Development of Green and High Throughput Microplate Reader-Assisted Universal Microwell Spectrophotometric Assay for Direct Determination of Tyrosine Kinase Inhibitors in Their Pharmaceutical Formulations Irrespective the Diversity of Their Chemical Structures
Abstract
1. Introduction
2. Results and Discussion
2.1. Strategy for Assay Development
2.2. Development of MW-UV-SPA
2.2.1. UV-Absorption Spectra and Selection of Proper Wavelength for Measurement
2.2.2. Optimization of Assay Conditions
2.3. Validation of MW-UV-SPA
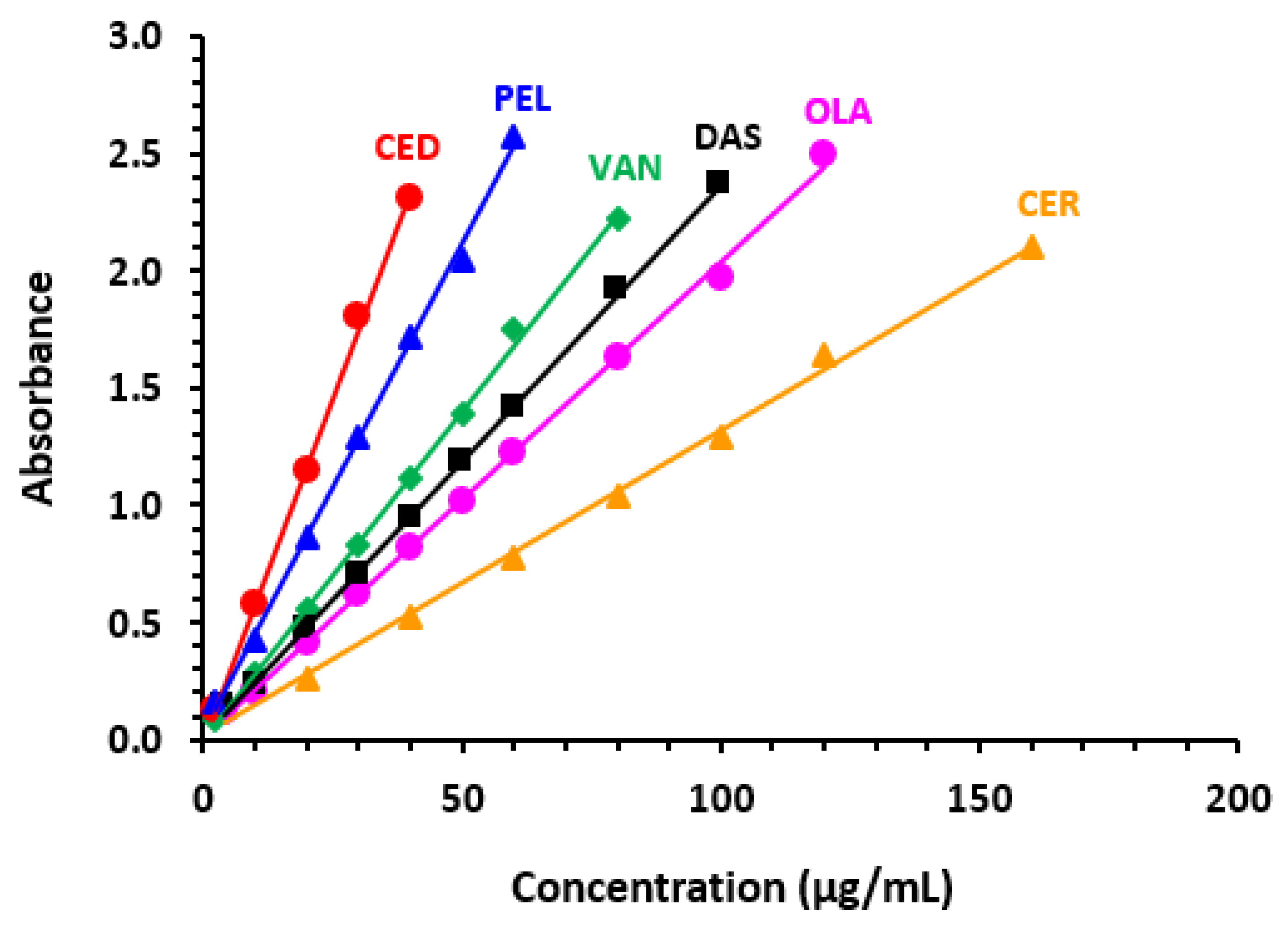
2.3.1. Linear Range and Sensitivity
2.3.2. Precision and Accuracy
2.3.3. Selectivity
2.3.4. Robustness and Ruggedness
2.4. Application of MW-UV-SPA to the Analysis of Pharmaceutical Formulations
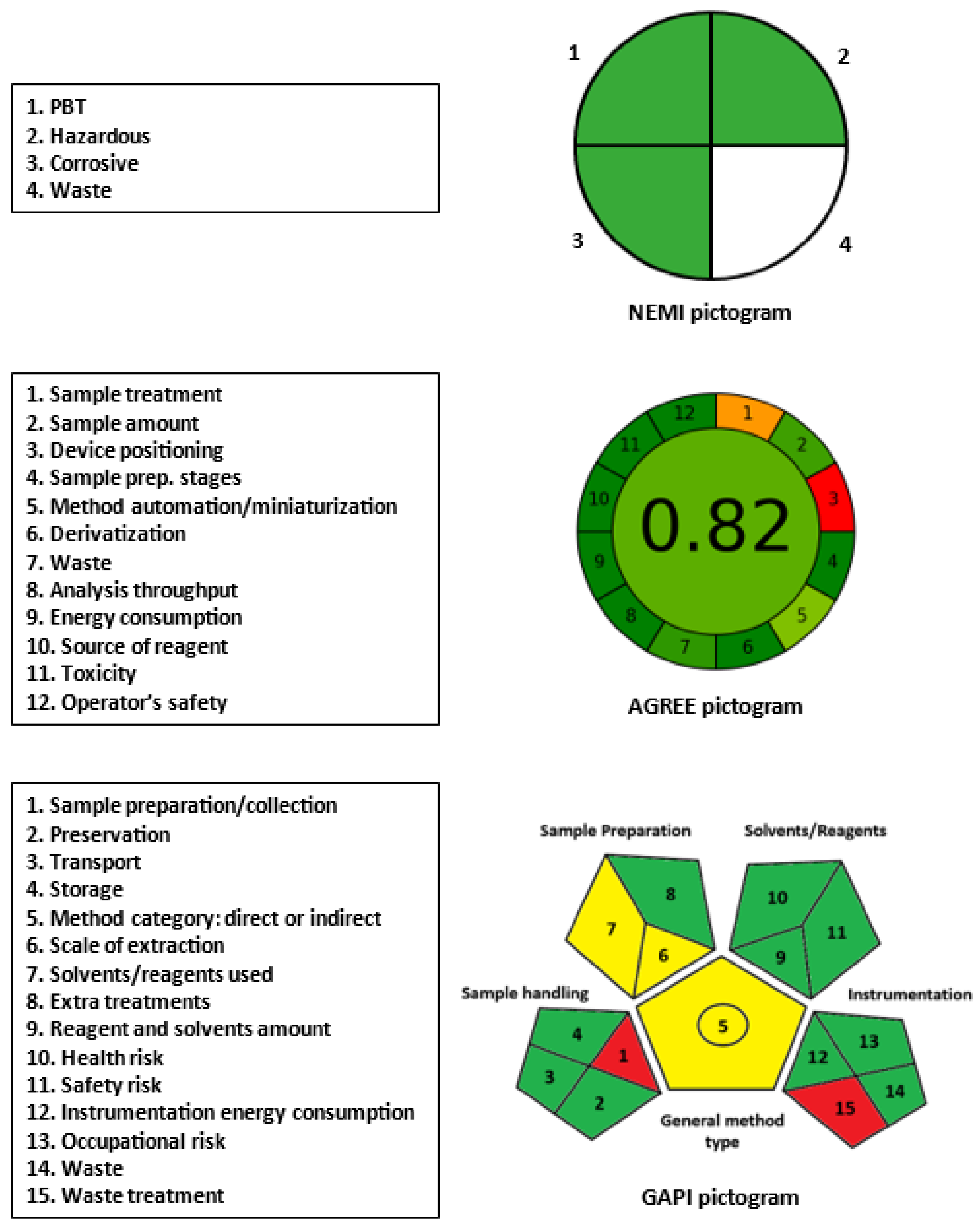
2.5. Greenness of MW-UV-SPA
2.6. Advantages of the Proposed Assay over the Previous Assays
3. Experimental
3.1. Instruments
3.2. Materials, Tools and Pharmaceutical Formulations
3.3. Preparation of Standard TKI Solution
3.4. Preparation of Pharmaceutical Formulation Solution
3.5. Procedure of MW-UV-SPA and Construction of Calibration Curves
3.6. Validation of MW-UV-SPA Procedure
3.6.1. Assessment of Linearity Range and Sensitivity
3.6.2. Estimation of Precision and Accuracy
3.6.3. Evaluation of Selectivity
3.6.4. Assessment of Robustness and Ruggedness
3.7. Greenness Assessment Procedures
3.7.1. National Environmental Method Index
3.7.2. Eco-Scale Assessment Tool
3.7.3. Green Analytical Procedure Index (GAPI)
3.7.4. Analytical Greenness Metric Tool (AGREE)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization, Geneva, Report 12 September 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 20 March 2023).
- Milaat, W.A. Knowledge of secondary-school female students on breast cancer and breast self-examination in Jeddah, Saudi Arabia. East. Mediterr. Health J. 2000, 6, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, A.A.; Ibrahim, E.M.; Raja, M.A.; Al-Sobhi, S.; Rostom, A.; Stuart, R.K. Locally advanced breast cancer in Saudi Arabia: High frequency of stage III in a young population. Med. Oncol. 1999, 16, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hashim, T.J. Adolescents and cancer: A survey of knowledge and attitudes about cancer in eastern province of Saudi Arabia. J. Fam. Community Med. 2000, 7, 29–35. [Google Scholar]
- Prager, G.W.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E.; Sonke, G.S.; Martínez, G.; Frenel, J.S.; Karamouzis, M.; et al. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef]
- Nwagbara, U.I.; Ginindza, T.G.; Hlongwana, K.W. Health systems influence on the pathways of care for lung cancer in low- and middle-income countries: A scoping review. Glob. Health 2020, 16, 23. [Google Scholar] [CrossRef]
- Bertino, J.R.; Hait, W. Principles of cancer therapy. In Textbook of Medicine, 22nd ed.; Golden, L., Ausiello, D., Eds.; WB Saunders: Philadelphia, PA, USA, 2004; pp. 1137–1150. [Google Scholar]
- Whittaker, S.; Marais, R.; Zhu, A.X. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010, 29, 4989–5005. [Google Scholar] [CrossRef]
- Agarwal, E.; Brattain, M.G.; Chowdhury, S. Cell survival and metastasis regulation by Akt signaling in colorectal cancer. Cell Signal. 2013, 25, 1711–1719. [Google Scholar] [CrossRef]
- Wang, Z.; Cole, P.A. Catalytic mechanisms and regulation of protein kinases. Methods Enzymol. 2014, 548, 1–21. [Google Scholar]
- Drake, J.M.; Lee, J.K.; Witte, O.N. Clinical targeting of mutated and wild-type protein tyrosine kinases in cancer. Mol. Cell Biol. 2014, 34, 1722–1732. [Google Scholar] [CrossRef]
- Knosel, T.; Kampmann, E.; Kirchner, T.; Altendorf-Hofmann, A. tyrosine kinases in soft tissue tumors. Pathologe 2014, 35 (Suppl. S2), 198–201. [Google Scholar]
- Winkler, G.C.; Barle, E.L.; Galati, G.; Kluwe, W.M. Functional differentiation of cytotoxic cancer drugs and targeted cancer therapeutics. Regul. Toxicol. Pharmacol. 2014, 70, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.C.; Grant, W.; John, R.J.; John, D.; Jogarao, G.; Atiqur, R.; Kimberly, B.; John, L.; Sung, K.K.; Rebecca, W.; et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin. Cancer Res. 2002, 8, 935–942. [Google Scholar]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Christopher, F. Targeted chronic myeloid leukemia therapy: Seeking a cure. J. Mang. Care Pharm. 2007, 13, S8–S12. [Google Scholar]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 36. [Google Scholar] [CrossRef]
- Darwish, I.A.; Khalil, N.Y.; AlZeer, M. ICH/FDA Guidelines-Compliant Validated Stability-Indicating HPLC-UV Method for the Determination of Axitinib in Bulk and Dosage Forms. Curr. Anal. Chem. 2020, 16, 1106–1112. [Google Scholar] [CrossRef]
- Khandare, B.; Musle, A.C.; Arole, S.S.; Popalghat, P.V. Analytical method development and validation of olmutinib bulk drug as per ICH Q2 guidelines by using RP-HPLC Method. J. Drug Deliv. Ther. 2019, 9, 608–611. [Google Scholar]
- Khalil, N.Y.; Darwish, I.A.; Alshammari, M.F.; Wani, T.A. ICH Guidelines-compliant HPLC-UV Method for Pharmaceutical Quality Control and Therapeutic Drug Monitoring of the Multi-targeted Tyrosine Kinase Inhibitor Pazopanib. S. Afr. J. Chem. 2017, 70, 60–66. [Google Scholar] [CrossRef]
- Latha, S.T.; Thangadurai, S.A.; Jambulingam, M.; Sereya, K.; Kamalakannan, D.; Anilkumar, M. Development and validation of RP-HPLC method for the estimation of Erlotinib in pharmaceutical formulation. Arab. J. Chem. 2017, 10, S1138–S1144. [Google Scholar] [CrossRef]
- Ashok, G.; Mondal, S.; Ganapaty, S.; Bandla, J. Development and validation of stability indicating method for the estimation of pazopanib hydrochloride in pharmaceutical dosage forms by RP-HPLC. Der Pharm. Lett. 2015, 7, 234–241. [Google Scholar]
- Bende, G.; Kollipara, S.; Kolachina, V.; Saha, R. Development and validation of a stability indicating RP-LC method for determination of imatinib mesylate. Chromatographia 2007, 66, 859–866. [Google Scholar] [CrossRef]
- Hajmalek, M.; Goudarzi, M.; Ghaffari, S.; Attar, H.; Mazlaghan, M.G. Development and validation of a HPTLC method for analysis of sunitinib malate. Braz. J. Pharm. Sci. 2016, 52, 595–601. [Google Scholar] [CrossRef]
- Dutta, D.; Das, S.; Ghosn, M. Validated HPTLC method for the determination of nintedanib in bulk drug. Proceedings 2019, 9, 22. [Google Scholar]
- Vadera, N.; Subramanian, G.; Musmade, P. Stability-indicating HPTLC determination of imatinib mesylate in bulk drug and pharmaceutical dosage form. J. Pharm. Biomed. Anal. 2007, 43, 722–726. [Google Scholar] [CrossRef]
- Mhaske, D.V.; Dhaneshwar, S.R. Stability indicating HPTLC and LC determination of dasatinib in pharmaceutical dosage form. Chromatographia 2007, 66, 95–102. [Google Scholar] [CrossRef]
- Reddy, C.N.; Prasad, P.; Sreedhar, N.Y. Voltammetric behavior of gefitinib and its adsorptive stripping voltammetric determination in pharmaceutical formulations and urine samples. Int. J. Pharm. Pharm. Sci. 2011, 3, 141–145. [Google Scholar]
- Rajesh, V.; Jagathi, V.; Sindhuri, K.; Devala Rao, G. Spectrofluorimetric method for the estimation of Erlotinib hydrochloride in pure and pharmaceutical formulations. E-J. Chem. 2011, 8, S304–S308. [Google Scholar] [CrossRef]
- Mandal, B.; Balabathula, P.; Mittal, N.; Wood, G.C. Himanshu Bhattacharjee. Development and validation of a spectrofluorimetric method for the determination of Erlotinib in spiked human plasma. J. Fluoresc. 2012, 22, 1425–1429. [Google Scholar] [CrossRef]
- Zawaneh, A.H.; Khalil, N.N.; Ibrahim, S.A.; Al-Dafiri, W.N.; Maher, H.M. Micelle-enhanced direct spectrofluorimetric method for the determination of linifanib: Application to stability studies. Luminescence 2017, 32, 1162–1168. [Google Scholar] [CrossRef]
- Maher, H.M.; Alzoman, N.Z.; Shehata, S.M. An eco-friendly direct spectrofluorimetric method for the determination of irreversible tyrosine kinase inhibitors, neratinib and pelitinib: Application to stability studies. Luminescence 2017, 32, 149–158. [Google Scholar] [CrossRef]
- Padmalatha, H.; Vidyasagar, G. Development and validation of UV spectrophotometric method for the determination of erlotinib in tablet formulation. Imperial. J. Med. Org. Chem. 2011, 1, 26–30. [Google Scholar]
- Sankar, F.G.; Latha, P.V.; Krishna, M.V. UV-spectrophotometric determination of imatinib mesylate. Asian J. Chem. 2006, 18, 1543–1544. [Google Scholar]
- Sankar, D.G.; Rajeswari, A.; Babu, A.N.; Krishna, M.V. UV-spectrophotometric determination of dasatinib in pharmaceutical dosage forms. Asian J. Chem. 2009, 21, 5777–5779. [Google Scholar]
- Annapurna, M.M.; Venkatesh, B.; Chaitanya, R.K. Analytical techniques for the determination of erlotinib HCl in pharmaceutical dosage forms by spectrophotometry. Chem. Sci. Trans. 2014, 3, 840–846. [Google Scholar]
- Khandare, B.; Dudhe, P.B.; Upasani, S.; Dhoke, M. Spectrophotometric determination of vandetanib in bulk by area under curve and first order derivative methods. Int. J. PharmTech Res. 2019, 12, 103–110. [Google Scholar] [CrossRef]
- Annapurna, M.M.; Venkatesh, B.; Chaitanya, R.K. New derivative spectrophotometric methods for the determination of erlotinib hydrochloride (a tyrosine kinase inhibitor). Indo Am. J. Pharm. Res. 2013, 3, 9270–9276. [Google Scholar]
- Khandare, B.; Musle, A.C.; Arole, S.S.; Pravin, V.; Popalghat, V. Spectrophotometric determination of olmutinib in bulk by area under curve and first order derivative methods and its validation as per ICH guidelines. J. Drug Deliv. Ther. 2019, 9, 349–354. [Google Scholar] [CrossRef]
- Souria, E.; Amoon, E.; Ravarib, N.S.; Keyghobadia, F.; Tehrania, M.B. Spectrophotometric methods for determination of sunitinib in pharmaceutical dosage forms based on ion-pair complex formation. Iran. J. Pharm. Res. 2020, 19, 103–109. [Google Scholar]
- Rani, G.U.; Chandrasekhar, B.; Devanna, N. Extractive colorimetric method development and validation for erlotinib in bulk and tablet dosage form. J. Appl. Pharm. Sci. 2011, 1, 176–179. [Google Scholar]
- Balaram, V.M.; Rao, J.V.; Khan, M.M.; Sharma, J.V.; Anupama, K. Visible spectrophotometric determination of imatinib mesylate in bulk drug and pharmaceutical formulations. Asian J. Chem. 2009, 21, 5241–5244. [Google Scholar]
- Abdel Karim, S.E.; Farghaly, R.A.; El-Nashar, R.M.; Abadi, A.H. Spectrophotometric determination of imatinib mesylate using charge transfer complexs in pure form and pharmaceutical formulation. Chem. Rapid Commun. 2014, 2, 55–63. [Google Scholar]
- Syamittra, B.; Parasuraman, S.; Yeng, W.Y.; Ping, Y.W.; Thujithra, J.; Kumar, J.; Dhanaraj, S.A. A review on adverse health effects of laboratory volatile solvents. Int. J. Clin. Ther. Diagn. 2014, 2, 59–63. [Google Scholar]
- Wennborg, H.; Bonde, J.P.; Stenbeck, M.; Olsen, J. Adverse reproduction outcomes among employee in biomedical research laboratories. Scand. J. Work Environ. Health 2002, 28, 5–11. [Google Scholar] [CrossRef]
- Lindbohm, M.L.; Taskinen, H.T.; Sallman, M.; Hemminki, K. Spontaneous abortions among women exposed to organic solvents. Am. J. Indust. Med. 2007, 17, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Wennborg, H.; Lennart, B.; Harri, V.; Gösta, A. Pregnancy outcome of personnel in Swedish biomedical research laboratories. J. Occup. Environ. Med. 2000, 42, 38–446. [Google Scholar] [CrossRef]
- Kristensen, P.; Hilt, B.; Svendsen, K.; Grimsrud, T.K. Incidence of lymphohaematopoietic cancer at university laboratory: A cluster investigation. Eur. J. Epidemiol. 2008, 23, 11–15. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Perry, G.W. High Throughput Analysis in the Pharmaceutical Industry; CRC/Taylor & Francis: Portland, OR, USA, 2009; pp. 1–72. [Google Scholar]
- Darwish, I.A.; Darwish, H.W.; Khalil, N.Y.; Sayed, A.Y. Experimental and computational evaluation of chloranilic acid as an universal chromogenic reagent for the development of a novel 96-microwell spectrophotometric assay for tyrosine kinase inhibitors. Molecules 2021, 26, 744. [Google Scholar] [CrossRef]
- Görög, S. Ultraviolet-Visible Spectrophotometry in Pharmaceutical Analysis; CRC Press: New York, NY, USA, 2018; pp. 1–504. [Google Scholar]
- Ahmed, S.; Rasul, A.; Masood, Z. Spectrophotometry in Pharmaceutical Analysis; LAP Lambert Academic Publishing: London, UK, 2011. [Google Scholar]
- Gore, M.G. (Ed.) Spectrophotometry and Spectrofluorimetry: A Practical Approach, 2nd ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Mennen, S.M.; Alhambra, C.; Allen, C.L.; Barberis, M.; Berritt, S.; Brandt, T.A.; Campbell, A.D.; Castañón, J.; Cherney, A.H.; Christensen, M.; et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process Res. Dev. 2019, 23, 1213–1242. [Google Scholar] [CrossRef]
- Welch, C.J. High throughput analysis enables high throughput experimentation in pharmaceutical process research. React. Chem. Eng. 2019, 4, 1895. [Google Scholar] [CrossRef]
- Dunn, P.J.; Wells, A.S.; Williams, M.T. (Eds.) Green Chemistry in the Pharmaceutical Industry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Agbenyega, J. Green Chemistry in the Pharma Industry: Sustainable Pastures for Those Who Innovate. Available online: https://www.cas.org/resources/cas-insights/sustainability/green-chemistry-pharma-industry (accessed on 20 March 2023).
- Msingh, R.; Pramanik, R.; Hazra, S. Role of green chemistry in pharmaceutical industry: A review. J. Univ. Shanghai Sci. Technol. 2021, 23, 291–299. [Google Scholar] [CrossRef]
- Darwish, I.A.; Almehizia, A.A.; Sayed, A.Y.; Khalil, N.Y.; Alzoman, N.Z.; Darwish, H.W. Synthesis, spectroscopic and computa-tional studies on hydrogen bonded charge transfer complex of duvelisib with chloranilic acid: Application to development of novel 96-microwell spectrophotometric assay. Spectrochim. Acta A 2021, 264, 120287. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Khalil, N.Y.; Darwish, H.W.; Alzoman, N.Z.; Al-Hossaini, A.M. Synthesis, spectroscopic and computational characterization of charge transfer complex of remdesivir with chloranilic acid: Application to development of novel 96-microwell spectrophotometric assay. J. Mol. Struct. 2022, 1263, 133104. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.Y.; Al Qhatani, M.N.; Al Qubaisi, K.A.; Sayed, A.Y.; Darwish, I.A. Development of two innovative 96-microwell-based spectrophotometric assays with high throughput for determination of fluoroquinolone antibiotics in their pharmaceutical formulations. J. Appl. Spectrosc. 2022, 89, 66–74. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline, Validation of Analytical Procedure: Q2(R2); ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green analytical methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef]
- Gałuszka, A.; Konieczka, P.; Migaszewski, Z.M.; Namieśnik, J. Analytical eco-scale for assessing the greenness of analytical procedures. Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-analytical greenness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
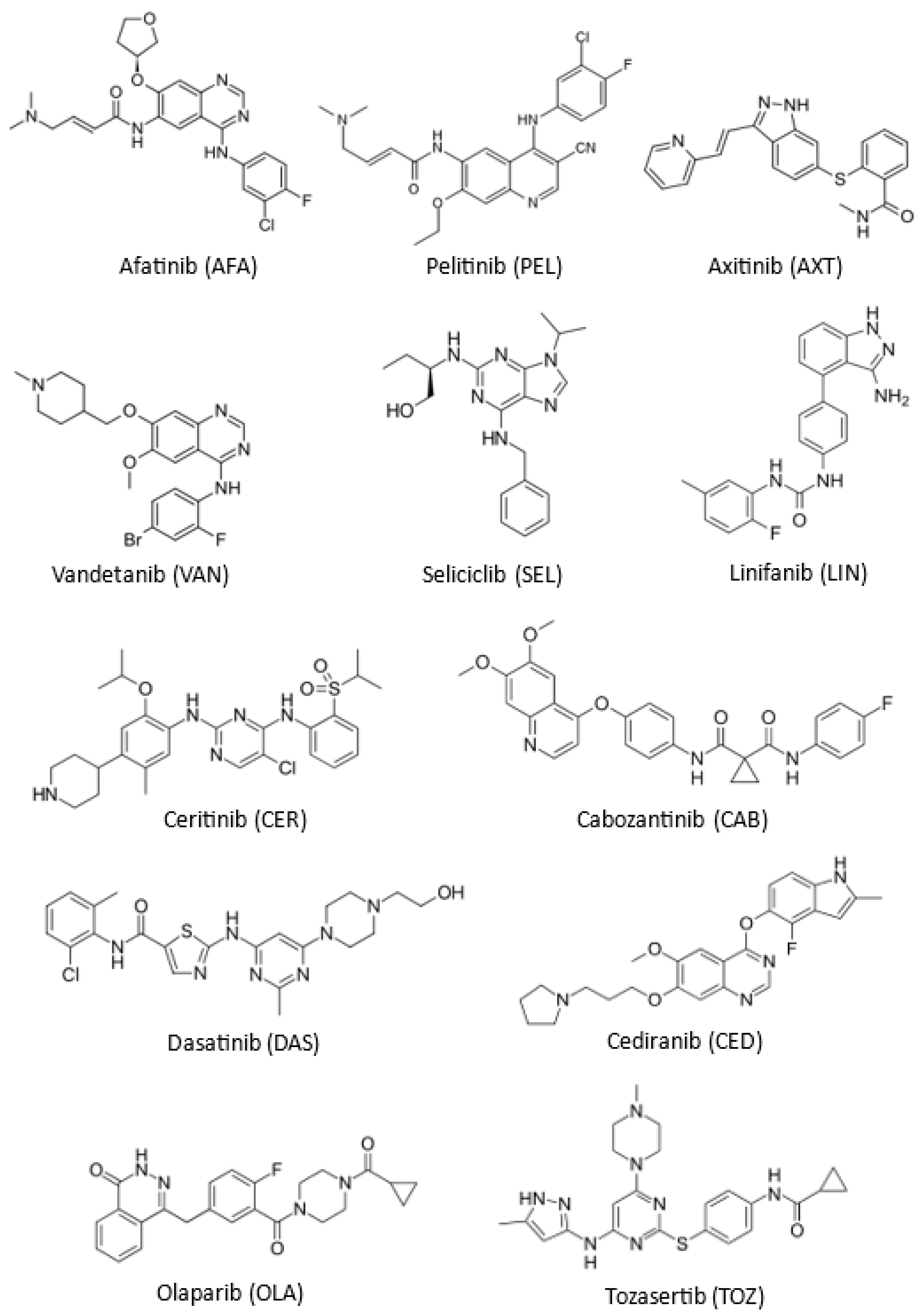



| TKI Name | Abbreviation | IUPAC Name | Molecular Formula | Molecular Weight |
|---|---|---|---|---|
| Afatinib | AFA | (Z)-but-2-enedioic acid;(E)-N-[4-(3-chloro-4-fluoroanilino)-7-[(3S)-oxolan-3-yl]oxyquinazolin-6-yl]-4-(dimethylamino)but-2-enamide | C24H25ClFN5O3 | 485.94 |
| Pelitinib | PEL | (2E)-N-{4-[(3-chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxyquinolin-6-yl}-4-(dimethylamino)but-2-enamide | C24H23ClFN5O2 | 467.90 |
| Cediranib | CED | 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline | C25H27FN4O3 | 450.51 |
| Axitinib | AXT | N-methyl-2-[[3-[(E)-2-pyridin-2-ylethenyl]-1H-indazol-6-yl]sulfanyl] benzamide | C22H18N4OS | 386.47 |
| Ceritinib | CER | 5-chloro-2-N-(5-methyl-4-piperidin-4-yl-2-propan-2-yloxyphenyl)-4-N-(2-propan-2-ylsulfonylphenyl)pyrimidine-2,4-diamine | C28H36ClN5O3S | 558.14 |
| Cabozantinib | CAB | N’1-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]phenyl}-N1-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide | C28H24FN3O5 | 501.50 |
| Linifanib | LIN | 1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-5-methylphenyl)urea | C17H15FN5O | 324.34 |
| Olaparib | OLA | 4-[[3-[4-(cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorophenyl]methyl]-2H-phthalazin-1-one | C24H23FN4O3 | 434.47 |
| Seliciclib | SEL | (2R)-2-{[6-(benzylamino)-9-(propan-2-yl)-9H-purin-2-yl]amino}butan-1-ol | C19H26N6O | 354.46 |
| Vandetanib | VAN | N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl)methoxy]quinazolin-4-amine | C22H24BrFN4O2 | 475.40 |
| Dasatinib | DAS | N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide | C22H26ClN7O2S | 488.01 |
| Tozasertib | TOZ | N-[4-[4-(4-methylpiperazin-1-yl)-6-[(5-methyl-1H-pyrazol-3-yl)amino] pyrimidin-2-yl]sulfanylphenyl]cyclopropanecarboxamide | C23H28N8OS | 464.59 |
| TKIs | λmax (nm) | Absorbance | ε × 104 (L mol−1 cm−1) | ||
|---|---|---|---|---|---|
| At λmax | At 230 nm | At λmax | At 230 nm | ||
| AFA | 254 | 0.678 | 0.559 | 3.29 | 2.72 |
| PEL | 213 | 3.854 | 0.437 | 18.00 | 2.19 |
| CED | 236 | 1.338 | 0.259 | 6.03 | 1.45 |
| AXT | 330 | 0.741 | 0.556 | 2.86 | 1.80 |
| CER | 277 | 0.426 | 0.408 | 2.38 | 1.77 |
| CAB | 242 | 0.716 | 0.573 | 3.59 | 2.03 |
| LIN | 204 | 1.414 | 0.555 | 4.59 | 2.64 |
| OLA | 277 | 0.124 | 0.628 | 0.54 | 2.43 |
| SEL | 232 | 0.577 | 1.153 | 2.05 | 5.19 |
| VAN | 250 | 0.745 | 0.473 | 3.54 | 2.31 |
| DAS | 322 | 0.716 | 0.859 | 3.49 | 4.02 |
| TOZ | 201 | 3.089 | 1.033 | 14.40 | 4.80 |
| Condition | Studied Range | Optimum Value a |
|---|---|---|
| Solvent | Different b | Methanol |
| pH of buffer solution | 3–10 | Without buffer |
| Sample volume (µL/well) | 50–200 | 200 |
| Measuring wavelength (nm) | 200–400 | 230 |
| TKIs | Linear Range a | Intercept | SDa b | Slope | SDb b | r b | LOD a | LOQ a |
|---|---|---|---|---|---|---|---|---|
| AFA | 2–80 | 0.0123 | 0.0099 | 0.0278 | 0.0044 | 0.9994 | 1.18 | 3.56 |
| PEL | 4–100 | 0.0341 | 0.0098 | 0.0217 | 0.0067 | 0.9992 | 1.49 | 4.52 |
| CED | 4–160 | 0.0061 | 0.0202 | 0.0128 | 0.0092 | 0.9996 | 5.21 | 15.78 |
| AXT | 2–90 | 0.0038 | 0.0100 | 0.0274 | 0.0050 | 0.9991 | 1.20 | 3.65 |
| CER | 2–120 | 0.0213 | 0.0099 | 0.0200 | 0.0021 | 0.9994 | 1.63 | 4.95 |
| CAB | 2–80 | 0.0130 | 0.0099 | 0.0279 | 0.0035 | 0.9995 | 1.17 | 3.55 |
| LIN | 2–80 | 0.0196 | 0.0099 | 0.0275 | 0.0044 | 0.9995 | 1.19 | 3.60 |
| OLA | 2–80 | 0.0281 | 0.0098 | 0.0313 | 0.0032 | 0.9991 | 1.03 | 3.13 |
| SEL | 2–40 | 0.0423 | 0.0097 | 0.0575 | 0.0045 | 0.9992 | 0.56 | 1.69 |
| VAN | 4–100 | 0.0115 | 0.0099 | 0.0233 | 0.0044 | 0.9994 | 1.40 | 4.25 |
| DAS | 2–60 | 0.0256 | 0.0098 | 0.0422 | 0.0037 | 0.9997 | 0.77 | 2.32 |
| TOZ | 2–50 | 0.0629 | 0.0097 | 0.0500 | 0.0080 | 0.9992 | 0.64 | 1.94 |
| TKIs | Relative Standard Deviation (%) | Recovery (% ± SD) c | |
|---|---|---|---|
| Intra−Assay (n = 5) a | Inter−Assay (n = 6) b | ||
| AFA | 0.64 | 1.54 | 100.4 ± 1.4 |
| PEL | 1.24 | 2.13 | 101.2 ± 0.8 |
| CED | 1.62 | 1.85 | 99.4 ± 1.2 |
| AXT | 1.05 | 1.62 | 101.8 ± 1.5 |
| CER | 1.12 | 1.82 | 102.9 ± 1.1 |
| CAB | 2.03 | 2.14 | 97.8 ± 2.4 |
| LIN | 1.42 | 1.42 | 99.4 ± 2.2 |
| OLA | 1.32 | 1.24 | 101.5 ± 2.1 |
| SEL | 1.51 | 0.89 | 99.6 ± 1.2 |
| VAN | 1.42 | 1.52 | 98.5 ± 2.1 |
| DAS | 1.15 | 2.13 | 102.8 ± 1.6 |
| TOZ | 1.82 | 1.72 | 101.9 ± 1.4 |
| TKI | Recovery (% ± SD) a | |||
|---|---|---|---|---|
| MCC (50) b | Starch (10) b | LMH (5) b | MS (5) b | |
| AFA | 100.2 ± 1.4 | 99.2 ± 1.2 | 99.4 ± 0.8 | 102.6 ± 1.3 |
| PEL | 101.4 ± 1.2 | 101.4 ± 1.9 | 100.2 ± 1.2 | 99.4 ± 1.4 |
| CED | 100.5 ± 0.8 | 99.5 ± 1.2 | 99.6 ± 0.9 | 100.1 ± 1.5 |
| AXT | 102.2 ± 1.6 | 101.4 ± 1.6 | 98.5 ± 2.1 | 103.2 ± 0.9 |
| CER | 100.4 ± 1.4 | 103.5 ± 2.2 | 103.1 ± 1.2 | 99.3 ± 1.6 |
| CAB | 99.3 ± 1.7 | 99.2 ± 1.4 | 99.4 ± 1.4 | 101.2 ± 1.2 |
| LIN | 101.8 ± 1.3 | 102.4 ± 1.5 | 102.1 ± 1.5 | 98.8 ± 1.8 |
| OLA | 100.5 ± 0.9 | 100.6 ± 1.2 | 101.2 ± 1.2 | 102.5 ± 1.6 |
| SEL | 98.6 ± 1.2 | 98.5 ± 0.8 | 100.3 ± 0.8 | 100.8 ± 1.2 |
| VAN | 99.4 ± 1.8 | 101.8 ± 1.4 | 98.8 ± 1.4 | 99.6 ± 2.1 |
| DAS | 103.4 ± 1.1 | 98.9 ± 1.2 | 102.5 ± 1.5 | 102.6 ± 1.2 |
| TOZ | 98.4 ± 1.4 | 97.5 ± 2.1 | 100.8 ± 1.6 | 99.4 ± 1.5 |
| Tablets (TKI, mg/Tablet) | Label Claim (% ± SD) |
|---|---|
| Inlyta (AXT, 50) | 102.1 ± 2.4 |
| Recentin (CED, 30) | 99.8 ± 1.2 |
| Gilotrif (AFA, 40) | 100.8 ± 2.4 |
| Cabometyx (CAB, 40) | 101.2 ± 1.6 |
| Caprelsa (VAN, 300) | 100.4 ± 1.8 |
| Sprycel (DAS, 50) | 98.9 ± 2.1 |
| Lynparza (OLA, 150) | 100.5 ± 1.5 |
| LM-Ceritinib (CER, 50) | 101.2 ± 2.1 |
| LM-Linifanib (LIN, 25) | 99.6 ± 1.8 |
| LM-Tozasertib (TOZ, 100) | 101.8 ± 1.6 |
| Eco-Scale Score Parameters | Penalty Points (PPs) |
|---|---|
| Reagents/solvent | |
| Methanol | 6 |
| ∑ = 6 | |
| Instrument: Energy used (kWh per sample) | |
| Microplate reader | 0 |
| pH meter | 0 |
| Vortex mixer | 0 |
| Sonicator | 0 |
| Centrifuge | 0 |
| ∑ = 0 | |
| Occupational hazardous | |
| Analytical process hermetic | 0 |
| Emission of vapors and gases to the air | 0 |
| ∑ = 0 | |
| Waste | |
| Production (<1 mL (g) per sample) | 0 |
| Treatment (No treatment involved) | 3 |
| ∑ = 3 | |
| Total PPs | 9 |
| Total Eco-Scale score | 92 |
| Trade Name of Tablets | TKI | Strength (mg/Tablet) | Manufacturer (Address) |
|---|---|---|---|
| Inlyta | AXT | 50 | Pfizer (New York, NY USA) |
| Recentin | CED | 30 | AstraZeneca (Cambridge, UK) |
| Gilotrif | AFA | 40 | Boehringer Ingelheim (Ingelheim am Rhein, Germany) |
| Cabometyx | CAB | 40 | Exelixis, Inc. (Alameda, CA, USA) |
| Caprelsa | VAN | 300 | (AstraZeneca, Cambridge, UK) |
| Sprycel | DAS | 50 | (Bristol Myers Squibb, New York, NY, USA) |
| Lynparza | OLA | 150 | (AstraZeneca, Cambridge, UK)) |
| Ceritinib | CED | 50 | Lab-made: Prepared in the laboratory a |
| Linifanib | LIN | 25 | Lab-made: Prepared in the laboratory a |
| Tozasertib | TOZ | 100 | Lab-made: Prepared in the laboratory a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, I.A.; Alzoman, N.Z. Development of Green and High Throughput Microplate Reader-Assisted Universal Microwell Spectrophotometric Assay for Direct Determination of Tyrosine Kinase Inhibitors in Their Pharmaceutical Formulations Irrespective the Diversity of Their Chemical Structures. Molecules 2023, 28, 4049. https://doi.org/10.3390/molecules28104049
Darwish IA, Alzoman NZ. Development of Green and High Throughput Microplate Reader-Assisted Universal Microwell Spectrophotometric Assay for Direct Determination of Tyrosine Kinase Inhibitors in Their Pharmaceutical Formulations Irrespective the Diversity of Their Chemical Structures. Molecules. 2023; 28(10):4049. https://doi.org/10.3390/molecules28104049
Chicago/Turabian StyleDarwish, Ibrahim A., and Nourah Z. Alzoman. 2023. "Development of Green and High Throughput Microplate Reader-Assisted Universal Microwell Spectrophotometric Assay for Direct Determination of Tyrosine Kinase Inhibitors in Their Pharmaceutical Formulations Irrespective the Diversity of Their Chemical Structures" Molecules 28, no. 10: 4049. https://doi.org/10.3390/molecules28104049
APA StyleDarwish, I. A., & Alzoman, N. Z. (2023). Development of Green and High Throughput Microplate Reader-Assisted Universal Microwell Spectrophotometric Assay for Direct Determination of Tyrosine Kinase Inhibitors in Their Pharmaceutical Formulations Irrespective the Diversity of Their Chemical Structures. Molecules, 28(10), 4049. https://doi.org/10.3390/molecules28104049







