Magnetic CoFe2O4 and NiFe2O4 Induced Self-Assembled Graphene Nanoribbon Framework with Excellent Properties for Li-Ion Battery
Abstract
1. Introduction
2. Results and Discussion
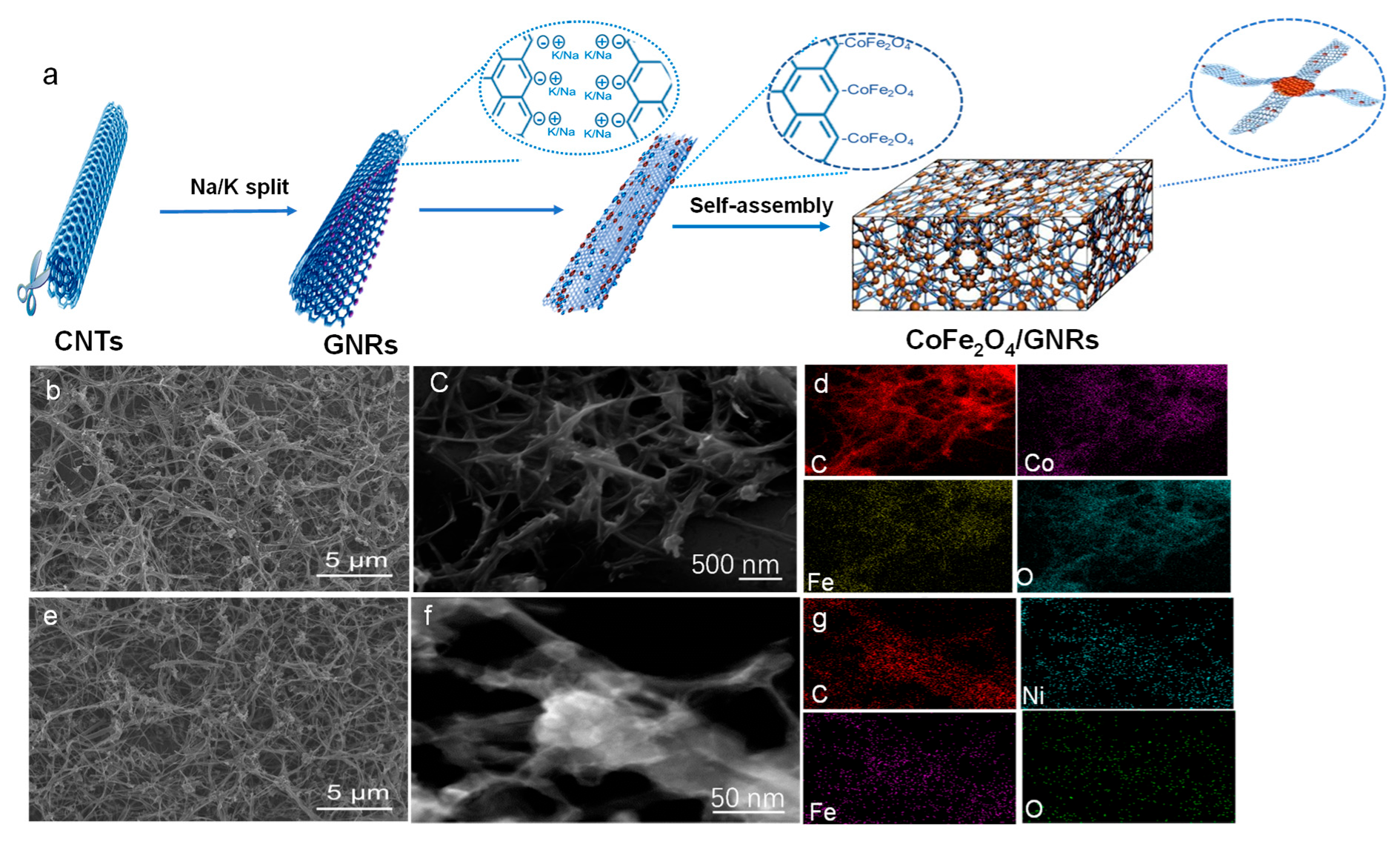
2.1. SEM and TEM Analysis
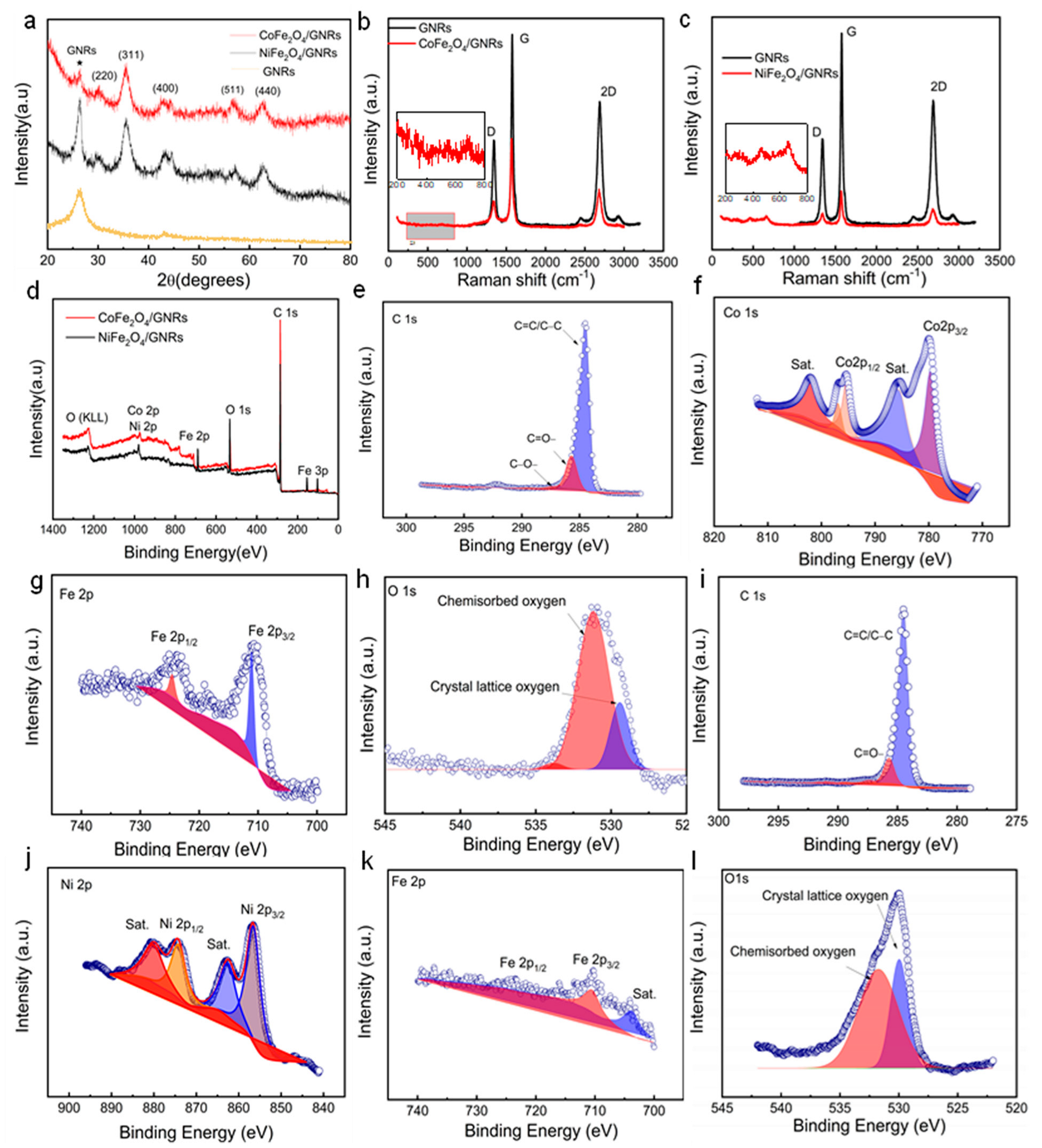
2.2. XRD, Raman, and XPS Analysis
2.3. BET and TGA Analysis
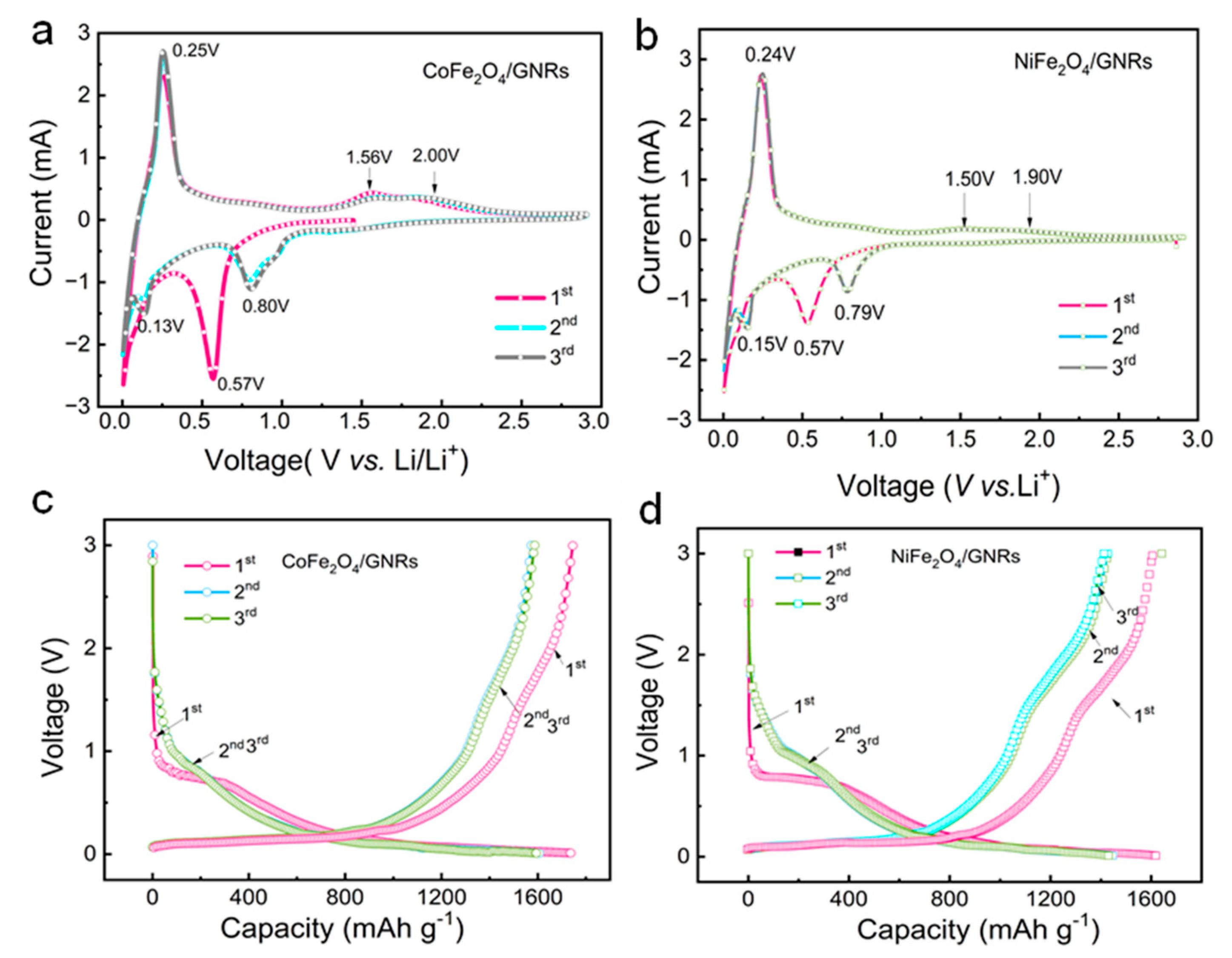
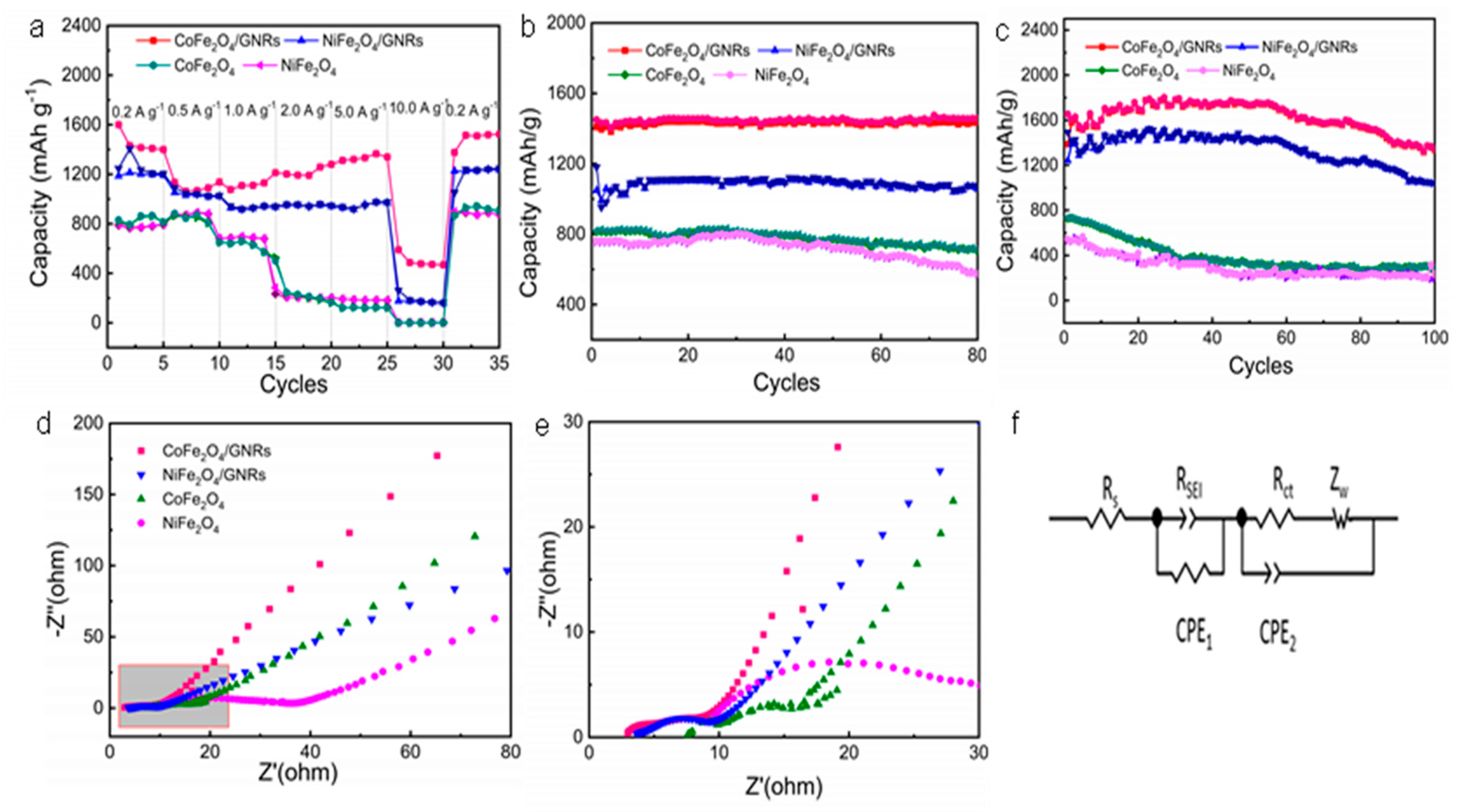
2.4. Electrochemistry Performance
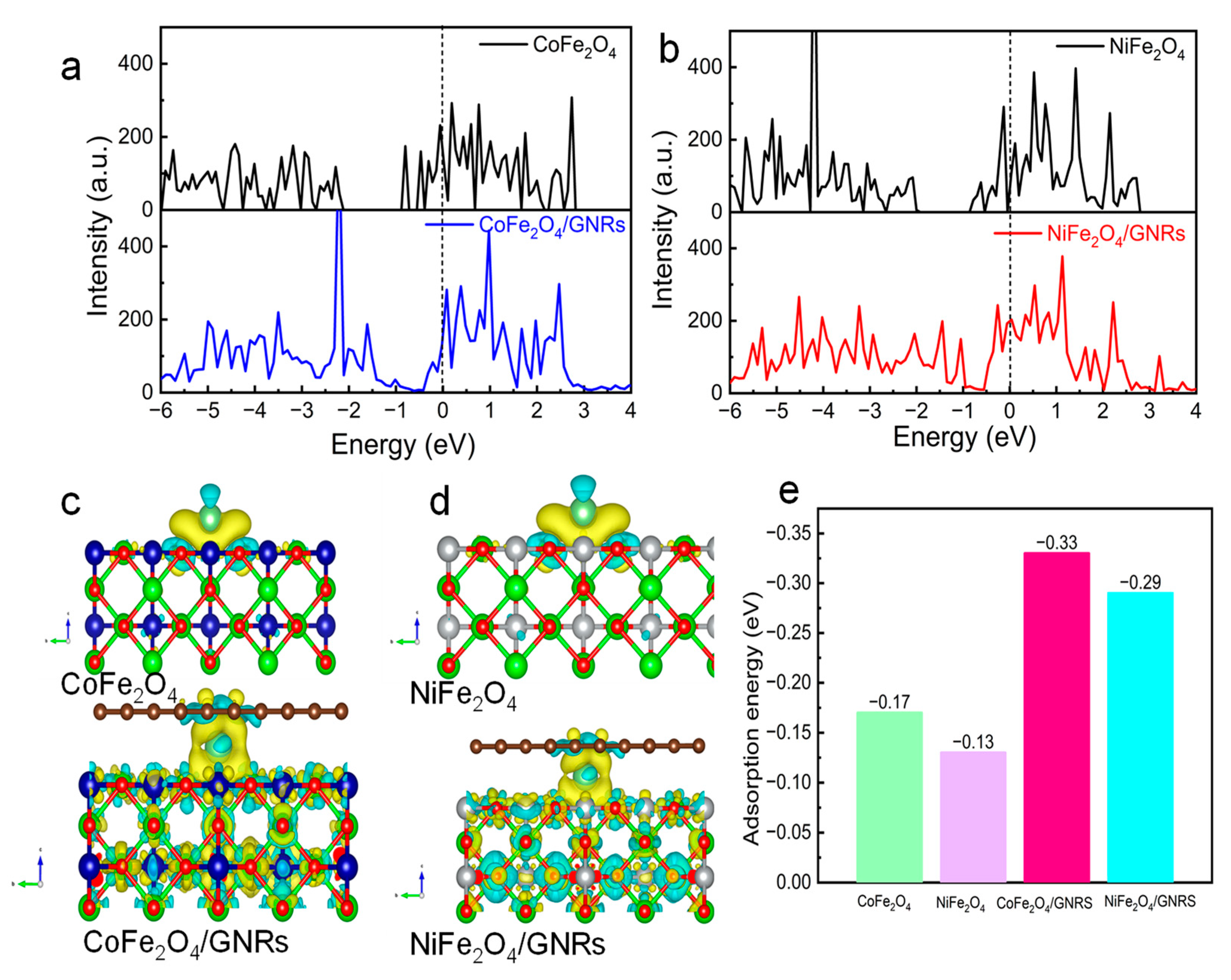
2.5. DFT Analysis
2.6. Magnetic Performance Analysis
3. Materials and Methods
3.1. Synthesis
3.2. Electrochemical Measurement
3.3. Sample Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, W.; Son, D.Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A. 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Lv, W.; Li, Z.; Deng, Y.; Yang, Q.; Kang, F. Graphene-based materials for electrochemical energy storage devices: Opportunities and challenges. Energy Stor. Mater. 2016, 2, 107–138. [Google Scholar] [CrossRef]
- Lebrouhi, B.E.; Baghi, S.; Lamrani, B.; Schall, E.; Kousksou, T. Critical materials for electrical energy storage: Li-ion batteries. J. Energy Stor. 2022, 55, 105471. [Google Scholar] [CrossRef]
- Chen, M.; Shao, L.L.; Wang, X.Q.; Qian, X.; Yuan, Z.Y.; Fang, L.X.; Ding, A.X.; Lv, X.W.; Wang, Y.N. Controlled synthesis of highly active nonstoichiometric tin phosphide/carbon composites for electrocatalysis and electrochemical energy storage applications. ACS Sustain. Chem. Eng. 2022, 10, 1482–1498. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.F.; Kang, Y.; Zhang, Y.H.; Yang, D.X.; Shi, F.N. Co3O4/LaCoO3 nanocomposites derived from MOFs as anodes for high-performance lithium-ion batteries. Inorg. Chem. Commun. 2022, 140, 109447. [Google Scholar] [CrossRef]
- Yang, D.; Feng, Y.; Gao, J.; Wu, K.; He, M. A simple fabrication for nanoscale SnO2-Fe2O3-C lithium-ion battery anode material with tubular network structure. Ionics 2022, 28, 2185–2196. [Google Scholar] [CrossRef]
- Dorri, M.; Zamani, C.; Babaei, A. Insights into temperature-induced evolution of spinel MnCo2O4 as anode material for Li-ion batteries with enhanced electrochemical performance. J. Alloys Compd. 2023, 941, 169034. [Google Scholar] [CrossRef]
- Alhakemy, A.Z.; Elseman, A.M.; Fayed, M.G.; Ahmed Amine Nassr, A.B.; El-Hady Kashyout, A.; Wen, Z. Hybrid electrocatalyst of CoFe2O4 decorating carbon spheres for alkaline oxygen evolution reaction. Ceram. Int. 2022, 48, 5442–5449. [Google Scholar] [CrossRef]
- Du, W.; Zheng, Y.; Liu, X.; Cheng, J.; Chenna Krishna Reddy, R.; Zeb, A.; Lin, X.; Luo, Y. Oxygen-enriched vacancy spinel MFe2O4/carbon (M = Ni, Mn, Co) derived from metal-organic frameworks toward boosting lithium storage. Chem. Eng. J. 2023, 451, 138626. [Google Scholar] [CrossRef]
- Zhao, Q.; Xia, Z.; Qian, T.; Rong, X.; Zhang, M.; Dong, Y.; Chen, J.; Ning, H.; Li, Z.; Hu, H.; et al. PVP-assisted synthesis of ultrafine transition metal oxides encapsulated in nitrogen-doped carbon nanofibers as robust and flexible anodes for sodium-ion batteries. Carbon 2021, 174, 325–334. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.; Yang, D.; Deng, B.; Huang, Z.; Ling, C.D.; Liu, H.; Wang, G.; Guo, Z.; Zheng, R. Bimetallic metal-organic frameworks derived Ni-Co-Se@C hierarchical bundle-like nanostructures with high-rate pseudocapacitive lithium ion storage. Energy Storage Mater. 2019, 17, 374–384. [Google Scholar] [CrossRef]
- Song, Y.; Liu, T.; Yao, B.; Kou, T.; Feng, D.; Liu, X.; Li, Y. Amorphous mixed-valence vanadium oxide/exfoliated carbon cloth structure shows a record high cycling stability. Small 2017, 13, 1700067. [Google Scholar] [CrossRef]
- Fan, H.; Chen, S.; Chen, X.; Tang, Q.; Hu, A.; Luo, W.; Dou, S.X. 3D Selenium sulfide@carbon nanotube array as long-life and high-rate cathode material for lithium storage. Adv. Funct. Mater. 2018, 28, 1805018. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Ding, Q.; Lin, X.; Han, T.; Hu, C. Free-standing nanowires growing on ginkgo biloba as high areal capacity Li-ion battery anode at high and low temperatures. Appl. Surf. Sci. 2023, 620, 156841. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yu, L.; Lou, X.W. Hierarchical tubular structures composed of Co3O4 hollow nanoparticles and carbon nanotubes for lithium storage. Angew. Chem. 2016, 55, 5990–5993. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, Z.; Yang, L.; Cheng, S.; Liu, M. A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J. Mater. Chem. 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- Han, W.; Qin, X.; Wu, J.; Li, Q.; Liu, M.; Xia, Y.; Kang, F. Electrosprayed porous Fe3O4/carbon microspheres as anode materials for high-performance lithium-ion batteries. Nano Res. 2018, 11, 892–904. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Wu, Z.; Han, L.; Huang, T.; Xin, H.L.; Wang, D. High-rate and long-life lithium-ion battery performance of hierarchically hollow-structured NiCo2O4/CNT nanocomposite. Electrochim. Acta 2017, 244, 8–15. [Google Scholar] [CrossRef]
- Xue, Z.; Li, L.; Cao, L.; Zheng, W.; Yang, W.; Yu, X. A simple method to fabricate NiFe2O4/NiO@Fe2O3 core-shelled nanocubes based on prussian blue analogues for lithium ion battery. J. Alloys Compd. 2020, 825, 153966. [Google Scholar] [CrossRef]
- Pham, T.V.; Guo, H.P.; Luo, W.; Chou, S.; Wang, J.; Liu, H. Carbon- and binder-free 3D porous perovskite oxide air electrode for rechargeable lithium–oxygen batteries. Small 2016, 12, 3031–3038. [Google Scholar] [CrossRef]
- Yin, Z.; Cho, S.; You, D.; Ahn, Y.; Yoo, J.; Kim, Y.S. Copper nanowire/multi-walled carbon nanotube composites as all-nanowire flexible electrode for fast-charging/discharginglithium-ionbattery. Nano Res. 2017, 11, 769–779. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Yang, X.; Gu, J.; Niu, M.; Yang, L.; Bai, Z. In situ synthesis of CoFe2O4 nanoparticles embedded in N-doped carbon nanotubes for efficient electrocatalytic oxygen reduction reaction. Int. J. Hydrogen Energy 2022, 47, 6059–6066. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Huang, J.; Li, J.; Zhou, P.; Liu, J.; Huang, X. Three-dimensional graphene-based nanocomposites for high energy density Li-ion batteries. J. Mater. Chem. A 2017, 5, 5977–5994. [Google Scholar] [CrossRef]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Wang, Q.; Zhang, H.; Guo, S. A universal strategy for hollow metal oxide nanoparticles encapsulated into B/N Co-doped graphitic nanotubes as high-performance lithium-ion battery anodes. Adv. Mater. 2018, 30, 1705441. [Google Scholar] [CrossRef]
- Yuan, D.; Ji, C.; Zhuge, X.; Chai, A.; Pan, L.; Li, Y.; Luo, Z.; Luo, K. Organic-inorganic interlayer enabling the stability of PVDF-HFP modified Li metal for lithium-oxygen batteries. Appl. Surf. Sci. 2023, 613, 155863. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Yang, F. Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup. J. Mater. Chem. A 2013, 1, 3446–3453. [Google Scholar] [CrossRef]
- Carbonellsanroma, E.; Hieulle, J.; Vilasvarela, M.; Brandimarte, P.; Iraola, M.; Barragan, A.; Pascual, J.I. Doping of graphene nanoribbons via functional group edge modification. ACS Nano 2017, 11, 7355–7361. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhang, S.; Long, J.; Huang, Y.; Wang, R. A three-dimensional sponge of graphene nanoribbons crosslinked by Fe3O4 nanoparticles for Li+ storage. J. Mater. Chem. A 2017, 5, 23592–23599. [Google Scholar] [CrossRef]
- Genorio, B.; Lu, W.; Dimiev, A.M.; Zhu, Y.; Raji, A.O.; Novosel, B. In situ intercalation replacement and selective functionalization of graphene nanoribbon stacks. ACS Nano 2012, 6, 4231–4240. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Yu, Z.; Meng, Q.; Zhang, T.; Liu, Q.; Zhang, D. A facile fabrication of Fe3O4/graphene nanosheets for lithium-ion battery. Sci. Adv. Mater. 2014, 6, 283–289. [Google Scholar] [CrossRef]
- Li, L.; Kovalchuk, A.; Fei, H.; Peng, Z.; Li, Y.; Kim, N.D.; Tour, J.M. Enhanced cycling stability of lithium-ion batteries using graphene-wrapped Fe3O4-graphene nanoribbons as anode materials. Adv. Energy Mater. 2015, 5, 1500171. [Google Scholar] [CrossRef]
- Agudo, A.L.; Gil, F.J.; Calleja, J.M.; Fernández, V. Raman spectroscopic study of MoO3/γ-Al2O3 and CoO(or NiO)-MoO3/γ-Al2O3 oxide catalysts prepared by different methods. J. Raman Spectrosc. 1981, 11, 454–458. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J. Identification of cobalt oxides with raman scattering and fourier transform infrared spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Wang, Y.; Liang, Y.; Majeed, A.; Yang, Z.; Yao, S.; Shen, X.; Li, T.; Qin, S. CoFe2O4 nanoparticles loaded N-doped carbon nanofibers networks as electrocatalyst for enhancing redox kinetics in Li-S batteries. Appl. Surf. Sci. 2021, 560, 149908. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M.A. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surf. Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Ma, J.; Yang, J.; Jiao, L.; Mao, Y.; Wang, T.; Duan, X.; Zheng, W. NiO nanomaterials: Controlled fabrication, formation mechanism and the application in lithium-ion battery. Crystengcomm. 2011, 14, 453–459. [Google Scholar] [CrossRef]
- Chu, Y.; Xiong, S. Mixed transition-metal oxides@carbon core-shell nanostructures derived from heterometallic clusters for enhanced lithium storage. Chin. Chem. Lett. 2022, 33, 486–490. [Google Scholar] [CrossRef]
- Ramadevi, P.; Shanmugavadivu, R.; Venkatesan, R.; Mayandi, J.; Sagadevan, S. Photocatalytic dye degradation efficiency and reusability of aluminium substituted nickel ferrite nanostructures for wastewater remediation. Inorg. Chem. Commun. 2023, 150, 110532. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Liu, K.; Lan, L.; Song, H.; Chen, X. Free-standing cobalt hydroxide nanoplatelet array formed by growth of preferential-orientation on graphene nanosheets as anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 20706–20713. [Google Scholar] [CrossRef]
- Varghese, B.; Reddy, M.V.; Zhu, Y.; Lit, C.S.; Hoong, T.C.; Subba Rao, G.V.; Chowdari, B.V.; Wee, A.T.; Lim, C.T.; Sow, C.H. Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chem. Mater. 2008, 20, 3360–3367. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 46, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Fan, H.J. Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef]
- Yang, C.; Peng, C.; Chen, P.; Ma, C.; Guo, K.; Cheng, Y. Insights into electrochemical performances of NiFe2O4 for lithium-ion anode materials. J. Alloys Compd. 2022, 896, 163079. [Google Scholar] [CrossRef]
- Ortiz, G.F.; Lavela, P.; Knauth, P.; Djenizian, T.; Alcántara, R.; Tirado, J.L. Tin-based composite materials fabricated by anodic oxidation for the negative electrode of Li-ion batteries. J. Electrochem. Soc. 2011, 158, A1094. [Google Scholar] [CrossRef]
- Han, D.H.; Wang, J.P.; Luo, H.L. Crystallite size effect on saturation magnetization of fine ferrimagnetic particles. J. Magn. Magn. Mater. 1994, 136, 176–182. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Surendranath, Y.; Kanan, M.W.; Nocera, D.G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 2010, 132, 16501–16509. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; He, C.; Bai, Q.; Miao, X.; Cao, C.; Wu, T. Magnetic CoFe2O4 and NiFe2O4 Induced Self-Assembled Graphene Nanoribbon Framework with Excellent Properties for Li-Ion Battery. Molecules 2023, 28, 4069. https://doi.org/10.3390/molecules28104069
Zhao X, He C, Bai Q, Miao X, Cao C, Wu T. Magnetic CoFe2O4 and NiFe2O4 Induced Self-Assembled Graphene Nanoribbon Framework with Excellent Properties for Li-Ion Battery. Molecules. 2023; 28(10):4069. https://doi.org/10.3390/molecules28104069
Chicago/Turabian StyleZhao, Xiyu, Chunyang He, Qiujv Bai, Xiangwen Miao, Cheng Cao, and Tianli Wu. 2023. "Magnetic CoFe2O4 and NiFe2O4 Induced Self-Assembled Graphene Nanoribbon Framework with Excellent Properties for Li-Ion Battery" Molecules 28, no. 10: 4069. https://doi.org/10.3390/molecules28104069
APA StyleZhao, X., He, C., Bai, Q., Miao, X., Cao, C., & Wu, T. (2023). Magnetic CoFe2O4 and NiFe2O4 Induced Self-Assembled Graphene Nanoribbon Framework with Excellent Properties for Li-Ion Battery. Molecules, 28(10), 4069. https://doi.org/10.3390/molecules28104069







