iz-otechestvennogo-rastitelnogo-syrya-sofory-tolstoplodnoy-soph-ra-pachyc-rpa-i-omely-beloy-viscum-album/viewer (accessed on 14 May 2023). (In Russian).
- Köse, B.; Erentürk, S. Drying characteristics of mistletoe (Viscum album L.) in convective and UV combined convective type dryers. Ind. Crops Prod. 2010, 32, 394–399. [Google Scholar] [CrossRef]
- Hah, Y.S.; Kim, E.J.; Goo, Y.M.; Kil, Y.S.; Sin, S.M.; Kim, S.G.; Yoon, T.J. Depigmenting Effects of Mistletoe (Viscum album var. coloratum) Extracts. J. Life Sci. 2022, 32, 355–361. [Google Scholar] [CrossRef]
- Harati, K.; Behr, B.; Daigeler, A.; Hirsch, T.; Jacobsen, F.; Renner, M.; Becerikli, M. Curcumin and Viscum album extract decrease proliferation and cell viability of soft-tissue sarcoma cells: An in vitro analysis of eight cell lines using real-time monitoring and colorimetric assays. Nutr. Cancer 2017, 69, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, S.J.; Choi, Y.N.; Kim, S.D.; Kwag, E.B.; Song, S.Y.; Park, J.H.; Kim, J.K.; Seo, C.; Choi, J.J.; et al. Selective Immune Modulating Activities of Viscum album and Its Components; A Possibility of Therapeutics on Skin Rash Induced by EGFR Inhibitors. Integr. Cancer Ther. 2022, 21, 1–11. [Google Scholar] [CrossRef]
- Palfi, M.C.; Racea, R.C.; Drăghici, G.; Seclaman, E.P.; Munteanu, M.; Mușat, O.; Ungureanu, E.; Milcu, A.; Boruga, M.; Rusu, L.; et al. Polyphenols Content and In Vitro Antitumor Activity of Hydroalcoholic Extract of Viscum album in Two Pigmented and Unpigmented Skin Cancer Cell Lines. Pharmaceuticals 2022, 70, 5. [Google Scholar] [CrossRef]
- Mamedov, N.; Gardner, Z.; Craker, L. Medicinal Plants Used in Russia and Central Asia for the Treatment of Selected Skin Conditions. J. Herbs Spices Med. Plants 2005, 11, 191–222. [Google Scholar] [CrossRef]
- Ustenova, G.O.; Beisembayeva, U.T.; Tuleeva, A.M. The use of medicinal plants of the flora of Kazakhstan in the treatment of dermatoses. Bull. KazNMU 2014, 207–209. [Google Scholar]

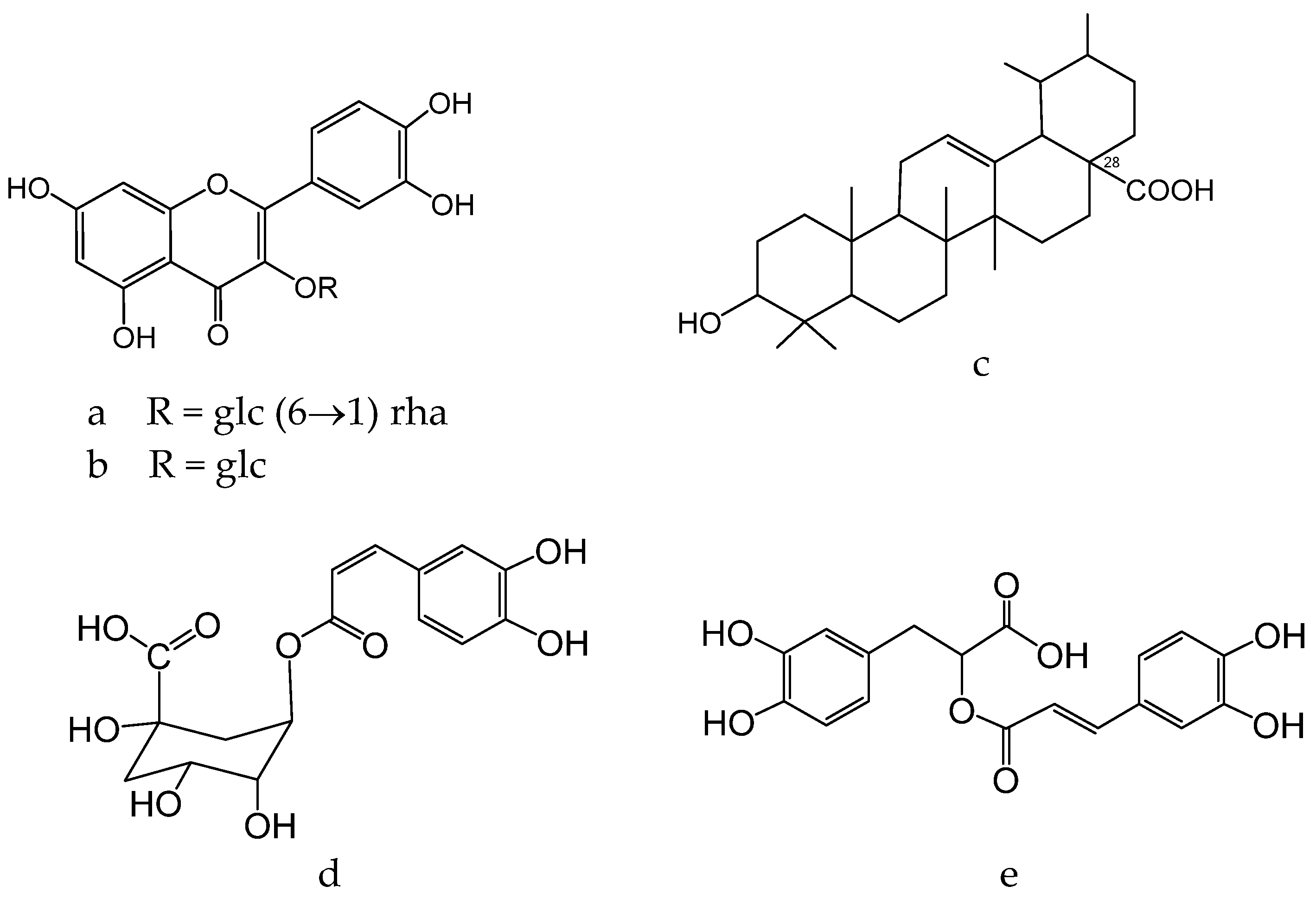

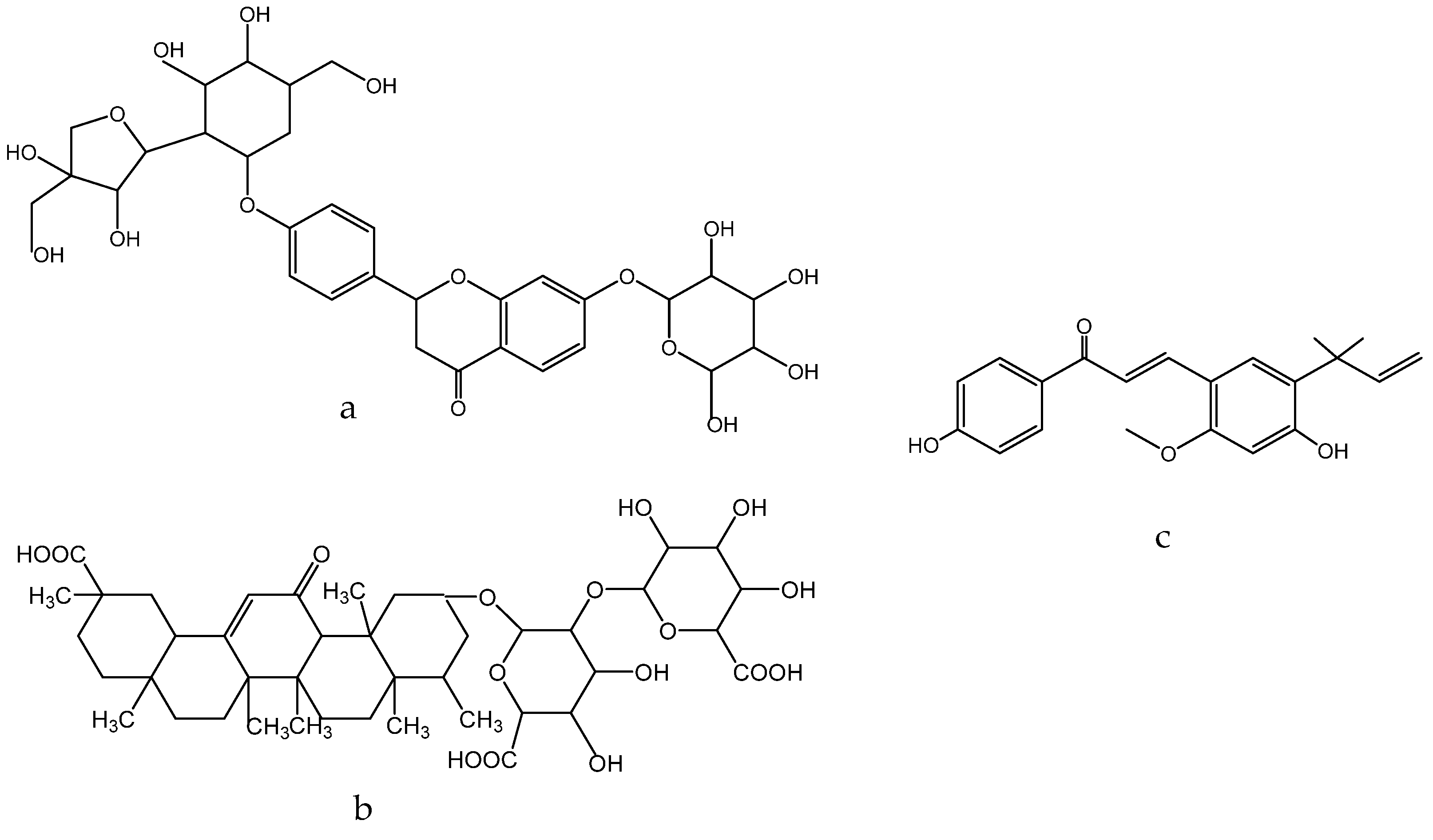
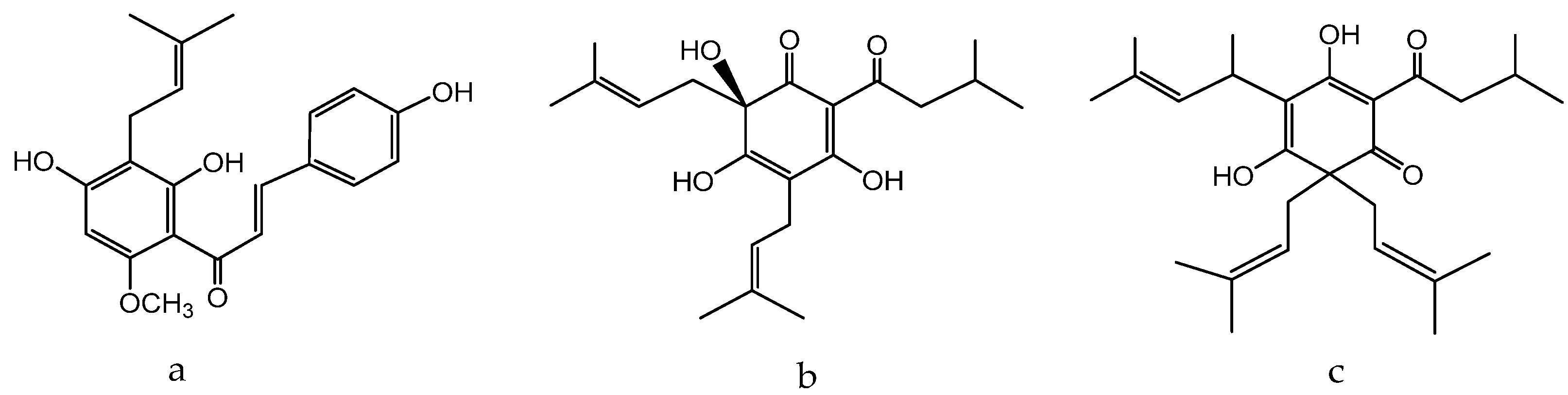

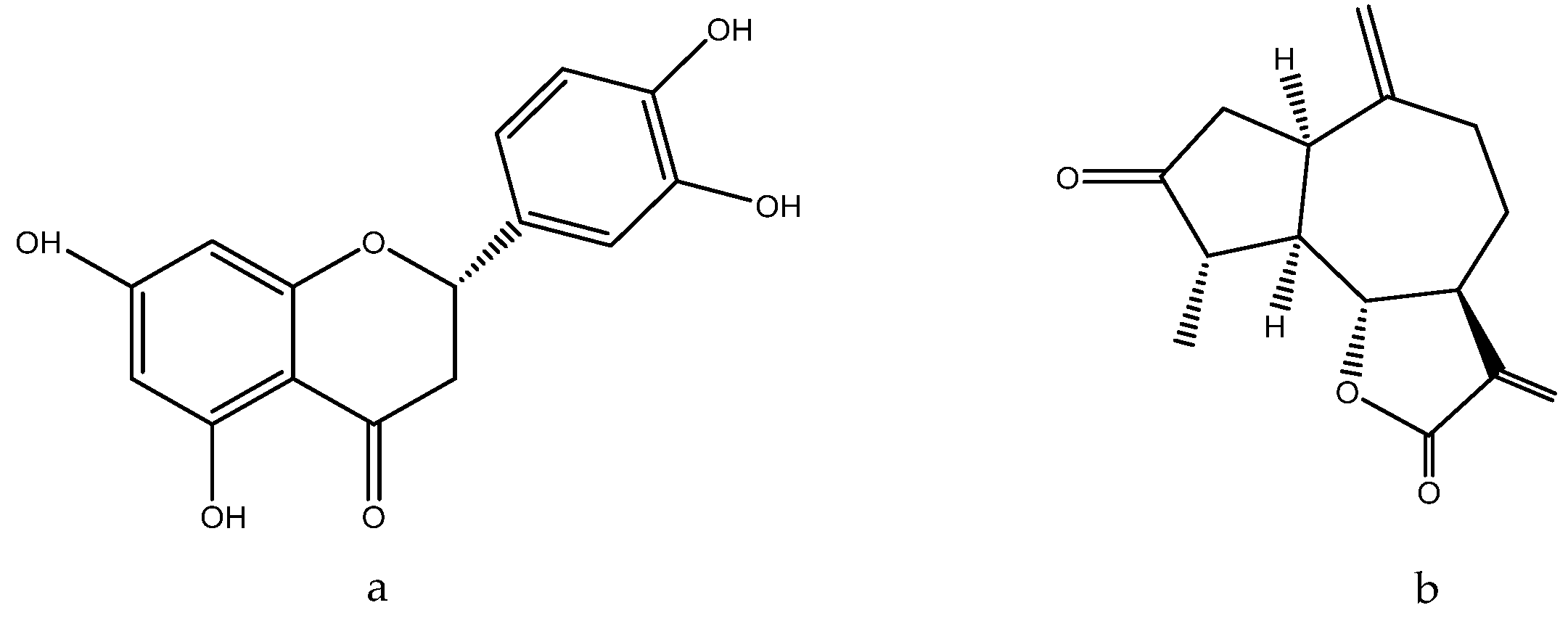


| Family and Scientific Name | Traditional Use | Biologically Active Compounds | Biological Activity | References |
|---|---|---|---|---|
| Аrасеае: Acorus calamus | pyoderma, acne vulgaris, alopecia eczema | essential oil, tannins, flavone, β-azarone, terpenes (cineol, limonene), proazulene | antioxidant, anti-inflammatory, antiulcer, antimicrobial, wound healing | [30,31,32,36] |
| Asteraceae: Achillea millefolium | acne, eczema, neurodermatitis, urticarial, vasculitis | sesquiterpenes (chamazulene) monoterpenes (camphor, thujol), flavone glycosides (apigenin, luteolin) | disinfectant, anti-inflammatory, antibacterial, antioxidant, antimicrobial, antiulcer | [18,22,23,26] |
| Artemisia absinthium | dermal fibroblasts, supporting or restoring elastin in the skin | flavonoids, phenols, and tannins | antibacterial, anti-inflammatory, antimicrobial, antiviral, antioxidant | [53,54,55,56,57] |
| Bidens tripartita L. | skin diseases such as acne and boils | essential oil, chlorophylls, flavonoids, cinnamic acid derivatives, tannins, polysaccharides, carotenoids, ascorbic acid, coumarins, chalcones, enzyme | anti-inflammatory, hemostatic, antiseptic, sedative, wound healing, antioxidant | [72,73,74,75] |
| Cichorium intybus L. | inflammation of the skin | inulin, glycoside intibin, proteins, sugars, pectin, sesquiterpene lactones, tannins and resinous substances, choline, carotene, vitamins B, B2, PP and C, taraxasterol, phenolic acids: chlorogenic, isochlorogenic, neochlorogenic, caffeic and chicory acids | antiseptic, anti-inflammatory, moisturizing and nourishing | [101,102,108,109,110,111] |
| Gnaphalium uliginosum L. | eczema and skin cancer | flavonoids, flavonols, sesquiterpenes, diterpenes, triterpenes, phytosterols, anthraquinones, caffeylquinic and caffeylglucaric acids, and carotenoids | antioxidant, antibacterial, antifungal, | [172,173,174] |
| Matricaria recutita L. | inflammatory conditions and lesions of the skin, skin irritation | flavonoids and their glycosides, coumarins, essential oil, terpenoids | sedative, antispasmodic, antiseptic, and antiemetic properties | [22,130,220] |
| Onopordum acanthium L. | UV protection, activity against itching, wounds | saponins, alkaloids, sesquiterpene lactones, flavonoids, quercetin, triterpenoids, sterols, nitrogen-containing compounds, phenolic acids, coumarins, inulin, fatty acids, eriodictyol | antioxidant, anti-inflammatory, antibacterial | [235,236,238] |
| Tanacetum vulgare L. | skin disorders such as acne, rosacea, and photoaging | phenolic acids, flavonoids, and their derivatives, caffeic acid, glycosides, sterols, cholesterol, campesterol, triterpenes | antibacterial, antiviral, antifungal, anti-inflammatory, and immunomodulatory | [350,351,352,353,354,355,356] |
| Taraxacum officinale Web. | Acne, warts | Taraxasterol, phenolic acids, polyphenolic compounds | Melanoma, tyrosinase inhibition, antioxidant properties | [372,373,374,375,376,377,378,379,380] |
| Apiaceae: Eryngium planum L. | skin wounds | flavonoids and phenolic acids, coumarin derivatives, terpene aldehyde esters, essential oils, and oligosaccharides | antioxidant, antimicrobial, anti-inflammatory | [142,144,145] |
| Pastinaca sativa L. | Stimulatory effect on melanogenesis, psoriasis, treatment of leukoderma | essential oil, heraclenol, oxypeucedanine hydrate, furanocoumarins | antioxidant | [257,258,260] |
| Boraginaceae: Symphytum officinale L. | therapeutic agent for wound healing, skin healing | Allantoin, rosmarinic acid, caffeic acid, chlorogenic acid | antimicrobial, anti-inflammatory, antioxidant | [335,337,340] |
| Brassicaceae: Capsella bursa-pastoris L. | skin diseases | flavonoids, polypeptides, choline, acetylcholine, histamine, tyramine, fatty acids, sterols, organic acids, amino acids, sulforaphane, vitamins, various trace elements | anti-inflammatory, antimicrobial, antioxidant | [88,90,91] |
| Cannabaceae: Humulus lupulus L. | inflammatory skin disorders in adolescents | lupulin, myrcene, linalool, kaempferol, quercetin, catechins, prenylnaringenin, geraniol, kaempferol, quercetin, catechins, prenylnaringenin | antitumor, anti-inflammatory, antiallergic, antipsoriatic, anti-collagenase | [175,180,182] |
| Equisetaceae: Equisetum arvense L. | skin cells and enhance skin texture | alkaloids, carbohydrates, proteins and amino acids, phytosterols, saponins, sterols, ascorbic acid, silicic acid, phenolic compounds and their glycosides, tannins, flavonoids (apigenin, genquanin, luteolin, kaempferol, quercetin), triterpenoids | antioxidant, anti-inflammatory, antibacterial, antimicrobial, antifungal | [125,126,127,128,129,130] |
| Ericaceae: Vaccinium myrtillus L. | skin-related ailments, eczema, burns, bruises, rashes, varicose veins, and acne | phenolic acids, flavonoids, resveratrol, polyphenols, phenolic acids, anthocyanins, organic acids, sugars, vitamins, fibers, glycosides, pyrogallic tannins, free hydroquinone, ascorbic acid, carotene, retinol acetate, thiamine bromide, pectin | antioxidant, anti-inflammatory, lipid-lowering | [393,404,405] |
| Fabaceae: Glycyrrhiza glabra L. | skin diseases, skin hyperpigmentation, eczema, psoriasis, dermocosmetics | Triterpene saponins glycyrrhizin, flavonoids, rhamnoliquirilin, liquiritigenin, lycoarylcoumarin, coumarin-GU-12, isoflavonoids, and chaconne oxyresveratrol, glabridin, liquiritin, apioside glucoliquiritin | anti-inflammatory, antiviral, antimicrobial, antioxidant, dermatology for treating skin diseases | [154,157,160,161] |
| Ononis spinosa L. | Wound healing and eczema, dermatitis and pruritus, treatment of burns | Isoflavonoids, pterocarpans, and dihydroisoflavonoids, comprising formononetin, calicosin, pseudobaptigenin, medicarpin, maakiain, onogenin, and sativanon, glucosides, glucoside malonates, glucoside acetates, and free aglycones | anti-inflammatory, antiviral, antimicrobial, antioxidant, anticancer, | [221,222,226] |
| Gramineae: Agropyron repens L. | inflammatory skin diseases, atopic dermatitis and acne | carbohydrates, pectin, triticin, thianogenic glycosides, flavonoids, saponins, essential oil, monoterpenes | skin diseases, antioxidant | [44,45] |
| Juglandaceae: Juglans regia L. | Skin inflammation, the treatment of acne, warts, eczema | flavonoids, quercetin, tannins, α-tocopherol | antioxidant effect | [200,201,202,203] |
| Lamiaceae: Thymus serpyllum L. | skin diseases | thymol, carvacrol, n-cymol, α-terpineol, borneol, ursolic, oleanolic acids, flavonoids, tannins, bitterness, gum | antioxidant, antiseptic, disinfectant | [382,390,391,392] |
| Lorantliaceae: Viscum album L. | skin toxicity | Lectin, viscumneoside III, viscumneoside V | antioxidant, antibacterial, antitumor | [421,423,424,426] |
| Orchidaceae: Orchis maculata L. | skin-preserving, | alkaloids, saponins, tannins, phenolic compounds, terpenes, sterols, flavonoids, anthocyanins | anti-inflammatory, antimicrobial, antioxidant | [244,245,251] |
| Papaveraceae: Chelidonium majus L. | warts, calluses, and eczema, skin rashes | Alkaloids, flavonoids, saponins, vitamins, phytosterols, aromatic and aliphatic acids, polysaccharides, alcohols, choline, tyramine, histamine, saponosides. | anti-inflammatory, antimicrobial, anticancer, antioxidant | [93,94,97] |
| Plantaginaceae: Plantago major L. | skin diseases, wounds, bruises, burns, and furunculosis | carbohydrates, nitrogen compounds, flavonoids, terpenoids, alicyclic compounds such as loliolid, tyrosol, tannins, vitamin K, organic acids, fatty oil. | wound healing, antibacterial, antiviral, antioxidant | [271,274,277] |
| Rosaceae: Rosa sinnamotea | Skincare–wound healing | of vitamins (C, B, P, PP, E, K), flavonoids, carotenes, carbohydrates, organic acids (tartaric, citric), polyunsaturated fatty acids, trace elements, alcohols, monoterpenes, sesquiterpenes | anti-inflammatory, antioxidant | [294,295] |
| Sorbus aucuparia L. | Skin ailments, wound-healing properties | Phenolic acids, flavonoids, proanthocyanidins, iridoids, coumarins, hydrolysable tannins, carotenoids, and anthocyanins, ascorbic acid, α-tocopherol, B1, B2, P, PP, K, and folic acid glucose, fructose, sucrose, sorbitol alcohol, phospholipids, pectin, organic acids, parasorbic acids, essential oil, macro- and microelements, glycoside amygdalin, | anti-inflammatory, antimicrobial, antifungal effect | [320,325,326] |
| Saxifragaceae: Ribes Nigrum L. | Exudative diathesis, eczema, furunculosis, atopic dermatitis, allergic pruritic dermatoses: neurodermatitis, itching, psoriasis, scleroderma, lichen planus, vasculitis and acne vulgaris | Soluble sugars, flavonoids, organic acids, vitamins, polyamino acids, macro- and microelements, and unsaturated fatty acids, gamma-linolenic acid (γ-C18:3), stearidonic acid (C18:4), tocochromanols (primarily γ-tocopherol and α-tocopherol), and sitosterol | antioxidant, antimicrobial, anti-inflammatory | [278,287,288,289,290] |
| Solanaceae: Solanum dulcamara L. | Treat skin mycotic infections | Solanine, saponins, phenolic compounds, flavonoids, anthocyanins, carotenoids, coumarins, phenolic acids | Antioxidant, skin diseases | [311,312] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berganayeva, G.; Kudaibergenova, B.; Litvinenko, Y.; Nazarova, I.; Sydykbayeva, S.; Vassilina, G.; Izdik, N.; Dyusebaeva, M. Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases. Molecules 2023, 28, 4192. https://doi.org/10.3390/molecules28104192
Berganayeva G, Kudaibergenova B, Litvinenko Y, Nazarova I, Sydykbayeva S, Vassilina G, Izdik N, Dyusebaeva M. Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases. Molecules. 2023; 28(10):4192. https://doi.org/10.3390/molecules28104192
Chicago/Turabian StyleBerganayeva, Gulzat, Bates Kudaibergenova, Yuliya Litvinenko, Irada Nazarova, Sandugash Sydykbayeva, Gulzira Vassilina, Nazerke Izdik, and Moldyr Dyusebaeva. 2023. "Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases" Molecules 28, no. 10: 4192. https://doi.org/10.3390/molecules28104192
APA StyleBerganayeva, G., Kudaibergenova, B., Litvinenko, Y., Nazarova, I., Sydykbayeva, S., Vassilina, G., Izdik, N., & Dyusebaeva, M. (2023). Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases. Molecules, 28(10), 4192. https://doi.org/10.3390/molecules28104192






