N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties
Abstract
1. Introduction
2. Results and Discussion
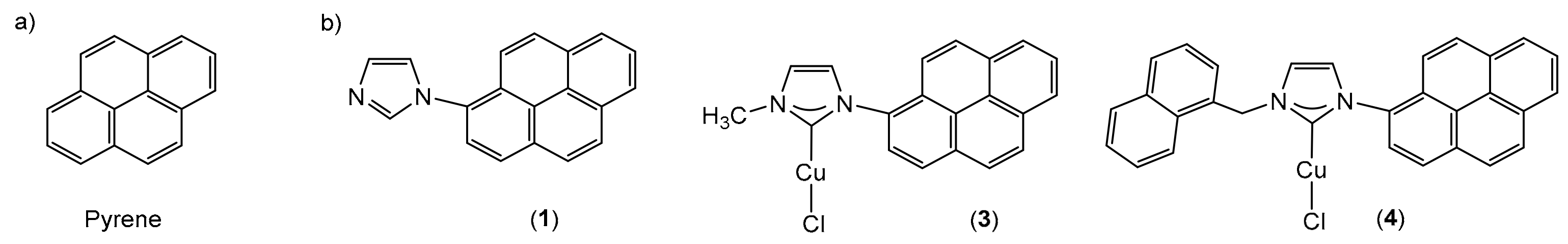
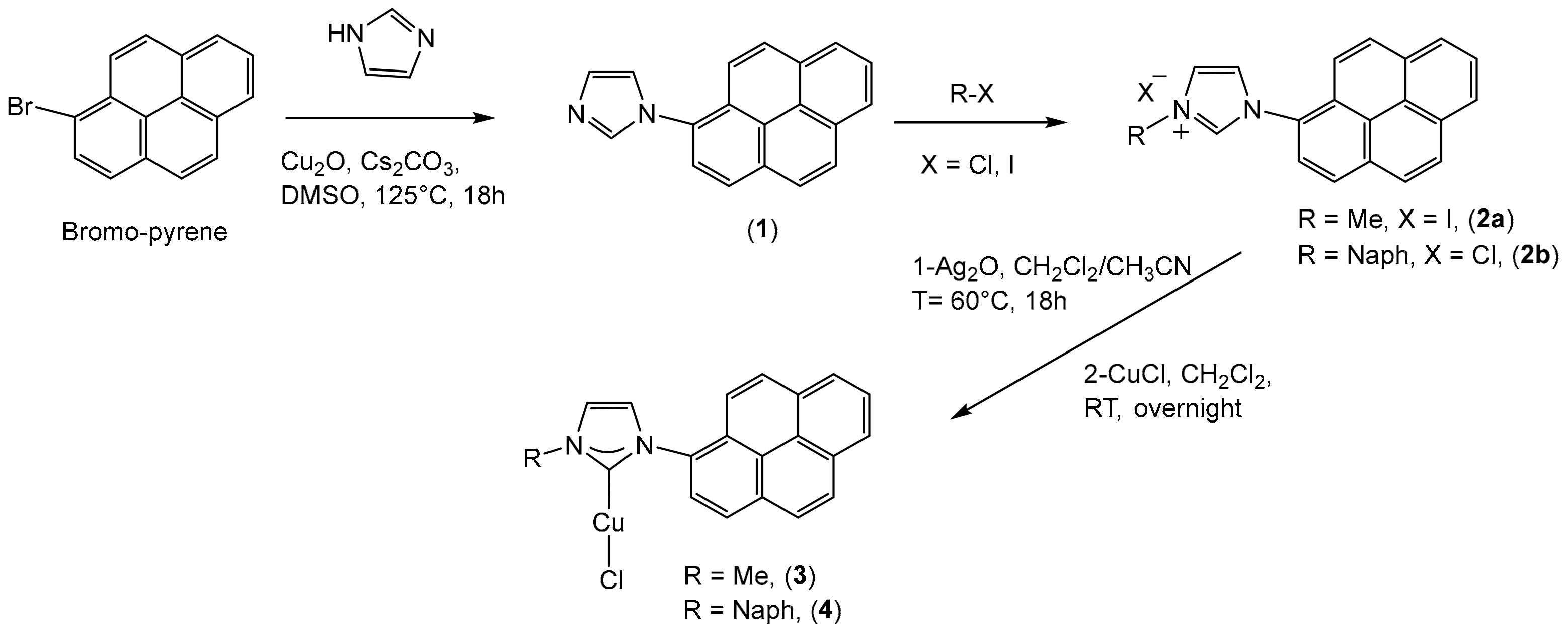
2.1. Synthesis and Characterization
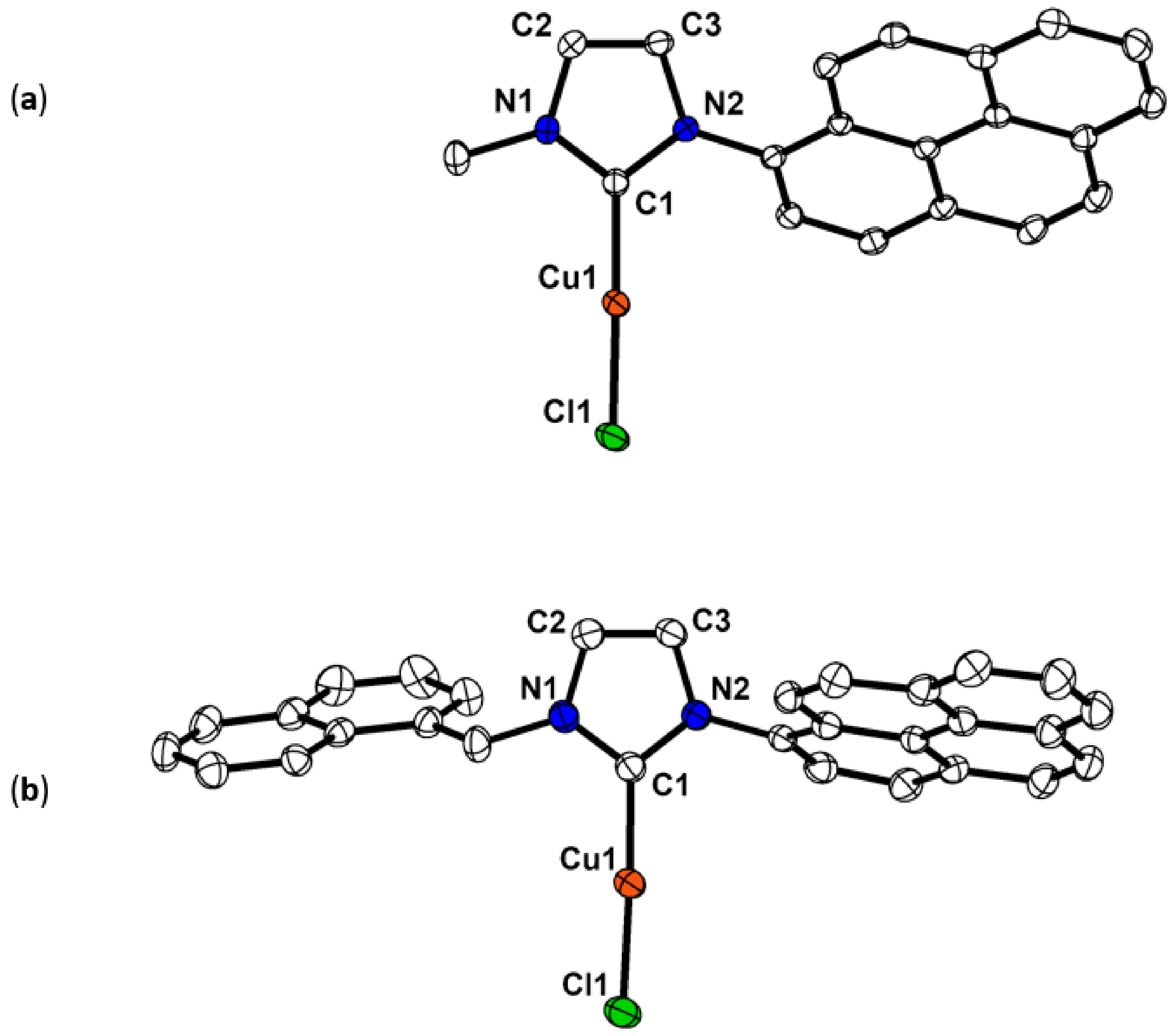
2.2. X-ray Molecular Structures of (Pyrenyl-NHC-R)-Cu-Cl, (R= Me, 3; R = Naph, 4)
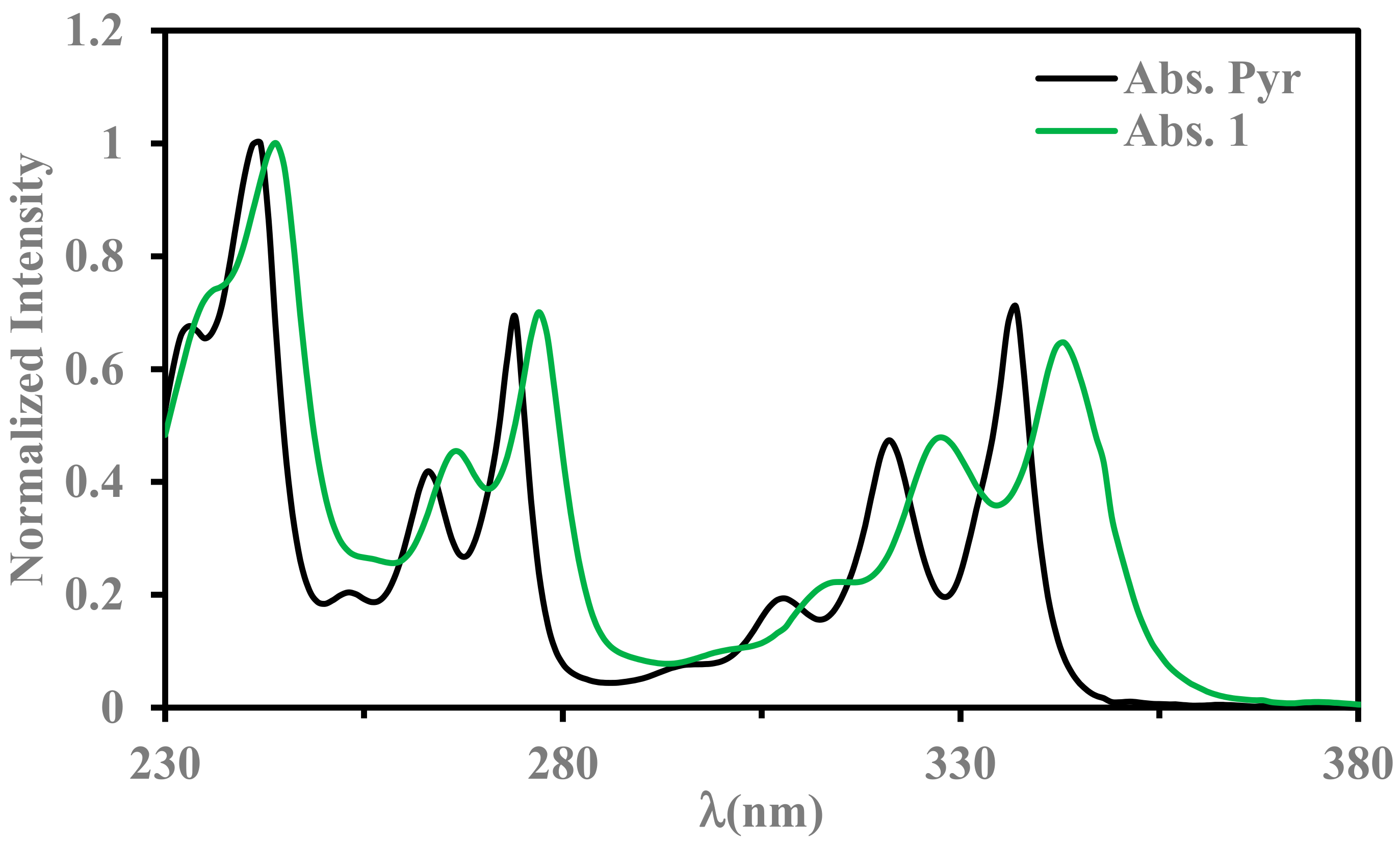
2.3. Absorption Properties
2.4. Luminescence Properties
3. Conclusions
4. Materials and Methods
- Synthesis of 1-(pyren-1-yl)-1H-imidazole (1)
- Synthesis of 3-methyl-1-(pyren-1-yl)-1H-imidazole-3-ium iodine (2a).
- Synthesis of 3-(naphthalen-1-ylmethyl)-1-(pyren-1-yl)-1H-imidazole-3-ium chloride (2b).
- General procedure for the preparation of Cu(I) N-heterocyclic carbene complexes (3, 4).
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; Wiley: Chichester, UK, 2017. [Google Scholar]
- Bourissou, D.; Guerret, O.; Gabbaie, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–91. [Google Scholar] [CrossRef]
- Herrmann, W.A. N-heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Neuditschko, B.; Zappe, K.; Schaier, M.; Gerner, M.C.; Schmetterer, K.G.; Del Favero, G.; Bonsignore, R.; Cichna-Markl, M.; Koellensperger, G.; et al. An Organometallic Gold(I) Bis-N-Heterocyclic Carbene Complex with Multimodal Activity in Ovarian Cancer Cells. Chem. Eur. J. 2020, 26, 15528–15537. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M.C. N-heterocyclic carbene metal complexes: Photoluminescence and applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Strassner, T. Phosphorescent Platinum(II) Complexes with CC Cyclometalated NHC Ligands. Acc. Chem. Res. 2016, 49, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Hossain, J.; Akhtar, R.; Khan, S. Luminescent coinage metal complexes of carbenes. Polyhedron 2021, 201, 115151–115187. [Google Scholar] [CrossRef]
- Amouri, H. Luminescent Complexes of Platinum, Iridium, and Coinage Metals Containing N-Heterocyclic Carbene Ligands: Design, Structural Diversity, and Photophysical Properties. Chem. Rev. 2023, 123, 230–270. [Google Scholar] [CrossRef] [PubMed]
- Groue, A.; Montier-Sorkine, E.; Cheng, Y.P.; Rager, M.N.; Jean, M.; Vanthuyne, N.; Crassous, J.; Lopez, A.C.; Moncada, A.S.; Barbieri, A.; et al. Enantiopure, luminescent, cyclometalated Ir(III) complexes with N-heterocyclic carbene-naphthalimide chromophore: Design, vibrational circular dichroism and TD-DFT calculations. Dalton Trans. 2022, 51, 2750–2759. [Google Scholar] [CrossRef]
- Lanoe, P.-H.; Chan, J.; Groue, A.; Gontard, G.; Jutand, A.; Rager, M.-N.; Armaroli, N.; Monti, F.; Barbieri, A.; Amouri, H. Cyclometalated N-heterocyclic carbene iridium(III) complexes with naphthalimide chromophores: A novel class of phosphorescent heteroleptic compounds. Dalton Trans. 2018, 47, 3440–3451. [Google Scholar] [CrossRef]
- Lanoe, P.-H.; Chan, J.; Gontard, G.; Monti, F.; Armaroli, N.; Barbieri, A.; Amouri, H. Deep-Red Phosphorescent Iridium(III) Complexes with Chromophoric N-Heterocyclic Carbene Ligands: Design, Photophysical Properties, and DFT Calculations. Eur. J. Inorg. Chem. 2016, 2016, 1631–1634. [Google Scholar] [CrossRef]
- Czerwieniec, R.; Leitl, M.J.; Homeier, H.H.H.; Yersin, H. Cu(I) complexes—Thermally activated delayed fluorescence. Photophysical approach and material design. Coord. Chem. Rev. 2016, 325, 2–28. [Google Scholar] [CrossRef]
- Leitl, M.J.; Zink, D.M.; Schinabeck, A.; Baumann, T.; Volz, D.; Yersin, H. Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties. Top. Curr. Chem. 2016, 374, 141–147. [Google Scholar] [CrossRef]
- Lanoe, P.-H.; Najjari, B.; Hallez, F.; Gontard, G.; Amouri, H. N-heterocyclic carbene coinage metal complexes containing naphthalimide chromophore: Design, structure, and photophysical properties. Inorganics 2017, 5, 58. [Google Scholar] [CrossRef]
- Romanov, A.S.; Di, D.; Yang, L.; Fernandez-Cestau, J.; Becker, C.R.; James, C.E.; Zhu, B.; Linnolahti, M.; Credgington, D.; Bochmann, M. Highly photoluminescent copper carbene complexes based on prompt rather than delayed fluorescence. Chem. Commun. 2016, 52, 6379–6382. [Google Scholar] [CrossRef]
- Gutierrez-Blanco, A.; Fernandez-Moreira, V.; Gimeno, M.C.; Peris, E.; Poyatos, M. Tetra-Au(I) Complexes Bearing a Pyrene Tetraalkynyl Connector Behave as Fluorescence Torches. Organometallics 2018, 37, 1795–1800. [Google Scholar] [CrossRef]
- Vogler, A. Luminescence of (NHC)Cu(I)Cl with NHC = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene under ambient conditions. UV phosphorescence in solution and in the solid state. Inorg. Chem. Commun. 2017, 84, 81–83. [Google Scholar]
- Gernert, M.; Muller, U.; Haehnel, M.; Pflaum, J.; Steffen, A. A Cyclic Alkyl(amino)carbene as Two-Atom pi-Chromophore Leading to the First Phosphorescent Linear Cu-I Complexes. Chem. Eur. J. 2017, 23, 2206–2216. [Google Scholar] [CrossRef]
- De Robillard, G.; Makni, O.; Cattey, H.; Andrieu, J.; Devillers, C.H. Towards sustainable synthesis of pyren-1-yl azoliums via electrochemical oxidative C-N coupling. Green Chem. 2015, 17, 4669–4679. [Google Scholar] [CrossRef]
- Pinto, A.; Echeverri, M.; Gomez-Lor, B.; Rodriguez, L. Highly emissive supramolecular gold(I)-BTD materials. Dalton Trans. 2022, 51, 8340–8349. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, E.S.; Kaczmarczyk, D.; Del Fre, S.; Favereau, L.; Caytan, E.; Cordier, M.; Vanthuyne, N.; Williams, J.A.G.; Srebro-Hooper, M.; Crassous, J. Helicenic N-heterocyclic carbene copper(I) complex displaying circularly polarized blue fluorescence. Dalton Trans. 2022, 51, 15571–15578. [Google Scholar] [CrossRef]
- Ren, X.; Wesolek, M.; Braunstein, P. Cu(I), Ag(I), Ni(II), Cr(III) and Ir(I) complexes with tritopic NimineCNHCNamine pincer ligands and catalytic ethylene oligomerization. Dalton Trans. 2019, 48, 12895–12909. [Google Scholar] [CrossRef]
- Liu, C.; Shen, H.-Q.; Chen, M.-W.; Zhou, Y.-G. C2-Symmetric Hindered “Sandwich” Chiral N-Heterocyclic Carbene Precursors and Their Transition Metal Complexes: Expedient Syntheses, Structural Authentication, and Catalytic Properties. Organometallics 2018, 37, 3756–3769. [Google Scholar] [CrossRef]
- Hermann, H.L.; Boche, G.; Schwerdtfeger, P. Metallophilic interactions in closed-shell copper(I) compounds—A theoretical study. Chem. Eur. J. 2001, 7, 5333–5342. [Google Scholar] [CrossRef]
- Harisomayajula, N.V.S.; Makovetskyi, S.; Tsai, Y.-C. Cuprophilic Interactions in and between Molecular Entities. Chem.-Eur. J. 2019, 25, 8936–8954. [Google Scholar] [CrossRef]
- Niko, Y.; Kawauchi, S.; Otsu, S.; Tokumaru, K.; Konishi, G.-i. Fluorescence Enhancement of Pyrene Chromophores Induced by Alkyl Groups through sigma-pi Conjugation: Systematic Synthesis of Primary, Secondary, and Tertiary Alkylated Pyrenes at the 1, 3, 6, and 8 Positions and Their Photophysical Properties. J. Org. Chem. 2013, 78, 3196–3207. [Google Scholar] [CrossRef]
- Sriyab, S.; Jorn-Iat, K.; Prompinit, P.; Wolschann, P.; Hannongbua, S.; Suramitr, S. Photophysical properties of 1-pyrene-based derivatives for nitroaromatic explosives detection: Experimental and theoretical studies. J. Lumin. 2018, 203, 492–499. [Google Scholar] [CrossRef]
- Chow, A.L.-F.; So, M.-H.; Lu, W.; Zhu, N.-Y.; Che, C.-M. Synthesis, Photophysical Properties, and Molecular Aggregation of Gold(I) Complexes Containing Carbon-Donor Ligands. Chem.-Asian J. 2011, 6, 544–553. [Google Scholar] [CrossRef]
- Birks, J.B. Excimers. Rep. Prog. Phys. 1975, 38, 903–974. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Q.; Gong, Y.; Liao, Q.; Song, G.; Li, Q.; Li, Z. Precise Regulation of Distance between Associated Pyrene Units and Control of Emission Energy and Kinetics in Solid State. CCS Chem. 2021, 3, 274–286. [Google Scholar] [CrossRef]

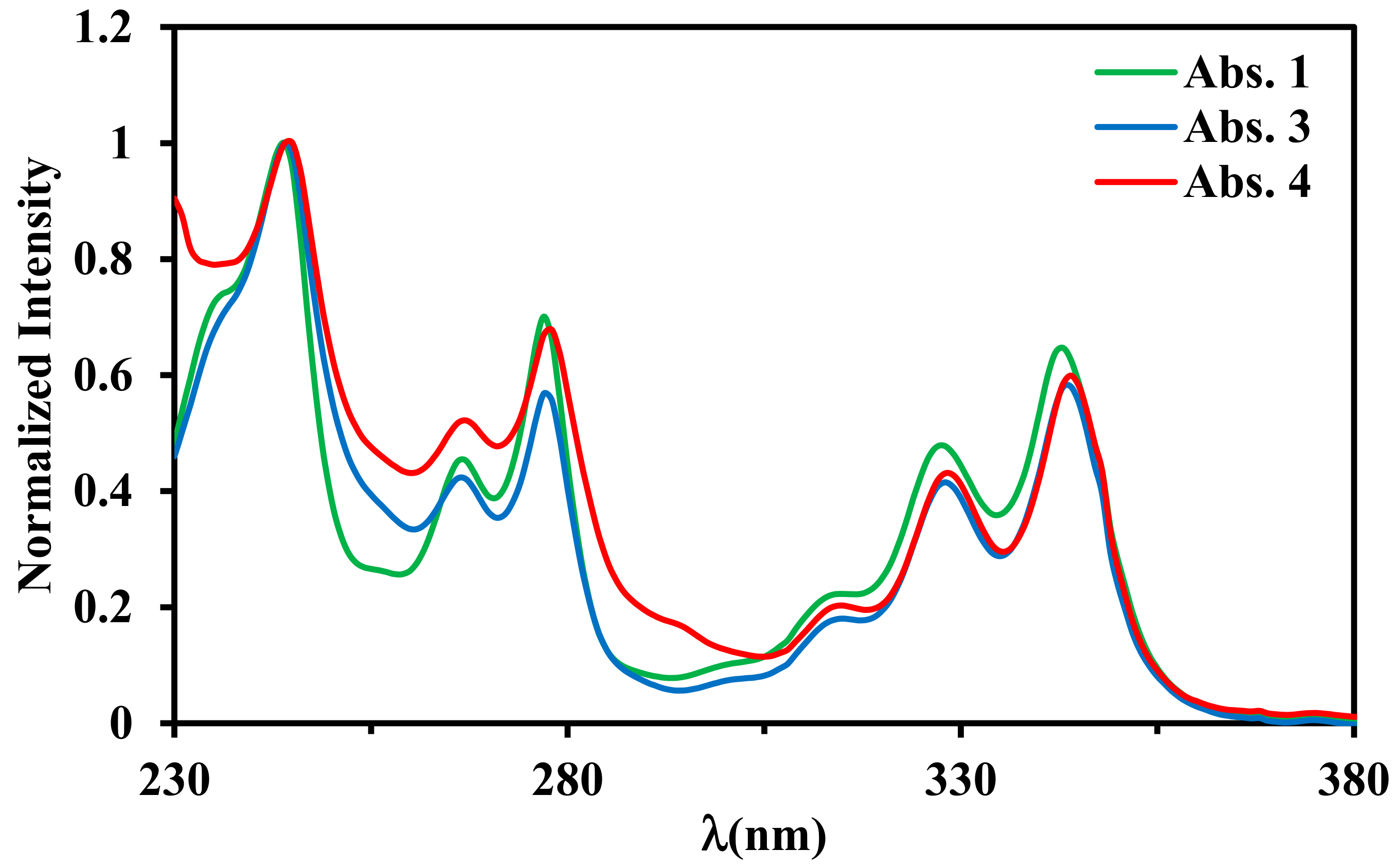
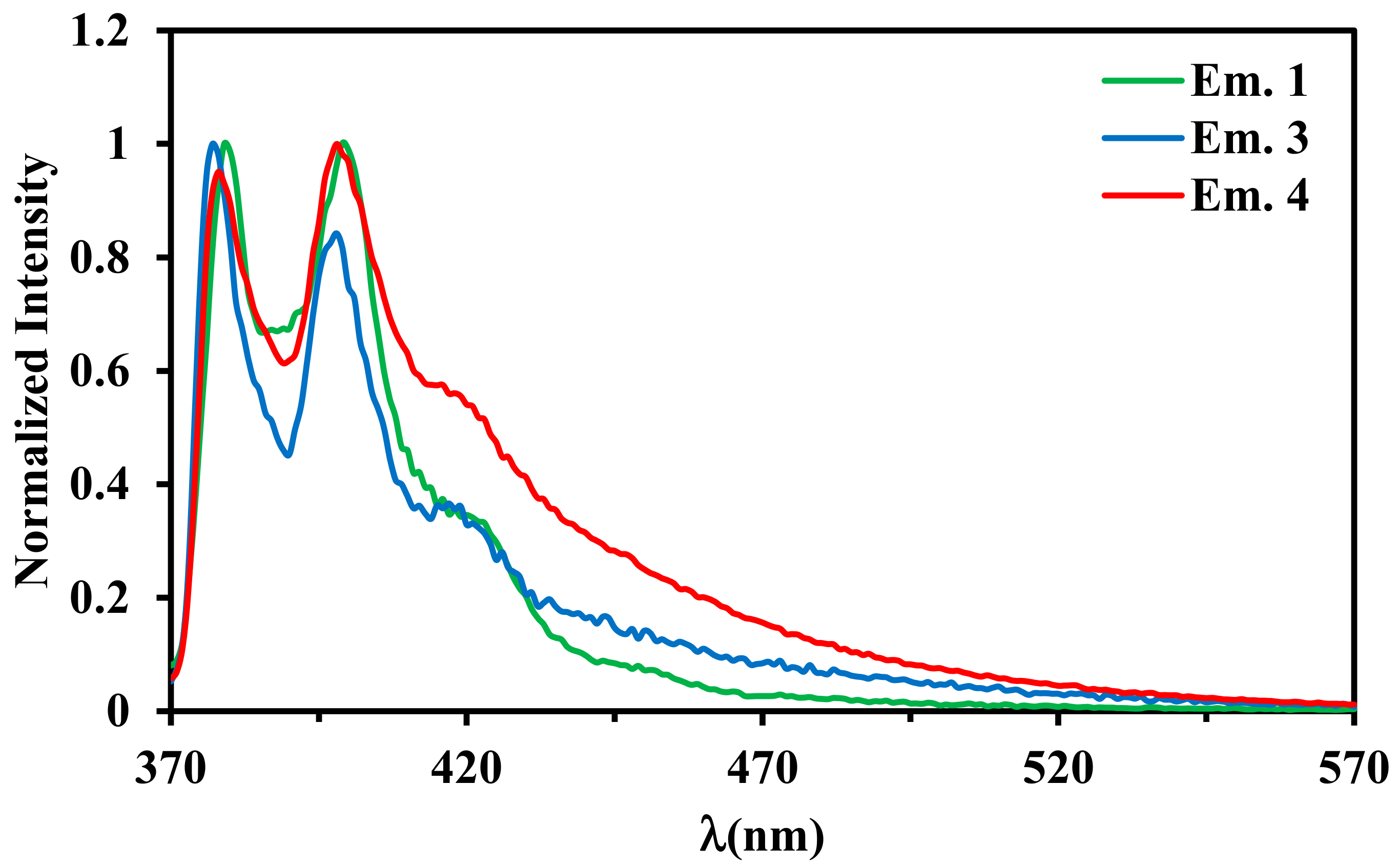
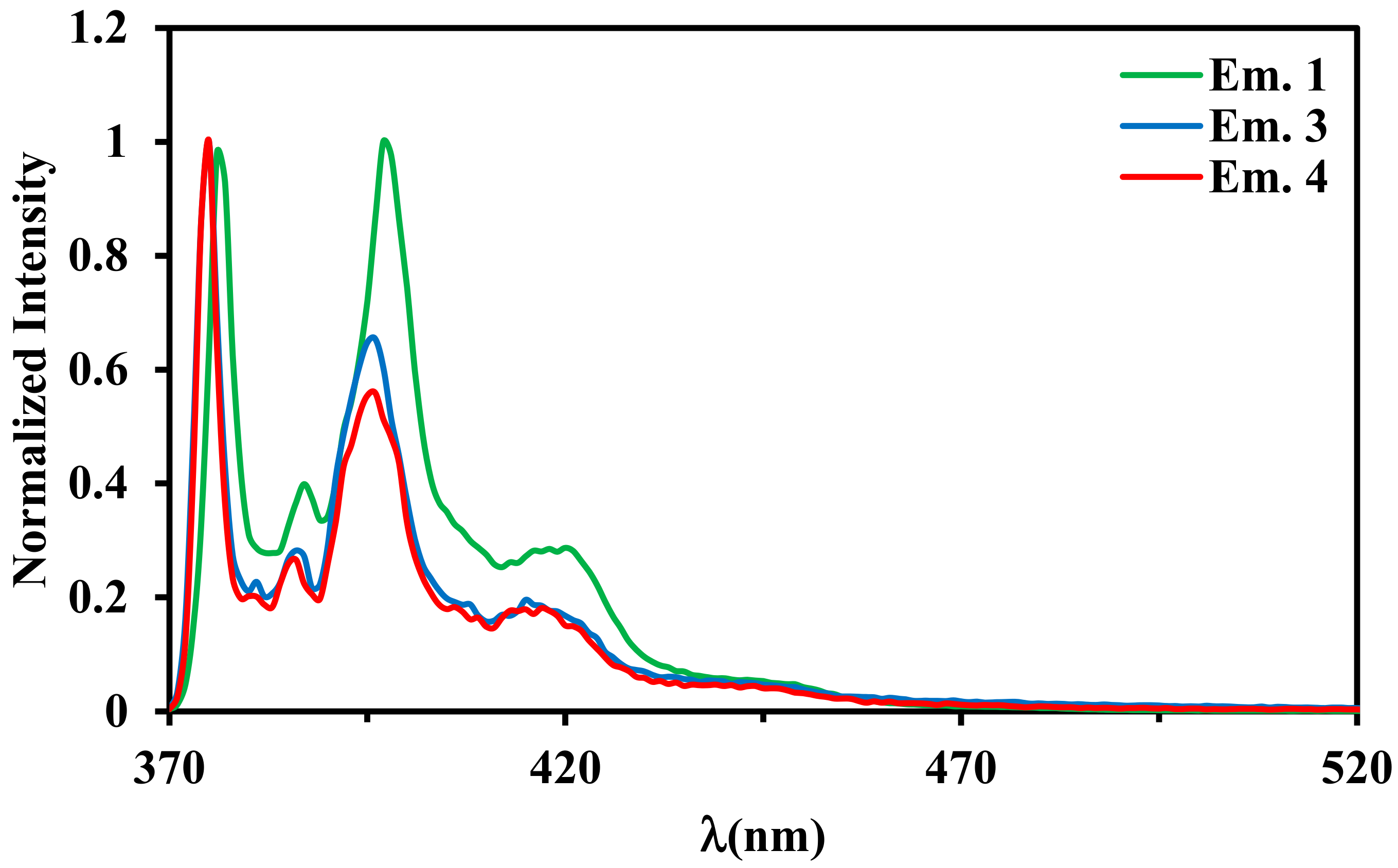
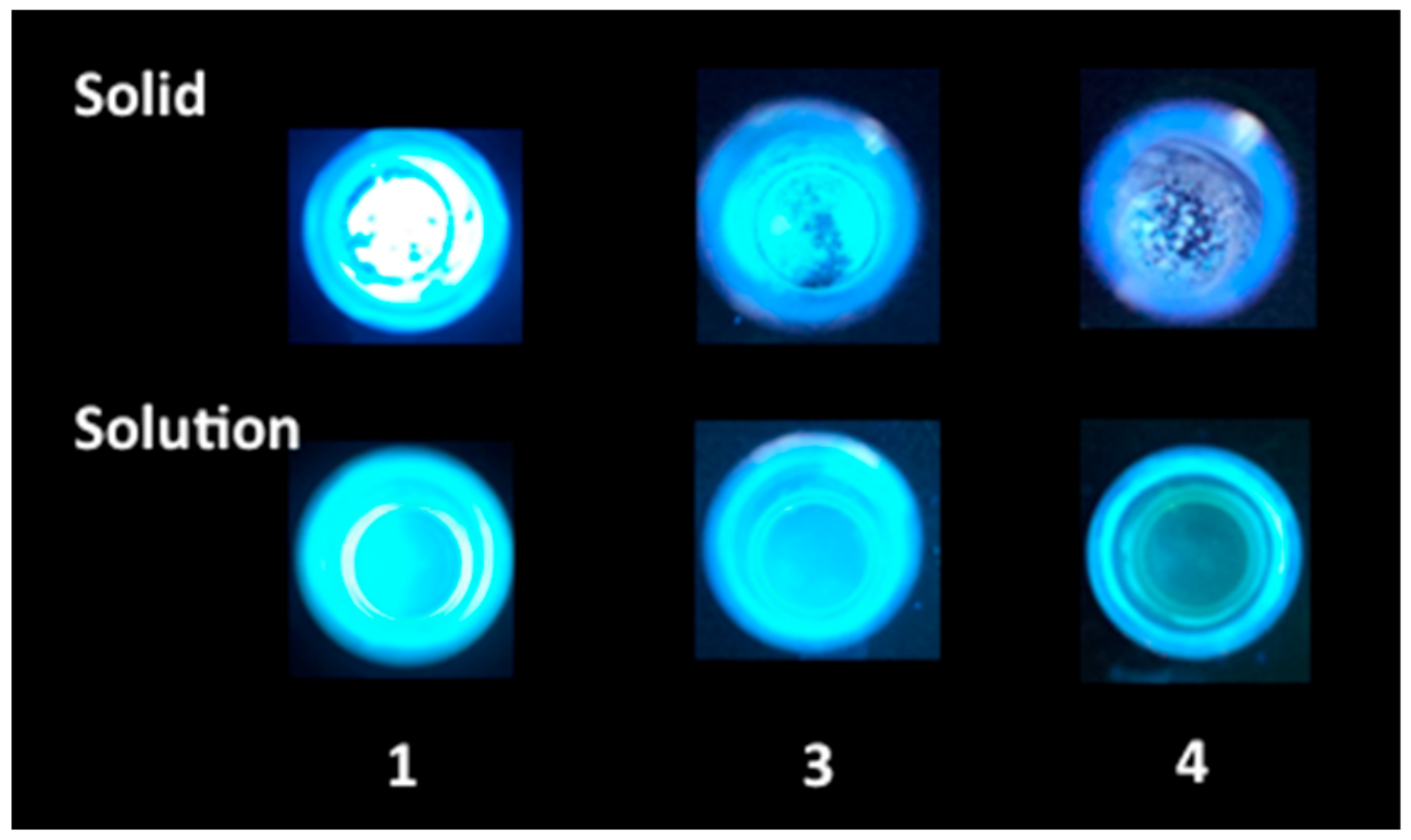
| λmax, nm (ε × 10−3 M−1 cm−1) a | |
|---|---|
| 1 | 235 sh (45), 244 (62.2), 267 (28.4),277 (43.6), 313 (13.5), 327 (30.1), 343 (40.6), 374 (0.6) |
| 3 | 236 sh (35), 244 (49.6), 267 (21.1), 277 (28.3), 314 (8.9), 328 (20.6), 344 (29.2), 375 (0.5) |
| 4 | 245 (52.4), 268 (27.2), 278 (35.4), 314 (10.8), 328 (22.5), 344 (21.2), 375 (1.1) |
| rt | 77 K | |||
|---|---|---|---|---|
| λmax, nm a | ϕ (%) a | τ, ns a | λmax, nm b | |
| 1 | 379 | 39 | 32.13 | 397 |
| 3 | 377 | 28 | 8.72 | 375 |
| 4 | 377 | 42 | 7.79 | 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Gontard, G.; Khatyr, A.; Knorr, M.; Amouri, H. N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties. Molecules 2023, 28, 4025. https://doi.org/10.3390/molecules28104025
Cheng Y, Gontard G, Khatyr A, Knorr M, Amouri H. N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties. Molecules. 2023; 28(10):4025. https://doi.org/10.3390/molecules28104025
Chicago/Turabian StyleCheng, Yaping, Geoffrey Gontard, Abderrahim Khatyr, Michael Knorr, and Hani Amouri. 2023. "N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties" Molecules 28, no. 10: 4025. https://doi.org/10.3390/molecules28104025
APA StyleCheng, Y., Gontard, G., Khatyr, A., Knorr, M., & Amouri, H. (2023). N-Heterocyclic Carbene Copper (I) Complexes Incorporating Pyrene Chromophore: Synthesis, Crystal Structure, and Luminescent Properties. Molecules, 28(10), 4025. https://doi.org/10.3390/molecules28104025








