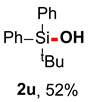Abstract
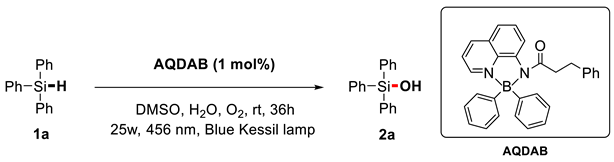
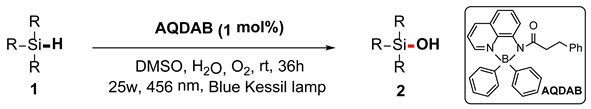
Herein, a four-coordinated organoboron compound, aminoquinoline diarylboron (AQDAB), is utilized as the photocatalyst in the oxidation of silane to silanol. This strategy effectively oxidizes Si–H bonds, affording Si–O bonds. Generally, the corresponding silanols can be obtained in moderate to good yields at room temperature under oxygen atmospheres, representing a green protocol to complement the existing preparation methods for silanols.
1. Introduction
Silanols are widely used in the silicone industry [1,2]. Furthermore, in organic synthesis [2,3], silanols also play an important role as nucleophiles in cross-coupling reactions [3,4], as directing groups to guide C–H bond activation [5,6], or as catalysts to activate the carbonyl moiety [7,8]. In the field of pharmaceutical chemistry, compounds containing the Si–OH moiety are widely used in enzyme inhibitors [9] and isosteres of pheromones [10,11]. Because of these important applications of silanol compounds, their synthesis has become the focus of continuous attention for the organic community. In the past half-century, silanol has been usually prepared by hydrolysis of chlorosilanes (Scheme 1a) [12,13], nucleophilic substitution [14] of siloxanes, or oxidation of silanes (Scheme 1b) [15,16,17,18]. However, these synthetical strategies generally require strict buffer conditions to avoid the production of siloxane, transition metal catalysts, and/or strong oxidants such as permanganates, silver salts, and osmium tetroxides. This damages the atomic economy of such strategies and limits their substrate scope and practical application [15,16,17,18,19,20,21]. Specifically, the use of transition metals can lead to products containing metal residues, which are difficult to clear away and seriously influence the bioactive application of the obtained silanols [22,23,24,25,26,27,28,29,30,31,32,33]. In this regard, the development of new strategies for silanols is highly desired, especially metal-free and more atom-economic and sustainable strategies.
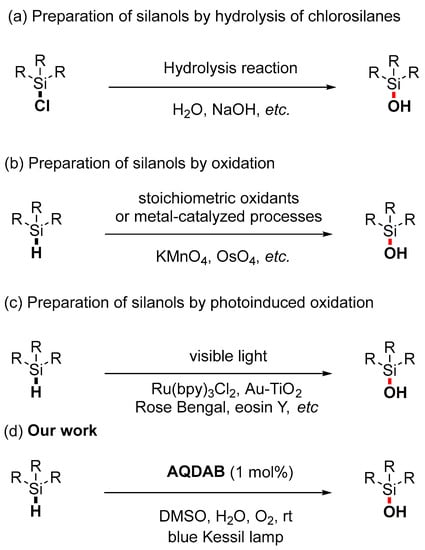
Scheme 1.
Different strategies for the synthesis of silanols. (a) Preparation of silanols by hydrolysis of chlorosilanes. (b) Preparation of silanols by oxidation. (c) Preparation of silanols by photoinduced oxidation. (d) Our developed one-pot synthesis of Silanols from Silanes.
With the development of photoredox catalysis in the last decade, the photo-induced oxidation of silane has also progressed significantly (Scheme 1c) [34,35,36,37]. In 2021, Chen, Fan, and their colleagues reported that under the irradiation of white light, Ru(bpy)3Cl2 could catalyze the formal dehydrogenative reactions between silanes and water to produce silanols [38]. In 2022, Zhang, Li, and their colleagues reported that silanes could be oxidized to silanols using Au-TiO2 as a photocatalyst [35]. In 2018, Wang reported that the conversion of silanes to silanols could be accomplished using Rose Bengal as a photocatalyst, oxygen as an oxidant, and water as an additive [37]. Subsequently, He and Zhang reported the conversion of silane to silanol using eosin Y as a photocatalyst [34]. The study of photoinduced synthesis of silanol inspired us to consider whether the photocatalyst aminoquinolate B,B-diphenyl complex AQDAB [39,40], which was developed by our group and applied in photooxidation reactions [40,41,42], could induce such transformations. Herein, the success of this hypothesis is reported. A range of diverse silanols can be obtained via the catalysis of this boron-based photocatalyst in the absence of metals and additives like strong bases, acids, and oxidants (Scheme 1d).
2. Results and Discussion
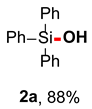
We initiated the study by investigating the hydroxylation reaction of triphenylsilane 1a (Table 1). Through screening different reaction conditions, the optimal reaction conditions are obtained as follows: aminoquinolate B,B-diphenyl complex AQDAB as the photocatalyst (1.0 mol%), O2 atmosphere, irradiation by a 456 nm blue Kessil lamp, in DMSO/H2O (1 mL/50 µL) at room temperature for 36 h (Entry 1). Under optimal conditions, triphenylsilanol 2a can be isolated with a yield of 88%. Then, the effect of each factor under these conditions was explored through control experiments. In the absence of AQDAB, O2, and light sources (Entries 2–4), the reaction will not take place. This indicates that these factors play an important role in the photocatalysis process. Using air instead of O2 caused the yield to drop to 17% (Entry 5). Then, we also explored the role of the solvent. Several other polar aprotic solvents, such as DMF and DMA, also afforded the product, albeit in lower yields (entries 6, 7). The use of DCM and MeCN as solvents results in very low reaction yields (Entries 8, 9). When the reaction time was reduced to 24 h, the yield dropped to 68% (Entry 10). This is because the triphenylsilane didn’t react completely. When white light is used as the light source, the reaction cannot proceed at all (Entry 11). Increasing or decreasing the amount of catalyst equivalent decreases the yield of product 2a (Entry 12, 13).

Table 1.
Optimization of the reaction conditions (a).
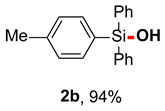
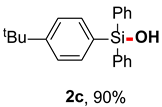
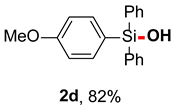
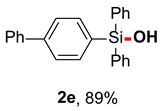
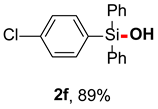
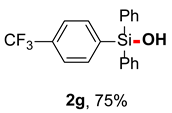
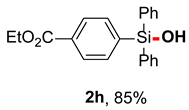
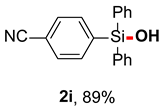
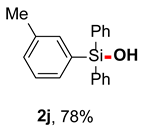
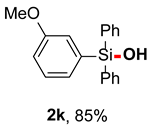
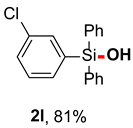
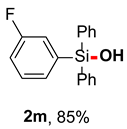
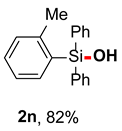
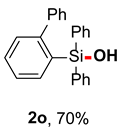
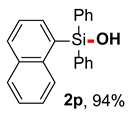
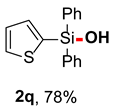
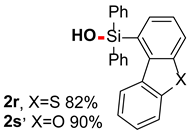
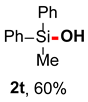
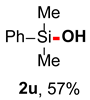
After obtaining the optimal reaction conditions, we began to explore the substrate scope of this transformation. As summarized in Table 2, generally, the reaction conditions showed good compatibility with diverse silanes and led to the corresponding silanols in moderate to excellent yields. In the beginning, triaryl silanes were explored. For triphenyl silanes, when one phenyl was substituted at para-positions, the corresponding silanols could be obtained in good to excellent yields (2b–2i, 75–94%), regardless of the electron-rich (-Me, -OMe) or electron-deficient (-CF3, -CN, -COOEt) properties of the attached substituents. In addition, the meta-substituted triphenyl silanes could also be converted into desired products with good yields (2j–2m, 78–85%). The compatibility with chloride and cyano groups also provided powerful scaffolds to enable further decoration of the obtained silanols. In addition, diphenyl(o-tolyl)silanol 2n was obtained in 82% yield, and [1,1′-biphenyl]-2-yldiphenylsilanol 2o with a sterically bulky group was obtained in 70% yield, demonstrating this protocol was not sensitive to the steric environment of the silicon-centers. In addition to phenyl-substituted silanes, naphthyl substrate also led to high yield (2p, 94%). Heteroaromatic substituents, such as thiophene, dibenzothiophene, and dibenzofuran cycles, had also been found to be compatible with this photooxidation process, resulting in products 2q–2s in 78–90% yields. Finally, methyldiphenyl silane and tert-butyldiphenyl silane were also effective substrates to generate silanols 2t and 2u in 60% and 52% yields, respectively. Dimethyl(phenyl)silanol 2v could also be obtained in a 57% yield.

Table 2.
Substrate scope of silanes.
The success of this photooxidation process prompted us to investigate the possibility of a larger-scale synthesis. Delightedly, taking triphenylsilane (1a) as a prototype, the yield of triphenylsilanol (2a) was 82% when the oxidation was performed using gram-scale starting materials (Scheme 2). This could prove the efficiency and demonstrate the application potential of this protocol.
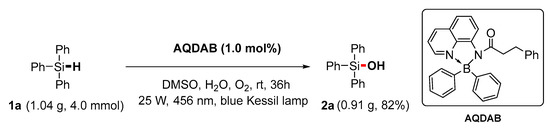
Scheme 2.
Gram-scale photocatalytic oxidation reaction.
Subsequently, we conducted a series of controlled experiments to elucidate the mechanism of this transformation. First, when the radical quenchers TEMPO (2,2,6,6-tetramethyl-1-piperi-dinyloxy) or BHT (butylated hydroxytoluene) were present in the mixture, the target product 2a could be obtained in only 23% isolated yield, implying that free radical species might be involved in the reaction pathway (Scheme 3a,b). When the reaction was performed under N2, the reaction could not proceed at all (Scheme 3c), indicating oxygen could participate in the reaction. When newly-opened dry DMSO was used as the solvent, the isolated yield decreased to 15% (Scheme 3d), showing H2O might also play an important role in the conversion from silanes to silanols. Furthermore, an 18O labeling experiment was carried out using triphenylsilane (1a) as the substrate with H218O under standard conditions (Scheme 3e). HRMS (ESI) analysis [see Supplementary Materials; m/z calcd for C18H1518OSi− (M − H)− 277.0940, found 277.0938; m/z calcd for C18H15OSi− (M − H)− 275.0898, found 275.0900] clearly verified H2O and O2 as the oxygen sources. Moreover, the on and off reaction of light showed that light was always required to promote the formation of the product during the reaction process (Figure 1).
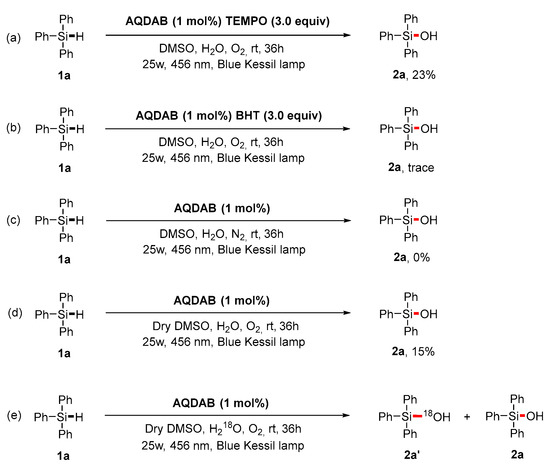
Scheme 3.
Mechanistic studies. (a,b) Radical trap experiments. (c,d) Control experiment. (e) 18O labeling experiment.
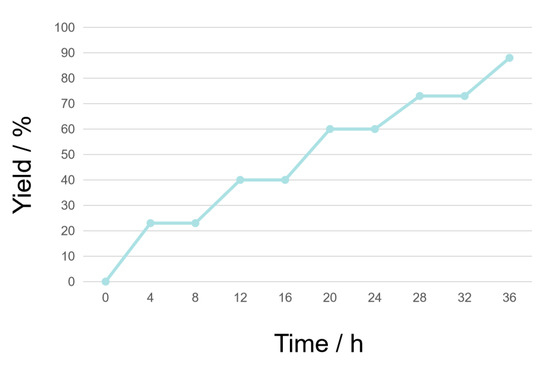
Figure 1.
Summary of the on and off reaction of light.
Based on the above observations and previous reports [43,44], a plausible mechanism for this photooxidation process was proposed, which is shown in Scheme 4. First, AQADB was excited to generate AQADB* species under visible light irradiation. Then, AQADB* interacted with 3O2 to generate 1O2 through the energy transfer (ET) process. Through this pathway, the excited state of the used photocatalyst AQADB* returned to its ground state. Subsequently, the generated 1O2 would react directly with silanes 1a, abstracting a hydrogen atom and forming a transient silil radical A plus a hydroperoxy radical HOO•. These two radical species would recombine to generate the Si–O bond, leading to the production of silylperoxide B. H2O might act as a nucleophile to attack silylperoxide B, thus forming a pentavalent ate complex C, which could decompose into silanol 2. The proposal that C was involved in was based on the observed different yields between the reactions with or without external water.
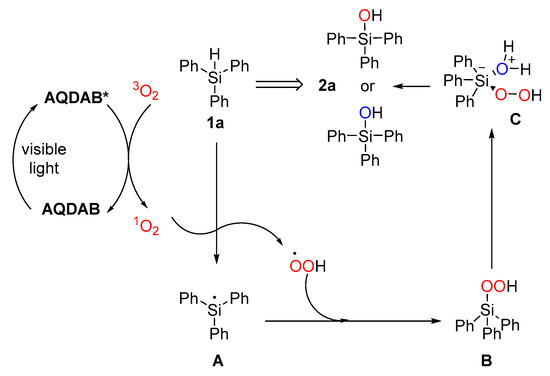
Scheme 4.
A plausible reaction mechanism.
3. Materials and Methods
3.1. Materials and Instruments
Unless otherwise noted, all the reactions of silanes to silanes were carried out under an oxygen atmosphere and a 25 W blue kessil lamp, as well as room temperature. Analytical thin layer chromatography (TLC) was performed on a glass plate uniformly coated with 0.25 mm 230–400 mesh silica gel containing a fluorescence indicator. Visualization was accomplished by exposure to a UV lamp. All the products in this article are compatible with standard silica gel chromatography. Column chromatography was performed on silica gel (200–300 mesh) using standard methods. NMR spectra were measured on a Bruker Ascend 400 spectrometer, and chemical shifts (δ) are reported in parts per million (ppm). 1H NMR spectra were recorded at 400 MHz in NMR solvents and referenced internally to the corresponding solvent resonance; 13C NMR spectra were recorded at 101 MHz and referenced to the corresponding solvent resonance; 19F NMR spectra were recorded at 376 MHz and referenced to corresponding solvent resonance. Coupling constants are reported in Hz, with multiplicities denoted as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad). Commercial reagents and solvents were purchased from Adamas, J&K, Energy, Sigma-Aldrich, Alfa Aesar, Acros Organics, Innochem, Matrix, Trc, Apinno, Macklin, Ark, Aladdin, Achem-block, Acmec, Coolpharm, Key Organics, and TCI and used as received unless otherwise stated.
3.2. General Procedure for the Synthesis of Silanols
A flame-dried 25-mL quartz reaction tube was placed on a magnetic stir bar. Then, silane 1 (0.2 mmol, 1.0 equiv.) were added to the flame-dried 25 mL quartz reaction tube, A triple oxygen replacement process was then performed using a double row of tubes. After that, a mixture of AQDAB (0.9 mg, 0.002 mmol, 1.0 mol%), DMSO (1 mL) and H2O (50 μL) was rapidly added into the flame-dried 25 mL quartz reaction tube. The reaction tube was placed on a 25 w blue Kessil reactor. Then the reaction mixture was stirred at 400–500 RPM and exposed to a blue case lamp at room temperature for 36 h. After taking out the reaction tube, transfer the reaction mixture to the separator funnel and add 10 mL of water to the separator funnel. Then, the reaction mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic phase was washed with brine (2 × 5.0 mL) and then dried over anhydrous Na2SO4. After concentration, the silanol crude product was purified by column chromatography (silica gel) to give silanol 2, using petroleum ether/ethyl acetate (20:1) as the eluent.
3.3. General Procedure for the Synthesis of AQDAB
The preparation methods of photocatalyst AQDAB used in this paper are methods disclosed by us in the previous literature [39,41]. In order to facilitate the synthesis of AQDAB, the preparation process of AQDAB was recorded in detail in this paper. In addition, UV-vis, CV, and fluorescence data are disclosed in the references [39].
3.3.1. Method A for the Synthesis of AQDAB
A flame-dried 25-mL quartz reaction tube was placed on a magnetic stir bar. After that, 3-phenyl-N-(quinolin-8-yl)propanamide (41.4 mg, 0.15 mmol, 1.0 equiv), phenyl trifluoroborate (138.0 mg, 0.75 mmol, 5.0 equiv), Mn (24.7 mg, 0.45 mmol, 3.0 equiv), 4-toluenesulfonyl chloride (71.5 mg, 0.375 mmol, 2.5 equiv.), Na2CO3 (7.9 mg, 0.075 mmol, 0.5 equiv) and CH3CN (1.5 mL) were added. Then the reaction mixture was stirred at 400–500 RPM at 130 °C for 24 h. After concentration, the AQDAB crude product was purified by column chromatography (silica gel) to give 62.7 mg of the photocatalyst in 95% yield, using petroleum ether/ethyl acetate (3:1) as the eluent.
3.3.2. Method B for the Synthesis of AQDAB
A flame-dried 125-mL quartz reaction tube was placed on a magnetic stir bar. Then, 3-phenyl-N-(quinolin-8-yl)propanamide (276.3 mg, 1.0 mmol, 1.0 equiv.), phenylboronic acid (1100.0 mg, 9.0 mmol, 9.0 equiv.), K3PO4 (636.8 mg, 3.0 mmol, 3.0 equiv.) and 1,4-dioxane (15 mL) were added. After that, the reaction mixture was stirred at 300–400 RPM at 130 °C for 36 h. After concentration, the AQDAB crude product was purified by column chromatography (silica gel) to give 286.2 mg of the photocatalyst in 65% yield, using petroleum ether/ethyl acetate (3:1) as the eluent.
3.3.3. Characterization Data of the AQDAB
1H NMR (400 MHz, CDCl3) δ 8.99 (d, J = 7.6 Hz, 1H), 8.43 (dd, J = 5.2, 0.8 Hz, 1H), 8.38 (d, J = 8.4 Hz, 1H), 7.80 (t, J = 8.4 Hz, 1H), 7.56−7.52 (m, 1H), 7.52−7.46 (m, 5H), 7.30−7.24 (m, 6H), 7.13 (t, J = 7.2 Hz, 2H), 7.10−7.03 (m, 1H), 6.83 (d, J = 6.8 Hz, 2H), 2.60 (dd, J = 9.5, 4.9 Hz, 2H), 2.57−2.49 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 176.2, 142.0, 141.5, 139.5, 139.1, 137.7, 133.5, 132.6, 128.5, 128.1, 127.9, 127.6, 127.2, 125.5, 122.5, 119.0, 117.2, 39.9, 31.5.
3.4. General Procedure for the Synthesis of Starting Materials [45]
3.4.1. The Synthesis of the Starting Materials of 2a–2f, 2j, 2k, 2m–2r, 2v
A flame-dried 100-mL round-bottom flask was placed on a magnetic stir bar. Three nitrogen replacement operations were performed on the round-bottomed flask. Then aryl bromide (5.0 mmol, 1.0 equiv.) was dissolved in THF (10 mL) and injected into a round-bottomed flask. After that, the round-bottomed flask is placed on the cryogenic reactor and cooled to −78 °C. (-BuLi (3.2 mL, 1.6 M THF solution, 6.0 mmol, 1.2 equiv.) was slowly injected into a round-bottomed flask over 30 min. The reaction mixture was stirred at 400–500 RPM at −78 °C for 2 h. Then slow injection of chlorodiphenylsilane (6.0 mmol, 1.2 equiv.) into a round-bottomed flask. Heat the reactor to room temperature and stir overnight. The reaction mixture was quenched with NH4Cl (15 mL, saturated aqueous solution), and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic phase was washed with brine (2 × 5.0 mL) and then dried over anhydrous Na2SO4. After concentration, the crude product was purified by column chromatography (silica gel) to give silane, using petroleum ether/ethyl acetate (200:1) as the eluent.
3.4.2. The Synthesis of the Starting Materials of 2g–2i, 2l
A flame-dried 100-mL round-bottom flask was placed on a magnetic stir bar. Three nitrogen replacement operations were performed on the round-bottomed flask. Then aryl iodide (5.0 mmol, 1.0 equivalent) was dissolved in THF (10 mL) and injected into a round-bottomed flask. After that, the round-bottomed flask is placed on the cryogenic reactor and cooled to −78 °C. i-PrMgCl (3 mL, 2.0 M THF solution, 6.0 mmol, 1.2 equiv.) was slowly injected into a round-bottomed flask over 15 min. The resulting mixture was heated to −40 °C within 2 h and held at −40 °C for another 2 h. Then, slow injection of chlorodiphenylsilane (6.0 mmol, 1.2 equiv.) into a round-bottomed flask. Heat the reactor to room temperature and stir overnight. The reaction mixture was quenched with NH4Cl (15 mL, saturated aqueous solution), and the mixture was extracted with CH2Cl2 (3 × 10 mL). The combined organic phase was washed with brine (2 × 5.0 mL) and then dried over anhydrous Na2SO4. After concentration, the crude product was purified by column chromatography (silica gel) to give silane, using petroleum ether/ethyl acetate (200:1) as the eluent.
3.5. Characterization Data of Products
Triphenylsilanol (2a) [45]: Following the General Procedure with triphenylsilane (52.0 mg, 0.2 mmol), 2a was obtained as colorless oil (48.6 mg, 88%).1H NMR (400 MHz, CDCl3) δ 7.71–7.61 (m, 6H), 7.49–7.44 (m, 3H), 7.43–7.36 (m, 6H), 2.63 (s, 1H).13C NMR (101 MHz, CDCl3) δ 135.1, 135.0, 130.1, 127.9.
Diphenyl(p-tolyl)silano (2b) [45]: Following the General Procedure with diphenyl(p-tolyl)silane (54.8 mg, 0.2 mmol), 2b was obtained as pale yellow oil (54.5 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 7.62–7.55 (m, 4H), 7.52–7.47 (m, 2H), 7.43–7.37 (m, 2H), 7.36–7.30 (m, 4H), 7.17 (d, J = 7.6 Hz, 2H), 2.85 (s, 1H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 140.0, 135.3, 135.0, 134.9, 131.4, 129.9, 128.6, 127.7, 21.5.
(4-(tert-butyl)phenyl)diphenylsilanol (2c) [45]: Following the General Procedure with (4-(tert-butyl)phenyl)diphenylsilane (62.2 mg, 0.2 mmol), 2c was obtained as colorless oil (59.8 mg, 90%). 1H NMR (400 MHz, CDCl3) δ 7.69–7.63 (m, 4H), 7.61–7.57 (m, 2H), 7.49–7.36 (m, 8H), 2.69 (s, 1H), 1.34 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 153.1, 135.4, 135.0, 134.9, 131.6, 130.0, 127.9, 124.9, 34.8, 31.2.
(4-methoxyphenyl)diphenylsilanol (2d) [45]: Following the General Procedure with (4-methoxyphenyl)diphenylsilane (58.0 mg, 0.2 mmol), 2d was obtained as pale yellow oil (50.0 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 7.63–7.58 (m, 4H), 7.54–7.50 (m, 2H), 7.39–7.32 (m, 6H), 6.93–6.87 (m, 2H), 3.79 (s, 3H), 2.66 (s, 1H).13C NMR (101 MHz, CDCl3) δ 161.2, 136.6, 135.5, 134.9, 130.0, 127.9, 126.1, 113.7, 55.0.
[1,1’-biphenyl]-4-yldiphenylsilanol (2e) [45]: Following the General Procedure with [1,1’-biphenyl]-4-yldiphenylsilane (67.2 mg, 0.2 mmol), 2e was obtained as white solid (62.7 mg, 89%). 1H NMR (400 MHz, CDCl3) δ 7.76–7.68 (m, 6H), 7.67–7.61 (m, 4H), 7.53–7.46 (m, 4H), 7.46–7.36 (m, 5H), 3.08 (s, 1H).13C NMR (101 MHz, CDCl3) δ 142.7, 140.8, 135.5, 135.1, 135.0, 133.8, 130.1, 128.8, 127.9, 127.5, 127.1, 126.6.
2-methyl-4-phenylquinoline (2f) [45]: Following the General Procedure with (4-chlorophenyl)diphenylsilane (58.8 mg, 0.2 mmol), 2f was obtained as pale yellow oil (52.7 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.64–7.57 (m, 4H), 7.56–7.44 (m, 4H), 7.42–7.33 (m, 6H), 3.16 (s, 1H).13C NMR (101 MHz, CDCl3) δ 136.5, 136.3, 134.9, 134.6, 133.5, 130.2, 128.1, 128.0.
Diphenyl(4-(trifluoromethyl)phenyl)silanol (2g) [45]: Following the General Procedure with diphenyl(4-(trifluoromethyl)phenyl)silane (65.6 mg, 0.2 mmol), 2g was obtained as pale yellow oil (51.6 mg, 75%). 1H NMR (400 MHz, CDCl3) δ 7.75 (d, J = 7.6 Hz, 2H), 7.65–7.57 (m, 6H), 7.51–7.45 (m, 2H), 7.44–7.36 (m, 4H), 2.92 (s, 1H).13C NMR (101 MHz, CDCl3) δ 140.1, 135.2, 134.9, 134.2, 131.9 (q, J = 32.2 Hz), 130.5, 128.1, 124.4 (q, J = 3.7 Hz), 124.1 (q, J = 273.7 Hz). 19F NMR (376 MHz, CDCl3) δ −62.91.
Ethyl 4-(hydroxydiphenylsilyl)benzoate (2h) [45]: Following the General Procedure with ethyl 4-(diphenylsilyl)benzoate (66.4 mg, 0.2 mmol), 2h was obtained as colorless oil (59.2 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 8.04–7.96 (m, 2H), 7.73–7.68 (m, 2H), 7.65–7.58 (m, 4H), 7.48–7.35 (m, 6H), 4.36 (q, J = 7.2 Hz, 2H), 3.59 (s, 1H), 1.38 (t, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 166.8, 141.3, 134.90, 134.88, 134.6, 131.5, 130.2, 128.5, 127.9, 61.1, 14.2.
4-(hydroxydiphenylsilyl)benzonitrile (2i) [45]: Following the General Procedure with methyl 4-(diphenylsilyl)benzonitrile (57.0 mg, 0.2 mmol), 2i was obtained as colorless oil (51.78 mg, 86%). 1H NMR (400 MHz, CDCl3) δ 7.70–7.69 (m, 2H), 7.64–7.54 (m, 6H), 7.51–7.25 (m, 2H), 7.43–7.36 (m, 4H), 3.38 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 142.1, 135.3, 134.8, 133.8, 131.0, 130.5, 128.1, 118.7, 113.3.
Diphenyl(m-tolyl)silanol (2j) [45]: Following the General Procedure with diphenyl(m-tolyl)silane (54.8 mg, 0.2 mmol), 2j was obtained as white solid (45.3 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 7.63–7.58 (m, 4H), 7.46–7.39 (m, 4H), 7.38–7.32 (m, 4H), 7.28–7.22 (m, 2H), 2.76 (s, 1H), 2.31 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 137.3, 135.4, 135.3, 135.0, 132.1, 130.9, 130.0, 127.9, 127.8, 21.5.
(4-methoxyphenyl)diphenylsilanol (2k) [34]: Following the General Procedure with (3-methoxyphenyl)diphenylsilane (58.0 mg, 0.2 mmol), 2k was obtained as white solid (52.0 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.67–7.58 (m, 4H), 7.45–7.40 (m, 2H), 7.39–7.34 (m, 4H), 7.33–7.28 (m, 1H), 7.21–7.12 (m, 2H), 7.00–6.93 (m, 1H), 3.75 (s, 3H), 2.61 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 159.0, 136.7, 135.00, 134.95, 130.1, 129.2, 127.9, 127.3, 120.1, 115.7, 55.1.
(3-chlorophenyl)diphenylsilanol (2l) [34]: Following the General Procedure with (3-chlorophenyl)diphenylsilane (58.8 mg, 0.2 mmol), 2l was obtained as colorless oil (50.2 mg, 81%). 1H NMR (400 MHz, CDCl3) δ 7.65–7.58 (m, 5H), 7.52–7.44 (m, 3H), 7.44–7.38 (m, 5H), 7.35–7.30 (m, 1H), 2.62 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 137.9, 134.9, 134.5, 134.4, 134.3, 134.2, 132.9, 130.4, 130.2, 129.4, 128.1.
(3-fluorophenyl)diphenylsilanol (2m) [45]: Following the General Procedure with (3-fluorophenyl)diphenylsilane (55.6 mg, 0.2 mmol), 2m was obtained as white solid (50.0 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 7.66–7.59 (m, 4H), 7.50–7.44 (m, 2H), 7.43–7.31 (m, 7H), 7.17–7.10 (m, 1H), 2.97 (s, 1H).13C NMR (101 MHz, CDCl3) δ 162.5 (d, J = 248.6 Hz), 138.3 (d, J = 4.2 Hz), 134.9, 134.4, 130.5 (d, J = 3.1 Hz), 130.3, 129.8 (d, J = 6.9 Hz), 128.0, 121.3 (d, J = 19.1 Hz), 117.1 (d, J = 21.1 Hz). 19F NMR (376 MHz, CDCl3) δ −113.04.
Diphenyl(o-tolyl)silanol (2n) [45]: Following the General Procedure with diphenyl(o-tolyl)silane (54.8 mg, 0.2 mmol), 2n was obtained as pale yellow oil (47.6 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 7.61–7.54 (m, 4H), 7.46–7.39 (m, 3H), 7.38–7.31 (m, 5H), 7.20–7.11 (m, 2H), 2.65 (s, 1H), 2.30 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 144.6, 136.6, 135.6, 134.9, 133.5, 130.4, 130.0, 129.9 127.9, 124.8, 23.3.
[1,1′-biphenyl]-2-yldiphenylsilanol (2o) [37]: Following the General Procedure with [1,1’-biphenyl]-2-yldiphenylsilane (67.2 mg, 0.2 mmol), 2o was obtained as white solid (49.3 mg, 70%).1H NMR (400 MHz, CDCl3) δ 7.51–7.44 (m, 6H), 7.42–7.37 (m, 2H), 7.36–7.30 (m, 6H), 7.28–7.20 (m, 3H), 7.19–7.14 (m, 2H).13C NMR (101 MHz, CDCl3) δ 149.0, 143.6, 136.6, 136.4, 134.8, 134.1, 129.8, 129.72, 129.69, 129.0, 128.2, 127.8, 127.5, 126.3.
Naphthalen-1-yldiphenylsilanol (2p) [45]: Following the General Procedure with naphthalen-1-yldiphenylsilane (62.0 mg, 0.2 mmol), 2p was obtained as pale yellow oil (61.3 mg, 94%). 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 8.4 Hz, 1H), 7.97 (d, J = 8.0 Hz, 1H), 7.90 (d, J = 8.0 Hz, 1H), 7.72–7.63 (m, 5H), 7.49–7.36 (m, 9H), 2.84 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 137.1, 136.5, 135.7, 135.0, 133.4, 132.9, 131.1, 130.1, 128.9, 128.8, 128.0, 126.1, 125.6, 125.0.
Diphenyl(thiophen-2-yl)silanol (2q) [45]: Following the General Procedure with diphenyl(thiophen-2-yl)silane (53.2 mg, 0.2 mmol), 2q was obtained as colorless oil (44.0 mg, 78%). 1H NMR (400 MHz, CDCl3) δ 7.71–7.54 (m, 5H), 7.44–7.24 (m, 7H), 7.22–7.14 (m, 1H), 3.06 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 137.6, 134.9, 134.7, 134.4, 132.5, 130.3, 128.3, 127.9.
Dibenzo[b,d]thiophen-4-yldiphenylsilanol (2r) [45]: Following the General Procedure with dibenzo[b,d]thiophen-4-yldiphenylsilane (73.2 mg, 0.2 mmol), 2r was obtained as white solid (62.7 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 8.25 (d, J = 8.0 Hz, 1H), 8.20–8.15 (m, 1H), 7.77–7.69 (m, 5H), 7.65 (d, J = 7.2 Hz, 1H), 7.52–7.39 (m, 9H), 3.07 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 145.8, 139.7, 135.2, 134.9, 134.8 134.0, 130.4, 129.4, 128.0, 126.6, 124.2, 123.8, 123.5, 122.6, 121.4.
Dibenzo[b,d]furan-4-yldiphenylsilanol (2s) [45]: Following the General Procedure with dibenzo[b,d]furan-4-yldiphenylsilane (70.0 mg, 0.2 mmol), 2s was obtained as white solid (65.9 mg, 90%). 1H NMR (400 MHz, CDCl3) δ 8.07 (d, J = 7.6 Hz, 1H), 7.99 (d, J = 7.6 Hz, 1H), 7.74 (d, J = 8.0 Hz, 4H), 7.56–7.35 (m, 11H), 3.41 (s, 1H).13C NMR (101 MHz, CDCl3) δ 160.8, 155.9, 135.0, 134.7, 134.2, 130.3, 127.9, 127.1, 123.9, 123.1, 122.8, 122.77, 122.69, 120.6, 118.2, 111.8.
Methyldiphenylsilanol (2t) [45]: Following the General Procedure with methyldiphenylsilane (39.6 mg, 0.2 mmol), 2t was obtained as colorless oil (25.7 mg, 60%). 1H NMR (400 MHz, CDCl3) δ 7.64–7.60 (m, 4H), 7.44–7.38 (m, 6H), 2.40 (s, 1H), 0.68 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 137.0, 133.9, 129.9, 127.9, −1.3.
Tert-butyldiphenylsilanol (2u) [37]: Following the General Procedure with tert-butyldiphenylsilane (48.0 mg, 0.2 mmol), 2t was obtained as colorless oil (26.6 mg, 52%). 1H NMR (400 MHz, CDCl3) δ 7.77–7.70 (m, 4H), 7.44–7.36 (m, 6H), 2.17 (s, 1H), 1.08 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 135.1, 134.8, 129.6, 127.7, 26.5, 19.0.
Dimethyl(phenyl)silanol (2v) [45]: Following the General Procedure with dimethyl(phenyl)silane (27.2 mg, 0.2 mmol), 2u was obtained as colorless oil (17.3 mg, 57%). 1H NMR (400 MHz, CDCl3) δ 7.63–7.58 (m, 2H), 7.42–7.36 (m, 3H), 1.90 (s, 1H), 0.41 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 139.1, 133.0, 129.6, 127.9, 0.0.
4. Conclusions
In conclusion, we have developed a photocatalytic oxidation strategy to achieve silanol synthesis. Four-coordinate aminoquinolate diarylboron compounds are used as photocatalysts for this conversion, which can produce 1O under visible light irradiation. This transformation bypasses the use of noble metal-based photocatalysts or oxidants. The boron-based photocatalyst is demonstrated herein to be a sustainable supplement to the noble metal-based photocatalysts. Research on its further application is also underway in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28104082/s1, copies of 1H-NMR, 13C-NMR, and 19F-NMR spectra of the products are included in the Supplementary Materials.
Author Contributions
Investigation, J.Y. and X.C.; Project administration, J.Z. (Jianshu Zhang) and J.Z. (Jinli Zhang); Validation, L.W.; Writing—original draft, L.X.; Writing—review and editing, P.L. (Ping Liu) and P.L. (Pengfei Li). All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (21963010) funded this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 2a–2v are available from the authors.
References
- Chandrasekhar, V.; Boomishankar, R.; Nagendran, S. Recent developments in the synthesis and structure of organosilanols. Chem. Rev. 2004, 104, 5847–5910. [Google Scholar] [CrossRef]
- Murugavel, R.; Voigt, A.; Walawalkar, M.G.; Roesky, H.W. Hetero-and metallasiloxanes derived from silanediols, disilanols, silanetriols, and trisilanols. Chem. Rev. 1996, 96, 2205–2236. [Google Scholar] [CrossRef]
- Denmark, S.E.; Regens, C.S. Palladium-catalyzed cross-coupling reactions of organosilanols and their salts: Practical alternatives to boron-and tin-based methods. Acc. Chem. Res. 2008, 41, 1486–1499. [Google Scholar] [CrossRef]
- Denmark, S.E. The interplay of invention, discovery, development, and application in organic synthetic methodology: A case study. J. Org. Chem. 2009, 74, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Mewald, M.; Schiffner, J.A.; Oestreich, M. A New Direction in C–H Alkenylation: Silanol as a Helping Hand. Angew. Chem. Int. Ed. 2012, 51, 1763–1765. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, G.; Breit, B. Entfernbare dirigierende Gruppen in der organischen Synthese und Katalyse. Angew. Chem. 2011, 123, 2498–2543. [Google Scholar] [CrossRef]
- Tran, N.T.; Min, T.; Franz, A.K. Silanediol hydrogen bonding activation of carbonyl compounds. Chem. A Eur. J. 2011, 17, 9897–9900. [Google Scholar] [CrossRef]
- Schafer, A.G.; Wieting, J.M.; Mattson, A.E. Silanediols: A new class of hydrogen bond donor catalysts. Org. Lett. 2011, 13, 5228–5231. [Google Scholar] [CrossRef]
- Franz, A.K.; Wilson, S.O. Organosilicon molecules with medicinal applications. J. Med. Chem. 2013, 56, 388–405. [Google Scholar] [CrossRef]
- Tacke, R.; Schmid, T.; Hofmann, M.; Tolasch, T.; Francke, W. Sila-linalool as a pheromone analogue: A study on C/Si bioisosterism. Organometallics 2003, 22, 370–372. [Google Scholar] [CrossRef]
- Kim, J.K.; Sieburth, S.M. Synthesis and properties of a sterically unencumbered δ-silanediol amino acid. J. Org. Chem. 2012, 77, 2901–2906. [Google Scholar] [CrossRef]
- Cella, J.A.; Carpenter, J.C. Procedures for the preparation of silanols. J. Organomet. Chem. 1994, 480, 23–26. [Google Scholar] [CrossRef]
- Cho, H.M.; Jeon, S.H.; Lee, H.K.; Kim, J.H.; Park, S.; Choi, M.-G.; Lee, M.E. Facile syntheses, structural characterizations, and isomerization of disiloxane-1, 3-diols. J. Organomet. Chem. 2004, 689, 471–477. [Google Scholar] [CrossRef]
- McN, S. The study of methods to synthesize silylated calixarenes in the 1, 3-alternate conformation. J. Org. Chem 1993, 58, 7584–7586. [Google Scholar]
- Spialter, L.; Austin, J.D. Ozone: A New Cleavage Reagent for Organosilanes. J. Am. Chem. Soc. 1965, 87, 4406. [Google Scholar] [CrossRef]
- Sommer, L.; Ulland, L.A.; Parker, G. Stereochemistry of asymmetric silicon. XX. Hydroxylation and carbene insertion reactions of R3SiH. J. Am. Chem. Soc. 1972, 94, 3469–3471. [Google Scholar] [CrossRef]
- Adam, W.; Mello, R.; Curci, R. O-Atom Insertion into Si–H Bonds by Dioxiranes: A Stereospecific and Direct Conversion of Silanes into Silanols. Angew. Chem. Int. Ed. Engl. 1990, 29, 890–891. [Google Scholar] [CrossRef]
- Valliant-Saunders, K.; Gunn, E.; Shelton, G.R.; Hrovat, D.A.; Borden, W.T.; Mayer, J.M. Oxidation of tertiary silanes by osmium tetroxide. Inorg. Chem. 2007, 46, 5212–5219. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Lucas, R. Oxidation of sterically hindered organosilicon hydrides using potassium permanganate. J. Organomet. Chem. 1996, 521, 229–234. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Montanari, V.; Resnati, G. Oxyfunctionalization reactions by perfluoro cis-2, 3-dialkyloxaziridines. Enantioselective conversion of silanes into silanols. Tetrahedron Lett. 1994, 35, 6329–6330. [Google Scholar] [CrossRef]
- Spialter, L.; Pazdernik, L.; Bernstein, S.; Swansiger, W.A.; Buell, G.R.; Freeburger, M.E. Mechanism of the reaction of ozone with the silicon-hydrogen bond. J. Am. Chem. Soc. 1971, 93, 5682–5686. [Google Scholar] [CrossRef]
- Schubert, U.; Lorenz, C. Conversion of Hydrosilanes to Silanols and Silyl Esters Catalyzed by [Ph3PCuH]6. Inorg. Chem. 1997, 36, 1258–1259. [Google Scholar] [CrossRef]
- Ison, E.A.; Corbin, R.A.; Abu-Omar, M.M. Hydrogen production from hydrolytic oxidation of organosilanes using a cationic oxorhenium catalyst. J. Am. Chem. Soc. 2005, 127, 11938–11939. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ko, S.; Chang, S. Highly selective and practical hydrolytic oxidation of organosilanes to silanols catalyzed by a ruthenium complex. J. Am. Chem. Soc. 2000, 122, 12011–12012. [Google Scholar] [CrossRef]
- Lee, Y.; Seomoon, D.; Kim, S.; Han, H.; Chang, S.; Lee, P.H. Highly efficient iridium-catalyzed oxidation of organosilanes to silanols. J. Org. Chem. 2004, 69, 1741–1743. [Google Scholar] [CrossRef]
- Mitsudome, T.; Noujima, A.; Mizugaki, T.; Jitsukawa, K.; Kaneda, K. Supported gold nanoparticle catalyst for the selective oxidation of silanes to silanols in water. Chem. Commun. 2009, 35, 5302–5304. [Google Scholar] [CrossRef]
- John, J.; Gravel, E.; Hagège, A.; Li, H.; Gacoin, T.; Doris, E. Catalytic oxidation of silanes by carbon nanotube–gold nanohybrids. Angew. Chem. 2011, 33, 7675–7678. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Kuroda, Y.; Yamaguchi, K.; Mizuno, N. Diamond-Shaped [Ag4]4+ Cluster Encapsulated by Silicotungstate Ligands: Synthesis and Catalysis of Hydrolytic Oxidation of Silanes. Angew. Chem. Int. Ed. 2012, 51, 2434–2437. [Google Scholar] [CrossRef]
- Adam, W.; Corma, A.; García, H.; Weichold, O. Titanium-Catalyzed Heterogeneous Oxidations of Silanes, Chiral Allylic Alcohols, 3-Alkylcyclohexanes, and Thianthrene 5-Oxide: A Comparison of the Reactivities and Selectivities for the Large-Pore Zeolite Ti-β, the Mesoporous Ti-MCM-41, and the Layered Alumosilicate Ti-ITQ-2. J. Catal. 2000, 196, 339–344. [Google Scholar]
- Adam, W.; Mitchell, C.M.; Saha-Möller, C.R.; Weichold, O. Host−Guest Chemistry in a Urea Matrix: Catalytic and Selective Oxidation of Triorganosilanes to the Corresponding Silanols by Methyltrioxorhenium and the Urea/Hydrogen Peroxide Adduct. J. Am. Chem. Soc. 1999, 121, 2097–2103. [Google Scholar] [CrossRef]
- Adam, W.; Saha-Möller, C.R.; Weichold, O. NaY zeolite as host for the selective heterogeneous oxidation of silanes and olefins with hydrogen peroxide catalyzed by methyltrioxorhenium. J. Org. Chem. 2000, 65, 2897–2899. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, R.; Kamata, K.; Mizuno, N. Highly selective oxidation of organosilanes to silanols with hydrogen peroxide catalyzed by a lacunary polyoxotungstate. Angew. Chem. 2009, 121, 9062–9066. [Google Scholar] [CrossRef]
- Limnios, D.; Kokotos, C.G. Organocatalytic oxidation of organosilanes to silanols. ACS Catal. 2013, 3, 2239–2243. [Google Scholar] [CrossRef]
- He, P.; Zhang, F.; Si, X.; Jiang, W.; Shen, Q.; Li, Z.; Zhu, Z.; Tang, S.; Gui, Q.-W. Visible-Light-Induced Aerobic Oxidation of Tertiary Silanes to Silanols using Molecular Oxygen as an Oxidant. Synthesis 2022, 55, 765–772. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Duan, P.; Zhang, W. Highly Active and Selective Photocatalytic Oxidation of Organosilanes to Silanols. ACS Sustain. Chem. Eng. 2022, 10, 4642–4649. [Google Scholar] [CrossRef]
- Cao, J.; Yang, X.; Ma, L.; Lu, K.; Zhou, R. Metal-free hydrogen evolution cross-coupling enabled by synergistic photoredox and polarity reversal catalysis. Green Chem. 2021, 23, 8988–8994. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Liu, L.-C.; Jiang, C.; He, T.; He, W. Metal-free visible-light-mediated aerobic oxidation of silanes to silanols. Sci. China Chem. 2018, 61, 1594–1599. [Google Scholar] [CrossRef]
- Lv, H.; Laishram, R.D.; Chen, J.; Khan, R.; Zhu, Y.; Wu, S.; Zhang, J.; Liu, X.; Fan, B. Metal-Free Visible-Light-Induced Atom-Transfer Radical Addition Reaction of Alkenes/Alkynes with ICH2CN. Chem. Commun. 2021, 57, 3660–3663. [Google Scholar] [CrossRef] [PubMed]
- Zu, W.; Day, C.; Wei, L.; Jia, X.; Xu, L. Dual aminoquinolate diarylboron and nickel catalysed metallaphotoredox platform for carbon–oxygen bond construction. Chem. Commun. 2020, 56, 8273–8276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zu, W.; Tian, Q.; Cao, Z.; Wei, Y.; Xu, L. A nickel/organoboron catalyzed metallaphotoredox platform for C (sp 2)–P and C (sp 2)–S bond construction. Org. Chem. Front. 2022, 9, 1070–1076. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Xu, L. 3D porous copper skeleton supported zinc anode toward high capacity and long cycle life zinc ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 13894–13899. [Google Scholar] [CrossRef]
- Wei, L.; Wei, Y.; Zhang, J.; Xu, L. Visible-light-mediated organoboron-catalysed metal-free dehydrogenation of N-heterocycles using molecular oxygen. Green Chem. 2021, 23, 4446–4450. [Google Scholar] [CrossRef]
- Cui, H.; Wei, W.; Yang, D.; Zhang, Y.; Zhao, H.; Wang, L.; Wang, H. SIRT5 desuccinylates and activates pyruvate kinase M2 to block macrophage IL-1β production and to prevent DSS-induced colitis in mice. Green Chem. 2017, 19, 3520–3524. [Google Scholar] [CrossRef]
- Rahaman, R.; Das, S.; Barman, P. Visible-light-induced regioselective sulfenylation of imidazopyridines with thiols under transition metal-free conditions. Green Chem. 2018, 20, 141–147. [Google Scholar] [CrossRef]
- Liang, H.; Wang, L.J.; Ji, Y.X.; Wang, H.; Zhang, B. Selective electrochemical hydrolysis of hydrosilanes to silanols via anodically generated silyl cations. Angew. Chem. Int. Ed. 2021, 60, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).