Chemical Composition and In Vitro Antioxidant Activity of Salvia aratocensis (Lamiaceae) Essential Oils and Extracts
Abstract
1. Introduction
2. Results
2.1. Essential Oil and Extract Yields
2.2. Essential Oil Chemical Characterization
2.3. Extract Chemical Characterization
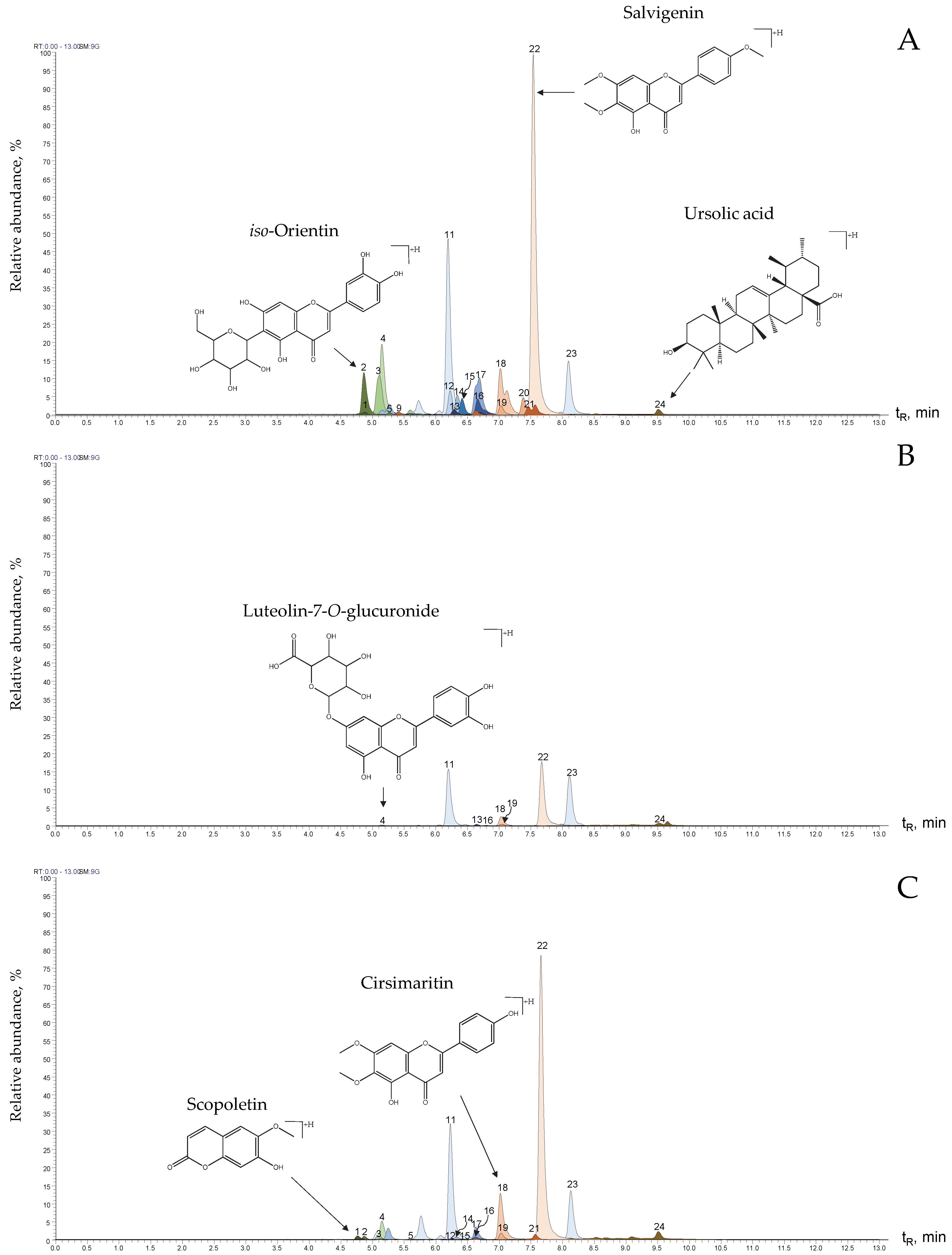
| Peak N° Figure 4 | Compound | Formula | Exact Experimental Mass, m/z | ∆ ppm | HCD, eV | Product Ions | Formula | m/z, I (%) | Identification Criteria | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Scopoletin | C10H8O4 | [M+H]+, 193.04939 | 0.78 | 20 | [(M+H)-CH3]+• | C9H6O4 | 178.02588 (5%) | a, b, c | |
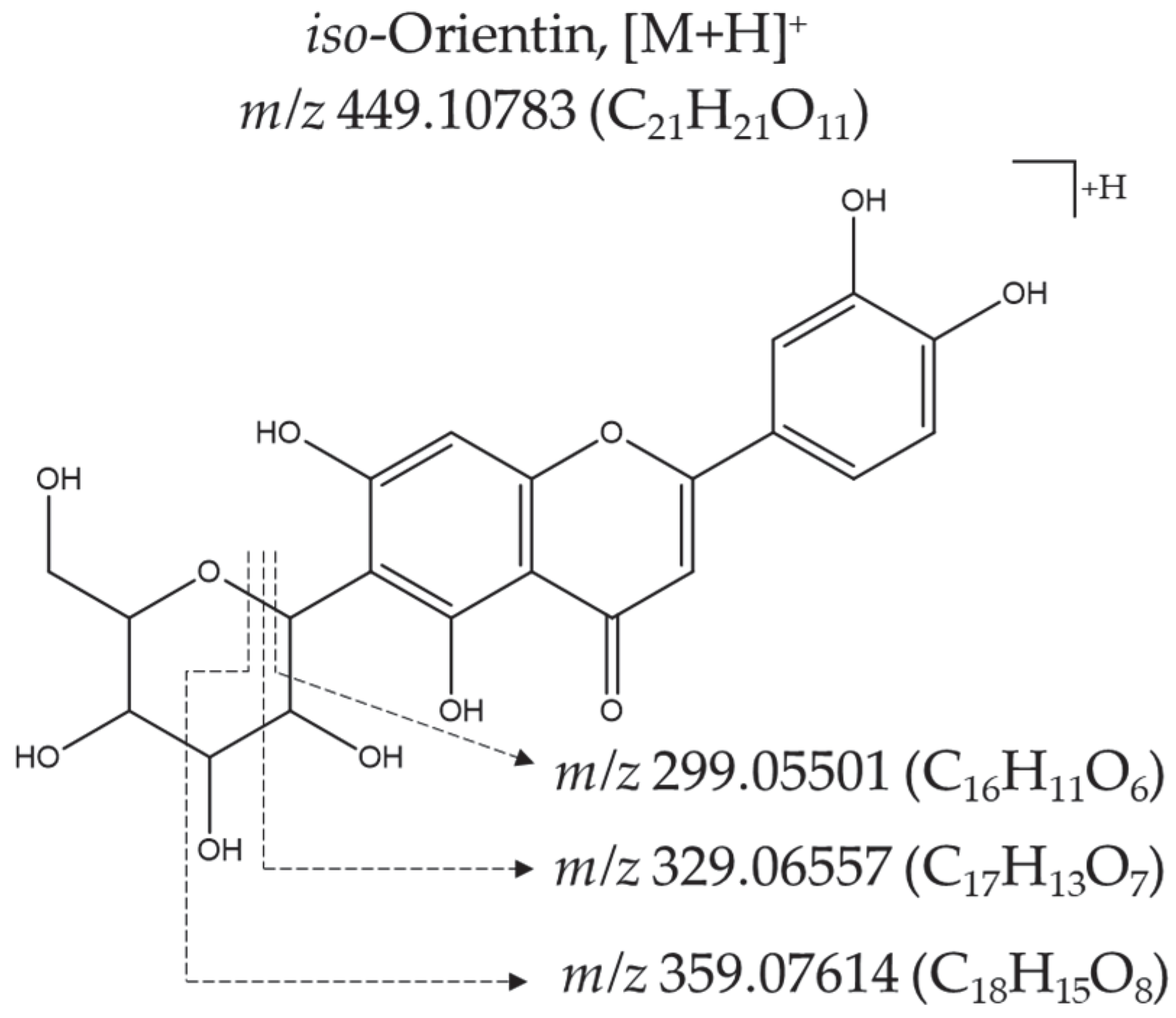
| 2 | iso-Orientin | C21H20O11 | [M+H]+, 449.10739 | 1 | 30 | [(M+H)-H2O]+ | C21H19O10 | 431.09753 (42%) | a, b | [18] |
| [(M+H)-2H2O]+ | C21H17O9 | 413.08694 (64%) | ||||||||
| [(M+H)-3H2O]+ | C21H15O8 | 395.07559 (25%) | ||||||||
| [(M+H)-C3H6O3]+ | C18H15O8 | 359.07562 (15%) | ||||||||
| [(M+H)-2H2O-C2H4O2]+ | C19H13O7 | 353.06500 (30%) | ||||||||
| [(M+H)-C4H8O4]+ | C17H13O7 | 329.06589 (100%) | ||||||||
| [(M+H)-C5H10O5]+ | C16H11O6 | 299.05457 (38%) | ||||||||
| [M−H]−, 447.09341 | 1 | 40 | [(M−H)-C3H6O3]− | C18H13O8 | 357.06131 (15%) | |||||
| [(M−H)-C4H8O4]− | C17H11O7 | 327.05112 (100%) | ||||||||
| [(M−H)-C5H8O5]− | C16H11O6 | 299.05576 (37%) | ||||||||
| 3 | Vitexin | C21H20O10 | [M+H]+, 433.11313 | 0.2 | 30 | [(M+H)-H2O]+ | C21H19O9 | 415.10272 (47%) | a, b, c | [21] |
| [(M+H)-2H2O]+ | C21H17O8 | 397.09216 (69%) | ||||||||
| [(M+H)-C4H8O4]+ | C17H13O6 | 313.07089 (100%) | ||||||||
| [(M+H)-C5H10O5]+ | C16H11O5 | 283.05991 (40%) | ||||||||
| [M−H]−, 431.09833 | 0.1 | 20 | [(M−H)-C3H6O3]− | C18H13O7 | 341.06644 (9%) | |||||
| [(M−H)-C4H8O4]− | C17H11O6 | 311.05588 (100%) | ||||||||
| [(M−H)-C5H8O5]− | C16H11O5 | 283.06094 (18%) | ||||||||
| 4 | Luteolin-7-O-glucuronide | C21H18O12 | [M+H]+, 463.08655 | 1.19 | 10 | [(M+H)-C6H8O6]+ | C15H11O6 | 287.05501 (34%) | a, b, c | [20] |
| [M−H]−, 461.07275 | 0.4 | 10 | [(M−H)-C6H8O6]− | C15H9O6 | 285.04047 (25%) | |||||
| 5 | Luteolin-7-O-glucoside | C21H20O11 | [M+H]+, 449.10730 | 1 | 10 | [(M+H)-C6H10O5]+ | C15H11O6 | 287.05460 (74%) | a, b, c | [9,19,20,21,22] |
| [M−H]−, 447.09332 | 1 | 10 | [(M−H)-C6H10O5]− | C15H9O6 | 285.04047 (15%) | |||||
| 6 | Salvianolic acid A | C26H22O10 | [M−H]−, 493.11432 | 0.6 | 20 | [(M−H)-C9H10O5]− | C17H11O5 | 295.06122 (14%) | a, b | [23,24,25] |
| [(M−H)-C15H16O7]− | C11H5O3 | 185.02362 (37%) | ||||||||
| 7 | Salvianolic acid B | C36H30O16 | [M−H]−, 717.14611 | 0.6 | 20 | [(M−H)-C9H8O4]− | C27H21O12 | 537.10437 (43%) | a, b | [21,23,24,25,26] |
| [(M−H)-C9H10O5]− | C27H19O11 | 519.09265 (36%) | ||||||||
| [(M−H)-C18H14O8]− | C18H15O8 | 359.07706 (100%) | ||||||||
| [(M−H)-C18H20O10]− | C18H9O6 | 321.04056 (37%) | ||||||||
| [(M−H)-C19H18O11]− | C17H11O5 | 295.06061 (34%) | ||||||||
| 8 | Rutin | C27H30O16 | [M+H]+, 611.16034 | 0.5 | 20 | [(M+H)-C6H10O4]+ | C21H21O12 | 465.10254 (5%) | a, b, c | [11,21] |
| [(M+H)-C6H10O4-C6H10O5]+ | C15H11O7 | 303.04941 (100%) | ||||||||
| [M−H]−, 609.14673 | 0.5 | 20 | [(M−H)-C6H10O4]− | C21H19O12 | 463.08844 (16%) | |||||
| [(M−H)-C12H20O9]− | C15H9O7 | 301.03513 (12%) | ||||||||
| 9 | Quercetin-3-O-glucoside | C21H20O12 | [M+H]+, 465.10236 | 0.9 | 30 | [(M+H)-C6H10O5]+ | C15H11O7 | 303.04941 (100%) | a, b, c | [9,22] |
| [M−H]−, 463.08829 | 0.2 | 20 | [(M−H)-C6H10O5]− | C15H9O7 | 301.03513 (12%) | |||||
| 10 | Rosmarinic acid | C18H16O8 | [M+H]+, 361.09143 | 1 | 10 | [(M+H)-C9H10O5]+ | C9H7O3 | 163.03868 (26%) | a, b, c | [19,21,22] |
| [M−H]−, 359.07736 | 0.3 | 10 | [(M−H)-C9H10O5]− | C9H5O3 | 161.02362 (100%) | |||||
| 11 | N.I. (Figure S3) | C20H22O5 | [M+H]+, 343.15353 | 1.3 | 20 | [(M+H)-H2O]+ | C20H21O4 | 325.14294 (69%) | ||
| [(M+H)-H2O-CO]+ | C19H21O3 | 297.14810 (47%) | ||||||||
| [(M+H)-H2O-CO-H2O]+ | C19H19O2 | 279.13757 (20%) | ||||||||
| 12 | Naringenin | C15H12O5 | [M+H]+, 273.07553 | 0.8 | 20 | [(M+H)-C8H8O]+ | C7H5O4 | 153.0181 (100%) | a, b, c | [19] |
| [M−H]−, 271.06134 | 0.5 | 20 | [(M−H)-C8H8O]− | C7H3O4 | 151.00285 (70%) | |||||
| [(M−H)-C7H4O4]− | C8H7O | 119.04929 (56%) | ||||||||
| 13 | Methyl-luteolin | C16H12O6 | [M+H]+, 301.07089 | 0.76 | 30 | [(M+H)-CH3]+• | C15H10O6 | 286.04736 (99%) | a, b | [10] |
| [(M+H)-C9H8O2]+ | C7H5O4 | 153.01846 (20%) | ||||||||
| [M−H]−, 299.05609 | 0.06 | 20 | [(M−H)-CH3]−• | C15H8O6 | 284.03287 (99%) | |||||
| 14 | Luteolin | C15H10O6 | [M+H]+, 287.05478 | 0.82 | 30 | [(M+H)-C8H6O2]+ | C7H5O4 | 153.01837(38%) | a, b, c | [10,11] |
| [M−H]−, 285.04046 | 0.3 | 30 | [(M−H)-C5H2O3]− | C10H7O3 | 175.03937 (11%) | |||||
| [(M−H)-C8H6O2]− | C7H3O4 | 151.00281 (20%) | ||||||||
| [(M−H)-C7H4O4]− | C8H5O2 | 133.02858 (72%) | ||||||||
| 15 | N.I. (Figure S4) | C31H26O15 | [M+H]+, 639.13336 | 1.69 | 20 | [(M+H)-H2O]+ | C31H25O14 | 621.12427 (63%) | ||
| [(M+H)-2H2O]+ | C31H23O13 | 603.11332 (17%) | ||||||||
| [(M+H)-C14H14O8]+ | C17H13O7 | 329.06497 (79%) | ||||||||
| [(M+H)-C15H16O9]+ | C16H11O6 | 299.05444 (7%) | ||||||||
| [(M+H)-C21H20O11]+ | C10H7O4 | 191.03363 (40%) | ||||||||
| [M−H]−, 637.11989 | 0.35 | 20 | [(M−H)-C10H8O5]− | C21H17O10 | 429.08243 (9%) | |||||
| [(M−H)-C13H12O7]− | C18H13O8 | 357.06137 (83%) | ||||||||
| [(M−H)-C14H14O8]− | C17H11O7 | 327.05075 (100%) | ||||||||
| [(M−H)-C15H16O9]− | C16H9O6 | 297.04013 (3%) | ||||||||
| 16 | Apigenin | C15H10O5 | [M+H]+, 271.06030 | 0.74 | 30 | [(M+H)-C8H6O]+ | C7H5O4 | 153.01848 (36%) | a, b, c | [10] |
| [M−H]−, 269.04565 | 0.4 | 30 | [(M−H)-C8H6O]− | C7H3O4 | 151.00281 (8%) | |||||
| [(M−H)-C7H4O4]− | C8H5O | 117.03355 (50%) | ||||||||
| 17 | Jaceosidin | C17H14O7 | [M+H]+, 331.08122 | 0.06 | 30 | [(M+H)-CH3]+• | C16H12O7 | 316.05811 (22%) | a, b | [10] |
| [(M+H)-CH3-H2O]+• | C16H10O6 | 298.04739 (100%) | ||||||||
| [(M+H)-C9H8O2]+ | C8H7O5 | 183.02911 (10%) | ||||||||
| [M−H]−, 329.06680 | 0.38 | 20 | [(M−H)-CH3]−• | C16H10O7 | 314.04330 (32%) | |||||
| [(M−H)-CH3-CH3]− | C15H7O7 | 299.01984 (36%) | ||||||||
| 18 | Cirsimaritin | C17H14O6 | [M+H]+, 315.08646 | 0.45 | 40 | [(M+H)-CH3]+• | C16H12O6 | 300.06302 (5%) | a, b, c | [10,19,22,27] |
| [(M+H)-CH3-H2O]+• | C16H10O5 | 282.05249 (5%) | ||||||||
| [(M+H)-CH3-C9H5O2]+• | C7H6O4 | 154.02632 (31%) | ||||||||
| [M−H]−, 313.07193 | 0.5 | 20 | [(M−H)-CH3]−• | C16H10O6 | 298.04831 (13%) | |||||
| [(M−H)-CH3-CH3]− | C15H7O6 | 283.02496 (23%) | ||||||||
| 19 | Eupatilin | C18H16O7 | [M+H]+, 345.09686 | 0.57 | 30 | [(M+H)-CH3]+• | C17H14O7 | 330.07269 (55%) | a, b, c | [10,24] |
| [(M+H)-CH3-CH3]+ | C16H11O7 | 315.04953 (<1%) | ||||||||
| [M−H]−, 343.08237 | 0.11 | 30 | [(M−H)-CH3]−• | C17H12O7 | 328.05847 (100%) | |||||
| [(M−H)-CH3-CH3]− | C16H9O7 | 313.03516 (37%) | ||||||||
| 20 | Cirsilineol | C18H16O7 | [M+H]+, 345.09686 | 0.05 | 30 | [(M+H)-CH3]+• | C17H14O7 | 330.07278 (32%) | a, b | [24] |
| [(M+H)-CH3-CH3]+ | C16H11O7 | 315.04950 (16%) | ||||||||
| [M−H]−, 343.08243 | 0.29 | 30 | [(M−H)-CH3]−• | C17H12O7 | 328.05383 (84%) | |||||
| [(M−H)-CH3-CH3]− | C16H9O7 | 313.03571 (5%) | ||||||||
| 21 | Acacetin | C16H12O5 | [M+H]+, 285.07547 | 0.98 | 30 | [(M+H)-CH3]+• | C15H10O5 | 270.05185 (21%) | b, c | [24] |
| [(M+H)-CH3-CO]+• | C14H10O4 | 242.05701 (43%) | ||||||||
| [M−H]−, 283.06199 | 0.03 | 20 | [(M−H)-CH3]−• | C15H8O5 | 268.03662 (21%) | |||||
| 22 | Salvigenin | C18H16O6 | [M+H]+, 329.10190 | 0.2 | 30 | [(M+H)-CH3]+• | C17H14O6 | 314.07843 (17%) | b, c | |
| [(M+H)-CH3-H2O]+• | C17H12O5 | 296.06790 (16%) | ||||||||
| [(M+H)-CH3-H2O-CO]+• | C16H12O4 | 268.07309 (5%) | ||||||||
| [(M+H)-CH3-H2O-2CO]+• | C15H12O3 | 240.07835 (100%) | ||||||||
| 23 | N.I. (Figure S5) | C20H22O5 | [M+H]+, 343.15372 | 0.8 | 10 | [(M+H)-C3H8]+ | C17H15O5 | 299.09088 (15%) | ||
| [(M+H)-C10H12O3]+ | C10H11O2 | 163.07530 (100%) | ||||||||
| [(M+H)-C10H12O3-C3H6O]+ | C7H5O | 105.03375 (7%) | ||||||||
| 24 | Ursolic acid | C30H48O3 | [M+H]+, 457.36713 | 1.1 | 10 | [(M+H)-H2O]+ | C30H47O2 | 439.35706 (48%) | b, c | [24] |
| [M−H]−, 455.35333 | 0.6 | - | - | - | - |
2.4. Antioxidant Activity
3. Discussion
3.1. Salvia aratocensis Essential Oil Chemical Characterization
3.2. Salvia aratocensis Extract’s Chemical Characterization
3.3. Antioxidant Activity of Essential Oil and Extracts
4. Materials and Methods
4.1. Reagents
4.2. Plant Material
4.3. Essential Oil Distillation
4.4. Extraction
4.5. GC/FID and GC/MS Essential Oil Analysis
4.6. UHPL-ESI(+/−)-Orbitrap-HRMS Analysis of the Extracts
4.7. Antioxidant Activity
4.7.1. ABTS+• Assay
4.7.2. ORAC Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- González-Gallegos, J.; Bedolla-García, B.; Cornejo-Tenorio, G.; Fernández-Alonso, J.; Fragoso-Martínez, I.; García-Peña, M.; Harley, R.; Klitgaard, B.; Martínez-Gordillo, M.; Wood, J.; et al. Richness and Distribution of Salvia Subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 2020, 181, 831–856. [Google Scholar] [CrossRef]
- Adımcılar, V.; Kalaycıoğlu, Z.; Aydoğdu, N.; Dirmenci, T.; Kahraman, A.; Bedia-Erim, F. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic of 14 Salvia species from Anatolia. J. Pharm. Biomed. Anal. 2019, 175, 112763. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Protección Social. Vademécum Colombiano de Plantas Medicianles, 1st ed.; Artes y Sistemas Integrados Ltda: Bogotá, Colombia, 2008; pp. 1–311. [Google Scholar]
- Fernández-Alonso, J.L. Studies in Colombian Labiatae IV. Novelties in Salvia and synopsis of sections Angulatae and Purpureae José Luis Fernández-Alonso. Caldasia 2003, 25, 235–281. [Google Scholar]
- Fernández-Alonso, J.L. Catálogo de Plantas y Líquenes de Colombia; Bernal, R., Gradstein, S.R., Celis, M., Eds.; Instituto de Ciencias Naturales de la Universidad Nacional de Colombia: Bogotá, Colombia, 2018; pp. 1436–1441. [Google Scholar]
- Bueno, J.; Escobar, P.; Martínez, J.R.; Leal, S.M.; Stashenko, E.E. Composition of three essential oils, and their mammalian cell toxicity and antimycobacterial activity against drug resistant-tuberculosis and nontuberculous mycobacteria strains. Nat. Prod. Commun. 2011, 6, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.; Mejía, J.; Córdoba, Y.; Martínez, J.R.; Stashenko, E.E.; del Valle, J.M. Optimization of flavonoids extraction from Lippia graveolens and Lippia origanoides chemotypes with ethanol-modified supercritical CO2 after steam distillation. Ind. Crop. Prod. 2020, 146, 112170. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Celano, R.; Piccinelli, A.L.; Pagano, I.; Roscigno, G.; Campone, L.; De Falco, E.; Rastrelli, L. Oil distillation wastewaters from aromatic herbs as new natural source of antioxidant compounds. Food Res. Int. 2017, 99, 298–307. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.W.; Lee, M.K.; Kim, Y.J.; Asamenew, G.; Cha, Y.S.; Kim, J.B. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Technol. 2018, 244, 1637–1646. [Google Scholar] [CrossRef]
- Ivanov, M.; Božunović, J.; Gašić, U.; Drakulić, D.; Stevanović, M.; Rajčević, N.; Stojković, D.Y. Bioactivities of Salvia nemorosa L. inflorescences are influenced by the extraction solvents. Ind. Crop. Prod. 2022, 175, 114260. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Cardeño, V.; Molina, C.; García, T.; Stashenko, E.E. Antioxidant activity and total phenol content of the ethanolic extracts of Salvia aratocensis, S. sochensis, Bidens reptons, and Montanoa ovalifolia. Sci. Tech. 2007, 1, 205–207. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011, 40, 043101. [Google Scholar] [CrossRef]
- Nist Standard Reference Database. NIST/EPA/NIH Spectral Library with Search Program, Version 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–804. [Google Scholar]
- McLafferty, F.W.; Douglas, B.S. The Wiley/NBS Registry of Mass Spectral Data, 2nd ed.; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Li, Q.M.; Van den Heuvel, H.; Dillen, L.; Claeys, M. Differentiation of 6-C- and 8-C-glycosidic flavonoids by positive ion fast atom bombardment and tandem mass spectrometry. Biol. Mass Spectrom. 1992, 21, 213–221. [Google Scholar] [CrossRef]
- Zúñiga-López, M.C.; Maturana, G.; Campmajó., G.; Saurina., J.; Núñez, O. Determination of bioactive compounds in sequential extracts of chia leaf (Salvia hispanica L.) using UHPLC-HRMS (Q-Orbitrap) and a global evaluation of antioxidant in vitro capacity. Antioxidants 2021, 10, 1151. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and S. fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef] [PubMed]
- Shojaeifard, A.R.; Hemmateenejad, Z.; Jassbi, B. Chemometrics-based LC-UV-ESIMS analyses of 50 Salvia species for detecting their antioxidant constituents. J. Pharma. Biomed. Anal. 2021, 193, 113745. [Google Scholar] [CrossRef]
- Samani, M.R.; D’Urso, G.; Montoro, P.; Ghasemi, P.A.; Piacente, S. Effects of bio-fertilizers on the production of specialized metabolites in Salvia officinalis L. leaves: An analytical approach based on LC-ESI/LTQ-Orbitrap/MS and multivariate data analysis. J. Pharm. Biomed. Anal. 2021, 197, 113951. [Google Scholar] [CrossRef] [PubMed]
- PubChem, National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 24 May 2022).
- FOODB. Available online: https://foodb.ca/ (accessed on 23 May 2022).
- Seong, G.U.; Chung, S.K. Marker compound contents and antioxidant capacities of the taproot and lateral root of danshen (Salvia miltiorrhiza Radix). J. Appl. Biol. Chem. 2020, 63, 23–28. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lin, T.S.; Wang, C.S.; Chien, H.J.; Juang, Y.M.; Chen, C.J.; Lai, C.C. Rapid determination of bioactive compounds in the different organs of Salvia miltiorrhiza by UPLC-MS/MS. J. Chromatogr. B 2019, 1104, 81–88. [Google Scholar] [CrossRef]
- Phenol-Explorer. Available online: http://phenol-explorer.eu/ (accessed on 14 April 2022).
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules 2016, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Jullien, F.; Moja, S.; Bony, A.; Legrand, S.; Petit, C.; Benabdelkader, T.; Poirot, K.; Fiorucci, S.; Guitton, Y.; Nicolè, F.; et al. Isolation and functional characterization of a τ-cadinol synthase, a new sesquiterpene synthase from Lavandula angustifolia. Plant Mol. Biol. 2014, 84, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wu, S.; Fu, X.; Lai, C.; Guo, D. De novo biosynthesis of τ-cadinol in engineered Escherichia coli. Bioresour. Bioprocess 2022, 9, 29. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2021, 37, 20–32. [Google Scholar] [CrossRef]
- Farhat, M.B.; Sotomayor, J.A.; Jordán, M.J. Salvia verbenaca L. essential oil: Variation of yield and composition according to collection site and phenophase. Biochem. Syst. Ecol. 2018, 82, 35–43. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Dhibi, S.; Dhifi, W.; Saad, R.B.; Brini, F.; Hfaidh, N.; Mnif, W. Essential oil from halophyte Lobularia maritima: Protective effects against CCL4-induced hepatic oxidative demage in rats and inhibition of the production of proinflammatory gene expression by lipopolysaccharide-stimulated RAW 264.7 macrophages. RSC Adv. 2019, 9, 36758–36770. [Google Scholar] [CrossRef]
- de Souza, T.S.; Ferreira, M.F.S.; Menini, L.; Souza, J.R.C.L.; Bernardes, C.O.; Ferreira, A. Chemotype diversity of Psidium guajava L. Phytochemistry 2018, 153, 129–137. [Google Scholar] [CrossRef]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Cioni, P.L.; Lachaâl, M.; Flamini, G. Essential oil and volatile emissions of basil (Ocimum basilicum) leaves exposed to NaCl or Na2SO4 salinity. J. Plant Nutr. Soil Sci. 2013, 176, 748–755. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Borowski, B.; Dzida, K. Essential oil composition of sweet basil cultivars as affected by nitrogen and potassium fertilization. Turk. J. Agric. For. 2013, 37, 427–436. [Google Scholar] [CrossRef]
- Ijaz, A.; Anwar, F.; Tufail, S.; Sherazi, H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar]
- Baldovino, S.; Rojas, J.; Rojas, L.B.; Lucena, M.; Buitrago, A.; Morales, A. Chemical composition and antibacterial activity of the essential oil of Monticalia andicola (Asteraceae) collected in Venezuela. Nat. Prod. Commun. 2009, 4, 2–5. [Google Scholar] [CrossRef]
- Nyegue, M.; Amvam-Zollo, P.H.; Etoa, F.; Agnanniet, H.; Chantal, M. Chemical and biological investigations of essential oils from stem barks of Enantia Chlorantha Oliv. and Polyalthia suaveolens Engler. & Diels. from Cameroon. Nat. Libr. Med. 2008, 3, 16–18. [Google Scholar]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and T. chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Viana, F.A.; Andrade-Neto, M.; Pouliquen, Y.B.M.; Uchoa, D.E.A.; Sobral, M.M.S.Z.; de Morais, S.M. Essential oil of Siparuna guianensis aublet from the amazon region of Brazil. J. Essent. Oil Res. 2002, 14, 60–62. [Google Scholar] [CrossRef]
- Claeson, P.; Andersson, R.; Samuelsson, G. τ-Cadinol: A pharmacologically active constituent of scented myrrh: Introductory pharmacological C-NMR Data. Planta Med. 1991, 57, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P.; Zygmunt, P.; Hogestiitt, E.D. Calcium antagonistic properties of the sesquiterpene τ-cadinol: A comparison with nimodipine in the isolated rat aorta. Pharmacol. Toxicol. 1991, 69, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Claeson, P.; Rhdstrom, P.; Skold, O. Bactericidal effect of the sesquiterpene τ-cadinol on Staphylococcus aureus. Phyther. Res. 1992, 6, 94–98. [Google Scholar] [CrossRef]
- Takei, M.; Umeyama, A.; Arihara, S. τ-Cadinol and calamenene induce dendritic cells from human monocytes and drive Th1 polarization. Eur. J. Pharmacol. 2006, 537, 190–199. [Google Scholar] [CrossRef]
- López-Hortas, L.; Pérez-Larrán, P.; González-Muñoz, M.J.; Falqué, E.; Domínguez, H. Recent developments on the extraction and applications of ursolic acid. A review. Food Res. Int. 2018, 103, 130–149. [Google Scholar] [CrossRef]
- Ma, C.; Cai, S.; Cui, J.; Wang, R.; Tu, P. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Nataraju, A.; Gowda, C.D.R.; Rajesh, R.; Vishwanath, B.S. Group IIA secretory PLA2 inhibition by ursolic acid: A potent anti- inflammatory molecule. Curr. Top. Med. Chem. 2007, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Harmand, P.O.; Duval, R.; Liagre, B.; Jayat-Vignoles, C.; Beneytout, J.L.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through caspase-3 activation and cell cycle arrest in HaCat cells. Int. J. Oncol. 2003, 23, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Anachatchairatana, T.T.; Remner, B.B.; Hokchaisiri, R.C.; Uksamrarn, A.S. Antimycobacterial activity of cinnamate-based esters of the triterpenes betulinic, oleanolic and ursolic acids. Chem. Pharm. Bull. 2008, 56, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bai, Y.; Liu, K.; Han, Y.; Zhang, J.; Liu, Y.; Hou, X.; Hao, E.; Hou, Y.; Bai, G. Ursolic acid inhibits the cholesterol biosynthesis and alleviates high fat diet-induced hypercholesterolemia via irreversible inhibition of HMGCS1 in vivo. Phytomedicine 2022, 103, 154233. [Google Scholar] [CrossRef]
- Ghasemzadeh, F.; Darzi, G.N.; Mohammadi, M. Extraction and purification of ursolic acid from the apple peel and in vitro assessment of the biochemical antibacterial, antioxidant and wound healing characteristics. Appl. Food Biotechnol. 2022, 9, 17–30. [Google Scholar]
- Dapkevicius, A.; van Beek, T.A.; Lelyveld, G.P.; van Veldhuizen, A.; de Groot, A.; Linssen, J.P.; Venskutonis, R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J. Nat. Prod. 2002, 65, 892–896. [Google Scholar] [CrossRef]
- Orhan, F.; Çeker, S.; Anar, M.; Agar, G.; Arasoglu, T.; Gulluce, M. Protective effects of three luteolin derivatives on aflatoxin B1-induced genotoxicity on human blood cells. Med. Chem. Res. 2016, 25, 2567–2577. [Google Scholar] [CrossRef]
- Cho, Y.C.; Park, J.; Cho, S. Anti-Inflammatory and antioxidative effects of luteolin-7-O-glucuronide in LPS-stimulated murine macrophages through TAK1 inhibition and Nrf2 activation. Int. J. Mol. Sci. 2020, 21, 2007. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Duran, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]


| Essential Oil Yield, % | Extract Yield, % | |||
|---|---|---|---|---|
| SD | MWHD | Dry Material before Distillation | Dry Residual Material after Distillation | |
| SD | MWHD | |||
| 0.07 | 0.08 | 19 | 4.6 | 4.2 |
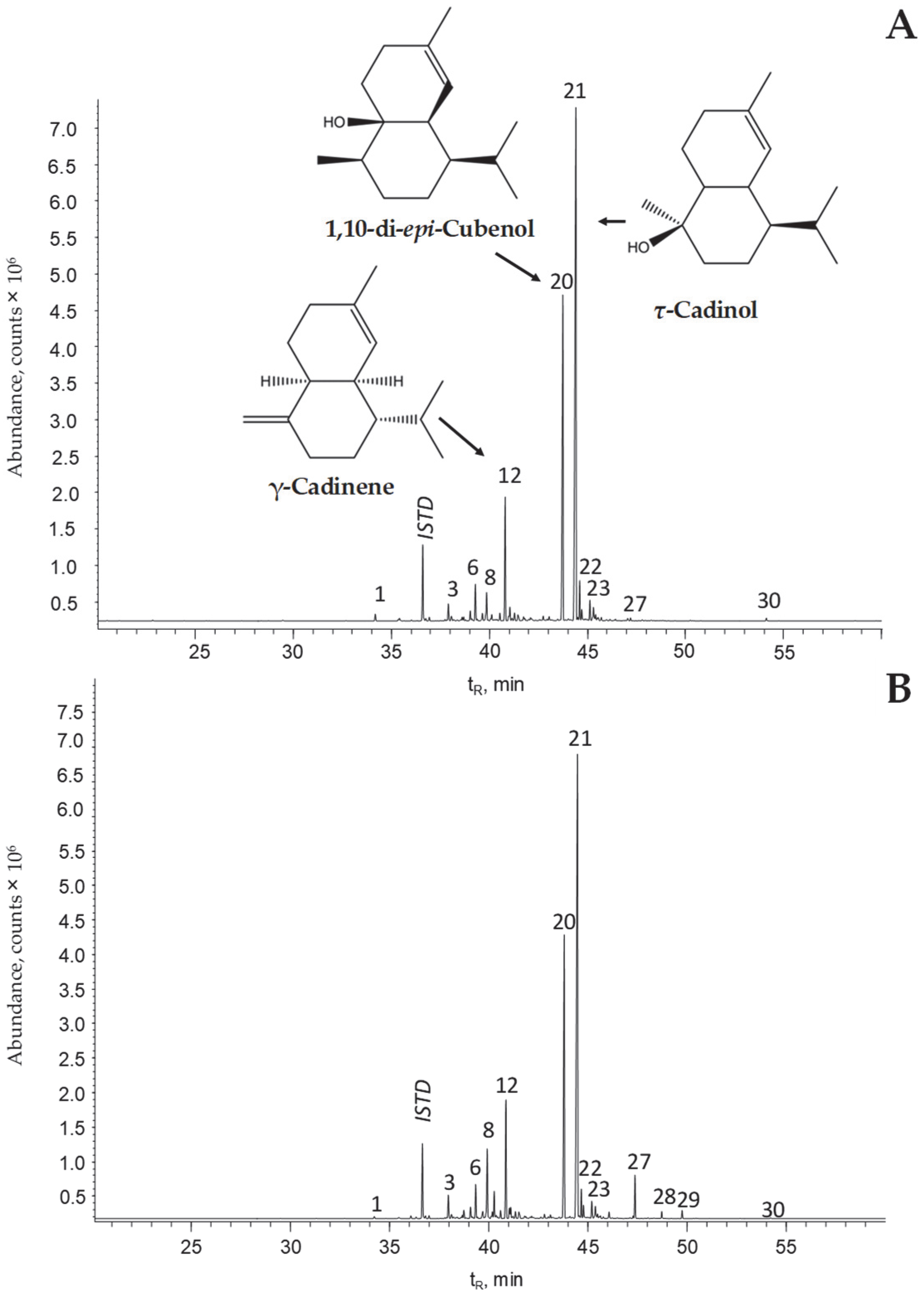
| Peak N° Figure 2 | Compound | Linear Retention Indices | Relative GC/FID Area (DB-5 Column), % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DB-5 | DB-WAX | ||||||||||
| Exp. | Lit. | Exp. | Lit. | SD (±S, n = 3) | MWHD (±S, n = 3) | ||||||
| 1 | Myrtenyl acetate a,b | 1325 | 1328 [14] | 1691 | 1698 [15] | 0.170 | ± | 0.001 | 0.48 | ± | 0.02 |
| 2 | β-Elemene a,b | 1396 | 1390 [14] | 1595 | 1590 [14] | 0.202 | ± | 0.007 | 0.215 | ± | 0.007 |
| 3 | (E)-β-Caryophyllene a,b,c | 1432 | 1420 [14] | 1606 | 1598 [14] | 1.60 | ± | 0.04 | 1.13 | ± | 0.03 |
| 4 | (Z)-Muurola-3,5-diene a,b | 1456 | 1454 [15] | 1645 | 1630 [15] | 0.191 | ± | 0.001 | 0.225 | ± | 0.008 |
| 5 | α-Humulene a,b,c | 1468 | 1472 [6] | 1680 | 1666 [14] | 0.726 | ± | 0.002 | 0.61 | ± | 0.01 |
| 6 | γ-Muurolene a,b | 1474 | 1477 [15] | 1696 | 1689 [14] | 2.17 | ± | 0.01 | 2.32 | ± | 0.06 |
| 7 | γ-Curcumene a,b | 1486 | 1490 [6] | 1474 | 1474 [15] | 0.716 | ± | 0.006 | 0.54 | ± | 0.03 |
| 8 | Germacrene D a,b | 1493 | 1481 [6] | 1718 | 1708 [14] | 4.85 | ± | 0.01 | 2.10 | ± | 0.01 |
| 9 | Valencene a,b | 1498 | 1496 [16] | 1724 | 1728 [14] | 0.442 | ± | 0.001 | 0.56 | ± | 0.02 |
| 10 | α-Farnesene a,b | 1506 | 1504 [15] | 1745 | 1743 [14] | 1.861 | ± | 0.006 | - | ||
| 11 | Germacrene A a,b | 1514 | 1518 [6] | 1754 | 1743 [14] | 0.520 | ± | 0.001 | 0.50 | ± | 0.01 |
| 12 | γ-Cadinene a,b | 1525 | 1532 [6] | 1767 | 1763 [14] | 7.63 | ± | 0.03 | 7.64 | ± | 0.09 |
| 13 | (Z)-Calemenene a,b | 1531 | 1537 [6] | 1835 | 1834 [14] | 0.728 | ± | 0.008 | 0.92 | ± | 0.01 |
| 14 | (Z)-Muurol-5-en-4-β-ol a,b | 1541 | 1550 [16] | 1974 | - | 0.466 | ± | 0.003 | 0.511 | ± | 0.002 |
| 15 | α-Cadinene a,b | 1546 | 1550 [6] | 1797 | 1815 [15] | 0.608 | ± | 0.005 | 0.56 | ± | 0.01 |
| 16 | Selina-3,7(11)-diene a,b | 1549 | 1545 [16] | 1785 | 1792 [15] | - | - | ||||
| 17 | Elemol a,b | 1556 | 1548 [16] | 2079 | 2078 [14] | 0.116 | ± | 0.001 | 0.217 | ± | 0.004 |
| 18 | Spirojatamol a,b | 1593 | 1592 [15] | - | - | 0.275 | ± | 0.009 | 0.318 | ± | 0.001 |
| 19 | Gleenol a,b | 1605 | 1610 [6] | 2039 | 2051 [15] | 0.296 | ± | 0.003 | 0.326 | ± | 0.002 |
| 20 | 1,10-di-epi-Cubenol a,b | 1631 | 1632 [6] | 2067 | 2074 [14] | 20.6 | ± | 0.1 | 23.75 | ± | 0.05 |
| 21 | τ-Cadinol a,b | 1660 | 1667 [6] | 2182 | 2169 [15] | 44.4 | ± | 0.3 | 48.8 | ± | 0.3 |
| 22 | C15H24O d (N.I., Figure S1) | 1665 | - | 2337 | - | 1.56 | ± | 0.02 | 2.06 | ± | 0.02 |
| 23 | α-Cadinol a,b | 1668 | 1677 [6] | 2234 | 2227 [14] | 0.82 | ± | 0.05 | 0.64 | ± | 0.08 |
| 24 | C15H24O e (N.I., Figure S2) | 1683 | - | 2377 | - | 1.10 | ± | 0.01 | 1.14 | ± | 0.01 |
| 25 | α-Bisabolol a,b | 1694 | 1699 [6] | 2217 | 2213 [14] | 0.92 | ± | 0.01 | 1.006 | ± | 0.005 |
| 26 | (2E, 6Z)-Farnesol a,b,c | 1718 | 1722 [15] | 2353 | 2356 [15] | 0.223 | ± | 0.001 | 0.302 | ± | 0.005 |
| 27 | Benzyl benzoate a,b,c | 1780 | 1762 [15] | 2648 | 2638 [14] | 2.52 | ± | 0.04 | 0.19 | ± | 0.01 |
| 28 | Farnesyl acetate a,b | 1834 | 1843 [15] | 2260 | 2260 [15] | 0.404 | ± | 0.008 | - | ||
| 29 | Benzyl salicylate a,b,c | 1884 | 1869 [15] | - | 2784 [15] | 0.50 | ± | 0.01 | - | ||
| 30 | Phytol a,b | 2107 | 2114 [15] | 2620 | 2622 [15] | - | 0.189 | ± | 0.005 | ||
| Standard Compound | Linear Dynamic Range, μg mg−1 | Equation | R2 | LOD, μg mg−1 | LOQ, μg mg−1 |
|---|---|---|---|---|---|
| Scopoletin | 0.02–0.8 | y = 463800386x + 2528726 | 0.998 | 0.04 | 0.13 |
| Vitexin | 0.06–0.8 1 | y = 29902795x − 234075 | 0.996 | 0.05 | 0.18 |
| 1–10 2 | y = 23632604x + 16237131 | 0.993 | |||
| Luteolin-7-O-glucuronide | 0.02–0.8 1 | y = 11017361x + 29367 | 0.997 | 0.04 | 0.15 |
| 1–10 2 | y = 13477938x − 3948338 | 0.993 | |||
| Luteolin-7-O-glucoside | 0.04–0.8 | y = 30330792x + 943824 | 0.995 | 0.06 | 0.21 |
| Rutin | 0.06–0.8 | y = 7754585x + 157454 | 0.992 | 0.07 | 0.23 |
| Quercetin-3-O-glucoside | 0.04–0.8 | y = 12031321x − 113291 | 0.997 | 0.05 | 0.16 |
| Rosmarinic acid | 0.06–0.8 | y = 31520429x − 1206359 | 0.991 | 0.08 | 0.28 |
| Naringenin | 0.06–0.8 1 | y = 29932955x + 344622 | 0.997 | 0.04 | 0.14 |
| 0.6–6 2 | y = 18445875x+ 9232124 | 0.995 | |||
| Luteolin | 0.04–0.8 | y = 62182328x + 1738473 | 0.996 | 0.06 | 0.21 |
| Apigenin | 0.06–0.8 | y = 68451624x + 3092256 | 0.994 | 0.08 | 0.26 |
| Cirsimaritin | 0.1–0.8 | y = 426952447x + 16851979 | 0.990 | 0.09 | 0.30 |
| Eupatilin | 0.02–0.8 | y = 197167110x − 1075471 | 0.997 | 0.04 | 0.13 |
| Acacetin | 0.04–0.8 | y = 228671251x + 1965791 | 0.995 | 0.06 | 0.21 |
| Salvigenin | 0.06–0.8 | y = 910743362x − 14489550 | 0.994 | 0.07 | 0.23 |
| Ursolic acid | 0.04–0.8 | y = 852032x + 73796 | 0.995 | 0.06 | 0.20 |
| Compound | Amount, mg gextract −1 (±S, n = 3) | ||
|---|---|---|---|
| Plant Material | |||
| Dry, before Distillation | After Distillation | ||
| SD | MWHD | ||
| Scopoletin | <LOQ | <LOQ | <LOQ |
| Vitexin | 3.07 ± 0.06 | 0.087 ± 0.007 | 0.35 ± 0.01 |
| Luteolin-7-O-glucuronide | 25.3 ± 0.3 | 1.16 ± 0.06 | 3.9 ± 0.2 |
| Luteolin-7-O-glucoside | 0.155 ± 0.003 | <LOQ | <LOQ |
| Rutin | <LOQ | N.D. | N.D. |
| Quercetin-3-O-glucoside | 0.3 ± 0.1 | N.D. | N.D. |
| Rosmarinic acid | 2.0 ± 0.4 | <LOQ | 0.73 ± 0.01 |
| Naringenin | 1.84 ± 0.08 | <LOQ | <LOQ |
| Luteolin | 0.70 ± 0.06 | <LOQ | <LOQ |
| Apigenin | 0.423 ± 0.002 | <LOQ | <LOQ |
| Cirsimaritin | <LOQ | <LOQ | <LOQ |
| Eupatilin | 0.27 ± 0.06 | <LOQ | <LOQ |
| Acacetin | <LOQ | <LOQ | <LOQ |
| Salvigenin | 0.8 ± 0.1 | 0.26 ± 0.08 | 0.43 ± 0.07 |
| Ursolic acid | 37 ± 3 | 28.9 ± 0.6 | 39.8 ± 0.6 |
| Sample | Plant Material | Antioxidant Activity, μmol Trolox® g−1 (±S, n = 3) | |
|---|---|---|---|
| ABTS+• | ORAC | ||
| EO-SD | Fresh | 49 ± 1 | 1520 ± 9 |
| EO-MWHD | Fresh | 32.1 ± 0.1 | 1610 ± 67 |
| Extract | Before distillation (dried) | 82 ± 4 | 1303 ± 14 |
| Biomass residue after SD (dried) | 51 ± 4 | 720 ± 75 | |
| Biomass residue after MWHD (dried) | 73 ± 5 | 1205 ± 12 | |
| BHT | 4990 ± 60 | 98 ± 5 | |
| α-Tocoferol | 2310 ± 40 | 450 ± 50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henríquez, J.C.; Duarte, L.V.; Sierra, L.J.; Fernández-Alonso, J.L.; Martínez, J.R.; Stashenko, E.E. Chemical Composition and In Vitro Antioxidant Activity of Salvia aratocensis (Lamiaceae) Essential Oils and Extracts. Molecules 2023, 28, 4062. https://doi.org/10.3390/molecules28104062
Henríquez JC, Duarte LV, Sierra LJ, Fernández-Alonso JL, Martínez JR, Stashenko EE. Chemical Composition and In Vitro Antioxidant Activity of Salvia aratocensis (Lamiaceae) Essential Oils and Extracts. Molecules. 2023; 28(10):4062. https://doi.org/10.3390/molecules28104062
Chicago/Turabian StyleHenríquez, Juan C., Laura V. Duarte, Lady J. Sierra, José L. Fernández-Alonso, Jairo R. Martínez, and Elena E. Stashenko. 2023. "Chemical Composition and In Vitro Antioxidant Activity of Salvia aratocensis (Lamiaceae) Essential Oils and Extracts" Molecules 28, no. 10: 4062. https://doi.org/10.3390/molecules28104062
APA StyleHenríquez, J. C., Duarte, L. V., Sierra, L. J., Fernández-Alonso, J. L., Martínez, J. R., & Stashenko, E. E. (2023). Chemical Composition and In Vitro Antioxidant Activity of Salvia aratocensis (Lamiaceae) Essential Oils and Extracts. Molecules, 28(10), 4062. https://doi.org/10.3390/molecules28104062







