Molecular Design of Porous Organic Polymer-Derived Carbonaceous Electrocatalysts for Pinpointing Active Sites in Oxygen Reduction Reaction
Abstract
1. Introduction
2. General Principles for ORR
2.1. Fundamental Mechanisms of Electrocatalytic ORR
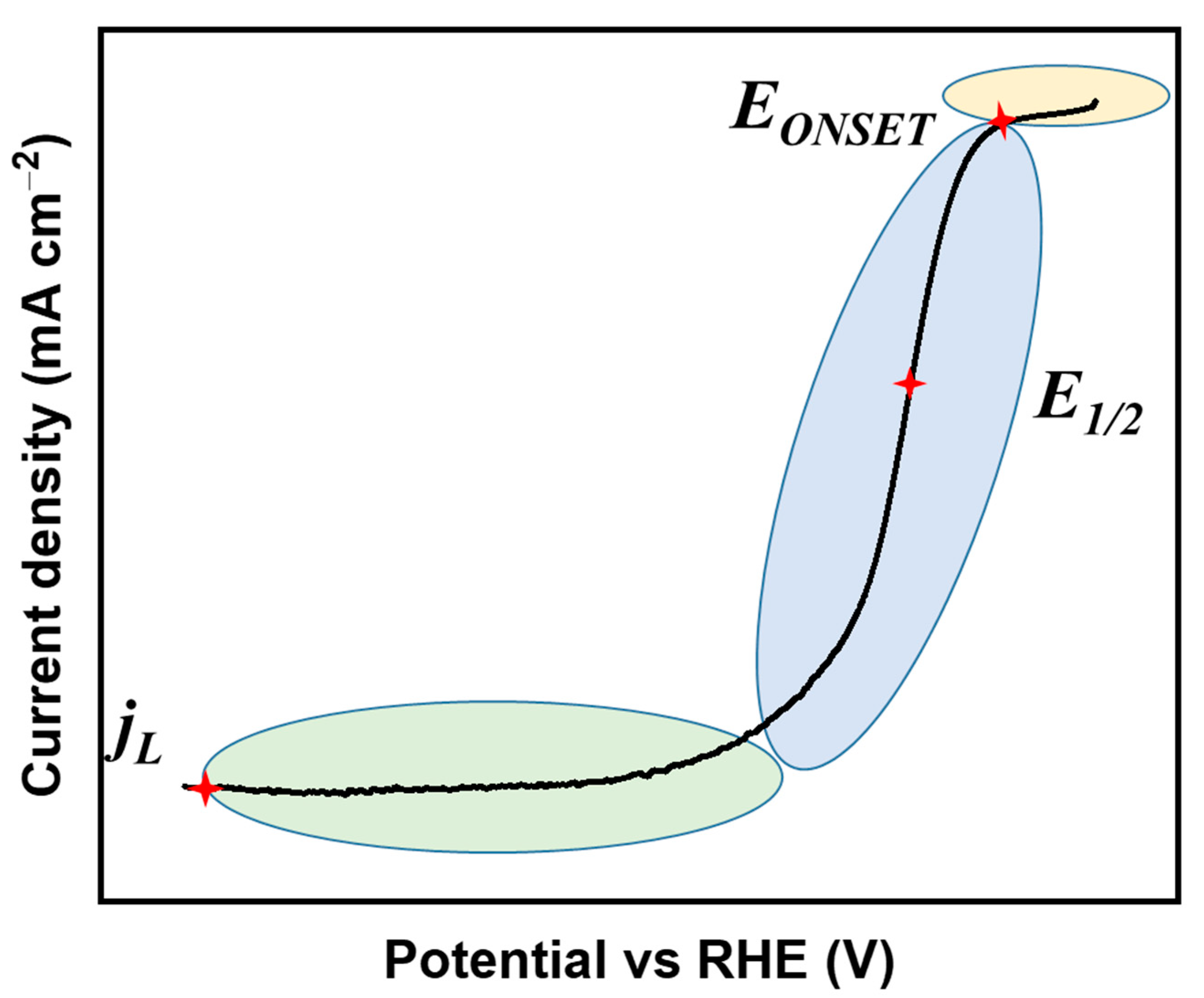
2.2. Electrochemical Evaluation for the ORR
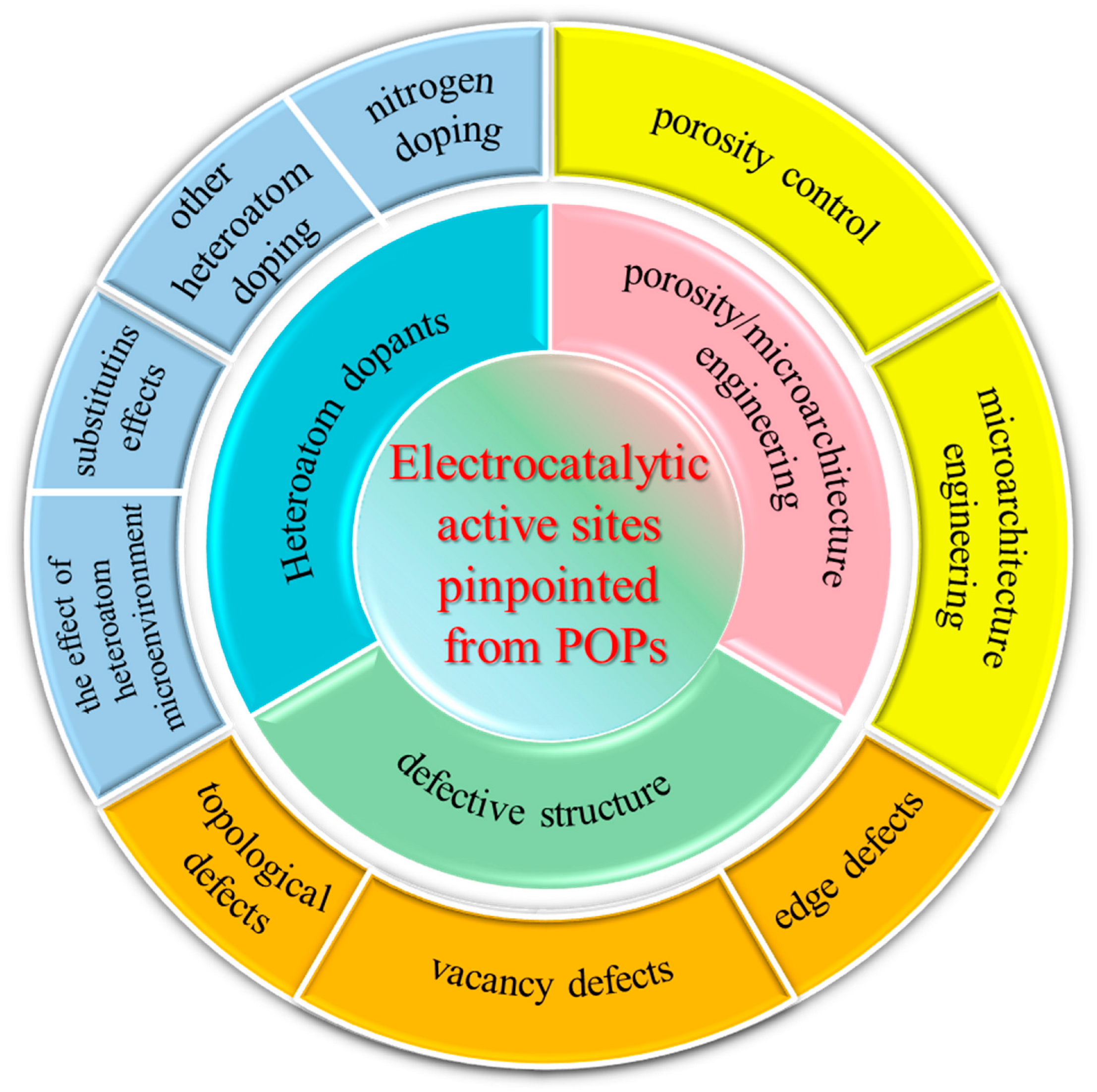
3. Electrocatalytic Active Sites Pinpointed from POPs
3.1. Heteroatom Dopants
3.1.1. Nitrogen Doping
3.1.2. Other Heteroatom Doping

3.1.3. Substitutions Effects
3.1.4. The Effect of Heteroatom Microenvironment
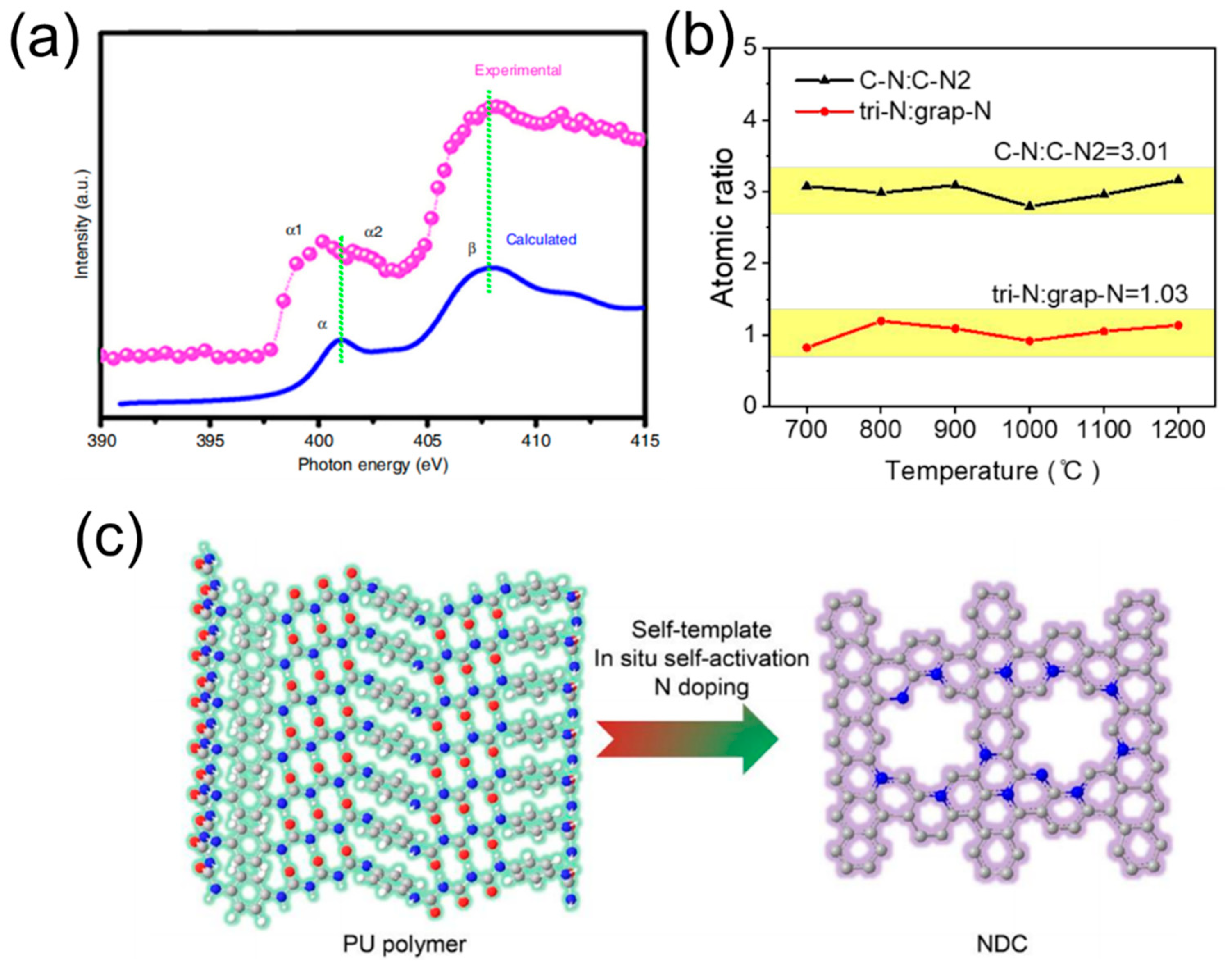
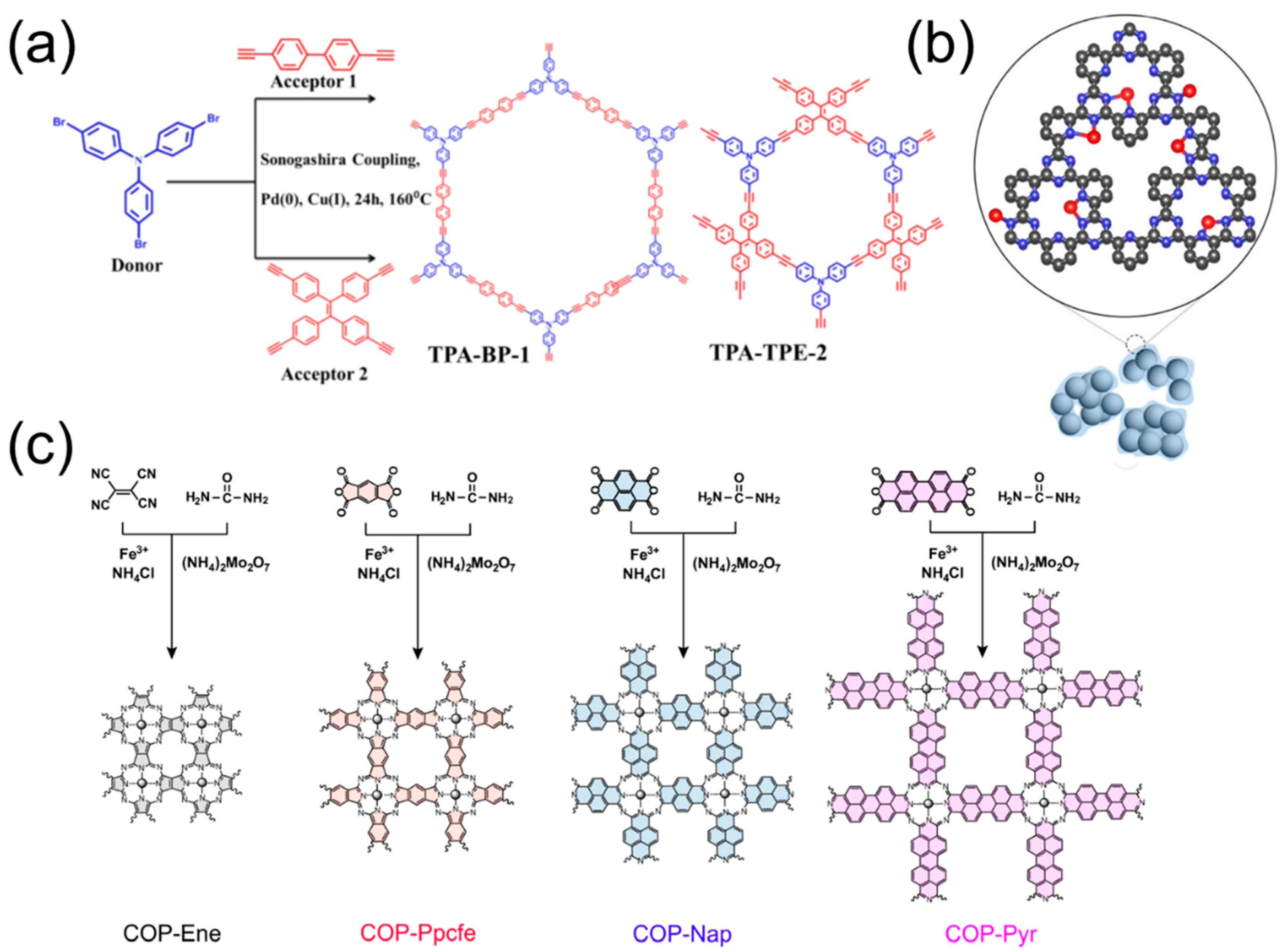
3.2. Microarchitecture Engineering
3.2.1. Porosity Modulation
3.2.2. Morphology Control
3.2.3. Dimension Adjustment
3.3. Defective Structure
3.3.1. Topological Defects
3.3.2. Vacancy Defects
3.3.3. Edge Defects

4. Structure—Property Relationship Constructed by POP-Derived Carbocatalysts
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive fuel cells. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ficca, V.C.A.; Santoro, C.; Placidi, E.; Arciprete, F.; Serov, A.; Atanassov, P.; Mecheri, B. Exchange Current Density as an Effective Descriptor of Poisoning of Active Sites in Platinum Group Metal-free Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2023, 13, 2162–2175. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Zhang, L.H.; Shi, Y.; Wang, Y.; Shiju, N.R. Nanocarbon Catalysts: Recent Understanding Regarding the Active Sites. Adv. Sci. 2020, 7, 1902126. [Google Scholar] [CrossRef]
- Dessalle, A.; Quilez-Bermejo, J.; Fierro, V.; Xu, F.; Celzard, A. Recent progress in the development of efficient biomass-based ORR electrocatalysts. Carbon 2023, 203, 237–260. [Google Scholar] [CrossRef]
- Wang, L.; Su, Y.; Gu, C. Solution Processing of Cross-Linked Porous Organic Polymers. Acc. Mater. Res. 2022, 3, 1049–1060. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.-B.; Dai, L. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212. [Google Scholar] [CrossRef]
- Lin, L.; Miao, N.; Wallace, G.G.; Chen, J.; Allwood, D.A. Engineering Carbon Materials for Electrochemical Oxygen Reduction Reactions. Adv. Energy Mater. 2021, 11, 2100695. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadnnap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef]
- Lazaridis, T.; Stühmeier, B.M.; Gasteiger, H.A.; El-Sayed, H.A. Capabilities and limitations of rotating disk electrodes versus membrane electrode assemblies in the investigation of electrocatalysts. Nat. Catal. 2022, 5, 363–373. [Google Scholar] [CrossRef]
- Xu, H.; Chen, L.; Shi, J. Advanced electrocatalytic systems for enhanced atom/electron utilization. Energy Environ. Sci. 2023, 16, 1334–1363. [Google Scholar] [CrossRef]
- Bouleau, L.; Perez-Rodriguez, S.; Quilez-Bermejo, J.; Izquierdo, M.T.; Xu, F.; Fierro, V.; Celzard, A. Best practices for ORR performance evaluation of metal-free porous carbon electrocatalysts. Carbon 2022, 189, 349–361. [Google Scholar] [CrossRef]
- Xie, C.; Yan, D.; Chen, W.; Zou, Y.; Chen, R.; Zang, S.; Wang, Y.; Yao, X.; Wang, S. Insight into the design of defect electrocatalysts: From electronic structure to adsorption energy. Mater. Today 2019, 31, 47–68. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Wu, B.; Meng, H.; Morales, D.M.; Zeng, F.; Zhu, J.; Wang, B.; Risch, M.; Xu, Z.J.; Petit, T. Nitrogen-Rich Carbonaceous Materials for Advanced Oxygen Electrocatalysis: Synthesis, Characterization, and Activity of Nitrogen Sites. Adv. Funct. Mater. 2022, 32, 2204137. [Google Scholar] [CrossRef]
- Tang, J.; Su, C.; Shao, Z. Covalent Organic Framework (COF)-Based Hybrids for Electrocatalysis: Recent Advances and Perspectives. Small Methods 2021, 5, e2100945. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, Z. Mechanisms of Oxygen Reduction Reaction on Nitrogen-Doped Graphene for Fuel Cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Roy, S.; Bandyopadhyay, A.; Das, M.; Ray, P.P.; Pati, S.K.; Maji, T.K. Redox-active and semi-conducting donor-acceptor conjugated microporous polymers as metal-free ORR catalysts. J. Mater. Chem. A 2018, 6, 5587–5591. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Zhu, P.; Cullen, D.A.; Hu, Y.; Yan, Q.-Q.; Shen, S.-C.; Chen, F.-Y.; Yu, H.; Shakouri, M.; Arregui-Mena, J.D.; et al. A general synthesis of single atom catalysts with controllable atomic and mesoporous structures. Nat. Synth. 2022, 1, 658–667. [Google Scholar] [CrossRef]
- Gao, J.; Feng, L.; Ma, R.; Su, B.-J.; Alenad, A.M.; Liu, Y.; Beller, M.; Jagadeesh, R.V. Cobalt single-atom catalysts for domino reductive amination and amidation of levulinic acid and related molecules to N-heterocycles. Chem. Catal. 2022, 2, 178–194. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Wang, X.; Wang, W.; Ma, T.; Liu, X. Fully π-conjugated dense topological salphen organic frameworks with atomic dispersed tetradentate cobalt sites for high-efficiency electrocatalytic oxygen reduction. Appl. Catal. B Environ. 2022, 315, 121590. [Google Scholar] [CrossRef]
- Kamiya, K.; Kamai, R.; Hashimoto, K.; Nakanishi, S. Platinum-modified covalent triazine frameworks hybridized with carbon nanoparticles as methanol-tolerant oxygen reduction electrocatalysts. Nat. Commun. 2014, 5, 5040. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Z. Identifying the impact of the covalent-bonded carbon matrix to FeN4 sites for acidic oxygen reduction. Nat. Commun. 2022, 13, 57. [Google Scholar] [CrossRef]
- Yang, H.; Gao, S.; Rao, D.; Yan, X. Designing superior bifunctional electrocatalyst with high-purity pyrrole-type CoN4 and adjacent metallic cobalt sites for rechargeable Zn-air batteries. Energy Storage Mater. 2022, 46, 553–562. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, W.; Li, Y.; Xu, Y.; Zhao, M.; Deng, M.; Wang, Y. Facile regulation of porous N-doped carbon-based catalysts from covalent organic frameworks nanospheres for highly-efficient oxygen reduction reaction. Carbon 2021, 180, 92–100. [Google Scholar] [CrossRef]
- Nayak, B.; Mukherjee, A.; Basu, S.; Bhanja, P.; Jena, B.K. Metal-Free Triazine-Based Porous Organic Polymer-Derived N-Doped Porous Carbons as Effective Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2022, 5, 15899–15908. [Google Scholar] [CrossRef]
- Xu, X.; Gao, Y.; Yang, Q.; Liang, T.; Luo, B.; Kong, D.; Li, X.; Zhi, L.; Wang, B. Regulating the activity of intrinsic sites in covalent organic frameworks by introducing electro-withdrawing groups towards highly selective H2O2 electrosynthesis. Nano Today 2023, 49, 101792. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Q.; Guo, R.; Kong, D.; Zhou, S.; Huang, X.; Iqbal, R.; Zhi, L. Nitrogen-Enriched Carbon/CNT Composites Based on Schiff-Base Networks: Ultrahigh N Content and Enhanced Lithium Storage Properties. Small 2018, 14, 1703569. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xu, Y.; Guo, Y.; Zhang, L.; Hu, Y.; Qian, J.; Huang, S. Highly graphitized N-doped carbon nanosheets from 2-dimensional coordination polymers for efficient metal-air batteries. Carbon 2022, 188, 135–145. [Google Scholar] [CrossRef]
- Haque, E.; Zavabeti, A.; Uddin, N.; Wang, Y.; Rahim, M.A.; Syed, N.; Xu, K.; Jannat, A.; Haque, F.; Zhang, B.Y.; et al. Deciphering the Role of Quaternary N in O2 Reduction over Controlled N-Doped Carbon Catalysts. Chem. Mater. 2020, 32, 1384–1392. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the Role of Quaternary-N of BINOL Covalent Triazine-Based Frameworks in Oxygen Reduction and Hydrogen Evolution Reactions. ACS Appl. Mater. Interfaces 2020, 12, 44689–44699. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, S.; Yu, X.; Li, K.; Ni, X.; Ye, L. Nitrogen-doped carbon spheres with precisely-constructed pyridinic-N active sites for efficient oxygen reduction. Appl. Surf. Sci. 2022, 598, 153786. [Google Scholar] [CrossRef]
- Cai, Y.; Tao, L.; Huang, G.; Zhang, N.; Zou, Y.; Wang, S. Regulating carbon work function to boost electrocatalytic activity for the oxygen reduction reaction. Chin. J. Catal. 2021, 42, 938–944. [Google Scholar] [CrossRef]
- Yang, C.; Maenosono, S.; Duan, J.; Zhang, X. COF-Derived N,P Co-Doped Carbon as a Metal-Free Catalyst for Highly Efficient Oxygen Reduction Reaction. ChemNanoMat 2019, 5, 957–963. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Z.; Kong, D.; Iqbal, R.; Yang, Q.-H.; Zhi, L. N,P co-doped hollow carbon nanofiber membranes with superior mass transfer property for trifunctional metal-free electrocatalysis. Nano Energy 2019, 64, 103879. [Google Scholar] [CrossRef]
- Lei, W.; Deng, Y.-P.; Li, G.; Cano, Z.P.; Wang, X.; Luo, D.; Liu, Y.; Wang, D.; Chen, Z. Two-Dimensional Phosphorus-Doped Carbon Nanosheets with Tunable Porosity for Oxygen Reactions in Zinc-Air Batteries. ACS Catal. 2018, 8, 2464–2472. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Chhetri, M. Borocarbonitrides as Metal-Free Catalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, 1803668. [Google Scholar] [CrossRef]
- Zheng, M.; Shi, J.; Yuan, T.; Wang, X. Metal-Free Dehydrogenation of N-Heterocycles by Ternary h-BCN Nanosheets with Visible Light, Angew. Chem. Int. Ed. 2018, 57, 5487–5491. [Google Scholar] [CrossRef] [PubMed]
- Kahan, R.J.; Hirunpinyopas, W.; Cid, J.; Ingleson, M.J.; Dryfe, R.A.W. Well-Defined Boron/Nitrogen-Doped Polycyclic Aromatic Hydrocarbons Are Active Electrocatalysts for the Oxygen Reduction Reaction. Chem. Mater. 2019, 31, 1891–1898. [Google Scholar] [CrossRef]
- Wang, X.; Han, C.; Li, H.; Su, P.; Ta, N.; Ma, Y.; Huang, Z.; Liu, J. Fabrication of monodispersed B, N co-doped hierarchical porous carbon nanocages through confined etching to boost electrocatalytic oxygen reduction. Nano Res. 2023, 16, 290–298. [Google Scholar] [CrossRef]
- Ishii, T.; Philavanh, M.; Negishi, J.; Inukai, E.; Ozaki, J.-I. Preparation of Chemically Structure-Controlled BN-Doped Carbons for the Molecular Understanding of Their Surface Active Sites for Oxygen Reduction Reaction. ACS Catal. 2022, 12, 1288–1297. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, D.; Liu, W.; Li, X.; Chen, Q. Fluorine enhanced pyridinic-N configuration as an ultra-active site for oxygen reduction reaction in both alkaline and acidic electrolytes. Carbon 2022, 187, 67–77. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Yang, J.; Ding, X.; Ji, W.; Jaworski, A.; Hedin, N.; Han, B.-H. Synergetic contribution of nitrogen and fluorine species in porous carbons as metal-free and bifunctional oxygen electrocatalysts for zinc–air batteries. Appl. Catal. B Environ. 2021, 297, 120448. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Li, Z.; Hao, L.; Wang, P.; Zou, X.; Liu, Z.; Zhang, G.; Zhang, C.-Y. Iron and Iodine Co-doped Triazine-Based Frameworks with Efficient Oxygen Reduction Reaction in Alkaline and Acidic Media. ACS Sustain. Chem. Eng. 2019, 7, 11787–11794. [Google Scholar] [CrossRef]
- Su, Z.; Ye, G.; Liu, S.; He, Z. A template-free I-2-assisted pyrolysis strategy to synthesize coral-like nitrogen-doped carbon with a regulated hierarchical porous structure toward efficient oxygen reduction. Chem. Eng. J. 2022, 440, 135852. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Zhang, L.; Li, H.; Zhu, L.; Yang, D.; Fang, Q.; Qiu, S.; Yao, X. Metal-Free Thiophene-Sulfur Covalent Organic Frameworks: Precise and Controllable Synthesis of Catalytic Active Sites for Oxygen Reduction. J. Am. Chem. Soc. 2020, 142, 8104–8108. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; He, R.; Peng, L.; Cai, D.; Qiao, J. Large-scale defect-engineering tailored tri-doped graphene as a metal-free bifunctional catalyst for superior electrocatalytic oxygen reaction in rechargeable Zn-air battery. Appl. Catal. B Environ. 2021, 285, 119811. [Google Scholar] [CrossRef]
- Zheng, Y.; Song, H.; Chen, S.; Yu, X.; Zhu, J.; Xu, J.; Zhang, K.A.I.; Zhang, C.; Liu, T. Metal-Free Multi-Heteroatom-Doped Carbon Bifunctional Electrocatalysts Derived from a Covalent Triazine Polymer. Small 2020, 16, e2004342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, W.; Liang, Y.; Yang, X.; Cao, M.; Cao, R. Template-free synthesis of non-noble metal single-atom electrocatalyst with N-doped holey carbon matrix for highly efficient oxygen reduction reaction in zinc-air batteries. Appl. Catal. B Environ. 2021, 285, 119780. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, D.; Zhao, M.; Chen, H.; Fan, X.; Gao, B.; Gu, L.; Guo, Y.; Qin, J.; Wei, J.; et al. Cross-Linked Polyphosphazene Hollow Nanosphere-Derived N/P-Doped Porous Carbon with Single Nonprecious Metal Atoms for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2020, 132, 14747–14754. [Google Scholar] [CrossRef]
- You, Z.; Wang, B.; Zhao, Z.; Zhang, Q.; Song, W.; Zhang, C.; Long, X.; Xia, Y. Metal-Free Carbon-Based Covalent Organic Frameworks with Heteroatom-Free Units Boost Efficient Oxygen Reduction. Adv. Mater. 2023, 35, e2209129. [Google Scholar] [CrossRef]
- Singh, A.; Verma, P.; Samanta, D.; Singh, T.; Maji, T.K. Bimodal Heterogeneous Functionality in Redox-Active Conjugated Microporous Polymer toward Electrocatalytic Oxygen Reduction and Photocatalytic Hydrogen Evolution. Chemistry 2020, 26, 3810–3817. [Google Scholar] [CrossRef]
- Wu, S.; Li, M.; Phan, H.; Wang, D.; Herng, T.S.; Ding, J.; Lu, Z.; Wu, J. Toward Two-Dimensional π-Conjugated Covalent Organic Radical Frameworks. Angew. Chem. Int. Ed. 2018, 57, 8007–8011. [Google Scholar] [CrossRef]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W. Advanced Electrocatalysts for the Oxygen Reduction Reaction in Energy Conversion Technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Cui, X.; Shi, J. The ORR kinetics of ZIF-derived Fe-N-C electrocatalysts. J. Catal. 2019, 372, 174–181. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.; Chu, Y.; Gao, N.; Jin, Z.; Ge, J.; Liu, C.; Xing, W. Construction and Regulation of a Surface Protophilic Environment to Enhance Oxygen Reduction Reaction Electrocatalytic Activity. ACS Appl. Mater. Interfaces 2020, 12, 41269–41276. [Google Scholar] [CrossRef]
- Peles-Strahl, L.; Zion, N.; Lori, O.; Levy, N.; Bar, G.; Dahan, A.; Elbaz, L. Bipyridine Modified Conjugated Carbon Aerogels as a Platform for the Electrocatalysis of Oxygen Reduction Reaction. Adv. Funct. Mater. 2021, 31, 2100163. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, X.; Yang, Q.; Ma, Y.; Zhao, N.; Huang, X.; Shaislamov, U.; Kong, D.; Zhi, L. Electrifying Schiff-based networks as model catalysts towards deeply understanding the crucial role of sp2-carbon in nitrogen-doped carbocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2022, 599, 153961. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; You, Z.; Zhang, Q.; Song, W.; Long, X. Heterocyclic Modulated Electronic States of Alkynyl-Containing Conjugated Microporous Polymers for Efficient Oxygen Reduction. Small 2023, 19, 2207298. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yun, W.; Wu, H.; Liu, X.; Wang, L.; Yu, Q.; Li, A.; Wang, H.; Song, C.; Gao, Z.; et al. Construction of sp3/sp2 carbon interface in 3D N-doped nanocarbon for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2019, 58, 15089–15097. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Lee, J.Y. Graphynes as Promising Cathode Material of Fuel Cell: Improvement of Oxygen Reduction Efficiency. J. Phys. Chem. C 2014, 118, 12035–12040. [Google Scholar] [CrossRef]
- Chen, X. Graphyne nanotubes as electrocatalysts for oxygen reduction reaction: The effect of doping elements on the catalytic mechanisms. Phys. Chem. Chem. Phys. 2015, 17, 29340–29343. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Ju, Q.; Ma, R.; Hu, Y.; Guo, B.; Liu, Q.; Thomas, T.; Zhang, T.; Yang, M.; Chen, W.; Wang, J. Highly Localized C-N2 Sites for Efficient Oxygen Reduction. ACS Catal. 2020, 10, 9366–9375. [Google Scholar] [CrossRef]
- Wollbrink, A.; Volgmann, K.; Koch, J.; Kanthasamy, K.; Tegenkamp, C.; Li, Y.; Richter, H.; Kaemnitz, S.; Steinbach, F.; Feldhoff, A.; et al. Amorphous, turbostratic and crystalline carbon membranes with hydrogen selectivity. Carbon 2016, 106, 93–105. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, J.; Tang, Z.; Bi, D.; Zhao, J.; Liang, Y. Optimal Configuration of N-Doped Carbon Defects in 2D Turbostratic Carbon Nanomesh for Advanced Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 11999–12006. [Google Scholar] [CrossRef]
- Tan, H.; Tang, J.; Henzie, J.; Li, Y.; Xu, X.; Chen, T.; Wang, Z.; Wang, J.; Ide, Y.; Bando, Y.; et al. Assembly of Hollow Carbon Nanospheres on Graphene Nanosheets and Creation of Iron–Nitrogen-Doped Porous Carbon for Oxygen Reduction. ACS Nano 2018, 12, 5674–5683. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Xu, R.; Wu, Y.; Jiang, Y.; Yu, Y. Binding Sulfur-Doped Nb2O5 Hollow Nanospheres on Sulfur-Doped Graphene Networks for Highly Reversible Sodium Storage. Adv. Funct. Mater. 2018, 28, 1800394. [Google Scholar] [CrossRef]
- Tang, D.; Wang, T.; Zhang, W.; Zhao, Z.; Zhang, L.; Qiao, Z.-A. Liquid Na/K Alloy Interfacial Synthesis of Functional Porous Carbon at Ambient Temperature. Angew. Chem. Int. Ed. 2022, 61, e202203967. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, S.; Song, H.; Guo, H.; Zhang, K.A.I.; Zhang, C.; Liu, T. Nitrogen-doped hollow carbon nanoflowers from a preformed covalent triazine framework for metal-free bifunctional electrocatalysis. Nanoscale 2020, 12, 14441–14447. [Google Scholar] [CrossRef]
- Li, N.; Tang, R.; Su, Y.; Lu, C.; Chen, Z.; Sun, J.; Lv, Y.; Han, S.; Yang, C.; Zhuang, X. Isometric Covalent Triazine Framework-Derived Porous Carbons as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. ChemSusChem 2023, 16, e202201937. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Xu, Z.; Yan, D.; Xiao, G. Hierarchically porous carbons fabricated by dual pore-forming approach for the oxygen reduction reaction. Carbon 2022, 189, 634–641. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Chen, Z.; Shi, C.; Yang, H.; Tang, Y.; Cen, Z.; Liu, S.; Fu, R.; Wu, D. Molecular Engineering toward High-Crystallinity Yet High-Surface-Area Porous Carbon Nanosheets for Enhanced Electrocatalytic Oxygen Reduction. Adv. Sci. 2022, 9, e2103477. [Google Scholar] [CrossRef]
- Jiao, L.; Hu, Y.; Ju, H.; Wang, C.; Gao, M.-R.; Yang, Q.; Zhu, J.; Yu, S.-H.; Jiang, H.-L. From covalent triazine-based frameworks to N-doped porous carbon/reduced graphene oxide nanosheets: Efficient electrocatalysts for oxygen reduction. J. Mater. Chem. A 2017, 5, 23170–23178. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Zhang, K.A.I.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Ultrasound-Triggered Assembly of Covalent Triazine Framework for Synthesizing Heteroatom-Doped Carbon Nanoflowers Boosting Metal-Free Bifunctional Electrocatalysis. ACS Appl. Mater. Interfaces 2021, 13, 13328–13337. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, T.; Lu, K.; Zhou, L.; Li, D.S.; Wang, R. Engineering Synergistic Edge-N Dipole in Metal-Free Carbon Nanoflakes toward the Intensified Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2021, 31, 2103187. [Google Scholar] [CrossRef]
- Zou, L.; Hou, C.-C.; Wang, Q.; Wei, Y.-S.; Liu, Z.; Qin, J.-S.; Pang, H.; Xu, Q. A Honeycomb-Like Bulk Superstructure of Carbon Nanosheets for Electrocatalysis and Energy Storage. Angew. Chem. Int. Ed. 2020, 59, 19627–19632. [Google Scholar] [CrossRef]
- Hou, C.C.; Zou, L.; Xu, Q. A Hydrangea-Like Superstructure of Open Carbon Cages with Hierarchical Porosity and Highly Active Metal Sites. Adv. Mater. 2019, 31, e1904689. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, C.; Liu, Y.; Yang, M.; Li, X.; He, Y.; Li, H.; Chen, H.; Lin, Z. Dual-Shelled Multidoped Hollow Carbon Nanocages with Hierarchical Porosity for High-Performance Oxygen Reduction Reaction in Both Alkaline and Acidic Media. Nano Lett. 2020, 20, 5639–5645. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.; Giuliani, A.; Melchionna, M.; Marchesan, S.; Criado, A.; Nasi, L.; Bevilacqua, M.; Tavagnacco, C.; Vizza, F.; Prato, M.; et al. N-Doped Graphitized Carbon Nanohorns as a Forefront Electrocatalyst in Highly Selective O2 Reduction to H2O2. Chem 2018, 4, 106–123. [Google Scholar] [CrossRef]
- Zhu, H.J.; Lu, M.; Wang, Y.R.; Yao, S.J.; Lan, Y.Q. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef]
- Royuela, S.; Martínez-Perián, E.; Arrieta, M.P.; Martínez, J.; Ramos, M.M.; Zamora, F.; Lorenzo, E.; Segura, J.L. Oxygen reduction using a metal-free naphthalene diimide-based covalent organic framework electrocatalyst. Chem. Commun. 2020, 56, 1267–1270. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, Y.; Zhang, X.; Oshima, Y.; Chen, Q.; Jiang, D. Template Conversion of Covalent Organic Frameworks into 2D Conducting Nanocarbons for Catalyzing Oxygen Reduction Reaction. Adv. Mater. 2018, 30, e1706330. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Li, H.; Chen, J.; Fei, J.; Yu, Z.; Yuan, Z.; Wang, C.; Zheng, H.; Liu, Z.; et al. One-Dimensional van der Waals Heterostructures as Efficient Metal-Free Oxygen Electrocatalysts. ACS Nano 2021, 15, 3309–3319. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Yin, C.; Wei, L.; Wu, W.; Hu, Z.; Wu, M. Synergistic Effects between Doped Nitrogen and Phosphorus in Metal-Free Cathode for Zinc-Air Battery from Covalent Organic Frameworks Coated CNT. ACS Appl. Mater. Interfaces 2017, 9, 44519–44528. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Tang, Y.; Li, L.; Hu, X.; Han, M.; Guo, Z.; Zhan, H.; Chen, B. Reticular Synthesis of One-Dimensional Covalent Organic Frameworks with 4-c sql Topology for Enhanced Fluorescence Emission. Angew. Chem. Int. Ed. 2023, 62, e202213268. [Google Scholar]
- An, S.; Li, X.; Shang, S.; Xu, T.; Yang, S.; Cui, C.-X.; Peng, C.; Liu, H.; Xu, Q.; Jiang, Z.; et al. One-Dimensional Covalent Organic Frameworks for the 2e− Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2023, 62, e202218742. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Z.; Fan, Y.; Liu, C.; Sun, H.; Liang, W.; Li, A. Calcination of Porphyrin-Based Conjugated Microporous Polymers Nanotubes As Nanoporous N-Rich Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2020, 3, 5260–5268. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant Contribution of Intrinsic Carbon Defects to Oxygen Reduction Activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, Y.; Mei, W.; Zhao, C.; Zhang, C.; Zhang, J.; Amiinu, I.S.; Mu, S. Effects of Intrinsic Pentagon Defects on Electrochemical Reactivity of Carbon Nanomaterials. Angew. Chem. Int. Ed. 2019, 58, 3859–3864. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Mao, X.; Zhang, L.; Liu, D.; Song, L.; Yan, X.; Chen, J.; Yang, D.; Zhou, J.; et al. A Directional Synthesis for Topological Defect in Carbon. Chem 2020, 6, 2009–2023. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Ge, B.; Zheng, F.; Zhang, N.; Zuo, M.; Yang, Y.; Chen, Q. Constructing Graphitic-Nitrogen-Bonded Pentagons in Interlayer-Expanded Graphene Matrix toward Carbon-Based Electrocatalysts for Acidic Oxygen Reduction Reaction. Adv. Mater. 2021, 33, 2103133. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, X.; Jia, Y.; Yao, X. Defective carbon-based materials: Controllable synthesis and electrochemical applications. EnergyChem 2021, 3, 100059. [Google Scholar] [CrossRef]
- Choi, K.; Kim, S. Theoretical Study of Oxygen Reduction Reaction Mechanism in Metal-Free Carbon Materials: Defects, Structural Flexibility, and Chemical Reaction. ACS Nano 2022, 16, 16394–16401. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Hu, H.; Huang, S.; Li, Y.; Lin, L.; Duan, F.; Zhu, H.; Du, M.; Lu, S. Versatile hyper-cross-linked polymer derived porous carbon nanotubes with tailored selectivity for oxygen reduction reaction. Carbon 2023, 202, 81–89. [Google Scholar] [CrossRef]
- Wee, J.-H.; Kim, C.H.; Lee, H.-S.; Choi, G.B.; Kim, D.-W.; Yang, C.-M.; Kim, Y.A. Enriched Pyridinic Nitrogen Atoms at Nanoholes of Carbon Nanohorns for Efficient Oxygen Reduction. Sci. Rep. 2019, 9, 20170. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Jiang, Z.Q.; Chen, W.H. The role of holes in improving the performance of nitrogen-doped holey graphene as an active electrode material for supercapacitor and oxygen reduction reaction. J. Power Sources 2014, 251, 55–65. [Google Scholar] [CrossRef]
- Bian, Y.R.; Wang, H.; Hu, J.T.; Liu, B.W.; Liu, D.; Dai, L.M. Nitrogen-rich holey graphene for efficient oxygen reduction reaction. Carbon 2020, 162, 66–73. [Google Scholar] [CrossRef]
- Ye, G.; Liu, S.; Huang, K.; Wang, S.; Zhao, K.; Zhu, W.; Su, Y.; Wang, J.; He, Z. Domain-Confined Etching Strategy to Regulate Defective Sites in Carbon for High-Efficiency Electrocatalytic Oxygen Reduction. Adv. Funct. Mater. 2022, 32, 2111396. [Google Scholar] [CrossRef]
- Yan, D.; Guo, L.; Xie, C.; Wang, Y.; Li, Y.; Li, H.; Wang, S. N, P-dual doped carbon with trace Co and rich edge sites as highly efficient electrocatalyst for oxygen reduction reaction. Sci. China Mater. 2018, 61, 679–685. [Google Scholar] [CrossRef]
- Yu, L.; Tang, L.; Guo, W.; Li, C.; Shin, D.; Liu, Z.; Lin, Y. Disclosing the natures of carbon edges with gradient nanocarbons for electrochemical hydrogen peroxide production. Matter 2022, 5, 1909–1923. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, L.; Ma, J.; Wang, Y.; Dai, L.; Zhang, J. Edge-doping modulation of N, P-codoped porous carbon spheres for high-performance rechargeable Zn-air batteries. Nano Energy 2019, 60, 536–544. [Google Scholar] [CrossRef]
- Jin, C.; Deng, H.; Zhang, J.; Hao, Y.; Liu, J. Jagged carbon nanotubes from polyaniline: Strain-driven high-performance for Zn-air battery. Chem. Eng. J. 2022, 434, 134617. [Google Scholar] [CrossRef]
- Xia, W.; Tang, J.; Li, J.; Zhang, S.; Wu, K.C.-W.; He, J.; Yamauchi, Y. Defect-Rich Graphene Nanomesh Produced by Thermal Exfoliation of Metal–Organic Frameworks for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 13354–13359. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Y.; Zhang, C.; Sun, Q.; Cheng, D.; Huang, X.; Feng, J.; Wan, J.; Zou, J.; Liu, C.; et al. Superstructured Macroporous Carbon Rods Composed of Defective Graphitic Nanosheets for Efficient Oxygen Reduction Reaction. Adv. Sci. 2021, 8, e2100120. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, X.; Song, Y.; Huang, G.; Wei, J.; Song, X.; Xiao, Q.; Zhao, T.; Jiang, W.; Li, X.; et al. Bifunctional Edge-Rich Nitrogen Doped Porous Carbon for Activating Oxygen and Sulfur. Adv. Funct. Mater. 2023, 33, 2213395. [Google Scholar] [CrossRef]
- Chang, F.; Su, P.; Guharoy, U.; Ye, R.; Ma, Y.; Zheng, H.; Jia, Y.; Liu, J. Edge-enriched N, S co-doped hierarchical porous carbon for oxygen reduction reaction. Chin. Chem. Lett. 2023, 34, 107462. [Google Scholar] [CrossRef]
- Yang, Q.; Xiao, Z.; Kong, D.; Zhang, T.; Duan, X.; Zhou, S.; Niu, Y.; Shen, Y.; Sun, H.; Wang, S.; et al. New insight to the role of edges and heteroatoms in nanocarbons for oxygen reduction reaction. Nano Energy 2019, 66, 104096. [Google Scholar] [CrossRef]

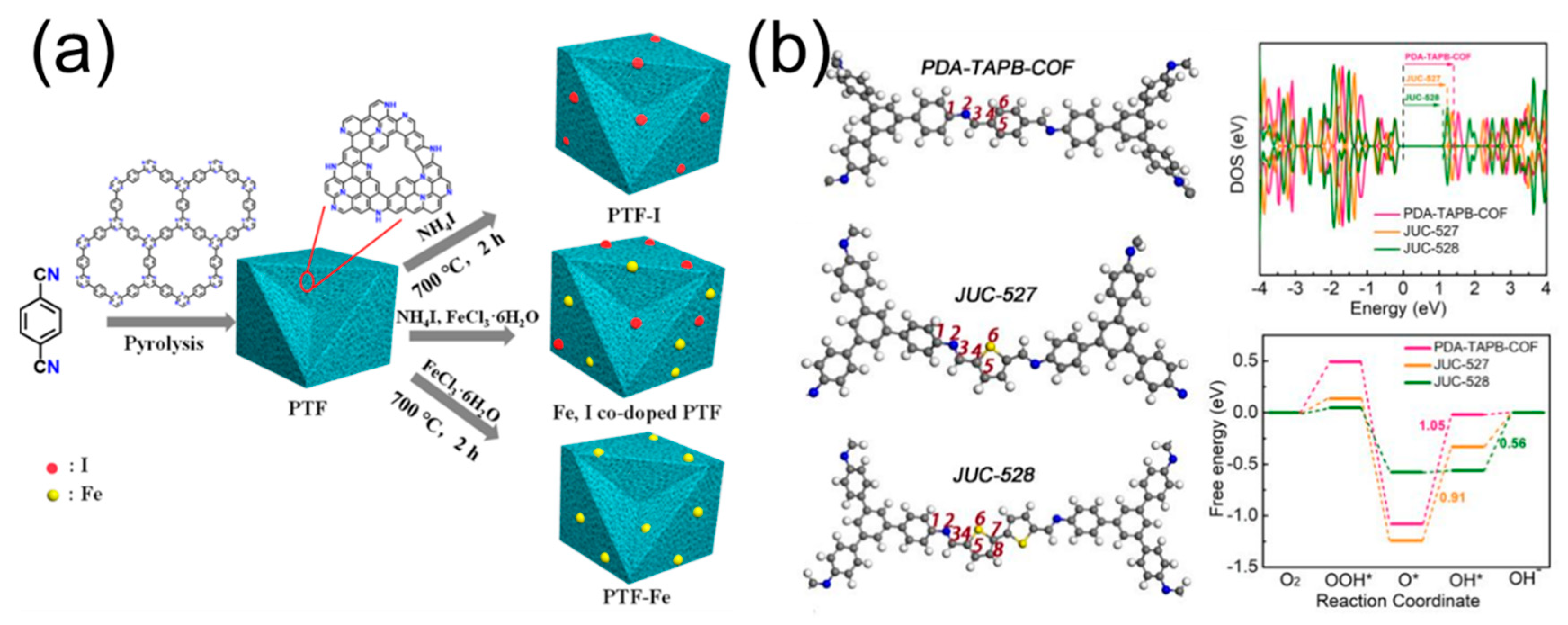
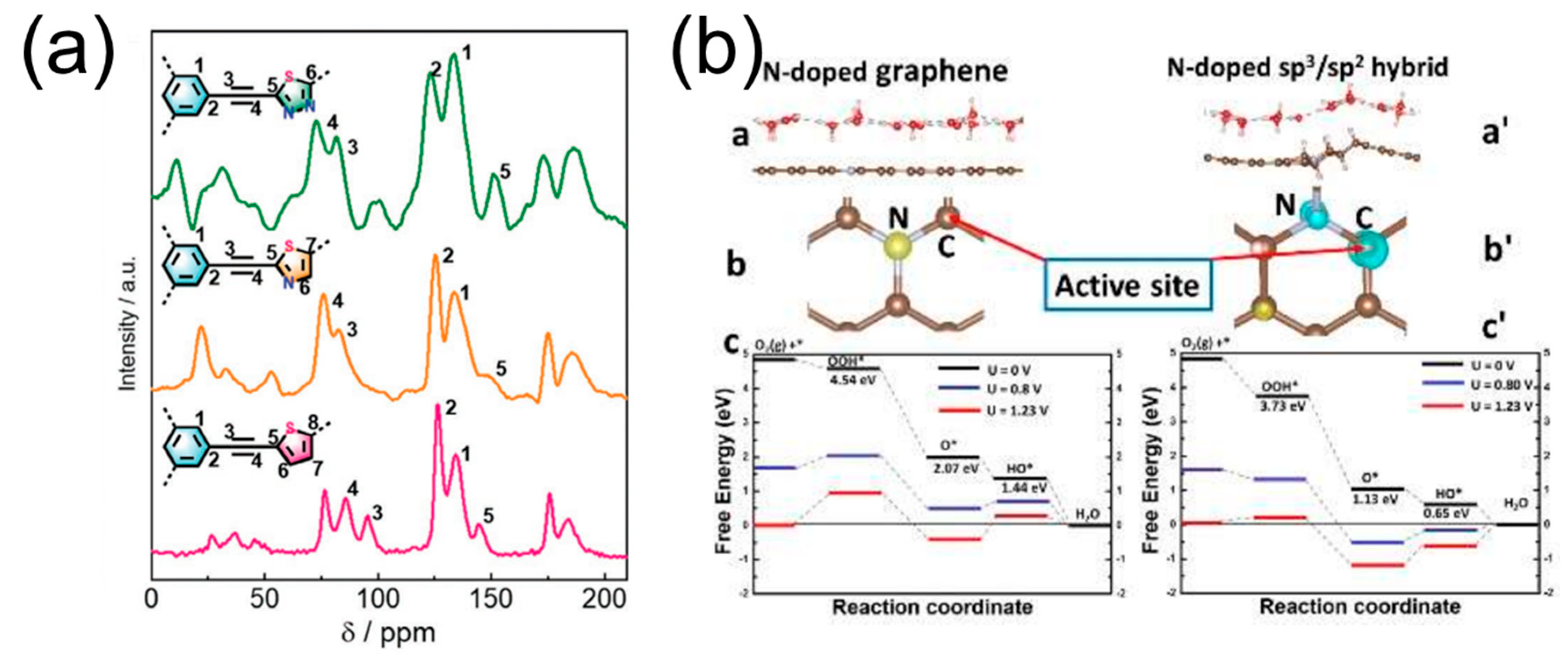
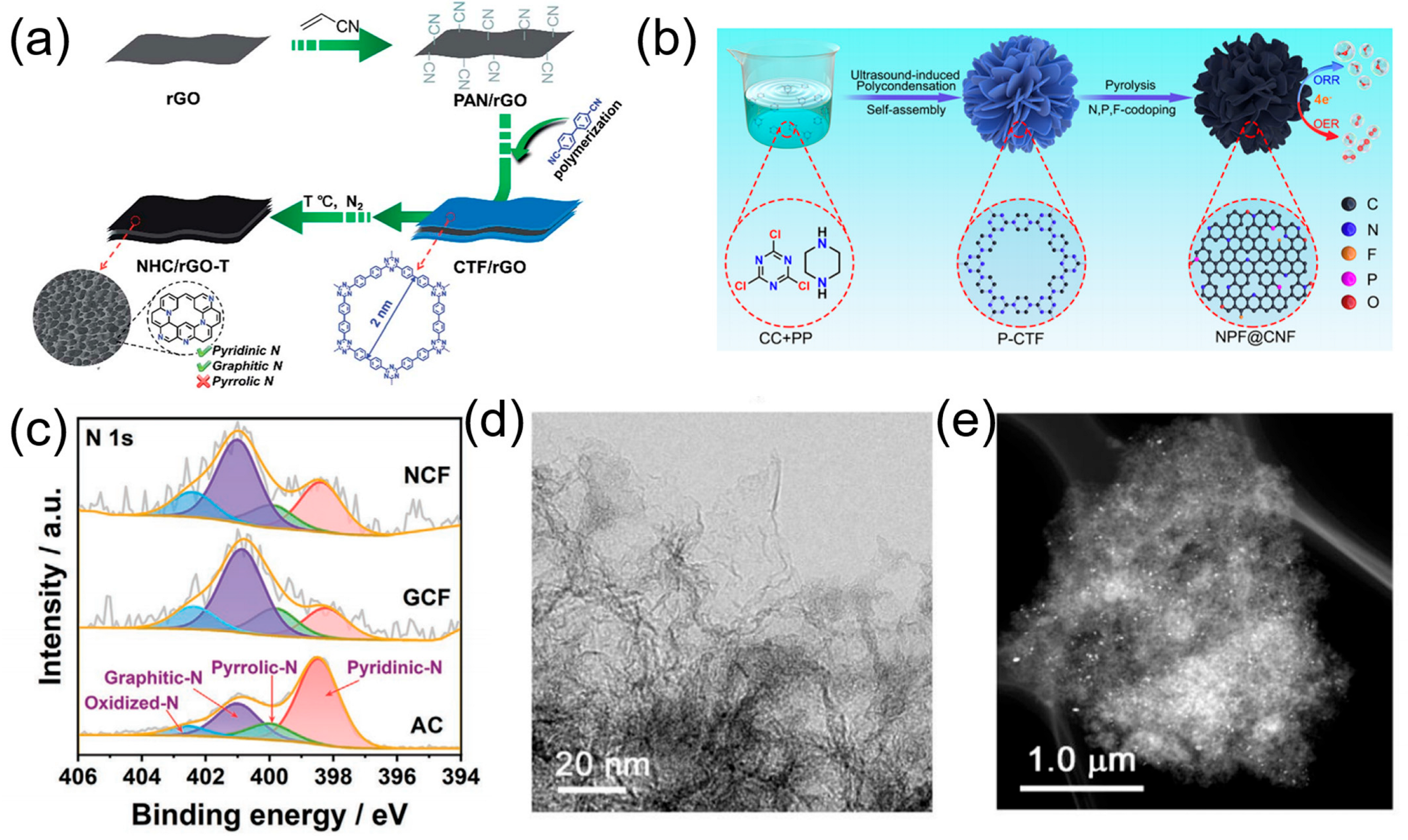
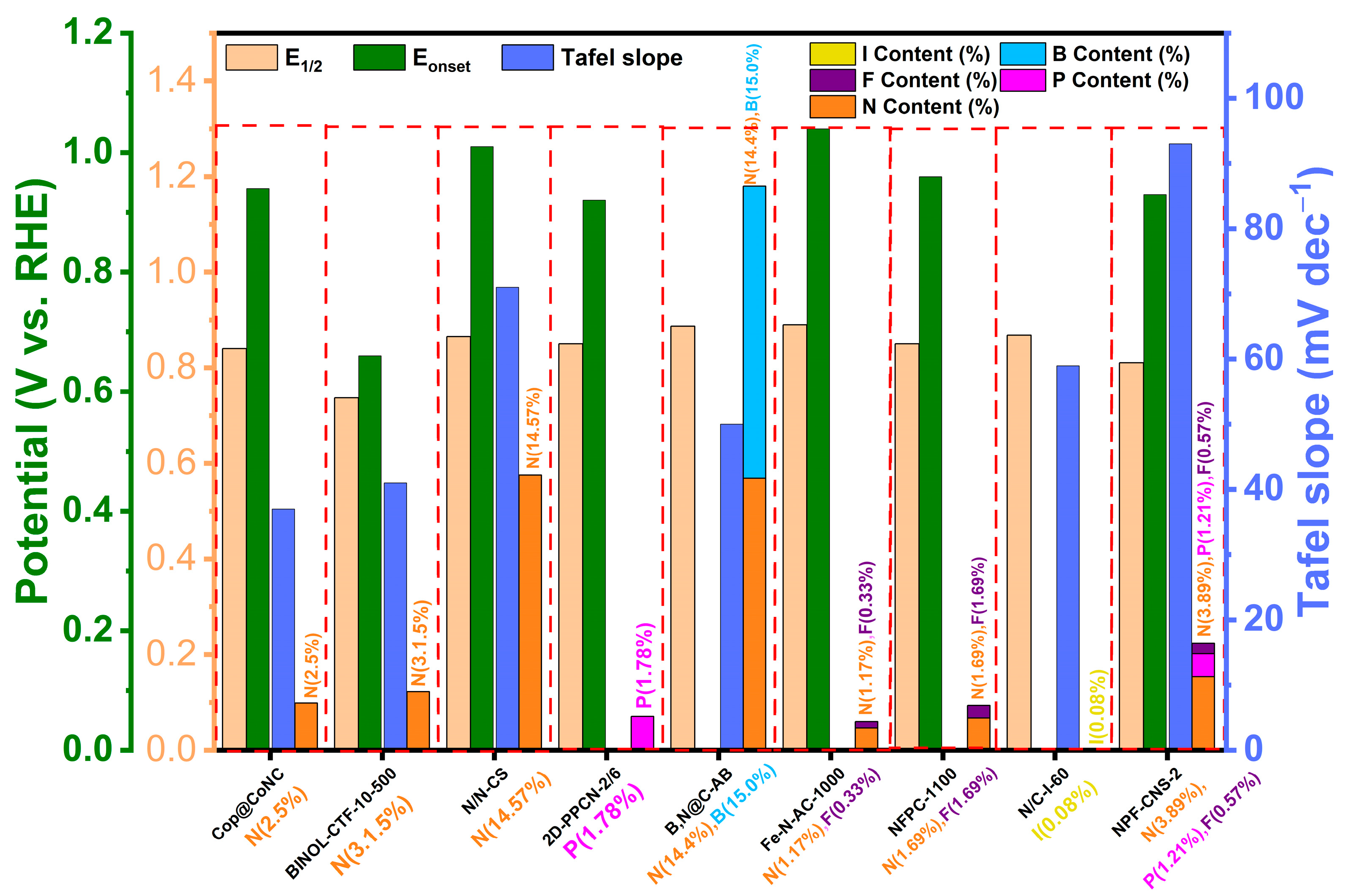
| Name of POPs-Derived Carbon | Electrolyte | Eonset/V (vs. RHE) | E1/2/V (vs. RHE) | Tafel Slope/mV dec−1 | Heteroatom Content/% | Ref. |
|---|---|---|---|---|---|---|
| COP−Ppcfe | 0.1 M HClO4 | 0.888 | 0.748 | 65.1 | NA | [26] |
| Cop@CoNC | 0.1 M KOH | 0.94 | 0.84 | 37 | ~2.5 (N) | [27] |
| N/POPQ800 | 0.1 M KOH | 0.832 | 0.728 | NA | NA | [29] |
| COF-CN | 0.1 M KOH | 0.72 | NA | 61 | NA | [30] |
| BINOL-CTF-10-500 | 0.1 M KOH | 0.66 | 0.737 | 41.04 | 3.105 (N) | [34] |
| N/N-CS | 0.1 M KOH | 1.01 | 0.865 | 71 | 14.57 (N) | [35] |
| N,P−HCNF-8 | 0.1 M KOH | 0.93 | 0.82 | 47 | 2.62–3.08 (N) | [38] |
| 2D−PPCN-2/6 | 0.1 M KOH | 0.92 | 0.85 | NA | 1.78 (P) | [39] |
| B,N@C−AB | 0.1 M KOH | NA | 0.887 | 50.2 | 14.4 (N); 15.0 (B) | [43] |
| Fe-N-AC-1000 | 0.1 M KOH | 1.04 | 0.89 | NA | 1.17 (N); 0.33 (F) | [45] |
| NFPC-1100 | 0.1 M KOH | 0.96 | 0.85 | NA | 0.68 (F); 1.69 (N) | [46] |
| N/C-I-60 | 0.1 M KOH | NA | 0.868 | 59 | 0.08 (I) | [48] |
| NPF-CNS-2 | 0.1 M KOH | 0.93 | 0.81 | 93 | 3.89 (N); 1.21 (P); 0.57 (F) | [51] |
| Name of POP-Derived Carbon | Electrolyte | Eonset/V (vs. RHE) | E1/2/V (vs. RHE) | Tafel Slope/mV dec−1 | BET SSA/m2 g−1 | Pore Volume/cm3 g−1 | Pore Size Distribution/nm | Ref. |
|---|---|---|---|---|---|---|---|---|
| N-HCNF-2-1000 | 0.1 M KOH | 1.01 | 0.84 | 111 | 368 | NA | NA | [73] |
| NHCS-2 | 0.1 M KOH | 1.002 | 0.893 | 77 | 918 | 1.18 | 27 | [75] |
| GPCNSs | 0.1 M KOH | 0.958 | 0.897 | 48 | 1342 | NA | 0.7, 1.2 and 4.0 | [76] |
| NHC/rGO-950 | 0.1 M KOH | 0.95 | 0.83 | 74 | 1344 | NA | 4–10 | [77] |
| NPF@CNF-800 | 0.1 M KOH | 0.97 | 0.85 | 88 | 533.3 | 0.349 | <1.0 | [78] |
| NCF | 0.1 M KOH | 1.00 | 0.85 | 71 | 897.5 | 1.64 | 0.7, 1.5 and 25 | [79] |
| Fe-SCNS | 0.1 M KOH | 0.99 | 0.89 | NA | 957 | 1.52 | ~20 | [80] |
| CoFe20@CC | 0.1 M KOH | 1.02 | 0.86 | 58.8 | 342 | 0.438 | NA | [81] |
| Co-N/S-DSHCN-3.5 | 0.1 M KOH | 0.989 | 0.878 | 67 | 429 | 0.4 | 2–100 | [82] |
| PA@TAPT-DHTA-COF 1000NH3 | 0.1 M KOH | 0.957 | 0.847 | 110 | 1160 | 0.59 | 0.5–6 | [86] |
| CC-3 | 0.1 M KOH | ~0.90 | 0.828 | 101 | 436 | NA | ~2.5 | [87] |
| 800-N, P-CNT | 0.1 M KOH | ~0.87 | 0.805 | NA | 181.9 | 1.26 | 16.43 | [88] |
| PYTA-TPEDH-COF | 0.1 M KOH | 0.69 | NA | 70 | 598 | 0.51 | 0.86 | [90] |
| TPP-CMP−900 | 0.1 M KOH | 0.95 | 0.83 | NA | 624 | 0.36 | 1.93 | [91] |
| Name of POP-Derived Carbon | Electrolyte | Eonset/V (vs. RHE) | E1/2/V (vs. RHE) | Tafel Slope/mV dec−1 | Defect Type | Ref. |
|---|---|---|---|---|---|---|
| PD-C | 0.1 M KOH | ~0.87 | 0.78 | NA | C5 | [93] |
| D-CM | 0.1 M KOH | ~0.90 | 0.81 | 74 | A-C5 | [94] |
| S-1-900 | 0.1 M HClO4 | ~0.92 | 0.81 | NA | C5 | [95] |
| N-O-SWNH | 0.1 M KOH | 0.91 | ~0.80 | NA | holes defects | [99] |
| N-hG6 | 0.1 M KOH | 0.91 | 0.833 | 78 | N-doping on the edge of holes | [101] |
| N/C-Br0.3 | 0.1 M KOH | ~0.95 | 0.903 | 57 | C5 | [102] |
| JCNT-0.5 | 0.1 M KOH | ~0.95 | 0.88 | 61 | zig-zag/arm-chair edge | [106] |
| PTA-1000 | 0.1 M KOH | ~0.90 | 0.78 | 74.2 | edge defects | [108] |
| COF800 | 0.1 M KOH | 0.86 | 0.79 | NA | edge defects | [109] |
| NCF | 0.1 M KOH | 1.0 | 0.85 | 71 | edge defects | [79] |
| NSCNT-6 | 0.1 M KOH | 0.92 | 0.78 | NA | edge defects | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, X.; Xin, X.; Dong, Y.; Zhao, B.; Gao, R.; Liu, T.; Li, N.; Liu, H.; Xiao, Z. Molecular Design of Porous Organic Polymer-Derived Carbonaceous Electrocatalysts for Pinpointing Active Sites in Oxygen Reduction Reaction. Molecules 2023, 28, 4160. https://doi.org/10.3390/molecules28104160
Mou X, Xin X, Dong Y, Zhao B, Gao R, Liu T, Li N, Liu H, Xiao Z. Molecular Design of Porous Organic Polymer-Derived Carbonaceous Electrocatalysts for Pinpointing Active Sites in Oxygen Reduction Reaction. Molecules. 2023; 28(10):4160. https://doi.org/10.3390/molecules28104160
Chicago/Turabian StyleMou, Xiaofeng, Xiaoyu Xin, Yanli Dong, Bin Zhao, Runze Gao, Tianao Liu, Na Li, Huimin Liu, and Zhichang Xiao. 2023. "Molecular Design of Porous Organic Polymer-Derived Carbonaceous Electrocatalysts for Pinpointing Active Sites in Oxygen Reduction Reaction" Molecules 28, no. 10: 4160. https://doi.org/10.3390/molecules28104160
APA StyleMou, X., Xin, X., Dong, Y., Zhao, B., Gao, R., Liu, T., Li, N., Liu, H., & Xiao, Z. (2023). Molecular Design of Porous Organic Polymer-Derived Carbonaceous Electrocatalysts for Pinpointing Active Sites in Oxygen Reduction Reaction. Molecules, 28(10), 4160. https://doi.org/10.3390/molecules28104160







