Photocatalysis in Water-Soluble Supramolecular Metal Organic Complex
Abstract
1. Introduction
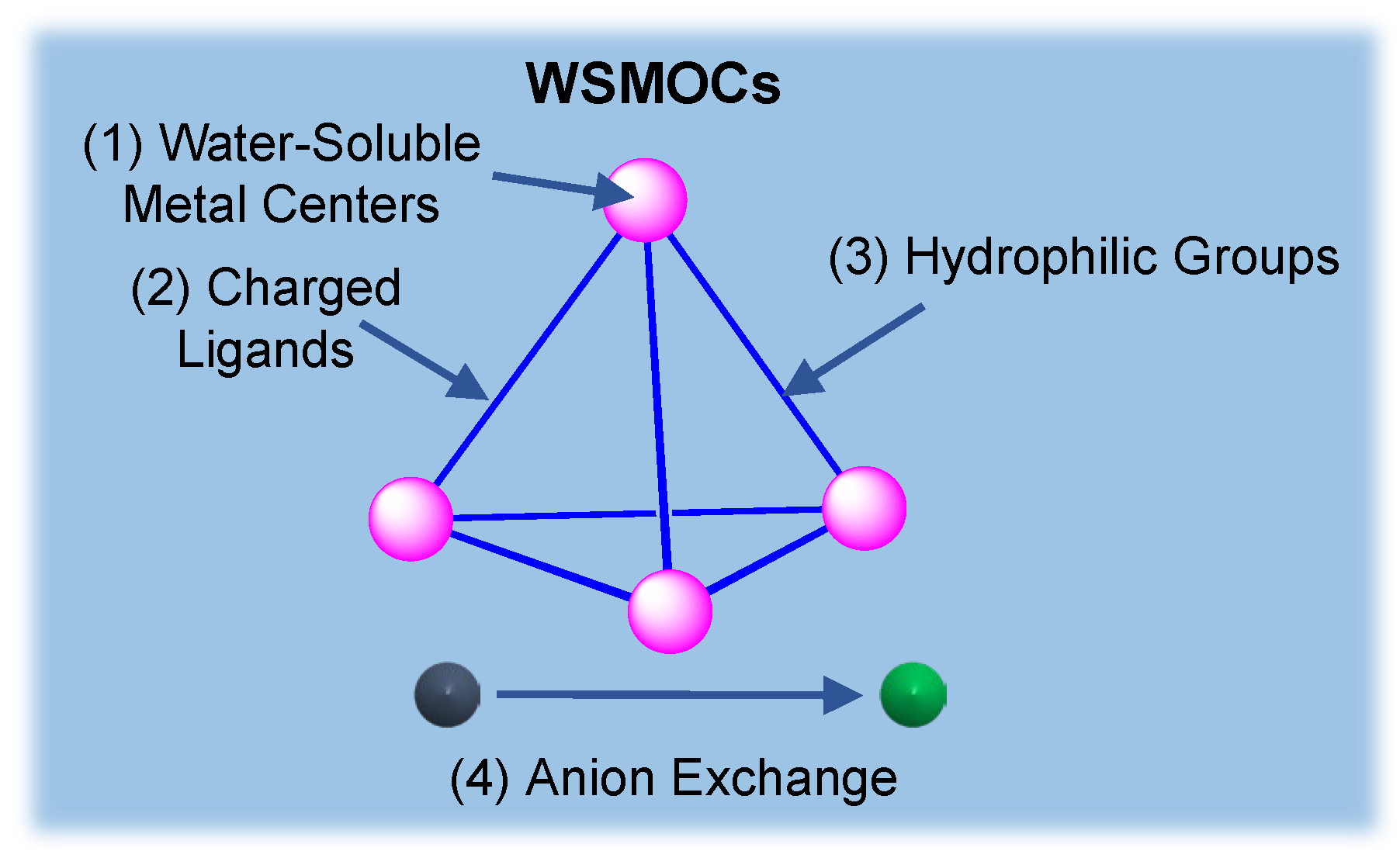
2. Strategies to Prepare Water-Soluble Metal-Organic Cages
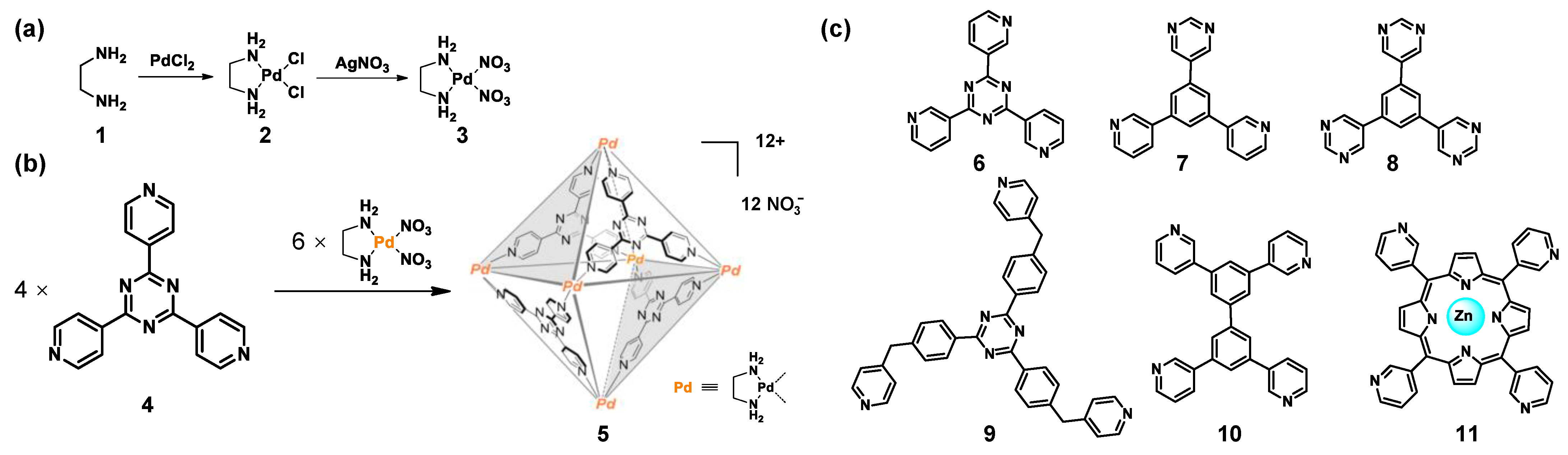
2.1. Metal Centers
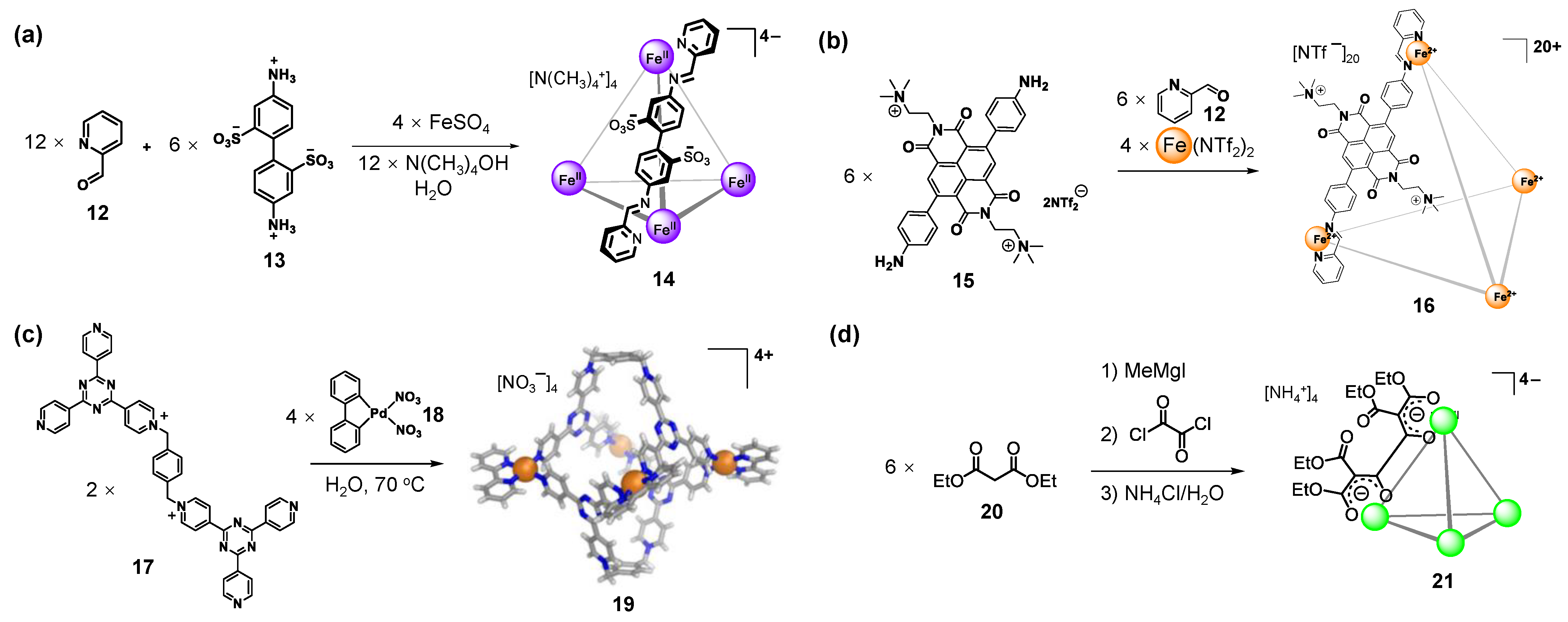
2.2. Charged Ligands
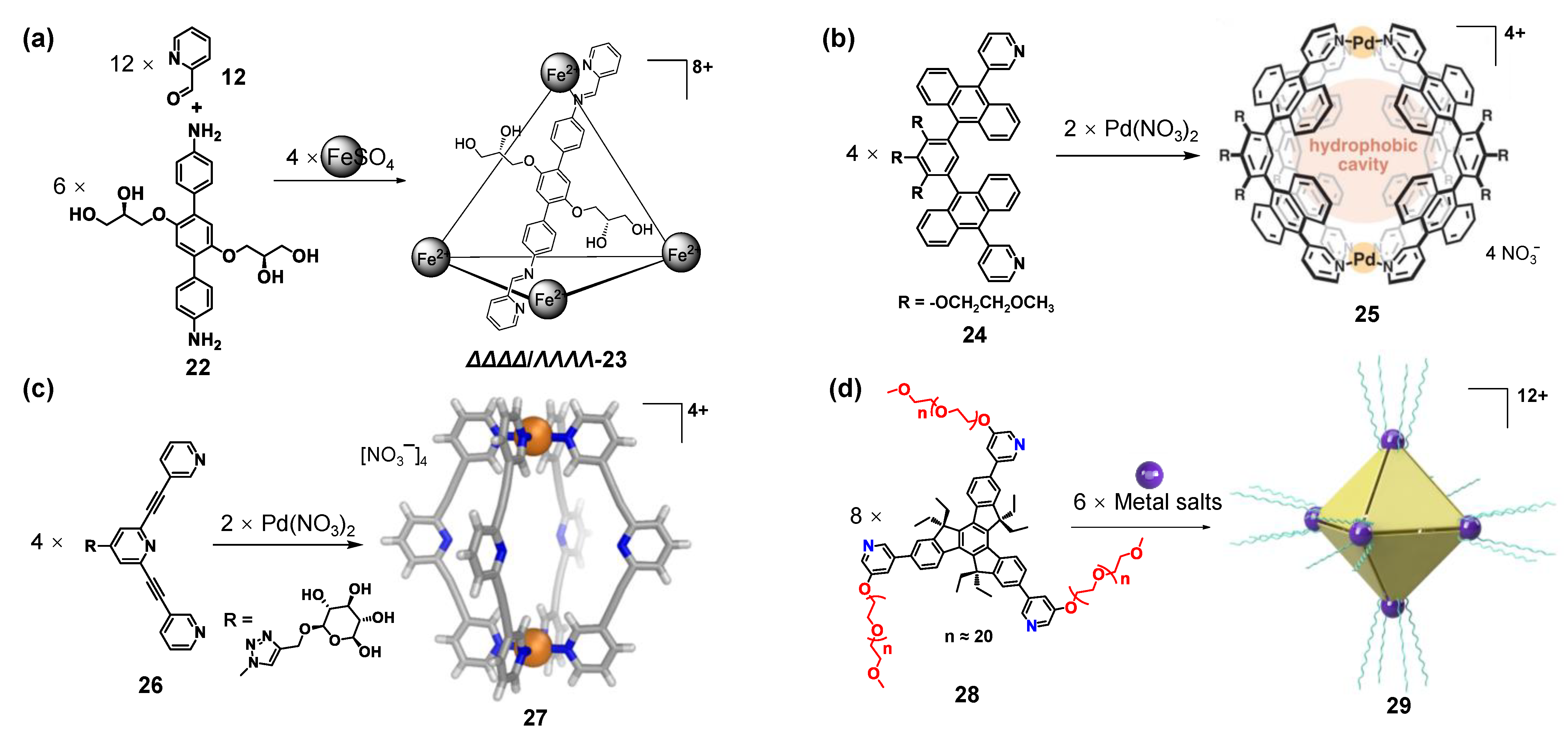
2.3. Hydrophilic Groups
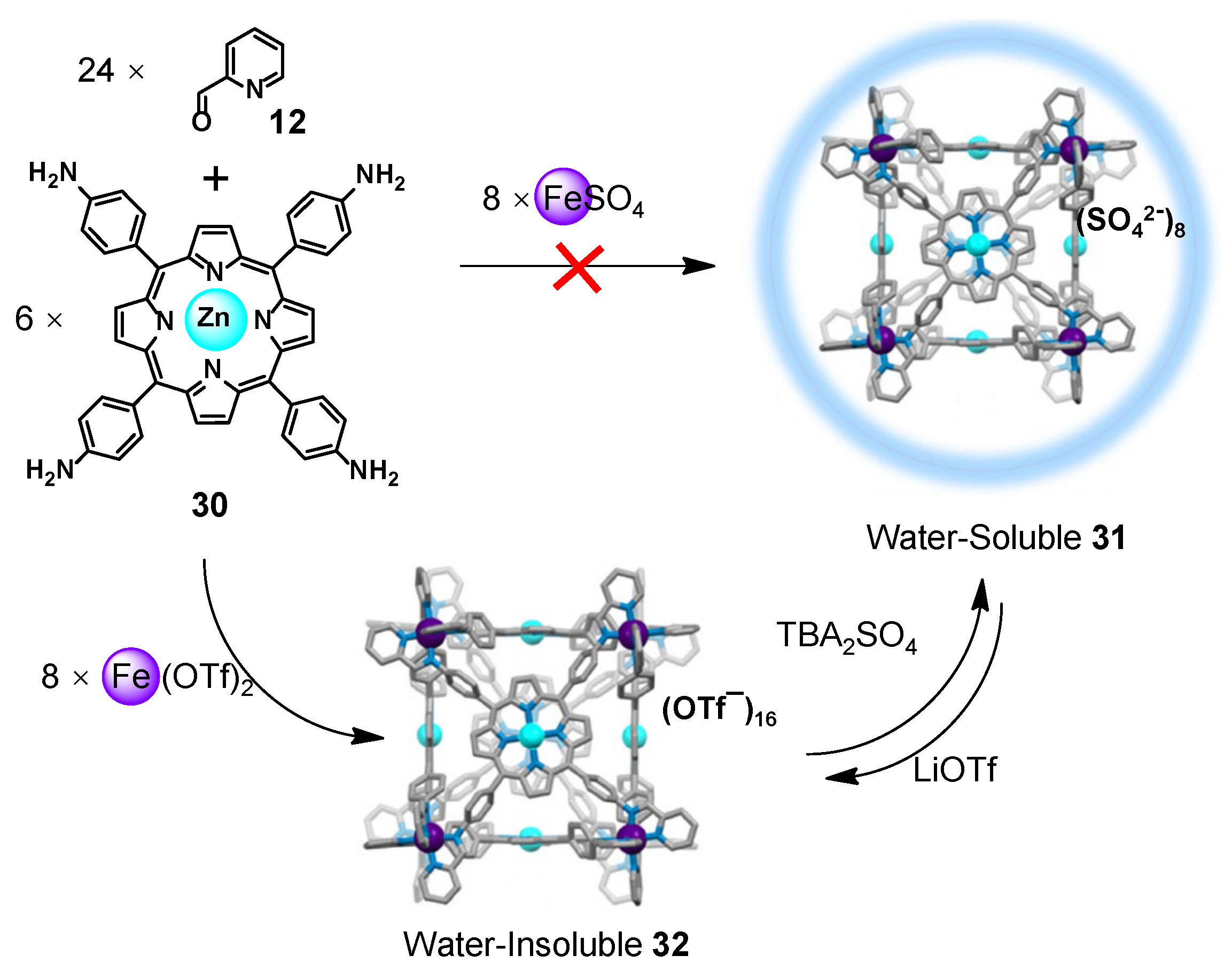
2.4. Anion Exchange
3. Photocatalysis in WSMOCs
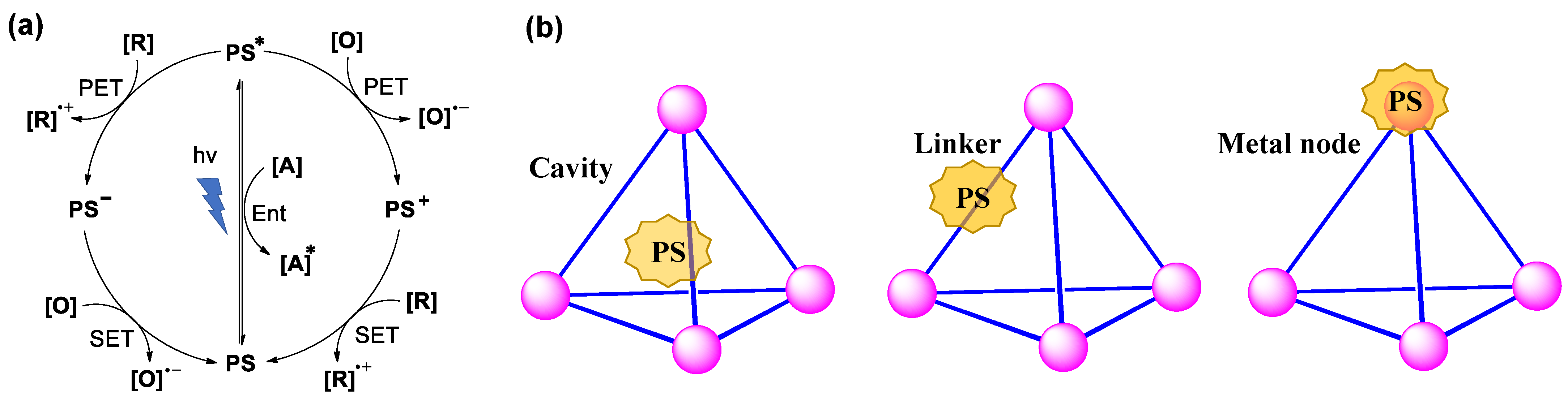
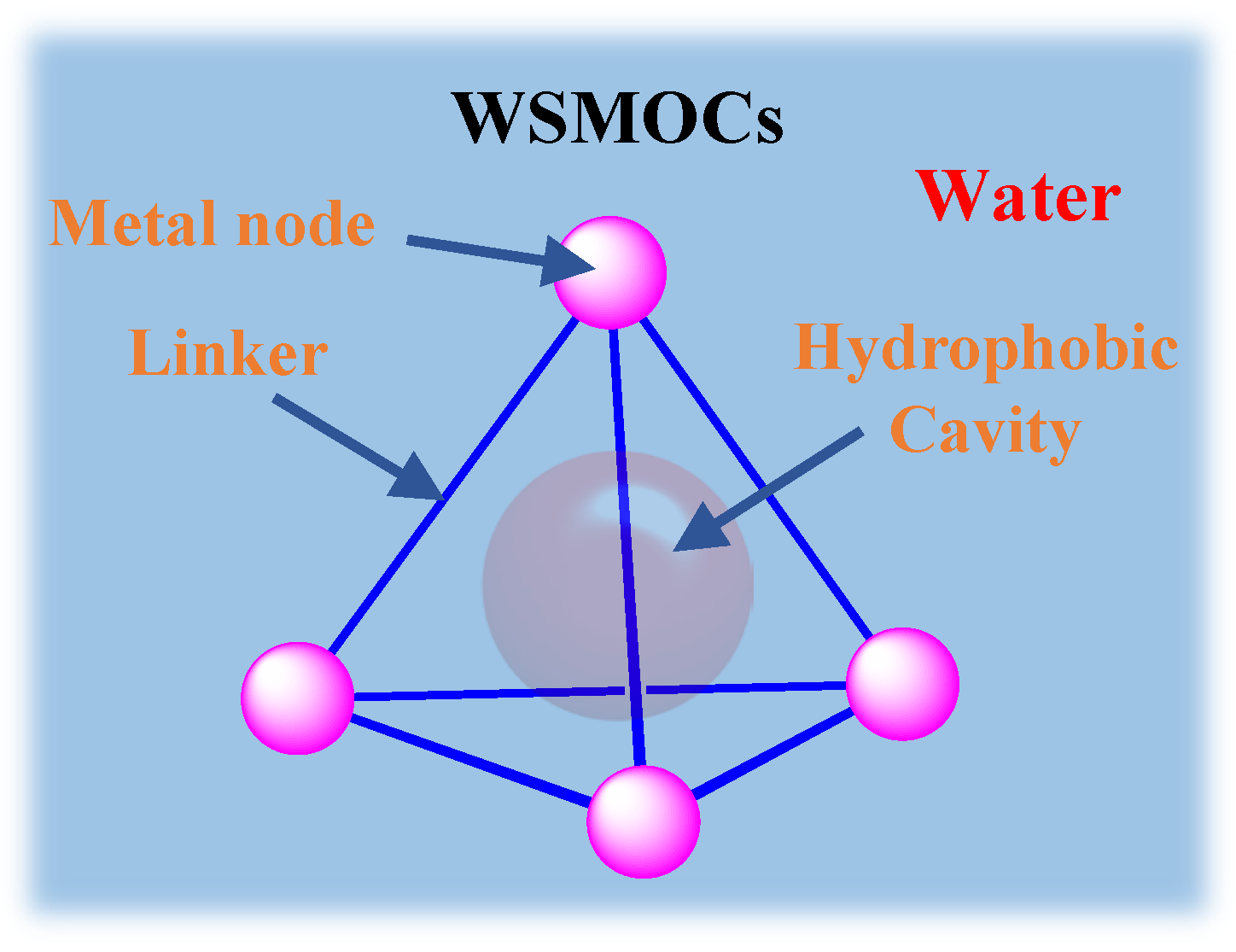
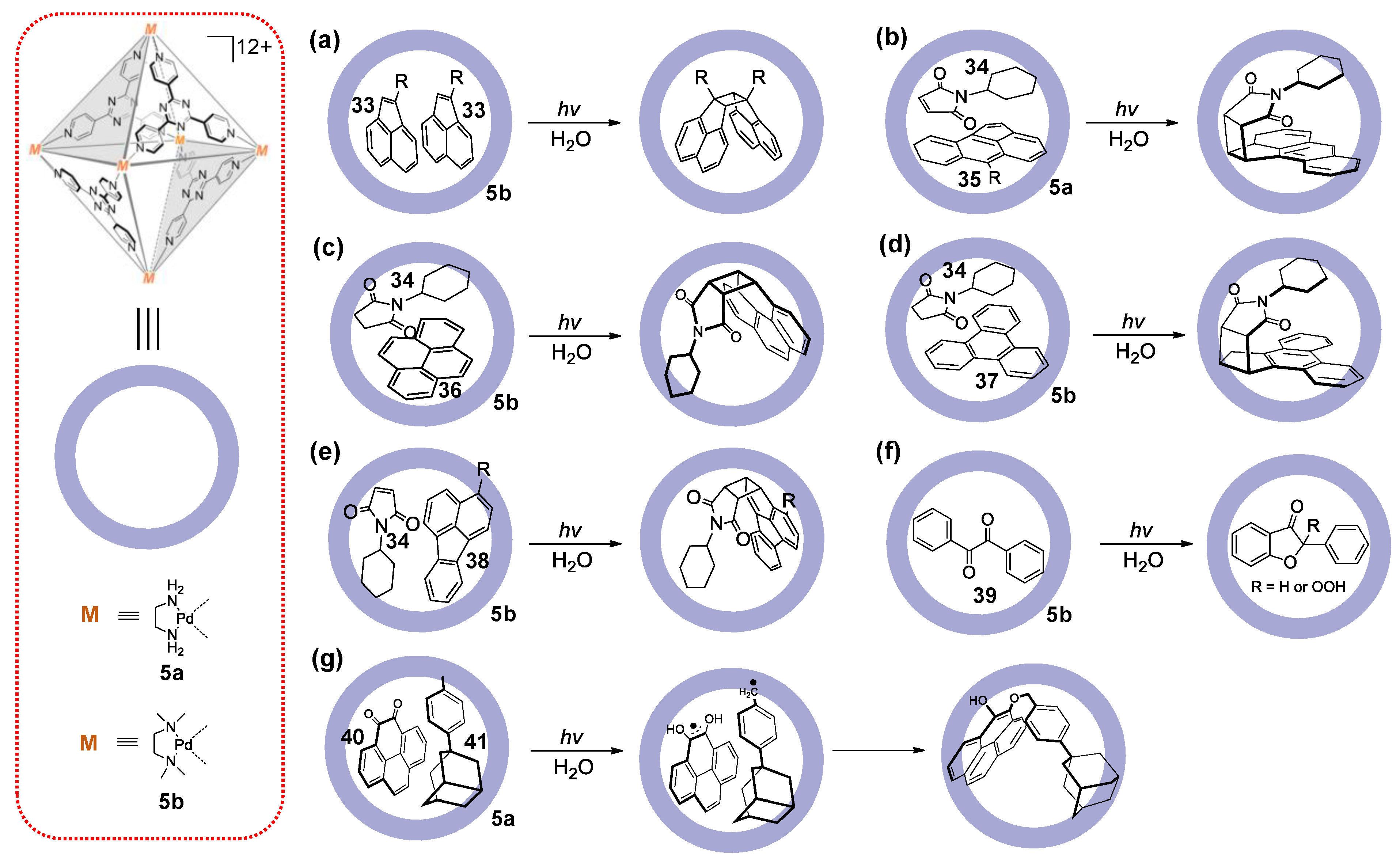
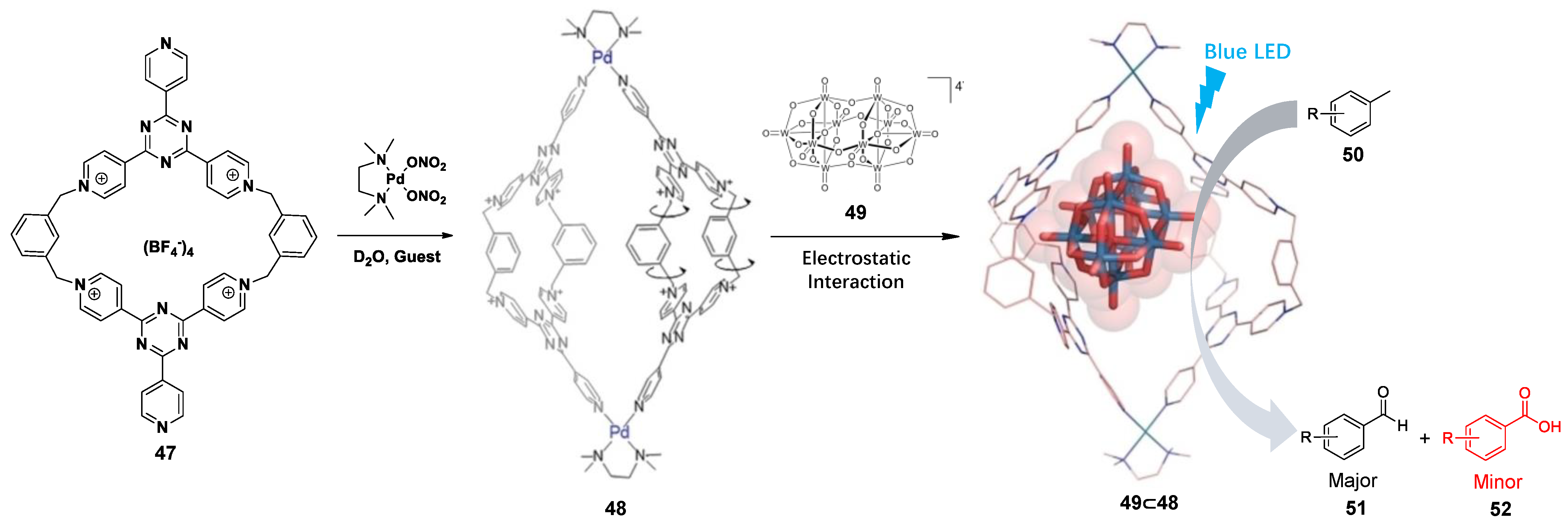
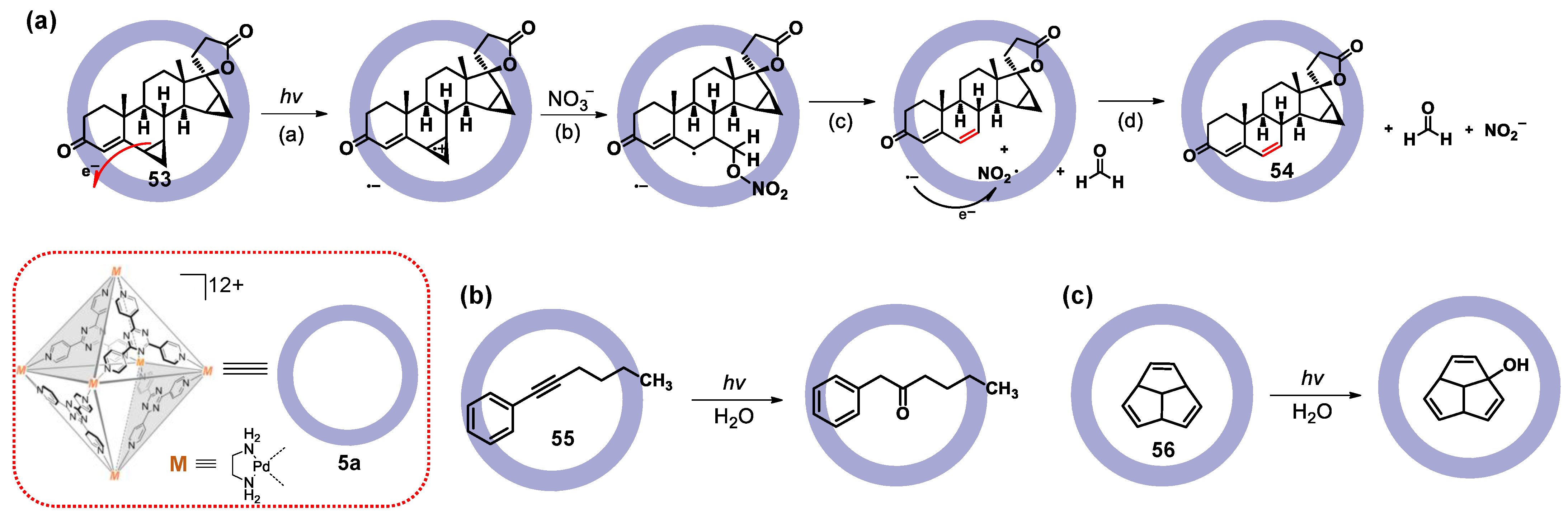
3.1. Photoredox Catalysis Mediated by the Cavity of WSMOCs
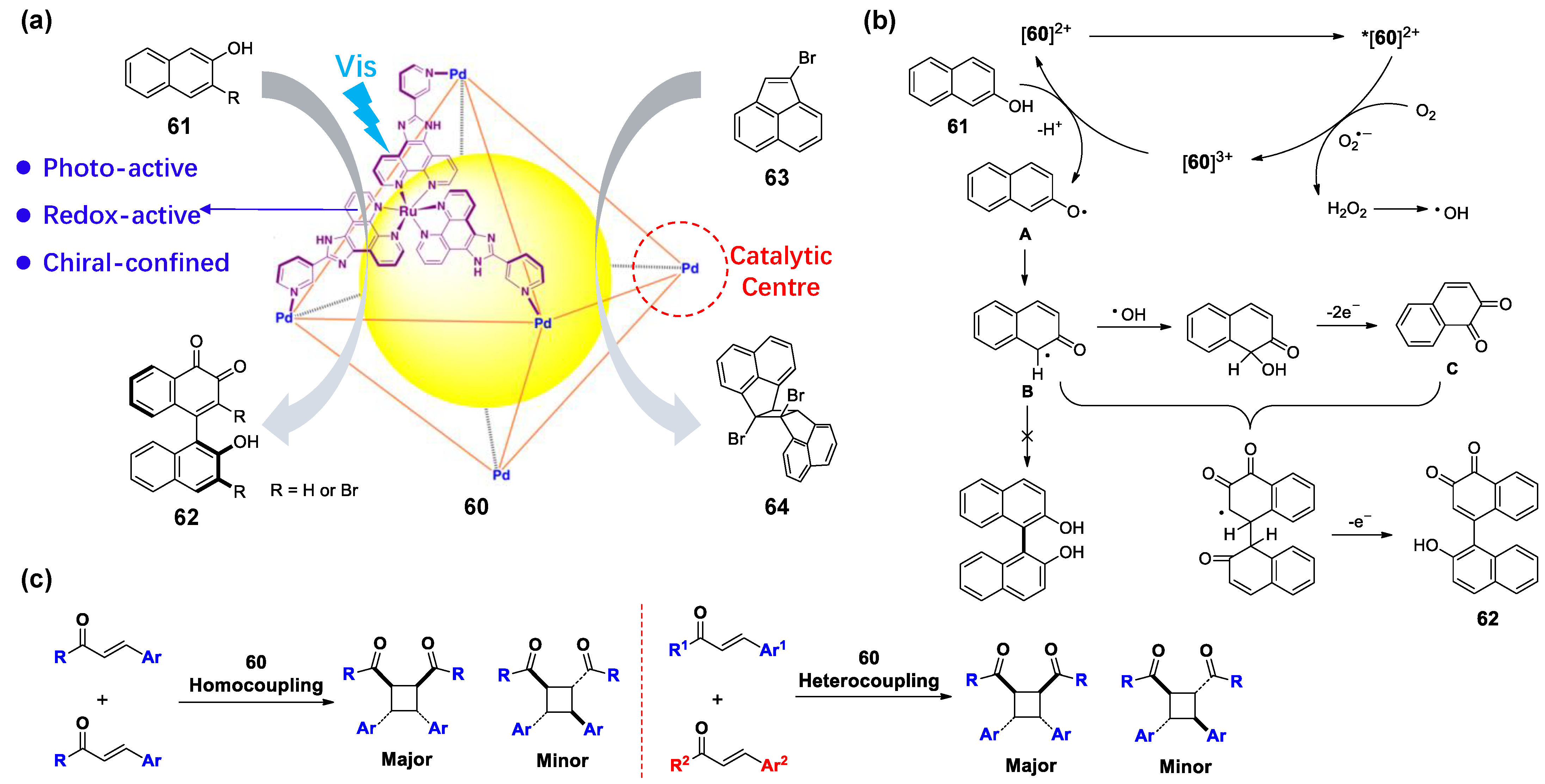
3.2. Photoredox Catalysis Mediated by the Ligand or Metal of WSMOC
3.3. Photoredox Catalysis Mediated by the WSMOC-Based Light-Harvesting System
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barber, J. Photosynthetic Energy Conversion: Natural and Artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, L. Artificial Photosynthesis: Opportunities and Challenges of Molecular Catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.J.; Odella, E.; Moore, G.F.; Gust, D.; Moore, T.A.; Moore, A.L. Proton-Coupled Electron Transfer in Artificial Photosynthetic Systems. Acc. Chem. Res. 2018, 51, 445–453. [Google Scholar] [CrossRef] [PubMed]
- McDermott, G.; Prince, S.; Freer, A.; Hawthornthwaite-Lawless, A.; Papiz, M.; Cogdell, R.; Isaacs, N. Crystal Structure of an Integral Membrane Light-Harvesting Complex from Photosynthetic Bacteria. Nature 1995, 374, 517–521. [Google Scholar] [CrossRef]
- Scholes, G.D.; Fleming, G.R.; Olaya-Castro, A.; van Grondelle, R. Lessons from Nature about Solar Light Harvesting. Nat. Chem. 2011, 3, 763–774. [Google Scholar] [CrossRef]
- Hak, C.H.; Sim, L.C.; Leong, K.H.; Lim, P.F.; Chin, Y.H.; Saravanan, P. M/g-C3N4 (M=Ag, Au, and Pd) Composite: Synthesis via Sunlight Photodeposition and Application towards the Degradation of Bisphenol A. Environ. Sci. Pollut. Res. 2018, 25, 25401–25412. [Google Scholar] [CrossRef]
- Millet, A.; Cesana, P.T.; Sedillo, K.; Bird, M.J.; Schlau-Cohen, G.S.; Doyle, A.G.; MacMillan, D.W.C.; Scholes, G.D. Bioinspired Supercharging of Photoredox Catalysis for Applications in Energy and Chemical Manufacturing. Acc. Chem. Res. 2022, 55, 1423–1434. [Google Scholar] [CrossRef]
- Shaffer, D.W.; Xie, Y.; Concepcion, J.J. O-O Bond Formation in Ruthenium-Catalyzed Water Oxidation: Single-Site Nucleophilic Attack vs. O-O Radical Coupling. Chem. Soc. Rev. 2017, 46, 6170–6193. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Long, Z.H.; Wang, X.Z.; Zhou, J.Y.; Wang, X.S.; Zhou, X.P.; Li, D. Cobalt-Based Metal-Organic Cages for Visible Light-Driven Water Oxidation. Inorg. Chem. 2021, 60, 10380–10386. [Google Scholar] [CrossRef]
- Noll, N.; Würthner, F. A Calix[4]Arene-Based Cyclic Dinuclear Ruthenium Complex for Light-Driven Catalytic Water Oxidation. Chem. Eur. J. 2021, 27, 444–450. [Google Scholar] [CrossRef]
- Ji, C.; Wang, W.; El-Sayed, E.S.M.; Liu, G.; Si, Y.; Su, K.; Ju, Z.; Wu, F.; Yuan, D. A High-Efficiency Dye-Sensitized Pt(II) Decorated Metal-Organic Cage for Visible-Light-Driven Hydrogen Production. Appl. Catal. B 2021, 285, 119782. [Google Scholar] [CrossRef]
- Kalamaras, E.; Maroto-Valer, M.M.; Andresen, J.M.; Wang, H.; Xuan, J. Thermodynamic Analysis of the Efficiency of Photo electrochemical CO2 Reduction to Ethanol. Energy Procedia 2019, 158, 767–772. [Google Scholar] [CrossRef]
- Lee, H.S.; Jee, S.; Kim, R.; Bui, H.T.; Kim, B.; Kim, J.K.; Park, K.S.; Kim, W.; Choi, K.M. A Highly Active, Robust Photocatalyst Heterogenized in Discrete Cages of Metal-Organic Polyhedra for CO2 Reduction. Energy Environ. Sci. 2020, 13, 519–526. [Google Scholar] [CrossRef]
- Ghosh, A.C.; Legrand, A.; Rajapaksha, R.; Craig, G.A.; Sassoye, C.; Balázs, G.; Farrusseng, D.; Furukawa, S.; Canivet, J.; Wisser, F.M. Rhodium-Based Metal-Organic Polyhedra Assemblies for Selective CO2 Photoreduction. J. Am. Chem. Soc. 2022, 144, 3626–3636. [Google Scholar] [CrossRef]
- Ma, G.; Yan, H.; Zong, X.; Ma, B.; Jiang, H.; Wen, F.; Li, C. Photocatalytic Splitting of H2S to Produce Hydrogen by Gas-Solid Phase Reaction. Chin. J. Catal. 2008, 29, 313–315. [Google Scholar] [CrossRef]
- Oladipo, H.; Yusuf, A.; Al Jitan, S.; Palmisano, G. Overview and Challenges of the Photolytic and Photocatalytic Splitting of H2S. Catal. Today 2021, 380, 125–137. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Xu, K.J.; Yang, W.B.; Wang, Z.J.; Zhong, F.F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef]
- Ravetz, B.D.; Tay, N.E.S.; Joe, C.L.; Sezen-Edmonds, M.; Schmidt, M.A.; Tan, Y.; Janey, J.M.; Eastgate, M.D.; Rovis, T. Development of a Platform for Near-Infrared Photoredox Catalysis. ACS Cent. Sci. 2020, 6, 2053–2059. [Google Scholar] [CrossRef]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of Small Molecule Aza-BODIPY: From Rational Structural Design to In Vivo Investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef]
- Rana, P.; Singh, N.; Majumdar, P.; Singh, S.P. Evolution of BODIPY/Aza-BODIPY Dyes for Organic Photoredox/Energy Transfer Catalysis. Coord. Chem. Rev. 2022, 470, 214698. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-Soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hang, X.; Ding, J.; Li, B.; Zhu, R.; Pang, H.; Xu, Q. Catalysis within Coordination Cages. Coord. Chem. Rev. 2021, 430, 213656. [Google Scholar] [CrossRef]
- Miao, L.; Zhu, X.; Liu, G.; Han, X.; Xie, W.; Lu, S.; Zhang, L.; Wang, K.; Shi, L.; Lu, S.; et al. Tunable aggregation-induced fluorescent and pressure-responsive luminescence supramolecular cages achieved by subcomponent self-assembly. Chin. Chem. Lett. 2023, 34, 107921. [Google Scholar] [CrossRef]
- Zhang, L.; Das, R.; Li, C.-T.; Wang, Y.-Y.; Hahn, F.E.; Hua, K.; Sun, L.-Y.; Han, Y.-F. C3-Symmetric Assemblies from Trigonal Polycarbene Ligands and MI Ions for the Synthesis of Three-Dimensional Polyimidazolium Cations. Angew. Chem. Int. Ed. 2019, 58, 13360–13364. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Z.; Yuan, C.; Guo, Z.; Liu, Y.; Zhao, D.; Liu, K.; Zhao, J.; Tan, H.; Yan, X. Trackable Supramolecular Fusion: Cage to Cage Transformation of Tetraphenylethylene-Based Metalloassemblies. Angew. Chem. Int. Ed. 2020, 59, 10013–10017. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Wang, X.; Ma, D.; Huang, F. Three-dimensional organic cage with aggregation-induced delayed fluorescence. Chin. Chem. Lett. 2021, 32, 1017–1019. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Lawrence, A.L.; Lusby, P.J. High Activity and Efficient Turnover by a Simple, Self-Assembled “Artificial Diels–Alderase”. J. Am. Chem. Soc. 2018, 140, 2862–2868. [Google Scholar] [CrossRef]
- Feng, T.; Li, X.; Wu, J.; He, C.; Duan, C. Cavity-directed nitroaromatics sensing within a carbazole-based luminescent supramolecular M2L3 cage. Chin. Chem. Lett. 2020, 34, 95–98. [Google Scholar] [CrossRef]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly Efficient Catalysis of the Kemp Elimination in the Cavity of a Cubic Coordination Cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef]
- Cullen, W.; Metherell, A.J.; Wragg, A.B.; Taylor, C.G.P.; Williams, N.H.; Ward, M.D. Catalysis in a Cationic Coordination Cage Using a Cavity-Bound Guest and Surface-Bound Anions: Inhibition, Activation, and Autocatalysis. J. Am. Chem. Soc. 2018, 140, 2821–2828. [Google Scholar] [CrossRef]
- Murase, T.; Nishijima, Y.; Fujita, M. Cage-Catalyzed Knoevenagel Condensation under Neutral Conditions in Water. J. Am. Chem. Soc. 2012, 134, 162–164. [Google Scholar] [CrossRef]
- Sheng, T.-P.; He, C.; Wang, Z.; Zheng, G.-Z.; Dai, F.-R.; Chen, Z.-N. Precise Assembly and Supramolecular Catalysis of Tetragonal- and Trigonal-Elongated Octahedral Coordination Containers. CCS Chem. 2022, 4, 1098–1107. [Google Scholar] [CrossRef]
- Li, S.-C.; Cai, L.-X.; Hong, M.; Chen, Q.; Sun, Q.-F. Combinatorial Self-Assembly of Coordination Cages with Systematically Fine-Tuned Cavities for Efficient Co-Encapsulation and Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202204732. [Google Scholar]
- Yoshizawa, M.; Tamura, M.; Fujita, M. Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis. Science 2006, 312, 251–255. [Google Scholar] [CrossRef]
- Kaphan, D.M.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Enabling New Modes of Reactivity via Constrictive Binding in a Supramolecular-Assembly-Catalyzed Aza-Prins Cyclization. J. Am. Chem. Soc. 2015, 137, 9202–9205. [Google Scholar] [CrossRef]
- Horiuchi, S.; Murase, T.; Fujita, M. Diels-Alder Reactions of Inert Aromatic Compounds within a Self-Assembled Coordination Cage. Chem. Asian J. 2011, 6, 1839–1847. [Google Scholar] [CrossRef]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Catalysis in Metal-Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef]
- Zhao, L.; Jing, X.; Li, X.; Guo, X.; Zeng, L.; He, C.; Duan, C. Catalytic Properties of Chemical Transformation within the Confined Pockets of Werner-Type Capsules. Coord. Chem. Rev. 2019, 378, 151–187. [Google Scholar] [CrossRef]
- Takezawa, H.; Shitozawa, K.; Fujita, M. Enhanced Reactivity of Twisted Amides inside a Molecular Cage. Nat. Chem. 2020, 12, 574–578. [Google Scholar] [CrossRef]
- Fiedler, D.; Leung, D.H.; Bergman, R.G.; Raymond, K.N. Selective Molecular Recognition, C-H Bond Activation, and Catalysis in Nanoscale Reaction Vessels. Acc. Chem. Res. 2005, 38, 349–358. [Google Scholar] [CrossRef]
- Koblenz, T.S.; Wassenaar, J.; Reek, J.N.H. Reactivity within a Confined Self-Assembled Nanospace. Chem. Soc. Rev. 2008, 37, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Kaphan, D.M.; Levin, M.D.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. A Supramolecular Microenvironment Strategy for Transition Metal Catalysis. Science 2015, 350, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic Reactions within the Cavity of Coordination Cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef]
- Jansze, S.; Cecot, M.G.; Severin, K. Reversible Disassembly of Metallasupramolecular Structures Mediated by a Metastable-State Photoacid. Chem. Sci. 2018, 9, 4253–4257. [Google Scholar] [CrossRef]
- Percastegui, E.G.; Mosquera, J.; Ronson, T.K.; Plajer, A.J.; Kieffer, M.; Nitschke, J.R. Waterproof Architectures through Subcomponent Self-Assembly. Chem. Sci. 2019, 10, 2006–2018. [Google Scholar] [CrossRef]
- Regeni, I.; Chen, B.; Frank, M.; Baksi, A.; Holstein, J.J.; Clever, G.H. Coal-Tar Dye-based Coordination Cages and Helicates. Angew. Chem. Int. Ed. 2021, 60, 5673–5678. [Google Scholar] [CrossRef]
- Yi, J.W.; Barry, N.P.E.; Furrer, M.A.; Zava, O.; Dyson, P.J.; Therrien, B.; Kim, B.H. Delivery of Floxuridine Derivatives to Cancer Cells by Water-Soluble Organometallic Cages. Bioconjugate Chem. 2012, 23, 461–471. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular Chemotherapy Based on Host-Guest Molecular Recognition: A Novel Strategy in the Battle against Cancer with a Bright Future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef]
- Howlader, P.; Mondal, S.; Ahmed, S.; Mukherjee, P.S. Guest-Induced Enantioselective Self-Assembly of a Pd6 Homochiral Octahedral Cage with a C3-Symmetric Pyridyl Donor. J. Am. Chem. Soc. 2020, 142, 20968–20972. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, B.; Drechsler, C.; Holstein, J.J.; Clever, G.H. Backbone-Bridging Promotes Diversity in Heteroleptic Cages. Angew. Chem. Int. Ed. 2021, 60, 6403–6407. [Google Scholar] [CrossRef]
- Mal, P.; Breiner, B.; Rissanen, K.; Nitschke, J.R. White Phosphorus Is Air-Stable within a Self-Assembled Tetrahedral Capsule. Science 2009, 324, 1697–1700. [Google Scholar] [CrossRef]
- Horiuchi, S.; Murase, T.; Fujita, M. Noncovalent Trapping and Stabilization of Dinuclear Ruthenium Complexes within a Coordination Cage. J. Am. Chem. Soc. 2011, 133, 12445–12447. [Google Scholar] [CrossRef]
- Takezawa, H.; Akiba, S.; Murase, T.; Fujita, M. Cavity Directed Chromism of Phthalein Dyes. J. Am. Chem. Soc. 2015, 137, 7043–7046. [Google Scholar] [CrossRef]
- Howlader, P.; Mondal, B.; Purba, P.C.; Zangrando, E.; Mukherjee, P.S. Self-Assembled Pd(II) Barrels as Containers for Transient Merocyanine Form and Reverse Thermochromism of Spiropyran. J. Am. Chem. Soc. 2018, 140, 7952–7960. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.Y.; Yan, C.; Wei, S.C.; Pan, M.; Zhang, L.; Su, C.Y. Stepwise Assembly of Pd6(RuL3)8 Nanoscale Rhombododecahedral Metal-Organic Cages via Metalloligand Strategy for Guest Trapping and Protection. J. Am. Chem. Soc. 2014, 136, 4456–4459. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt(IV) Prodrugs within a Pt(II) Cage for Drug Delivery. Chem. Sci. 2015, 6, 1189–1193. [Google Scholar] [CrossRef]
- Samanta, S.K.; Moncelet, D.; Briken, V.; Isaacs, L. Metal–Organic Polyhedron Capped with Cucurbit[8]uril Delivers Doxorubicin to Cancer Cells. J. Am. Chem. Soc. 2016, 138, 14488–14496. [Google Scholar] [CrossRef]
- Samanta, S.K.; Quigley, J.; Vinciguerra, B.; Briken, V.; Isaacs, L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc. 2017, 139, 9066–9074. [Google Scholar] [CrossRef]
- Haynes, C.J.E.; Zhu, J.; Chimerel, C.; Hernández-Ainsa, S.; Riddell, I.A.; Ronson, T.K.; Keyser, U.F.; Nitschke, J.R. Blockable Zn10L15 Ion Channels through Subcomponent Self-Assembly. Angew. Chem. Int. Ed. 2017, 56, 15388–15392. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Horike, N.; Hijikata, Y.; Kondo, M.; Carné-Sánchez, A.; Larpent, P.; Ikemura, S.; Osaki, T.; Kamiya, K.; Kitagawa, S.; et al. Metal-Organic Cuboctahedra for Synthetic Ion Channels with Multiple Conductance States. Chem 2017, 2, 393–403. [Google Scholar] [CrossRef]
- Li, Y.; Dong, J.; Gong, W.; Tang, X.; Liu, Y.; Cui, Y.; Liu, Y. Artificial Biomolecular Channels: Enantioselective Transmembrane Transport of Amino Acids Mediated by Homochiral Zirconium Metal−Organic Cages. J. Am. Chem. Soc. 2021, 143, 20939–20951. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.A.G.; Zarra, S.; Turner, R.M.; Nitschke, J.R. A Self-Organizing Chemical Assembly Line. J. Am. Chem. Soc. 2013, 135, 19143–19146. [Google Scholar] [CrossRef]
- Kumar, K.S.; Mane, V.S.; Yadav, A.; Kumbhar, A.S.; Boomishankar, R. Photochemical Hydrogen Evolution from Water by a 1D-Network of Octahedral Ni6L8 Cages. Chem. Commun. 2019, 55, 13156–13159. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Zhou, L.-Y.; Xu, L. Photocatalysis in Supramolecular Fluorescent Metallacycles and Metallacages. Chem. Asian J. 2021, 16, 3805–3816. [Google Scholar] [CrossRef]
- Morimoto, M.; Bierschenk, S.M.; Xia1, K.T.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. Advances in Supramolecular Host-Mediated Reactivity. Nat. Catal. 2020, 3, 969. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-X.; Chen, L.-J.; Yang, H.-B. Construction of Multiferrocenyl Metallacycles and Metallacages via Coordination-Driven Self-Assembly: From Structure to Functions. Chem. Soc. Rev. 2015, 44, 2148–2167. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Liuc, J.; Stang, P.J. Recent Developments in the Construction and Applications of Platinum-Based Metallacycles and Metallacages via Coordination. Chem. Soc. Rev. 2020, 49, 3889–3919. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Wang, Y.-T.; Shi, X.; Yang, H.-B.; Xu, L. Switchable Metallacycles and Metallacages. Chem. Soc. Rev. 2023, 52, 1129–1154. [Google Scholar] [CrossRef]
- Shi, L.; Wang, B.; Lu, S. Efficient bottom-up synthesis of graphene quantum dots at an atomically precise level. Matter 2023, 6, 728–760. [Google Scholar] [CrossRef]
- Tuo, W.; Xu, Y.; Fan, Y.; Li, J.; Qiu, M.; Xiong, X.; Li, X.; Sun, Y. Biomedical Applications of Pt(II) Metallacycle/Metallacage-based Agents: From Mono-Chemotherapy to Versatile Imaging Contrasts and Theranostic Platforms. Coord. Chem. Rev. 2021, 443, 214017. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; He, H.; Sun, L.; Wang, H.; Fang, X.; Zhao, Y.; Zheng, D.; Qi, Y.; Li, Z.; et al. In Situ Photodeposition of Platinum Clusters on a Covalent Organic Framework for Photocatalytic Hydrogen Production. Nat. Commun. 2022, 13, 1355. [Google Scholar] [CrossRef]
- Weng, W.; Guo, J. The Effect of Enantioselective Chiral Covalent Organic Frameworks and Cysteine Sacrificial Donors on Photocatalytic Hydrogen Evolution. Nat. Commun. 2022, 13, 5768. [Google Scholar] [CrossRef]
- Ma, S.; Deng, T.; Li, Z.; Zhang, Z.; Jia, J.; Li, Q.; Wu, G.; Xia, H.; Yang, S.-W.; Liu, X. Photocatalytic Hydrogen Production on a sp2-Carbon-Linked Covalent Organic Framework. Angew. Chem. Int. Ed. 2022, 61, e202208919. [Google Scholar] [CrossRef]
- Kondo, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Design of Metal-Organic Framework Catalysts for Photocatalytic Hydrogen Peroxide Production. Chem 2022, 8, 2924–2938. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, M.; Liu, R.; Zhang, X.; Li, G. State-of-the-Art Advancements in Photocatalytic Hydrogenation: Reaction Mechanism and Recent Progressin Metal-Organic Framework (MOF)-Based Catalysts. Adv. Sci. 2022, 9, 2103361. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for Binding Multiple Guests in Metal-Organic Cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Busschaert, N.; Caltagirone, C.; van Rossom, W.; Gale, P.A. Applications of Supramolecular Anion Recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical Applications of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Fujita, M.; Yazaki, J.; Ogura, K. Preparation of a Macrocyclic Polynuclear Complex, [(en)Pd(4,4,-bpy)]4(NO3)8, Which Recognizes an Organic Molecule in Aqueous Media. J. Am. Chem. Soc. 1990, 112, 5645–5647. [Google Scholar] [CrossRef]
- Fujita, M.; Oguro, D.; Miyazawa, M.; Oka, H.; Yamaguchi, K.; Ogura, K. Self-Assembly of Ten Molecules into Nanometer-Sized Host Frameworks. Nature 1995, 378, 469–471. [Google Scholar] [CrossRef]
- Hayes, R.; Bernard, S.A.; Imberti, S.; Warr, G.G.; Atkin, R. Solvation of Inorganic Nitrate Salts in Protic Ionic Liquids. J. Phys. Chem. C 2014, 118, 21215–21225. [Google Scholar] [CrossRef]
- Thøgersen, J.; Réhault, J.; Odelius, M.; Ogden, T.; Jena, N.K.; Jensen, S.J.K.; Keiding, S.R.; Helbing, J. Hydration Dynamics of Aqueous Nitrate. J. Phys. Chem. B 2013, 117, 3376–3388. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Tominaga, M.; Hori, A.; Therrien, B. Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res. 2005, 38, 369–378. [Google Scholar] [CrossRef]
- Tian, C.-B.; Sun, Q.-F. Combinatorial Coordination Self-Assembly for Organopalladium Cages with Fine-Tuned Structure and Function. Chem. Eur. J. 2023, e202300195. [Google Scholar] [CrossRef]
- Mal, P.; Schultz, D.; Beyeh, K.; Rissanen, K.; Nitschke, J.R. An Unlockable-Relockable Iron Cage by Subcomponent Self-Assembly. Angew. Chem. Int. Ed. 2008, 47, 8297–8301. [Google Scholar] [CrossRef]
- Ronson, T.K.; Giri, C.; Kodiah Beyeh, N.; Minkkinen, A.; Topic, F.; Holstein, J.J.; Rissanen, K.; Nitschke, J.R. Size-Selective Encapsulation of Hydrophobic Guests by Self-Assembled M4L6 Cobalt and Nickel Cages. Chem. Eur. J. 2013, 19, 3374–3382. [Google Scholar] [CrossRef]
- Smulders, M.M.J.; Zarra, S.; Nitschke, J.R. Quantitative Understanding of Guest Binding Enables the Design of Complex Host-Guest Behavior. J. Am. Chem. Soc. 2013, 135, 7039–7046. [Google Scholar] [CrossRef]
- Roukala, J.; Zhu, J.; Giri, C.; Rissanen, K.; Lantto, P.; Telkki, V.V. Encapsulation of Xenon by a Self-Assembled Fe4L6 Metallosupramolecular Cage. J. Am. Chem. Soc. 2015, 137, 2464–2467. [Google Scholar] [CrossRef]
- Du, K.; Zemerov, S.D.; Hurtado Parra, S.; Kikkawa, J.M.; Dmochowski, I.J. Paramagnetic Organocobalt Capsule Revealing Xenon Host-Guest Chemistry. Inorg. Chem. 2020, 59, 13831–13844. [Google Scholar] [CrossRef]
- Lu, Z.; Ronson, T.K.; Nitschke, J.R. Reversible Reduction Drives Anion Ejection and C60 Binding within an FeII4L6 Cage. Chem. Sci. 2020, 11, 1097–1101. [Google Scholar] [CrossRef]
- Cai, L.-X.; Li, S.-C.; Yan, D.-N.; Zhou, L.-P.; Guo, F.; Sun, Q.-F. Water-Soluble Redox-Active Cage Hosting Polyoxometalates for Selective Desulfurization Catalysis. J. Am. Chem. Soc. 2018, 140, 4869–4876. [Google Scholar] [CrossRef]
- Saalfrank, R.W.; Stark, A.; Peters, K.; von Schnering, H.G. The First “Adamantoid” Alkaline Earth Metal Chelate Complex: Synthesis, Structure, and Reactivity. Angew. Chem. Int. Ed. Engl. 1988, 27, 851–853. [Google Scholar] [CrossRef]
- Bolliger, J.L.; Belenguer, A.M.; Nitschke, J.R. Enantiopure Water-Soluble [Fe4L6] Cages: Host-Guest Chemistry and Catalytic Activity. Angew. Chem. Int. Ed. 2013, 52, 7958–7962. [Google Scholar] [CrossRef]
- Kishi, N.; Li, Z.; Yoza, K.; Akita, M.; Yoshizawa, M. An M2L4 Molecular Capsule with an Anthracene Shell: Encapsulation of Large Guests up to 1 nm. J. Am. Chem. Soc. 2011, 133, 11438–11441. [Google Scholar] [CrossRef]
- Yamashina, M.; Sei, Y.; Akita, M.; Yoshizawa, M. Safe Storage of Radical Initiators within a Polyaromatic Nanocapsule. Nat. Commun. 2014, 5, 4662. [Google Scholar] [CrossRef]
- Wang, L.-J.; Bai, S.; Han, Y.-F. Water-Soluble Self-Assembled Cage with Triangular Metal−Metal Bonded Units Enabling the Sequential Selective Separation of Alkanes and Isomeric Molecules. J. Am. Chem. Soc. 2022, 144, 16191–16198. [Google Scholar] [CrossRef]
- Lewis, J.E.M.; Crowley, J.D. Exo-and Endo-hedral Interactions of Counteranions with Tetracationic Pd2L4 Metallosupramolecular Architectures. Supramol. Chem. 2014, 26, 173–181. [Google Scholar] [CrossRef]
- Preston, D.; McNeill, S.M.; Lewis, J.E.M.; Giles, G.I.; Crowley, J.D. Enhanced Kinetic Stability of [Pd2L4]4+ Cages through Ligand Substitution. Dalton Trans. 2016, 45, 8050–8060. [Google Scholar] [CrossRef]
- Lewis, J.E.M.; Elliott, A.B.S.; McAdam, C.J.; Gordon, K.C.; Crowley, J.D. “Click” to Functionalise: Synthesis, Characterisation and Enhancement of the Physical Properties of a Series of Exo- and Endo-Functionalised Pd2L4 Nanocages. Chem. Sci. 2014, 5, 1833–1843. [Google Scholar] [CrossRef]
- Han, X.; Guo, C.; Xu, C.; Shi, L.; Liu, B.; Zhang, Z.; Bai, Q.; Song, B.; Pan, F.; Lu, S.; et al. Water-Soluble Metallo-Supramolecular Nanoreactors for Mediating Visible-Light Promoted Cross-Dehydrogenative Coupling Reactions. ACS Nano 2023, 17, 3723–3736. [Google Scholar] [CrossRef] [PubMed]
- Percástegui, E.G.; Mosquera, J.; Nitschke, J.R. Anion Exchange Renders Hydrophobic Capsules and Cargoes Water Soluble. Angew. Chem. Int. Ed. 2017, 56, 9136–9140. [Google Scholar] [CrossRef] [PubMed]
- Grommet, A.B.; Hoffman, J.B.; Percástegui, E.G.; Mosquera, J.; Howe, D.J.; Bolliger, J.L.; Nitschke, J.R. Anion Exchange Drives Reversible Phase Transfer of Coordination Cages and Their Cargoes. J. Am. Chem. Soc. 2018, 140, 14770–14776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Lavendomme, R.; Nitschke, J.R. Selective Separation of Polyaromatic Hydrocarbons by Phase Transfer of Coordination Cages. J. Am. Chem. Soc. 2019, 141, 18949–18953. [Google Scholar] [CrossRef]
- Sinha, I.; Mukherjee, P.S. Chemical Transformations in Confined Space of Coordination Architectures. Inorg. Chem. 2018, 57, 4205. [Google Scholar] [CrossRef]
- Tang, J.-H.; Li, Y.; Wu, Q.; Wang, Z.; Hou, S.; Tang, K.; Sun, Y.; Wang, H.; Wang, H.; Lu, C.; et al. Single-Molecule Level Control of Host-Guest Interactions in Metallocycle-C60 Complexes. Nat. Commun. 2019, 10, 4599. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Catti, L. Bent Anthracene Dimers as Versatile Building Blocks for Supramolecular Capsules. Acc. Chem. Res. 2019, 52, 2392. [Google Scholar] [CrossRef]
- Roy, B.; Ghosh, A.K.; Srivastava, S.; D’Silva, P.; Mukherjee, P.S. A Pd8 Tetrafacial Molecular Barrel as Carrier for Water Insoluble Fluorophore. J. Am. Chem. Soc. 2015, 137, 11916. [Google Scholar] [CrossRef]
- Acharyya, K.; Bhattacharyya, S.; Sepehrpour, H.; Chakraborty, S.; Lu, S.; Shi, B.; Li, X.; Mukherjee, P.S.; Stang, P.J. Self-Assembled Fluorescent Pt(II) Metallacycles as Artificial Light-Harvesting Systems. J. Am. Chem. Soc. 2019, 141, 14565. [Google Scholar] [CrossRef]
- Li, Z.; Yan, X.; Huang, F.; Sepehrpour, H.; Stang, P.J. NearInfrared Emissive Discrete Platinum (II) Metallacycles: Synthesis and Application in Ammonia Detection. Org. Lett. 2017, 19, 5728. [Google Scholar] [CrossRef]
- Zhang, M.; Saha, M.L.; Wang, M.; Zhou, Z.; Song, B.; Lu, C.; Yan, X.; Li, X.; Huang, F.; Yin, S.; et al. Multicomponent Platinum(II) Cages with Tunable Emission and Amino Acid Sensing. J. Am. Chem. Soc. 2017, 139, 5067. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wu, L.; Lu, S.; Ling, S.; Li, G.; Xu, L.; Ma, L.; Hou, Y.; Wang, X.; et al. Emissive Platinum(II) Cages with Reverse Fluorescence Resonance Energy Transfer for Multiple Sensing. J. Am. Chem. Soc. 2020, 142, 2592. [Google Scholar] [CrossRef]
- Chen, L.-J.; Yang, H.-B. Construction of Stimuli-Responsive Functional Materials via Hierarchical Self-Assembly Involving Coordination Interactions. Acc. Chem. Res. 2018, 51, 2699. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Chowdhury, A.; Saha, R.; Mukherjee, P.S. Multifunctional Self-Assembled Macrocycles with Enhanced Emission and Reversible Photochromic Behavior. Inorg. Chem. 2019, 58, 3968. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Maity, M.; Chowdhury, A.; Saha, M.L.; Panja, S.K.; Stang, P.J.; Mukherjee, P.S. Coordination-Assisted Reversible Photoswitching of Spiropyran-Based Platinum Macrocycles. Inorg. Chem. 2020, 59, 2083. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Klosterman, J.K.; Fujita, M. Functional Molecular Flasks: New Properties and Reactions within Discrete, Self-Assembled Hosts. Angew. Chem. Int. Ed. 2009, 48, 3418–3438. [Google Scholar] [CrossRef]
- Raynal, M.; Ballester, P.; Vidal-Ferran, A.; Van Leeuwen, P.W.N.M. Supramolecular Catalysis. Part 2: Artificial Enzyme Mimics. Chem. Soc. Rev. 2014, 43, 1734–1787. [Google Scholar] [CrossRef]
- Yao, W.; Wang, J.; Zhong, A.; Wang, S.; Shao, Y. Transition-Metal-Free Catalytic Hydroboration Reduction of Amides to Amines. Org. Chem. Front. 2020, 7, 3515–3520. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, C.-J.; Feng, Q.-Z.; Zhai, J.-J.; Qi, S.-S.; Zhong, A.-G.; Chu, M.-M.; Xu, D.-Q. Copper-Catalyzed Asymmetric 1,6-Conjugate Addition of in Situ Generated Para-Quinone Methides with β-Ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.-F.; Cheng, J.-Z.; Zhong, A.-G.; Wen, H.-R.; Liu, S.-Y. Novel Diketopyrrolopyrrole-Based π-Conjugated Molecules Synthesized Via One-Pot Direct Arylation Reaction. Molecules 2019, 24, 1760. [Google Scholar] [CrossRef]
- Yao, W.; He, L.; Han, D.; Zhong, A. Sodium Triethylborohydride-Catalyzed Controlled Reduction of Unactivated Amides to Secondary or Tertiary Amines. J. Org. Chem. 2019, 84, 14627–14635. [Google Scholar] [CrossRef]
- Mozaceanu, C.; Taylor, C.G.P.; Piper, J.R.; Argent, S.P.; Ward, M.D. Catalysis of an Aldol Condensation Using a Coordination Cage. Chemistry 2020, 2, 22–32. [Google Scholar] [CrossRef]
- Bender, T.A.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. A Supramolecular Strategy for Selective Catalytic Hydrogenation Independent of Remote Chain Length. J. Am. Chem. Soc. 2019, 141, 11806–11810. [Google Scholar] [CrossRef] [PubMed]
- Bierschenk, S.M.; Bergman, R.G.; Raymond, K.N.; Toste, F.D. A Nanovessel-Catalyzed Three-Component Aza-Darzens Reaction. J. Am. Chem. Soc. 2020, 142, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Cavity-Directed, Highly Stereoselective [2 + 2] Photodimerization of Olefins within Self-Assembled Coordination Cages. Angew. Chem. Int. Ed. 2002, 41, 1347–1349. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Takeyama, Y.; Okano, T.; Fujita, M. Cavity Directed Synthesis within a Self-Assembled Coordination Cage: Highly Selective [2 + 2] Cross-Photodimerization of Olefins. J. Am. Chem. Soc. 2003, 125, 3243–3247. [Google Scholar] [CrossRef]
- Nishioka, Y.; Yamaguchi, T.; Yoshizawa, M.; Fujita, M. Unusual [2 + 4] and [2 + 2] Cycloadditions of Arenes in the Confined Cavity of Self-Assembled Cages. J. Am. Chem. Soc. 2007, 129, 7000–7001. [Google Scholar] [CrossRef]
- Nishioka, Y.; Yamaguchi, T.; Kawano, M.; Fujita, M. Asymmetric [2 + 2] Olefin Cross Photoaddition in a Self-Assembled Host with Remote Chiral Auxiliaries. J. Am. Chem. Soc. 2008, 130, 8160–8161. [Google Scholar] [CrossRef]
- Horiuchi, S.; Nishioka, Y.; Murase, T.; Fujita, M. Both [2 + 2] and [2 + 4] Additions of Inert Aromatics via Identical Ternary Host Guest Complexes. Chem. Commun. 2010, 46, 3460–3462. [Google Scholar] [CrossRef]
- Furusawa, T.; Kawano, M.; Fujita, M. The Confined Cavity of a Coordination Cage Suppresses the Photocleavage of α-Diketones to Give Cyclization Products through Kinetically Unfavorable Pathways. Angew. Chem. Int. Ed. 2007, 46, 5717–5719. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fujita, M. Highly Selective Photomediated 1,4-Radical Addition to o-Quinones Controlled by a Self-Assembled Cage. Angew. Chem. Int. Ed. 2008, 47, 2067–2069. [Google Scholar] [CrossRef]
- Gera, R.; Das, A.; Jha, A.; Dasgupta, J. Light-Induced Proton Coupled Electron Transfer is idea Nanocage. J. Am. Chem. Soc. 2014, 136, 15909–15912. [Google Scholar] [CrossRef]
- Das, A.; Mandal, I.; Venkatramani, R.; Dasgupta, J. Ultrafast Photoactivation of C-H Bonds inside Water-Soluble Nanocages. Sci. Adv. 2019, 5, eaav4806. [Google Scholar] [CrossRef]
- Gera, R.; Dasgupta, J. Photochemistry Using a Host-Guest Charge Transfer Paradigm: DMABN as a Dynamical Probe of Ground and Excited States. Phys. Chem. Chem. Phys. 2021, 23, 9280–9284. [Google Scholar] [CrossRef]
- Guo, X.-Q.; Zhou, L.-P.; Hu, S.-J.; Cai, L.-X.; Cheng, P.-M.; Sun, Q.-F. Hexameric Lanthanide–Organic Capsules with Tertiary Structure and Emergent Functions. J. Am. Chem. Soc. 2021, 143, 6202–6210. [Google Scholar] [CrossRef]
- Yan, D.N.; Cai, L.X.; Cheng, P.M.; Hu, S.J.; Zhou, L.P.; Sun, Q.F. Photooxidase Mimicking with Adaptive Coordination Molecular Capsules. J. Am. Chem. Soc. 2021, 143, 16087–16094. [Google Scholar] [CrossRef]
- Yan, D.N.; Cai, L.X.; Hu, S.J.; Zhou, Y.F.; Zhou, L.P.; Sun, Q.F. An Organo-Palladium Host Built from a Dynamic Macrocyclic Ligand: Adaptive Self-Assembly, Induce-Fit Guest Binding, and Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202209879. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Miyagi, S.; Kawano, M.; Ishiguro, K.; Fujita, M. Alkane Oxidation via Photochemical Excitation of a Self-Assembled Molecular Cage. J. Am. Chem. Soc. 2004, 126, 9172–9173. [Google Scholar] [CrossRef]
- Cullen, W.; Takezawa, H.; Fujita, M. Demethylenation of Cyclopropanes via Photoinduced Guest-to-Host Electron Transfer in an M6L4 Cage. Angew. Chem. Int. Ed. 2019, 58, 9171–9173. [Google Scholar] [CrossRef]
- Murase, T.; Takezawa, H.; Fujita, M. Photo-Driven Anti Markovnikov Alkyne Hydration in Self-Assembled Hollow Complexes. Chem. Commun. 2011, 47, 10960–10962. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Nishijima, Y.; Fujita, M. Unusual Photoreaction of Triquinacene within Self-Assembled Hosts. Chem. Asian J. 2012, 7, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.M.; Ellis, S.R.; Nichols, E.M.; Mathies, R.A.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular Ga4L612− Cage Photosensitizes 1,3-Rearrangement of Encapsulated Guest via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2015, 137, 10128–10131. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.W.; Li, K.; Xiao, L.M.; Chen, S.; Wu, K.; Chen, X.D.; Fan, Y.Z.; Liu, J.M.; Su, C.-Y. Regio- and Enantioselective Photodimerization within the Confined Space of a Homochiral Ruthenium/Palladium Heterometallic Coordination Cage. Angew. Chem. Int. Ed. 2017, 56, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fan, Y.Z.; Lu, Y.L.; Zheng, S.P.; Su, C.-Y. VisibleLight Photocatalysis of Asymmetric [2 + 2] Cycloaddition in Cage Confined Nanospace Merging Chirality with Triplet-State Photosensitization. Angew. Chem. Int. Ed. 2020, 59, 8661–8669. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wu, K.; Yin, C.; Li, K.; Huang, Y.; Ruan, J.; Feng, X.; Hu, P.; Su, C.-Y. Cage-Confined Photocatalysis for Wide-Scope Unusually Selective [2 + 2] Cycloaddition through Visible-Light Triplet Sensitization. Nat. Commun. 2020, 11, 4675. [Google Scholar] [CrossRef]
- Jin, Y.; Jiang, H.; Tang, X.; Zhang, W.; Liu, Y.; Cui, Y. Coordination-Driven Self-Assembly of Anthraquinone-Based MetalOrganic Cages for Photocatalytic Selective [2 + 2] Cycloaddition. Dalton Trans. 2021, 50, 8533–8539. [Google Scholar] [CrossRef]
- Wu, K.; Li, K.; Chen, S.; Hou, Y.-J.; Lu, Y.-L.; Wang, J.-S.; Wei, M.-J.; Su, C.-Y. The Redox Coupling Effect in a Photocatalytic RuII-PdII Cage with TTF Guest as Electron Relay Mediator for Visible-Light Hydrogen-Evolving Promotion. Angew. Chem. Int. Ed. 2020, 59, 2639–2643. [Google Scholar] [CrossRef]
- Jing, Y.; Li, S.; Su, M.; Bao, H.; Wan, W. Barbier Hyperbranching Polymerization-Induced Emission toward Facile Fabrication of White Light-Emitting Diode and Light-Harvesting Film. J. Am. Chem. Soc. 2019, 141, 16839–16848. [Google Scholar] [CrossRef]
- Lee, S.H.; Matula, A.J.; Hu, G.; Troiano, J.L.; Karpovich, C.J.; Crabtree, R.H.; Batista, V.S.; Brudvig, G.W. Strongly Coupled Phenazine-Porphyrin Dyads: Light-Harvesting Molecular Assemblies with Broad Absorption Coverage. ACS Appl. Mater. Interfaces 2019, 11, 8000–8008. [Google Scholar] [CrossRef]
- Closs, G.L.; Miller, J.R. Intramolecular Long-Distance Electron Transfer in Organic Molecules. Science 1988, 240, 440. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.-J.; Zhang, Y.; Hu, Y.-X.; Jiang, W.-L.; Yin, G.-Q.; Tan, H.; Li, X.; Xu, L.; Yang, H.-B. Photoswitchable Förster resonance energy transfer (FRET) within a heterometallic Ir–Pt macrocycle. Chem. Commun. 2019, 55, 11119–11122. [Google Scholar] [CrossRef]
- Catti, L.; Kishida, N.; Kai, T.; Akita, M.; Yoshizawa, M. Polyaromatic Nanocapsules as Photoresponsive Hosts in Water. Nat. Commun. 2019, 10, 1948. [Google Scholar] [CrossRef]
- Jiang, N.; Yuan, Z.; Li, T.; Zhu, Y.; Chen, Y.-S.; Lin, L.; Zhang, J.; Chan, Y.-T.; Wang, J. Synthesis and Characterization of Ferrocene Based Hemicages. J. Org. Chem. 2018, 83, 4824–4830. [Google Scholar] [CrossRef]
- Huo, G.-F.; Shi, X.; Tu, Q.; Hu, Y.-X.; Wu, G.-Y.; Yin, G.-Q.; Li, X.; Xu, L.; Ding, H.-M.; Yang, H.-B. Radical-Induced Hierarchical Self-Assembly Involving Supramolecular Coordination Complexes in Both Solution and Solid States. J. Am. Chem. Soc. 2019, 141, 16014–16023. [Google Scholar] [CrossRef]
- He, Y.-Q.; Fudickar, W.; Tang, J.-H.; Wang, H.; Li, X.; Han, J.; Wang, Z.; Liu, M.; Zhong, Y.-W.; Linker, T.; et al. Capture and Release of Singlet Oxygen in Coordination-Driven Self-Assembled Organoplatinum(II) Metallacycles. J. Am. Chem. Soc. 2020, 142, 2601–2608. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Hou, Y.; Wang, H.; Li, X.; He, G.; Zhang, M. Aqueous Platinum(II)-Cage-Based Light-Harvesting System for Photocatalytic Cross-Coupling Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2019, 58, 8862–8866. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, R.; Mukherjee, P.S. Self-Assembled Metallasupramolecular Cages towards Light Harvesting Systems for Oxidative Cyclization. Chem. Sci. 2021, 12, 5319–5329. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.; Shi, L.; Liu, X.; Ya, H.; Han, X. Photocatalysis in Water-Soluble Supramolecular Metal Organic Complex. Molecules 2023, 28, 4068. https://doi.org/10.3390/molecules28104068
Hong D, Shi L, Liu X, Ya H, Han X. Photocatalysis in Water-Soluble Supramolecular Metal Organic Complex. Molecules. 2023; 28(10):4068. https://doi.org/10.3390/molecules28104068
Chicago/Turabian StyleHong, Dongfeng, Linlin Shi, Xianghui Liu, Huiyuan Ya, and Xin Han. 2023. "Photocatalysis in Water-Soluble Supramolecular Metal Organic Complex" Molecules 28, no. 10: 4068. https://doi.org/10.3390/molecules28104068
APA StyleHong, D., Shi, L., Liu, X., Ya, H., & Han, X. (2023). Photocatalysis in Water-Soluble Supramolecular Metal Organic Complex. Molecules, 28(10), 4068. https://doi.org/10.3390/molecules28104068




